Reliance on history and description of episodes of collapse to differentiate seizures from syncope can be misleading. Syncope can have features of seizures or can be the cause of seizures. Clinical and neurologic examinations can also be misleading. High‐grade atrioventricular (AV) block can be intermittent in cats and interictal neurologic examination can be normal in patients with epilepsy. In this report we describe high‐grade AV dysfunction that mimicked epilepsy in 3 cats.
Case 1
A 12‐year‐old male neutered domestic shorthaired cat was evaluated after 12 seizure‐like episodes in a 24‐hour period. During the episodes, the cat assumed a crouched posture, developed tonicity in all 4 limbs, fell into lateral recumbency, had head tremors, experienced impaired consciousness and did not respond to visual stimuli. The episodes lasted between 10 and 20 seconds. For 1 year before presentation, the cat had experienced other episodes in which it swayed from side to side and had spontaneous nystagmus, the direction and nature of which was not recorded. Between the episodes, general physical examination and neurologic examinations were unremarkable. Based on the history, the neurolocalization was prosencephalon.
A CBC was normal. Serum biochemistry abnormalities consisted of hypoproteinemia 54.6 g/L (reference range, 61–81 g/L) and mild hypoglobulinemia 21.1 g/L (reference range, 25–46 g/L). Total T4 concentration was not measured. Systolic blood pressure measured by Doppler sphygmomanometry was 190 mmHg and an ECG showed normal sinus rhythm with a heart rate of 200 beats/min (bpm). Results of magnetic resonance (MR) a imaging of the brain and analysis of cerebrospinal fluid (CSF) from the cisterna magna were unremarkable. The CSF white blood cell (WBC) count was 3 cells/μL (normal, <5 cells/μL) and protein concentration was 0.17 g/L (normal, <0.2 g/L). Treatment with phenobarbitone b was initiated with 5 mg/kg IV boluses every 5 hours up to a total of 20 mg/kg in 24 hours. The maintenance dosage of phenobarbitone was 2 mg/kg PO q12h.
The cat was presented again 24 hours later for vomiting, diarrhea, and vacant episodes during which it would lift its left thoracic limb and collapse. Physical and neurologic examinations were unremarkable between the episodes. Phenobarbitone was increased to 3 mg/kg PO q12h. No additional episodes of vomiting or diarrhea were observed.
The episodes continued over 3 weeks and the cat was re‐examined. On physical examination the cat was bradycardic with an irregular rhythm and heart rate of 120 bpm, and had a grade II/VI left apical systolic murmur. An ECG was consistent with sinus bradycardia and an echocardiogram c showed a slight increase in left ventricular diastolic diameter and additional moderator bands within the left ventricle. No structural cause for the murmur was found. A 24‐hour ECG (Holter monitor d ) documented periods of high‐grade AV block without any ventricular escape activity for up to 19 seconds preceding an apparent seizure‐like episode (Fig 1). Phenobarbitone was discontinued and Terbutaline e was prescribed. However, the owner elected not to start the terbutaline and the cat was euthanized.
Figure 1.
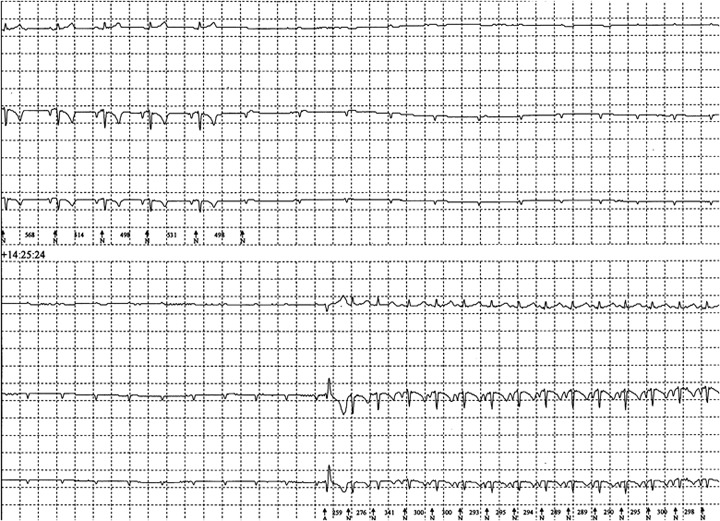
ECG from “Case 1” showing nonconducted P waves followed by a wide and bizarre complex consistent with a ventricular escape complex before returning to sinus rhythm.
