Abstract
Respiratory infections are very common in Kuwait, yet little is known about the cause of severe lower respiratory tract infections. This study was designed to investigate the viral cause of lower respiratory tract infections using sensitive molecular methods. PCR was applied to investigate 10 respiratory viruses in respiratory samples from 1,014 patients aged between 3 days to 76 years with acute lower respiratory tract infections. Of the 1,014 patients with lower respiratory tract infections, 288 (28.4%) had a viral infection. One hundred fifty‐five (53.8%) presented with bronchiolitis, 100 (43.7%) with pneumonia, and 33 (11.5%) with croup. One hundred six (36.8%) and 99 (34.4%) patients had evidence of respiratory syncytial virus and human rhinoviruses infections, respectively. Adenoviruses were detected in 44 (15.2%) patients, while influenza A virus in 21 (7.3%) patients. The majority of respiratory syncytial virus infections (84%) were among patients aged <1 year. Similarly, of the 99 patients infected by human rhinoviruses, 50 (50.5%) were also among this age group. In contrast, most of influenza A virus infections, 12 of 21 (57.1%), were among patients aged over 16 years. Parainfluenza virus‐2 and human coronaviruses were not detected in any of the patients' samples. Over the 3‐year period, most of the hospitalized patients were seen during the autumn and winter months from October through March. These data show that respiratory syncytial virus and human rhinoviruses may be the major causes of lower respiratory tract infections in children admitted to hospital in Kuwait. J. Med. Virol. 82:1462–1467, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: viral infections, PCR, respiratory infections
INTRODUCTION
Respiratory infections are a major cause of morbidity and mortality in developing countries especially in infants, children, and the elderly [Gwatkin, 1980; Leowski, 1987; Campbell, 1995; Hwang et al., 2007]. In industrialized countries, acute respiratory infections cause enormous direct and indirect healthcare costs, in addition to being a major cause of morbidity. They can also be an important cause of mortality among children, the elderly, and immunocompromised patients [Glezen et al., 2000; Barsaoui et al., 2001; Bianco et al., 2002]. The causative agents of acute respiratory infections encompass a wide variety of microorganisms. Viral causes may be responsible for more than 35% of lower respiratory tract infections [Barsaoui et al., 2001; Siritantikorn et al., 2002]. Respiratory syncytial virus, followed by parainfluenza, and influenza viruses are the most common viruses to cause lower respiratory tract infections [Shann, 1986; Puthavathana et al., 1990; Siritantikorn et al., 2002]. Indeed, respiratory syncytial virus may be responsible for more than 78% of lower respiratory tract infections among children [Barsaoui et al., 2001; Carballal et al., 2001; Hall et al., 2001; Zambon et al., 2001; Siritantikorn et al., 2002].
In developing countries, respiratory syncytial virus was shown to be responsible for up to 40% of cases with pneumonia and/or bronchiolitis [Barsaoui et al., 2001; Siritantikorn et al., 2002]. Influenza viruses may also be an important cause of lower respiratory tract infections in both children and the elderly, but with a shorter duration of illness [Zambon et al., 2001]. Adenoviruses, generally, are quite notorious in causing outbreaks among military recruits [Sanchez et al., 2001] but have also been reported in children <5 years of age [Larranaga et al., 2000]. Although human rhinoviruses are the most common upper respiratory pathogens, inducing the majority of common colds worldwide, recent evidence has suggested an association between human rhinoviruses with asthma exacerbations, including severe episodes requiring hospitalization. This indicates that human rhinovirus infections can result in serious disease [Miller et al., 2007; Louie et al., 2009]. Current evidence supports the notion that human rhinoviruses infect the lower airways, inducing a local inflammatory response [Papadopoulos et al., 2000]. Such evidence suggests that the role of human rhinoviruses in other lower respiratory diseases, such as pneumonia, bronchitis, bronchiolitis, and cystic fibrosis, should be re‐examined with more sensitive molecular techniques.
Very few studies have been conducted to document the role of viral infections and lower respiratory tract disease in the Gulf region despite the fact that acute respiratory disease and pneumonia are very common in countries like Kuwait [Hijazi et al., 1997, 1996]. Previous studies have shown that the molecular techniques based on PCR were far more sensitive and reliable than conventional virus isolation in tissue culture or viral antigen detection by immunofluoresence tests [Wendt, 1997; Macek et al., 1999; Weigl et al., 2000; Drosten et al., 2003; Ksiazek et al., 2003; Mackay et al., 2003; Rota et al., 2003; Simpson et al., 2003]. In this study sensitive and reliable PCR techniques were used to investigate the role of 10 different respiratory viruses in causing serious lower respiratory tract infections in patients admitted to hospital in Kuwait.
