Abstract
Background
Patients with pediatric cancer have a higher risk of morbidity and mortality because of respiratory viral infections than other patient populations.
Objectives
To investigate the causative viruses of respiratory infections and their burden among patients with pediatric cancer in Lebanon.
Study design
Nasopharyngeal swabs along with clinical and demographic data were collected from patients with pediatric cancer presenting febrile episodes with upper respiratory tract symptoms. Total nucleic acid was extracted from specimens followed by the real‐time PCR analysis targeting 14 respiratory viruses to estimate the frequency of infections.
Results
We obtained 89 nasopharyngeal swabs from patients with pediatric cancer (mean age, 5.8 ± 4.2 years). Real‐time PCR confirmed viral infection in 77 swabs (86.5%). Among these, 151 respiratory viruses were detected. Several viruses cocirculated within the same period; respiratory syncytial virus (RSV) being the most common (45.45%), followed by parainfluenza virus (PIV; 26%), influenza type B (26%), human metapneumovirus (24.6%), and human coronavirus (HCoV; 24.6%). Coinfections were detected in 55% of the subjects, and most of them involved RSV with one or more other viruses. A strong correlation was found between PIV, Flu (influenza of any type), RSV, and HCoV with the incidence of coinfections. RSV was associated with lower respiratory tract infections, nasal congestion, bronchitis, and bacteremia. HCoV was associated with bronchiolitis; rhinovirus was associated with hospital admission.
Conclusion
Patients with pediatric cancer have a high burden of respiratory viral infections and a high incidence of coinfections. Molecular diagnostics can improve management of febrile episodes and reduce antibiotic use.
Keywords: coinfection, patients with pediatric cancer, prevalence, real‐time PCR, respiratory tract infections, virus infections
Highlights
Respiratory viruses are leading cause of ARTI in pediatric cancer patients.
Coinfections are common among febrile pediatric cancer patients.
RSV was the most common in mono‐ and coinfections among pediatric cancer patients.
RSV, PIV, Flu, HCoV are associated with coinfections.
Molecular diagnostics permit rapid and sensitive diagnostics and limit antibiotic abuse.
1. INTRODUCTION
Immunocompromised patients, such as those with cancer and hematopoietic stem cell transplantation, have a higher risk for respiratory infections,1 and single or mixed respiratory viruses are frequently detected in those with acute respiratory symptoms.2, 3, 4, 5, 6 Defects in innate and adaptive immunity3 coupled with damage in the mucosal membrane and frequent exposure to a healthcare environment contribute to increased morbidity7, 8 and mortality of respiratory infections in these patients.2, 3, 7, 9, 10, 11 In healthy children, respiratory viruses are usually confined to the upper respiratory tract; in immunocompromised patients, progression to the lower respiratory tract is a more frequent and feared complication.2, 12, 13 Despite advances in cancer therapy and outcomes during the last decade, respiratory viral infections and complications are frequent barriers to the success of antineoplastic treatment.11, 12, 14
Respiratory infections are major causes of febrile episodes in patients with pediatric cancer.3 These patients often are initiated on broad‐spectrum antibiotics to cover serious bacterial diseases, leading to unnecessary increased exposure to antibiotics and the potential emergence of antibiotic resistance.3, 15, 16 Accurate respiratory viral diagnosis and early access to treatment can improve outcomes, allow the prompt initiation of infection control measures, and limit antibiotic use.10 Molecular diagnostic assays for respiratory virus detection and identification are becoming increasingly popular because they outperform traditional viral detection methods, such as antigen detection and cell culture‐based assays in terms of speed, efficiency, specificity, and sensitivity.10, 12 Altogether, these facts highlight the need for surveillance studies that utilize molecular diagnostic tools to elucidate the role of viral pathogens during respiratory infections, their risk factors, and outcomes.12, 17, 18, 19
Epidemiological studies of respiratory viral infections in patients with pediatric cancer in low‐resource settings are scarce.3, 11, 17, 18, 19, 20, 21 The main purpose of this study was to screen for viral etiologic agents and associated risk factors and complications during respiratory infections in patients with pediatric cancer in Lebanon to address the joint need for surveillance studies using molecular diagnostic tools and additional studies of respiratory infections in developing countries.
2. MATERIALS AND METHODS
2.1. Study design and data collection
Between October 2014 and December 2015, nasopharyngeal swabs were collected from cancer patients with acute respiratory tract infection (ARTI) at the Children’s Cancer Center in Lebanon. Patients were considered eligible for this study if they were patients with pediatric cancer having febrile episodes with upper respiratory tract symptoms. The inclusion criteria were as follows: age <18 years, received cancer treatment within the last three months, fever ≥38°C within the previous 72 hours, and having one or more of the following symptoms: cough, sore throat, nasal congestion, rhinorrhea, or respiratory distress. The following data were obtained from enrolled participants: age, sex, demographics, use of influenza vaccines and antivirals, date of onset of symptoms, and hospital admission and length of stay. Medical charts were reviewed to determine health complications, bacterial infection, and absolute neutrophil and lymphocyte counts. Another chart review was performed one month after the initial febrile illness to monitor potential complications. The project was approved by the institutional review boards at the American University of Beirut and St. Jude Children’s Research Hospital. Parental informed consent and participant assent, when applicable, was obtained.
2.2. Sample collection and screening
Nasopharyngeal swabs from each patient were collected by health care providers, preserved in virus transport media, and transported to the laboratory for further analysis. Viral nucleic acid was extracted by using PureLink Viral RNA/DNA mini Kit (Invitrogen, Carlsbad, CA). The AgPath‐ID One‐Step RT‐PCR kit (Applied Biosystems, Austin, TX) was used to screen extracted RNA samples for 14 respiratory viruses: human metapneumovirus (HMPV), respiratory syncytial virus (RSV), influenza A virus (Flu A), influenza B virus (Flu B), rhinovirus (RhV), adenovirus (AdV), parainfluenza viruses 1 to 4 parainfluenza virus (PIV1–4), and human coronaviruses (HCoV‐HKU1, HCoV‐229E, HCoV‐OC43, and HCoV‐NL63). The sequences of primers and probes were obtained from the Center for Disease Control and Prevention (CDC). All runs were performed in the presence of a no‐template control (NTC) and positive control for each target. Extraction controls were screened to exclude cross‐contamination during extraction. Flu A‐positive samples were further subtyped via real‐time PCR using the CDC‐established protocol. RSV‐positive samples were subtyped by conventional PCR followed by 1.5% gel electrophoresis using RSV‐A and RSV‐B primers specific for the G gene hypervariable region.22, 23
2.3. Statistical analysis
The univariate regression analysis was performed to determine the association between viral mono‐ and coinfections with variables and outcomes, including demographics, hospital/ICU admission, lower respiratory tract infection (LRTI), and other clinical symptoms, such as bronchitis, fever, mechanical ventilation, nasal congestion, respiratory distress, vomiting, neutropenia (absolute neutrophil count [ANC] < 1500 cells/µL), and lymphopenia (absolute lymphocyte count < 2000 cells/µL). The χ2 test and odds ratio were computed to test the association between the categorical variables. All variables that were statistically associated with severe outcomes in the univariate models were included in multivariate logistic regression using the backward selection method; a significance level of 0.10 or less was required for a covariate to stay in the model. Then, odds ratio estimates with P values for tested variables were monitored after adjusting for age and sex. Similarly, the correlation analysis between variables was also included, and correlation coefficients were calculated to measure the strength of the relationship between different variables. For these tests, P value < 0.05 was considered significant. Statistical analysis was performed using SAS 9.4 software.