Case 2
A 12‐year‐old female spayed domestic shorthaired cat was presented for collapsing episodes with increasing frequency with up to 20 occurring during the 48 hours before presentation. The episodes were described by the owner to be preceded by a period of sneezing; the cat would then shake its head and fall into lateral recumbency before returning to normal in <60 seconds. Clinical examination revealed mild otitis externa in the left ear but was otherwise unremarkable. On neurologic examination, there was decreased facial sensation on the left side and tactile placing deficits in the left thoracic limb. The lesion was localized to the right prosencephalon. The episodes of collapse continued while the cat was hospitalized although these episodes were different from those previously described. During the episodes the cat vocalized, had increased muscle tone, fell into lateral recumbency, had facial automatisms consisting of lip chewing and hypersalivation, exhibited running automatisms, then stood up and seemed to be disorientated for a few seconds before returning to normal (Fig 2).
Figure 2.

Screen capture from a video of one of the seizure‐like episodes that occurred during hospitalization in “Case 2.” Full video as supporting information in the online version of this article.
CBC, serum biochemistry, and urine analysis results were unremarkable. Feline immunodeficiency virus and feline leukemia virus tests were negative by immunochromatography. Total thyroxine (T4) concentration was normal: 33 nmol/L (reference range, 19–65 nmol/L). Systolic blood pressure measured by Doppler sphygmomanometry was 144 mmHg and an abdominal ultrasound examination was unremarkable. Results of MR imaging of the brain and CSF analysis were also unremarkable. The CSF WBC count was 1 cells/μL (normal, <5 cells/μL) and the protein concentration was 0.11 g/L (normal, <0.2 g/L). Real‐time polymerase chain reactions for detection of Toxoplasma gondii, Borna virus, and feline coronavirus were negative. Treatment with phenobarbitone was started with a loading dose of 3 mg/kg IV boluses every 2 hours up to a total dose of 20 mg/kg phenobarbitone in a 24‐hour period. The maintenance dosage of phenobarbitone was 2.5 mg/kg PO q12h. The episodes initially appeared to stop, but after 24 hours they began again. The cat was treated with leviteracetam f 20 mg/kg PO q8h and the episodes stopped.
The cat was presented again 10 days later after 20 seizure‐like episodes in 24 hours. On clinical examination, there was an irregularly irregular bradyarrhythmia with a heart rate of 120 bpm. On neurologic examination, the cat was ataxic, tetraparetic, and had decreased menace response in the right eye. A 3‐lead ECG revealed high‐grade 2nd‐degree and 3rd‐degree AV block, with the majority of QRS complexes showing a right bundle branch block morphology with some normal sinus complexes interspersed. No abnormalities were seen on 2‐dimensional and M‐mode echocardiography. An electroencephalogram (EEG) was performed and no epileptiform activity was recorded during the start of the seizure‐like episode, but during the episode there was movement artifact that would have obscured any activity (Fig 3). A permanent pacemaker g was implanted via a celiotomy‐transdiaphragmatic approach using an epicardial lead with the pulse generator placed in the abdomen. The pacemaker was programmed in VVI mode (ventricular demand pacing) with the rate set at 120 bpm. The cat was weaned off phenobarbitone and leviteracetam. At a 15‐week re‐examination, the cat had suffered no additional seizure‐like episodes.
Figure 3.
