Abstract
Background: The microscopic agglutination test (MAT) is commonly used for the diagnosis of canine leptospirosis. In dogs it is sometimes suggested that the serogroup with the highest MAT titer is the infecting serogroup; however, this is not true in humans with confirmed leptospirosis. We sought to investigate the value of MAT results in predicting the infecting serogroup by comparing results across several laboratories and within individual dogs over time.
Objectives: To examine the variability in MAT results across different laboratories in dogs recently vaccinated against leptospirosis, and in dogs with clinical leptospirosis, and to investigate variability over time in MAT results in individual dogs with leptospirosis.
Animals: Eighteen dogs from a research colony, 9 of which had been vaccinated against leptospirosis, and 17 dogs clinically diagnosed with leptospirosis.
Methods: Serum samples were submitted to up to 5 veterinary diagnostic laboratories for MAT titers from each dog on at least 1 occasion. MAT results also were followed over time in 6 dogs diagnosed with leptospirosis.
Results: MAT results were discordant across different laboratories in dogs recently vaccinated against leptospirosis and in dogs with clinical leptospirosis. MAT results varied over time in individual dogs with the disease.
Conclusions and Clinical Importance: The MAT is a valuable test for the diagnosis of leptospirosis in dogs, but it is unlikely that test results can be used to predict the infecting serogroup. Laboratories offering the MAT should consider participation in a proficiency scheme.
Keywords: Antibody, Diagnosis, Laboratory, Serology
Abbreviations:
- ILS
International Leptospirosis Society
- MAT
microscopic agglutination test
- PCR
polymerase chain reaction
- SPF
specific pathogen free
- VDL
veterinary diagnostic laboratory
Leptospirosis is an important zoonotic bacterial disease of dogs caused by the pathogenic species Leptospira interrogans and Leptospira kirschneri. 1 , 2 , 3 Antigenically related leptospires of these species are divided into numerous serovars, and related serovars are grouped into serogroups. 1 , 4 Leptospiral serovars are maintained in the environment by mammalian reservoir hosts that shed leptospires in the urine. Clinical disease may result when dogs are exposed to organisms by direct contact with infected urine, or indirect transmission may occur by contact with contaminated water or soil. Other less common forms of direct transmission include venereal or transplacental, and through bite wounds or ingestion of infected tissues. 1 The serovars most commonly reported to be associated with disease in dogs are Canicola, Icterohaemorrhagiae, Pomona, Bratislava, and Grippotyphosa. 1 , 5 The manifestations of leptospirosis in dogs range from subclinical to peracute disease, and infection can result in sudden death, acute renal failure, hepatic disease, vasculitis, or uveitis. 1 , 2 , 6 Bivalent bacterin vaccines containing inactivated serovars Icterohaemorrhagiae and Canicola were developed in the 1970s. 7 Over the past 10 years, vaccines containing the additional serovars Pomona and Grippotyphosa have been developed. 8
Definitive diagnosis of leptospirosis can be difficult. Confirmation of the diagnosis may be based on the results of serology, polymerase chain reaction (PCR)‐based assays, fluorescent antibody testing of urine or tissue samples, or organism isolation. 1 Serology does not distinguish between natural exposure and vaccination, although postvaccinal titers are usually low (≤1 : 400) and typically are present for <3 months. 1 , 5 , 8 , 9 , 10 A titer of ≥1 : 800 in a dog with compatible clinical signs and no history of vaccination against leptospirosis often is regarded as supportive of leptospirosis. 1 , 2 , 5 , 10 , 11 Because titers of 1 : 1600, or higher, can be detected in the early postvaccination period in some dogs vaccinated against leptospirosis, 8 a cut‐off of 1 : 1600 may be used to increase the specificity of a single high‐MAT titer for the diagnosis of leptospirosis, particularly if the vaccination history is unknown. A 4‐fold change in antibody titer over 2–4 weeks also can be used to confirm the diagnosis. 1 , 10 Fluorescent antibody testing of urine and culture of the organism are specific but insensitive. 10 Culture of the organisms is particularly challenging because leptospires require specific growth media, and are slow growing. 4 , 12 Conventional PCR testing of urine has been shown to be highly sensitive although somewhat less specific for the diagnosis of leptospirosis in dogs. 13 Real‐time, or quantitative, PCR is also increasingly used to detect pathogenic leptospires in the blood or urine of humans and dogs. 14 , 15 , 16 Other diagnostic tests, such as IgM antibody detection by ELISA, have been used in human medicine. 4
