Abstract
Objectives
Canine infectious respiratory disease (CIRD) is a disease of multifactorial aetiology, where multiple pathogens act sequentially or synergistically to cause disease. It is common within large dog populations, such as those in re‐homing or training kennels. Vaccines are vital in its management of CIRD, but they often fail to prevent disease. Recently, a number of novel pathogens have been identified in CIRD outbreaks and represent new targets for vaccination.
Key findings
Innate immune responses provide a vital first line of defence against the infectious agents involved in the development of CIRD. Once breeched, adaptive mucosal immunity is necessary to prevent infection and limit spread. Current vaccines target only a few of the agents involved in CIRD. Evidence, from the limited amount of published data, indicates that although vaccinating against these agents reduces infection rates, duration of shedding and severity of disease, it does not induce sterilising immunity; and this has important consequences for the management of the disease, and the future of CIRD vaccine development.
Summary
In the process of considering the development of novel CIRD vaccines, this paper focuses on the immunological mechanisms that provide protection for the respiratory tract, the current recommendations for canine vaccination, and the challenges surrounding existing CIRD vaccines, and their future development.
Keywords: canine infectious respiratory disease, kennel cough, review, vaccine
Introduction: canine infectious respiratory disease
Canine infectious respiratory disease (CIRD), also known as ‘kennel cough’, is a common disease syndrome that is particularly prevalent within large dog populations, such as those in re‐homing or training kennels. CIRD represents a major welfare issue for kennel facilities, pet owners and veterinarians globally, with outbreaks resulting in delays to re‐homing and training and expensive treatment costs. It is characterised by clinical signs such as coughing, nasal discharge and dyspnoea, which can persist for several weeks and may result in severe disease, such as bronchopneumonia, and even, on occasions, lead to death or euthanasia.[1]
Akin to respiratory syndromes in cattle (bovine respiratory disease complex)[2] and pigs (porcine respiratory disease complex),[3] CIRD is a complex infection of multifactorial aetiology, where multiple pathogens act sequentially or synergistically to cause disease. In addition, environmental factors, age, stress and underlying health problems can also contribute to disease spread and susceptibility. Kennelled dogs are particularly at risk of CIRD because of the high population density, and, in re‐homing shelters, there is often a constant influx of susceptible animals and pathogens compounding the situation.[4]
The pathogens traditionally associated with CIRD, include canine parainfluenza virus (CPIV or PIV5),[5] canine adenovirus type 2 (CAV‐2),[6] canine herpes virus 1 (CHV‐1)[7] and Bordetella bronchiseptica (Bb).[8, 9] They are spread through aerosolised droplets from coughing and sneezing, and by contact with fomites present on items such as bedding, bowls and clothing. Methods for preventing CIRD include both (1) good biosecurity such as quarantine of new and sick animals, and high levels of sanitation and (2) efficacious vaccination.[10] Often however, shelters do not have the space for implementing quarantine procedures, and in busy kennel environments, maintaining high levels of sanitation is challenging. Thus, vaccination is vital in managing this disease, and as such, several mono‐ and multivalent vaccines are available (Table 1).
Table 1.
Current canine vaccine (NOAH Compendium 2014) and VMD listings
| Manufacturer | Name | Target | Core/Non‐core | Possible Co‐administration | Administration |
|---|---|---|---|---|---|
| C+ = Core + CPIV | |||||
| Merial | Eurican DHPPi | CDV, CAV, CPV, CPiV | C | Eurican L | Subcutaneous |
| Eurican P | CPV | C | Eurican L | Subcutaneous | |
| Eurican L | Leptospira | C | Eurican DHppi / Eurican P | Subcutaneous | |
| Rabisin | Rabies subunit | N | N/A | Subcutaneous | |
| Eurican Herpes 205 | CHV subunit | N | N/A | Subcutaneous | |
| MSD Animal Health | Nobivac KC | Bordetella bronchiseptica, CPiV | N | N/A | Intranasal |
| Nobivac DHP | CDV, CAV, CPV | C | Nobivac Rabies/ Nobivac L | Subcutaneous | |
| Nobivac DHPPI | CDV, CAV, CPV, CPiV | C+ | Nobivac Rabies/ Nobivac L | Subcutaneous | |
| Nobivac Parvo‐C | CPV | C | Nobivac Rabies/ Nobivac L | Subcutaneous | |
| Nobivac Rabies | Inactivated rabies | N | Nobivac DHP/ Nobivac DHPPI/ Nobivac Parvo‐C | Subcutaneous | |
| Nobivac L | Killed Leptospira | C | Nobivac DHP/ Nobivac DHPPI/ Nobivac Parvo‐C | Subcutaneous | |
| Nobivac Lepto 2 | Subunit Leptospira | C | N/A | Subcutaneous | |
| Nobivac Pi | CPiV | N | N/A | Subcutaneous | |
| Zoetis Animal Health | Bronchi‐ Shield | Bordetella bronchiseptica | N | N/A | Intranasal |
| Duramune DAP | CDV, CAV, CPV | C | N/A | Subcutaneous | |
| Duramune DAP+L | CDV, CAV, CPV, Leptospira | C | N/A | Subcutaneous | |
| Duramune DAPPi | CDV, CAV, CPV, CPiV, | C+ | N/A | Subcutaneous | |
| Duramune DAPPi+L | CDV, CAV, CPV, CPiV, Leptospira | C+ | N/A | Subcutaneous | |
| Duramune DAPPi+LC | CDV, CAV, CPV, CPiV, CCoV, Leptospira | C+ | N/A | Subcutaneous | |
| Duramune Puppy DP+C | CDV, CPV, CCoV | C | N/A | Subcutaneous | |
| DuramunePi | CPiV | N | N/A | Subcutaneous | |
| DuramunePi +L | CPiV, Leptospira | C+ | N/A | Subcutaneous | |
| DuramunePi +LC | CPiV, CCoV, Leptospira | C+ | N/A | Subcutaneous | |
| Vanguard 7 | CDV, CAV, CPV, CPiV, Leptospira | C+ | N/A | Subcutaneous | |
| Vanguard CPV | CPV | C | N/A | Subcutaneous | |
| Vanguard CPV‐L | CPV, Leptospira | C | N/A | Subcutaneous | |
| Vanguard L | Leptospira | C | N/A | Subcutaneous | |
| Vanguard Rabies | Inactivated rabies | N | N/A | Subcutaneous | |
| Virbac | Canigen KC | Bordetella bronchiseptica, CPiV | N | N/A | Intranasal |
| Canigen DHP | CDV, CAV, CPV | C | Canigen Rabies/ Canigen Lepto | Subcutaneous | |
| Canigen DHPi | CDV, CAV, CPV, CPiV | C+ | Canigen Rabies/ Canigen Lepto | Subcutaneous | |
| Canigen ParvoC | CPV | C | Canigen Rabies/ Canigen Lepto | Subcutaneous | |
| Canigen Pi | CPiV | N | Canigen Rabies/ Canigen Lepto | Subcutaneous | |
| Canigen Rabies | Inactivated rabies | N | Canigen DHP/ Canigen DHPi/ Canigen ParvoC/ Canigen Pi | Subcutaneous | |
| Canigen Lepto 2 | Killed Leptospira | C | Canigen DHP/ Canigen DHPi/ Canigen ParvoC/ Canigen Pi | Subcutaneous | |
| Canixin DHPPi/L | CDV, CAV, CPV, CPiV, Leptospira | C+ | N/A | Subcutaneous | |
| CaniLeish | Leishmania subunit | C | N/A | Subcutaneous |
CAV, canine adenovirus; CDV, canine distemper virus; CHV, canine herpes virus; CPiV, canine parainfluenza virus; CPV, canine parvovirus; NOAH, National Office of Animal Health; VMD, Veterinary Medicines Directorate.