MATERIALS AND METHODS
Study Population
The study included both children and adult patients (n = 1,014) suffering from lower respiratory tract infections. The WHO criteria for lower respiratory tract infections in children were used [WHO, 2003]. Furthermore, the American Thoracic Society criteria were used for lower respiratory tract infections in adults [American Thoracic Society, 2001, 2005]. Patients were admitted to Mubarak Al‐Kabir Hospital, Kuwait. The specimens included nasopharyngeal aspirate, nasopharyngeal swabs, bronchoalveolar lavage, and tracheal aspirate. They were collected in the hospital and processed at the Virology Unit, Faculty of Medicine, Kuwait University for the presence of viral nucleic acid using PCR techniques. The study population included 542 male and 472 female patients. Of the 1,014 patients with lower respiratory tract infections, 155 (53.8%) presented with bronchiolitis, 100 (34.7%) with pneumonia, and 33 (11.5%) with croup. The age of the patients ranged between 3 days and 76 years. The majority of samples were collected during autumn and winter. Autumn in Kuwait lies between September and November and Winter lies between December and March.
RNA and DNA Isolation
Nasopharyngeal aspirate, bronchoalveolar lavage, and tracheal aspirate were processed directly for nucleic acid isolation; as for the nasopharyngeal swab, Tris–EDTA buffer was added before carrying the nucleic acid isolation procedure. Nucleic acid was extracted from the reference strains and from the clinical specimens using QIAamp mini kit (Qiagen Ltd. West Sussex, UK) according to manufacturer's instructions and stored at −80°C if they were not processed in the same day.
Duplex (dPCR) and Triplex PCR (tPCR)
Upon extraction of the RNA from clinical specimens, determination of the presence of viral nucleic acid was done. Three PCR formats were designed in this study. Duplex PCR (dPCR) was designed to detect influenza A and B viruses. Primers used in dPCR were targeting genes for non‐structural proteins of influenza A virus (from nucleotides 467 to 656) and influenza B (from nucleotides 732 to 977) [Grondahl et al., 1999]. Triplex PCR (tPCR1) contained three other primer sets, which targeted the fusion protein gene of respiratory syncytial virus (from nucleotides 6227 to 6466), the hemagglutinin–neuraminidase gene of parainfluenza virus‐1 (from nucleotides 7372 to 7551), and the fusion protein gene of parainfluenza virus‐3 (from nucleotides 5073 to 4907) [Grondahl et al., 1999]. The third PCR was also a tPCR (tPCR2), and was designed to detect human rhinoviruses, human coronavirus‐229E, and human coronavirus‐OC43. tPCR2 contained three other primer sets which targeted the VP4 protein of human rhinoviruses (from nucleotides 452 to 566) [Pitkäranta et al., 1997], and N protein of human coronavirus‐299E, human coronavirus‐OC43 (nucleotides 762–1219 and 655–1025, respectively) [Vabret et al., 2001].
Single PCR
Single PCR was used to detect adenoviruses and parainfluenza‐2 [Echevarría et al., 1998; Grondahl et al., 1999]. Adenovirus primers targeted genes for hexon protein of adenoviruses (from nucleotides 2755 to 2888) [Grondahl et al., 1999] and the hemagglutinin–neuraminidase gene of parainfluenza virus‐2 (from nucleotides 803 to 822) [Echevarría et al., 1998] were not incorporated in the dPCR or tPCR as it affected the sensitivity and specificity of the test (data not shown). Adenovirus and parainfluenza virus‐2 primers were therefore used in a single PCR mix and RT‐PCR mix, respectively.
PCR and RT‐PCR Conditions
The RT step was performed for 60 min at 37°C in 10‐µl reaction volume containing 1× Gene Amp RNA PCR buffer, 5 µl of 25 mM MgCl2, 1 mM of each deoxynucleoside triphosphates, 2.5 µM of random hexamers, 0.5 µl RNAse inhibitor, 3 µl of viral nucleic acid, and 2.5 U/µl of reverse transcriptase enzyme (Gene Amp RNA Core Kit; Applied Biosystems, Chicago, IL). Following heat inactivation of the reverse transcriptase at 90°C for 5 min, the entire reaction mixture was used for PCR in a total volume of 50 µl. The reaction mix composition was 2 mM MgCl2 solution, 1× PCR buffer (0.02 pg) of each forward and reverse primer, and 0.05 µl of 5 U/µl Ampli Taq DNA Pol. PCR was performed as follows: 94°C denaturation for 1 min followed by 40 cycles of denaturation at 94°C for 30 sec, annealing at 50°C for 30 sec, elongation at 72°C for 30 sec, and final extension at 72°C for 7 min. Water was used, instead of nucleic acid, as a negative control. ATCC reference virus strains of adenoviruses (VR‐4), influenza A (VR‐544), influenza B (VR‐523), parainfluenza 1 (VR‐105), parainfluenza 2 (VR‐92), parainfluenza 3 (VR‐93), respiratory syncytial virus (VR‐26), coronavirus type 229E (VR‐740), and coronavirus type OC43 (VR‐759), and rhinovirus type 14 (VR‐284) were included as positive controls to validate each test.
