Abstract
Limited information is available on the non‐influenza etiology and epidemiology of influenza‐like illness (ILI) in China. From April 2011 to March 2014, we collected oropharyngeal swabs from children less than 5 years of age with symptoms of ILI who presented to the outpatient departments of Suzhou University Affiliated Children's Hospital (SCH). We used reverse transcription polymerase chain reaction (rt‐PCR) or PCR to detect 11 respiratory viruses. Among 3,662 enrolled ILI patients, 1,292 (35.3%) tested positive for at least one virus. Influenza virus (16.9%) was detected most frequently (influenza A 7.4%, influenza B 9.5%), followed by respiratory syncytial virus (RSV) (5.6%), parainfluenza virus (PIV) types 1–4 (4.8%), human bocavirus (HBoV) (3.8%), human metapneumovirus (HMPV) (3.5%), and adenovirus (ADV) (3.0%). Co‐infections were identified in 108 (2.9%) patients. Influenza virus predominantly circulated in January–March and June–July. The 2013–2014 winter peaks of RSV and influenza overlapped. Compared with other virus positive cases, influenza positive cases were more likely to present with febrile seizure, and RSV positive cases were more likely to present with cough and wheezing, and were most frequently diagnosed with pneumonia. These data provide a better understanding of the viral etiology of ILI among children less than 5 years of age in Suzhou, China. Influenza is not only the most frequently identified pathogen but it is also the only vaccine preventable illness among the 11 pathogens tested. Such findings suggest the potential value of exploring value of influenza vaccination among this influenza vaccination target group. J. Med. Virol. 88:1334–1340, 2016 . © 2016 Wiley Periodicals, Inc.
Keywords: influenza virus, respiratory syncytial virus, parainfluenza virus
INTRODUCTION
Acute respiratory infections (ARI) are one of the leading causes of morbidity and mortality in children, especially in developing countries [Razanajatovo et al., 2011]. The etiology of ARIs is often unknown, because viral testing among patients with respiratory symptoms is uncommon [Ju et al., 2014]. Many countries conduct influenza‐like illness (ILI) surveillance, and monitor cases of respiratory illness with fever. Data from these surveillance systems suggest that ILI is caused by a wide range of respiratory viruses, including seasonal influenza A and B viruses (FLU A and FLU B), respiratory syncytial viruses A and B (RSV A and RSV B), parainfluenza viruses 1–4 (PIVs 1–4), human rhinovirus (HRV), adenovirus (ADV), and others [Ren et al., 2009; Buecher et al., 2010; Razanajatovo et al., 2011; Thiberville et al., 2012]. Recent advances in molecular detection techniques have facilitated the identification of several novel respiratory viruses that could also cause influenza‐like illness, such as human metapneumovirus (HMPV), human bocavirus (HBoV), human coronavirus (HCoV‐NL63 and HKU1), and more [van der Hoogen et al., 2001; van der Hoek et al., 2004; Woo et al., 2005; Allander et al., 2007].
In 2000, China launched a nationwide surveillance network for influenza. This ILI surveillance system provides timely, though nonspecific, data on influenza activity. In China, as in other countries, only 10–30% of influenza‐like illnesses are caused by influenza virus [Thomas, 2014; Otomaru et al., 2015]. Identifying ILI causative agents based on clinical signs and symptoms is difficult, because most respiratory illnesses, regardless of etiology, lead to similar clinical presentations. In addition, there are limited data on the incidence and clinical presentation of respiratory infections caused by viruses other than influenza in China [Peng et al., 2012; Yang et al., 2012; Li et al., 2013; Ju et al., 2014; Fu et al., 2015].
Building upon the influenza surveillance system established in Suzhou University Affiliated Children's Hospital (SCH) in 2009, we investigated ILI cases from April 2011 to March 2014 among children less than 4 years of age presenting to the outpatient department in SCH, Suzhou to better understand how frequently 11 common viral respiratory pathogens cause ILI, and to describe their associated epidemiological and clinical characteristics.
MATERIALS AND METHODS
Study Site
This study was conducted at SCH in Jiangsu Province, China. SCH is the single tertiary children's hospital serving Suzhou district. An investigation of medical records showed that in 2011 the total number of outpatient visits among children less than 5 years old at this hospital was 396,568, which accounted for approximately 28.5% of all outpatient visits among children less than 5 years of age in the municipal district of Suzhou [Wang et al., 2013].
