Abstract
Ebola virus disease (EVD), caused by Ebola viruses, resulted in more than 11 500 deaths according to a recent 2018 WHO report. With mortality rates up to 90%, it is nowadays one of the most deadly infectious diseases. However, no Food and Drug Administration‐approved Ebola drugs or vaccines are available yet with the mainstay of therapy being supportive care. The high fatality rate and absence of effective treatment or vaccination make Ebola virus a category‐A biothreat pathogen. Fortunately, a series of investigational countermeasures have been developed to control and prevent this global threat. This review summarizes the recent therapeutic advances and ongoing research progress from research and development to clinical trials in the development of small‐molecule antiviral drugs, small‐interference RNA molecules, phosphorodiamidate morpholino oligomers, full‐length monoclonal antibodies, and vaccines. Moreover, difficulties are highlighted in the search for effective countermeasures against EVD with additional focus on the interplay between available in silico prediction methods and their evidenced potential in antiviral drug discovery.
Keywords: advancement, antiviral, discovery, drugs, Ebola virus, in silico methods, therapeutic
1. INTRODUCTION
The Filoviridae family consists of three genera, namely Marburgvirus, Cuevavirus, and Ebolavirus (EBOV).1 The Ebolavirus includes five virus species included Sudan ebolavirus, Tai Forest ebolavirus, Bundibugyo ebolavirus, Reston ebolavirus, and Zaire ebolavirus.2, 3
High mortality rates associated with Ebola virus disease (EVD) outbreaks in humans illustrate their extreme vulnerability as host for filoviruses. Bodily fluids of an infected animal or individual serve as a mode of transmission, through exposure to cuts, wounds, and mucous membranes, direct or accidental injection,4, 5, 6, 7 and recently suspected cases of sexual transmission.8, 9 During disease prognosis, macrophages, monocytes, and dendritic cells are generally infected first, which later progressed to major cellular targets.10, 11, 12, 13 The action of viral proteins (VP24 and VP35) causes suppression of type‐I interferon resulting in dysregulation of the immune response and activation of T‐cells as result of EBOV infection,14, 15 with various disease manifestations.16, 17, 18, 19
Among clinical signs, initial nonspecific symptoms arise (malaise, fever, and gastrointestinal infection19, 20 followed by the state of shock, severe uveitis, vision loss,7, 21 organ failure, and ultimately death.22, 23 Disease progression towards the EBOV disease accounts for 90% mortality rate in humans.24, 25, 26, 27 Due to hypovolemic shock and multiorgan damage, death typically occurs between 6 and 16 days after the chronic symptoms of hemorrhagic illness.28
EVD outbreaks tend to rely on supportive care measures with fluid and electrolyte replacement. During the 2014‐2016 outbreak (which resulted in 28 616 infections and 11 310 deaths), a coordinated and collaborative global effort with some stakeholders and agencies have been carried out resulting in some vaccine candidates and therapeutics capable of protecting high‐risk populations with unprecedented efficiency. In 2018, the Ministry of Heath of Democratic Republic of Congo (DRC) reported two consecutive EVD outbreaks, of which the first on 8 May (May‐June 2018 Equateur province DRC outbreak) and the second on 1 August (August‐present, 2018, Kivu province DRC outbreak). The first outbreak resulted in 54 EVD cases (38 confirmed and 16 probable) and 33 deaths (overall case‐fatality ratio of 61%) as of 24 July, 2018 (declared the end of this outbreak),29 while the latter is ongoing, which includes 238 cases (203 confirmed and 35 probable) and 155 deaths resulting in a case‐fatality rate of 65.1% (as of 21 October, 2018).30 Preclinical efforts toward specific EBOV countermeasures have been enduring for years with first treatment and vaccine clinical trials conducted during the 2013 to 2016 EBOV outbreak in West‐Africa.31, 32, 33, 34, 35, 36, 37 During the DRC outbreaks, the countermeasures were evaluated in West‐Africa EVD outbreak and demonstrated their clinical efficacy. In addition, a small number of studies (case reports) exists for the experi countermeasures has been enduring mental use of anti‐Ebola antiviral and vaccine leads.38, 39, 40, 41 EBOV vaccine development has already been covered in previously published reviews.34, 42, 43, 44, 45 In brief, the recent update includes the development of replication‐competent rVSV‐ZEBOV (rVSV) used in May‐June 2018 Equateur DRC outbreak in a ring vaccination trial (Phase III; NCT03161366), which proved to be well tolerated with improved efficacy and safety.35, 36 Currently, rVSV is being investigated along with other investigational therapeutics in the ongoing Kivu DRC outbreak (Phase II; NCT03719586). A second update includes Ad26‐ZEBOV/MVA‐BN‐Filo prime‐boost vaccine evidenced as safe and well tolerated.46, 47 Both rVSV and Ad26‐ZEBOV/MVA‐BN‐Filo prime‐boost vaccine have been evaluated in Phases I, II, and III clinical trials. Other nonreplicating vaccines are virus‐vector‐based evidenced to be highly effective in non‐human primates (NHPs).48, 49, 50, 51, 52, 53 The current study is focused on the therapeutic advancement towards EVD and the challenges encountered.
1.1. Biological targets of EBOV
Identification of suitable biological drug targets is the primary step towards therapeutic development.54, 55 Validated through a combination of genetic,56 biochemical,57, 58 structural, and computational strategies,54, 55 a variety of drug targets have been identified in both host and pathogen.59
Encased in a lipid envelope, filoviruses are filamentous in shape.60 Linear, nonsegmented, negative‐sense single‐stranded RNA encodes a 19‐kb genome containing the genetic information for seven structural proteins and considered as potential drug targets, namely transcription activator VP30, polymerase cofactor VP35, matrix proteins VP40 and VP24, nucleoprotein (NP), glycoprotein (GP), and RNA‐dependent RNA polymerase (L).61, 62 The structure and function of these proteins aided in deciphering the molecular mechanisms of filovirus lifecycle and have been explanatorily reviewed in a series of three reviews by Martin et al., describing aspects of filovirus entry,63 replication cycle,64 assembly, and budding.65 Briefly, GP, a heterodimeric complex of GP1,2 surface protein, orchestrates viral entry into the host cell and participates in virus egress.66 Due to the major role of GP1,2 in viral entry, numerous approaches targeting the entry process have been explored to block EBOV replication at an early stage, namely immune‐based therapies,67, 68, 69, 70 peptide‐based antiviral molecules, a broad range of small molecules, reviewed by Rhein and Maury,71 and more specific entry inhibitors targeting the fusion events characterized by the GP2/NPC1 (Niemann‐Pick C1) interaction.72, 73
After entry by macropinocytosis, replication and transcription cycles involve the releasing of viral nucleocapsids into the host cell cytoplasm, resulting in the synthesis of new viral proteins and genomes. The whole process of assembly and budding is coordinated by NP, VP24, and VP40, and enhanced by GP.74 NP binds to the viral RNA and creates RNP complex with polymerase L and viral proteins VP24, VP30, and VP35.75 VP30 and NP play an important role in the RNA‐binding activity.75 The multifunctional protein, VP35, suppresses host‐innate immunity and has been found to be involved in transcription/replication processes together with nucleocapsid assembly.76, 77 The interactions between these proteins (ie, NP‐NP and NP‐VP35) are essential to regulate the formation of the EBOV replication complex for efficient transcription/replication. Blocking this nucleocapsid formation by protein‐protein interaction inhibitors can lead to potential inhibition of EBOV. for example, VP35‐derived peptide (first identified NP ligand) that specifically binds to NP, blocks NP oligomerization and causes the release of RNA from NP‐RNA complex.78, 79 Other studies identified 18β‐glycyrrhetinic acid and licochalcone‐A80 to potentially disrupt the association of NP‐RNA complexes, aptamers,81 pyrrolidinone compounds,82 and recently MCCB4 (ene‐thiazolidinedione group‐containing compound),83 that specifically lead to specific VP35‐NP interaction. VP24 functions as nucleocapsid maturation factor84 and transcription/replication modulator.85 Additionally, it has been identified as a target for protein‐protein interaction inhibitors, namely for a macrocyclic peptide inhibitor VP24‐KPNA5 (karyopherin α5) which specifically disrupt VP24‐KPNA interaction.86 VP40 is a matrix protein which coordinates with VP24 toward viral assembly, budding,87 and regulation of viral transcription.88 All structural/functional features and recent advances for these crucial regulatory proteins, essential in viral messenger RNA (mRNA) synthesis and genome replication, have been critically reviewed64 and therefore constitute key targets for designing EBOV‐specific drugs.
1.2. Current experimental therapeutics against EVD
Key strategies recognized to combat EBOV include: (i) directly targeting the virus, (ii) modulating the host factors or immune response, and (iii) disease management. Critically targeting the viral lifecycle is among the most popular strategies for EBOV therapeutics.61, 63, 89, 90, 91 This is done either by targeting the initial binding and/or entry of the virus into the host cells or the later viral replication and packaging. EBOV antiviral compounds mainly encompass small molecules, antisense therapies, and immunotherapeutic drugs.
Current treatment of EVD is centered upon modulating coagulation and maintaining oxygen levels in Ebola patients.13, 92, 93, 94 Because of these therapeutic interventions, sustenance and recovery have been reported. However, significant improvement might be observed as a result of an adequate level of support care.27, 92, 93, 95, 96 To date, no commercial vaccines or specific therapies are available for EBOV. However, various studies have been reported that explain the comprehensive development of vaccines,11, 42, 43, 61 small‐molecule inhibitors,90, 91, 97, 98, 99, 100, 101, 102, 103 and repurposed Food and Drug Administration (FDA) approved drugs against Ebola.90, 100, 104, 105, 106, 107, 108, 109, 110 Table 1 provides an overview of anti‐EBOV compounds and their level of efficacy, reported in either IC50 or EC50, in vitro assays against EBOV, clinical status, and observational studies.
Table 1.