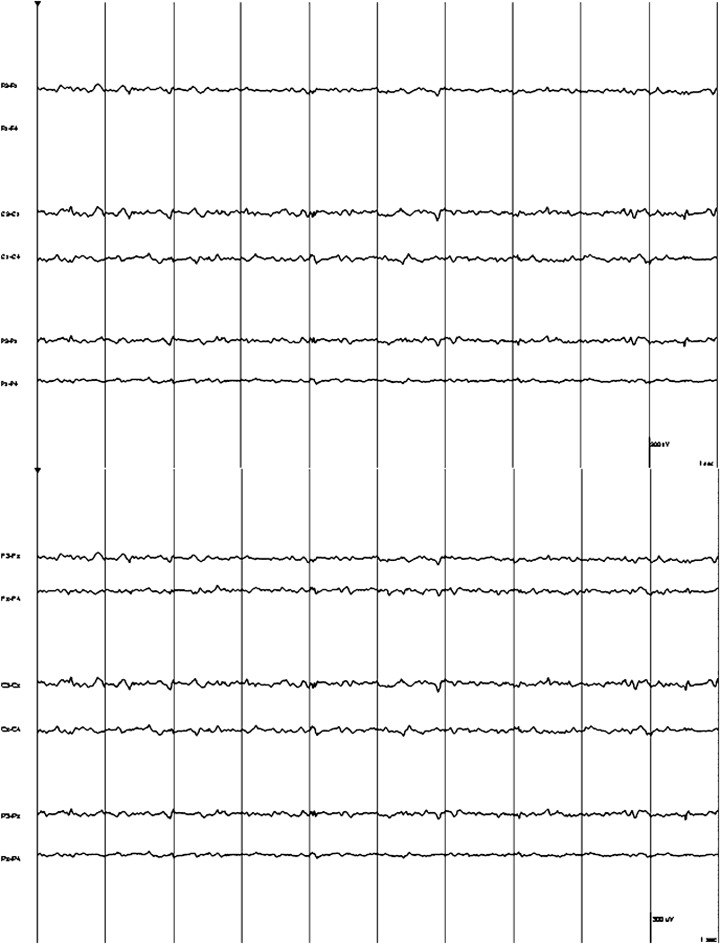
An electroencephalogram of “Case 2” just before the seizure‐like episode before movement obscured the trace.
Case 3
A 14‐year‐old female Birman cat was presented for collapsing episodes that lasted 60 seconds. During the episodes, the cat had increased respiratory effort, became paretic, collapsed into lateral recumbency and urinated. It also had a 2‐month history of bradycardia that was preceded by a 2‐year history of a cardiac rhythm disturbance; no further characterization of the rhythm could be made. The cat had suffered from gradual weight loss over the preceding 2 years; it had become more lethargic and would wander aimlessly around the house. On physical examination, body condition score was 3/9, the cat had mild ocular and nasal discharge and on thoracic auscultation was bradycardic with a heart rate of 85 bpm with synchronous, good quality pulses. On neurologic examination, there was mild pelvic limb paresis only.
Results of a CBC were unremarkable, serum biochemistry abnormalities consisted of mild hypoalbuminemia 27.1 g/L (reference range, 28–42 g/L) and mild hyponatremia 149 mmol/L (reference range, 153–162 mmol/L). Total serum T4 concentration was 15 nmol/L (reference range, 19–65 nmol/L) and was considered consistent with a sick euthyroid state. On abdominal ultrasound examination, both kidneys were slightly small at 32 mm in length (reference range, 38–46 mm) 1 and had bright corticomedullary bands of unknown clinical relevance. An ECG confirmed bradycardia and 3rd‐degree AV block. An echocardiogram identified dilatation of all 4 chambers consistent with chronic bradycardia‐induced neurohormonal activation leading to sodium and water retention. The cat was treated with 0.3 mg terbutaline PO q8h. A transient decrease in systolic blood pressure to 80 mmHg was identified by Doppler sphygmomanometry but blood pressure stabilized between 120 and 140 mmHg after 2 days. Three months after discharge, the cat was reported to have more energy and had no additional collapsing episodes.
