Abstract
Summary. Telbivudine is an orally bioavailable L‐nucleoside with potent and specific anti‐hepatitis B virus activity. The higher rate of hepatitis B e antigen (HBeAg) seroconversion during telbivudine treatment than other potent anti‐HBV agents suggests a potential immunomodulatory effect. We sought to determine the effects of telbivudine on the immune system, particularly on cytokine production and T‐cell response, using an animal model with mouse hepatitis virus strain 3 (MHV‐3)‐induced hepatitis. The effects of telbivudine on virus replication and cytokine production were investigated in vitro using MHV‐3‐infected macrophages, and the effects on T‐cell response were investigated in vivo in an MHV‐3‐induced viral hepatitis model. Telbivudine had no effect on MHV‐3 replication in macrophages. However, the production of tumour necrosis factor‐α and interleukin‐12 was increased significantly in MHV‐3‐induced macrophages treated with telbivudine. In vivo survival was enhanced in telbivudine‐treated mice, with marked normalization in clinical conditions and histological lesions. Serum levels of interferon‐γ were elevated significantly after telbivudine treatment in MHV‐3‐infected C3H mice. In contrast, serum interleukin‐4 levels were decreased significantly. Furthermore, telbivudine treatment enhanced the ability of T cells to undergo proliferation and secrete cytokines but did not affect cytotoxicity of infected hepatocytes. Of note, we found that telbivudine treatment suppressed programmed death ligand 1 expression on T cells. The results demonstrate the immunomodulatory properties of telbivudine, independent of its antiviral activity, in a mouse model of MHV‐3‐induced hepatitis.
Keywords: hepatitis B virus, interleukin, oral nucleos(t)ide analogue, programmed death ligand 1, tumour necrosis factor
Abbreviations:
- CHB
chronic hepatitis B
- FasL
Fas ligand
- HBeAg
hepatitis B e antigen
- HBV
hepatitis B virus
- MHV‐3
mouse hepatitis virus strain 3
- PD‐1
programmed death 1
- PD‐L1
programmed death ligand 1
Introduction
Hepatitis B virus (HBV) infection is a significant cause of liver‐related morbidity and mortality, particularly in regions of high endemicity [1]. The natural course and clinical outcome of HBV infection is mediated by a complex interaction between the virus and the host’s immune response. Successful control of HBV replication is associated with a strong, multi‐specific CD4+ and CD8+ T‐cell response in conjunction with a humoral immune response [2, 3, 4, 5]. Based on current understanding of host–virus interactions and the natural course of HBV infection, management strategies have focussed on enhancement of the host’s HBV‐specific T‐cell response and direct suppression of HBV replication to attain sustained viral control and remission of liver disease [6, 7]. Of the currently available anti‐HBV therapies, only interferon (IFN)‐α and pegylated IFN‐α have demonstrated an immunomodulatory effect on HBV [8]. The immunomodulatory effect has been reported with the nucleoside ribavirin; the mechanisms of action are considered to be Th1 cell differentiation and upregulation of activity of double‐stranded (ds) RNA‐activated protein kinase [9, 10, 11]. Very little is known about the influence of newer oral nucleosides on the T‐cell‐specific immune response.
Telbivudine, an L‐nucleoside analogue of thymidine, is a potent and specific inhibitor of HBV DNA polymerase, and it is approved for the treatment of patients with chronic hepatitis B (CHB) [12, 13, 14, 15, 16]. In the 2‐year analysis of the GLOBE study, the rate of hepatitis B e antigen (HBeAg) seroconversion during telbivudine administration was higher than during lamivudine administration in patients with serum alanine aminotransferase (ALT) levels greater than two times the upper limit of normal (ULN) [17]. The relatively high HBeAg seroconversion rate attained with telbivudine differs from the rates obtained with other potent anti‐HBV agents and suggests that telbivudine potentially has immunomodulatory activities.
The aims of the present study were to characterize the effects of telbivudine on cytokine profile and T‐cell response in vitro and in vivo using a previously characterized mouse model of viral hepatitis induced by the coronavirus mouse hepatitis virus strain 3 (MHV‐3) [18, 19]. The effect of telbivudine on the programmed cell death pathway, a possible mechanism by which CD8+ T‐cell function was influenced, was also investigated.
Materials and methods
Telbivudine and lamivudine
Telbivudine (pure substance) was obtained from Novartis Pharma (Basel, Switzerland) and lamivudine was purchased from Moravek Biochemical (Brea, CA, USA). Substance concentrations were determined by high‐performance liquid chromatography.
Virus
Mouse hepatitis virus strain 3 was originally obtained from the American Type Culture Collection (Rockville, MD, USA) and it was plaque purified on monolayers of DBT cells and grown to titres of 2 × 107 plaque‐forming units (p.f.u.)/mL. Virus was harvested by centrifugation at 4500 g for 1 h at 4 °C and was assayed on monolayers of L2 cells in a standard plaque assay.
Mice
Female BALB/cJ and C3H mice at 6–8 weeks of age were obtained from the Hubei Provincial Institute of Science and Technology (Wuhan, China) and the Vital River Company (Beijing, China), respectively. All animal studies were conducted in accordance with the Chinese Council on Animal Care and they were approved by Tongji Hospital (Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China).