Patient Enrollment
We conducted surveillance for influenza‐like‐illness (ILI) among children less than five years old who presented to the outpatient department of SCH from 2011 to 2014. ILI was defined as the presence of fever (axillary temperature ≥38°C) and cough or sore throat (inflamed or red pharynx was used to judge sore throat in young children), with onset of symptoms within the last 3 days. At the beginning of each month, we randomly selected 1–3 trained attending physicians among the 20 serving within the outpatient department and emergency department (ED) to enroll patients five working days per week. After obtaining informed consent from the parent or guardian, physicians enrolled all children less than 5 years of age who met the ILI case definition on any data collection day. At the time of enrollment, the investigator or physician collected an oropharyngeal swab specimen, and conducted a brief questionnaire to collect contact information, demographic data, past medical history, and a description of clinical manifestations.
Specimen Management and Laboratory Tests
After collection, each oropharyngeal swab was immediately placed into viral transport media (Youkang Technology Co., Beijing, China). The media was then divided into two tubes; one was stored at 4°C, while the other was stored at −80°C. Within 72 hr, the first tube was sent by cold chain to the National Influenza Surveillance Network laboratory of Suzhou CDC for influenza virus testing; the second tube was sent to the molecular laboratory of the Department of Epidemiology, School of Public Health, Fudan University to conduct testing for respiratory viruses other than influenza.
Viral RNA was extracted using High Pure Viral RNA Kits (Roche, Shanghai, China) according to the manufacturer's instructions. Viral DNA was extracted using MagGene Viral DNA/RNA Kits (Tiangen Biotech, Beijing, China). We used reverse transcription‐polymerase chain reaction (RT‐PCR) or polymerase chain reaction (PCR) methods to detect 11 common respiratory viruses, including influenza A and B, RSV A and B, PIVs 1–4, ADV, HMPV, and HBoV. For influenza virus testing, we performed rRT‐PCR using influenza virus A/B dual fluorescent quantitative RT‐PCR kits (BioPerfectus Technology Co., Jiangsu, China). For other respiratory virus testing, we used methods obtained by literature review (Supplement Table). The primers were synthesized by Sangon Biotech Company, and reverse transcriptase and Taq polymerase were obtained from Takara Company (PrimeScript® RT Master Mix and Premix Taq® Version 2.0).
Statistical Analysis
Data analysis were performed using SPSS statistical package version 17.0 (SPSS Inc., Chicago, IL). Descriptive statistics were used to summarize the continuous variables and discrete variables. Categorical variables were presented as numbers or percentages, and Chi‐square or, when appropriate, two‐tailed Fisher's exact test were used to compare groups. Continuous variables were presented as the mean with standard deviation (S.D.) or the mean with 95% confidence interval (CI) or as the median with inter‐quartile range (IQR). Student's t test or nonparametric test was used to compare groups.
Ethics Statement
This study was approved by the Institutional Review Board (IRB) of the School of Public Health, Fudan University. Verbal informed consent was obtained from parents or guardians of children before specimen collection and questionnaire administration.
RESULTS
Demographics of Enrolled Patients
From April 2011 to March 2014, we enrolled 3,662 patients with ILI; throat swabs were collected and respiratory virus detection was completed on all. Among these, 2,882 (78.7%) were from the outpatient department, while 780 (21.3%) were from the ED. The male to female ratio was 1.28:1 and the median age was 19.5 months (IQR: 10.5–36.0). The distributions of gender, age, district, and medical insurance were similar across the outpatient department and the ED.
Detection of Respiratory Viruses
Among the 3,662 ILI patients, 1,292 (35.3%) tested positive for at least one virus, and among these, 846 (65.5%) tested positive for a respiratory virus other than influenza. The percent positive was highest for influenza virus (16.9%, influenza A 7.4%, influenza B 9.5%), followed by RSV (5.6%), PIV types 1–4 (4.8%), HBoV (3.8%), HMPV (3.5%), and ADV (3.0%). The majority of patients with RSV had RSV type A (96.1%). Among the patients with PIV, all four types were identified, with PIV types three and one accounting for the majority (63.1% and 26.1%, respectively). Co‐infection was identified among 108 (2.9%) patients, and of these, 51 (47.2%) were co‐infected with influenza and other viruses. (Table I). The age and gender distribution of different virus positive ILI cases was shown in Table II. The majority of RSV, PIV, ADV, and HBoV positive cases were aged 12–35 months, but HMPV positive cases mainly distributed in 36–47 months age group.