Confirmatory Tests
In order to confirm the result of the PCR product, a hybridization technique was performed as previously described by Grondahl et al. 1999.
Testing PCR Specificity
The specificity of PCR was established for each PCR format using a panel of ATCC reference viruses to check for cross reactivity.
RESULTS
Of the 1,014 patients investigated, 28.4% had evidence of a virus infection. All positive samples were confirmed by hybridization (Table I). Respiratory syncytial virus was detected in 36.8%, human rhinoviruses in 34.4%, adenoviruses in 15.2%, and influenza A virus in 7.3% in samples from these patients. The incidence of parainfluenza virus‐1, influenza B, and parainfluenza virus‐3 infections were low and accounted for 3.1%, 2.1%, and 1% of these patients, respectively. On the other hand, human coronavirus‐229E, human coronavirus‐OC43, and parainfluenza virus‐2 were not detected among any of the samples from patients investigated.
Table I.
Viruses Detected in Patients With Lower Respiratory Tract Viral Infections in Kuwait
| Type of viral infection | Positivity rate, n (%) |
|---|---|
| Respiratory syncytial virus | 106 (36.8) |
| Human rhinoviruses | 99 (34.4) |
| Influenza A virus | 21 (7.3) |
| Adenoviruses | 44 (15.2) |
| Influenza B virus | 6 (2.1) |
| Parainfluenza virus‐1 | 9 (3.1) |
| Parainfluenza virus‐3 | 3 (1.0) |
| Parainfluenza virus‐2 | 0 |
| Human coronavirus‐229E and ‐OC43 | 0 |
| Total positives | 288 (28.4) |
| Total negatives | 726 (71.6) |
One hundred sixty‐eight of the 288 (58.3%) patients with lower respiratory tract infections were aged <1 year (Table II). Of these, respiratory syncytial virus was detected in 53%, human rhinoviruses in 30%, and adenoviruses in 10% of these infants. The number of males with lower respiratory tract infections in this group (<1 year) was slightly higher than females, with a M/F ratio of 1.5:1. The M/F ratios of patients from 1 to 5 years of age also were higher in males than females (1.4:1). The majority (57.1%) of influenza virus infections were among those older than 16 years of age (Table II). The male‐to‐female ratio in this age group of patients (≥16 years) was slightly lower than the younger patient group (M/F ratio of 1.2:1). Overall, the majority of lower respiratory tract infections (74.6%) were among children <5 years of age. Table III shows the clinical syndromes and the etiological agents. The majority of the 69.7% patients who presented with croup and 30.3% with bronchiolitis had respiratory syncytial virus in their samples. On the other hand, 36.8% and 42% of those presenting with bronchiolitis and pneumonia had human rhinoviruses in their samples, respectively.
Table II.
Distribution of Patients With Lower Respiratory Tract Infection in Relation to Age and Causative Agent
| Type of virus | <1 year, n (%) | 1–5 years, n (%) | 5–16 years, n (%) | >16 years, n (%) | Total |
|---|---|---|---|---|---|
| Respiratory syncytial virus | 89 (52.9) | 13 (27.6) | 1 (7.7) | 3 (5) | 106 |
| Human rhinoviruses | 50 (29.7) | 17 (36.1) | 5 (38.5) | 27 (45) | 99 |
| Influenza A virus | 4 (2.3) | 3 (6.4) | 2 (15.4) | 12 (20) | 21 |
| Adenoviruses | 17 (10) | 11 (23.4) | 3 (23.1) | 13 (21.7) | 44 |
| Influenza B virus | 1 (0.6) | 3 (6.4) | 1 (7.7) | 1 (1.6) | 6 |
| Parainfluenza virus‐1 | 4 (2.3) | — | 1 (7.7) | 4 (6.7) | 9 |
| Parainfluenza virus‐3 | 3 (1.8) | — | — | — | 3 |
| M/F ratioa | 1.5:1 | 1.4:1 | 1.2:1 | 1:1 | 1.3:1 |
| Total positives | 168 (58.3%) | 47 (16.3%) | 13 (4.5%) | 60 (20.8%) | 288 |
Male to female ratio.
Table III.