In vitro study
Macrophage isolation
Peritoneal macrophages were harvested from BALB/cJ mice 4 days after an intraperitoneal injection of 1.5 mL of 3% thioglycollate, as outlined previously [20]. Macrophages were ≥95% pure, as determined by morphology. Viability exceeded 95% by Trypan blue exclusion.
Effect of telbivudine on the replication of MHV‐3 in macrophages
Macrophages were incubated in the presence of indicated concentrations of telbivudine (10, 50 or 100 μg/mL) with MHV‐3. Macrophages were harvested 10 h after infection and viral titre was determined in a standard plaque assay.
Cytokine level measurement by PCR and enzyme‐linked immunosorbent assay
To determine the effect of telbivudine on tumour necrosis factor (TNF)‐α and interleukin (IL)‐12 cytokine production, one million macrophages from BALB/cJ mice were stimulated with MHV‐3 (multiplicity of infection, 2.5) and telbivudine was added at indicated concentrations. Relative quantitative real‐time polymerase chain reaction (PCR) was performed to measure the mRNA levels on a Roche LightCycler® (Roche Diagnostics, Nutley, NJ, USA) following the manufacturers’ instructions. Cytokine protein levels were determined in cell supernatants following appropriate dilution using enzyme‐linked immunosorbent assay (ELISA) (R&D Systems, Abingdon, UK). The cytokine levels in lipopolysaccharide (LPS)‐stimulated macrophages with or without telbivudine added from the same strain of mice were also investigated.
In vivo study
Animal model
Fifteen C3H mice each infected with 10 p.f.u. MHV‐3 received telbivudine 60 mg/kg in 100 μL phosphate‐buffered saline (PBS) via intraperitoneal injection for 15 days, starting 2 h before infection and daily thereafter. An additional 15 MHV‐3‐infected mice in each group received lamivudine 60 mg/kg per day or PBS as a control. Clinical conditions and survival were observed. Animals were killed on Day 15 after infection, and spleens, livers and blood were collected for experiments.
Clinical conditions
The effect of treatment with telbivudine on MHV‐3‐induced liver disease was determined by monitoring mice twice daily for changes in clinical signs, including abnormalities of fur texture, increased respiration, and the presence of tremor. Conditions were graded on a scale of 1–5, with 1 indicating the marked presence of an abnormality and 5 indicating normalcy. A composite score for all parameters was calculated by two independent examiners who were blinded to the treatment regimens to minimize bias.
Liver histology
At the time the mice were killed, livers were removed, weighed and fixed in 10% buffered formalin for a minimum of 4 h. Tissues were processed and paraffin embedded by routine methods, and they were stained with haematoxylin and eosin.
Isolation of splenocytes and CD8+ T cells
Spleens from each group of mice were harvested 15 days after MHV‐3 infection. Spleens were dissected and single‐cell suspensions were made by passage through a nylon cell strainer. After density gradient centrifugation and lysis of red cells in ammonium chloride solution, cells were prepared for study. CD8+ T cells were purified from single‐cell splenocyte suspension using an isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany).
Hepatocytes
Mouse hepatitis virus strain 3‐infected C3H mice were anaesthetized and their livers were perfused in situ with a Ca2+‐free Hank’s balanced salt solution containing 1 mm ethylenediaminetetraacetic acid for 4 min at 37 °C, followed by a 0.05% collagenase solution (type IV; Sigma‐Aldrich, St Louis, MO, USA) for 10 min. After digestion, the livers were removed and placed into a sterile Petri dish. Single‐cell suspensions were prepared. The viability of hepatocytes both before and after treatment was ≥95%, as assessed by Trypan blue staining.
Serum collection and cytokine measurements
On the day the mice were killed, sera were collected and assayed for IL‐4 and IFN‐γ using the appropriate ELISA (R&D Systems).
Intracellular staining for cytokines
Mice were sacrificed at the indicated time points. Purified cells were cultured at 2 × 106 cells/mL in the presence of 25 ng/mL phorbol myristate acetate plus 1 μg/mL ionomycin for 5 h in 24‐well plates. Cells were then stained with monoclonal antibodies specific for surface markers, permeabilized, and fixed using Cytofix/Cytoperm™ (BD, Franklin Lakes, NJ, USA) according to the manufacturer’s instructions. Different cell subsets were identified using antibodies to IL‐4, IL‐10, IFN‐γ, TNF‐α, perforin, granzyme B (eBioscience, San Diego, CA, USA) and their isotypes.
Flow cytometry
The antibodies used for staining were anti‐CD3 (PE‐Cy5.5), anti‐CD8 (APC), anti‐CD4 [fluorescein isothiocyanate (FITC)], anti‐programmed death 1(PD‐1), anti‐programmed death ligand 1 (PDL‐1), anti‐Fas ligand (FasL), anti‐perforin, anti‐granzyme B, anti‐TNF‐α‐R1/2, anti‐TNF‐α, anti‐IFN‐γ (PE), and isotype control (PE) (eBioscience). All analyses were performed on a FACSAria™ cell sorter (BD), and subsequent analysis was performed with FACSDiva software (BD).