There is, however widespread, if anecdotal, recognition that current vaccines often fail to prevent CIRD, and this has sparked a resurgence of interest in the prevention of this disease. Stimulating studies centred on pathogen identification, surveillance and management, and the findings of which have important implications for future CIRD vaccine design.[13, 14, 15, 16, 17, 18, 19, 20, 21, 22]
Perhaps the most important findings are the number of newly emerging or re‐emerging pathogens identified in CIRD outbreaks. These novel agents include: canine respiratory coronavirus (CRCoV),[13] canine pneumovirus (CnPnV),[20] canine influenza virus (CIV),[23] pan‐tropic canine coronavirus,[24] Streptococcus zooepidemicus [15] and Mycoplasma cynos,[18] all of which are the subject of a recent review.[25] With the exception of CIV (for which a vaccine has been recently licensed in the USA), these novel agents are not currently targeted by vaccination, and because they are likely to play an important role in the development and persistence of CIRD in otherwise well‐vaccinated kennels, they are obvious targets for future vaccine development.
In the process of considering novel CIRD vaccines, this is the first review paper to focus on the immunological mechanisms that provide protection for the respiratory tract, the current recommendations for canine vaccination and, most importantly, the challenges surrounding existing CIRD vaccines and their future development. We highlight within this review how the lack of published data within the field has hampered CIRD vaccine development, and hope to raise the question about how best to address this in the future.
Innate and adaptive immunity at the respiratory mucosa
The viral and bacterial pathogens associated with CIRD infect dogs through the mucosal lining of the respiratory tract.[9, 26, 27] In addition to infection with pathogenic organisms, the lower respiratory tract, which must remain sterile, is constantly being challenged by the microbial flora that exists in the upper airways (i.e. mouth, buccal cavity and pharynx). There are two major mechanisms that provide protection for the respiratory tract: the innate immune system and the adaptive immune system. Both are critically important, but have different roles to play.
Essentially, the innate immune system of the respiratory tract provides the first line of defence and is comprised of physical, chemical and cellular components. It is the subject of recent reviews[28, 29, 30] and outlined in Figure 1. Briefly: (1) physical protection is provided by the presence of an epithelial barrier, and the action of mucociliary clearance mechanisms. The epithelial barrier is composed of pseudo‐stratified columnar epithelial cells joined by tight junctions, which regulate the movement of particulate matter across the barrier. Mucociliary clearance occurs as a result of the constant shedding of ‘sticky’ mucus (in which pathogens become trapped) and the continual movement of this mucus by the ciliated epithelial cells away from the lower respiratory tract and into the pharynx. (2) Chemical components of the innate immune system include antimicrobial agents such as, defensins, lysozymes and proteins of the compliment cascade that inhibit or kill invading pathogens. The elements are produced by epithelial cells and other cellular components of the innate immune system, and they modify the composition of the airway mucus. (3) A range of cellular components are essential for effective antiviral or bacterial responses and includes not only the epithelial cells, but also the phagocytic immune cells (e.g. macrophage, dendritic cells (DCs) and neutrophils), which engulf and digest microorganisms using a range of lytic enzymes, proteases and reactive oxygen and nitrogen species. Macrophage and DCs also act as antigen presenting cells in stimulating adaptive immune responses.
Figure 1.
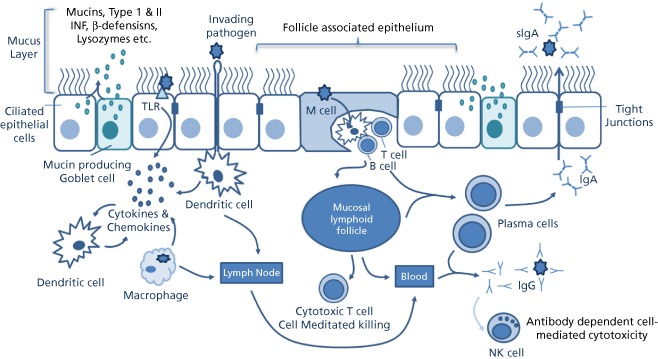
The epithelial barrier is composed of ciliated pseudo‐stratified columnar epithelial cells joined by tight junctions, mucus containing a range of antimicrobial agents traps pathogens that are transported out of the airways by cilia beating. Mucosal epithelial cells detect pathogens using pattern recognition receptors and signal to epithelial DCs via cytokines and chemokines. DCs beneath the epithelium extend dendrites between epithelial cells to sample the lumen. In the FAE of the MALT, M‐cells transport antigen to DCs residing in M‐cell pockets, which present antigens to intraepithelial T and B lymphocytes. Activated DCs migrate to lymphoid follicles or nodes to initiate adaptive immune responses. Plasma cells migrate from the lymphoid follicles and produce IgA, which is transported across the epithelium into the lumen.