Distribution of Patients With Lower Respiratory Tract Infection in Relation to Symptom Presentation
| Type of virus | Croup, n (%) | Bronchiolitis, n (%) | Pneumonia, n (%) |
|---|---|---|---|
| Respiratory syncytial virus (n = 106) | 23 (69.7) | 47 (30.3) | 36 (36) |
| Human rhinoviruses (n = 99) | — | 57 (36.8) | 42 (42) |
| Influenza A virus (n = 21) | 2 (6.1) | 12 (7.7) | 7 (7) |
| Adenoviruses (n = 44) | — | 32 (20.7) | 12 (12) |
| Influenza B virus (n = 6) | 2 (6.1) | 3 (1.9) | 1 (1) |
| Parainfluenza virus‐1 (n = 9) | 4 (12.1) | 3 (1.9) | 2 (2) |
| Parainfluenza virus‐3 (n = 3) | 2 (6.1) | 1 (0.7) | — |
| Total positives (n = 288) | 33 (11.5) | 155 (53.8) | 100 (34.7) |
Table IV shows the distribution of viruses detected in relation to the type of sample collected from the patients. One hundred twenty‐one (42%) of the viruses were detected in nasopharyngeal aspirate samples while 29.2% were detected in nasopharyngeal swabs, 23.6% in bronchoalveolar lavages, and 5.2% in tracheal aspirates.
Table IV.
Distribution of Detected Viruses in Relation to the Type of Specimen Collected
| Type of virus | Nasopharyngeal swab, n (%) | Nasopharyngeal aspirate, n (%) | Bronchoalveolar lavage, n (%) | Tracheal aspirate, n (%) |
|---|---|---|---|---|
| Respiratory syncytial virus (n = 106) | 33 (31.1) | 47 (44.3) | 20 (18.9) | 6 (5.7) |
| Human rhinoviruses (n = 99) | 29 (29.3) | 45 (45.5) | 21 (21.2) | 4 (4.0) |
| Influenza A virus (n = 21) | 4 (19) | 5 (23.8) | 10 (47.6) | 2 (9.5) |
| Adenoviruses (n = 44) | 13 (29.5) | 16 (36.4) | 13 (29.5) | 2 (4.5) |
| Influenza B virus (n = 6) | 2 (33.3) | 2 (33.3) | 2 (33.3) | — |
| Parainfluenza virus‐1 (n = 9) | 2 (22.2) | 4 (44.4) | 2 (22.2) | 1 (11.1) |
| Parainfluenza virus‐3 (n = 3) | 1 (33.3) | 2 (66.6) | — | — |
| Total positives (n = 288) | 84 (29.2) | 121 (42.0) | 68 (23.6) | 15 (5.2) |
Figure 1 shows that the majority of the patients with viral lower respiratory tract infections over the 3‐year period of the study were hospitalized during autumn and winter (October to March) with a peak incidence during December and January.
Figure 1.
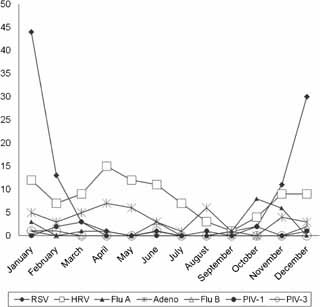
Seasonal distribution of respiratory viruses in patients with lower respiratory tract infections in Kuwait.
DISCUSSION
This is the first study in this region that investigated the role of 10 different respiratory viruses in lower respiratory tract infections in hospitalized patients using sensitive molecular methods over a 3‐year period. Therefore, data from this study would be of interest both locally and internationally.
In this study, 74.6% of the patients were under the age of 5 years, 58.3% were less than 1 year of age. This age distribution is similar to that reported by other studies from elsewhere [Denny and Clyde, 1983; Al‐Hajjar et al., 1998; Farshad et al., 2008]. Male patients under the age of 5 years in this study tended to have a slightly higher incidence of lower respiratory tract infections when compared to female patients but over the age of 6 years the male‐to‐female sex ratio was almost similar. This is in general agreement with findings by other studies [Glezen and Denny, 1973]. Bronchiolitis and pneumonia were the most frequent clinical features found to be associated with lower respiratory tract viral infections among patients in this study. This is similar to that reported from other parts of the world [Hijazi et al., 1996; Al‐Hajjar et al., 1998; Barsaoui et al., 2001].