Table I.
Respiratory Virus Test Results From Outpatients Aged Less Than 5 Years With Influenza‐Like Illness in Suzhou, China From April 2011 to March 2014 (N = 3662)
| Viral type a | Number of positive cases | Positive rate (%) |
|---|---|---|
| Influenza virus | 619 | 16.9 |
| FLU A | 270 | 7.4 |
| FLU B | 349 | 9.5 |
| RSV | 206 | 5.6 |
| RSV A | 198 | 5.4 |
| RSV B | 8 | 0.2 |
| PIV | 176 | 4.8 |
| PIV 1 | 46 | 1.3 |
| PIV 2 | 4 | 0.1 |
| PIV 3 | 111 | 3.0 |
| PIV 4 | 15 | 0.4 |
| HMPV | 128 | 3.5 |
| ADV | 109 | 3.0 |
| HBoV | 138 | 3.8 |
| Co‐infection b | 108 | 2.9 |
| At least one virus detected c | 1292 | 35.3 |
| At least one virus other than influenza detected d | 846 | 23.1 |
Influenza viruses (FLU), respiratory syncytial viruses (RSV), parainfluenza viruses (PIV), human metapneumovirus (HMPV), adenovirus (ADV), and human bocavirus (HBoV).
More than one respiratory virus detected.
At least one respiratory virus tested positive.
At least one respiratory virus detected and influenza virus not detected.
Table II.
Demographic Characteristics of Influenza‐Like Illness Outpatients by Virus Type, Suzhou Children's Hospital, Suzhou, China, April 2011–March 2014, n (%)
| Flu(+) (N = 619) | RSV(+) (N = 206) | PIV(+) (N = 176) | HMPV(+) (N = 128) | ADV(+) (N = 109) | HBoV(+) (N = 138) | Virus(−) (N = 2370) | P‐value | |
|---|---|---|---|---|---|---|---|---|
| Gender | 0.910 | |||||||
| Male | 359 (58.0) | 120 (58.1) | 98 (55.4) | 67 (52.7) | 61 (56.1) | 77 (55.8) | 1323 (55.8) | |
| Age | <0.001 | |||||||
| 0m‐ | 30 (4.8) | 5 (2.3) | 7 (4.1) | 5 (4.1) | 1 (1.0) | 11 (7.7) | 288 (12.2) | |
| 6 m‐ | 77 (12.5) | 31 (15.1) | 36 (20.3) | 16 (12.2) | 26 (24.2) | 29 (21.2) | 693 (29.2) | |
| 12 m‐ | 169 (27.3) | 91 (44.2) | 57 (32.4) | 29 (23.0) | 35 (31.8) | 40 (28.8) | 581 (24.5) | |
| 24 m‐ | 125 (20.2) | 53 (25.6) | 26 (14.9) | 26 (20.3) | 14 (13.6) | 24 (17.3) | 330 (13.9) | |
| 36 m‐ | 106 (17.1) | 22 (10.5) | 29 (16.2) | 40 (31.1) | 20 (18.2) | 19 (13.5) | 283 (11.9) | |
| 48–59 m | 112 (18.1) | 5 (2.3) | 21 (12.2) | 12 (9.5) | 13 (12.1) | 16 (11.5) | 194 (8.2) | |
| District | 0.001 | |||||||
| Gusu district | 108 (17.4) | 27 (13.1) | 34 (19.2) | 28 (21.9) | 18 (16.9) | 38 (27.5) | 432 (18.2) | |
| New urban district | 429 (69.3) | 135 (65.5) | 101 (57.5) | 82 (64.4) | 74 (67.7) | 78 (56.9) | 1593 (67.2) | |
| County‐level district | 73 (11.8) | 37 (17.9) | 31 (17.8) | 14 (11.0) | 10 (9.2) | 19 (13.7) | 273 (11.5) | |
| Other district | 9 (1.4) | 7 (3.6) | 10 (5.5) | 4 (2.7) | 7 (6.2) | 3 (2.0) | 68 (2.9) | |
| Health insurance | <0.001 | |||||||
| Yes | 259 (47.7) | 69 (33.7) | 52 (29.7) | 54 (41.9) | 48 (43.9) | 50 (36.5) | 1077 (45.5) |
Seasonal Distribution
The proportions testing positive for at least one virus were 43.3%, 31.0%, and 33.2% in April 2011–March 2012, April 2012–March 2013, and April 2013–March 2014, respectively (χ 2 = 35.6, P < 0.001). During the 3 years, respiratory viruses were detected in every month of the 3 years, with the overall percent positive specimens among all ILI specimens collected ranging between 10% and 60%. The percent positive for respiratory viruses demonstrated an obvious seasonal pattern, highest in December–March and lowest in September–November (Fig. 1). During the 3 years, influenza had three peaks, the first in January–March 2012 when influenza B virus predominated, the second in July and August 2012 when influenza A virus predominated, and the third in December 2013–January 2014 when influenza A virus predominated. RSV infection occurred predominantly from October to December 2011, March to April 2012, December 2012 to February 2013, and November 2013 to February 2014. The 2013–2014 winter peak of RSV overlapped with the influenza virus winter peak. PIV types 1–3 circulated year round, but peaked in summer and autumn. The monthly distribution of the other respiratory viruses was relatively constant, with no clear seasonal pattern.