Innate immune responses require no prior exposure and thus are particularly important when a dog encounters a new pathogen for the first time. However many respiratory pathogens have evolved strategies for overcoming these primary defences and cause widespread damage to the epithelium, resulting in loss of epithelial barrier integrity. This may be characterised by morphological changes to the cell (as a result of cytotoxic effects of viral replication, toxins, induction of apoptosis) including the loss of cilia or cilia function, disruption to tight junctions, increased mucus production and the down regulation of the pro‐inflammatory cytokines responsible for the recruitment and activation of phagocytic immune cells. Pathogens that can compromise these innate mechanisms are likely to facilitate deeper penetration of the airways by both itself and other ‘bystander’ microorganisms, and thus, appropriate adaptive immune responses are also required.[31, 32, 33]
In contrast to innate responses, adaptive immune responses are antigen specific and result in an immunological memory that helps to prevent recurrent infections by the same pathogen. Importantly, it is also able to differentiate between antigens that enter the body through mucosal surfaces (such as those involved in CIRD) and those that enter via injection or injury, and tailors its response accordingly. During infection, CIRD pathogens trigger both mucosal and systemic immune responses. In terms of disease prevention, it is thus safe to assume that preventative immunity for respiratory pathogens is highly dependent upon both mucosal and systemic immunity, and both should be carefully considered during CIRD vaccine development. It is however predominantly the mucosal immune response that provides protection against pathogen adherence, colonisation and invasion at the mucosal surface, whereas the systemic response is more largely involved in containing and clearing infection once an infection takes hold, suggesting that vaccines that preferentially stimulate mucosal immunity may be of greater benefit.
Mucosal‐associated lymphoid tissues
Immunity at the mucosal surface is mediated by the mucosa‐associated lymphoid tissue (MALT), where strategically placed lymphoid follicles serve as the principle immune induction sites.[34] The MALT is distinct from the systemic immune system, although immune cells activated at the mucosa can trigger a systemic response. The main function of MALT is (1) to protect mucus membranes against pathogenic microbes, (2) to distinguish and tolerate innocuous antigens (e.g. ingested food, airborne matter and commensal organisms) and (3) to prevent the development of harmful immune responses to antigens that cross the mucosa and enter the body.[35] The MALT is subdivided according to the mucosal tissue with which it is associated (i.e. gastro‐intestinal, respiratory, cervical), and its composition differs between species. In dogs, the palatine, lingual and nasopharyngeal tonsils are the most clearly identified components of the respiratory MALT.[34, 36]
There are a number of ways in which mucosal immune responses may be initiated, but all entail pathogen sensing, and the engagement of specialised mucosal DCs, which help govern the nature of the ensuing mucosal immune response (details of which can be found in the following reviews[37, 38, 39, 40] and outlined in Figure 1).
Briefly, DC activation may occur as follows: (1) Mucosal epithelial cells may detect pathogens using pattern recognition receptors such as Toll‐like receptors and RNA‐sensing molecules. Toll‐like receptors are integral membrane proteins that recognise a variety of pathogen‐associated molecular patterns and play a crucial role in the initiation of immune responses in the respiratory epithelium through the activation of several transcription factors (e.g. nuclear factor kappa beta (NK‐κB), Interferon regulatory factor 3 (IRF‐3) and Interferon regulatory factor 7 (IRF‐7)). These in turn induce the production of pro‐inflammatory cytokines and Type I interferons (IFN α and β). These are the principal mediators of the innate immune response and signal to the underlying epithelial DCs. (2) In areas of the respiratory mucosa where lymphoid follicles are absent, motile DCs residing beneath the epithelium migrate into the epithelial layer, or extend dendrites between epithelial cells into the lumen to capture antigens. (3) Where the mucosal lymphoid follicles are present, the epithelium is differentiated into the follicle‐associated epithelium (FAE), which contains specialised M‐cells. M‐cells transport antigens and bacteria across the FAE where they are captured by DCs residing in special pockets situated on the basal side of the M‐cell.
Once activated, DCs migrate to adjacent lymphoid follicles or draining lymph nodes where they present antigen to T and B lymphocytes to initiate adaptive immune responses. In the FAE of the MALT, DCs may also present antigens to intra‐epithelial T and B lymphocytes, which co‐reside with DCs in the pockets of M‐cells. Depending on the initial stimulus (i.e. viral or bacterial) DCs will direct either cell‐mediated (T‐lymphocytes) or humoral (B lymphocytes) responses.[39] This is achieved through the activation of T‐helper (Th) cells (a subset of T‐lymphocytes), which produce cytokines that act accordingly on B‐ or T‐lymphocytes and other immune cells.
The cytokines and chemokines produced by epithelial and DCs also promote the recruitment and activation of the other innate immune cells including neutrophils, macrophage and natural killer cells, which are crucial for mounting an efficient immune response.
Humoral immunity at the mucosa
The majority of pathogen‐specific protective immunity at the mucosa is mediated through the humoral branch of the adaptive immune response, and specifically, through the secreted antibody: IgA (sIgA).[35, 41] The production of Th2 type cytokines (e.g. interleukin (IL)‐4, IL‐10 and transforming growth factor β) by Th cells stimulates the maturation of IgA‐committed B lymphocytes, which migrate from the lymphoid follicles to the effector sites (typically the laminar propria or the epithelium of the mucosal surface) where they differentiate into IgA‐producing plasma cells. At the effector sites, IgA is processed by epithelial cells into secretory IgA and transported across the epithelium into the lumen where it elicits its effects in multiple ways: (1) traps antigens and pathogens in the mucus, (2) inhibits bacterial adhesion to the mucosal surface, (3) neutralises virus (both intra‐ and extracellular) and toxins, (4) eliminates antigens in tissues via immune complex receptor‐mediated transport mechanisms through epithelial cells and (4) enhances innate immune responses through antibody dependent cell‐mediated cytotoxicity.