Respiratory syncytial virus was detected in 36.8% of the patients. Of these 84% were aged <1 year, and thus, respiratory syncytial virus was by far the most common virus detected in infants with lower respiratory tract infections in Kuwait. Significantly, perhaps, 69.7% of those in whom respiratory syncytial virus was detected, had croup and 30.3% had bronchiolitis. This is also in agreement with a number of published studies from the Gulf region and elsewhere [Al‐Hajjar et al., 1998; Wahab et al., 2001; Al‐Toum et al., 2006; Farshad et al., 2008; Kaplan et al., 2008]. It was important to find in this study that human rhinoviruses were the second viruses detected most commonly in samples from patients with lower respiratory tract infections, since 34.4% of patients appear to have been infected with this virus. Also very importantly that 50.5% who had rhinoviruses were infants aged <1 year. Significantly, 42% of the patients infected with rhinoviruses had pneumonia and 36.8% had bronchiolitis. Others have also reported human rhinoviruses in children admitted to hospital with lower respiratory tract infections [Calvo et al., 2007; Chung et al., 2007; Peltola et al., 2008]. In contrast, adenoviruses were detected in only 15.2% of the patients, 38.6% of whom were infants aged <1 year. Nearly 23% of adenovirus infections were associated with bronchiolitis and 12% with pneumonia. In a number of recent reports, adenoviruses were detected in infants suffering from lower respiratory tract infections [Hong et al., 2001; Al‐Toum et al., 2006; Zhang et al., 2007; Kaplan et al., 2008]. In Turkey, both respiratory syncytial virus and adenoviruses were detected with a frequency of 31.5%, and these were the most common agents causing disease in infants [Yüksel et al., 2008].
The overall incidence of influenza A virus infection detection in clinical samples was relatively low (7.3%) and the majority (57.1%) were among patients older than 16 years of age. This generally correlated well with the published data on the incidence of similar infections elsewhere [Al‐Toum et al., 2006; Kaplan et al., 2008; Yüksel et al., 2008; Zaraket et al., 2009]. Influenza B, parainfluenza virus‐1, and parainfluenza virus‐3 accounted for a relatively small number of lower respiratory tract infections among the patients in this study. However, human coronavirus‐229E, human coronavirus‐OC43, or parainfluenza virus‐2 was not detected in any of the patients' samples. Other studies, albeit few, have reported the detection of coronaviruses and parainfluenza virus‐2 in infants and children with lower respiratory tract infections [Vabret et al., 2001; Drosten et al., 2003; Ksiazek et al., 2003; Rota et al., 2003].
In this study, virus was most frequently (42%) detected in nasopharyngeal aspirates than in nasopharyngeal swabs (29.2%), bronchoalveolar lavage (23.6%), or in tracheal aspirate (5.2%). Indeed, in adult and mixed adult–pediatric populations, nasopharyngeal aspirates were shown to have advantages over nasopharyngeal swabs for the identification of influenza viruses [Covalciuc et al., 1999; Lieberman et al., 2009]. Also Gruteke et al. 2004 found that nasopharyngeal aspirates were important for the isolation and detection of any respiratory virus.
Most of the patients were admitted to hospital during the autumn and winter months of October to March. This is generally in line with reports published previously [Al‐Hajjar et al., 1998; Wahab et al., 2001; Al‐Toum et al., 2006; Farshad et al., 2008; Kaplan et al., 2008].
A major observation in this study was that in 71.6% of patients admitted to hospital, the etiological agents could not be detected although very sensitive methods were used. Clearly, therefore, it would be very important for us to investigate the role of a number of other viruses such as human metapneumovirus, bocavirus, the new human coronavirus‐NL 63, and polyomaviruses WU and KI that have been described recently [Naghipour et al., 2007; Bialasiewicz et al., 2008; Foulongne et al., 2008; Hindiyeh et al., 2008; Ren et al., 2008]. Indeed, in a very small pilot study involving 54 negative samples from this study, 16.7% were found to be positive for human metapneumovirus by PCR (data not shown).
Acknowledgements
We are very grateful to Dr. Wassin Chehadeh for critically reviewing this article.