Cytotoxicity against hepatocytes
Freshly isolated hepatocytes from MHV‐3‐infected C3H mice were used as target cells and CD8+ T cells as effector cells. Cytolytic activity was determined with the CytoTox 96® non‐radioactive cytotoxicity assay (Promega, Madison, WI, USA). For each effector:target cell ratio (ranging from 40.0:1 to 2.5:1), specific target cell lysis was calculated as follows: [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. Each effector:target cell ratio was measured in quadruplicate.
Statistical analysis
Statistical analysis was conducted with a Student’s t‐test, and a P‐value of ≤0.05 was considered statistically significant. Results were reported as the mean ± standard deviation for three or more separate experiments, each performed in triplicate.
Results
Telbivudine does not affect MHV‐3 replication in macrophages
First, we investigated whether telbivudine has no cytotoxic effect on macrophages. The addition of up to 100 μg/mL telbivudine to peritoneal macrophages freshly isolated from BALB/cJ mice had no toxic effects, as demonstrated by Trypan blue exclusion (95% viable cells).
To examine the efficacy of telbivudine on viral replication directly, isolated macrophages were treated with telbivudine at indicated concentrations. The starting concentration (10 μg/mL) was chosen based on maximal concentration and area under the concentration–time curve from zero to infinity, and it was as close to twice the dose as that for patient administration [21, 22]. Telbivudine had no effect on MHV‐3 replication from the starting dose of 10 μg/mL to doses as high as 50 and 100 μg/mL (Fig. 1).
Figure 1.
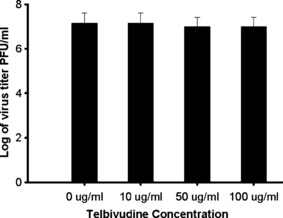
Telbivudine does not affect MHV‐3 replication in BALB/cJ macrophages in vitro. Confluent monolayers of macrophages were infected with 2 × 106 MHV‐3 in the presence of indicated concentrations of telbivudine for 10 h. Viral titres were measured on monolayers of L2 cells. Data are presented as mean ± SD for three separate experiments done in duplicate.
Telbivudine enhanced the production of TNF‐α and IL‐12 in macrophages after MHV‐3 infection but not in LPS‐induced macrophages
Experiments were performed to test the ability of telbivudine to modulate the immune response of MHV‐3‐infected macrophages. Macrophages produced significantly higher levels of TNF‐α and IL‐12 in response to MHV‐3 infection in comparison with basal values. Telbivudine significantly enhanced the production of TNF‐α and IL‐12 in MHV‐3‐infected macrophages compared with controls and this effect occurred in a dose‐dependent manner (Figs 2b,d). A similar pattern of increase in TNF‐α and IL‐12 mRNA production was also observed in response to telbivudine (Figs 2a,c). However, telbivudine had no effect on TNF‐α and IL‐12 levels or mRNA production in LPS‐stimulated macrophages (Figs 2a,b).
Figure 2.
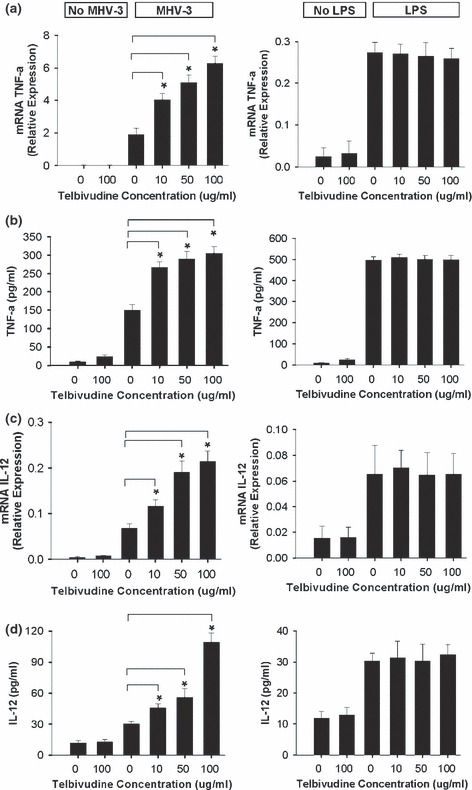
Effect of telbivudine on MHV‐3‐infected macrophages in vitro. The left panels show that telbivudine enhanced the production and mRNA transcription of TNF‐α (a,b) and IL‐12 (c,d) in macrophages after MHV‐3 infection. The right panels show that telbivudine has no effect on TNF‐α (a,b) or IL‐12 (c,d) in LPS‐induced macrophages. Data are presented as mean ± SD for three separate experiments. *P < 0.01.
Telbivudine improves survival and clinical outcome in vivo
Telbivudine increased the survival rate in MHV‐3‐infected C3H mice from 18.2% to 63.6% compared with the PBS‐treated controls (Fig. 3a). A composite clinical score was obtained by combining the clinical scores for all measured symptoms. Telbivudine‐treated mice maintained improved composite clinical scores in all conditional categories to the end of the study (Fig. 3b).
