Abstract
Understanding etiological role and epidemiological profile is needed to improve clinical management and prevention of acute respiratory infections (ARIs). A 5‐year prospective study about active surveillance for outpatients and inpatients with ARIs was conducted in Gansu province, China, from January 2011 to November 2015. Respiratory specimens were collected from patients and tested for eight respiratory viruses using polymerase chain reaction (PCR) or reverse transcription polymerase chain reaction (RT‐PCR). In this study, 2768 eligible patients with median age of 43 years were enrolled including pneumonia (1368, 49.2%), bronchitis (435, 15.7%), upper respiratory tract infection or URTI (250, 9.0%), and unclassified ARI (715, 25.8%). Overall, 29.2% (808/2768) were positive for any one of eight viruses, of whom 130 cases were identified with two or more viruses. Human rhinovirus (HRV) showed the highest detection rate (8.6%), followed by influenza virus (Flu, 7.3%), respiratory syncytial virus (RSV, 6.1%), human coronavirus (hCoV, 4.3%), human parainfluenza (PIV, 4.0%), adenovirus (ADV, 2.1%), human metapneumovirus (hMPV, 1.6%), and human bocavirus (hBoV, 0.7%). Compared with URTI, RSV was more likely identified in pneumonia (χ2 = 12.720, P < 0.001) and hCoV was more commonly associated with bronchitis than pneumonia (χ2 = 15.019, P < 0.001). In patients aged less than 5 years, RSV showed the highest detection rate and hCoV was the most frequent virus detected in adults and elderly. The clear epidemical seasons were observed in HRV, Flu, and hCoV infections. These findings could serve as a reference for local health authorities in drawing up further plans to prevent and control ARIs associated with viral etiologies.
Keywords: acute respiratory infections, epidemiology, molecular detection, respiratory virus
1. INTRODUCTION
Acute respiratory infections (ARIs) were the significant cause of morbidity and mortality throughout the world, especially in infants and young children.1, 2 The most frequently reported etiological agents responsible for ARIs were respiratory viruses including respiratory syncytial virus (RSV), influenza virus (Flu), human parainfluenza virus (PIV), human coronavirus (hCoV), human rhinovirus (HRV), adenovirus (ADV), human metapneumovirus (hMPV), and human bocavirus (hBoV). In terms of geographical distribution, 70% of children who died from ARIs were in Southeast Asia and Africa.2
In China, despite the mortality of live births reduced significantly in past decades, ARIs were still the main cause of children mortality.3 Several studies have reported the prevalence of respiratory viruses causing ARIs in China4, 5, 6, 7, 8; however most of these studies were conducted in developed regions of eastern coastal China. Thus, this paper presents data on the epidemiology of viral etiologies associated with ARIs in Gansu Province, which located in a relatively undeveloped area of China and aims to provide basic data of viral etiologies of ARIs to direct local disease prevention and control.
2. METHODS
2.1. Ethical approval
This study was approved by the Ethics Review Committee of Chinese Center for Disease Control and Prevention (CDC) and all participants were informed of the study objectives, and written consent was obtained from patients or guardians.
2.2. Study design and patient enrollment
From January 2011 to November 2015, active surveillance was conducted for inpatients and outpatients with ARIs in 14 sentinel hospitals in Gansu province of China. Considering the capacities of surveillance, these sentinel site were chosen very carefully. The surveillance protocol developed by Chinese CDC, including patient enrollment, data and specimen collection; laboratory testing, was used by all participating hospitals and laboratories.
Inpatients and outpatients were first screened by physicians of sentinel hospitals for ARIs and if they met inclusion criteria as follows, these patients would be enrolled into our study. A patient was considered to be having ARIs if they had: (1) at least one of listed manifestation of acute infection: measured fever (≥38°C), abnormal white blood cell (WBC) differential, leukocytosis (a WBC count more than 10 000/µL) or leucopenia (a WBC count less than 4000/µL), and chill; (2) at least one of listed signs/symptoms: cough, sputum, shortness of breath, lung auscultation abnormality (rale or wheeze), tachypnoea, and chest pain. Among ARIs patients, those with a chest radiograph demonstrating punctuate, patchy or uniform density opacity were defined as having radiographic evidence of pneumonia.5
The diagnosis for each patient admitted in this study was made by attending physicians and based on standard clinical criteria. Thus, pneumonia was diagnosed with fever, tachypnoea, chest pain, and respiratory distress where focal or diffuse crackles or decreased vesicular sounds were present on auscultation. Chest radiograph was used to distinguish pneumonia and other ARIs, but some diagnosis of pneumonia were based on clinical criteria alone. Bronchitis was diagnosed in whom upper respiratory symptoms preceded lower respiratory symptoms of wheeze, dyspnea, and signs of respiratory distress. Upper respiratory tract infections were diagnosed based on symptoms such as cough, runny nose, sore throat, and coryza. ARIs other than pneumonia, bronchitis, and upper respiratory tract infections were defined as unclassified ARIs.