3. RESULTS
3.1. Characteristics of the study population
During the 14‐month study period, 89 febrile episodes were recorded in 67 individual patients. The median age of patients was 4.5 years (IQR 3‐8 years), and 54% of the patients were male (Table 1); 31.5% of the patients had solid tumors, and 68.5% had liquid tumors (Table 1). The most prevalent respiratory symptoms in this population were cough (84.3%), rhinorrhea (85.4%), and nasal congestion (74.2%). In our sample population, 33.3% of the children were admitted to the hospital.
Table 1.
Baseline characteristics of enrolled pediatric cancer patients with ARTI
| Patient characteristics | n (N = 89) | % | |
|---|---|---|---|
| Age, y | 0‐2 | 15 | 16.9 |
| 2‐6 | 41 | 46.1 | |
| >6 | 33 | 37.1 | |
| Sex | Male | 48 | 54 |
| Female | 41 | 46 | |
| Clinical findings | Fever | 89 | 100.0 |
| Bacterial coinfection | 1 | 1.1 | |
| Pneumonia | 4 | 4.5 | |
| Respiratory distress | 19 | 21.3 | |
| Cough | 75 | 84.3 | |
| Rhinorrhea | 76 | 85.4 | |
| Nasal congestion | 66 | 74.2 | |
| Sore throat | 15 | 16.9 | |
| Vomiting | 10 | 11.2 | |
| Diarrhea | 5 | 5.6 | |
| Tumor type | Solid tumor | 28 | 31.5 |
| Liquid tumor | 61 | 68.5 | |
Abbreviation: ARTI, acute respiratory tract infection
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.2. Prevalence and seasonal distribution of respiratory viruses
A total of 151 respiratory viruses were detected in 86.5% (77/89) nasal swabs obtained from 67 patients with pediatric cancer presenting fever. Most patients (55%, n = 49) had coinfections with two or more viruses; 31.5% (n = 28) had monoinfections (ie, a one‐virus infection). According to the chart review, none of the patients had coinfection with bacteria. RSV was the most common virus in the 77 febrile episodes with at least one detected virus, followed by PIV, Flu B, and HMPV. The most prevalent viruses in the 28 monoinfections were HMPV, RSV, and RhV, whereas the most commonly detected viruses in the 49 coinfections were RSV, FluB, and PIV3 (Figure 1 ). Respiratory viral infections were detected mainly during the winter season (December to March) and, to a lesser extent, during the spring (Figure 2). Sporadic infections were detected during the summer and fall seasons. Several respiratory viruses were cocirculating during the same period.
Figure 1.
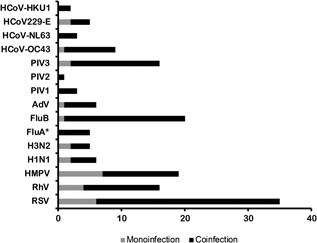
Viruses detected in the respiratory specimens. The frequency of respiratory viruses detected among cancer patients with monoinfections (n = 28) or coinfections (n = 49; 123 detected viruses among coinfections). (FluA*: influenza A viruses that were not subtyped). AdV, adenovirus; Flu A, influenza A virus, Flu B, influenza B virus, HCoV, human coronaviruses; HMPV, human metapneumovirus; PIV 1 to 4, parainfluenza viruses; RhV, rhinovirus; RSV, respiratory syncytial virus
Figure 2.
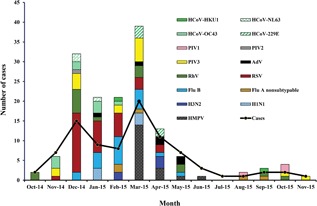
Monthly distribution of the ARTI cases and the detected viruses. Respiratory viruses were detected throughout the year, with a peak in winter. AdV, adenovirus; ARTI, acute respiratory tract infection; Flu A, influenza A virus; Flu B, influenza B virus; HCoV, human coronaviruses; HMPV, human metapneumovirus; PIV 1 to 4, parainfluenza viruses; RhV, rhinovirus; RSV, respiratory syncytial virus
The influenza vaccination rate was relatively low: only 44.7% (n = 30) of the patients with febrile episodes who were eligible to receive the vaccine (ie, age > 6 months; n = 67) did. Of this vaccine‐eligible group, 22% (n = 15) had Flu A and 28% (n = 19) had Flu B. In the vaccinated group, 43.3% (n = 13) had influenza A and/or B infection; 16.7% (n = 5) had both influenza A and B.
3.3. Risk factors and clinical outcomes of ARTI with viral etiology
The univariate analysis was used to assess the association between demographic variables, clinical findings, and respiratory viruses with ARTI (Table 2). Children younger than 2 years were at a significantly higher risk of developing respiratory distress than were those aged 2 to 6 years (P < 0.01, OR, 0.077 [CL 0.015‐0.400]) or those older than 6 years (P = 0.0053, OR, 0.086 [CL 0.015‐0.482]). Neutropenia (ANC < 1500 cells/µL) was identified as a risk factor for hospital admission (P = 0.0192, OR, 3.625 [CL 1.234‐10.65]). No statistically significant association was observed with respect to sex, cancer type and treatment, lymphopenia, or antiviral drug administration and the tested variables. The type of cancer, receiving an anticancer drug, neutropenia, and lymphopenia was not associated with an increased risk of coinfection. In addition, the presence of viral coinfection did not seem to correlate with any of the recorded clinical symptoms (Table 2).
Table 2.