Figure 3.
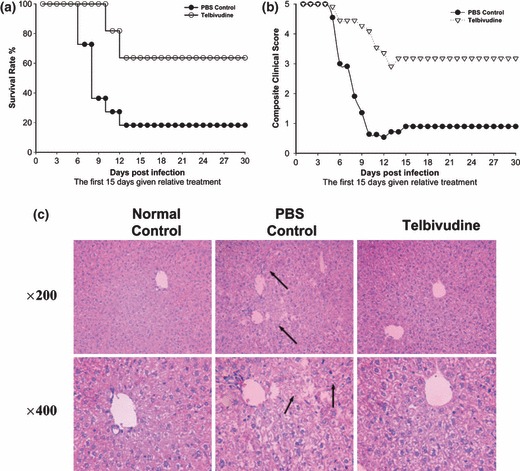
Telbivudine therapy improves survival rate, clinical conditions and histological lesions in MHV‐3‐infected C3H mice. Fifteen animals in each group received either telbivudine or PBS for 15 days. (a) Survival rates in the telbivudine group increased to 63.6% when compared with the PBS group (18.2%). (b) Composite clinical scores: scores were calculated for fur texture, respiration and tremor. Data are the mean examination of three animals per group scored in a blinded fashion. (c) Histological improvement: at 15 days after infection, hepatic necrosis was minimized post telbivudine treatment (arrows).
Telbivudine reduces histological lesions in the liver
In addition to improving overall survival and clinical scores, telbivudine reduced the histological signs associated with MHV‐3 infection (Fig. 3c). The livers recovered from the PBS‐treated mice showed remarkable necrosis by 15 days after infection compared with the livers obtained from the uninfected control mice (the arrows in Fig. 3c indicate necrosis). In contrast, although not completely free of disease, the livers recovered from the telbivudine‐treated mice showed a marked reduction in histological evidence of hepatic necrosis (Fig. 3c).
Telbivudine promotes IFN‐γ production and inhibits IL‐4 production in vivo
Telbivudine treatment resulted in an additional 1.8‐fold increase in IFN‐γ production in sera compared with MHV‐3‐infected controls (Table 1). In contrast, a 1.6‐fold inhibition of IL‐4 production was observed in the telbivudine‐treated group compared with the controls.
Table 1.
Effect of telbivudine on cytokine release following MHV‐3 infection in vivo
| Days after MHV‐3 infection in C3H mice | No infection control | Treatment | ||||
|---|---|---|---|---|---|---|
| PBS | Telbivudine | |||||
| IFN‐γ (ng/mL) | IL‐4 (pg/mL) | IFN‐γ (ng/mL) | IL‐4 (pg/mL) | IFN‐γ (ng/mL) | IL‐4 (pg/mL) | |
| 15 | 17.7 ± 1.3 | 48.2 ± 2.0 | 67.4 ± 3.5 | 44.6 ± 2.6 | 125.9* ± 4.3 | 26.6* ± 1.8 |
Concentrations of IFN‐γ and IL‐4 were measured in sera of C3H mice 15 days after infection. Data are presented as mean ± SD from three separate experiments with three mice in each group. *P < 0.01 compared with PBS‐treated mice.
Telbivudine increases the total number of T cells, the percentage of IFN‐γ‐ and TNF‐α‐producing CD4+ T cells, and the percentage of IFN‐γ‐producing CD8+ T cells
Phenotypic analysis of spleen cells from telbivudine‐treated mice revealed an increase in the total number of T cells compared with PBS‐ and lamivudine‐treated controls (Fig. 4a). The frequency of IFN‐γ‐producing CD4+ and CD8+ T cells in the telbivudine‐treated group was significantly increased compared with the PBS‐ and lamivudine‐treated groups (Figs 4b,c,f). The frequency of TNF‐α‐producing CD4+ T cells in the telbivudine‐treated group was increased significantly compared with the PBS‐ and lamivudine‐treated groups (Figs 4d,g). The frequency of the TNF‐α‐producing CD8+ T cells was similar among all of the treatment groups (Figs 4e,g). No difference was observed in the frequency of IL‐4‐ and IL‐10‐producing CD4+ and CD8+ T cells (data not shown).
Figure 4.