2.3. Specimen collection and testing
Respiratory specimens (nasopharyngeal swab or aspirate, sputum, bronchoalveolar lavage, or lung puncture aspirate) were collected in ARIs patients and placed immediately in viral transport media (VTM). Collected specimens were stored at 4‐8°C at the local hospital and were transferred to the sentinel laboratories for diagnostic testing. Viral molecular tests were completed within 24 h after collection; otherwise specimens in VTM should be stored at −70°C.
Every specimen from patients was detected for eight viruses. The viral nucleic acid was directly extracted from specimens by commercial kits (QIAmpMiniElute Virus Spin kit, QIAamp Viral RNA Mini kit or RNeasy Mini kit, Qiagen, Valencia, CA) recommended by surveillance protocol. ADV and hBoV were determined by Polymerase chain reaction (PCR).9, 10 Reverse transcription‐Polymerase chain reaction (RT‐PCR) was performed to detect the other six viruses.11, 12, 13, 14 The primer sequences of PCR or RT‐PCR were shown in Table 1. If any one of the targeted viruses was detected in the specimens, the patient was considered to be positive for that viral etiology. The cases where only one virus identified were labelled as single infection, two etiologies were co‐infection, and three or more were multiple‐infection.
Table 1.
Primer sequences of detected respiratory viruses
| Viruses | Sequences of primers(5′‐3′) | Target genes | Products (bp) |
|---|---|---|---|
| Flu A, B, and C | FluAC1: GAACTCRTYCYWWATSWCAAWGRRGAAAT
FluB1: ACAGAGATAAAGAAGAGCGTCTACAA FluABC2: ATKGCGCWYRAYAMWCTYARRTCTTCAWAIGC FluAB3: GATCAAGTGAKMGRRAGYMGRAAYCCAGG FluC3: AAATTGGAATTTGTTCCTTTCAAGGGACA FluAC4: TCTTCAWATGCARSWSMAWKGCATGCCATC FluB4: CTTAATATGGAAACAGGTGTTGCCATATT |
NP | Flu A: 301 bp
Flu B: 226 bp Flu C: 111 bp |
| RSV A, B | RSVAB1: ATGGAGYTGCYRATCCWCARRRCAARTGCAAT
RSVAB2: AGGTGTWGTTACCCTGCATTRACACTRAATTC RSVA3: TTATACACTCAACAATRCCAAAAAWACC RSVB3: ATCTTCCTAACTCTTGCTRTTAATGCATTG RSVA4: AAATTCCCTGGTAATCTCTAGTAGTAGTCTGT RSVB4: GATGCGACAGCTCTGTTGATTTACTATG |
F | RSV A: 363 bp
RSV B: 611 bp |
| PIV 1‐4 | 1PIV13: AGGWTGYSMRGATATAGGRAARTCAT
1PIV2: TAATTCCTCTTAAAATTGACAGTATCGA 1PIV4: ATCCAGARRGACGTCACATCAACTCAT 2PIV13: CTWGTATATATATRTAGATCTTKTTRCCTAGT 2PIV24: TRAGRCCMCCATAYAMRGGAAATA 3PIV13: ACGACAAYAGGAARTCATGYTCT 3PIV24: CYMAYGGRTGYAYTMGAATWCCATCATT 4PIV1: GACAACAATCTTTGGCCTATCAGATA 4PIV2: GCTAGATCAGTTGTGGCATAATCT 4PIV3: GAGTTGACCATCCTYCTRTCTGAAAAC 4PIV4: TGACTATRCTCGACYTTRAAATAAGG |
HA | PIV 1: 439 bp
PIV 2: 297 bp PIV 3: 390 bp PIV 4: 174 bp |
| HRV | 1HRVF: CTCCGGCCCCTGAATRYGGCTAA
2HRVR: TCIGGIARYTTCCASYACCAICC 3HRVF: ACCRASTACTTTGGGTRWCCGTG 4HRVR: CTGTGTTGAWACYTGAGCICCCA |
HA | HRV: 110 bp |
| hMPV | hMPVLF: CATGCCCACTATAAAAGGTCAG
hMPVLR: CACCCCAGTCTTTCTTGAAA |
L | hMPV: 171 bp |
| hCoV | hCoVFc: GGTTGGGACTATCCTAAGTGTGA
hCoVRc: CCATCATCAGATAGAATCATCATA |
POL | hCoV: 440 bp |
| ADV | 1‐ADVF: GCCSCARTGGKCWTACATGCACATC
1‐ADVR: CAGCACSCCICGRATGTCAAA |
Hexon | ADV: 301 bp |
| hBoV | hBoVF: GACCTCTGTAAGTACTATTAC
hBoVR: CTCTGTGTTGACTGAATACAG |
NP1 | hBoV: 354 bp |
2.4. Data collection and statistical analysis
Demographic characteristics, clinical symptoms were collected by staff of sentinel hospitals through a standardized questionnaire of protocol.