The reference method for the serologic diagnosis of leptospirosis is the microscopic agglutination test (MAT). 2 , 4 , 17 To perform this test, dilutions of the patient's serum are added to suspensions of live leptospiral serovars. The highest dilution of serum in which 50% agglutination of organisms occurs is reported as the titer. 17 The results of the MAT are thought to be serogroup specific. 4 Sera from dogs with leptospirosis commonly react with multiple serogroups, but the serogroup with the highest titer generally is assumed to be the cause of the infection. 1 , 2 , 5 , 18 , 19 , 20 Cross‐reaction is thought to primarily occur during the 1st 6 weeks of disease. 21 Paradoxical reactions have been described in people with leptospirosis, in which the highest initial titer develops against a noninfecting serogroup. 4 This finding is common in people, and can prevent identification of the infecting serogroup from the results of MAT titers alone. A study of culture‐positive humans with leptospirosis showed that the MAT correctly predicted the infecting serogroup in only 46% of cases. 22 When followed over time in infected people, cross‐reacting titers tend to decrease and the titer to the infecting serogroup usually predominates. 4 In addition, due to the subjective nature of interpretation of the MAT, variations in results among different laboratories and between different observers within the same laboratory may occur. 4 , 23 , 24
To our knowledge, the utility of the highest MAT titer in predicting the infecting serogroup in dogs has not been directly tested. Also, the variability of the MAT among laboratories or over time in individual dogs has not been investigated. The first objective of this study was to examine the variability in MAT results among different laboratories in dogs recently vaccinated against leptospirosis, and in dogs with clinical leptospirosis. The second objective of the study was to investigate variability over time in MAT results in individual dogs with leptospirosis.
Materials and Methods
Animals
The MAT was performed on serum from specific pathogen‐free (SPF) dogs, at either 4 or 5 veterinary diagnostic laboratories (VDLs A–E), [Link] , [Link] , [Link] , [Link] , [Link] depending on sample volume obtained. A subset of these dogs was vaccinated with a 4‐serovar (Canicola, Icterohaemorrhagiae, Pomona, Grippotyphosa) leptospirosis subunit vaccine. f The vaccine also contained an inactivated coronavirus fraction. Fourteen days after vaccination, a serum sample was submitted to 5 VDLs for the MAT.
Dogs that were suspected to have leptospirosis were prospectively enrolled in the study. An initial serum sample was collected from these dogs. A portion of the sample was submitted for the MAT at 1 VDL and the remaining serum (if available) was frozen for future analysis at multiple VDLs. Convalescent samples were obtained between 1 and 4 weeks after the initial sample and were frozen for future analysis at multiple VDLs.
The study protocol was approved by the Colorado State University Institutional Animal Care and Use Committee.
Sample Handling and Submission
For sample submission to the VDLs, standard laboratory submission forms were used and the laboratories were not alerted that a study was being performed. Serum samples were frozen and stored at −20°C, and were subsequently shipped overnight on cold packs for morning delivery.
Diagnostic Criteria
Diagnosis of leptospirosis for the purposes of this study required the presence of typical clinical signs (lethargy, anorexia, vomiting, fever, abdominal pain) and laboratory abnormalities consistent with leptospirosis (azotemia, increased liver enzyme activities, hyperbilirubinemia, thrombocytopenia), with no other diagnosis obtained for these signs, together with one of the following: a 4‐fold change in MAT titers from at least 1 VDL, or a single MAT titer ≥1 : 1600 from at least 1 VDL, with no history of vaccination against leptospirosis. 2 A titer of 1 : 1600 was used as the cut‐off in order to increase the specificity of a single titer for diagnosis of leptospirosis. Serum was sent to either 4 or 5 VDLs for the MAT, depending on sample volume obtained. Fourteen dogs had initial serum samples submitted to multiple VDLs. Twelve dogs had convalescent samples submitted to multiple VDLs. Six dogs had MAT titers followed at 5 VDLs over time with at least 3 samples analyzed over a period of >30 days.
Serogroups Evaluated
For all dogs in this study, titers were requested for the serogroups Bratislava, Canicola, Grippotyphosa, Hardjo, Icterohaemorrhagiae, Pomona, and Autumnalis. The MAT for serogroup Hardjo was not performed at VDL E, and the MAT for the serogroup Autumnalis was not performed at VDLs A and B. For each dog's sample, the serogroup with the highest MAT titer was recorded for each VDL. When >1 serogroup was recorded as having the highest titer, these results were considered to be “tied.” Tied results were considered to be different between VDLs if at least 1 of the serogroups was different. For example, a highest and equal reported titer to both Grippotyphosa and Autumnalis serogroups at 1 VDL was regarded as different from a highest and equal reported titer to both Grippotyphosa and Pomona serogroups at another VDL. Titers were reported as negative if <1 : 100 at VDLs A, B, D, and E, and if <1 : 50 at VDL C.