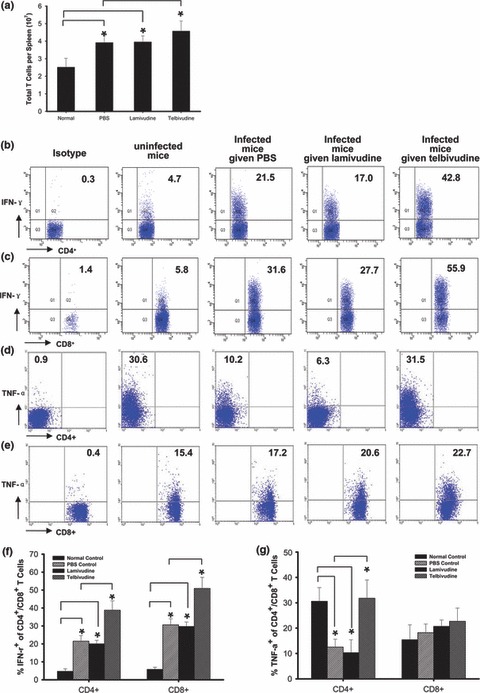
Effect of telbivudine treatment on the total number of T cells and the percentage of IFN‐γ and TNF‐α in CD4+ and CD8+ T cells. Mice were sacrificed 15 days after infection, and single‐cell suspensions were prepared from their spleen. Cells were stained with anti‐CD3, anti‐CD4 or anti‐CD8, and IFN‐γ or TNF‐α. Telbivudine treatment increased the total number of T cells, which was determined by the percentage of CD3+ cells in counted splenocytes (a). Telbivudine increased the percentage of IFN‐γ‐ and TNF‐α‐producing CD4+ T cells (b,d,f,g) and the percentage of IFN‐γ‐producing CD8+ T cells (c,f,g). The numbers in plots were assessed on CD4+ or CD8+ T cells. The results are a combination of three independent experiments. (b–e) are representative of three similar experiments. *P < 0.01.
Telbivudine has no effect on cytolytic function but downregulates PD‐L1 expression on T cells
To determine whether telbivudine treatment enhanced killer T‐cell cytotoxicity, we performed cytotoxicity assays using spleen‐isolated CD8+ T cells as effector cells. An increase in cytotoxicity (Fig. 5a) was observed in all infected groups, but no differences were observed among groups with or without telbivudine treatment, suggesting that telbivudine has no effect on the cytolytic function of T cells. PD‐1 and FasL expression were increased in infected groups compared with the uninfected group, but no differences were observed among infected groups (Fig. 5b). There were no significant differences for tumour necrosis factor receptor (TNFR)‐1, TNFR‐2, perforin or granzyme B expression on the CD8+ T cells among infected groups and the uninfected group (Fig. 5c). To further understand this phenomenon, we investigated the expression of PD‐L1 and found that telbivudine treatment significantly downregulated the expression of PD‐L1 on CD3+ and CD8+ T cells compared with PBS and lamivudine treatment (Figs 5d,e).
Figure 5.
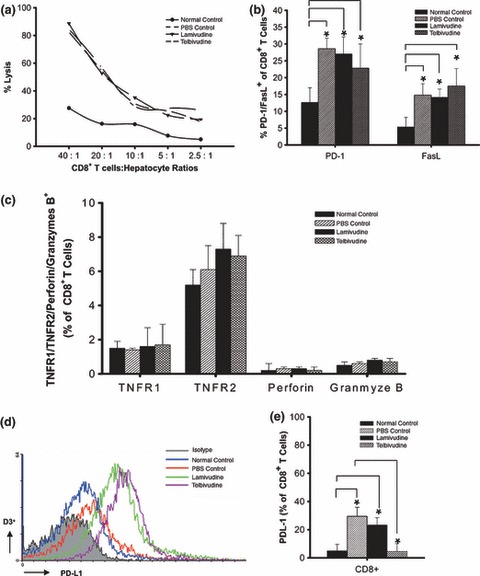
Telbivudine has no effect on cytotoxicity. Hepatocytes were isolated from MHV3‐infected C3H mice on Day 15 as target cells. As effector cells, CD8+ T cells (pooled from three mice per experimental group) were obtained from each group. (a) Cytotoxicity to hepatocytes and (b) PD‐1 and Fas ligand expression on CD8+ T cells were increased in infected groups. There were no differences between the PBS‐, lamivudine‐ and telbivudine‐treated groups. (c) TNFR1, TNFR2, perforin and granzyme B expression on CD8+ T cells were similar in each group. (d) Histograms show PD‐L1 expression levels on CD3+ cells from each group. (e) The percentage of PD‐L1 expression on CD8+ T cells is shown. The results are a combination of three independent experiments. (d) is representative of three similar experiments. *P < 0.01.
Discussion
This is the first study to demonstrate, both in vitro and in vivo, that telbivudine has immunomodulatory effects, independent of its potent antiviral activity. Our data show that telbivudine has no inhibitory effects on the replication of MHV‐3 in macrophages. This finding is consistent with the fact that telbivudine is highly selective for HBV and lacks activity against other viruses [12]. This study showed that telbivudine, at concentrations that are achieved in vivo, enhanced the production of TNF‐α and IL‐12 in BALB/cJ macrophages in vitro when challenged with MHV‐3. Telbivudine was unable to cause a similar effect in LPS‐induced inflammatory cytokines. This may be because the mechanism of induction of TNF‐α and IL‐12 by LPS is different from that by virus [23].