Data were analyzed using SPSS (v20.0, SPSS, Chicago, IL). Two tailed Mann‐Whitney test was used to compare median of two groups and comparison of median in more than two groups used Kruskal‐Wallis test. Categorical data was performed using Chi‐square test or Fisher exact test. P‐value < 0.05 was considered to be statistically significant.
3. RESULTS
3.1. Characteristics of patients with ARIs
Between January 2011 and November 2015, 2768 eligible patients with ARIs were enrolled in this study. Among all ARIs patients, 29.0% were children aged ≤5 years and 25.1% were elderly aged ≥65 years, with a median age of 43 years (interquartile rang [IQR], 4‐63 years). A total of 1834 patients (66.3% of total) were male. The distribution of respiratory viruses in males and females had no difference (χ2 = 1.612, P > 0.05). A temperature ≥38°C were documented in 47.8% of ARIs cases. A total of 85.0% of patients were suffered from cough, which was the most common clinical symptom and 33.7% were reported to show abnormal chest radiography (Table 2).
Table 2.
Demographic and clinical characteristics of patients with ARIs
| Characteristics | Virus positive (%) N = 808 | Total (%) N = 2768 |
|---|---|---|
| Gender | ||
| Male | 521 (64.5) | 1834 (66.3) |
| Female | 287 (35.5) | 934 (33.7) |
| Age groups | ||
| <1 year | 105 (13.0) | 339 (12.2) |
| 1‐5 years | 182 (22.5) | 464 (16.8) |
| 6‐14 years | 49 (6.1) | 170 (6.1) |
| 15‐49 years | 135 (16.7) | 580 (21.0) |
| 50‐64 years | 137 (17.0) | 519 (18.8) |
| ≥65years | 200 (24.8) | 696 (25.1) |
| Clinical symptoms | ||
| T ≥ 38°C | 366 (45.3) | 1323 (47.8) |
| Cough | 736 (91.1) | 2353 (85.0) |
| Expectoration | 482 (59.7) | 1536 (55.5) |
| Pulmonary rale | 284 (35.1) | 806 (29.1) |
| Runny nose | 145 (18.0) | 433 (15.6) |
| Sore throat | 119 (14.7) | 375 (13.5) |
| Chest pain | 73 (9.0) | 298 (10.8) |
| Fatigue | 129 (16.0) | 504 (18.2) |
| Tachypnoea | 143 (17.7) | 440 (15.9) |
| Dyspnea | 123 (15.2) | 425 (15.4) |
| Headache | 53 (6.6) | 215 (7.8) |
| Abnormal chest radiography | 288 (35.6) | 932 (33.7) |
T, temperature.
3.2. Viral etiologies detected in different type of patients
Of all 2768 ARIs patients tested for eight viruses, 808 (29.2%) were positive for at least one virus. The median age of these patients was lower than patients who were negative for any respiratory viruses (P < 0.05, Mann‐Whitney test). HRV showed the highest detection rate (8.6%, 237/2768), followed by Flu (7.3%, 201/2768), RSV (6.1%, 169/2768), hCoV (4.3%, 120/2768), PIV (4.0%, 112/2768), ADV (2.1%, 57/2768), hMPV (1.6%, 45/2768), and hBoV (0.7%, 20/2768) (Table 3).