Ebola virus (EBOV) drug candidates, their efficacies (EC50 or IC50) and clinical status
| Ebola preclinical data | |||||
|---|---|---|---|---|---|
| Compound/drug | EBOV target and description | EBOV clinical trial phase | Results/status | In vitro efficacy data | Animal efficacy data |
| BCX4430 | A novel adenosine nucleotide analogue that inhibits RNA polymerase L activity by incorporating into new viral RNA chains and cause chain termination | Phase I (NCT02319772) | Phase I complete; generally safe and well tolerated up to 10 mg/kg daily for 7 d | EC50: 11.8 μM | Mice: 90% survival at 150 mg/kg BID PO; |
| EC90: 25.4 μM (IM Kikwit) | 100% survival at 150 mg/kg BID IM; | ||||
| NHP: 100% survival at 25 mg/kg BID; days 0‐14; | |||||
| EC50:3.4 μM | 100% survival rate at 100 mg/kg loading dose BID; days 2‐3; | ||||
| EC90: 10.5 μM (Boniface)89 | 67% survival at maintenance dose of 25 mg/kg BID till days 3‐17111 | ||||
| GS‐5734 | a novel monophosphoramidate prodrug of adenosine analogues, it selectively inhibits EBOV replication by targeting its RNA‐dependent RNA polymerase and converts it into active triphosphate nucleotides in efficient cells | Phase II (NCT02818582) | Phase I complete; Phase II, given intravenously in survivors with viral perseverance in their semen | EC50 replication: 0.021‐0.066 μM | NHP: 100% protection at 10 mg/kg IV days 3‐15 (extensively summarized in Warren et al113 |
| EC90: 0.053‐0.203 μM (Mayinga) | |||||
| EC50: 0.014 μM | |||||
| EC90: 0.045 μM (Makona) | |||||
| VTR: | |||||
| EC50: 0.003 μM | |||||
| Reporter Assay | |||||
| EC90: 0.013 μM (Makona)112 | |||||
| Favipiravir (T‐705; avian) | a pyrazine derivative that was discovered during a screen of compounds against influenza virus A/PR/8/34 (H1N1); it modified intracellularly to purine derivative and inhibits RNA polymerase L with significant efficacy | Phase III (NCT02329054 and NCT02363322) | Limited efficacy in patients with lower to moderate levels of virus (C t > 20); T‐705 was well tolerated114 | EC50: 44.2 μg/mL114 | Mice: 300 mg/kg qD PO; day 1; 90% survival; |
| 150, 75, and 37.5 mg/kg qD PO; days 0 ‐14; 100% survival | |||||
| 300, 150, 75, and 37.5 mg/kg BID PO; days 0‐14; 100% survival (While mice gained weight) | |||||
| 8, 1.6, 0.325 mg/kg PO qD; survival rate 90%, 10%, and 0%, respectively115 | |||||
| NHP: 400/200 qD PO; days 0‐10; 17% survival | |||||
| 250/150 mg/kg PO BID; days 0‐14; and 125/75 mg/kg PO BID; days 0‐14; All NHPs succumbed between days 11‐15 and days 8‐11115 | |||||
| 150 and 180 mg/kg BID days 1‐21 (with 1‐d loading dose of 250 mg/kg); ~50% survival | |||||
| Amiodarone | Cationic amphiphilic drug (CAD) amiodarone is a widely used antiarrhythmic drug which inhibits EBOV infection (in vitro) at an early stage of viral replication | Phase II (NCT02307591) | Terminated: unknown statistically significant | IC50 entry: 5.6 μM | Mice: No survival rate of 60 mg/kg. |
| IC50 entry (lentivirus): 2.2 μM; | 0% to 40% survival at 90 mg/kg.101 | ||||
| IC50 replication: 0.4 μM90 | NPH: N/A | ||||
| Amodiaquine | CAD103 | Preclinical | Showed significantly lower mortality (50.7%) for amodiaquine compared with lumefantrine (64.4%) | EC50 entry: 2.6 μM; | Mice: No increased survival at 60 mg/kg BID IP; days 0‐7.101 |
| EC50 replication: 34 μM | NPH: N/A | ||||
| Chloroquine | CAD | N/A | Well tolerated | EC50 entry: 4.7 μM | Mice: Mixed results across several dose/studies; IP and PO with 0% to 80% survival rate. |
| EC50 replication: 16 μM116 | Hamsters: No efficacy at 50 mg/kg IP in combination with doxycycline (2.5 mg/kg) and azithromycin (50 mg/kg). Guinea pigs: no protection up to 100 mg/kg. | ||||
| Hydroxychloroquine | CAD | N/A | N/A | EC50 replication: 22 μM | N/A |
| EC50 entry: 9.5 μM117 | |||||
| Clomiphene | CAD; entry inhibitor | N/A | Used in combination treatment together with irbesartan and atorvastatin for some patients. Well tolerated at prescribed doses | EC50 replication: 11 μM | Mice: 10% survival at 60 mg/kg IP BID.116 |
| EC50 entry:1.3 μM116 | NPH: N/A | ||||
| Toremifene | CAD; entry inhibitor | N/A | N/A | EC50: 1.73 μM (Kikwit) | Mice: 50% survival at 60 mg/kg IP qD on days 0, 1, 3, 5, 7, 9.100 |
| EC50:0.973 μM (Mayinga)100 | |||||
| EC50:1.10 μM (Makona)118 | NPH: N/A | ||||
| Amiodarone | CAD; entry inhibitor | Phase II (NCT02307591) | Terminated early; reduction in case‐fatality rate; not statistically significant | IC50 entry: 5.6 μM | Mice: 90 mg/kg; 0‐40% survival.101 |
| IC50 entry (lentivirus): 2.2 μM | |||||
| IC50 replication: 0.4 μM90 | NPH: N/A | ||||
| Azithromycin | CAD | Registered as NCT02380625 (not yet open to recruitment) | Well tolerated in critically ill patients | EC50 replication: 5.1 μM101 | Mice: 10%‐60% survival at 100 mg/kg BID IP. |
| EC50 VLP entry: 2.79 μM | 0% survival by PO route. | ||||
| EC90 VLP entry: 15.8 μM119 | Guinea pigs: no efficacy.101 | ||||
| Sertraline | CAD; entry inhibitor | N/A | Well tolerated in healthy adults and children | IC50: 3.13 μM (Vero) | Mice: 70% survival at 10 mg/kg PO qD. |
| IC50: 1.44 μM (HepG2) | NPH: N/A | ||||
| Bepridil | Glycoprotein120 | N/A | N/A | IC50: 5.08 μM (Vero) | Mice: 100% survival at 12 mg/kg |
| IC50: 3.21 μM (HepG2) | |||||
| (Mayinga)120 | NPH: N/A | ||||
| Brincidofovir (BCV, CMX001) | Monophosphoramidate prodrug of an adenosine analogue selectively inhibits viral RNA (L) polymerase | Phase II (NCT02271347) | Clinical trial halted due to low enrollment | EC50: 0.88 μM (Makona) | No preclinical efficacy reported so far31 |
| And withdrawn by the company for further development as EBOV therapeutic31 | EC50: 0.66‐0.79 μM (kikwit, Huh7)121 | ||||
| TKM‐100802/TKM‐130803 | Small interfering RNA‐lipid nanoparticle product that targets viral RNA polymerase L, VP35, and VP24 | TKM‐100802: Phase I (NCT02041715) | TKM‐100802: Terminated | EC50: 50 ng/mL (Makona) | NHP: 100% survival against Kikwit (0.5 mg/kg qD IV) and Makona; (0.5 mg/kg qD IV) after viral challenge123 |
| TKM‐130803: Phase II (PACTR201501000997429) | TKM‐130803: Terminated; failure to achieve a survival probability and did not demonstrate efficacy122 | EC50: 50‐100 ng/mL (Kikwit) | |||
| EC50: 100‐250 ng/mL (Makona) | |||||
| EC50: 1‐50 ng/mL (Makona)123, 124 | |||||
| ZMapp | A cocktail of three human chimeric neutralizing mAbs (c13C6, c2G4, and 4G7) selected from MB‐003 and ZMab antibody cocktails. Selectively targets the viral glycoprotein | Phase II (NCT02363322) | Suspended: due to no conclusion on efficacy available | EC50: <0.1‐1 μg/mL (Gueckedou) | NHP: 100% survival at 50 mg/kg, 3 doses with 3‐d interval IV70 |
| Convalescent whole blood/plasma (Ebola‐Tx) | ABO‐compatible plasma from a separate convalescent donor, Targets whole virus/glycoprotein | Phase II/III (NCT02342171) | Completed; No adverse reactions associated and found no significant improvement in efficacy in survival125 | N/A | NHP: No efficacy observed with whole blood; efficacy of concentrated IgG from survivors |
| AVI‐7537 | VP24 | Phase I (NCT01593072) | Withdrawn before enrollment; further development has been suspended due to funding constraints | EC50: 0.585 μM | NHP: 75% survival at 40 mg/kg qD IV.126 |
| Tilorone hydrochloride (tilorone) | N/A | N/A | N/A | EC50: 0.23 μM116 | Mice: 25 and 50 mg/kg qD IP D0‐8; proved efficacious in protecting 90% of mice from a lethal EBOV challenge116 |
Abbreviations: BID, twice daily; IM, intramuscular injection; IP, intraperitoneal injection; IV, intravenous injection; mAb, monoclonal antibody; NHP, non‐human primate; PO, oral administration; qD, once daily; siRNA, small interfering RNA; VTR, herpesvirus telomerase RNA.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
1.3. Small molecules: direct EBOV inhibitors
Development of potent small molecules directed against the RNA‐dependent RNA polymerase L required for viral replication is the most promising therapeutics. The three most potent nucleotide viral polymerase inhibitors, GS‐5734, BCX4430, and favipiravir (T‐705), have demonstrated in vitro and animal efficacy in EBOV‐infected mice and NHPs. They have reported antiviral effects due to intracellular conversion to their corresponding nucleoside triphosphate for incorporation into the viral genome and inhibition of viral polymerase.
By the end of the West‐Africa EBOV outbreak, GS‐5734 clearly indicated 100% protection of rhesus monkeys following lethal EBOV challenge113 and an improved highly potent in vitro efficacy against Mayinga and Makona strains as compared to favipiravir.112 Moreover, GS‐5734 has been recently administered for the first time to a newborn baby.127 BCX4430 has been tested against for its broad‐spectrum inhibition of various viruses including, arenaviruses, bunyaviruses, coronaviruses, paramyxoviruses, and flaviviruses.89, 128, 129 Additionally, animal survival efficacy accounted for 90% to 100% in mice128 and 67% to 100% in rhesus monkeys using increased doses of BCX4430.111 Favipiravir (T‐705) remained a potential anti‐EBOV candidate during the West‐Africa outbreak94, 130 and has reported up to 100% survival rate in EBOV‐infected mice even with the lowest oral dose of 37.5 mg/kg once daily. In comparison, NHP resulted in 17% to 50% survival rate.94, 115
Among various FDA screens, multiple compounds including amodiaquine, diphenylpyraline, ketotifen, diphenoxylate were reported to inhibit EBOV replication, while others including, verapamil, dronedarone, sertraline, toremifene, chloroquine, teicoplanin, and amiodarone were considered EBOV entry inhibitors.90, 100, 104, 110 Very recently, tilorone (EC50 = 0.23 μM) was reported to be the most potent small‐molecule inhibitor with a single‐daily dose of 25 and 50 mg/kg intraperitoneal and proved efficacious in protecting 90% of mice from a lethal EBOV challenge.116 Some of these inhibitors are shown in Figure 1. Other studies include the identification of coumarin‐based antihistamine‐like molecules,131 benzoquinoline compounds (SW456 compound reported to be the most potent compound in the infectious EBOV assay, IC50 = 0.5 μM),132 ellagic acid (IC50 = 1.4 μM),133 and vindesine (IC50 = 0.34 μM),134 that proved to have potential EBOV inhibition.
Figure 1.
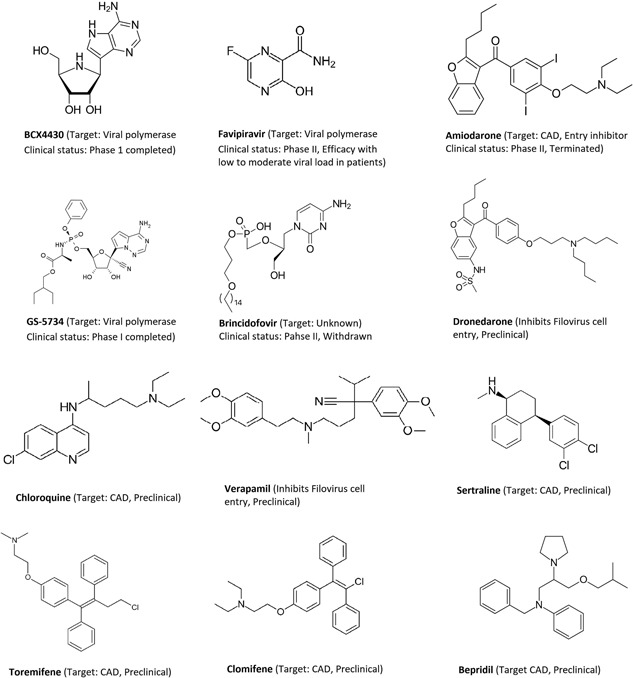
Few drug candidates to treat Ebola virus disease
1.4. Immunotherapeutics and other treatments
Direct antivirals preventing viral entry incorporate large numbers of immune‐therapeutics under development.64, 98, 135 EVD treatment has recently been linked with the modulation of the immune system. Cytokines and chemokines play an immunomodulatory role during EBOV infection promote viral clearance by an enhanced immune response. However, an overwhelming inflammatory cytokine release can cause an undesirable effect. Therefore, numerous research groups have currently identified and studied a variety of anti‐Ebola monoclonal antibodies (mAbs), leading to the development of several commercialized mAb cocktails as recently reviewed.136
EBOV GP constitutes a prime target for therapeutic antibodies. ZMAb, a cocktail of three mouse mAbs (1H3, 2G4, and 4G7), resulted in 50% to 100% protection,67, 69 while MB‐003, a cocktail of three mouse‐human chimeric mAbs (13C6, 13F6, and 6D8), demonstrated 67% protection after EBOV (Kikwit strain) infection in rhesus monkeys.68 To identify the most protective combination, ZMapp was introduced as a combination of three EBOV‐GP mAbs (13C6, 2G4, and 4G7) and demonstrated 100% protection for rhesus monkeys.70 The functional mechanism of binding of ZMapp has been described for its neutralizing activity targeting the GP base and glycan cap.137 Further studies reported the further optimization of ZMapp against NHPs by using two chimeric mAbs (13C6 and 2G4), designated as MIL77, which protected all EBOV (Makona strain) infected rhesus monkeys.138
Very recently, adeno‐associated virus‐mediated mAb 5D2 or 7C9 delivers 100% protection against mouse‐adapted EBOV infection, whereas neutralizing mAb 2G4 revealed 83% protection.139 Also, a coformulated cocktails REGN3470‐3471‐3479 (three human 3‐mAb cocktails REGN3470, REGN3471, and REGN3479 in 1:1:1 ratio) is currently being evaluated and proved safe and well tolerated with no observed immunogenicity after a randomized, first‐in‐human Phase I clinical trial (NCT002777151).37
Other therapeutics include the nucleic acid‐based inhibitors with phosphorodiamidate morpholino oligomers (PMOs) and small‐interference RNAs (siRNAs), involved in promoting degradation of mRNA transcripts and constitute two essential classes of antisense therapies.102, 140, 141 Both compounds target vital proteins involved in EBOV transcription/translation processes VP24, VP35, and viral polymerase L. The development underlying the modification of PMOs and siRNA has been elaborated142 and recently reviewed.143 PMO combination, as in AVI‐6002 (AVI‐7537 and AVI‐7539), has shown significant efficacy and safety in NHP126 when directed against both VP35/VP24 (63% protection) and VP24 alone (75% protection)126 without further development. TKM‐100803, a lipid siRNA nanoparticle product that targets viral RNA polymerase L, VP35, and VP24 demonstrated no survival advantage after Phase II single‐arm trial122 without further development of TKM‐EBOV modifications.