Previous studies of the immune response during antiviral treatment with nucleoside or nucleotide analogues in patients with CHB have shown that potent suppression of HBV replication is associated with enhanced T‐cell reactivity and cytokine production [24, 25, 26]. However, these studies in patients with CHB do not allow dissection of whether the effect on adaptive immunity is due to direct immunomodulatory properties of the antiviral agent or a secondary to reduced viraemia and antigen levels. The advantage of our mouse model is that it allows investigation of potential immunomodulatory effects independent of antiviral activity. The use of MHV‐3, which produces a strain‐dependent pattern of viral hepatitis in inbred strains of mice, has brought insights into the pathogenesis of viral hepatitis [27, 28, 29, 30, 31]. A previous study using an MHV‐3 virus‐induced hepatitis model from our group showed that ribavirin preserves Th1 cytokine production but inhibits Th2 cytokine response; the study explored the remarkable increased sustained virological response post interferon and ribavirin combination therapy for hepatitis C patients [9].
The results from the in vitro experiments on the MHV‐3‐infected macrophages support a further investigation of the immunomodulatory effect of telbivudine in vivo. Because MHV‐3 belongs to the coronavirus family of positive‐stranded RNA viruses, it is a suitable model with which to study the pure immunomodulatory effect of telbivudine without the influence of its viral inhibitory effect, which in turn may indirectly affect the immune system. In this study, telbivudine treatment significantly improved survival in MHV‐3‐infected C3H mice and it improved clinical conditions and histological outcomes. The treatment of MHV‐3‐infected C3H mice with telbivudine resulted in the inhibition of IL‐4 production, and similar doses of telbivudine enhanced the production of IFN‐γ, and furthermore increased total T cell numbers, IFN‐γ‐producing CD4+ and CD8+ T cells and TNF‐α‐producing CD4+ T cells, indicating a predominantly Th1 response post telbivudine treatment.
One mechanism of the immune response involves naive CD4+ T cells, which, through the production of cytokines, control the development of immune–effector mechanisms [32]. A Th1 response has been associated with host resistance whereas a Th2 response has been associated with susceptibility in murine models of MHV‐3. Studies have suggested that resistance to MHV‐3 is associated with a predominant Th1 response, the production of IFN, and cytotoxic T cells [33, 34]. Cytotoxic lymphocytes kill target cells by two major pathways: one is dependent on the exocytosis of granule effector molecules, including perforin and granzymes A and B; the other is dependent on the engagement of target cell TNFR‐1 family death receptors (e.g. Fas, TNFR‐1, TNFR‐2) by effector cell‐expressed FasL, membrane‐bound or secreted TNF, or TNF‐related apoptosis‐inducing ligand [35, 36, 37]. Our results demonstrate that FasL plays an important role in cytotoxic T lymphocyte (CTL) cytotoxicity to hepatocytes in MHV‐3‐infected C3H mice. However, telbivudine treatment did not promote CTL cytotoxicity. Previous studies have established that TNF‐α and IFN‐γ are key cytokines for non‐cytolytic control of HBV replication [38, 39, 40]. The release of these cytokines from T cells leads to inactivation of HBV without killing the infected hepatocytes. Our results suggest that telbivudine is altering the balance of Th1/Th2 cytokines which is independent of the direct antiviral effect against MHV‐3.
Programmed death‐1 is a receptor that is expressed on a subset of activated T and B cells and PD‐L1 (also known as B7‐H1) is expressed on both haematopoietic and parenchymal cells [41, 42]. In chronic viral infection, CD8 T cells express high levels of PD‐1, but its ligand PD‐L1 is also highly upregulated [43]. In vivo blockade of the interaction of PD‐1 with its ligand PD‐L1 has been reported to enhance T‐cell responses [43]. Reduction of PD‐1 during the treatment of CHB was shown to correlate with reduction of HBV DNA levels [26]. Given the role of PD‐1/PD‐L1 is an important inhibitory pathway in T‐cell function, the effect of telbivudine on the expression of PD‐1 and PD‐L1 was investigated to further define the effect of telbivudine on T‐cell response. Although telbivudine does not alter the expression of PD‐1, of note is that it significantly downregulated PD‐L1 expression on T cells in MHV‐3‐infected C3H mice. This result provides initial evidence for a role of telbivudine in this inhibitory pathway in regulating T‐cell function that may favour the development of immune clearance of virus infection. However, the effects of telbivudine on PD‐1 and PD‐L1 expression on other cell types should be clarified in further studies. Also, the underlying molecular mechanism requires further investigation. One of the proposed mechanisms is upregulation of the activity of cellular kinases to facilitate efficient phosphorylation. On the other hand, many nucleoside analogue‐like substances [e.g. CpG‐containing DNA, as found in DNA viruses and bacteria; viral dsRNA; the viral mimic polyinosinic:polycytidylic acid (polyI:C)] can exert immunomodulatory activities via toll‐like receptors. As telbivudine is a thymidine nucleoside analogue, it may exert immunomodulatory activities via toll‐like receptors.
In summary, the present study demonstrates that the beneficial effects of telbivudine in MHV‐3‐induced hepatitis may be mediated by its effect on the immune response rather than the inhibition of viral replication in this animal model. The immunomodulatory effect of telbivudine may be explained partly by a shift in the balance between Th1‐ and Th2‐like immune responses. This in turn may explain the benefit of telbivudine on the amelioration of MHV‐3 infection and increased survival as well as improvements in clinical scores and liver histology. These data identify an immunomodulatory mechanism of telbivudine treatment in the MHV‐3‐induced viral hepatitis model and provide an insight into a potential additional mode of action for the management of viral hepatitis infection.