Table 3.
Viral etiologies detected in inpatients and outpatients
| Items | Total (%) N = 2768 | Inpatients (%) N = 2336 | Outpatients (%) N = 432 | P‐value |
|---|---|---|---|---|
| Gender (male/female) | 1834/934 | 1548/788 | 286/146 | >0.05 |
| Median age (IQR) | 43 (4‐63) | 40 (10‐63) | 38.63 (6‐65) | >0.05 a |
| Positive for anyvirus | 808 (29.2) | 697 (29.8) | 111 (25.7) | >0.05 |
| Single infection | 678 (24.5) | 584 (25.0) | 94 (21.8) | >0.05 |
| Co‐infection | 111 (4.0) | 96 (4.1) | 15 (3.5) | >0.05 |
| Multiple‐infection | 19 (0.7) | 17 (0.7) | 2 (0.5) | >0.05 b |
| Virus | ||||
| HRV | 237 (8.6) | 195 (8.3) | 42 (9.7) | >0.05 |
| Flu | 201 (7.3) | 175 (7.5) | 26 (6.0) | >0.05 |
| RSV | 169 (6.1) | 152 (6.5) | 17 (3.9) | < 0.05 |
| PIV | 112 (4.0) | 102 (4.3) | 10 (2.3) | < 0.05 |
| hCoV | 120 (4.3) | 101 (4.3) | 19 (4.4) | >0.05 |
| ADV | 57 (2.1) | 52 (2.2) | 5 (1.2) | >0.05 |
| hMPV | 45 (1.6) | 37 (1.6) | 8 (1.9) | >0.05 |
| hBoV | 20 (0.7) | 17 (0.7) | 3 (0.7) | >0.05 |
IQR, interquartile rang.
Mann‐Whitney test.
Fisher exact probability.
Of 2336 inpatients, 29.8% tested positive for at least one virus. This rate was similar to that of outpatients (χ2 = 3.027, P > 0.05). The most common virus was HRV, with 8.3% (195/2336) detected in inpatients and 9.7% (42/432) in outpatients, whereas the detection rate of HRV had no difference between two groups (χ2 = 0.880, P > 0.05). Compared with outpatients, only RSV and PIV were more likely detected in inpatients (RSV: χ2 = 4.206, P < 0.05; PIV: χ2 = 4.849, P < 0.05) (Table 3).
In 808 positive patients, 678 detected with one virus were single infections (24.5%, 678/2768). Two or more respiratory viruses were identified in 130 cases of whom 111 (4.0%, 111/2768), 19 (0.7%, 19/2768) were co‐infections and multiple‐infections, respectively (Table 3).
3.3. Viral etiologies detected in different diagnosis of ARIs patients
Overall, 49.3% (1366/2768) were diagnosed with pneumonia, 15.7% (435/2768) with bronchitis, 9.2% (254/2768) with upper respiratory tract infections (URTI), and 25.8% (713/2768) were unclassified ARIs cases. The percentage of positive patients differed in those groups (χ2 = 24.026, P < 0.001) and median age did so (P < 0.001, Kruskal‐Wallis test). Viruses detected in each of four groups were broadly similar. Only RSV was more likely identified in pneumonia than URTI or unclassified ARIs (partition of χ2 method: pneumonia vs URTI, χ2 = 12.720, P < 0.001; pneumonia vs unclassified ARIs, χ2 = 13.118, P < 0.001) and hCoV infection was more commonly associated with bronchitis than pneumonia or unclassified ARIs (partition of χ2 method: bronchitis vs pneumonia, χ2 = 15.019, P < 0.001; bronchitis vs unclassified ARIs, χ2 = 25.219, P < 0.001) (Table 4).
Table 4.