Calculation of Agreement
Agreement between the laboratories was expressed as a percentage agreement with 95% confidence limits. These values were calculated from the number of samples in which the serogroup with the highest titer was the same at all VDLs, expressed as a percentage of the total number of samples tested. These calculations were performed separately for vaccinates, initial titers, and convalescent titers. Confidence limits were calculated with an online calculator (http://faculty.vassar.edu/lowry/prop1.html, accessed October 7, 2010). Serogroups Autumnalis and Hardjo were excluded from the calculations because these were not reported in the MAT results from all VDLs in the study. The acute titers from dog 4 were removed from the analysis, because all titers initially were negative in this patient, and convalescent titers from dog 9 were removed because results were only available from 3 VDLs. Similarly, all results from VDL E were removed from the calculations, because titers were not performed at this laboratory in all patients.
Results
MAT titers were performed on 18 SPF dogs. Seventeen of the 18 SPF dogs had negative prevaccination MAT titers at all VDLs (<1 : 100 at VDLs A, B, D, and E, and <1 : 50 at VDL C). One SPF dog had a MAT titer of 1 : 200 against serogroup Pomona at VDL C. Nine SPF dogs were vaccinated, and all 9 developed positive titers at all VDLs. Positive titers ranged from 1 : 50 to 1 : 6400. Seven dogs had a highest titer of ≥1 : 800 from at least 1 VDL and 3 dogs had a highest titer ≥1 : 800 at all 5 VDLs. Seven vaccinated dogs developed a highest titer to a nonvaccinal serogroup from at least 1 VDL. In all 9 vaccinated dogs, the identity of the serogroup with the highest titer differed between VDLs. The serogroups with the highest titer for each vaccinated dog at each VDL are summarized in Table 1. After exclusion of incomplete data sets, there was agreement between all VDLs in 1 of 9 samples, giving a percentage agreement of 11% (95% confidence interval: 2–41%) for the samples from the vaccinated dogs. The serogroup in which there was agreement among the 4 VDLs included in the calculation was Icterohaemorrhagiae.
Table 1.
Number of SPF dogs with the highest MAT titer to each tested serogroup at 5 different VDLs (A–E), 14 days after vaccination with a 4‐serovar leptospira vaccine.
| VDL | A | B | C | D | E | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serogroup | Total Number of Dogs | Dog ID | Total Number of Dogs | Dog ID | Total Number of Dogs | Dog ID | Total Number of Dogs | Dog ID | Total Number of Dogs | Dog ID |
| Br | 2 | a, b | 0 | 1 | a | 0 | 1 | c | ||
| Ha | 0 | 0 | 0 | 0 | 0 | |||||
| Ic | 1 | h | 6 | a, b, c, g, h, i | 3 | e, h, i | 0 | 1 | i | |
| Ca | 1 | i | 0 | 0 | 0 | 0 | ||||
| Gr | 1 | g | 0 | 0 | 0 | 0 | ||||
| Po | 0 | 0 | 0 | 0 | 0 | |||||
| Au | 0 | 0 | 1 | c | 4 | a, c, e, f | 4 | a, b, d, h | ||
| Mult | 4 | c, d, e, f | 3 | d, e, f | 4 | b, d, f, g | 5 | b, d, g, h, i | 3 | e, f, g |
| Neg | 0 | 0 | 0 | 0 | 0 | |||||
Br, Bratislava; Ha, Hardjo; Ic, Icterohaemorrhagiae; Ca, Canicola; Gr, Grippotyphosa; Po, Pomona; Au, Autumnalis; Mult, highest titers to multiple serovars; Neg, negative titer (<1 : 100 at VDLs A, B, D, and E, and <1 : 50 at VDL C); SPF, specific pathogen free; VDL, veterinary diagnostic laboratory; MAT, microscopic agglutination test.
Dog ID: identification code for each individual dog. Data represent a total of 9 dogs.