Statement of interests
Authors’ declaration of personal interests: The authors have no financial conflict of interest.
Declaration of funding interests: This work was funded by the National Key Basic Research Program of China (grant no. 2007CB512900) and the Eleventh 5‐Year Plan Key Project (grant no. 2008ZX10002‐004).
Acknowledgements
We thank Jinshang Hu for secretarial work and members of our institution for discussion and technical help.
References
- 1. World Health Organization . Hepatitis B Fact Sheet. Available at: http://www.who.int/mediacentre/factsheets/fs204/en/ (accessed 31 January 2009).
- 2. Ferrari C, Penna A, Bertoletti A et al. Cellular immune response to hepatitis B virus‐encoded antigens in acute and chronic hepatitis B virus infection. J Immunol 1990; 145: 3442–3449. [PubMed] [Google Scholar]
- 3. Rehermann B, Fowler P, Sidney J et al. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J Exp Med 1995; 181: 1047–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thimme R, Wieland S, Steiger C et al. CD8 (+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol 2003; 77: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Penna A, Artini M, Cavalli A et al. Long‐lasting memory T cell responses following self‐limited acute hepatitis B. J Clin Invest 1996; 98: 1185–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. European Association for the Study of the Liver . EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol 2009; 50: 227–242. [DOI] [PubMed] [Google Scholar]
- 7. Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45: 507–539. [DOI] [PubMed] [Google Scholar]
- 8. Tilg H. New insights into the mechanisms of interferon alfa: an immunoregulatory and anti‐inflammatory cytokine. Gastroenterology 1997; 112: 1017–1021. [DOI] [PubMed] [Google Scholar]
- 9. Ning Q, Brown D, Parodo J et al. Ribavirin inhibits viral‐induced macrophage production of TNF, IL‐1, the procoagulant fgl2 prothrombinase and preserves Th1 cytokine production but inhibits Th2 cytokine response. J Immunol 1998; 160: 3487–3493. [PubMed] [Google Scholar]
- 10. Ning Q, Lakatoo S, Liu M et al. Induction of prothrombinase fgl2 by the nucleocapsid protein of virulent mouse hepatitis virus is dependent on host hepatic nuclear factor‐4 alpha. J Biol Chem 2003; 278: 15541–15549. [DOI] [PubMed] [Google Scholar]
- 11. Liu WL, Su WC, Cheng CW et al. Ribavirin up‐regulates the activity of double‐stranded RNA‐activated protein kinase and enhances the action of interferon‐alpha against hepatitis C virus. J Infect Dis 2007; 196: 425–434. [DOI] [PubMed] [Google Scholar]
- 12. Bryant ML, Bridges EG, Placidi L et al. Antiviral L‐nucleosides specific for hepatitis B virus infection. Antimicrob Agents Chemother 2001; 45: 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Golitsina N, Danehy F, Fellows R. Telbivudine phosphorylation by three enzymes: implications for antihepatitis B virus activity in vitro and in the clinic. Hepatology 2006; 44: 561A. 16941686 [Google Scholar]
- 14. Hernandez‐Santiago B, Placidi L, Cretton‐Scott E et al. Pharmacology of beta‐L‐thymidine and beta‐L‐2′‐deoxycytidine in HepG2 cells and primary human hepatocytes: relevance to chemotherapeutic efficacy against hepatitis B virus. Antimicrob Agents Chemother 2002; 46: 1728–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seifer M, Patty A, Dukhan D. Telbivudine (LDT) preferentially inhibits second (+) strand HBV DNA synthesis. J Hepatol 2005; 42: 151. [Google Scholar]
- 16. Lai CL, Gane E, Liaw YF et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med 2007; 357: 2576–2588. [DOI] [PubMed] [Google Scholar]
- 17. Liaw YF, Gane E, Leung N et al. 2‐Year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology 2009; 136: 389–392. [DOI] [PubMed] [Google Scholar]
- 18. Qin XM, Zhang JG, Su K, Yang C, Yan WM, Ning Q. Establishment and characterization of a chronic viral hepatitis model induced by murine hepatitis virus type 3. Virol Sin 2006; 21: 417–420. [Google Scholar]
- 19. Zhang J, Qin X, Wang XJ et al. A primary study of the subgroups of T lymphocytes in MHV‐3 induced chronic viral hepatitis. Virol Sin 2007; 22: 339–346. [Google Scholar]
- 20. Levy GA, Adamson G, Phillips MJ et al. Targeted delivery of ribavirin improves outcome of murine viral fulminant hepatitis via enhanced anti‐viral activity. Hepatology 2006; 43: 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu P, Jiang J, Wang H et al. Single‐dose and multiple‐dose pharmacokinetics and safety of telbivudine after oral administration in healthy Chinese subjects. J Clin Pharmacol 2006; 46: 999–1007. [DOI] [PubMed] [Google Scholar]