Additionally, managing EVD includes treatment of clinical manifestations like hemorrhage or coagulation abnormalities.24, 92 Electrolyte balance gets disturbed as soon as the virus starts to reproduce and spreads across the body. Patients require fluid intake (intravenous or oral) rich in electrolytes, to treat dehydration and to restore electrolyte balance.21, 92, 93, 144 Moreover, EBOV infection results in disturbed blood clotting. For that purpose, anticoagulants like recombinant nematode anticoagulant protein c2 (rNAPc2) and recombinant human‐activated protein C (rhAPC)145 known to affect coagulation pathways have been investigated and resulted in reduced morbidity and fatality.146
1.5. 2014‐2016 EBOV outbreak—an overview
Regardless of no effective treatment against EVD, potential drug candidates indicated promising results in animal models after the West‐Africa 2014‐2016 outbreak.31, 64, 81, 89, 90, 94, 99, 100, 113, 126, 128, 130, 147, 148 Safety and efficacy concerns arose due to the limited duration of the outbreak. For this reason, EBOV therapeutics in advanced development had only been evaluated in the initial two phases of the clinical trials.31, 32, 149 Bafilomycin, chlorpromazine, cytochalasin B, mannose‐binding lectin, and ZMapp were reported to inhibit viral entry,70, 100, 150, 151, 152 while other mAbs and cocktails are under development and most are now in preclinical stages.138, 139, 153, 154, 155, 156 Because of successful preclinical NHPs data,70, 138 ZMapp was recently used on four patients evacuated from West‐Africa157 and successfully treated two patients repatriated to the United States.93 In 2015, a randomized controlled trial (Prevail II; NCT02363322) of 72 patients demonstrated 22% deaths (8 of 36 patients), who were treated with ZMapp compared with the group of patients who received standard care alone (37% deaths; 13 of 35 patients).
During the outbreak, two prominent vaccines, rVSVΔG‐ZEBOV‐GP (rVSV) and cAd3‐EBO, were tested in clinical trials and proved to be efficacious.35, 36, 158, 159 rVSV is currently in Phase II (NCT02876328 and NCT02788227) and is being tested in the United States and Africa. Against viral polymerase, several drugs have been reported and tested.81, 99, 128 BCX4430 and GS‐5734 have successfully crossed Phase I clinical trials (Table 1). In male EBOV patients, GS‐5734 is currently being tested for the reduction of viral load in semen.89, 113 Very recently, the antiviral activity of drugs was discovered, and selected molecular probes have been identified for EBOV infection. Clomiphene and toremifene, selective estrogen receptor modulators (SERMs), have been identified as a result of this in vitro screening.100 SERMs block Ebola entry by causing a reduction in the accumulation of endolysosomal calcium and the concentration of cellular sphingosine160 and have been approved by the FDA for treating EBOV infections.
Although the 2014‐2016 outbreak highlighted several therapeutic compounds161 that successfully crossed Phase I clinical trials, some of them indicated safety concerns.34, 44, 117, 162 The clinical trials conducted during the outbreak lacked proper controls and statistical power due to the severity and urgency of the disease.163 TKM‐130803 and amiodarone reached Phase II of clinical trials in Sierra Leone, which were later terminated due to lack of demonstrating efficacy and statistical power.122, 163 FDA halted TKM‐100802 because of the release of cytokines triggered by the action of the siRNA causing flu‐like symptoms in treated individuals.164, 165 Likewise, brincidofovir, an oral bioactive molecule, failed to cross the Phase II clinical trials for efficacy, safety, and tolerability, resulting in discontinuation.31 Another study, the JIKI trial, was carried out in 2014 to test the efficacy of favipiravir. The current favipiravir data suggests its efficacy for low to moderate viral loads.32 This trial which was conducted in four Ebola treatment centers in Guinea and relied on the use of historical controls. Because of the ambiguity of the results, JIKI was criticized for its design.32 Another clinical trial of nonrandomized Ebola‐Tx, carried out in Guinea evaluating convalescent plasma, failed to demonstrate an improved survival rate.125, 166 Similarly, amodiaquine showed good invitro efficacy in inhibiting EBOV activity104 but showed liver‐related toxic effects during artesunate‐amodiaquine combinatory treatment.167
1.6. 2018 EBOV outbreak (May‐June 2018, August‐present)—an update
Two consecutive outbreaks are reported in the following year. On 8 May 2018, the Government of the Democratic Republic of Congo (DRC) reported an EVD outbreak (Zaire EBOV strain) in the north‐west of the country. According to the last updated situation report of Equateur DRC outbreak (24 July 2018), a total of 54 EVD cases (38 laboratories confirmed and 16 probable) were reported since the beginning of this outbreak on 4 April 2018 till 2 June. Of these 54 cases, (29 from Iboko, 21 from Bikoro and 4 from Wangata health zones), 33 died resulting in a case‐fatality ratio of 61%.29 This is the ninth outbreak in DRC in the last four decades (since 1976), with the last outbreak dated in May 2017 (8 cases with 4 deaths). Due to improved efficacy and safety of rVSV (evaluated in more than 10 000 individuals),35, 36 the test treatment was administered during the May‐June 2018 DRC outbreak, currently being investigated in a ring vaccination trial (Phase III; NCT03161366). After the declaration of the end of Equateur DRC outbreak, another EVD outbreak was reported in Kivu and Ituri provinces of DRC on 1st August 2018. As of 21st October 2018, a total of 238 cases (203 laboratories confirmed and 35 probable) have been reported, including 155 deaths, resulting in a case‐fatality ratio of 65.1%.30 To date, the 10th ongoing outbreak in DRC has raised serious concerns due to the spread in surrounding regions. In this outbreak, investigational therapeutics including ZMapp, remdesivir(GS‐5734), and mAB114 (a monoclonal antibody used for the very first time to treat infected individuals) are being investigated together with rVSV (Phase II; NCT03719586).
1.7. In silico methods for anti‐EBOV drug discovery
In silico methods in drug discovery hold great potential and may prove beneficial at any stage in the preclinical development of drug candidates.168 Especially, areas like target validation, the design of compound libraries,169, 170 hit identification,171, 172, 173 hit‐to‐lead optimization,174 and preclinical candidate identification can essentially benefit from exponentially increasing in silico tools, with unprecedented accuracy. A report from Bayer HealthCare175 illustrates the significance of integrating computational drug design in pharmaceutical companies. This report states that computer‐aided design methods (CADD) have aided in approximately half of the 20 new chemical entities currently being tested in Phase I clinical trials. Figure 2 highlights the interconnected stages and different phases in the drug discovery process mapped with EBOV updates.
Figure 2.
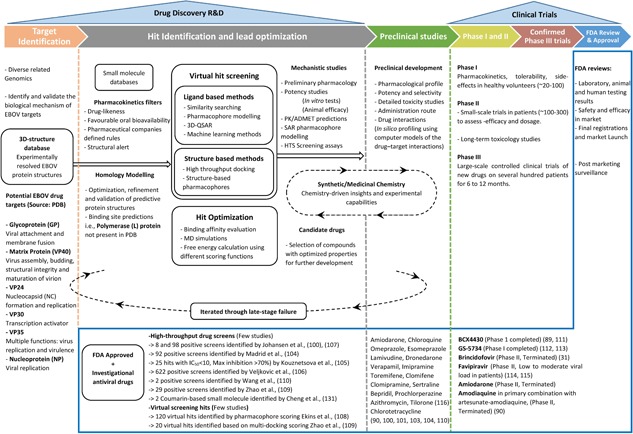
Stepwise drug discovery process from target identification, hit‐to‐lead optimization, and clinical trials along with the progress in the development of small‐molecule inhibitors against EBOV. ADMET, absorption, distribution, metabolism, excretion, and toxicity; HTS, high through‐put screen; PDB, protein data bank; PK, pharmacokinetics; QSAR, quantitative structure‐activity relationship; R&D, research and development; SAR, structure‐activity relationship
1.8. Homology modeling
Protein modeling plays a significant role in the drug discovery process. The goal of homology modeling is structure prediction from a known sequence with accuracy comparable to experimentally resolved structures.176, 177, 178 Restrictions linked with this technique are the presence of inserts and loop sequences, which cannot be accurately predicted in the absence of a three‐dimensional (3D) crystal structure.179 The gap between known protein sequences and identified protein structures is significantly growing. Given an enormous amount of data through a vast array of DNA sequencing techniques available, experimental structure identification techniques require attention.180 Computational techniques are actively exploited in the pharmaceutical industry for the prediction of 3D protein models.124, 168 To expand the scope of computational methods and to improve model accuracy, efforts are being made continuously. These approaches help to predict the tertiary structure of a protein through its amino acid sequence to combat this issue.176 Depending on the available information, these methods can be characterized as either de novo or homology modeling. Template‐based modeling also referred to as homology modeling or comparative modeling, is the most trusted method for model design.176 Similar folding properties of the members of a protein family with the core structure unaffected by modifications in the sequence are fundamental criteria governing homology modeling.181 Models are generated given target‐protein sequences and X‐ray, cryo‐EM, or NMR‐determined structures. Even with a low sequence similarity (~20%), accurate models can be obtained using homology modeling.182, 183, 184, 185 For this, a template structure is initially selected to identify similar experimentally determined structures, after which template‐target sequence alignment is performed. The 3D model is then energetically refined to optimize model quality. The refinement of the model includes optimizing bond lengths and angles and removing clashes in geometry. If required, additional structural modifications can be applied, until a relevant and accurate model is obtained. However, the refinement of the model often does not meet the desired level of accuracy.186 A number of potential in silico studies have been documented over last few years which include homology modeling of unresolved EBOV polymerase187, 188 and docking‐based virtual screening of compounds that have potential to bind with important residues lining the binding pocket of EBOV VP40, VP24, VP30, and VP35.82, 108, 189, 190, 191, 192, 193, 194
1.9. Molecular dynamics simulation to elucidate ligand‐protein interactions
Although optimal ligand‐receptor interactions can be predicted through molecular docking,195 not all key interactions between the ligand and the active site of the receptor will be accurately depicted. Hence, molecular docking followed by molecular dynamics (MD) simulations of the obtained complexes can help in understanding interaction modes. Rastelli et al196 reported sulfonamide derivatives to bind effectively within the active site of aldose reductase. Contrary to these predictions, the experiments demonstrated lower activity and binding potential of these compounds, therefore, negating the prediction made. Later, the in silico refinements of these compounds using MD revealed the interruption of key interactions between sulphonamide ligands and the receptor due to an additional water molecule. The migration of this water molecule from outside explained the reduced activity of these compounds when tested experimentally.196 In another study by Cavalli et al,197 MD simulations were used as a platform to discern several different docked complexes of propidium and human acetylcholinesterase and most stable structures identified which correlated with the experimentally verified binding modes.