REFERENCES
- Al‐Hajjar S, Akhter J, Al Jumaah S, Hussain Qadri SM. 1998. Respiratory viruses in children attending a major referral centre in Saudi Arabia. Ann Trop Paediatr 18: 87–92. [DOI] [PubMed] [Google Scholar]
- Al‐Toum R, Bdour S, Ayyash H. 2006. Epidemiology and clinical characteristics of respiratory syncytial virus infections in Jordan. J Trop Pediatr 52: 282–287. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society . 2001. Guidelines for the management of adults with community‐acquired pneumonia. Am J Respir Crit Care Med 163: 1730–1754. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society . 2005. Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia. Am J Respir Crit Care Med 171: 388–416. [DOI] [PubMed] [Google Scholar]
- Barsaoui S, Oubich F, Kechrid A, Zidi F, Arrouji Z, Ben Rejab S. 2001. Clinical aspects and etiology of lower respiratory tract infections in hospitalized children. Tunis Med 79: 361–365. [PubMed] [Google Scholar]
- Bialasiewicz S, Whiley DM, Lambert SB, Jacob K, Bletchly C, Wang D, Nissen MD, Sloots TP. 2008. Presence of the newly discovered human polyomaviruses KI and WU in Australian patients with acute respiratory tract infection. J Clin Virol 41: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco A, Mazzarella G, Bresciani M, Paciocco G, Spiteri MA. 2002. Virus induced asthma. Monaldi Arch Chest Dis 57: 188–190. [PubMed] [Google Scholar]
- Calvo C, García‐García ML, Blanco C, Pozo F, Flecha IC, Pérez‐Breña P. 2007. Role of rhinovirus in hospitalized infants with respiratory tract infections in Spain. Pediatr Infect Dis J 26: 904–908. [DOI] [PubMed] [Google Scholar]
- Campbell H. 1995. Acute respiratory infections: A global challenge. Arch Dis Child 73: 281–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballal G, Videla CM, Espinosa MA, Savy V, Uez O, Sequeira MD, Knez V, Requeijo PV, Posse CR, Miceli I. 2001. Multicentered study of viral acute lower respiratory infections in children from four cities of Argentina, 1993–1994. J Med Virol 64: 167–174. [DOI] [PubMed] [Google Scholar]
- Chung JY, Han TH, Kim SW, Hwang ES. 2007. Respiratory picornavirus infections in Korean children with lower respiratory tract infections. Scand J Infect Dis 39: 250–254. [DOI] [PubMed] [Google Scholar]
- Covalciuc KA, Webb KH, Carlson CA. 1999. Comparison of four clinical specimen types for detection of influenza A and B viruses by optical immunoassay (FLU OIA test) and cell culture methods. J Clin Microbiol 37: 3971–3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny FW, Clyde WA. 1983. Acute respiratory tract infections; an overview. Workshop on acute respiratory disease among children of the world. Paediatr Res 17: 1026–1029. [Google Scholar]
- Drosten C, Gunther S, Preiser W, Van Der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguiere AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Muller S, Rickerts V, Sturmer M, Vieth S, Klenk HD, Osterhaus AD, Schmitz H, Doerr HW. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 348: 1967–1976. [DOI] [PubMed] [Google Scholar]
- Echevarría JE, Erdman DD, Swierkosz EM, Holloway BP, Anderson LJ. 1998. Simultaneous detection and identification of human parainfluenza viruses 1, 2, and 3 from clinical samples by multiplex PCR. J Clin Microbiol 36: 1388–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farshad N, Saffar MJ, Khalilian AR, Saffar H. 2008. Respiratory viruses in hospitalized children with acute lower respiratory tract infections, Mazandaran Province, Iran. Indian Pediatr 45: 590–592. [PubMed] [Google Scholar]
- Foulongne V, Brieu N, Jeziorski E, Chatain A, Rodière M, Segondy M. 2008. KI and WU polyomaviruses in children, France. Emerg Infect Dis 14: 523–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezen P, Denny FW. 1973. Epidemiology of acute lower respiratory disease in children. N Engl J Med 288: 498–505. [DOI] [PubMed] [Google Scholar]
- Glezen WP, Greenberg SB, Atmar RL, Peidra PA, Couch RB. 2000. Impact of respiratory virus infections on person with chronic underlying conditions. J Am Med Assoc 283: 499–505. [DOI] [PubMed] [Google Scholar]