Figure 1.
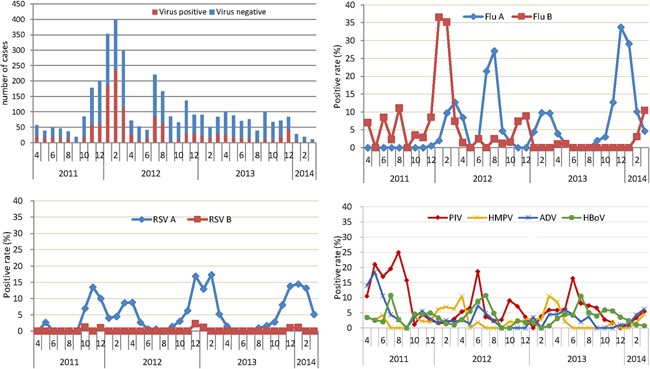
Monthly distribution of respiratory virus detection in five most commonly detected viruses among children less than 5 years of age seeking outpatient care for influenza‐like illness, Suzhou China, 2011–2014. RSV, Respiratory syncytial viruses; PIV, parainfluenza viruses; HMPV, human metapneumovirus; ADV, adenoviruses; and HBoV, human bocavirus.
Age and Gender Distribution
The proportion of cases testing positive for at least one virus increased with age, from 25.8% in <6 months children to 47.0% in 48–59 months children (P < 0.001). Influenza virus, especially type B, contributed to the high percent testing positive for at least one virus in children ≥2 years old. For RSV, the positive rate in children aged 12–35months was higher than that of very young infants and older children. There was no difference in the percent positive for at least one virus between males and females (35.5% vs. 35.0%, P = 0.074).
Clinical Characteristics
The clinical characteristics of infections caused by different respiratory viruses are shown in Table III. The influenza positive ILI cases were more likely to have convulsion than other virus positive ILI cases (2.3% vs. 0.7%, χ 2 = 5.20, P = 0.02). Compared to other virus positive ILI cases, the RSV positive cases were more likely to have cough (82.6% vs. 68.2%, χ 2 = 17.0, P < 0.001) and wheezing symptoms (15.1% vs. 5.8%, χ 2 = 21.9, P < 0.001), and were more frequently diagnosed with pneumonia (57.3% vs. 15.5%, χ 2 = 175.6, P < 0.001) (The pneumonia diagnosed mainly depended on auscultation of lungs). The PIV positive cases had the highest proportion presenting with vomiting (23.3% vs. 12.3%, χ 2 = 15.5, P < 0.001). Most of the ADV positive ILI cases were identified from the ED, and were more likely to present with diarrheal symptoms than other virus positive cases (18.2% vs. 10.0%, P = 0.016). The HBoV positive ILI cases had the highest proportion presenting with rhinorrhea (46.2% vs. 26.9%, χ2 = 22.6, P < 0.001).
Table III.