Interestingly, MD simulation assisted in the discovery and development of antiviral drugs.198, 199, 200 For the first time, a combination of MD refinements of postdocking complexes and ensemble‐based molecular docking has helped to reveal a unique symmetrical binding mode of daclatasvir with hepatitis C virus (HCV) NS5A protein. This drug is currently in Phase III clinical trials and is being tested for different HCV genotypes.201 Moreover, through MD simulations, identification of a trench adjacent to the active site of HIV‐1 integrase has been made possible.202 The role of the trench in ligand binding later became evident when a site‐directed mutagenesis study was carried out. These findings helped in the design of potent HIV‐1 integrase inhibitors with enhanced antiviral activity. A 3D structure model of major coreceptor of HIV‐1, CCR5, has been constructed through the use of MD simulations.203 Furthermore, the development of antiviral drugs against influenza virus (IFV) has also benefitted from MD simulations. Through the use of this method, a universal cavity (150‐cavity) adjacent to the binding site of the natural substrate has been reported with neuraminidase (NA) proteins of human 2009 pandemic H1N1, avian H5N1, and human H2N2 strains.204
Recently, MD simulations have been used to study the molecular behavior of ZIKV NS3‐helicase, both in the presence and absence of single‐stranded RNA, and the potential implications for NS3‐helicase activity/inhibition.205 Recent studies have reported notable examples of MD‐driven drug discovery.199 These studies prove the usefulness of MD simulation in understanding molecular interactions and the mechanism of drug binding,206, 207 especially against the drug‐resistant viruses.206, 208, 209
1.10. Hit‐to‐lead optimization
Hit‐to‐lead optimization is the most essential phase to closely examine the chemical scaffold concerning its absorption, distribution, metabolism, and excretion (ADME) challenges in the drug discovery process. In silico ligand‐profiling210, 211 benefited from the boost of repurposing drugs91, 104, 108, 194 and the notion of designing drugs with controlled selectivity profiles.212 This approach is aimed at: (i) The utilization of phenotypic screening hits to predict potential targets and their mechanism of action, (ii) identifying off‐targets potentially responsible for adverse reactions and side effects, and (iii) careful analysis of ADMET (absorption, distribution, metabolism, excretion, and toxicity) parameters to propose potential hits.
Another useful in silico method, particularly beneficial for lead optimization, is quantitative structure‐property relationship (QSPR) modeling which is useful for the identification of key structural features responsible for interacting with the target protein. For many ADME endpoints measured in the pharmaceutical industry, QSPR models have prospectively shown their ability to extract knowledge from a wide variety of chemical scaffolds proving their utility as predictive models.213 QSPR models, based on machine learning techniques, are desirable to achieve the optimal potency and ADME properties. To reduce the risk of failure in trials, a useful QSAR/QSPR model is necessary to accurately predict the activity of a compound for each drug discovery project. However, these models do not provide adequate information about the modifications that should be made to the tested compound in the next cycle of drug design. To address this issue, the matched molecular pair analysis technique is another promising approach. This method assesses the mean effect of different substituents on various ADME parameters, such as: (i) permeability,214 (ii) solubility,215 (iii) clearance,214 and (iv) cytochrome P450 inhibition.147 The design of a new scaffold that interacts with the desired pharmacological target can be benefitted based on these findings. Molecular substitutions that are closely linked with the molecular properties can guide the design of such scaffolds. Several studies are reporting the use of quantitative structure‐activity relationship modeling in lead optimization.216, 217, 218, 219
Computational drug discovery has proven to accelerate the challenging process of designing and optimizing new drug candidates. Hierarchical virtual screening of ligand‐based and structure‐based methods delineated their validity in finding potential hits, even in the early phase of drug discovery. Because of the increased efficiency on Ebola hit‐to‐lead optimization, an interplay between the several stages of in silico drug design has been depicted in Figure 3. Because of the rapid development of faster architectures and comprehensive algorithms for high‐level computations, the impact of computational structure‐based drug design on antiviral drug discovery and lead optimization will have a more profound impact in future years. Not only hit identification but also elucidation of its biological target has provided information for use in drug discovery research. In this perspective, Perilla et al,220 have described the physical properties of the HIV‐1 capsid protein using the all‐atom MD simulations. Andoh et al,221 performed the all‐atom MD calculation study of entire poliovirus and found rapid equilibrium exchange of water molecules across the capsid, finally concluded the capsid to function as a semipermeable membrane. The next study by Le et al,222 less restrictive to a single target, studied drug interactions of Tamiflu and Relenza to multiple evolutionary correlated proteins. More specifically swine influenza A/H1N1, Spanish H1N1, and avian H5N1 flu N1 NAs were investigated using MD techniques for possible drug resistance mechanisms, in combination with electrostatic analysis. The research group created a molecular model of the swine influenza A/H1N1 type‐I NA based on the avian H5N1 type‐I NA, after which all three NAs were simulated as apo‐conformation and compared with its bound state with oseltamivir (Tamiflu) or zanamivir (Relenza). When compared with each other the simulations identified conserved and unique drug‐protein interactions across all three proteins mediated by hydrogen bonds. This elucidation of key molecular interactions was used to predict mutations that could lead to drug resistance.
Figure 3.
Detailed comprehensive workflow toward efficient anti‐EBOV drug discovery. ADMET, absorption, distribution, metabolism, excretion, and toxicity; CoMFA, comparative molecular field analysis; CoMSIA, comparative molecular similarity indices analysis; GBSA, generalized born surface area; MM, molecular mechanics; PAINS, filters for removal of pan assay interference compounds; PBSA, Poisson‐Boltzmann surface area; PCA, principal component analysis; PDB, protein data bank; QM; quantum mechanics; QSAR, quantitative structure‐activity relationship; Rg, radius gyration; RMSD, root‐mean‐square deviation; RMSF, root‐mean‐square fluctuation
1.11. Some advances in MD simulations
More advanced nudge elastic band or catalytic MD techniques identify reaction or conformational transition paths.223 Advanced hybrid QM/MM MD simulations have been proven extremely beneficial to gain more profound insights into the reaction mechanisms involved in the investigated biosystems. To more specifically asses which amino acids are interacting, per‐residue energy decoupling has the potential to identify key interactions with the target. Many drug candidates are known to bind to less populated structures within the target's conformational space. In this view, ensemble‐based docking describes a method in which the ligand is docked to multiple conformational forms a biomolecular target instead of only one. Based on the hypothesis of the influence of induced fit in enzymes, the normal‐mode analysis could prove to be helpful in the elucidation of the collective motions of protein domains that underlie their conformational changes upon binding a ligand.224 In other research, slow motions have been extracted from MD trajectories by using principal component analysis and MD simulation clustering.225, 226 The same technique might be incorporated in the elucidation of interacting amino acids of the target with the ligand of interest, as described using per‐residue energy decoupling. Steered MD simulations applying predefined degrees of freedom can be used after identifying the catalytic important domain movements of the target and the relation with ligand binding. The refinement quality of postdocking complexes is generally assessed by plotting the root‐mean square deviation and root‐mean square fluctuation of obtained trajectories. For comparison purposes between MD‐refined complex systems, binding‐free energy calculations using the molecular mechanics Poisson‐Boltzmann surface area/generalized born surface area can be incorporated in the workflow. Like these, numerous recent studies have been performed with the aid of MD simulations in search of direct antivirals205, 218, 226, 227, 228, 229, 230, 231, 232, 233 and investigating drug resistance mechanisms.226, 227, 234, 235, 236, 237
Furthermore, computational power has increased exponentially over the past 30 years with the sequential development of more powerful supercomputer units (high‐performance computing). With the assumption of a continuation of Moore's law, which is reasonable given the latest advancements in computing power, a one–million‐fold increase in processing power is expected. However, until quantum computing becomes a reality, a maximum level of processing power is expected due to limitations in computational resources. Specialized supercomputers designed especially for all‐atom MD simulations have been developed that show to the ability to reach millisecond timescales that represent interesting biological processes.238, 239 Efforts being made in quantum computing offer an exciting outlook.240 To date, actual quantum computers are still in their initial stage of development with quantum computational operations executed only experimentally on a very small number of quantum bits. With these timewise developments, it might become possible to simulate large biological systems and determine, computationally, the 3D folding of proteins starting from their amino acid sequence.241 In drug discovery processes the applicability is very promising, especially in target identification and interaction analysis.
2. PROPOSED CHALLENGES
The success rates for drug discovery pipeline involving target identification, screening, hit‐to‐lead optimization, and preclinical candidate selection are within the range of 69% to 85%.242 Failure of a discovery project accounts for several reasons; notably, (i) unclear underlying mechanism of target protein, (ii) lack of leads, (iii) poor potency, (iv) lack of efficacy, (v) inappropriate drug‐like properties, and (vi) unexpected high animal‐toxicity levels. However, the success rates of the clinical development of drugs vary distinctly. An experimental drug in Phase I clinical trials has approximately 10% chance to reach the market.242 Inadequate efficacy of drugs accounts for two‐thirds of recent Phase III clinical trial failures, half of the trials for Phase II trials and approximately 16% for Phase I trials.242 Most drugs fail during clinical trials even though all experimental drugs enter human clinical trials based on extensive preclinical data indicating the efficacy in vivo. Moreover, experiments with Ebola strains require a BSL4 safety level, which narrows the possibilities and slows the progress in anti‐EBOV drug research. The complexities of human biology, amplified by the limitations of target‐based drug discovery approach243 poses a significant challenge.
The magnitude of the recent EBOV outbreak, coupled with drug discovery and development challenges has necessitated the need to explore broad‐spectrum alternative strategies. Despite no current countermeasures, experimental drug and vaccine development is progressing with some in the earliest stages of product development. Repurposing drugs provide an alternative way to accelerate the process of drug design and discovery. Investigation of FDA‐approved compounds in new directions opens avenues for devising a strategy against challenging diseases, in particular, EVD. Drugs gain FDA approval after rigorously being screened through a set of criteria including pharmacokinetic and pharmacodynamics, dosage, toxicity, safety, and efficacy. Repurposed drugs bypass Phase I of clinical trials accelerating the drug discovery process and additionally eliminating the logistic considerations like manufacturing and distribution. However, when repurposing such drugs, any unfavorable data gained from these clinical trials may not serve any purpose against the approved drug, it might cut down on the development timeline.
Furthermore, studying the mechanism of action of such repurposed drugs may be difficult. Therefore, experiments specifically designed to identify the mechanism of action of repurposed compounds may provide insights into the EBOV lifecycle, and can help devise new trials against EVD pandemic. Additionally, drug resistance can also be a major clinical problem for the treatment of EBOV‐infected individuals, as only a small number of mutations can drastically change the biological properties of RNA viruses,244 HIV virus,245 and influenza viruses.246
The statistical power of preclinical studies is crucial for the efficiency of clinical studies, preventing the unnecessary testing of a large number of compounds.247 A large proportion of drugs proceeding into clinical trials never showed animal efficacy which leads to a large number of useless therapeutics.248 Moreover, the statistical power of clinical studies poses an additional challenge to enroll a sufficient number of participants in a clinical trial to demonstrate a statistically significant study.249 EBOV‐infected animal models need to be reliable enough to reflect the patient's situation. Furthermore, the overall aspects of experimental procedures and efficacy should be estimated to a higher level.250
Additionally, pharmacokinetics properties particularly plasma half‐life, are representative of drug efficacy. With the use of animal models, the importance of interspecies translation of half‐life becomes more evident. In the light of recent clinical trials, although many treatment options for EVD have been proposed, there exists no FDA‐approved drug yet. The genetics and immunological profile vary from one population to another. This poses a further challenge for evaluating drug safety profile in different populations. Identifying viable hits through in silico hit identification and screening is almost achievable for any target, however, hit‐to‐lead optimization remains cumbersome. A reasonable argument is that computational methods that accurately predict binding constants for chemically diverse compounds and large datasets, still need to be optimized. Second, ADMET properties are difficult to predict for large datasets because it is impossible to simplify them to a single molecular event. Incontrovertibly, it is the ADMET properties that cause the failure of most drug candidates. Increased attention has been paid to the pharmacokinetic properties during lead optimization. As a result, poor pharmacokinetic properties, once a major issue, today account for only 10% of clinical failures, mostly in Phase I.251 With the joint efforts by regulatory institutions, meta‐analytic analysis with controlled experimental protocols and performance can yield safe and unprecedented predictive results for human clinical trials.