In addition to sIgA, locally produced antibodies, IgM and IgG in the lower respiratory tract, and serum‐derived IgG (derived from activation of the systemic adaptive immune response) may also contribute to immune defences.[41]
Cell‐mediated immunity at the mucosa
Although cytotoxic T‐lymphocytes do not prevent pathogen entry, they are crucial in the clearance and containment of viral pathogens once they have infected cells. Cytotoxic T‐lymphocytes monitor the cells in the body and are able to recognise viral antigens when presented on the cell surface coupled to specialised T‐lymphocyte binding protein (MHC class 1 molecules). In the presence of Th1 type cytokines (e.g. IFN‐γ, IL‐2); cytotoxic T‐lymphocytes induce the apoptotic destruction of infected target cells, thereby also destroying the pathogen.[35, 38]
Current canine vaccines
There is currently a range of single and multivalent vaccines available for use in dogs (Table 1), which facilitate different vaccination regimes depending on the dogs' environment and risk factors.[11, 12] They are classed as either ‘Core’ vaccines (recommended for all dogs; in the UK, this includes canine distemper virus (CDV), canine parvovirus (CPV), canine adenovirus (CAV 1+2), Leptospira canicola and Leptospira icterohaemorrhagiae) or ‘Non‐Core’ Vaccines (Optional, depending on lifestyle (vacation kennels for overseas holidays) and risk factors. In the UK, this includes rabies and the respiratory vaccines including CPIV and Bb).
Live attenuated vaccines
The majority of canine vaccines are live attenuated vaccines, although some killed or subunit vaccines (rabies, Leptospira, parenteral Bb vaccines) are available (Table 1). Live attenuated vaccines contain a version of the virus or bacteria that has been altered so it is unable to cause serious or clinical disease; they are however able to replicate in the host and, as such, are processed by the immune system in much the same way the pathogen would be during natural infection. They elicit strong cellular and antibody responses, with a long duration of immunity and, for this reason, they are often superior to inactivated or subunit vaccines, especially in young dogs, where maternally derived antibody (MDA) may be present.[42]
Live attenuated vaccines for many viruses are relatively easy to create as they only have a small number of genes that can be easily manipulated. Attenuated vaccines are most often generated through the continuous culture of the virus in established laboratory cell lines (for typically 50–100 passages depending on the virus). As the virus grows and adapts to its new environment, it mutates into a strain that can then replicate only poorly its natural host. Because live attenuated vaccines replicate within the host, they do not require adjuvants, and in some situations, shedding of the vaccine strain by recently vaccinated animals, may be useful for generating herd immunity where vaccination of all individuals is challenging, but has the risk of inducing vaccine‐derived disease in immunocompromised contacts.[43]
The major disadvantage with live attenuated vaccines is the possibility that they may revert to a virulent form and cause disease, and may be unsafe for use in either immunocompromised or pregnant animals.[4, 44] For some viral and bacterial pathogens, their fastidious growth characteristics or complex genomes make live attenuated vaccines difficult to create and control, and as a result, killed or subunit vaccines are preferred. However, advances in technology are helping to address many of these issues.[45, 46]
Route of inoculation
With a few exceptions, canine vaccines are injected subcutaneously (Table 1). This is not surprising because the majority of vaccine research in both human and veterinary medicine is based largely on injected vaccines, which offer the benefit of delivering a known quantity of antigen and result in the generation of specific immune responses that can be easily measured in blood or serum samples.
By contrast, intranasal vaccination faces many challenges; in particular, they need to overcome many, if not all of the innate immune mechanisms present in the upper airways. For live vaccines, many of the inherent properties of the pathogen, which facilitate infection, may help to overcome this. In addition, recent advances in vaccine delivery vehicles are also proving to be promising,[45, 46] but the situation remains far from ideal, and studies continue to be hampered by the inability to accurately deliver or quantitate the amount of delivered antigen. In veterinary medicine, there is the added issue of delivering mucosal vaccines both safely and correctly. Delivering an oral or intranasal vaccine to a defensive, if not aggressive, large dog is not a trivial problem. Delivery of an intranasal vaccine also has the frequent and unpleasant outcome, of being snorted back out immediately after delivery. Therefore subcutaneous vaccination is both surer and safer for the veterinarian. Nevertheless, mucosal immunity is of paramount importance in the prevention of many respiratory agents. Although published data relating to canine vaccine development and efficacy is limited, the increased efficacy of mucosal over parenteral vaccination for Bb and CPIV has been shown (discussed later in this review),[47, 48] furthermore, in the face of MDA, a CAV‐2 vaccine administered intranasally was also shown to be superior to parenteral vaccination.[49]
Core vaccinations
In guidelines published by the World Small Animal Veterinary Association (WSAVA),[42] it is recommended that puppies are vaccinated with core vaccines at 8–9, 11–12 and 14–16 weeks old, followed by a booster at 12 months of age and every 3 years thereafter. This intensive vaccination regime during the first year of life is aimed at overcoming problems surrounding vaccine efficacy due to divergent levels of MDA in individual dogs. In general, dogs responded well to this regime, and numerous experimental studies have shown that antibodies to CDV, CPV and CAV‐1 & 2 are maintained for three or more years.[50, 51, 52, 53] In the context of CIRD, dogs receiving regular core vaccinations from puppyhood should therefore be adequately protected from CDV and CAV‐2 infection, two pathogens traditionally associated with canine respiratory disease. However, for those entering kennel facilities where no vaccination history is provided, and a rapid onset of immunity is vital, the WSAVA recommendations are to administer 1 dose of core vaccine before or upon arrival, with a booster 2 weeks later.[42]
Non‐core canine infectious respiratory disease vaccinations
The non‐core vaccines that are relevant to this paper are those against respiratory disease caused by Bb and CPIV. Vaccines against these pathogens are administered either subcutaniously or intranasally, although the choice of intranasal vaccines is limited (Table 1). WSAVA recommendations are to administer CPIV and Bb subcutaneous vaccines at various time points throughout puppyhood, and in adult dogs, two doses should be administered 3–4 weeks apart. In many instances, CPIV is now also included as a component of the multivalent core vaccines (Table 1). Whether administered as a monovalent or multivalent vaccine, the limited duration of immunity offered means booster vaccines are recommended every 12 months.[42] Intranasal formulations may be administered as early as 3 weeks of age with a second dose 3–4 weeks later, followed by another at 1 year. In adult dogs, two doses 3–4 weeks apart are recommended. Boosters are recommended annually or more frequently as required.[42, 54, 55]
Importantly for CPIV and Bb, the WSAVA recommends the use of intranasal over and above subcutaneous vaccination.[42] It is generally considered that although subcutaneous vaccines are good at inducing systemic immunity, they tend to be relatively poor inducers of mucosal immunity; conversely, mucosal vaccines have been shown to be good at inducing both mucosal and systemic immune responses.