Partial seizures are described as simple when consciousness is not impaired or complex when consciousness is impaired. Partial seizures of both types can develop into generalized seizures. 2 Partial seizures are variable in presentation but may include orofacial automatisms, repetitive movements of an extremity, repetitive vocalization, circling, and salivation. 3 Patients suffering from partial seizures may have identifiable pre‐ and postictal periods that can assist in the differentiation between seizures and syncope. There are exceptions however and the lack of identifiable pre‐ and postictal periods does not preclude the diagnosis of a seizure disorder. 4 Partial seizures occur commonly in cats secondary to a variety of pathologies including both intracranial disease such as inflammatory, infectious, neoplastic, traumatic, parasitic (cuterebral myiasis), vascular lesions and extracranial causes such as hyperthyroidism and hepatic encephalopathy. 3 Intracranial causes of seizures, especially neoplasia rather than primary epilepsy, are more common in cats >7 years of age. 3 , 5 , 6 During a generalized tonic clonic seizure, cats may exhibit opisthotonus, claw extension as well as running movements and loss of consciousness. 3 All 3 of the cats in this series had episodes that resembled clusters of complex partial seizures. There was no epileptiform activity seen on the EEG in 1 cat, which suggests the episodes were not because of seizure activity, but humans with frontal seizures may have no EEG abnormalities. 7 The EEG activity in the cat was obscured by movement artifact during actual collapse, and consequently epileptiform spikes may have been missed. 8 In humans, EEG recordings during syncope differ from those recorded during epileptiform activity in the following ways: the EEG tracing in syncope shows high amplitude generalized slow waves initially, the trace then becomes flat during the period of anoxia or hypoxia followed by the resumption of slow waves before the trace returns to normal. In humans there is no epileptiform activity during or between episodes of syncope. 8 , 9 Complex partial seizures as well as generalized seizures can be associated with preictal ECG changes, these most commonly are sinus tachycardia but sinus bradycardia is also reported. 10 Patients with increased intracranial pressure may also have bradycardia with systemic hypertension (Cushing's Reflex). 11 Therefore, the presence of ECG abnormalities should not preclude investigation of intracranial disease.
High‐grade AV block occurs with a transient (2nd degree) or permanent (3rd degree) failure of conduction through the AV node or Bundle of His. 12 It may be associated with structural cardiac disease such as hypertrophic cardiomyopathy in cats, 13 with severe systemic disease 12 or it can be idiopathic. 14 Third‐degree AV block in cats usually is persistent, although it can be intermittent. 14 Clinical signs include lethargy, episodic weakness, and syncope but AV block can also be an incidental finding. 13 , 14 Complete AV block with absence of ventricular escape complexes has been described in 2 previous case reports of individual cats that presented with syncope. 15 , 16 One of these cats also showed seizure‐like activity, 16 but collapsing episodes ceased in both after pacemaker implantation.