Risk factors and clinical outcomes of patients with respiratory infections
| Patient characteristics | Clinical Outcomes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fever | P | Nasal congestion | P | Respiratory distress | P | Bronchiolitis | P | Hospital admission | P | LRTI | P | Coinfection | P | |
| OR [95% CI] | OR [95% CI] | OR [95% CI] | OR [95% CI] | OR [95% CI] | OR [95% CI] | OR [95% CI] | ||||||||
| Age | ||||||||||||||
| 2‐6 y vs 0‐2 y | 0.15 [0.026‐ 0.85] | 0.03 * | 0.347 [0.038‐3.178] | 0.35 | 0.077 [0.015‐0.400] | 0.002 | 0.95 | 2.526 [0.457‐13.964] | 0.29 | 0.258 [0.031‐ 2.125] | 0.2 | 0.8 [0.19‐3.372] | 0.8 | |
| >6 y vs 0‐2 y | 0.395 [0.079‐ 1.962] | 0.26 | 0.422 [0.043‐4.165] | 0.46 | 0.086 [0.015‐0.482] | 0.005 | 0.818 [0.066‐10.196] | 0.87 | 2 [0.342‐ 11.703] | 0.44 | 1.333 [0.220‐ 8.099] | 0.8 | 0.788 [0.176‐3.526] | 0.8 |
| Sex (female vs male) | 1.2 [0.344‐ 4.188] | 0.78 | 0.828 [0.255‐2.691] | 0.75 | 0.437 [0.133‐1.438] | 0.173 | 0.567 [0.049‐ 6.568] | 0.65 | 0.599 [0.209‐1.719] | 0.34 | 1.920 [0.488‐ 7.549] | 0.4 | 0.762 [0.29‐2.004] | 0.6 |
| Treatment in last 3 mo | 1.913 [0.216‐16.932] | 0.56 | 0.469 [0.053‐4.123] | 0.49 | 2.857[0.329‐24.790] | 0.341 | 0.96 | 1.055 [0.237‐4.697] | 0.94 | 0.96 | 1.719 [0.417‐7.082] | 0.5 | ||
| Anticancer drug | 0.967 | 0.977 | 1.277 [0.132‐12.320] | 0.833 | 0.97 | 0.75 [0.116‐ 4.856] | 0.76 | 0.97 | 1.944 [0.303‐12.469] | 0.5 | ||||
| Tumor (Solid vs liquid) | 3.077 [0.844‐ 11.212] | 0.09** | 0.912 [0.243‐3.421] | 0.89 | 0.756 [0.209‐2.729] | 0.669 | 1.222 [0.104‐14.339] | 0.87 | 2.146 [0.708‐6.507] | 0.18 | 1.778 [0.440‐ 7.191] | 0.4 | 0.633 [0.216‐1.855] | 0.4 |
| Neutropenia (Yes vs no) | 0.453 | 1.929 [0.527‐7.061] | 0.32 | 0.736 [0.231, 2.345] | 0.605 | 0.648 [0.056, 7.529] | 0.73 | 3.625 [1.234‐ 10.65] | 0.0192 | 0.861 [0.218, 3.398] | 0.83 | 1.314 [ 0.485‐3.559] | 0.5916 | |
| Lymphopenia | 0.533 [0.118‐ 2.408] | 0.41 | 0.350 [0.041‐3.015] | 0.34 | 0.500 [0.125‐ 1.999] | 0.327 | 0.385 [0.032‐4.658] | 0.45 | 2.647 [0.519‐ 13.492] | 0.24 | 2.000 [0.227‐ 17.633] | 0.5 | 1.123 [0.305‐4.136] | 0.9 |
| Antiviral drug | 0.985 | 0.99 | 0.984 | 0.99 | 0.99 | 0.99 | 0.99 | |||||||
| Family fever in last 8 d | 0.9 [0.17‐4.76] | 0.9 | 1.395 [0.269‐7.246] | 0.69 | 1.077 [0.253‐4.578] | 0.92 | 0.96 | 0.691 [0.164‐ 2.915] | 0.61 | 0.465 [0.053‐ 4.062] | 0.5 | 1.167 [0.329‐4.131] | 0.8 | |
| Virus detected | ||||||||||||||
| Parainfluenza virus | 1.077 [0.253‐4.578] | 0.92 | 0.205 [0.057‐0.728] | 0.01 | 0.675 [0.166‐2.748] | 0.583 | 1.633 [0.138‐19.294] | 0.7 | 0.856 [0.255‐2.869] | 0.8 | 1.451 [0.328‐ 6.420] | 0.6 | 8.522 [1.754‐ 41.411] | 0.008 |
| Influenza virus | 0.147 [0.018‐1.224] | 0.08 | 0.395 [0.118‐1.319] | 0.13 | 0.909 [0.272‐3.039] | 0.877 | 0.94 | 1.598 [0.548‐4.66] | 0.39 | 0.463 [0.090‐ 2.390] | 0.4 | 6.158 [1.792‐21.159] | 0.004 | |
| Adenovirus | 0.97 | 0.780 [0.075‐8.129] | 0.84 | 1.067 [0.103‐ 11.032] | 0.957 | 0.98 | 0.97 | 0.97 | 0.97 | |||||
| Rhinovirus | 1.5 [0.343‐6.561] | 0.59 | 3.805 [0.451‐ 32.122] | 0.22 | 1.556 [0.407‐5.949] | 0.518 | 2.167 [0.181‐ 25.903] | 0.54 | 4.343 [1.214‐ 15.532] | 0.02 | 1.045 [0.194‐ 5.628] | 0.96 | 2.089 [0.573‐7.612] | 0.3 |
| Respiratory syncytial virus | 6.167 [1.486‐25.586] | 0.01 | 5.357 [1.091‐26.300] | 0.04 | 1.684 [0.543‐5.227] | 0.367 | 0.95 | 2.24 [0.786‐6.385] | 0.13 | 4.316 [1.005‐ 18.544] | 0.049 | 10.679 [3.075‐ 37.079] | 0.0002 | |
| Human coronavirus | 1.958 [0.434‐8.833] | 0.38 | 1.227 [0.233‐6.453] | 0.81 | 3.409 [0.877‐ 13.251] | 0.08 | 12.223 [1.002‐149.166] | 0.049 | 1.814 [0.485‐6.779] | 0.38 | 2.625 [0.560‐ 12.309] | 0.2 | 10.741 [1.287‐ 89.605] | 0.03 |
| Human metapneumovirus | 0.242 [0.029‐2.043] | 0.19 | 0.205 [0.057‐0.728] | 0.01 | 1.083 [0.294‐3.989] | 0.904 | 0.95 | 0.408 [0.102‐1.634] | 0.21 | 0.311 [0.036‐ 2.667] | 0.3 | 1.481 [0.468‐4.687] | 0.5 | |
Statistically significant values (P < 0.05) are indicated in bold.