3. CONCLUDING REMARKS
After the largest, most devastating Ebola outbreak (2014‐2016), efforts toward EVD treatment have gained vital importance. This outbreak has highlighted an urgent need to develop an efficacious treatment that can be used to curtail future outbreaks. As a result of clinical research, numerous countermeasures have been developed including vaccines (rVSV‐ZEBOV and Ad26‐ZEBOV/MVA‐BN‐Filo prime‐boost vaccine), nucleoside and nucleotide analogues (BCX4430, favipiravir, and GS‐5734), plasma transfusions (Ebola‐Tx), immunotherapeutics (Zmapp and MIL77), nucleic acid‐based drugs, and repurposed drugs. The scientific community has to overcome multiple challenges to ensure a licensed efficacious drug for future outbreaks. In this regard, it is required to widen the prospects in the development of therapeutic agents with broad‐spectrum activity against filoviruses like Marburg virus, Sudan virus, or other viral pathogens. However, the drug discovery and development pipeline lead to only a small number of compounds that enter clinical trials, thus making it not just a challenging but also a time‐consuming process. Until the next outbreak, drug development efforts rely on efficacy characterization in animal models of EVD. The EBOV outbreaks have also reconfirmed the significance of the immunological basis of vaccine protection to the scientific community. This will not only help to assist the progression of vaccine candidates in development, but also vaccine efficacy can be assessed for potential outbreaks of genetically diverse strains in the coming episodes.
Within the entire process from drug discovery to authorization, a great potential can be attributed to in silico methods of drug discovery and may prove beneficial at any stage in the preclinical development of drug candidates. In silico drug discovery methods have already changed the perception of drug design and development. Methods in computational chemistry, particularly MD simulations and QSPR, will significantly impact the trajectory of the drug discovery process in the pharmaceutical industry. With an increased understanding of human biology, clinical trials are expected to gain success. MD simulations, in this case, will make useful contributions in understanding the underlying molecular processes and biological functions. Through the application of QSPR modeling, improvising the ability to design better molecules is an achievable goal. With the presence of an enormous amount of data, computational approaches are the most sought after methods to answer biological problems. With advancement in understanding the mechanism and mode of action of EBOV, future in silico work will have an essential role in the development of drug candidates against the devastating EVD.
Mirza MU, Vanmeert M, Ali A, Iman K, Froeyen M, Idrees M. Perspectives towards antiviral drug discovery against Ebola virus. J Med Virol. 2019;91:2029‐2048. 10.1002/jmv.25357
References
REFERENCES
- 1. Kuhn JH, Bao Y, Bavari S, et al. Virus nomenclature below the species level: a standardized nomenclature for natural variants of viruses assigned to the family Filoviridae. Arch Virol. 2013;158:301‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuhn JH, Becker S, Ebihara H, et al. Proposal for a revised taxonomy of the family Filoviridae: classification, names of taxa and viruses, and virus abbreviations. Arch Virol. 2010;155:2083‐2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ladner JT, Wiley MR, Mate S, et al. Evolution and spread of Ebola virus in Liberia, 2014‐2015. Cell Host Microbe. 2015;18:659‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bausch DG, Towner JS, Dowell SF, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis. 2007;196(suppl 2):S142‐147. [DOI] [PubMed] [Google Scholar]
- 5. Weingartl HM, Embury‐Hyatt C, Nfon C, Leung A, Smith G, Kobinger G. Transmission of Ebola virus from pigs to non‐human primates. Sci Rep. 2012;2:811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Judson S, Prescott J, Munster V. Understanding ebola virus transmission. Viruses. 2015;7:511‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Varkey JB, Shantha JG, Crozier I, et al. Persistence of Ebola virus in ocular fluid during convalescence. N Engl J Med. 2015;372:2423‐2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mate SE, Kugelman JR, Nyenswah TG, et al. Molecular evidence of sexual transmission of Ebola virus. N Engl J Med. 2015;373:2448‐2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Christie A, Davies‐Wayne GJ, Cordier‐Lassalle T, et al. Possible sexual transmission of Ebola virus—Liberia, 2015. Morb Mortal Wkly Rep. 2015;64:479‐481. [PMC free article] [PubMed] [Google Scholar]
- 10. Dimitrov DS. Virus entry: molecular mechanisms and biomedical applications. Nat Rev Microbiol. 2004;2:109‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feldmann H, Jones S, Klenk HD, Schnittler HJ. Ebola virus: from discovery to vaccine. Nat Rev Immunol. 2003;3:677‐685. [DOI] [PubMed] [Google Scholar]
- 12. Singh G, Kumar A, Singh K, Kaur J. Retracted: Ebola virus: an introduction and its pathology. Rev Medi Virol. 2016;26:49‐56. [DOI] [PubMed] [Google Scholar]
- 13. Geisbert TW, Hensley LE, Larsen T, et al. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques—Evidence that dendritic cells are early and sustained targets of infection. Am J Pathol. 2003;163:2347‐2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cárdenas WB. Evasion of the interferon‐mediated antiviral response by filoviruses. Viruses. 2010;2:262‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kühl A, Pöhlmann S. How Ebola virus counters the interferon system. Zoonoses Public Health. 2012;59:116‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bixler S, Goff A. The role of cytokines and chemokines in filovirus infection. Viruses. 2015;7:5489‐5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vine V, Scott DP, Feldmann H. Ebolavirus: an overview of molecular and clinical pathogenesis. Methods Mol Biol. 2017;1628:39‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zampieri CA, Sullivan NJ, Nabel GJ. Immunopathology of highly virulent pathogens: insights from Ebola virus. Nature Immunol. 2007;8:1159‐1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chertow DS, Kleine C, Edwards JK, Scaini R, Giuliani R, Sprecher A. Ebola virus disease in West Africa—clinical manifestations and management. N Engl J Med. 2014;371:2054‐2057. [DOI] [PubMed] [Google Scholar]
- 20. Kreuels B, Wichmann D, Emmerich P, et al. A case of severe Ebola virus infection complicated by gram‐negative septicemia. N Engl J Med. 2014;371:2394‐2401. [DOI] [PubMed] [Google Scholar]
- 21. Alves DA, Honko AN, Kortepeter MG, et al. Necrotizing scleritis, conjunctivitis, and other pathologic findings in the left eye and brain of an Ebola virus‐infected Rhesus Macaque (Macaca mulatta) with apparent recovery and a delayed time of death. J Infect Dis. 2016;213:57‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ansari AA. Clinical features and pathobiology of Ebolavirus infection. J Autoimmun. 2014;55:1‐9. [DOI] [PubMed] [Google Scholar]
- 23. Martines RB, Ng DL, Greer PW, Rollin PE, Zaki SR. Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg viruses. J Pathol. 2015;235:153‐174. [DOI] [PubMed] [Google Scholar]
- 24. Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2011;377:849‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baize S, Pannetier D, Oestereich L, et al. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med. 2014;371:1418‐1425. [DOI] [PubMed] [Google Scholar]
- 26. Colebunders R, Jacob ST, Ariën KK, Weggheleire AD, Decroo T. High mortality in non‐Ebola virus disease cases: need to provide timely and effective care. Lancet Infect Dis. 2017;17:1021‐1022. [DOI] [PubMed] [Google Scholar]
- 27. Schieffelin JS, Shaffer JG, Goba A, et al. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med. 2014;371:2092‐2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wolf T, Kann G, Becker S, et al. Severe Ebola virus disease with vascular leakage and multiorgan failure: treatment of a patient in intensive care. Lancet. 2015;385:1428‐1435. [DOI] [PubMed] [Google Scholar]
- 29. World Health Organization . Ebola Virus Disease. Democratic Republic of Congo: Declaration of the end of the ninth EVD outbreak in Bikoro Health Zone, Équateur Province Author; 2018. Democratic Republic of the Congo: Health Emergency Information and Risk Assessment. [Google Scholar]
- 30. World Health Organization . Democratic Republic of Congo: Ebola Virus disease‐External Situation Report 12. Author; 2018. Democratic Republic of the Congo: Health Emergency Information and Risk Assessment. [Google Scholar]
- 31. Dunning J, Kennedy SB, Antierens A, et al. Experimental treatment of Ebola virus disease with Brincidofovir. PLOS One. 2016;11:e0162199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sissoko D, Laouenan C, Folkesson E, et al. Experimental treatment with Favipiravir for Ebola virus disease (the JIKI Trial): a historically controlled, single‐arm proof‐of‐concept trial in Guinea. PLOS Med. 2016;13:e1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Van Griensven J, Edwards T, De Lamballerie X, et al. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374:33‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang Y, Li J, Hu Y, Liang Q, Wei M, Zhu F. Ebola vaccines in clinical trial: The promising candidates. Hum Vac Immunother. 2017;13:153‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Henao‐Restrepo AM, Camacho A, Longini IM, et al. Efficacy and effectiveness of an rVSV‐vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open‐label, cluster‐randomised trial (Ebola Ça Suffit!). Lancet. 2017;389:505‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Samai M, Seward JF, Goldstein ST, et al. The Sierra Leone trial to introduce a vaccine against Ebola: an evaluation of rVSV∆ G‐ZEBOV‐GP vaccine tolerability and safety during the West Africa Ebola outbreak. J Infect Dis. 2018;217:S6‐S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sivapalasingam S, Kamal M, Slim R, et al. Safety, pharmacokinetics, and immunogenicity of a co‐formulated cocktail of three human monoclonal antibodies targeting Ebola virus glycoprotein in healthy adults: a randomised, first‐in‐human phase 1 study. Lancet Infect Dis. 2018;18:884‐893. [DOI] [PubMed] [Google Scholar]
- 38. Cnops L, Gerard M, Vandenberg O, et al. Risk of misinterpretation of Ebola virus PCR results after rVSV ZEBOV‐GP vaccination. Clin Infectious Dis. 2015;60:1725‐1726. [DOI] [PubMed] [Google Scholar]
- 39. Günther S, Feldmann H, Geisbert TW, et al. Management of accidental exposure to Ebola virus in the biosafety level 4 laboratory, Hamburg, Germany. J Infect Dis. 2011;204:S785‐S790. [DOI] [PubMed] [Google Scholar]
- 40. Jacobs M, Aarons E, Bhagani S, et al. Post‐exposure prophylaxis against Ebola virus disease with experimental antiviral agents: a case‐series of health‐care workers. Lancet Infect Dis. 2015;15:1300‐1304. [DOI] [PubMed] [Google Scholar]
- 41. Wong KK, Davey RT, Hewlett AL, et al. Use of postexposure prophylaxis after occupational exposure to Zaire ebolavirus. Clin Infect Dis. 2016;63:376‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Venkatraman N, Silman D, Folegatti PM, Hill A. Vaccines against Ebola virus. Vaccine. 2017;36:5454‐5459. [DOI] [PubMed] [Google Scholar]
- 43. Schnell MJ. Progress in Ebola Virus vaccine development. J Infect Dis. 2017;215:1775‐1776. [DOI] [PubMed] [Google Scholar]
- 44. Cardile AP, Warren TK, Martins KA, Reisler RB, Bavari S. Will there be a cure for Ebola? Annu Review Pharmacol Toxicol. 2017;57:329‐348. [DOI] [PubMed] [Google Scholar]
- 45. Ohimain EI. Recent advances in the development of vaccines for Ebola virus disease. Virus Res. 2016;211:174‐185. [DOI] [PubMed] [Google Scholar]