Although published studies for canine vaccines are extremely limited, in one study comparing intranasal and subcutaneous Bb vaccinations, dogs receiving intranasal vaccinations had higher sIgA levels (in nasal secretions) and were significantly better protected from challenge with a virulent Bb strain at 9 weeks postvaccination, when compared with dogs receiving a subcutaneous Bb or placebo vaccines.[47] In an experimental study where dogs were vaccinated with different doses of an intranasal Bb vaccine, shedding of the vaccine strain was detected up to 4 weeks postvaccination. Following challenge with virulent Bb, clinical signs of disease and shedding of the challenge strain were reduced in a vaccine dose‐dependent manner.[56] In another study, comparing CPIV intranasal and parenteral vaccines, dogs vaccinated via the intranasal route had significantly reduced clinical signs of disease following challenge. Furthermore, viral shedding was reduced from 70% in control dogs, to 50% in dogs vaccinated via the parenteral route, and to 1% in dogs vaccinated intranasally.[48]
In kennel situations, where speed of immunity is paramount, mucosal vaccines are also preferred.[42] Unfortunately, no detailed studies relating to onset of immunity following subcutaneous Bb vaccination have been published; however, in one study examining intranasal Bb vaccination, a steady increase in agglutinating antibody titres were observed 1–4 weeks postvaccination, with no significant difference observed in dogs receiving different vaccination doses.[56] In another study, the vaccine was shown to induce protective immunity 72 h postvaccination.[57] Although the reason for the rapid onset of immunity was not determined within the scope of that study, studies within the field of TB vaccination have shown that mucosal vaccines are able to provide non‐specific immunity immediately after vaccination by stimulating innate immune responses at the mucosal surface (reviewed in Beverly et al [58]), and it is possible that similar mechanisms were at play.
To date, published data relating to CPIV vaccine efficacy remain extremely limited;[59] its assessment is hampered by difficulties in reproducing disease in laboratory models (often mild or negligible) and by uncontrollable influences in field studies, such as the presence of other disease‐causing agents. Although results vary greatly, the general consensus from published data suggests that although vaccinating against CPIV or Bb reduces the rate of infection, the duration of shedding and the severity of disease, they do not induce sterilising immunity; and animals may still become infected.[48, 54, 60, 61, 62, 63, 64] Although there are obvious benefits to the use of these vaccines, the incomplete protection elicited has important ramifications for the control and eradication of this disease, particularly within kennel populations, by assisting in maintaining a reservoir for these pathogens.
An example of this was seen in a study carried out in a large re‐homing facility over three consecutive years. In this study, all dogs were vaccinated against CPIV upon arrival. However, despite vaccination, CPIV remained endemic within the kennel, and significant numbers of dogs become infected with CPIV 2–3 weeks postentry.[14] In busy kennel environments with a high turnover of dogs and the presence of other risk factors, underlying CPIV and Bb infections, may contribute to the development of more severe diseases.[65] An additional issue arising from the efficacy and safety perspectives is the vaccination of immunocompromised individuals. Particularly in the re‐homing or rescue kennel environment, dogs may be immunocompromised as a result of stress, malnutrition, disease or other underlying conditions. Trying to induce a rapid protective immune response in such animals poses additional challenges and leaves the animal susceptible to infection in the intervening period between booster vaccinations, increasing the demand for quarantine measures. From a safety perspective, the use of live attenuated vaccines in immunocompromised individuals may result in vaccine‐associated disease. Thus, the degree of protection elicited by vaccination versus the potential problems arising should be carefully considered.
Canine parainfluenza virus infection and immunity
The challenges associated with CPIV vaccine efficacy are not limited to vaccine design and delivery. Despite the lack of experimental data, it has long been recognised that the immune response to natural CPIV infections are slow and do not result in sterilising or long‐lasting immunity, leaving the dog susceptible to repeat infections. Indeed similar findings have been shown for human parainfluenza virus (PIV) infections, where multiple repeat infections occur and immunity correlates with higher (and likely more broadly cross‐reactive) neutralising antibody titres.[66, 67, 68] Although experimental intranasal vaccine studies in dogs support the idea that local mucosal immunity in CPIV infections is important, there is no published data characterising the mucosal immune response to CPIV in the canine host, and there are very little data relating to the characterisation and duration of cell‐mediated immune responses or CPIV serum antibodies following either natural or experimental infection, or vaccination.
There has also been very limited exploration of the genetic and antigenic diversity of circulating CPIV strains. PIV species possesses two major spike glycoproteins, one involved in cell attachment (hemagglutinin‐neuraminidase) and the other involved in mediating the fusion of viral host cell membranes (Fusion). It is against these two proteins that neutralising antibodies are targeted in convalescent serum.[69, 70, 71] Studies conducted in the 1960s using polyclonal serum revealed few antigenic differences; however, the study was limited to only three canine isolates.[72, 73] In a 1980s study, the use of monoclonal antibodies revealed minor differences in the HN and F proteins, although of the five PIV5 isolates used, only two were derived from dogs.[74] To date, only six complete CPIV genome sequences have been published, and very little additional sequence data for individual viral genes are available, much of which is based on cell culture passaged isolates.[75] In 2014, a study comparing the six CPIV genomes and nine additional PIV5 genomes from different species (six human, one simian and two porcine), revealed a remarkably low‐level diversity in the HN and F genes, regardless of host, year of isolation or geographic origin.[75] Interestingly, however, the highest degree of diversity was seen among the canine isolates,[75] and by analogy with human and bovine parainfluenza species, it is possible that the diversity among CPIV strains may be greater than current studies indicate.[76] Nevertheless, the authors suggested that the low level of variation observed was an indication that the virus is either not particularly immunogenic or that cell‐mediated immunity is more important.[75]
Indeed PIVs have evolved numerous mechanisms for suppressing host immune responses, thus promoting their survival within the cell and surrounding environment. Although this has not been studied for CPIV specifically, for other PIV species, this includes the suppression of type 1 IFN, and the potential masking of HN and F protein epitopes due to glycosylation.[77] As mentioned above, Type 1 IFN are the principal mediators of innate antiviral responses, and PIVs have evolved a range of mechanisms for suppressing its activity by hijacking and modifying cellular regulatory pathways through the activity of virally encoded IFN‐antagonist proteins (Reviewed in Audsley and Moseley, and Parks and Alexander‐Miller[78, 79]).