Syncope is defined as temporary loss of consciousness and postural control with spontaneous recovery. 17 In a study of humans, syncope was induced by hyperventilation, orthostasis, and Valsalva maneuver in 56/59 volunteers. 9 Interestingly, 90% experienced either focal, multifocal or generalized myoclonic jerks, or a combination; 79% experienced oral automatisms consisting of lip licking, chewing, and fumbling, which classically would be attributed to partial seizure activity. Forty percent experienced visual and auditory hallucinations and 1 patient experienced vertical nystagmus. In humans with syncope, myoclonic jerks and convulsions occur because of transient hypoxia and are the rule rather than the exception. The episode duration usually is shorter than what is observed during an epileptic event. 8 Oral automatisms during syncope tend to be of shorter duration than those observed during a seizure but there is substantial overlap, making clinical differentiation impossible. 8
Cardiac arrythmias cause cerebral hypoxia that can either induce true seizure activity or can have characteristics of seizure activity. This situation is referred to as anoxic‐epileptic seizures or anoxic seizures, respectively. 18 , 19 , 20 , 21 Anoxic seizures first were described in 1983 18 and are defined as nonepileptic events because of abrupt interruption of the energy supply to metabolically active cerebral neurons. 19 They occur in both children and adults and often are misdiagnosed as seizures. A review of 74 adults with apparent seizures, 36 of whom were receiving anticonvulsant therapy were reinvestigated. Almost 42% were found to be experiencing syncope secondary to prolonged bradycardia or severe hypotension. 22 If the period of anoxia is long enough, however, a generalized tonic clonic seizure can occur. A case report of a 3‐year‐old girl describes a generalized tonic clonic seizure lasting approximately 2 hours after she lost consciousness as a result of a prolonged breath holding incident. 23 A study of eye witness accounts of humans experiencing collapsing episodes found that humans were 5 times more likely to have had a seizure if they were seen to experience postictal signs such as disorientation after the collapse. Postictal signs were reported to be the most useful discriminating factor between seizures and syncope. 24 Another study of humans assigned a point system based on historical criteria to assist in the differentiation of syncope and seizures. The system had 94% sensitivity and 94% specificity. Patients experiencing a seizure were more likely to have had mood changes, hallucinations, or trembling characteristic of preictal signs before loss of consciousness. During loss of consciousness, seizure patients were unresponsive, had convulsive movements, urinated, injured their tongue, and were cyanotic. These patients experienced postictal confusion, muscle pain, and headaches. Patients collapsing because of syncope experienced presyncope (dizziness), diaphoresis (excessive sweating), dyspnea, chest pain and palpitations and feelings of warmth, nausea, or vertigo. Patients with syncope lost consciousness after prolonged sitting or standing, in response to needles, when in hot or cold environments or during exercise. 25 In humans suffering from prolonged syncopal episodes, the period of disorientation typically does not exceed 30 seconds. 8 The 2nd cat in this report appeared to have a period of disorientation after collapse that lasted several minutes. This finding normally would be indicative of a postictal period and hence indicate that a seizure had occurred, but in cats the period of disorientation after syncope can last 10 minutes. 26
Cerebral hypoxia secondary to cardiac arrest in humans has been reported to cause a variety of neurologic deficits including mild cognitive dysfunction, seizures, myoclonus, and persistent vegetative states. 27 The 2nd cat had different neurologic deficits on both routine examinations. This finding could indicate that cerebral dysfunction developed secondary to episodes of cerebral hypoxia. Thus, not only can hypoxia during a cardiac arrest lead to development of seizures 27 but it is also possible that the repeated periods of cerebral hypoxia could predispose cats to epilepsy.
Cats tend to experience complex partial seizures rather than generalized seizures. 3 It is possible that these cats were experiencing seizure activity secondary to hypoxia from their high‐grade AV block. In a recent retrospective study of 21 cats with 3rd‐degree AV block, only 2 cats had neurologic signs that were not clearly specified and they were not attributed to the arrhythmia. 14 Without diagnostic EEG recordings of the entire collapse episodes from all 3 cats, we cannot exclude seizure activity. However, because 2 of the cats responded to treatment for their underlying rhythm disturbance, treatment initially should be directed at this problem rather than at any presumed seizure activity secondary to the hypoxia.