- 46. Milligan ID, Gibani MM, Sewell R, et al. Safety and immunogenicity of novel adenovirus type 26‐ and modified vaccinia ankara‐ vectored ebola vaccines: a randomized clinical trial. JAMA. 2016;315:1610‐1623. [DOI] [PubMed] [Google Scholar]
- 47. Winslow RL, Milligan ID, Voysey M, et al. Immune responses to novel adenovirus type 26 and modified vaccinia virus Ankara–vectored Ebola vaccines at 1 year. JAMA. 2017;317:1075‐1077. [DOI] [PubMed] [Google Scholar]
- 48.Marceau CD, Negi SS, Hernandez H, et al. Novel neutralizing monoclonal antibodies protect rodents against lethal filovirus challenges. Trials Vaccinology. 2014;3:89‐94. [Google Scholar]
- 49. Dolzhikova IV, Zubkova OV, Tukhvatulin AI, et al. Safety and immunogenicity of GamEvac‐Combi, a heterologous VSV‐and Ad5‐vectored Ebola vaccine: an open phase I/II trial in healthy adults in Russia. Hum Vac Immunother. 2017;13:613‐620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Johnson RF, Kurup D, Hagen KR, et al. An inactivated Rabies virus–based Ebola Vaccine, FILORAB1, adjuvanted with glucopyranosyl lipid A in stable emulsion confers complete protection in nonhuman primate challenge models. J Infect Dis. 2016;214:S342‐S354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shukarev G, Callendret B, Luhn K, Douoguih M, consortium. A two‐dose heterologous prime‐boost vaccine regimen eliciting sustained immune responses to Ebola Zaire could support a preventive strategy for future outbreaks. Hum Vacc Immunother. 2017;13:266‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wong G, Richardson JS, Pillet S, et al. Adenovirus‐vectored vaccine provides postexposure protection to Ebola virus–infected nonhuman primates. J Infect Dis. 2015;212:S379‐S383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhu F‐C, Wurie AH, Hou L‐H, et al. Safety and immunogenicity of a recombinant adenovirus type‐5 vector‐based Ebola vaccine in healthy adults in Sierra Leone: a single‐centre, randomised, double‐blind, placebo‐controlled, phase 2 trial. The Lancet. 2017;389:621‐628. [DOI] [PubMed] [Google Scholar]
- 54. Butcher EC, Berg EL, Kunkel EJ. Systems biology in drug discovery. Nat Biotechnol. 2004;22:1253‐1259. [DOI] [PubMed] [Google Scholar]
- 55. Davidov E, Holland J, Marple E, Naylor S. Advancing drug discovery through systems biology. Drug Discov Today. 2003;8:175‐183. [DOI] [PubMed] [Google Scholar]
- 56. Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727‐730. [DOI] [PubMed] [Google Scholar]
- 57. Debouck C, Goodfellow PN. DNA microarrays in drug discovery and development. Nat Genet. 1999;21:48‐50. [DOI] [PubMed] [Google Scholar]
- 58. Schenone M, Dančík V, Wagner BK, Clemons PA. Target identification and mechanism of action in chemical biology and drug discovery. Nat Chem Biol. 2013;9:232‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Santos R, Ursu O, Gaulton A, et al. A comprehensive map of molecular drug targets. Nat Rev Drug Discov. 2017;16:19‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sanchez A, Kiley MP, Holloway BP, Auperin DD. Sequence analysis of the Ebola virus genome: organization, genetic elements, and comparison with the genome of Marburg virus. Virus Res. 1993;29:215‐240. [DOI] [PubMed] [Google Scholar]
- 61. Wilson JA, Bray M, Bakken R, Hart MK. Vaccine potential of Ebola virus VP24, VP30, VP35, and VP40 proteins. Virology. 2001;286:384‐390. [DOI] [PubMed] [Google Scholar]
- 62. Moller‐Tank S, Maury W. Ebola virus entry: a curious and complex series of events. PLOS Pathog. 2015;11:e1004731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Martin B, Hoenen T, Canard B, Decroly E. Filovirus proteins for antiviral drug discovery: a structure/function analysis of surface glycoproteins and virus entry. Antiviral Res. 2016;135:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Martin B, Canard B, Decroly E. Filovirus proteins for antiviral drug discovery: Structure/function bases of the replication cycle. Antiviral Res. 2017;141:48‐61. [DOI] [PubMed] [Google Scholar]
- 65. Martin B, Reynard O, Volchkov V, Decroly E. Filovirus proteins for antiviral drug discovery: Structure/function of proteins involved in assembly and budding. Antiviral Res. 2018;150:183‐192. [DOI] [PubMed] [Google Scholar]
- 66. Gustin JK, Bai Y, Moses AV, Douglas JL. Ebola virus glycoprotein promotes enhanced viral egress by preventing Ebola VP40 from associating with the host restriction factor BST2/tetherin. J Infect Dis. 2015;212:S181‐S190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Qiu X, Audet J, Wong G, et al. Successful treatment of ebola virus‐infected cynomolgus macaques with monoclonal antibodies. Sci transl Med. 2012;4:138ra181‐138ra81. [DOI] [PubMed] [Google Scholar]
- 68. Olinger GG, Jr. , Pettitt J, Kim D, et al. Delayed treatment of Ebola virus infection with plant‐derived monoclonal antibodies provides protection in rhesus macaques. Proc Nat Acad Sci USA. 2012;109:18030‐18035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Qiu X, Audet J, Wong G, et al. Sustained protection against Ebola virus infection following treatment of infected nonhuman primates with ZMAb. Sci Reports. 2013;3:3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Qiu XG, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514:47‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rhein BA, Maury WJ. Ebola virus entry into host cells: identifying therapeutic strategies. Curr Clin Microbiol Rep. 2015;2:115‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Basu A, Mills DM, Mitchell D, et al. Novel small molecule entry inhibitors of Ebola virus. J Infect Dis. 2015;212:S425‐S434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Côté M, Misasi J, Ren T, et al. Small molecule inhibitors reveal Niemann–Pick C1 is essential for Ebola virus infection. Nature. 2011;477:344‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hartlieb B, Weissenhorn W. Filovirus assembly and budding. Virology. 2006;344:64‐70. [DOI] [PubMed] [Google Scholar]
- 75. Noda T, Hagiwara K, Sagara H, Kawaoka Y. Characterization of the Ebola virus nucleoprotein–RNA complex. J Gen Virol. 2010;91:1478‐1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Feagins AR, Basler CF. Lloviu virus VP24 and VP35 proteins function as innate immune antagonists in human and bat cells. Virology. 2015;485:145‐152. [DOI] [PubMed] [Google Scholar]
- 77. Kirchdoerfer RN, Moyer CL, Abelson DM, Saphire EO. The Ebola virus VP30‐NP interaction is a regulator of viral RNA synthesis. PLOS Pathog. 2016;12:e1005937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kirchdoerfer RN, Abelson DM, Li S, Wood MR, Saphire EO. Assembly of the Ebola virus nucleoprotein from a chaperoned VP35 complex. Cell Rep. 2015;12:140‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Leung DW, Borek D, Luthra P, et al. An intrinsically disordered peptide from Ebola virus VP35 controls viral RNA synthesis by modulating nucleoprotein‐RNA interactions. Cell Rep. 2015;11:376‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fu X, Wang Z, Li L, et al. Novel chemical ligands to Ebola virus and Marburg virus nucleoproteins identified by combining affinity mass spectrometry and metabolomics approaches. Sci Rep. 2016;6:29680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Binning JM, Wang T, Luthra P, et al. Development of RNA aptamers targeting Ebola virus VP35. Biochemistry. 2013;52:8406‐8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Brown CS, Lee MS, Leung DW, et al. In silico derived small molecules bind the filovirus VP35 protein and inhibit its polymerase cofactor activity. J Mol Biol. 2014;426:2045‐2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Easton V, McPhillie M, Garcia‐Dorival I, et al. Identification of a small molecule inhibitor of Ebolavirus genome replication and transcription using in silico screening. Antiviral Res. 2018;156:46‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Noda T, Halfmann P, Sagara H, Kawaoka Y. Regions in Ebola virus VP24 that are important for nucleocapsid formation. J Infect Dis. 2007;196:S247‐S250. [DOI] [PubMed] [Google Scholar]
- 85. Watanabe S, Noda T, Halfmann P, Jasenosky L, Kawaoka Y. Ebola virus (EBOV) VP24 inhibits transcription and replication of the EBOV genome. J Infect Dis. 2007;196:S284‐S290. [DOI] [PubMed] [Google Scholar]
- 86. Song X, Lu L‐Y, Passioura T, Suga H. Macrocyclic peptide inhibitors for the protein–protein interaction of Zaire Ebola virus protein 24 and karyopherin alpha 5. Org Biomol Chem. 2017;15:5155‐5160. [DOI] [PubMed] [Google Scholar]
- 87. Dessen A, Volchkov V, Dolnik O, et al. Crystal structure of the matrix protein VP40 from Ebola virus. EMBO J. 2000;19:4228‐4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bornholdt ZA, Noda T, Abelson DM, et al. Structural rearrangement of ebola virus VP40 begets multiple functions in the virus life cycle. Cell. 2013;154:763‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Warren TK, Wells J, Panchal RG, et al. Protection against filovirus diseases by a novel broad‐spectrum nucleoside analogue BCX4430. Nature. 2014;508:402‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gehring G, Rohrmann K, Atenchong N, et al. The clinically approved drugs amiodarone, dronedarone and verapamil inhibit filovirus cell entry. J Antimicrob Chemother. 2014;69:2123‐2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yuan S. Possible FDA‐approved drugs to treat Ebola virus infection. Infect Dis Poverty. 2015;4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bah EI, Lamah MC, Fletcher T, et al. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N Engl J Med. 2015;372:40‐47. [DOI] [PubMed] [Google Scholar]
- 93. Lyon GM, Mehta AK, Varkey JB, et al. Clinical care of two patients with Ebola virus disease in the United States. N Engl J Med. 2014;371:2402‐2409. [DOI] [PubMed] [Google Scholar]
- 94. Zhang T, Zhai M, Ji J, Zhang J, Tian Y, Liu X. Recent progress on the treatment of Ebola virus disease with Favipiravir and other related strategies. Bioorg Med Chem Lett. 2017;27:2364‐2368. [DOI] [PubMed] [Google Scholar]
- 95. Fowler RA, Fletcher T, Fischer WA, 2nd , et al. Caring for critically ill patients with ebola virus disease. Perspectives from West Africa. Am J Resp Crit Care Med. 2014;190:733‐737. [DOI] [PubMed] [Google Scholar]
- 96. Keshtkar‐Jahromi M, Martins KAO, Cardile AP, Reisler RB, Christopher GW, Bavari S. Treatment‐focused Ebola trials, supportive care and future of Filovirus care. Expert Rev Anti Infect Ther. 2018;16:67‐76. [DOI] [PubMed] [Google Scholar]
- 97. Agnihotri P, Mishra AK, Mishra S, Sirohi VK, Sahasrabuddhe AA, Pratap JV. Identification of novel inhibitors of leishmania donovani γ‐glutamylcysteine synthetase using structure‐based virtual screening, docking, molecular dynamics simulation, and in vitro studies. J Chem Inf Model. 2017;57:815‐825. [DOI] [PubMed] [Google Scholar]
- 98. Aman MJ, Kinch MS, Warfield K, et al. Development of a broad‐spectrum antiviral with activity against Ebola virus. Antiviral Res. 2009;83:245‐251. [DOI] [PubMed] [Google Scholar]
- 99. Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T‐705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100:446‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Johansen LM, Brannan JM, Delos SE, et al. FDA‐approved selective estrogen receptor modulators inhibit Ebola virus infection. Sci Transl Med. 2013;5:190ra179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Madrid PB, Panchal RG, Warren TK, et al. Evaluation of Ebola virus inhibitors for drug repurposing. ACS infect Dis. 2015;1:317‐326. [DOI] [PubMed] [Google Scholar]
- 102. Wu W, Liu S. The drug targets and antiviral molecules for treatment of Ebola virus infection. Cur Top Med Chem. 2017;17:361‐370. [DOI] [PubMed] [Google Scholar]