Viral etiologies detected in different diagnosis of ARIs patients
| Items | Pneumonia (%) N = 1366 | Bronchitis (%) N = 435 | URTI (%) N = 254 | Unclassified ARIs (%) N = 713 | P‐value |
|---|---|---|---|---|---|
| Gender (male/female) | 890/476 | 300/135 | 159/95 | 485/228 | >0.05 |
| Median age (IQR) | 49 (8‐66) | 59 (37‐70) | 7 (3‐27) | 28 (5‐60) | <0.001 a |
| Positive for anyvirus | 427 (31.3) | 149 (34.3) | 72 (28.3) | 160 (22.7) | <0.001 |
| Virus | |||||
| HRV | 114 (8.3) | 44 (10.1) | 24 (9.4) | 56 (7.8) | >0.05 |
| Flu | 111 (8.1) | 37 (8.5) | 16 (6.3) | 37 (5.2) | >0.05 |
| RSV | 107 (7.8) | 24 (5.5) | 11 (4.3) | 28 (3.9) | <0.001 |
| hCoV | 57 (4.2) | 39 (9.0) | 7 (2.8) | 17 (2.4) | <0.001 |
| PIV | 54 (4.0) | 17 (3.9) | 12 (4.7) | 29 (4.1) | >0.05 |
| hMPV | 23 (1.7) | 11 (2.5) | 1 (0.4) | 10 (1.4) | >0.05 |
| ADV | 23 (1.7) | 10 (2.3) | 9 (3.5) | 15 (2.1) | >0.05 |
| hBoV | 8 (0.6) | 3 (0.7) | 2 (0.8) | 7 (1.0) | >0.05 b |
IQR, interquartile rang; URTI, upper respiratory tract infection.
Kruskal‐Wallis test.
Fisher exact probability.
3.4. Viral etiologies distribution in different age groups
All of ARIs patients were divided into six age groups. The overall detection rate between age groups had significant difference (χ2 = 35.268, P < 0.01) and the highest detection rate was observed in young children (1‐5 years, 45.3%). RSV infections were most frequent in infants (<1 year, 16.2%) and young children (1‐5 years, 9.3%). PIV showed the same detection rate as RSV in patients aged 1‐5 years. In older children group, the predominant viruses were HRV and Flu with equal detection rate (6‐14 years, 9.4%). HRV and Flu also showed the highest detection rate in adult (15‐49 years, 10.3% and 50‐64 years, 8.3%) and elderly patients (≥65 years, 9.5%), respectively. Apart from hMPV and hBoV, each of else respiratory viruses incidence differed among age groups (Table 5).
Table 5.
Age‐specific incidences of eight respiratory viruses
| Viruses | <1 year N = 339 (%) | 1‐5 N = 464 (%) | 6‐14 N = 170 (%) | 15‐49 N = 580 (%) | 50‐64 N = 519 (%) | ≥65 N = 696 (%) | P‐value |
|---|---|---|---|---|---|---|---|
| HRV | 22 (6.5) | 35 (7.5) | 16 (9.4) | 60 (10.3) | 43 (8.3) | 61 (8.9) | <0.05 |
| Flu | 12 (3.5) | 33 (7.1) | 16 (9.4) | 36 (6.2) | 38 (7.3) | 66 (9.5) | <0.05 |
| RSV | 55 (16.2) | 43 (9.3) | 6 (3.5) | 15 (2.6) | 20 (3.9) | 30 (4.3) | <0.01 |
| hCoV | 9 (2.7) | 7 (1.5) | 8 (4.7) | 34 (5.9) | 23 (4.4) | 39 (5.6) | <0.01 |
| PIV | 14 (4.1) | 43 (9.3) | 10 (5.9) | 13 (2.2) | 12 (2.3) | 20 (2.9) | <0.01 |
| ADV | 4 (1.2) | 27 (5.8) | 5 (2.9) | 5 (0.9) | 7 (1.3) | 9 (1.3) | <0.01 |
| hMPV | 2 (0.6) | 14 (3.0) | 1 (0.6) | 7 (1.2) | 10 (1.9) | 11 (1.6) | >0.05 |
| hBoV | 3 (0.9) | 8 (1.7) | 1 (0.6) | 2 (0.3) | 2 (0.4) | 4 (0.6) | >0.05 a |
| Total | 121 (35.7) | 210 (45.3) | 63 (37.1) | 172 (29.7) | 155 (29.9) | 240 (34.5) | <0.01 |
Fisher exact probability.
3.5. Temporal trends of viral etiologies
Over the 59 months study period, there were clear seasonal peaks for HRV, Flu, and hCoV infections. HRV infections were occurred each month throughout the year during 5 years with an annual a peak in September‐October (Figure 1, panel A). Flu and hCoV infections showed a similarly circulation of one peak annually, with the peak of Flu infections was observed during December‐January and hCoV during July to August (Figure 1, panels B and D). RSV and PIV did not show the clear epidemic season (Figure 1, panels C and E). Due to the infections of ADV, hMPV, and hBoV appeared sporadically among our study period, we did not present seasonal distributions of these viruses in Figure 1.