One of the cats reported here underwent successful placement of a pacemaker. Pacemaker placement is reported to be the treatment of choice for cats with symptomatic 3rd‐degree AV block. 12 , 14 , 16 One of the 3 cats appears to have experienced a decrease in the number of collapsing episodes with terbutaline, a β2 adrenergic agonist. More long‐term follow‐up would be required to confirm whether such treatment could be a successful alternative to pacemaker implantation. Terbutaline is transiently effective when used in pregnant women to treat fetuses with complete AV block. 28
This report highlights the difficulties in correctly differentiating complex partial seizures from syncope in cats with high‐grade AV block. Additional diagnostic tests including EEG and Holter monitoring should be utilized when older cats are presented for collapsing episodes and have no structural brain disease because cardiac disease is more prevalent in the geriatric cat population than is primary epilepsy. 29 , 30
Footnotes
a1.5 T Gyroscan NT, Phillips Medical Systems, Heindhoven, the Netherlands
bPhenobarbitone, Vetoquinol, Berkshire, UK
cGE Machine, model Vivid 7, GE Healthcare, Pollards Wood, Chalfont St Giles, Buckinghamshire, UK
dLifecard CF, Delmar Reynolds, Labcorp Corporation of America, Ambulatory Monitoring Services, Burlington, NC
eTerbutaline, AstraZeneca UK Limited, Luton, UK
fLeviteracetam, UCB Pharma Inc, Berkshire, UK
gMedtronic Sigma SSR 303, Medtronic World Headquarters, Minneapolis, MN
Supporting information
Video S1: A video of one of the seizure‐like episodes that occurred during hospitalization in "Case 2."
Please note: Wiley‐Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Acknowledgments
The authors thank Charlotte Pace and Kat Evans for their technical support.
References
- 1. Walter PA, Feeney DA, Johnston GR, et al Feline renal ultrasonography: Quantitative analyses of imaged anatomy. Am J Vet Res 1987;48:596–599. [PubMed] [Google Scholar]
- 2. Berendt M, Gram L. Epilepsy and seizure classification in 63 dogs: A reappraisal of veterinary epilepsy terminology. J Vet Intern Med 1999;13:14–20. [PubMed] [Google Scholar]
- 3. Kline KL. Feline epilepsy. Clin Tech Small Anim Pract 1998;13:152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berendt M. Epilepsy In: CH V, ed. Braund's clinical neurology in small animals: Localization, diagnosis and treatment. Ithaca, NY; 2004. Available at: http://www.ivis.org. Accessed March 1, 2008. [Google Scholar]
- 5. Platt SR. Feline seizure control. J Am Anim Hosp Assoc 2001;37:515–517. [DOI] [PubMed] [Google Scholar]
- 6. Barnes HL, Chrisman CL, Mariani CL, et al Clinical signs, underlying cause, and outcome in cats with seizures: 17 cases (1997–2002). J Am Vet Med Assoc 2004;225:1723–1726. [DOI] [PubMed] [Google Scholar]
- 7. Fejerman N. Nonepileptic disorders imitating generalized idiopathic epilepsies. Epilepsia 2005;46 (Suppl 9):80–83. [DOI] [PubMed] [Google Scholar]
- 8. Lempert T. Recognizing syncope: Pitfalls and surprises. J R Soc Med 1996;89:372–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lempert T, Bauer M, Schmidt D. Syncope: A videometric analysis of 56 episodes of transient cerebral hypoxia. Ann Neurol 1994;36:233–237. [DOI] [PubMed] [Google Scholar]
- 10. Mayer H, Benninger F, Urak L, et al EKG abnormalities in children and adolescents with symptomatic temporal lobe epilepsy. Neurology 2004;63:324–328. [DOI] [PubMed] [Google Scholar]
- 11. Fodstad H, Kelly PJ, Buchfelder M. History of the Cushing reflex. Neurosurgery 2006;59:1132–1137; discussion 1137. [DOI] [PubMed] [Google Scholar]