- 103. Salata C, Calistri A, Parolin C, Baritussio A, Palù G. Antiviral activity of cationic amphiphilic drugs. Exp Rev Anti Infect Ther. 2017;15:483‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Madrid PB, Chopra S, Manger ID, et al. A systematic screen of FDA‐approved drugs for inhibitors of biological threat agents. PLOS One. 2013;8:e60579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kouznetsova J, Sun W, Martínez‐Romero C, et al. Identification of 53 compounds that block Ebola virus‐like particle entry via a repurposing screen of approved drugs. Emerg Microbes Infect. 2014;3:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Veljkovic V, Loiseau PM, Figadere B, et al. Virtual screen for repurposing approved and experimental drugs for candidate inhibitors of EBOLA virus infection. F1000Res. 2015;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Johansen LM, DeWald LE, Shoemaker CJ, et al. A screen of approved drugs and molecular probes identifies therapeutics with anti–Ebola virus activity. Sci Transl Med. 2015;7:290ra289. [DOI] [PubMed] [Google Scholar]
- 108. Ekins S, Freundlich JS, Coffee M. A common feature pharmacophore for FDA‐approved drugs inhibiting the Ebola virus. F1000Res. 2014;3:277 10.12688/f1000research.5741.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhao Z, Martin C, Fan R, Bourne PE, Xie L. Drug repurposing to target Ebola virus replication and virulence using structural systems pharmacology. BMC Bioinformatics. 2016;17:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wang Y, Cui R, Li G, et al. Teicoplanin inhibits Ebola pseudovirus infection in cell culture. Antiviral Res. 2016;125:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Warren T, MacLennan S, Mathis A, et al. Efficacy of Galidesivir against Ebola virus disease in Rhesus monkeys. Open Forum Infect Dis. 2017;4:S302. [Google Scholar]
- 112. Lo MK, Jordan R, Arvey A, et al. GS‐5734 and its parent nucleoside analog inhibit Filo‐, Pneumo‐, and Paramyxoviruses. Sci Rep. 2017;7:43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Warren TK, Jordan R, Lo MK, et al. Therapeutic efficacy of the small molecule GS‐5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bixler SL, Bocan TM, Wells J, et al. Intracellular conversion and in vivo dose response of favipiravir (T‐705) in rodents infected with Ebola virus. Antiviral Res. 2018;151:50‐54. [DOI] [PubMed] [Google Scholar]
- 115. Bixler SL, Bocan TM, Wells J, et al. Efficacy of favipiravir (T‐705) in nonhuman primates infected with Ebola virus or Marburg virus. Antiviral Res. 2018;151:97‐104. [DOI] [PubMed] [Google Scholar]
- 116. Ekins S, Lingerfelt MA, Comer JE, et al. Efficacy of Tilorone Dihydrochloride against Ebola Virus Infection. Antimicrob Agents Chemother. 2018;62:e01711‐01717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Liu G, Wong G, Su S, et al. Clinical evaluation of Ebola virus disease therapeutics. Trends Mol Med. 2017;23:820‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Postnikova E, Cong Y, DeWald LE, et al. Testing therapeutics in cell‐based assays: factors that influence the apparent potency of drugs. PLOS One. 2018;13:e0194880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sun W, He S, Martínez‐Romero C, et al. Synergistic drug combination effectively blocks Ebola virus infection. Antiviral Res. 2017;137:165‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ren J, Zhao Y, Fry EE, Stuart DI. Target identification and mode of action of four chemically divergent drugs against Ebolavirus infection. J Med Chem. 2018;61:724‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. McMullan LK, Flint M, Dyall J, et al. The lipid moiety of brincidofovir is required for in vitro antiviral activity against Ebola virus. Antiviral Res. 2016;125:71‐78. [DOI] [PubMed] [Google Scholar]
- 122. Dunning J, Sahr F, Rojek A, et al. Experimental treatment of Ebola virus disease with TKM‐130803: a single‐arm Phase 2 clinical trial. PLOS Med. 2016;13:e1001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Madelain V, Nguyen THT, Olivo A, et al. Ebola virus infection: review of the pharmacokinetic and pharmacodynamic properties of drugs considered for testing in human efficacy trials. Clin Pharmacokinet. 2016;55:907‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Katsila T, Spyroulias GA, Patrinos GP, Matsoukas MT. Computational approaches in target identification and drug discovery. Comput Struct Biotechnol J. 2016;14:177‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. van Griensven J, Edwards T, de Lamballerie X, et al. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374:33‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Warren TK, Whitehouse CA, Wells J, et al. A single phosphorodiamidate morpholino oligomer targeting VP24 protects rhesus monkeys against lethal Ebola virus infection. mBio. 2015;6 10.1128/mBio.02344-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Dörnemann J, Burzio C, Ronsse A, et al. First newborn baby to receive experimental therapies survives Ebola virus disease. J Infect Dis. 2017;215:171‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Taylor R, Kotian P, Warren T, et al. BCX4430‐A broad‐spectrum antiviral adenosine nucleoside analog under development for the treatment of Ebola virus disease. J Infect Public Health. 2016;9:220‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Eyer L, Zouharová D, Širmarová J, et al. Antiviral activity of the adenosine analogue BCX4430 against West Nile virus and tick‐borne flaviviruses. Antiviral Res. 2017;142:63‐67. [DOI] [PubMed] [Google Scholar]
- 130. Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz‐Fontela C, Günther S. Successful treatment of advanced Ebola virus infection with T‐705 (favipiravir) in a small animal model. Antiviral Res. 2014;105:17‐21. [DOI] [PubMed] [Google Scholar]
- 131. Cheng H, Schafer A, Soloveva V, et al. Identification of a coumarin‐based antihistamine‐like small molecule as an anti‐filoviral entry inhibitor. Antiviral Res. 2017;145:24‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Luthra P, Liang J, Pietzsch CA, et al. A high throughput screen identifies benzoquinoline compounds as inhibitors of Ebola virus replication. Antiviral Res. 2018;150:193‐201. [DOI] [PubMed] [Google Scholar]
- 133. Cui Q, Du R, Anantpadma M, et al. Identification of ellagic acid from plant Rhodiola rosea L. as an anti‐Ebola virus entry inhibitor. Viruses. 2018;10:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Capuzzi SJ, Sun W, Muratov EN, et al. Computer‐aided discovery and characterization of novel Ebola virus inhibitors. J Med Chem. 2018;61:3582‐3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Falzarano D. Assessment of inhibition of Ebola virus progeny production by antiviral compounds. Methods Mol Biol. 2017;1628:203‐210. [DOI] [PubMed] [Google Scholar]
- 136. Cross RW, Mire CE, Feldmann H, et al. Post‐exposure treatments for Ebola and Marburg virus infections. Nat Rev Drug Discov. 2018;17(6):413‐434. [DOI] [PubMed] [Google Scholar]
- 137. Davidson E, Bryan C, Fong RH, et al. Mechanism of Binding to Ebola Virus Glycoprotein by the ZMapp, ZMAb, and MB‐003 Cocktail Antibodies. J Virol. 2015;89:10982‐10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Qiu X, Audet J, Lv M, et al. Two‐mAb cocktail protects macaques against the Makona variant of Ebola virus. Sci Transl Med. 2016;8:329ra333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Van Lieshout LP, Soule G, Sorensen D, et al. Intramuscular adeno‐associated virus–mediated expression of monoclonal antibodies provides 100% Protection Against Ebola virus infection in mice. J Infect Dis. 2018;217:916‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Spurgers KB, Silvestri LS, Warfield KL, Bavari S. Toward RNA interference‐based therapy for filovirus infections. Drug Dev Res. 2009;70:246‐254. [Google Scholar]
- 141. Trad MA, Naughton W, Yeung A, et al. Ebola virus disease: An update on current prevention and management strategies. J Clin Virol. 2017;86:5‐13. [DOI] [PubMed] [Google Scholar]
- 142. Kole R, Krainer AR, Altman S. RNA therapeutics: beyond RNA interference and antisense oligonucleotides. Nat Rev Drug Discov. 2012;11:125‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nan Y, Zhang YJ. Antisense phosphorodiamidate morpholino oligomers as novel antiviral compounds. Front Microbiol. 2018;9:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Qin E, Bi J, Zhao M, et al. Clinical features of patients wIth Ebola virus disease in Sierra Leone. Clin Infect Dis. 2015;61:491‐495. [DOI] [PubMed] [Google Scholar]
- 145. Hensley LE, Stevens EL, Yan SB, et al. Recombinant human activated protein C for the postexposure treatment of Ebola hemorrhagic fever. J Infect Dis. 2007;196(suppl 2):S390‐399. [DOI] [PubMed] [Google Scholar]
- 146. Geisbert TW, Hensley LE, Jahrling PB, et al. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. Lancet. 2003;362:1953‐1958. [DOI] [PubMed] [Google Scholar]
- 147. Pettitt J, Zeitlin L, Kim DH, et al. Therapeutic intervention of Ebola virus infection in rhesus macaques with the MB‐003 monoclonal antibody cocktail. Sci Transl Med. 2013;5:199ra113‐199ra113. [DOI] [PubMed] [Google Scholar]
- 148. Florescu DF, Kalil AC, Hewlett AL, et al. Administration of Brincidofovir and Convalescent Plasma in a Patient With Ebola Virus Disease. Clin Infect Dis. 2015;61:969‐973. [DOI] [PubMed] [Google Scholar]
- 149. Schibler M, Vetter P, Cherpillod P, et al. Clinical features and viral kinetics in a rapidly cured patient with Ebola virus disease: a case report. The Lancet Infectious diseases. 2015;15:1034‐1040. [DOI] [PubMed] [Google Scholar]
- 150. Wilson JA, Hevey M, Bakken R, et al. Epitopes involved in antibody‐mediated protection from Ebola virus. Science. 2000;287:1664‐1666. [DOI] [PubMed] [Google Scholar]
- 151. Bhattacharyya S, Warfield KL, Ruthel G, Bavari S, Aman MJ, Hope TJ. Ebola virus uses clathrin‐mediated endocytosis as an entry pathway. Virology. 2010;401:18‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Yonezawa A, Cavrois M, Greene WC. Studies of Ebola virus glycoprotein‐mediated entry and fusion by using pseudotyped human immunodeficiency virus type 1 virions: Involvement of cytoskeletal proteins and enhancement by tumor necrosis factor alpha. J Virol. 2005;79:918‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Corti D, Misasi J, Mulangu S, et al. Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science. 2016;351:1339‐1342. [DOI] [PubMed] [Google Scholar]
- 154. Holtsberg FW, Shulenin S, Vu H, et al. Pan‐ebolavirus and Pan‐filovirus mouse monoclonal antibodies: protection against Ebola and Sudan Viruses. J Virol. 2015;90:266‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Furuyama W, Marzi A, Nanbo A, et al. Discovery of an antibody for pan‐ebolavirus therapy. Sci Rep. 2016;6:20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Howell KA, Qiu X, Brannan JM, et al. Antibody treatment of Ebola and Sudan virus infection via a uniquely exposed epitope within the glycoprotein receptor‐binding site. Cell Rep. 2016;15:1514‐1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Uyeki TM, Mehta AK, Davey RT, Jr , et al. Clinical management of Ebola virus disease in the United States and Europe. N Engl J Med. 2016;374:636‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Samai M, Seward JF, Goldstein ST, et al. The sierra leone trial to introduce a vaccine against ebola: an evaluation of rVSV∆ G‐ZEBOV vaccine tolerability and safety during the West Africa Ebola outbreak. J Infect Dis. 2018;217:s6‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ledgerwood JE, DeZure AD, Stanley DA, et al. Chimpanzee adenovirus vector Ebola vaccine. N Engl J Med. 2017;376:928‐938. [DOI] [PubMed] [Google Scholar]