Clinical Characteristics of Illness by Virus Isolated Among Children Less Than 5 Years Old, Suzhou China, 2011–2014
| Virus(+)* (N = 1292) | Flu(+) (N = 619) | RSV(+) (N = 206) | PIV(+) (N = 176) | HMPV(+) (N = 128) | ADV(+) (N = 109) | HBoV(+) (N = 138) | |
|---|---|---|---|---|---|---|---|
| Clinic | |||||||
| Outpatient | 839 (64.9) | 463 (74.8) | 153 (74.4) | 108 (61.6) | 80 (62.2) | 46 (42.4) | 90 (65.4) |
| Emergency department | 453 (35.1) | 156 (25.2) c | 53 (25.6) c | 68 (38.4) | 48 (37.8) | 63 (57.6) c | 48 (34.6) |
| Past history | |||||||
| Underlying condition a | 63 (4.9) | 28 (4.6) | 12 (5.7) | 2 (1.4) | 20 (11.1) c | 5 (4.8) | 0 (0.0) |
| Immunosuppressive drug use b | 89 (6.9) | 84 (13.6) c | 10 (4.9) | 5 (2.8) | 20 (11.3) c | 5 (4.8) | 14 (10.0) |
| Premature birth | 129 (10.0) | 53 (8.5) | 25 (12.3) | 12 (7.0) | 15 (8.5) | 16 (14.5) | 11 (8.0) |
| Clinical symptoms | |||||||
| Fever T(°C) | 38.8 ± 0.6 | 38.9 ± 0.6 d | 38.6 ± 0.5 | 38.8 ± 0.5 | 38.9 ± 0.5 | 39.0 ± 0.6d | 38.7 ± 0.6 |
| Cough | 911 (70.5) | 446 (72.0) | 170 (82.6) c | 101 (57.5) c | 102 (79.7) c | 63 (57.6) c | 104 (75.0) |
| Rhinorrhea | 375 (29.0) | 152 (24.5) | 77 (37.2) c | 58 (32.9) | 55 (31.1) | 30 (27.3) | 64 (46.2) c |
| Wheezing | 94 (7.3) | 37 (5.9) | 31 (15.1) c | 10 (5.5) | 14 (8.1) | 3 (3.0) | 5 (3.8) |
| Vomiting | 178 (13.8) | 75 (12.1) | 29 (14.0) | 41 (23.3) c | 17 (9.5) | 18 (16.7) | 26 (19.2) |
| Diarrhea | 133 (10.3) | 62 (10.0) | 17 (8.1) | 29 (16.2) | 19 (10.8) | 20 (18.2) c | 13 (9.6) |
| Convulsion | 19 (1.5) | 14 (2.3) c | 1 (0.5) | 2 (1.4) | 2 (1.4) | 0 (0.0) | 0 (0.0) |
| Diagnosis | |||||||
| Bronchiolitis | 158 (12.2) | 110 (17.8) c | 23 (11.0) | 32 (18.3) c | 12 (7.0) | 3 (3.1) c | 14 (9.8) |
| Pneumonia | 286 (22.1) | 131 (21.1) | 118 (57.3) c | 17 (9.9) c | 59 (33.8) c | 10 (9.2) c | 41 (29.4) c |
| Asthma | 50 (3.9) | 19 (3.1) | 25 (12.2) c | 2 (1.4) | 12 (7.0) c | 1 (0.9) | 11 (7.8) c |
| Acute otitis media | 50 (3.9) | 3 (0.5) | 2 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
*ADV, adenovirus; HBoV, human bocavirus; HMPV, human metapneumovirus; FLU, Influenza viruses; PIV, parainfluenza viruses, RSV, respiratory syncytial viruses.
Underlying medical conditions include asthma, chronic pulmonary disease, congenital heart disease, neuromuscular disease, renal dysfunction, blood disorders, immunosuppression, etc.
Immunosuppressive drug use during the past 12 months.
Chi‐square test P < 0.05 in comparison with other virus positive cases.
Student t test P < 0.05 in comparison with other virus positive cases.
DISCUSSION
This study used surveillance data from an influenza network sentinel hospital to describe the frequency of ILI caused by 11 common respiratory viruses and their associated epidemiological and clinical characteristics in Chinese children. In our study, 35.3% of ILI cases among outpatient children less than 5 years old tested positive for at least one virus, and among these, 65.5% tested positive for a respiratory virus other than influenza. The most common respiratory virus causing ILI was influenza virus, followed by RSV, PIV types 1–4, HBoV, HMPV, and ADV. The percent of ILI cases testing positive for any respiratory virus was highest in December–March and lowest in September–November. Influenza is not only the most frequently identified pathogen but it is also the only vaccine preventable illness among the 11 pathogens tested.