Seventeen dogs with leptospirosis had MAT titers performed at multiple VDLs. Three dogs (dogs 3, 9, and 16) had initial MAT titers performed at only 1 VDL. Fourteen dogs with leptospirosis had initial samples submitted to multiple VDLs for the MAT. The serogroups with the highest titer for each of these 14 dogs at each VDL are summarized in Table 2. In 2 of 14 dogs the serogroup with the highest MAT titers was the same at all 4 VDLs tested (dogs 5 and 10). In 1 dog the initial MAT titer was negative at 4 of 5 VDLs (dog 4). For the remaining 11 dogs, at least 1 VDL had discordant results, in which the identity of the serogroup with the highest titer differed between VDLs. Specifically, in 2 dogs there was no agreement among any of the VDLs (dogs 11 and 14), in 4 dogs there was agreement between 2 VDLs (dogs 1, 7, 15, and 17), in 3 dogs there was agreement among 3 VDLs (dogs 2, 8, and 12), and in 1 dog there was agreement among 4 of 5 VDLs (dog 6). For dog 13, VDLs A and B reported highest titers to serogroups Grippotyphosa and Pomona whereas VDLs C and D reported highest titers to serogroups Grippotyphosa and Autumnalis.
Table 2.
Number of dogs with leptospirosis with the highest MAT titer to each tested serogroup from initial samples submitted to multiple VDLs (A–E).
| VDL | A | B | C | D | E | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serogroup | Total Number of Dogs | Dog ID | Total Number of Dogs | Dog ID | Total Number of Dogs | Dog ID | Total Number of Dogs | Dog ID | Total Number of Dogs | Dog ID |
| Br | 0 | 1 | 1 | 1 | 8 | 1 | 8 | 1 | 8 | |
| Ha | 0 | 0 | 0 | 0 | 0 | |||||
| Ic | 0 | 0 | 0 | 0 | 0 | |||||
| Ca | 1 | 2 | 1 | 2 | 1 | 2 | 0 | 0 | ||
| Gr | 3 | 5, 6, 10 | 5 | 5, 6, 10, 12, 15 | 5 | 5, 6, 10, 12, 17 | 5 | 1, 5, 6, 10, 12 | 3 | 1, 11, 13 |
| Po | 2 | 1, 7 | 0 | 0 | 1 | 7 | 0 | |||
| Au | 0 | 0 | 0 | 0 | 3 | 4, 6, 7 | ||||
| Mult | 5 | 12, 13, 14, 15, 17 | 6 | 7, 8, 11, 13, 14, 17 | 6 | 1, 7, 11, 13, 14, 15 | 6 | 2, 11, 13, 14, 15, 17 | 0 | |
| Neg | 3 | 4, 8, 11 | 1 | 4 | 1 | 4 | 1 | 4 | 0 | |
| NA | 0 | 0 | 0 | 0 | 7 | 2, 5, 10, 12, 14, 15, 17 | ||||
Br, Bratislava; Ha, Hardjo; Ic, Icterohaemorrhagiae; Ca, Canicola; Gr, Grippotyphosa; Po, Pomona; Au, Autumnalis; Mult, highest titers to multiple serovars; Neg, negative titer (<1 : 100 at VDLs A, B, D, and E, and <1 : 50 at VDL C); NA, analysis not performed; VDL, veterinary diagnostic laboratory; MAT, microscopic agglutination test.
Dog ID: identification number for each individual dog. Data represents a total of 14 dogs.
After exclusion of incomplete data sets, there was agreement among all VDLs in 4 of 13 samples, giving a percentage agreement of 31% (95% confidence interval, 13–58%) for the initial samples. The serogroups in which there was agreement among the 4 VDLs included in the calculation were Grippotyphosa (in 3 cases) and Canicola (in a single case).
Twelve dogs with leptospirosis had convalescent samples submitted to multiple VDLs for the MAT. The serogroups with the highest titer at each VDL in these dogs are summarized in Table 3. In 3 of the 12 dogs, the serogroup with the highest MAT titer was the same at all VDLs tested (dog 8: 5 VDLs; dog 9: 3 VDLs; dog 12: 4 VDLs). Nine of the 12 dogs had discordant results, in which the identity of the serogroup with the highest titer differed among VDLs. Specifically, in 3 dogs there was no agreement among any of the VDLs (dogs 4, 7, and 16). In 2 dogs there was agreement between 2 VDLs (dogs 3 and 14), in 3 dogs there was agreement among 3 VDLs (dogs 2, 11, and 17), and in 1 dog there was agreement among 4 of 5 VDLs (dog 10).
Table 3.