- 22. Zhou XJ, Lim SG, Lloyd DM, Chao GC, Brown NA, Lai CL. Pharmacokinetics of telbivudine following oral administration of escalating single and multiple doses in patients with chronic hepatitis B virus infection: pharmacodynamic implications. Antimicrob Agents Chemother 2006; 50: 874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shigemi S, Tsutsumi H, Takeuchi R, Matsuda K, Imai S, Ogra PL. Enhanced cytokine production by milk macrophages following infection with respiratory syncytial virus. J Leukoc Biol 1997; 61: 630–636. [DOI] [PubMed] [Google Scholar]
- 24. Boni C, Penna A, Bertoletti A et al. Transient restoration of anti‐viral T cell responses induced by lamivudine therapy in chronic hepatitis B. J Hepatol 2003; 39: 595–605. [DOI] [PubMed] [Google Scholar]
- 25. Cooksley H, Chokshi S, Maayan Y et al. Hepatitis B virus e antigen loss during adefovir dipivoxil therapy is associated with enhanced virus‐specific CD4+ T‐cell reactivity. Antimicrob Agents Chemother 2008; 52: 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Evans A, Riva A, Cooksley H et al. Programmed death 1 expression during antiviral treatment of chronic hepatitis B: impact of hepatitis B e‐antigen seroconversion. Hepatology 2008; 48: 759–769. [DOI] [PubMed] [Google Scholar]
- 27. Ding JW, Ning Q, Liu MF et al. Fulminant hepatic failure in murine hepatitis virus strain 3 infection: tissue‐specific expression of a novel fgl2 prothrombinase. J Virol 1997; 71: 9223–9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ding JW, Ning Q, Liu MF et al. Expression of the fgl2 and its protein product (prothrombinase) in tissues during murine hepatitis virus strain‐3 (MHV‐3) infection. Adv Exp Med Biol 1998; 440: 609–618. [DOI] [PubMed] [Google Scholar]
- 29. Levy GA, Liu M, Ding J et al. Molecular and functional analysis of the human prothrombinase gene (HFGL2) and its role in viral hepatitis. Am J Pathol 2000; 156: 1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marsden PA, Ning Q, Fung LS et al. The Fgl2/fibroleukin prothrombinase contributes to immunologically mediated thrombosis in experimental and human viral hepatitis. J Clin Invest 2003; 112: 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu C, Sun Y, Luo X, Yan W, Xi D, Ning Q. Novel mfgl2 antisense plasmid inhibits murine fgl2 expression and ameliorates murine hepatitis virus type 3‐induced fulminant hepatitis in BALB/cJ mice. Hum Gene Ther 2006; 17: 589–600. [DOI] [PubMed] [Google Scholar]
- 32. Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1989; 7: 145–173. [DOI] [PubMed] [Google Scholar]
- 33. Levy GA, Shaw R, Leibowitz JL, Cole E. The immune response to mouse hepatitis virus: genetic variation in antibody response and disease. Adv Exp Med Biol 1984; 173: 345–364. [DOI] [PubMed] [Google Scholar]
- 34. Pope M, Chung SW, Mosmann T, Leibowitz JL, Gorczynski RM, Levy GA. Resistance of naive mice to murine hepatitis virus strain 3 (MHV‐3) requires development of Th1, but not Th2 response, whereas pre‐existing antibody protects against primary infection. J Immunol 1996; 156: 3342–3349. [PubMed] [Google Scholar]
- 35. Kägi D, Vignaux F, Ledermann B et al. Fas and perforin pathways as major mechanisms of T cell‐mediated cytotoxicity. Science 1994; 265: 528–530. [DOI] [PubMed] [Google Scholar]
- 36. Braun MY, Lowin B, French L, Acha‐Orbea H, Tschopp J. Cytotoxic T cells deficient in both functional fas ligand and perforin show residual cytolytic activity yet lose their capacity to induce lethal acute graft‐versus host disease. J Exp Med 1996; 183: 657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Russell JH, Ley TJ. Lymphocyte‐mediated cytotoxicity. Annu Rev Immunol 2002; 20: 323–370. [DOI] [PubMed] [Google Scholar]
- 38. Chisari FV. Hepatitis B virus transgenic mice: insights into the virus and the disease. Hepatology 1995; 22: 1316–1325. [DOI] [PubMed] [Google Scholar]
- 39. Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol 2001; 19: 65–91. [DOI] [PubMed] [Google Scholar]
- 40. Suri D, Schilling R, Lopes AR et al. Non‐cytolytic inhibition of hepatitis B virus replication in human hepatocytes. J Hepatol 2001; 35: 790–797. [DOI] [PubMed] [Google Scholar]
- 41. Agata Y, Kawasaki A, Nishimura H et al. Expression of the PD‐1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 1996; 8: 765–772. [DOI] [PubMed] [Google Scholar]
- 42. Nishimura H, Honjo T. PD‐1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol 2001; 22: 265–268. [DOI] [PubMed] [Google Scholar]
- 43. Barber DL, Wherry EJ, Masopust D et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006; 439: 682–687. [DOI] [PubMed] [Google Scholar]