Borderline values (P < 0.05) are presented in bold italics.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Next, the association of the detected viruses with complications and coinfection was assessed. To account for the small sample size, genotypes or subtypes of the same virus were grouped together (eg, influenza virus for Flu A and B). RhV was significantly associated with hospital admission (P = 0.023, OR, 4.343 [CL 1.214‐15.532]), whereas RSV was significantly associated with LRTI (P = 0.0493, OR, 4.316 [CL 1.005‐18.544]) and nasal congestion (P = 0.0387, 5.357 [1.091‐26.300]). HCoV was significantly associated with bronchiolitis (P = 0.049, OR, 12.223 [CL 1.002‐149.166]), whereas PIV and HMPV were significantly associated with nasal congestion (P = 0.01, OR, 0.205 [CL 0.057‐0.728]). PIV, influenza virus, RSV, and HCoV were significantly associated with coinfection (P < 0.01, OR, 8.522 [CL 1.754‐ 41.411]; P = 0.0039, OR, 6.158 [CL 1.792‐21.159]; P < 0.01, OR, 10.679 [CL 3.075‐37.079]; P = 0.0283, OR, 10.741 [CL 1.287‐89.605], respectively). Among these, RSV had the strongest correlation with coinfection (Table 2).
Variables were further analyzed via multivariate logistic regression analysis with the selection method of backward elimination, whereby a P value of 0.1 was required for a covariate to stay in the model. Children aged 2 to 6 years and those older than 6 years had lower odds (94.8%, and 95.2%, respectively) of having respiratory distress than those younger than 2 years (P < 0.01). The odds of having a coinfection was 27.3 times greater (P < 0.001, OR, 27.3 [CL 2.8‐268]) in patients with influenza than in those without influenza. Furthermore, the patients with RSV infections were 70.7 times more likely to have a coinfection (P < 0.001, OR, 70.7 [CL7.4‐678]) than those with the other tested viruses. PIV remained significantly associated with coinfection (P < 0.001, OR, 41.5 [CL 3.3‐514.5]). Infection with RSV remained significantly associated with nasal congestion (P = 0.046). The odds of having nasal congestion were 5.1 (CL [1.03‐25.3]) times greater for patients with RSV than for those without RSV infection. After adjusting for age and sex, the estimates and significance did not change.
The correlation analysis showed that RSV (r = 0.5, P < 0.0001), influenza virus (r = 0.37, P < 0.0001), PIV (r = 0.36, P < 0.001), and HCoV (r = 0.32, P < 0.001) were significantly associated with coinfection. AdV (r = 0.23, P = 0.0648) showed a moderate relationship with coinfection. Neutropenia was positively associated with RhV (r = 0.34, P = 0.0053), but PIV had a negative association (r = −0.21, P = 0.095). RSV was positively correlated with HCoV (r = 0.29, P = 0.0161) but negatively correlated with HMPV (r = −0.39, P < 0.01; Figure 3A). Hospital admission and neutropenia were significantly correlated (r = 0.4, P < 0.001), as were hospital admission and RhV (r = 0.29, P = 0.018). Diarrhea was significantly associated with HCoV (r = 0.49, P < 0.0001) and with RSV (r = 0.26, and P = 0.0312). Nasal congestion was also significantly correlated with RSV (r = 0.27, P = 0.0256). HCoV (r = 0.22, and P = 0.0681) had a moderate relationship with respiratory distress (Figure 3B).
Figure 3.
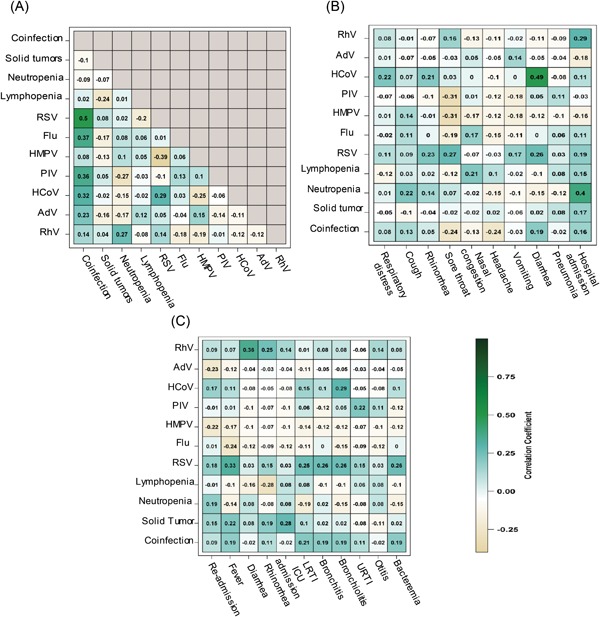
Heat maps representing (A) correlation matrix of infections (n = 67), (B) correlation between infections and events (n = 67), and (C) correlation between infections and events in follow‐up (n = 67). Negative correlations are shown in yellow, and positive correlations are shown in green. Neutropenia was defined as absolute neutrophil count < 1500 cells/µL, and lymphopenia was defined as lymphocyte count < 2000µL. AdV, adenovirus; Flu, influenza; HCoV, human coronaviruses; HMPV, human metapneumovirus; PIV 1 to 4, parainfluenza viruses; RhV, rhinovirus; RSV, respiratory syncytial virus
The follow‐up data revealed that recurrent fever was significantly related to the previous RSV infection (r = 0.33, P < 0.01) and moderately correlated with solid tumors (r = 0.22, P = 0.0821). Rhinorrhea was positively related to the previous RhV infection (r = 0.25, P = 0.040) but negatively correlated with lymphopenia (r = −0.28, P = 0.025). Both bronchitis (r = 0.26, P = 0.031) and bacteremia (r = 0.26, P = 0.0312) were significantly related to the prior RSV infection during the follow‐up period. LRTI was significantly associated with RSV (r = 0.25, P = 0.0384) but moderately associated with coinfections (r = 0.21, P = 0.0901). Moreover, positive correlations were observed between bronchiolitis and previous HCoV (r = 0.29, P = 0.016) and RSV infections (r = 0.26, P = 0.031), whereas URTI was moderately correlated with the previous PIV infection (r = 0.22, P = 0.074; Figure 3C).
3.4. Repeated detection of respiratory viruses and RSV genotyping
During the study period, 17 of the 67 patients (25.4%) had repeated febrile episodes, which often led to the detection of the same virus(es) associated with the first episode (Table 3). The median time between repeated febrile episodes with a confirmed viral infection was 61 days (range 10‐196 days). Repeated detection of the same virus was noted in nine patients. RSV was most frequently detected (6/17) in the repeated episodes. Using genotype‐specific PCR to detect RSV showed that three of these paired samples had RSVA; for the remaining three paired specimens, a genotype could not be determined in at least one of the specimens. We also detected one case for each of the repeated episodes of HCoV‐OC43, Flu B, RhV, PIV3, AdV, and HMPV.