- 12. Fox PR, Harpster NK. Diagnosis and management of feline arrhythmias In: Fox PR, Sisson D, Moise NS, eds. Textbook of canine and feline cardiology principles and clinical practice, 2nd ed Philadelphia, PA: Saunders; 1999:386–399. [Google Scholar]
- 13. Miller MS, Tilley LP, Smith FWK Jr Electrocardiography In: Fox PR, Sisson D, Moise NS, eds. Textbook of canine and feline cardiology principles and clinical practice, 2nd ed Philadelphia, PA: Saunders; 1999:82–83. [Google Scholar]
- 14. Kellum HB, Stepien RL. Third‐degree atrioventricular block in 21 cats (1997–2004). J Vet Intern Med 2006;20:97–103. [DOI] [PubMed] [Google Scholar]
- 15. Ferasin L, Van De Stad M, Rudorf H, et al Syncope associated with paroxysmal atrioventricular block an ventricular standstill in a cat. J Small Anim Pract 2002;43:124–128. [DOI] [PubMed] [Google Scholar]
- 16. Forterre S, Nürnberg J‐H, Forterre F, et al Transvenous demand pacemaker treatment for intermittent complete heart block in a cat. J Vet Cardiol 2001;3:21–26. [DOI] [PubMed] [Google Scholar]
- 17. Miller RH, Lehmkuhl LB, Bonagura JD, et al Retrospective analysis of the clinical utility of ambulatory electrocardiographic (Holter) recordings in syncopal dogs: 44 cases (1991–1995). J Vet Intern Med 1999;13:111–122. [DOI] [PubMed] [Google Scholar]
- 18. Stephenson JB. Anoxic seizures or epilepsy? Br Med J (Clin Res Ed) 1983;287:1302–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stephenson JB. Anoxic seizures: Self-terminating syncopes. Epileptic Disord 2001;3:3–6. [PubMed] [Google Scholar]
- 20. Stephenson J, Breningstall G, Steer C, et al Anoxic‐epileptic seizures: Home video recordings of epileptic seizures induced by syncopes. Epileptic Disord 2004;6:15–19. [PubMed] [Google Scholar]
- 21. McLeod KA, Wilson N, Hewitt J, et al Cardiac pacing for severe childhood neurally mediated syncope with reflex anoxic seizures. Heart 1999;82:721–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zaidi A, Clough P, Cooper P, et al Misdiagnosis of epilepsy: Many seizure-like attacks have a cardiovascular cause. J Am Coll Cardiol 2000;36:181–184. [DOI] [PubMed] [Google Scholar]
- 23. Kuhle S, Tiefenthaler M, Seidl R, et al Prolonged generalized epileptic seizures triggered by breath‐holding spells. Pediatr Neurol 2000;23:271–273. [DOI] [PubMed] [Google Scholar]
- 24. Hoefnagels WA, Padberg GW, Overweg J, et al Transient loss of consciousness: The value of the history for distinguishing seizure from syncope. J Neurol 1991;238:39–43. [DOI] [PubMed] [Google Scholar]
- 25. Sheldon R, Rose S, Ritchie D, et al Historical criteria that distinguish syncope from seizures. J Am Coll Cardiol 2002;40:142–148. [DOI] [PubMed] [Google Scholar]
- 26. Willis R, McLeod K, Cusack J, et al Use of an implantable loop recorder to investigate syncope in a cat. J Small Anim Pract 2003;44:181–183. [DOI] [PubMed] [Google Scholar]
- 27. Khot S, Tirschwell DL. Long‐term neurological complications after hypoxic‐ischemic encephalopathy. Semin Neurol 2006;26:422–431. [DOI] [PubMed] [Google Scholar]
- 28. Robinson BV, Ettedgui JA, Sherman FS. Use of terbutaline in the treatment of complete heart block in the fetus. Cardiol Young 2001;11:683–686. [DOI] [PubMed] [Google Scholar]
- 29. Miller MS TL, Smith FW Jr Cardiopulmonary disease in the geriatric dog and cat. Vet Clin North Am Small Anim Pract 1989;19:87–102. [DOI] [PubMed] [Google Scholar]
- 30. Parent JM QA. Seizures in cats. Vet Clin North Am Small Anim Pract 1996;26:811–825. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1: A video of one of the seizure‐like episodes that occurred during hospitalization in "Case 2."
Please note: Wiley‐Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