The proportion of children seeking outpatient care for ILI who tested positive for at least one virus in our study (35.3%) is similar to that found in a study in Beijing China among adults >16 years of age seeking care for ILI [Yang et al., 2012]. Our percent positive was lower than that observed in other studies conducted among children less than 5 years of age seeking outpatient care for ILI in southern China: Huizhou [Ju et al., 2014] (57.9%), Zhuhai [Li et al., 2013] (44.8%), and the central China city of Wuhan [Peng et al., 2012] (55.8% during 2008–2010, which covered the 2009 H1N1 pandemic period). While the differences in percent positive among studies might be explained by numerous factors, including case definition, specimen types, and population distribution, one factor that may have led to an underestimation of cases testing positive for at least one virus in our study is that, unlike the studies in Huizhou [Ju et al., 2014] and Wuhan [Peng et al., 2012], we did not used the commercial kit to test the viruses, which might affect the yield. In addition, we did not test for viral pathogens such as human rhinovirus and human coronavirus, which are common etiologies of ARIs in children [Cui et al., 2011; Song et al., 2013]. Therefore, the actual percent of viral infection among children with ILI in Suzhou is likely higher than 35.3%.
The virus detected most frequently in our study was influenza (16.9%), followed by RSV (5.6%), and PIV (4.8%). These data are consistent with the positive rates observed in other studies among children less than 5 years old in China [Peng et al., 2012; Li et al., 2013; Ju et al., 2014]. In Italy, the most common viruses causing ILI among young children in 2008–2009 were influenza virus A, followed by rhinovirus, and RSV [Razanajatovo et al., 2011]. In Belgium, the most commonly identified viruses causing ILI among young children in 2009 were influenza virus and RSV [Hombrouck et al., 2012], while in France 2009, the most frequent respiratory viruses were RSV and human metapneumovirus [Falchi et al., 2011]. Of note, these European studies were all carried out during the 2009 H1N1 influenza pandemic period, which may have influenced the spectrum of viruses circulating at the time. During the influenza pandemic period, the activity of several non‐influenza respiratory viruses was reduced or delayed compared to non‐pandemic periods.
RSV, which in many countries has been reported to be almost as common a cause of ARIs among children as influenza, causes the greatest burden of disease among children less than 5 years of age compared with older children [Nair et al., 2010]. In our study, RSV was the second most commonly detected virus after influenza virus. RSV infection occurred predominantly in the spring and winter seasons, and the 2013–2014 winter peak of RSV overlapped with the influenza virus peak. RSV commonly confounds the study of the epidemiology and disease burden of influenza. The co‐circulation of RSV with influenza virus impacts the sensitivity and positive predictive value of ILI surveillance for influenza activity [Tang et al., 2010; Ruf and Knuf, 2014]. Therefore, when capacity does not allow for laboratory confirmation of respiratory viruses, epidemiologists should consider how RSV may impact ILI surveillance data, as RSV is likely contributing to ILI disease burden, particularly in December–April.
In our study, we found that children infected with different respiratory viruses presented with different signs and symptoms. For example, compared with other viruses, influenza was more frequently associated with convulsion, RSV was more likely to cause cough and wheezing, HBoV more often caused rhinorrhea, while PIV and ADV were more likely to cause vomiting and diarrhea. These results are consistent with other studies, suggesting that, despite the common, non‐specific signs and symptoms associated with respiratory illness, infection with each virus can lead to differences in clinical presentation [Chen et al., 2004; Kesebir et al., 2006; Zhang et al., 2014], while these differences are probably too subtle to guide clinical diagnosis and empiric treatment.
This study has several limitations. First, we did not detect other viruses known to cause ILI in children, such as human rhinoviruses and human coronavirus, which likely led to an underestimation of our viral positive rate among ILI cases. Second, the lack of controls may be a more relevant issue given that some of these viruses could have been associated with carriage or shedding from a previous or impending illness event, particularly during co‐infections. Third, the loss of sensitivity inherent in the use of the ILI case definition (particularly as operationalized as measured fever) and throat swabs alone. It was reported that using ILI which requires measure fever could drop the sensitivity by >10% and that only obtaining throat swabs could drop the sensitivity by another 10% [Dawood et al., 2015].