Figure 1.
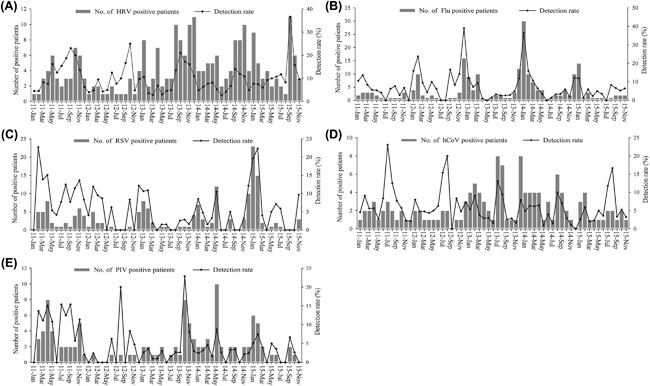
Monthly positive number and detection rate for individual viruses among the total patients. A,HRV, (B) Flu, (C) RSV, (D) hCoV, (E) PIV
4. DISCUSSION
Before this study there was a similar report about prevalence of children infected with respiratory viruses in Gansu province.15 However, that study was only based on children aged less than 12 years in 1 year, therefore we conducted the study aimed to describe the viral etiologies in patients with ARIs during 5 consecutive years in Gansu province, China. Of total 2768 patients admitted in this study, 29.2% were positive for at least one virus, which was lower than that reported in Shandong (35.75%), Shanghai (32.09%), and 22 provinces of China (36.6%),4, 7, 16 possibly because of the difference of regions and study design. The principal etiologies detected in ARIs patients were HRV (8.6%), Flu (7.3%), RSV (6.1%), hCoV (4.3%), and PIV (4.0%). Distribution of virus identification was similar not only in different type of patients but also in each of diagnosis groups. Highest detection rate (45.3%) in patients aged 1‐5 years indicated that ARIs still was a risk factor for younger children's health, although the mortality due to respiratory tract infections decreased more than 35% in this age population.17
Pneumonia, a leading cause of mortality of children less than 5 years especially in developing country,18 accounted for almost 50% of all ARIs patients in this study. Although detected in all the major clinical diagnosis of ARIs, RSV was more commonly detected in pneumonia patients. Published reports state that RSV was a well‐known cause of pneumonia in children younger than 5 years and elderly,19 but less common cause in adults, although few study examined RSV as an important etiology due to Community‐Acquired Pneumonia in this age group.20 In contrast with previous study that hCoV primarily infect upper respiratory and gastrointestinal tract,21 the result of our study that hCoV could be detected in 9.0% of all bronchitis may in part reflect it was also an important etiology for lower respiratory tract infections.
The finding that RSV was the major etiology in young children with ARIs under 5 years were consistent with studies from china and other countries,6, 7, 16, 22, 23, 24, 25, 26 and indicated that prevention strategies for RSV such immunization when a suitable vaccine is available in the future could have large public health impact in Gansu province. HRV, the etiology with highest detection rate in adults, was supported by previous studies that revealed common cold were mainly caused by HRV infections for adults people.27, 28 In addition, Flu was an important virus identified in older children and elderly, which suggested influenza virus was the significant cause of ARIs for those population in relatively undeveloped region of china like Gansu province. Influenza vaccine was commercially available, such as inactivated vaccine, which was administered intramuscularly or intranasally.29, 30, 31 However, it had an extremely low coverage rate in China.32 We considered Influenza vaccine should be used in widespread areas to reduce the incidence of influenza disease in Gansu. It should be noted that PIV was a another major virus for children aged 1‐5 years besides RSV, which was in agreement with studies conducted before.33, 34, 35 This finding indicated that local pediatricians not only took priority over RSV infections but also paid more attention to PIV, when younger children were infected with respiratory viruses.
For temporal Trend of viral etiologies, seasonality of respiratory virus was varied in different regions particularly for enormous territory country as china. This study described clearly seasonal distribution on HRV, Flu, and hCoV. HRV infections were identified each month throughout the study period while it most prevalent in the autumn (September‐October), which was consistent with published reports,36 but different from Beijing and Shandong in China.5, 7 The reason may be related to region's climate and demographic factors. Minor changes about influenza seasonality resulted in annual epidemic with winter peak in temperate regions.37 This was similar to our finding that the peak of Flu infections occurred in December‐January. hCoV showed an epidemic season annually in summer (July‐August), whereas previous studies demonstrated no clearly temporal trends for hCoV infections or hCoV more prevalent in winter‐spring.38, 39 The difference may be resulted from geographic location and alternating pattern of viral seasonality.