Table 3.
Viruses detected among patients with multiple febrile episodes
| Patient ID# | Virus(es) detected in each sample | Interval between specimen collection (days) | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 1 | RhV | ‐‐* | ‐‐ | ‐‐ | ‐‐ | |
| RSVA | HCoV‐OC43 | ‐‐ | ‐‐ | ‐‐ | 73 | |
| HCoV‐229E | RSV | ‐‐ | ‐‐ | ‐‐ | 82 | |
| PIV3 | PIV1 | ‐‐ | ‐‐ | ‐‐ | 196 | |
| 10 | HCoV‐OC43 | RSV | ‐‐ | ‐‐ | ‐‐ | |
| RSVA | HCoV‐OC43 | ‐‐ | ‐‐ | ‐‐ | 16 | |
| 12 | RSVA | RhV | PIV3 | ‐‐ | ‐‐ | |
| RSVA | ‐‐ | ‐‐ | ‐‐ | ‐‐ | 12 | |
| 13 | RSVA | RhV | ‐‐ | ‐‐ | ‐‐ | |
| RSVA | FluB | FluA | ‐‐ | ‐‐ | 51 | |
| 17 | RSVA | RhV | FluB | PIV2 | PIV3 | |
| RhV | PIV3 | ‐‐ | ‐‐ | ‐‐ | 80 | |
| 18 | RSVA | PIV3 | ‐‐ | ‐‐ | ‐‐ | |
| HMPV | ‐‐ | ‐‐ | ‐‐ | ‐‐ | 72 | |
| HMPV | RhV | HCoV‐229E | ‐‐ | ‐‐ | 11 | |
| ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | 22 | |
| 21 | RSVA | RhV | ‐‐ | ‐‐ | ‐‐ | |
| ‐‐ | FluB | H1N1 | ‐‐ | ‐‐ | 10 | |
| RSVA | FluB | H3N2 | ‐‐ | ‐‐ | 40 | |
| 23 | RSVA | ‐‐ | ‐‐ | ‐‐ | ‐‐ | |
| HCoV‐229E | ‐‐ | ‐‐ | ‐‐ | 89 | ||
| 26 | RSV** | AdV | ‐‐ | ‐‐ | ‐‐ | |
| AdV | ‐‐ | ‐‐ | ‐‐ | ‐‐ | 133 | |
| 28 | RSV | FluB | HCoV‐OC43 | ‐‐ | ‐‐ | |
| HMPV | RSV | ‐‐ | ‐‐ | ‐‐ | 65 | |
| 31 | RSV | FluB | PIV3 | ‐‐ | ‐‐ | |
| HCoV‐229E | ‐‐ | ‐‐ | ‐‐ | 57 | ||
| 32 | RSV | FluB | FluA | HCoV‐OC43 | HCoV‐HKU1 | |
| RSVB | ‐‐ | ‐‐ | ‐‐ | ‐‐ | 50 | |
| 35 | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | |
| ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | 43 | |
| 40 | HMPV | Flu B | PIV3 | ‐‐ | ‐‐ | |
| __ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | 127 | |
| 42 | HMPV | PIV3 | ‐‐ | ‐‐ | ‐‐ | |
| AdV | FluB | ‐‐ | ‐‐ | ‐‐ | 70 | |
| 45 | HMPV | ‐‐ | ‐‐ | ‐‐ | ‐‐ | |
| FluB | ‐‐ | ‐‐ | ‐‐ | ‐‐ | 37 | |
| 62 | HMPV | ‐‐ | ‐‐ | ‐‐ | ‐‐ | |
| HCoV‐HEKU1 | RhV | FluA | 76 | |||
Abbreviations: AdV, adenovirus; Flu A, influenza A virus; Flu B, influenza B virus; HCoV, human coronaviruses; HMPV, human metapneumovirus; PIV 1 to 4, parainfluenza viruses; RhV, rhinovirus; RSV, respiratory syncytial virus
No additional virus was detected in this specimen.
Subtype could not be determined.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
4. DISCUSSION
This study demonstrates that respiratory viruses are a leading cause of acute respiratory infections in pediatric cancer patients and should be considered an important etiology of febrile neutropenia and hospital admissions. The data showed that viral infections were associated with 86.5% of the febrile episodes in patients with pediatric cancer. Detection of a respiratory viral infection in this population has multiple implications, including persistence of infection and disease reactivation. These findings also highlight the urgent need for vaccines and effective drugs against the commonly circulating viral pathogens, which will limit unnecessary administration of antivirals and antibacterials, and best management of isolation precautions of infected patients.
A substantial subset (55%) of our study population had coinfections with multiple viruses. We did not find any association between coinfections and clinical symptoms/outcome or having neutropenia or lymphopenia in our patients. Consistent with our findings, Torres et al18 found no difference in clinical outcome in children with febrile neutropenia having mixed respiratory viral infections and those with monoinfections. Similar findings were reported by Rotzén‐Östlund et al24 who found that immunocompetent children with mixed or monoinfections had a similar duration of hospitalization, risk of pediatric ICU admission, and need for oxygen. However, other studies in immunocompetent children found that coinfection was associated with more‐severe lower respiratory tract infection and a higher risk of hospital admission than monoinfection was.25, 26 A meta‐analysis of 21 studies involving 4280 immunocompetent patients found no evidence of increased severity as a result of coinfections compared with monoinfections.27 Therefore, the clinical implications of respiratory viral coinfections need further investigation.