With such little knowledge about the natural course of CPIV infection and immune responses to the virus in the canine host, it is not surprising that vaccines capable of producing long‐lasting and protective immunity to CPIV continue to elude us. As the number of novel agents associated with CIRD continue to increase, all with differing characteristics and modes of pathogenicity, the need to comprehensively investigate the role of each agent within the CIRD complex and the host–pathogen interactions taking place become increasingly important to ensure that (1) the appropriate pathogens are targeted for vaccination and (2) vaccines stimulate appropriate and effective immune responses.
Future development
There are a number of challenges for future dog respiratory vaccines, possibly some shared by vaccines for other veterinary species and human medicine.
First, there is now increasing evidence that there are a number of newly emerging (newly discovered may be more accurate) pathogens that can contribute to multicomponent disease complexes such as respiratory diseases. Although this may appear overwhelmingly complicated for future vaccine designs, we are aware from our own research that certain viral pathogens are able to disable the innate immune responses, thereby facilitating other ‘superinfection’ with bystander agents. In our case, with CIRD, we have proposed that certain viruses, i.e. CRCoV and CnPnV, can allow mycoplasmas and bacteria to penetrate the deeper airways and cause clinical disease. Future vaccine design could be focussed on preventing these early events and thereafter protecting against the opportunist secondary infection.
Second, although mucosal vaccination holds many benefits, the debate about their efficacy compared with parenteral vaccines remain. The evidence is often that they are effective, but not long lived (months and not years). Possibly, their more immediate effect is by stimulating rapidly the innate immune response and giving some non‐specific but rapid protection. A way forward may be through a prime and boost schedule, which utilises both mucosal and parenteral vaccination strategies. Although recent studies in Bb vaccination have shown beneficial effects of this,[80] such regimes require further detailed experimental data. As our understanding of mucosal immune responses increases alongside technologies that support efficacious mucosal vaccine delivery, so do the possibilities for improving CIRD vaccination regimes. Nonetheless, the issue of an intranasal vaccine delivery in aggressive animal species must be considered fully before commercial companies will invest in development programmes.
In closing, the ultimate decision to develop a new vaccine against mucosal diseases, such as CIRD with its complex aetiologies, will ultimately rest with pharmaceutical companies. They have both the expertise and the funding to take discovery of novel pathogens through the rigorous demands of development to satisfy the need and the safety regulation for licensing of new vaccines.
References
- 1. Appel MJ, Binn LN. Canine infectious tracheobronchitis short review: kennel cough. In: Appel M., ed. Virus Infections of Carnivores. London: Elsevier Science Publishers, 1987: 201–211. [Google Scholar]
- 2. Taylor JD et al. The epidemiology of bovine respiratory disease: what is the evidence for predisposing factors? Can Vet J 2010; 51: 1095–1102. [PMC free article] [PubMed] [Google Scholar]
- 3. Opriessnig T et al. Polymicrobial respiratory disease in pigs. Anim Health Res Rev 2011; 12: 133–148. [DOI] [PubMed] [Google Scholar]
- 4. Pesavento PA, Murphy BG. Common and emerging infectious diseases in the animal shelter. Vet Pathol 2014; 51: 478–491. [DOI] [PubMed] [Google Scholar]
- 5. Appel MJ, Percy DH. SV‐5‐like parainfluenza virus in dogs. J Am Vet Med Assoc 1970; 156: 1778–1781. [PubMed] [Google Scholar]
- 6. Ditchfield J et al. Association of canine adenovirus (Toronto A 26/61) with an outbreak of laryngotracheitis (‘kennel cough’): a preliminary report. Can Vet J 1962; 3: 238–247. [PMC free article] [PubMed] [Google Scholar]
- 7. Karpas A et al. Canine tracheobronchitis: isolation and characterization of the agent with experimental reproduction of the disease. Proc Soc Exp Biol Med 1968; 127: 45–52. [DOI] [PubMed] [Google Scholar]
- 8. Bemis DA. Bordetella and Mycoplasma respiratory infections in dogs and cats. Vet Clin North Am Small Anim Pract 1992; 22: 1173–1186. [DOI] [PubMed] [Google Scholar]
- 9. Bemis DA et al. Pathogenesis of canine bordetellosis. J Infect Dis 1977; 135: 753–762. [DOI] [PubMed] [Google Scholar]
- 10. Appel M, Bemis DA. The canine contagious respiratory disease complex (kennel cough). Cornell Vet 1978; 68(Suppl. 7): 70–75. [PubMed] [Google Scholar]
- 11. Jeffs J et al. NOAH Compendium of Animal Medicines. UK: National Office of Animal Health Ltd., 2014. [Google Scholar]
- 12. VMD . Veterinary Medical Directorate Product Information Database. 2014. http://www.vmd.defra.gov.uk/ProductInformationDatabase/.