However, there were three limitations of this study. First, the subtype of virus was not performed in this study and subtype data were not collected. These data could provide more significant information by age group and seasonality in various regions. Second, this study did not included bacterial etiologies, which prevented us from obtaining more comprehensive data on etiologies associated with ARIs. Third, because a significant proportion of ARIs patients had no diagnostic result, we were unable to better characterize viral etiologies of other respiratory infectious disease like asthma which was an important category of ARIs.33
In summary, the findings presented in this study provided important background information of respiratory viral etiologies in Gansu province, China, which may contribute to health authorities for drawing up further plans to prevent and control of respiratory virus infections, and guided the further research in future. Moreover, active surveillance in sentinel hospitals was great helpful to understand viral spectrum of ARIs and estimate disease burden of ARIs associated with respiratory viruses.
CONFLICTS OF INTEREST
The authors have declared that no competing interests exist.
ACKNOWLEDGMENTS
This work was supported by National Science and Technology Major Project of China (Grant number: 2012ZX10004‐208). The sponsor had no role in the design, conduct, data analysis, and reporting of study. The authors of this paper would like to thank all staff members of sentinel hospitals for collecting data and specimens.
Li X, Li J, Meng L, et al. Viral etiologies and epidemiology of patients with acute respiratory infections based on sentinel hospitals in Gansu Province, Northwest China, 2011‐2015. J Med Virol. 2018;90: 828–835. 10.1002/jmv.25040
Contributor Information
Juansheng Li, Email: lijsh16@163.com.
Lei Meng, Email: mleicdc@163.com.
REFERENCES
- 1. Murray CJL, Lopez AD. Global mortality, disability, and the contribution of risk factors: global burden of disease study. Lancet. 1997; 349:1436–1442. [DOI] [PubMed] [Google Scholar]
- 2. Williams BG, Gouws E, Boschi‐Pinto C, et al. Estimates of world‐wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002; 2:25–32. [DOI] [PubMed] [Google Scholar]
- 3. Rudan I, Chan KY, Zhang JSF, et al. Causes of deaths in children younger than 5 years in China in 2008. Lancet. 2010; 375:1083–1089. [DOI] [PubMed] [Google Scholar]
- 4. Jing HE, Gong Y, Zhang WJ, et al. Study on the viral etiology of acute respiratory tract infections in Shanghai area during 2009–2010. J Microbes Infect. 2011; 6:90–96. [Google Scholar]
- 5. Ren L, Gonzalez R, Wang Z, et al. Prevalence of human respiratory viruses in adults with acute respiratory tract infections in Beijing, 2005–2007. Clin Microbiol Infect. 2009; 15:1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sung RYT, Chan PKS, Tsen T, et al. Identification of viral and atypical bacterial pathogens in children hospitalized with acute respiratory infections in Hong Kong by multiplex PCR assays. J Med Virol. 2009; 81:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu T, Li Z, Zhang S, et al. Viral Etiology of acute respiratory tract infections in hospitalized children and adults in Shandong Province, China. Virol J. 2015; 12:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang H, Zheng Y, Deng J, et al. Prevalence of respiratory viruses among children hospitalized from respiratory infections in Shenzhen, China. Virol J. 2016; 13:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allard A, Girones R, Juto P, et al. Polymerase chain reaction for detection of adenoviruses in stool samples. J Clin Microbiol. 1991; 29:2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chung JY, Han TH, Kim CK, et al. Bocavirus infection in hospitalized children, South Korea. Emerg Infect Dis. 2006; 12:1254–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coiras MT, Perez‐Brena P, Garcia ML, et al. Simultaneous detection of influenza A, B, and C viruses, respiratory syncytial virus, and adenoviruses in clinical samples by multiplex reverse transcription nested‐PCR assay. J Med Virol. 2003; 69:132–144. [DOI] [PubMed] [Google Scholar]