Most studies investigating respiratory viral infections in immunocompromised children were carried out in developed countries. In Sweden, respiratory viral infections were detected in 45% of cases of febrile neutropenia in children with mixed malignancies.19 A similar rate of respiratory viral infections in children with leukemia or mixed malignancies was reported in the USA, Germany, Finland, and Chile.2, 18, 28, 29 In contrast, in Spain, respiratory viral infections were detected in only 12% of patients with cancer, and all viral infections were monoinfections.11 In the mentioned studies, RSV, RhV, and Flu A were the most commonly detected viruses.2, 3, 11, 18, 19, 29 In our study group, RSV was the most prevalent virus, followed by PIV, and Flu B, with HMPV being the most frequent virus in monoinfections and RSV detected predominantly in coinfections. The higher rate of virus detection in our study population in Lebanon could be attributed to the use of real‐time PCR, the higher number of screened viruses, and including respiratory symptoms as an inclusion criterion. Some studies identified respiratory viruses via one or more of the following techniques: clinical symptoms and serology testing, virus culture, fluorescent antibody detection, and PCR for limited viral targets.2, 11, 18, 19, 28, 29 Nonetheless, even when compared with studies that utilized PCR as a diagnostic tool to screen a comparable number of viral targets (10 or more) in patients with cancer, this study has a higher incidence of virus detection.3, 17, 18, 19 This higher incidence of viral infection might be attributed to the cultural interaction that includes close contact, thus promoting transmission.30, 31
This study reflects the importance of using molecular diagnostic assays in clinical practice for better diagnosis and improving patient care.32, 33, 34 It is important to note that mutations in the region of the detection primers could result in reduced detection rates by PCR. Therefore, continuous monitoring of the circulating virus strains and updating of the detection primers to capture any novel, variant strains is necessary.
Accurate information on the frequency of respiratory viruses and their burden allows for accurate recommendations in prioritizing drug and vaccine development. For instance, children receiving chemotherapy developed life‐threating complications when infected with RSV.11, 35 Previous studies in patients with pediatric cancer reported that RSV can be associated with pneumonia and neutropenia leading to severe life‐threatening complications.11, 17, 35, 36 Mucositis was very common in patients with neutropenia, and this might be one of the predictive factors of the severity of respiratory viral infections, especially those because of RSV.11, 17, 37 The data showed that RSV was significantly associated with LRTI, fever, bronchiolitis, nasal congestion, and diarrhea but did not increase the risk of ICU admission. Similar to what was previously reported, the data suggests that encountering RSV infection in patients with pediatric cancer might add to their disease burden and affect their response to the treatment.35, 38, 39, 40 Moreover, this study revealed a high prevalence of RSV in coinfections and in repeated infections in patients with pediatric cancer, highlighting the urgent need for the development of RSV antivirals and vaccines. Currently, RSV vaccines under development are in phase III clinical trials, constituting a step in the right direction.41, 42, 43 In infants at high‐risk (preterm or having chronic illness), RSV neutralizing monoclonal antibodies, including the FDA‐approved palivizumab, are effective for prophylaxis but remain very expensive in developing countries.44, 45, 46 The emergence of RSV variants that are resistant to palivizumab is a concern that highlights the need for more therapeutic options.47, 48 Prophylaxis should be utilized in patients with cancer, especially in those with the highest risk for infection (in this study, younger children), to reduce potential complications.
In patients with cancer, influenza infections are usually accompanied by bacteremia and delays in chemotherapy.49, 50 A study conducted by Mendoza Sánchez et al11 showed that 40% of immunocompromised children (cancer and HIV; age ≤ 14 years) infected with influenza required hospitalization. Such outcomes would be effectively reduced by annual vaccinations, which are now recommended for patients and their health care providers. In this study, infection with influenza A and/or B was detected in 26.9% (24/89) of the cases. The vaccination rate among the children ≥6 months (recommended age for the vaccine) with febrile episodes was 44.7%, which is considered to be low for this high‐risk population. Therefore, emphasizing the importance of attaining universal vaccination in this population and their caregivers is likely to improve patient outcomes by preventing influenza infection.
The duration of shedding and frequency of recurrent virus detections have not been thoroughly investigated in immunocompromised children.19, 35 In the current study, 17 of the 67 patients had repeated detection of respiratory viruses in subsequent febrile episodes, and half of these patients had repeated detection of the same virus. RSV was the most common virus detected repeatedly in subsequent febrile episodes. It was not possible to rule out whether these infections were persistent or repeated infections because this study only included patients in whom a febrile episode developed rather than monitoring all patients on a routine basis. Hall et al35 reported prolonged RSV shedding that persisted for more than 20 days in some cases.35 Martin et al51 reported prolonged shedding of respiratory viruses in samples collected at least 7 days apart: the viruses involved in prolonged shedding included HMPV, RSV, AdV, HCoV, RhV, and PIV. A study by Soderman et al19 suggested that the shedding time of Flu, HMPV, RSV, and PIV is limited, and virus clearance was evident at a median follow‐up time of 28 days. In contrast, the same RhV genotypes were detected from subsequent follow‐up samples after 12 to 51 days, and some RhV‐positive patients reported the appearance of respiratory symptoms 6 days or more before fever onset, indicating the possibility of prolonged shedding. Longitudinal studies whereby patients are screened on a weekly basis until no detection of a given virus is confirmed are required to determine the frequency of repeated infections vs persistent infections among children with cancer and their impact on patient outcomes. A molecular approach using sequencing of the sequentially detected viruses should allow a better understanding of the extent of virus evolution in these patients.
The current study had several limitations, including the heterogeneity of cancer types in the studied population and the absence of a control group of patients with asymptomatic cancer. Another limitation was the lack of follow‐up sampling to monitor the status of the detected respiratory virus and differentiate between prolonged vs persistent shedding. Moreover, human bocavirus and enteroviruses were not analyzed, which could have further increased the overall detection rate. Despite the mentioned limitations, screening for respiratory viruses, and their related clinical manifestations is of great importance, especially in scarcely studied populations and particularly in developing countries, to guide better patient management and to develop evidence‐based infection control measures.
5. CONCLUSION
Respiratory infections can lead to serious complications and might become life‐threatening, particularly in the context of weakened immune systems of patients with cancer. Few studies have investigated respiratory infections in patients with cancer, especially in children. In this study, we detected a high incidence of respiratory viral infections among children with cancer in Lebanon via real‐time PCR. The results demonstrate the usefulness of real‐time PCR in diagnosis and, therefore, in guiding proper clinical management and infection control. Preventing respiratory viral infections in immunocompromised patients, including those with cancer, is critical for protecting these patients, especially given the absence of effective vaccines and antiviral drugs for most of these viruses.
FUNDING
This project was supported by the Children's Infection Defense Center fund, ALSAC, and the American University of Beirut Faculty of Medicine seed fund.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Soudani N, Caniza MA, Assaf‐Casals A, et al. Prevalence and characteristics of acute respiratory virus infections in pediatric cancer patients. J Med Virol. 2019;91:1191:1191‐1201. 10.1002/jmv.25432
Contributor Information
Ghassan Dbaibo, Email: gdbaibo@aub.edu.lb.
Hassan Zaraket, Email: hz34@aub.edu.lb.