Number of dogs with leptospirosis with the highest MAT titer to each tested serogroup from convalescent samples submitted to multiple VDLs (A–E).
| VDL | A | B | C | D | E | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serogroup | Total Number of Dogs | Dog ID | Total Number of Dogs | Dog ID | Total Number of Dogs | Dog ID | Total Number of Dogs | Dog ID | Total Number of Dogs | Dog ID |
| Br | 2 | 7, 8 | 1 | 8 | 2 | 4, 8 | 2 | 8, 11 | 1 | 8 |
| Ha | 0 | 0 | 0 | 0 | 0 | |||||
| Ic | 0 | 1 | 3 | 0 | 0 | 0 | ||||
| Ca | 1 | 3 | 0 | 0 | 0 | 0 | ||||
| Gr | 6 | 2, 9, 10, 11, 12, 17 | 3 | 9, 12, 17 | 9 | 2, 3, 9, 10, 11, 12, 14, 16, 17 | 4 | 2, 10, 12, 14 | 2 | 10, 11 |
| Po | 1 | 16 | 4 | 4, 10, 11, 14 | 0 | 0 | 0 | |||
| Au | 0 | 0 | 0 | 2 | 3, 16 | 3 | 2, 3, 4 | |||
| Mult | 2 | 4, 14 | 3 | 2, 7, 16 | 1 | 7 | 3 | 4, 7, 17 | 2 | 7, 14 |
| Neg | 0 | 0 | 0 | 0 | 0 | |||||
| NA | 0 | 0 | 0 | 1 | 9 | 4 | 9, 12, 16, 17 | |||
Br, Bratislava; Ha, Hardjo; Ic, Icterohaemorrhagiae; Ca, Canicola; Gr, Grippotyphosa; Po, Pomona; Au, Autumnalis; Mult, highest titers to multiple serovars; Neg, negative titer (<1 : 100 at VDLs A, B, D, and E, and <1 : 50 at VDL C); NA, analysis not performed; VDL, veterinary diagnostic laboratory; MAT, microscopic agglutination test.
Dog ID, identification number for each individual dog. Data represents a total of 12 dogs.
After exclusion of incomplete data sets, there was agreement among all VDLs in 3 of 11 samples, giving a percentage agreement of 27% (95% confidence interval, 10–56%) for the convalescent samples. The serogroups in which there was agreement among the 4 VDLs included in the calculation were Grippotyphosa (in 2 cases) and Bratislava (in a single case).
Six dogs (dogs 3, 4, 7, 8, 10, and 11) with leptospirosis had MAT titers followed at multiple VDLs over time. In dog 10, the serogroup with the highest titer changed over time at 1 of 5 VDLs. In dog 8, the serogroup with the highest titer changed over time at 2 of 5 VDLs. In dogs 3, 4, and 11, the serogroup with the highest titer changed over time at 3 of 5 VDLs. In dog 7, the serogroup with the highest titer changed over time at 5 of 5 VDLs. The MAT results for dog 11 at VDL D are illustrated in Figure 1. Initially, MAT titers in this dog were weakly positive to 2 serogroups. At day 15, the highest titer was to serogroup Bratislava. However, at day 35, the highest titer was to serogroup Autumnalis. In this dog, the presumptive infecting serogroup would change depending on the time after infection at which the MAT was performed.
Figure 1.
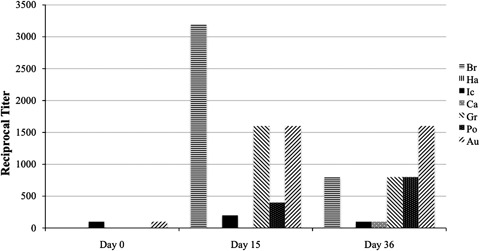
Change in MAT titers over time from dog 11 at VDL D. Br, Bratislava; Ha, Hardjo; Ic, Icterohaemorrhagiae; Ca, Canicola, Gr, Grippotyphosa; Po, Pomona; Au, Autumnalis; MAT, microscopic agglutination test.
Discussion
The results of this study demonstrate that MAT results are discordant among different laboratories in dogs recently vaccinated against leptospirosis and in dogs with clinical leptospirosis. The percentage agreement among the VDLs was as low as 11% for the vaccinated dogs, and was only 31% for initial titers from the clinical cases, and 27% for the results from the convalescent samples. In addition, the data show that MAT results vary over time in individual dogs with the disease. These observations suggest that MAT titers cannot be relied upon to predict the infecting serogroup in dogs with leptospirosis.