- 12. Coiras MT, Aguilar JC, Garcia ML, et al. Simultaneous detection of fourteen respiratory viruses in clinical specimens by two multiplex reverse transcription nested‐PCR assays. J Med Virol. 2004; 72:484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peiris JSM, Tang W‐H, Chan K‐H, et al. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis. 2003; 9:628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Woo PC, Lau SK, Chu CM, et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005; 79:884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang G, Yu D, Mao N, et al. Viral etiology of acute respiratory infection in Gansu Province, China, 2011. PLoS ONE. 2013; 8:e64254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feng L, Li Z, Zhao S, et al. Viral etiologies of hospitalized acute lower respiratory infection patients in China, 2009–2013. PLoS ONE. 2014; 9:e99419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Troeger C, Forouzanfar M, Rao PC, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017; 17:1133–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Niederman MS, Krilov LR. Acute lower respiratory infections in developing countries. Lancet. 2013; 381:1341–1342. [DOI] [PubMed] [Google Scholar]
- 19. Falsey AR, McElhaney JE, Beran J, et al. Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza‐like illness. J Infect Dis. 2014; 209:1873–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johnstone J, Majumdar SR, Fox JD, et al. Viral infection in adults hospitalized with community‐acquired pneumonia: prevalence, pathogens, and presentation. Chest. 2008; 134:1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li S‐W, Lin C‐W. Human coronaviruses: clinical features and phylogenetic analysis. Biomedicine. 2013; 3:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malasao R, Okamoto M, Chaimongkol N, et al. Molecular characterization of human respiratory syncytial virus in the Philippines, 2012–2013. PLoS ONE. 2015; 10:e0142192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaneko M, Watanabe J, Kuwahara M, et al. Impact of respiratory syncytial virus infection as a cause of lower respiratory tract infection in children younger than 3 years of age in Japan. J Infect. 2002; 44:240–243. [DOI] [PubMed] [Google Scholar]
- 24. Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta‐analysis. Infect Immun. 2010; 6:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stein RT, Bont LJ, Zar H, et al. Respiratory syncytial virus hospitalization and mortality: systematic review and meta‐analysis. Pediatr Pulmonol. 2017; 52:556–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pierangeli A, Gentile M, Di Marco P, et al. Detection and typing by molecular techniques of respiratory viruses in children hospitalized for acute respiratory infection in Rome, Italy. J Med Virol. 2007; 79:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Greenberg SB. Respiratory consequences of rhinovirus infection. Arch Intern Med. 2003; 163:278–284. [DOI] [PubMed] [Google Scholar]
- 28. Wat D. The common cold: a review of the literature. Eur J Intern Med. 2004; 15:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heikkinen T, Järvinen A. The common cold. Lancet. 2003; 361:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Glueck R. Review of intranasal influenza vaccine. Adv Drug Deliv Rev. 2001; 51:203–211. [DOI] [PubMed] [Google Scholar]
- 31. Glueck R. Pre‐clinical and clinical investigation of the safety of a novel adjuvant for intranasal immunization. Vaccine. 2001; 20:S42–S44. [DOI] [PubMed] [Google Scholar]
- 32. Feng L, Mounts AW, Feng Y, et al. Seasonal influenza vaccine supply and target vaccinated population in China, 2004‐2009. Vaccine. 2010; 28:6778–6782. [DOI] [PubMed] [Google Scholar]
- 33. Bezerra PGM, Britto MCA, Correia JB, et al. Viral and atypical bacterial detection in acute respiratory infection in children under five years. PLoS ONE. 2011; 6:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berkley JA, Munywoki P, Ngama M, et al. Viral etiology of severe pneumonia among Kenyan young infants and children. JAMA. 2010; 303:2051–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guerrier G, Goyet S, Chheng ET, et al. Acute viral lower respiratory tract infections in Cambodian children: clinical and epidemiologic characteristics. Pediatr Infect Dis J. 2013; 32:e8–13. [DOI] [PubMed] [Google Scholar]
- 36. Arruda E, Pitkaranta A, Witek TJ, Jr. , et al. Frequency and natural history of rhinovirus infections in adults during autumn. J Clin Microbiol. 1997; 35:2864–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ghebrehewet S, MacPherson P, Ho A. Influenza. BMJ. 2016; 355:6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ren L, Gonzalez R, Xu J, et al. Prevalence of human coronaviruses in adults with acute respiratory tract infections in Beijing, China. J Med Virol. 2011; 83:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gaunt ER, Hardie A, Claas EC, et al. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real‐time PCR method. J Clin Microbiol. 2010; 48:2940–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]