This study systematically investigated the frequency of 11 viral respiratory etiologies of medically attended ILI and their associated clinical characteristics among children less than 5 years of age in Suzhou City, Jiangsu Province. Although the majority of ILI cases testing positive for at least one virus tested positive for a respiratory virus other than influenza, it is important to consider the contributions of co‐circulating viruses when evaluating the burden and cost of influenza‐associated ILI outpatient visits. Influenza is the most common frequently identified pathogen and the unique vaccine preventable illness. The local children still would benefit from the improvement in influenza vaccination coverage.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Supporting Information.
ACKNOWLEDGMENTS
We would like to thank Dr. Carolyn Greeene, Suizan Zhou, and Azziz‐Baumgartner Eduardo from US Centers for Disease Control and Prevention for their assistance in the manuscript preparation.
Dan Wang, Liling Chen, and Yunfang Ding contributed equally to this work.
REFERENCES
- Allander T, Jartti T, Gupta S, Niesters HG, Lehtinen P, Osterback R, Vuorinen T, Waris M, Bjerkner A, Tiveljung‐Lindell A, van den Hoogen BG, Hyypia T, Ruuskanen O. 2007. Human bocavirus and acute wheezing in children. Clin Infect Dis 44:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buecher C, Mardy S, Wang W, Duong V, Vong S, Naughtin M, Vabret A, Freymuth F, Deubel V, Buchy P. 2010. Use of a multiplex PCR/RT‐PCR approach to assess the viral causes of influenza‐like illnesses in Cambodia during three consecutive dry seasons. J Med Virol 82:1762–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HL, Chiou SS, Hsiao HP, Ke GM, Lin YC, Lin KH, Jong YJ. 2004. Respiratory adenoviral infections in children: A study of hospitalized cases in southern Taiwan in 2001–2002. J Trop Pediatr 50:279–284. [DOI] [PubMed] [Google Scholar]
- Cui LJ, Zhang C, Zhang T, Lu RJ, Xie ZD, Zhang LL, Liu CY, Zhou WM, Ruan L, Ma XJ, Tan WJ. 2011. Human coronaviruses HCoV‐NL63 and HCoV‐HKU1 in hospitalized children with acute respiratory infections in Beijing. China Adv Virol 2011:129134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood FS, Jara J, Estripeaut D, Vergara O, Luciani K, Corro M, de Leon T, Saldana R, Castillo BJ, Rauda FR, Cazares RA, Brizuela DFY, Franco D, Gaitan M, Schneider E, Berman L, Azziz‐Baumgartner E, Widdowson MA. 2015. What is the added benefit of oropharyngeal swabs compared to nasal swabs alone for respiratory virus detection in hospitalized children aged <10 years? J Infect Dis 212:1600–1603. [DOI] [PubMed] [Google Scholar]
- Falchi A, Turbelin C, Andreoletti L, Arena C, Blanchon T, Bonmarin I, Hanslik T, Leruez‐Ville M, De Lamballerie X, Carrat F. 2011. “ Nationwide surveillance of 18 respiratory viruses in patients with influenza‐like illnesses: A pilot feasibility study in the French Sentinel Network.” J Med Virol 83:1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Pan L, Sun Q, Zhu W, Zhu L, Ye C, Xue C, Wang Y, Liu Q, Ma P, Qiu H. 2015. The clinical and etiological characteristics of influenza‐like illness (ILI) in outpatients in Shanghai, China, 2011 to 2013. PLoS ONE 10:e119513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombrouck A, Sabbe M, Van Casteren V, Wuillaume F, Hue D, Reynders M, Gerard C, Brochier B, Van Eldere J, Van Ranst M, Thomas I, . 2012. “ Viral aetiology of influenza‐like illness in Belgium during the influenza A(H1N1) 2009 pandemic.” Eur J Clin Microbiol Infect Dis 31:999–1007. [DOI] [PubMed] [Google Scholar]