- Grondahl B, Puppe W, Hoppe A, Kuhe I, Weigl JA, Schmitt HJ. 1999. Rapid identification of nine microorganisms causing acute respiratory tract infections by single‐tube multiplex reverse transcription‐PCR: Feasibility study. J Clin Microbiol 37: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruteke P, Glas AS, Dierdorp M, Vreede WB, Pilon JW, Bruisten SM. 2004. Practical implementation of a multiplex PCR for acute respiratory tract infections in children. J Clin Microbiol 42: 5596–5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwatkin DR. 1980. How many die? A set of demographic estimates of the annual number of infant and children death in the world. Am J Public Health 70: 1286–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CB, Long CE, Schnabel KC. 2001. Respiratory syncytial virus infections in previously healthy working adults. Clin Infect Dis 15: 792–796. [DOI] [PubMed] [Google Scholar]
- Hijazi Z, Pacsa A, Eisa S, el Shazli A, Abd el‐Salam RA. 1996. Laboratory diagnosis of acute lower respiratory tract viral infections in children. J Trop Pediatr 42: 276–280. [DOI] [PubMed] [Google Scholar]
- Hijazi Z, Pacsa A, el‐Gharbawy F, Chugh TD, Essa S, el Shazli A, Abd el‐Salam R. 1997. Acute lower respiratory tract infections in children in Kuwait. Ann Trop Paediatr 17: 127–134. [DOI] [PubMed] [Google Scholar]
- Hindiyeh MY, Keller N, Mandelboim M, Ram D, Rubinov J, Regev L, Levy V, Orzitzer S, Shaharabani H, Azar R, Mendelson E, Grossman Z. 2008. High rate of human bocavirus and adenovirus coinfection in hospitalized Israeli children. J Clin Microbiol 46: 334–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JY, Lee HJ, Piedra PA, Choi EH, Park KH, Koh YY, Kim WS. 2001. Lower respiratory tract infections due to adenovirus in hospitalized Korean children: Epidemiology, clinical features, and prognosis. Clin Infect Dis 32: 1423–1429. [DOI] [PubMed] [Google Scholar]
- Hwang SW, Cheung AM, Moineddin R, Bell CM. 2007. Population mortality during the outbreak of severe acute respiratory syndrome in Toronto. BMC Public Health 7: 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan NM, Dove W, Abd‐Eldayem SA, Abu‐Zeid AF, Shamoon HE, Hart CA. 2008. Molecular epidemiology and disease severity of respiratory syncytial virus in relation to other potential pathogens in children hospitalized with acute respiratory infection in Jordan. J Med Virol 80: 168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, Rollin PE, Dowell SF, Ling AE, Humphrey CD, Shieh WJ, Guarner J, Paddock CD, Rota P, Fields B, DeRisi J, Yang JY, Cox N, Hughes JM, LeDuc JW, Bellini WJ, Anderson LJ. 2003. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 348: 1953–1966. [DOI] [PubMed] [Google Scholar]
- Larranaga C, Kajon A, Villagra E, Avendano LF. 2000. Adenovirus surveillance on children hospitalized for acute lower respiratory infections in Chile (1988–1996). J Med Virol 60: 342–346. [PubMed] [Google Scholar]
- Leowski J. 1987. Mortality from acute respiratory infection in children under 5 years of age: Global estimates. World Health Stat Q 39: 138–144. [PubMed] [Google Scholar]
- Lieberman D, Lieberman D, Shimoni A, Keren‐Naus A, Steinberg R, Shemer‐Avni Y. 2009. Identification of respiratory viruses in adults: Nasopharyngeal versus oropharyngeal sampling. Clin Microbiol 47: 3439–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie JK, Roy‐Burman A, Guardia‐Labar L, Boston EJ, Kiang D, Padilla T, Yagi S, Messenger S, Petru AM, Glaser CA, Schnurr DP. 2009. Rhinovirus associated with severe lower respiratory tract infections in children. Pediatr Infect Dis J 28: 337–339. [DOI] [PubMed] [Google Scholar]
- Macek V, Dakhama A, Hogg JC, Green FH, Rubin BK, Hegele RG. 1999. PCR detection of viral nucleic acid in fatal asthma: Is the lower respiratory tract a reservoir for common viruses? Can Respir J 6: 37–43. [DOI] [PubMed] [Google Scholar]
- Mackay IM, Jacob KC, Woolhouse D, Waller K, Syrmis MW, Whiley DM, Siebert DJ, Nissen M, Sloots TP. 2003. Molecular assays for detection of human metapnuemovirus. J Clin Microbiol 41: 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Lu X, Erdman DD, Poehling KA, Zhu Y, Griffin MR, Hartert TV, Anderson LJ, Weinberg GA, Hall CB, Iwane MK, Edwards KM. 2007. Rhinovirus‐associated hospitalizations in young children. J Infect Dis 195: 773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghipour M, Cuevas LE, Bakhshinejad T, Dove W, Hart CA. 2007. Human bocavirus in Iranian children with acute respiratory infections. J Med Virol 79: 539–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos NG, Bates PJ, Bardin PG, Papi A, Leir SH, Fraenkel DJ, Meyer J, Lackie PM, Sanderson G, Holgate ST, Johnston SL. 2000. Rhinoviruses infect the lower airways. J Infect Dis 181: 1875–1884. [DOI] [PubMed] [Google Scholar]
- Peltola V, Waris M, Osterback R, Susi P, Hyypiä T, Ruuskanen O. 2008. Clinical effects of rhinovirus infections. J Clin Virol 43: 411–414. [DOI] [PubMed] [Google Scholar]