Dogs in this study that were vaccinated with a 4‐serovar subunit vaccine developed positive MAT titers at all VDLs. These dogs frequently developed the highest titers to nonvaccinal serogroups. This finding has been reported in a previous study in which dogs vaccinated with a subunit vaccine containing serovars Pomona and Grippotyphosa developed the highest MAT titers to serogroup Autumnalis. 8 In our study, we also identified vaccinated dogs that developed highest titers to the nonvaccinal serogroup Bratislava. MAT titers against serogroup Bratislava were not evaluated in the previous study. 8 Our study provides further evidence that leptospirosis subunit vaccines may produce immune responses that are not serogroup specific when measured by the MAT. Another potential explanation is contamination of the antigens in the MAT test with additional serogroups, but this is unlikely because highest titers were found to nonvaccinal serogroups at multiple VDLs. It is also possible that the vaccine used in the study was contaminated with antigens from serogroups Autumnalis and Bratislava, but this is also unlikely given the requirements for vaccine manufacturers to frequently submit type cultures to federal agencies for confirmatory analysis. 8
In dogs with leptospirosis, the serogroup with the highest titer from the MAT has been assumed to represent the infecting serogroup. 2 , 5 , 18 , 19 , 25 , 26 In a study of 4 dogs experimentally infected with serovar Grippotyphosa, all dogs developed antibodies to the infecting serovar, and 2 developed serologic cross reactions with serogroups Pomona and Bratislava; however, the magnitude of the titers was not reported. 27 Interpretation of the MAT is plagued by cross‐reaction among serogroups. In people, the cross‐reactions are more common in acute phase samples. Cross‐reactions occur because the MAT detects both IgG and IgM antibodies and because leptospires have several common antigens. 4 In addition, paradoxical reactions, where the highest titer develops against a noninfecting serogroup, occur commonly in people, and the MAT has been shown to predict the infecting serogroup in only 46% of people with leptospirosis. 22
In 29.7% of the initial sample MATs and in 20% of the convalescent sample MATs, tied highest titers to multiple serogroups were found. This relatively high percentage of tied results from the MAT also confounds interpretation of the potential infecting serogroup. For the purposes of this study, when >1 serogroup was recorded as having the highest titer, these were considered to be different among VDLs if at least one of the serogroups was different. This approach was chosen because most clinicians will only submit titers to a single VDL. If the results report that the highest titer is against >1 serogroup, it is usually assumed that the infecting serogroup is one of the ones with the highest titer, but it cannot be definitively identified. Thus, a result of the same and highest titers to serogroups Grippotyphosa and Autumnalis could be ascribed to infection with serogroups Grippotyphosa or Autumnalis, whereas a result of the same and highest titers to serogroups Grippotyphosa and Pomona could result from infection with serogroups Grippotyphosa or Pomona. When looking at results from multiple VDLs, as in this study, a different interpretation may be made. For example, for the initial MAT results in dog 14, Grippotyphosa was one of the tied highest serogroups at all of the VDLs to which samples were submitted. Thus, it might be concluded that Grippotyphosa was the infecting serogroup in this case. However, there is no published evidence to support this assumption.
Despite the phenomena of cross‐reactivity, paradoxical reactions, and equality of high titers, the MAT is a valuable test for the diagnosis of leptospirosis in dogs. It is relatively inexpensive and widely available, and a 4‐fold change in MAT titer can be used confirm infection in dogs with signs consistent with leptospirosis. 1 When managing individual patients that are suspected to have leptospirosis, confirmation of the identity of the infecting serogroup does not affect the treatment plan, and it should not influence the necessity to protect against transmission of this zoonotic disease to other animals or humans. Reliable identification of the infecting serovar in dogs with leptospirosis is most likely to be important for epidemiologic surveys, control of wildlife hosts, and vaccine planning. It has been suggested that the identity of the infecting serovar may influence prognosis in individual dogs, but these data are difficult to interpret because they were based on MAT results, and not on serovar identification by bacterial culture and molecular typing. 25
In this study, we demonstrate that the MAT performed at different laboratories in the same dog with leptospirosis can yield different results. In addition, we found that the serogroup with the highest titer identified by the MAT can change over time in individual dogs with leptospirosis. These findings challenge the suggestion that the serogroup with the highest MAT titer is the infecting serogroup. Studies involving leptospiral culture and molecular typing of isolates from dogs with leptospirosis are necessary to definitively determine the infecting serogroup and examine how these results compare with the MAT titers.
Interpretation of MAT results is subjective, and this may contribute to the variation in results among laboratories noted in the present study. 4 , 24 Although simple in principle, the MAT is a complex test that relies on the maintenance of live cultures of several leptospiral serovars. 4 These cultures may be subject to cross‐contamination, misidentification, strain switching, or deterioration over time. 24 , 28 Thus, quality control is important when considering the results of the MAT from individual laboratories. 23 , 28 The use of proficiency schemes can have a positive impact on the performance of the MAT in diagnostic laboratories. 24 One such proficiency scheme is provided by the International Leptospirosis Society (ILS) (http://www.med.monash.edu.au/microbiology/staff/adler/proftemp.html, accessed October 9, 2010). We did not investigate the quality control measures employed in the VDLs used in the present study. However, participation in proficiency schemes such as that provided by the ILS should be considered by all laboratories that perform the MAT.