- 13. Erles K et al. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology 2003; 310: 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Erles K et al. Longitudinal study of viruses associated with canine infectious respiratory disease. J Clin Microbiol 2004; 42: 4524–4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chalker VJ et al. The association of Streptococcus equi subsp. zooepidemicus with canine infectious respiratory disease. Vet Microbiol 2003; 95: 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Erles K, Brownlie J. Investigation into the causes of canine infectious respiratory disease: antibody responses to canine respiratory coronavirus and canine herpesvirus in two kennelled dog populations. Arch Virol 2005; 150: 1493–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mitchell JA et al. Detection of canine pneumovirus in dogs with canine infectious respiratory disease. J Clin Microbiol 2013; 51: 4112–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chalker VJ et al. Mycoplasmas associated with canine infectious respiratory disease. Microbiology 2004; 150(Pt 10): 3491–3497. [DOI] [PubMed] [Google Scholar]
- 19. Chalker VJ et al. Respiratory disease in kennelled dogs: serological responses to Bordetella bronchiseptica lipopolysaccharide do not correlate with bacterial isolation or clinical respiratory symptoms. Clin Diagn Lab Immunol 2003; 10: 352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Renshaw RW et al. Pneumovirus in dogs with acute respiratory disease. Emerg Infect Dis 2010; 16: 993–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kapoor A et al. Characterization of novel canine bocaviruses and their association with respiratory disease. J Gen Virol 2012; 93(Pt 2): 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Decaro N et al. European surveillance for pantropic canine coronavirus. J Clin Microbiol 2013; 51: 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crawford PC et al. Transmission of equine influenza virus to dogs. Science 2005; 310: 482–485. [DOI] [PubMed] [Google Scholar]
- 24. Buonavoglia C et al. Canine coronavirus highly pathogenic for dogs. Emerg Infect Dis 2006; 12: 492–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Priestnall SL et al. New and emerging pathogens in canine infectious respiratory disease. Vet Pathol 2014; 51: 492–504. [DOI] [PubMed] [Google Scholar]
- 26. Mitchell JA et al. Tropism and pathological findings associated with canine respiratory coronavirus (CRCoV). Vet Microbiol 2013; 162: 582–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Damian M et al. Immunohistochemical detection of antigens of distemper, adenovirus and parainfluenza viruses in domestic dogs with pneumonia. J Comp Pathol 2005; 133: 289–293. [DOI] [PubMed] [Google Scholar]
- 28. Ganesan S et al. Barrier function of airway tract epithelium. Tissue Barriers 2013; 1: e24997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parker D, Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol 2011; 45: 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vareille M et al. The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol Rev 2011; 24: 210–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anderton TL et al. Ciliostasis is a key early event during colonization of canine tracheal tissue by Bordetella bronchiseptica . Microbiology 2004; 150(Pt 9): 2843–2855. [DOI] [PubMed] [Google Scholar]
- 32. Priestnall SL et al. Quantification of mRNA encoding cytokines and chemokines and assessment of ciliary function in canine tracheal epithelium during infection with canine respiratory coronavirus (CRCoV). Vet Immunol Immunopathol 2009; 127: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Avadhanula V et al. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species‐ and cell type‐dependent manner. J Virol 2006; 80: 1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cesta MF. Normal structure, function, and histology of mucosa‐associated lymphoid tissue. Toxicol Pathol 2006; 34: 599–608. [DOI] [PubMed] [Google Scholar]
- 35. Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med 2005; 11(Suppl. 4): S45–S53. [DOI] [PubMed] [Google Scholar]
- 36. Casteleyn C et al. The tonsils revisited: review of the anatomical localization and histological characteristics of the tonsils of domestic and laboratory animals. Clin Dev Immunol 2011; 2011: 472460. doi: 10.1155/2011/472460 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol 2006; 6: 148–158. [DOI] [PubMed] [Google Scholar]
- 38. McGhee JR, Fujihashi K. Inside the mucosal immune system. PLoS Biol 2012; 10: e1001397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chang SY et al. Mucosal dendritic cells shape mucosal immunity. Exp Mol Med 2014; 46: e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim SH, Jang YS. Antigen targeting to M cells for enhancing the efficacy of mucosal vaccines. Exp Mol Med 2014; 46: e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine 2007; 25: 5467–5484. [DOI] [PubMed] [Google Scholar]
- 42. Day MJ et al. WSAVA guidelines for the vaccination of dogs and cats. J Small Anim Pract 2010; 51: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anderson EJ. Rotavirus vaccines: viral shedding and risk of transmission. Lancet Infect Dis 2008; 8: 642–649. [DOI] [PubMed] [Google Scholar]
- 44. Krakowka S et al. Canine parvovirus infection potentiates canine distemper encephalitis attributable to modified live‐virus vaccine. J Am Vet Med Assoc 1982; 180: 137–139. [PubMed] [Google Scholar]
- 45. Gerdts V et al. Mucosal delivery of vaccines in domestic animals. Vet Res 2006; 37: 487–510. [DOI] [PubMed] [Google Scholar]
- 46. Heegaard PM et al. Adjuvants and delivery systems in veterinary vaccinology: current state and future developments. Arch Virol 2011; 156: 183–202. [DOI] [PubMed] [Google Scholar]
- 47. Davis R et al. Comparison of the mucosal immune response in dogs vaccinated with either an intranasal avirulent live culture or a subcutaneous antigen extract vaccine of Bordetella bronchiseptica . Vet Ther 2007; 8: 32–40. [PubMed] [Google Scholar]
- 48. Kontor EJ et al. Canine infectious tracheobronchitis: effects of an intranasal live canine parainfluenza–Bordetella bronchiseptica vaccine on viral shedding and clinical tracheobronchitis (kennel cough). Am J Vet Res 1981; 42: 1694–1698. [PubMed] [Google Scholar]