References
REFERENCES
- 1. Corti M, Palmero D, Eiguchi K. Respiratory infections in immunocompromised patients. Curr Opin Pulm Med. 2009;15(3):209‐217. 10.1097/MCP.0b013e328329bd2c [DOI] [PubMed] [Google Scholar]
- 2. Hakim H, Dallas R, Zhou Y, et al. Acute respiratory infections in children and adolescents with acute lymphoblastic leukemia. Cancer. 2016;122(5):798‐805. 10.1002/cncr.29833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benites ECA, Cabrini DP, Silva ACB, et al. Acute respiratory viral infections in pediatric cancer patients undergoing chemotherapy. J Pediatr (Rio J). 2014;90(4):370‐376. 10.1016/j.jped.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fazekas T, Eickhoff P, Rauch M, et al. Prevalence and clinical course of viral upper respiratory tract infections in immunocompromised pediatric patients with malignancies or after hematopoietic stem cell transplantation. J Pediatr Hematol Oncol. 2012;34(6):442‐449. 10.1097/MPH.0b013e3182580bc8 [DOI] [PubMed] [Google Scholar]
- 5. Perlin E, Bang KM, Shah A, et al. The impact of pulmonary infections on the survival of lung cancer patients. Cancer. 1990;66(3):593‐596. [DOI] [PubMed] [Google Scholar]
- 6. Kohno S, Kola H, Oka M, et al. The pattern of respiratory infection in patients with lung cancer. Tohoku J Exp Med. 1994;173(4):405‐411. [DOI] [PubMed] [Google Scholar]
- 7. Lustberg MB. Management of neutropenia in cancer patients. Clin Adv Hematol Oncol HO. 2012;10(12):825‐826. [PMC free article] [PubMed] [Google Scholar]
- 8. Rolston KVI. Infections in cancer patients with solid tumors: a review. Infect Dis Ther. 2017;6(1):69‐83. 10.1007/s40121-017-0146-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aronchick JM. Pulmonary infections in cancer and bone marrow transplant patients. Semin Roentgenol. 2000;35(2):140‐151. 10.1053/ro.2000.6152 [DOI] [PubMed] [Google Scholar]
- 10. Godbole G, Gant V. Respiratory tract infections in the immunocompromised. Curr Opin Pulm Med. 2013;19(3):244‐250. 10.1097/MCP.0b013e32835f82a9 [DOI] [PubMed] [Google Scholar]
- 11. Mendoza Sánchez MC, Ruiz‐Contreras J, Vivanco L, et al. Respiratory virus infections in children with cancer or HIV infection. J Pediatr Hematol Oncol. 2006;28(3):154‐159. 10.1097/01.mph.0000210061.96075.8e [DOI] [PubMed] [Google Scholar]
- 12. von Lilienfeld‐Toal M, Berger A, Christopeit M, et al. Community acquired respiratory virus infections in cancer patients—guideline on diagnosis and management by the infectious diseases working party of the German society for haematology and medical oncology. Eur J Cancer. 2016;67(Suppl C):200‐212. 10.1016/j.ejca.2016.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hussain Z, Ansari JA, Salman M, Khan EA, Asghar J. An evaluation of acute respiratory infection surveillance systems in Gilgit‐Baltistan Pakistan. JPMA J Pak Med Assoc. 2016;66(6):682‐687. [PubMed] [Google Scholar]
- 14. Dizon DS, Krilov L, Cohen E, et al. Clinical cancer advances 2016: annual report on progress against cancer from the American society of clinical oncology. J Clin Oncol. 2016;34(9):987‐1011. 10.1200/JCO.2015.65.8427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akinosoglou KS, Karkoulias K, Marangos M. Infectious complications in patients with lung cancer. Eur Rev Med Pharmacol Sci. 2013;17(1):8‐18. [PubMed] [Google Scholar]
- 16. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2011;52(4):e56‐e93. 10.1093/cid/cir073 [DOI] [PubMed] [Google Scholar]
- 17. Christensen MS, Nielsen LP, Hasle H. Few but severe viral infections in children with cancer: a prospective RT‐PCR and PCR‐based 12‐month study. Pediatr Blood Cancer. 2005;45(7):945‐951. 10.1002/pbc.20469 [DOI] [PubMed] [Google Scholar]
- 18. Torres JP, De la Maza V, Kors L, et al. Respiratory viral infections and coinfections in children with cancer, fever and neutropenia: clinical outcome of infections caused by different respiratory viruses. Pediatr Infect Dis J. 2016;35(9):949‐954. 10.1097/INF.0000000000001209 [DOI] [PubMed] [Google Scholar]
- 19. Söderman M, Rhedin S, Tolfvenstam T, et al. Frequent respiratory viral infections in children with febrile neutropenia ‐ a prospective follow‐up study. PLoS One. 2016;11(6):e0157398 10.1371/journal.pone.0157398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vliora C, Syridou G, Papaevangelou V. Viral respiratory infections in children receiving chemotherapy or undergoing stem cell transplantation. Clin Microbiol Open Access. 2014;3(1):137 10.4172/2327-5073.1000137 [DOI] [Google Scholar]
- 21. Meerhoff TJ, Simaku A, Ulqinaku D, et al. Surveillance for severe acute respiratory infections (SARI) in hospitals in the WHO European region ‐ an exploratory analysis of risk factors for a severe outcome in influenza‐positive SARI cases. BMC Infect Dis. 2015;15:1 10.1186/s12879-014-0722-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peret TC, Golub JA, Anderson LJ, Hall CB, Schnabel KC. Circulation patterns of genetically distinct group A and B strains of human respiratory syncytial virus in a community. J Gen Virol. 1998;79(Pt 9):2221‐2229. 10.1099/0022-1317-79-9-2221 [DOI] [PubMed] [Google Scholar]
- 23. Sato M, Saito R, Sakai T, et al. Molecular epidemiology of respiratory syncytial virus infections among children with acute respiratory symptoms in a community over three seasons. J Clin Microbiol. 2005;43(1):36‐40. 10.1128/JCM.43.1.36-40.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rotzén‐Östlund M, Eriksson M, Tiveljung Lindell A, Allander T, Zweygberg Wirgart B, Grillner L. Children with multiple viral respiratory infections are older than those with single viruses. Acta Paediatr. 2014;103(1):100‐104. 10.1111/apa.12440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aberle JH, Aberle SW, Pracher E, Hutter H‐P, Kundi M, Popow‐Kraupp T. Single versus dual respiratory virus infections in hospitalized infants: impact on clinical course of disease and interferon‐gamma response. Pediatr Infect Dis J. 2005;24(7):605‐610. [DOI] [PubMed] [Google Scholar]