The present study has several potential limitations. None of the dogs had culture‐confirmed leptospirosis. Culture of leptospires from infected dogs is difficult and rarely performed in clinical patients. 5 Thus, we chose to confirm leptospirosis based on criteria frequently used in veterinary medicine. 1 , 2 , 5 , 10 The absence of culture data in the dogs in our study also precludes definitive identification of the infecting serogroup in these patients. However, the differences in the serogroup with the highest MAT titer between laboratories and in individual dogs over time cast further doubt on the utility of the MAT results in predicting the infecting serogroup in dogs.
We chose to test for the most common serogroups of leptospirosis that are believed to infect dogs in North America. The 7 serogroups selected were those most commonly evaluated in serological studies of canine leptospirosis, 29 , 30 although testing for certain serogroups was not readily available at all VDLs used in the present study. Some of the dogs in the study may have been infected with serogroups that were not tested for, which could also confound the interpretation of the results of the MAT.
In conclusion, this study demonstrates that there is variation in the results of the MAT in individual dogs when performed at different laboratories. In addition, the identity of the serogroup with the highest MAT titer in dogs with leptospirosis frequently changes over time. Future studies comparing isolation and typing of leptospires with MAT results in infected dogs are needed to determine the frequency with which MAT results predict the infecting serogroup in these patients.
Footnotes
aColorado State University Veterinary Diagnostic Laboratory, Fort Collins, CO
bKansas State Veterinary Diagnostic Laboratory, Manhattan, KS
cMichigan State University Diagnostic Center for Population and Animal Health, Lansing, MI
dCornell University Animal Health Diagnostic Center, Ithaca, NY
eIDEXX Laboratories Inc, Westbrook, ME
fCvK/4L, Fort Dodge Laboratories Inc, Fort Dodge, IA
Acknowledgments
The authors are grateful to M. Gill for providing the samples from SPF dogs, and to S. Rao for discussions regarding quantitation of the data. This study was supported by grants from the College Research Council of the College of Veterinary Medicine and Biomedical Sciences at Colorado State University, and from Fort Dodge Animal Health, Overland Park, KS.
This work was performed at Colorado State University. Preliminary tests of this study were presented at the 25th Annual American College of Veterinary Internal Medicine Forum, Seattle, WA, 2007.
References
- 1. Greene CE, Sykes JE, Brown CA, Hartman K. Leptospirosis In: Greene CE, ed. Infectious Diseases of the Dog and Cat, 3 ed St Louis, MO: Saunders Elsevier; 2006:402–417. [Google Scholar]
- 2. Langston CE, Heuter KJ. Leptospirosis: A re-emerging zoonotic disease. Vet Clin North Am Small Anim Pract 2003;33:791–807. [DOI] [PubMed] [Google Scholar]
- 3. Alton DA, Berke O, Reid‐Smith R, et al Increase in seroprevalence of canine leptospirosis and its risk factors, Ontario 1998–2006. Can J Vet Res 2009;73:167–175. [PMC free article] [PubMed] [Google Scholar]
- 4. Levett PN. Leptospirosis. Clin Microbiol Rev 2001;14:296–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bolin CA. Diagnosis of leptospirosis: A reemerging disease of companion animals. Sem Vet Med Surg 1996;11:166–171. [DOI] [PubMed] [Google Scholar]
- 6. Townsend WM, Stiles J, Krohne SG. Leptospirosis and panuveitis in a dog. Vet Ophth 2006;9:169–173. [DOI] [PubMed] [Google Scholar]
- 7. Jull DJ, Heath KR. The evaluation of a combined L. canicola and L. icterohaemorrhagiae vaccine on hamsters and dogs. J Small Anim Pract 1960;1:245–258. [Google Scholar]
- 8. Barr SC, McDonough PL, Scipioni‐Ball RL, Starr JK. Serologic response of dogs given a commercial vaccine against Leptospira interrogans serovar Pomona and Leptospira kirschneri serovar Grippotyphosa. Am J Vet Res 2005;66:1780–1784. [DOI] [PubMed] [Google Scholar]
- 9. van de Maele I, Claus A, Haesebrouck F, Daminet S. Leptospirosis in dogs: A review with emphasis on clinical aspects. Vet Rec 2008;163:409–413. [DOI] [PubMed] [Google Scholar]