- Pitkäranta A, Arruda E, Malmberg H, Hayden FG. 1997. Detection of rhinovirus in sinus brushings of patients with acute community‐acquired sinusitis by reverse transcription‐PCR. J Clin Microbiol 35: 1791–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthavathana P, Wasi C, Kositanont U, Suwanjutha S, Chantarojanasiri T, Kantakamalakul W, Kantawateera P, Thongcharoen P. 1990. A hospital based study of acute viral infections of the respiratory tract in children, with emphasis on laboratory diagnosis. Rev Infect Dis 12: S988–S994. [DOI] [PubMed] [Google Scholar]
- Ren L, Gonzalez R, Xie Z, Zhang J, Liu C, Li J, Li Y, Wang Z, Kong X, Yao Y, Hu Y, Qian S, Geng R, Yang Y, Vernet G, Paranhos‐Baccalà G, Jin Q, Shen K, Wang J. 2008. WU and KI polyomavirus present in the respiratory tract of children, but not in immunocompetent adults. J Clin Virol 43: 330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota PA, Oberste MS, Monroe SS, Nix WA, Campagnoli R, Icenogle JP, Penaranda S, Bankamp B, Maher K, Chen MH, Tong S, Tamin A, Lowe L, Frace M, DeRisi JL, Chen Q, Wang D, Erdman DD, Peret TC, Burns C, Ksiazek TG, Rollin PE, Sanchez A, Liffick S, Holloway B, Limor J, McCaustland K, Olsen‐Rassmussen M, Fouchier R, Gunther S, Osterhaus AD, Drosten C, Pallansch MA, Anderson LJ, Bellini WJ. 2003. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 300: 1394–1399. [DOI] [PubMed] [Google Scholar]
- Sanchez JL, Binn LN, Innis BL, Reynolds RD, Lee T, Mitchell‐Raymundo F, Craig SC, Marquez JP, Shepherd GA, Polyak CS, Conolly J, Kohlhase KF. 2001. Epidemic of adenovirus‐induced respiratory illness among military recruits: Epidemiologic and immunologic risk factors in healthy, young adults. J Med Virol 65: 710–718. [DOI] [PubMed] [Google Scholar]
- Shann F. 1986. Etiology of severe pneumonia in children in developing countries. Pediatr Infect Dis 5:247–252. [DOI] [PubMed] [Google Scholar]
- Simpson JL, Moric I, Wark PA, Johnston SL, Gibson PG. 2003. Use of induced sputum for the diagnosis of influenza and infections in asthma: A comparison of diagnostic techniques. J Clin Virol 26: 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siritantikorn S, Puthavathana P, Suwanjutha S, Chantarojanasiri T, Sunakorn P, Ratanadilok NA, Phuket T, Nawanopparatsakul S, Teeyapaiboonsilpa P, Taveepvoradej S, Pengmesri J, Pongpate S. 2002. Acute viral lower respiratory infections in children in a rural community in Thailand. J Med Assoc Thai 85: S1167–S1175. [PubMed] [Google Scholar]
- Vabret A, Mouthon F, Mourez T, Gouarin S, Petitjean J, Freymuth F. 2001. Direct diagnosis of human respiratory coronaviruses 229E and OC43 by the polymerase chain reaction. J Virol Methods 97: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahab AA, Dawod ST, Raman HM. 2001. Clinical characteristics of respiratory syncytial virus infection in hospitalized healthy infants and young children in Qatar. J Trop Pediatr 47: 363–366. [DOI] [PubMed] [Google Scholar]
- Weigl JA, Puppe W, Grondahl B, Schmitt HJ. 2000. Epidemiological investigation of nine respiratory pathogens in hospitalized children in Germany using multiple reverse‐transcriptase polymerase chain reaction. Eur J Clin Microbiol Infect Dis 19: 336–343. [DOI] [PubMed] [Google Scholar]
- Wendt CH. 1997. Community respiratory viruses: Organ transplant recipients. Am J Med 102: 31–36; discussion 42–43. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2003. Consultative meeting to review evidence and research priorities in the management of acute respiratory infections. Geneva: World Health Organization; WHO/ARI/04.2. [Google Scholar]
- Yüksel H, Yilmaz O, Akçali S, Söğüt A, Yilmaz Ciftdoğan D, Urk V, Ertan P, Sanlidağ T. 2008. Common viral etiologies of community acquired lower respiratory tract infections in young children and their relationship with long term complications. Mikrobiyol Bul 42: 429–435. [PubMed] [Google Scholar]
- Zambon MC, Stockton JD, Clewley JP, Fleming DM. 2001. Contribution of influenza and respiratory syncytial virus to community cases of influenza‐like illness: An observational study. Lancet 358: 1410–1416. [DOI] [PubMed] [Google Scholar]
- Zaraket H, Dbaibo G, Salam O, Saito R, Suzuki H. 2009. Influenza virus infections in Lebanese children in the 2007–2008 season. Jpn J Infect Dis 62: 137–138. [PubMed] [Google Scholar]
- Zhang H, Liu CY, Wang Y, Xie ZD. 2007. Detection of adenovirus‐IgM antibody in children hospitalized with lower respiratory tract infection in Beijing Children's Hospital. Zhonghua Liu Xing Bing Xue Za Zhi 28: 686–687. [PubMed] [Google Scholar]