- 26. Cilla G, Oñate E, Perez‐Yarza EG, Montes M, Vicente D, Perez‐Trallero E. Viruses in community‐acquired pneumonia in children aged less than 3 years old: high rate of viral coinfection. J Med Virol. 2008;80(10):1843‐1849. 10.1002/jmv.21271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Asner SA, Science ME, Tran D, Smieja M, Merglen A, Mertz D. Clinical disease severity of respiratory viral co‐infection versus single viral infection: a systematic review and meta‐analysis. PLoS One. 2014;9(6):e99392 10.1371/journal.pone.0099392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Katsimpardi K, Papadakis V, Pangalis A, et al. Infections in a pediatric patient cohort with acute lymphoblastic leukemia during the entire course of treatment. Support Care Cancer. 2006;14(3):277‐284. 10.1007/s00520-005-0884-6 [DOI] [PubMed] [Google Scholar]
- 29. Koskenvuo M, Möttönen M, Rahiala J, et al. Respiratory viral infections in children with leukemia. Pediatr Infect Dis J. 2008;27(11):974‐980. 10.1097/INF.0b013e31817b0799 [DOI] [PubMed] [Google Scholar]
- 30. Mossong J, Hens N, Jit M, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5(3):e74 10.1371/journal.pmed.0050074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stein ML, Van steenbergen JE, Buskens V, et al. Comparison of contact patterns relevant for transmission of respiratory pathogens in Thailand and the Netherlands using respondent‐driven sampling. PLoS One. 2014;9(11):e113711 10.1371/journal.pone.0113711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rhedin S, Lindstrand A, Rotzen‐Ostlund M, et al. Clinical utility of PCR for common viruses in acute respiratory illness. Pediatrics. 2014;133(3):e538‐e545. 10.1542/peds.2013-3042 [DOI] [PubMed] [Google Scholar]
- 33. Vallières E, Renaud C. Clinical and economical impact of multiplex respiratory virus assays. Diagn Microbiol Infect Dis. 2013;76(3):255‐261. 10.1016/j.diagmicrobio.2013.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Elden LJR, van Kraaij MGJ, Nijhuis M, et al. Polymerase chain reaction is more sensitive than viral culture and antigen testing for the detection of respiratory viruses in adults with hematological cancer and pneumonia. Clin Infect Dis. 2002;34(2):177‐183. 10.1086/338238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hall CB, Powell KR, MacDonald NE, et al. Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med. 1986;315(2):77‐81. 10.1056/NEJM198607103150201 [DOI] [PubMed] [Google Scholar]
- 36. Whimbey E, Englund JA, Couch RB. Community respiratory virus infections in immunocompromised patients with cancer. Am J Med. 1997;102(3A):10‐18. discussion 25‐26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anaissie EJ, Mahfouz TH, Aslan T, et al. The natural history of respiratory syncytial virus infection in cancer and transplant patients: implications for management. Blood. 2004;103(5):1611‐1617. 10.1182/blood-2003-05-1425 [DOI] [PubMed] [Google Scholar]
- 38. El Saleeby CM, Somes GW, DeVincenzo JP, Gaur AH. Risk factors for severe respiratory syncytial virus disease in children with cancer: the importance of lymphopenia and young age. Pediatrics. 2008;121(2):235‐243. 10.1542/peds.2007-1102 [DOI] [PubMed] [Google Scholar]
- 39. Salzer W, Dinndorf P, Dreyer Z, Hilden J, Reaman GH. Analysis of infectious complications in infants with acute lymphoblastic leukemia treated on the Children’s Cancer Group Protocol 1953: a report from the Children’s Oncology Group. J Pediatr Hematol Oncol. 2009;31(6):398‐405. 10.1097/MPH.0b013e3181a6dec0 [DOI] [PubMed] [Google Scholar]
- 40. Torres HA, Aguilera EA, Mattiuzzi GN, et al. Characteristics and outcome of respiratory syncytial virus infection in patients with leukemia. Haematologica. 2007;92(9):1216‐1223. [DOI] [PubMed] [Google Scholar]
- 41. Wu H, Pfarr DS, Johnson S, et al. Development of motavizumab, an ultra‐potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J Mol Biol. 2007;368(3):652‐665. 10.1016/j.jmb.2007.02.024 [DOI] [PubMed] [Google Scholar]
- 42. Weisman LE. Motavizumab, a second‐generation humanized mAb for the prevention of respiratory syncytial virus infection in high‐risk populations. Curr Opin Mol Ther. 2009;11(2):208‐218. [PubMed] [Google Scholar]
- 43. Neuzil KM. Progress toward a respiratory syncytial virus vaccine. Clin Vaccine Immunol. 2016;23(3):186‐188. 10.1128/CVI.00037-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Turner T, Kopp B, Paul G, Hayes jr D, Thompson R, Landgrave L. Respiratory syncytial virus: current and emerging treatment options. Clin Outcomes Res CEOR. 2014;6:217‐225. 10.2147/CEOR.S60710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Anderson LJ, Dormitzer PR, Nokes DJ, Rappuoli R, Roca A, Graham BS. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine. 2013;31(Suppl 2):B209‐B215. 10.1016/j.vaccine.2012.11.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Olchanski N, Hansen RN, Pope E, et al. Palivizumab prophylaxis for respiratory syncytial virus: examining the evidence around value. Open Forum Infect Dis. 2018;5(3). 10.1093/ofid/ofy031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Adams O, Bonzel L, Kovacevic A, Mayatepek E, Hoehn T, Vogel M. Palivizumab‐resistant human respiratory syncytial virus infection in infancy. Clin Infect Dis. 2010;51(2):185‐188. 10.1086/653534 [DOI] [PubMed] [Google Scholar]
- 48. Zhu Q, Patel NK, McAuliffe JM, et al. Natural polymorphisms and resistance‐associated mutations in the fusion protein of respiratory syncytial virus (RSV): effects on RSV susceptibility to palivizumab. J Infect Dis. 2012;205(4):635‐638. 10.1093/infdis/jir790 [DOI] [PubMed] [Google Scholar]
- 49. Joseph C, Togawa Y, Shindo N. Bacterial and viral infections associated with influenza. Influenza Other Respir Viruses. 2013;7(Suppl 2):105‐113. 10.1111/irv.12089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ljungman P, Gleaves CA, Meyers JD. Respiratory virus infection in immunocompromised patients. Bone Marrow Transplant. 1989;4(1):35‐40. [PubMed] [Google Scholar]
- 51. Martin ET, Fairchok MP, Stednick ZJ, Kuypers J, Englund JA. Epidemiology of multiple respiratory viruses in childcare attendees. J Infect Dis. 2013;207(6):982‐989. 10.1093/infdis/jis934 [DOI] [PMC free article] [PubMed] [Google Scholar]