- 10. Harkin KR. Leptospirosis In: Bonagura JD, Twedt DC, eds. Kirks Current Veterinary Therapy XIV. St Louis, MO: Saunders Elsevier; 2009:1237–1240. [Google Scholar]
- 11. Sessions JK, Greene CE. Canine leptospirosis: Epidemiology, pathogenesis, and diagnosis. Compend Contin Educ Pract Vet 2004;26:606–623. [Google Scholar]
- 12. Bharti AR, Nally JE, Ricaldi JN, et al Leptospirosis: A zoonotic disease of global importance. Lancet Infect Dis 2003;3:757–771. [DOI] [PubMed] [Google Scholar]
- 13. Harkin KR, Roshto YM, Sullivan JT. Clinical application of a polymerase chain reaction assay for diagnosis of leptospirosis in dogs. J Am Vet Med Assoc 2003;222:1224–1229. [DOI] [PubMed] [Google Scholar]
- 14. Smythe LD, Smith IL, Smith GA, et al A quantitative PCR (TaqMan) assay for pathogenic Leptospira spp. BMC Infect Dis 2002;2:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stoddard RA, Gee JE, Wilkins PP, et al Detection of pathogenic Leptospira spp through TaqMan polymerase reaction targeting the LipL32 gene. Diagn Microbiol Infect Dis 2009;64:247–255. [DOI] [PubMed] [Google Scholar]
- 16. Rojas P, Monahah AM, Schuller S, et al Detection and quantification of leptospires in urine of dogs: A maintenance host for the zoonotic disease leptospirosis. Eur J Clin Microbiol Infect Dis 2010;29:1305–1309. [DOI] [PubMed] [Google Scholar]
- 17. Cole JR, Ellinghausen HC, Rubin HL. Laboratory diagnosis of leptospirosis in domestic animals. Proc Annu Meet US Anim Health Assoc 1979;83:189–195. [PubMed] [Google Scholar]
- 18. Mastrorilli C, Dondi F, Agnoli C, et al Clinicopathological features and outcome predictors of Leptospira interrogans Australis serogroup infection in dogs: A retrospective study of 20 cases (2001–2004). J Vet Intern Med 2007;21:3–10. [DOI] [PubMed] [Google Scholar]
- 19. Ghneim GS, Viers JH, Chomel BB, et al Use of a case–control study and geographic information systems to determine environmental and demographic risk factors for canine leptospirosis. Vet Res 2007;38:37–50. [DOI] [PubMed] [Google Scholar]
- 20. Stokes JE, Kaneene JB, Schall WD, et al Prevalence of serum antibodies against six Leptospira serovars in healthy dogs. J Am Vet Med Assoc 2007;230:1657–1664. [DOI] [PubMed] [Google Scholar]
- 21. Ross LA, Rentko V Leptospirosis In: Bonagura JD, ed Kirks Current Veterinary Therapy XIII. Philadelphia, PA: WB Saunders; 2000:308–310. [Google Scholar]
- 22. Levett PN. Usefulness of serologic analysis as a predictor of the infecting serovar in patients with severe leptospirosis. Clin Infect Dis 2003;36:447–452. [DOI] [PubMed] [Google Scholar]
- 23. Ellinghausen HC. Laboratory practices involving the leptospiral microscopic agglutination microtiter test. Proc Annu Meet US Anim Health Assoc 1979;83:163–179. [PubMed] [Google Scholar]
- 24. Chappel RJ, Goris M, Palmer MF, Hartskeerl RA. Impact of proficiency testing on results of the microscopic agglutination test for diagnosis of leptospirosis. J Clin Microbiol 2004;42:5484–5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goldstein RE, Lin RC, Langston CE, et al Influence of infecting serogroup on clinical features of leptospirosis in dogs. J Vet Intern Med 2006;20:489–494. [DOI] [PubMed] [Google Scholar]
- 26. Geisen V, Stengel C, Brem S, et al Canine leptospirosis infections—clinical signs and outcome with different suspected Leptospira serogroups (42 cases). J Small Anim Pract 2007;48:324–328. [DOI] [PubMed] [Google Scholar]
- 27. Brown CA, Roberts AW, Miller MA, et al Leptospira interrogans serovar Grippotyphosa infection in dogs. J Am Vet Med Assoc 1996;209:1265–1267. [PubMed] [Google Scholar]
- 28. Cerqueira GM, McBride AJA, Queiroz A, et al Monitoring Leptospira strain collections: The need for quality control. Am J Trop Med Hyg 2010;82:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moore GE, Guptill LF, Glickman NW, et al Canine leptospirosis, United States. 2002–2004. Emerg Infect Dis 2006;12:501–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gautam R, Wu C‐C, Guptill LK, et al Detection of antibodies against Leptospira serovars via microscopic agglutination tests in dogs in the United States, 2000–2007. J Am Vet Med Assoc 2010;237:293–298. [DOI] [PubMed] [Google Scholar]