- 49. Appel M et al. Canine adenovirus type 2‐induced immunity to two canine adenoviruses in pups with maternal antibody. Am J Vet Res 1975; 36: 1199–1202. [PubMed] [Google Scholar]
- 50. Mouzin DE et al. Duration of serologic response to five viral antigens in dogs. J Am Vet Med Assoc 2004; 224: 55–60. [DOI] [PubMed] [Google Scholar]
- 51. Abdelmagid OY et al. Evaluation of the efficacy and duration of immunity of a canine combination vaccine against virulent parvovirus, infectious canine hepatitis virus, and distemper virus experimental challenges. Vet Ther 2004; 5: 173–186. [PubMed] [Google Scholar]
- 52. Larson LJ, Schultz RD. Three‐year serologic immunity against canine parvovirus type 2 and canine adenovirus type 2 in dogs vaccinated with a canine combination vaccine. Vet Ther 2007; 8: 305–310. [PubMed] [Google Scholar]
- 53. Roth JA, Spickler AR. Duration of immunity induced by companion animal vaccines. Anim Health Res Rev 2010; 11: 165–190. [DOI] [PubMed] [Google Scholar]
- 54. Jacobs AA et al. Protection of dogs for 13 months against Bordetella bronchiseptica and canine parainfluenza virus with a modified live vaccine. Vet Rec 2005; 157: 19–23. [DOI] [PubMed] [Google Scholar]
- 55. Lehar C et al. Demonstration of 1‐year duration of immunity for attenuated Bordetella bronchiseptica vaccines in dogs. Vet Ther 2008; 9: 257–262. [PubMed] [Google Scholar]
- 56. Iemura R et al. Simultaneous analysis of the nasal shedding kinetics of field and vaccine strains of Bordetella bronchiseptica . Vet Rec 2009; 165: 747–751. [PubMed] [Google Scholar]
- 57. Gore T et al. Intranasal kennel cough vaccine protecting dogs from experimental Bordetella bronchiseptica challenge within 72 hours. Vet Rec 2005; 156: 482–483. [DOI] [PubMed] [Google Scholar]
- 58. Beverley PC et al. Harnessing local and systemic immunity for vaccines against tuberculosis. Mucosal Immunol 2014; 7: 20–26. [DOI] [PubMed] [Google Scholar]
- 59. Ellis JA, Krakowka GS. A review of canine parainfluenza virus infection in dogs. J Am Vet Med Assoc 2012; 240: 273–284. [DOI] [PubMed] [Google Scholar]
- 60. Chladek DW et al. Canine parainfluenza–Bordetella bronchiseptica vaccine immunogenicity. Am J Vet Res 1981; 42: 266–270. [PubMed] [Google Scholar]
- 61. Edinboro CH et al. A placebo‐controlled trial of two intranasal vaccines to prevent tracheobronchitis (kennel cough) in dogs entering a humane shelter. Prev Vet Med 2004; 62: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Alkire LT, Chladek DW. Field evaluation of an intranasally administered canine parainfluenza–Bordetella bronchiseptica vaccine. Vet Med Small Anim Clin 1980; 75: 1003–1005. [PubMed] [Google Scholar]
- 63. Glickman LT, Appel MJ. Intranasal vaccine trial for canine infectious tracheobronchitis (kennel cough). Lab Anim Sci 1981; 31: 397–399. [PubMed] [Google Scholar]
- 64. Emery JB et al. A canine parainfluenza viral vaccine: immunogenicity and safety. Am J Vet Res 1976; 37: 1323–1327. [PubMed] [Google Scholar]
- 65. Wagener JS et al. Role of canine parainfluenza virus and Bordetella bronchiseptica in kennel cough. Am J Vet Res 1984; 45: 1862–1866. [PubMed] [Google Scholar]
- 66. Glezen WP et al. Parainfluenza virus type 3: seasonality and risk of infection and reinfection in young children. J Infect Dis 1984; 150: 851–857. [DOI] [PubMed] [Google Scholar]
- 67. Smith CB et al. Protective effect of antibody to parainfluenza type 1 virus. N Engl J Med 1966; 275: 1145–1152. [DOI] [PubMed] [Google Scholar]
- 68. Tremonti LP et al. Neutralizing activity in nasal secretions and serum in resistance of volunteers to parainfluenza virus type 2. J Immunol 1968; 101: 572–577. [PubMed] [Google Scholar]
- 69. Murphy BR et al. Current approaches to the development of vaccines effective against parainfluenza and respiratory syncytial viruses. Virus Res 1988; 11: 1–15. [DOI] [PubMed] [Google Scholar]
- 70. Spriggs MK et al. Immunization with vaccinia virus recombinants that express the surface glycoproteins of human parainfluenza virus type 3 (PIV3) protects patas monkeys against PIV3 infection. J Virol 1988; 62: 1293–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Spriggs MK et al. Expression of the F and HN glycoproteins of human parainfluenza virus type 3 by recombinant vaccinia viruses: contributions of the individual proteins to host immunity. J Virol 1987; 61: 3416–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Crandell RA et al. Isolation of a parainfluenza virus from sentry dogs with upper respiratory disease. Am J Vet Res 1968; 29: 2141–2147. [PubMed] [Google Scholar]
- 73. Lazar EC et al. Serologic and infectivity studies of canine SV‐5 virus. Proc Soc Exp Biol Med 1970; 135: 173–176. [DOI] [PubMed] [Google Scholar]
- 74. Randall RE et al. Isolation and characterization of monoclonal antibodies to simian virus 5 and their use in revealing antigenic differences between human, canine and simian isolates. J Gen Virol 1987; 68(Pt 11): 2769–2780. [DOI] [PubMed] [Google Scholar]
- 75. Rima BK et al. Stability of the parainfluenza virus 5 genome revealed by deep sequencing of strains isolated from different hosts and following passage in cell culture. J Virol 2014; 88: 3826–3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Terrier O et al. Characterization of naturally occurring parainfluenza virus type 2 (hPIV‐2) variants. J Clin Virol 2008; 43: 86–92. [DOI] [PubMed] [Google Scholar]
- 77. Komada H et al. N‐glycosylation contributes to the limited cross‐reactivity between hemagglutinin neuraminidase proteins of human parainfluenza virus type 4A and 4B. Med Microbiol Immunol (Berl) 2000; 189: 1–6. [DOI] [PubMed] [Google Scholar]
- 78. Audsley MD, Moseley GW. Paramyxovirus evasion of innate immunity: diverse strategies for common targets. World J Virol 2013; 2: 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Parks GD, Alexander‐Miller MA. Paramyxovirus activation and inhibition of innate immune responses. J Mol Biol 2013; 425: 4872–4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ellis JA et al. Effect of vaccination on experimental infection with Bordetella bronchiseptica in dogs. J Am Vet Med Assoc 2001; 218: 367–375. [DOI] [PubMed] [Google Scholar]