- Ju X, Fang Q, Zhang J, Xu A, Liang L, Ke C. 2014. Viral etiology of influenza‐like illnesses in Huizhou, China, from 2011 to 2013. Arch Virol 159:2003–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesebir D, Vazquez M, Weibel C, Shapiro ED, Ferguson D, Landry ML, Kahn JS. 2006. Human bocavirus infection in young children in the United States: Molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. J Infect Dis 194:1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wei Q, Tan A, Wang L. 2013. Epidemiological analysis of respiratory viral etiology for influenza‐like illness during 2010 in Zhuhai. China Virol J 10:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta‐analysis. Lancet 375:1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otomaru H, Kamigaki T, Tamaki R, Opinion J, Santo A, Daya E, Okamoto M, Saito M, Tallo V, Lupisan S, Suzuki A, Oshitani H. 2015. Influenza and other respiratory viruses detected by influenza‐like illness surveillance in leyte island, the Philippines, 2010–2013. PLoS ONE 10:e123755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Kong W, Guo D, Liu M, Wang Y, Zhu H, Pang B, Miao X, Yu B, Luo T, Hu Q, Zhou D. 2012. The epidemiology and etiology of influenza‐like illness in Chinese children from 2008 to 2010. J Med Virol 84:672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razanajatovo NH, Richard V, Hoffmann J, Reynes JM, Razafitrimo GM, Randremanana RV, Heraud JM. 2011. Viral etiology of influenza‐like illnesses in Antananarivo, Madagascar, July 2008 to June 2009. PLoS ONE 6:e17579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L, Gonzalez R, Wang Z, Xiang Z, Wang Y, Zhou H, Li J, Xiao Y, Yang Q, Zhang J, Chen L, Wang W, Li Y, Li T, Meng X, Zhang Y, Vernet G, Paranhos‐Baccala G, Chen J, Jin Q, Wang J. 2009. Prevalence of human respiratory viruses in adults with acute respiratory tract infections in Beijing, 2005–2007. Clin Microbiol Infect 15:1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf BR, Knuf M. 2014. The burden of seasonal and pandemic influenza in infants and children. Eur J Pediatr 173:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MH, Zhao LQ, Qian Y, Zhu RN, Deng J, Wang F, Sun Y, Tian R. 2013. [Different species of human rhinovirus infection in children with acute respiratory tract infections in Beijing]. Zhonghua Er Ke Za Zhi 51:903–908. [PubMed] [Google Scholar]
- Tang JW, Lai FY, Wong F, Hon KL. 2010. Incidence of common respiratory viral infections related to climate factors in hospitalized children in Hong Kong. Epidemiol Infect 138:226–235. [DOI] [PubMed] [Google Scholar]
- Thiberville SD, Ninove L, Vu HV, Botelho‐Nevers E, Gazin C, Thirion L, Salez N, de Lamballerie X, Charrel R, Brouqui P. 2012. The viral etiology of an influenza‐like illness during the 2009 pandemic. J Med Virol 84:1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RE. 2014. Is influenza‐like illness a useful concept and an appropriate test of influenza vaccine effectiveness? Vaccine 32:2143–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus AD. 2001. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med 7:719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoek L, Pyrc K, Jebbink MF, Vermeulen‐Oost W, Berkhout RJ, Wolthers KC, Wertheim‐van DP, Kaandorp J, Spaargaren J, Berkhout B. 2004. Identification of a new human coronavirus. Nat Med 10:368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Zhang T, Wu J, Jiang Y, Ding Y, Hua J, Li Y, Zhang J, Chen L, Feng Z, Iuliano D, McFarland J, Zhao G. 2013. Socio‐economic burden of influenza among children younger than 5 years in the outpatient setting in Suzhou, China. PLoS ONE 8:e69035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo PC, Lau SK, Chu CM, Chan KH, Tsoi HW, Huang Y, Wong BH, Poon RW, Cai JJ, Luk WK, Poon LL, Wong SS, Guan Y, Peiris JS, Yuen KY. 2005. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol 79:884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Yao Y, Chen M, Yang X, Xie Y, Liu Y, Zhao X, Gao Y, Wei L. 2012. Etiology and clinical characteristics of influenza‐like illness (ILI) in outpatients in Beijing, June 2010 to May 2011. PLoS ONE 7:e28786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Zhu Q, Zhang X, Ding Y, Steinhoff M, Black S, Zhao G. 2014. Clinical characteristics and direct medical cost of respiratory syncytial virus infection in children hospitalized in Suzhou, China. Pediatr Infect Dis J 33:337–341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Supporting Information.