- 160. Fan H, Du X, Zhang J, et al. Selective inhibition of Ebola entry with selective estrogen receptor modulators by disrupting the endolysosomal calcium. Sci Rep. 2017;7:41226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Mendoza EJ, Qiu X, Kobinger GP. Progression of Ebola therapeutics during the 2014‐2015 outbreak. Trends Mol Med. 2016;22:164‐173. [DOI] [PubMed] [Google Scholar]
- 162. Boisen ML, Hartnett JN, Goba A, et al. Epidemiology and management of the 2013‐16 West African Ebola outbreak. Annu Rev Virol. 2016;3:147‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Bellan SE, Pulliam JRC, Pearson CAB, et al. Statistical power and validity of Ebola vaccine trials in Sierra Leone: a simulation study of trial design and analysis. The Lancet Infectious diseases. 2015;15:703‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Kraft CS, Hewlett AL, Koepsell S, et al. The use of TKM‐100802 and convalescent plasma in 2 patients with Ebola virus disease in the United States. Clin Infect Dis. 2015;61:496‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Gao J, Yin L. Drug development for controlling Ebola epidemic—a race against time. Drug Discov Ther. 2014;8:229‐231. [DOI] [PubMed] [Google Scholar]
- 166. Mupapa K, Massamba M, Kibadi K, et al. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. International Scientific and Technical Committee. J Infect Dis. 1999;179(suppl 1):S18‐23. [DOI] [PubMed] [Google Scholar]
- 167. Gignoux E, Azman AS, De Smet M, et al. Effect of artesunate–amodiaquine on mortality related to Ebola virus disease. N Engl J Med. 2016;374:23‐32. [DOI] [PubMed] [Google Scholar]
- 168. Hung CL, Chen CC. Computational approaches for drug discovery. Drug Dev Res. 2014;75:412‐418. [DOI] [PubMed] [Google Scholar]
- 169. Ghose AK, Viswanadhan VN, Wendoloski JJ. A knowledge‐based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J Comb Chem. 1999;1:55‐68. [DOI] [PubMed] [Google Scholar]
- 170. Terrett NK, Gardner M, Gordon DW, Kobylecki RJ, Steele J. Combinatorial synthesis—the design of compound libraries and their application to drug discovery. Tetrahedron. 1995;51:8135‐8173. [Google Scholar]
- 171. Cavasotto C, W. orry A. Ligand docking and structure‐based virtual screening in drug discovery. Curr Top Med Chem. 2007;7:1006‐1014. [DOI] [PubMed] [Google Scholar]
- 172. Klebe G. Virtual ligand screening: strategies, perspectives and limitations. Drug Discov Today. 2006;11:580‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Kumar A, Zhang KYJ. Hierarchical virtual screening approaches in small molecule drug discovery. Methods. 2015;71:26‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Bleicher KH, Böhm H‐J, Müller K, Alanine AI. A guide to drug discovery: Hit and lead generation: beyond high‐throughput screening. Nat Rev Drug Discov. 2003;2:369‐378. [DOI] [PubMed] [Google Scholar]
- 175. Hillisch A, Heinrich N, Wild H. Computational chemistry in the pharmaceutical industry: from childhood to adolescence. ChemMedChem. 2015;10:1958‐1962. [DOI] [PubMed] [Google Scholar]
- 176. Cavasotto CN, Phatak SS. Homology modeling in drug discovery: current trends and applications. Drug Discov Today. 2009;14:676‐683. [DOI] [PubMed] [Google Scholar]
- 177. Hillisch A, Pineda LF, Hilgenfeld R. Utility of homology models in the drug discovery process. Drug Discov Today. 2004;9:659‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Larsson P, Wallner B, Lindahl E, Elofsson A. Using multiple templates to improve quality of homology models in automated homology modeling. Protein Sci. 2008;17:990‐1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Ohlson T, Wallner B, Elofsson A. Profile‐profile methods provide improved fold‐recognition: a study of different profile‐profile alignment methods. Proteins. 2004;57:188‐197. [DOI] [PubMed] [Google Scholar]
- 180. Petrey D, Xiang Z, Tang CL, et al. Using multiple structure alignments, fast model building, and energetic analysis in fold recognition and homology modeling. Proteins. 2003;53:430‐435. [DOI] [PubMed] [Google Scholar]
- 181. Chothia C, Lesk AM. The relation between the divergence of sequence and structure in proteins. EMBO J. 1986;5:823‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Fiser A, Šali A. Modeller: generation and refinement of homology‐based protein structure models. Methods Enzymol. 2003;374:461‐491. [DOI] [PubMed] [Google Scholar]
- 183. Fan H, Irwin JJ, Webb BM, Klebe G, Shoichet BK, Sali A. Molecular docking screens using comparative models of proteins. J Chem Inform Model. 2009;49:2512‐2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Huang YJ, Mao B, Aramini JM, Montelione GT. Assessment of template‐based protein structure predictions in CASP10. Proteins. 2014;82(suppl 2):43‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Eswar N, Eramian D, Webb B, Shen MY, Sali A. Protein structure modeling with MODELLER. Methods Mol. Biol. 2008;426:45‐159. [DOI] [PubMed] [Google Scholar]
- 186. MacCallum JL, Pérez A, Schnieders MJ, Hua L, Jacobson MP, Dill KA. Assessment of protein structure refinement in CASP9. Proteins. 2011;79(suppl 10):74‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Balmith M, Soliman MES. Potential Ebola drug targets–filling the gap: a critical step forward towards the design and discovery of potential drugs. Biologia. 2017;72:1‐13. [Google Scholar]
- 188. Ganugapati J, Akash S. Multi‐template homology based structure prediction and molecular docking studies of protein ‘L’ of Zaire ebolavirus (EBOV). Inform Med Unlocked. 2017;9:68‐75. [Google Scholar]
- 189. Tambunan USF, Nasution MAF. Identification of novel Ebola virus (EBOV) VP24 inhibitor from Indonesian natural products through in silico drug design approach. AIP Conf Proc. 2017;1862:030091. [Google Scholar]
- 190. Setlur AS, Naik SY, Skariyachan S. Herbal lead as ideal bioactive compounds against probable drug targets of Ebola virus in comparison with known chemical analogue: A computational drug discovery perspective. Interdiscip Sci. 2017;9:254‐277. [DOI] [PubMed] [Google Scholar]
- 191. Mirza M, Ikram N. Integrated computational approach for virtual hit identification against Ebola viral proteins VP35 and VP40. Inter J Mol Sci. 2016;17:1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Ren J‐X, Zhang R‐T, Zhang H, Cao XS, Liu LK, Xie Y. Identification of novel VP35 inhibitors: virtual screening driven new scaffolds. Biomed Pharmacother. 2016;84:199‐207. [DOI] [PubMed] [Google Scholar]
- 193. Ekins S, Freundlich JS, Clark AM, Anantpadma M, Davey RA, Madrid P. Machine learning models identify molecules active against the Ebola virus in vitro. F1000Res. 2015;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Ekins S, Williams AJ, Krasowski MD, Freundlich JS. In silico repositioning of approved drugs for rare and neglected diseases. Drug Discov Today. 2011;16:298‐310. [DOI] [PubMed] [Google Scholar]
- 195. Gschwend DA, Good AC, Kuntz ID. Molecular docking towards drug discovery. J Mol Recog. 1996;9:175‐186. [DOI] [PubMed] [Google Scholar]
- 196. Rastelli G, Ferrari AM, Costantino L, Gamberini MC. Discovery of new inhibitors of aldose reductase from molecular docking and database screening. Bioorganic Med Chem. 2002;10:1437‐1450. [DOI] [PubMed] [Google Scholar]
- 197. Cavalli A, Bottegoni G, Raco C, De Vivo M, Recanatini M. A computational study of the binding of propidium to the peripheral anionic site of human acetylcholinesterase. J Med Chem. 2004;47:3991‐3999. [DOI] [PubMed] [Google Scholar]
- 198. Ode H, Nakashima M, Kitamura S, Sugiura W, Sato H. Molecular dynamics simulation in virus research. Front Microbiol. 2012;3 10.3389/fmicb.2012.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Ganesan A, Coote ML, Barakat K. Molecular dynamics‐driven drug discovery: leaping forward with confidence. Drug Discov Today. 2017;22:249‐269. [DOI] [PubMed] [Google Scholar]
- 200. Harvey MJ, De Fabritiis G. High‐throughput molecular dynamics: the powerful new tool for drug discovery. Drug Discov Today. 2012;17:1059‐1062. [DOI] [PubMed] [Google Scholar]
- 201. Barakat KH, Anwar‐Mohamed A, Tuszynski JA, Robins MJ, Tyrrell DL, Houghton M. A refined model of the HCV NS5A protein bound to daclatasvir explains drug‐resistant mutations and activity against divergent genotypes. J Chem Inform Model. 2014;55:362‐373. [DOI] [PubMed] [Google Scholar]
- 202. Schames JR, Henchman RH, Siegel JS, Sotriffer CA, Ni H, McCammon JA. Discovery of a novel binding trench in HIV integrase. J Med Chem. 2004;47:1879‐1881. [DOI] [PubMed] [Google Scholar]
- 203. Maeda K, Das D, Yin PD, et al. Involvement of the second extracellular loop and transmembrane residues of CCR5 in inhibitor binding and HIV‐1 fusion: insights into the mechanism of allosteric inhibition. J Mol Biology. 2008;381:956‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Amaro RE, Swift RV, Votapka L, Li WW, Walker RC, Bush RM. Mechanism of 150‐cavity formation in influenza neuraminidase. Nat Commun. 2011;2:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Mottin M, Braga RC, da Silva RA, et al. Molecular dynamics simulations of Zika virus NS3 helicase: insights into RNA binding site activity. Biochem Biophys Res Commun. 2017;492:643‐651. [DOI] [PubMed] [Google Scholar]
- 206. Wang J, Ma C, Fiorin G, et al. Molecular dynamics (MD) simulation directed rational design of inhibitors targeting drug‐resistant mutants of influenza A virus M2. J Am Chem Soc. 2011;133:12834‐12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Asthana S, Shukla S, Vargiu AV, et al. Different molecular mechanisms of inhibition of bovine viral diarrhea virus and hepatitis C virus RNA‐dependent RNA polymerases by a novel benzimidazole. Biochemistry. 2013;52:3752‐3764. [DOI] [PubMed] [Google Scholar]
- 208. Wittayanarakul K, Aruksakunwong O, Saen‐oon S, et al. Insights into saquinavir resistance in the G48V HIV‐1 protease: quantum calculations and molecular dynamic simulations. Biophys J. 2005;88:867‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Perryman AL, Lin JH, McCammon JA. HIV‐1 protease molecular dynamics of a wild‐type and of the V82F/I84V mutant: Possible contributions to drug resistance and a potential new target site for drugs. Protein Sci. 2004;13:1108‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Rognan D. Structure‐based approaches to target fishing and ligand profiling. Mol Inform. 2010;29:176‐187. [DOI] [PubMed] [Google Scholar]
- 211. Westermaier Y, Barril X, Scapozza L. Virtual screening: an in silico tool for interlacing the chemical universe with the proteome. Methods. 2015;71:44‐57. [DOI] [PubMed] [Google Scholar]
- 212. Hopkins A, Mason J, Overington J. Can we rationally design promiscuous drugs? Curr Opin Struct Biol. 2006;16:127‐136. [DOI] [PubMed] [Google Scholar]
- 213. Guvench O. Computational functional group mapping for drug discovery. Drug Discov Today. 2016;21:1928‐1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Hussain J, Rea C. Computationally efficient algorithm to identify matched molecular pairs (MMPs) in large data sets. J Chem Inform Model. 2010;50:339‐348. [DOI] [PubMed] [Google Scholar]
- 215. Leach AG, Jones HD, Cosgrove DA, et al. Matched molecular pairs as a guide in the optimization of pharmaceutical properties; a study of aqueous solubility, plasma protein binding and oral exposure. J Med Chem. 2006;49:6672‐6682. [DOI] [PubMed] [Google Scholar]
- 216. Roy K, Leonard JT. QSAR modeling of HIV‐1 reverse transcriptase inhibitor 2‐amino‐6‐arylsulfonylbenzonitriles and congeners using molecular connectivity and E‐state parameters. Bioorg Med Chem. 2004;12:745‐754. [DOI] [PubMed] [Google Scholar]
- 217. Elfiky AA, Elshemey WM. IDX‐184 is a superior HCV direct‐acting antiviral drug: a QSAR study. Med Chem Res. 2016;25:1005‐1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Tu J, Li JJ, Shan ZJ, Zhai HL. Exploring the binding mechanism of Heteroaryldihydropyrimidines and Hepatitis B Virus capsid combined 3D‐QSAR and molecular dynamics. Antiviral Res. 2017;137:151‐164. [DOI] [PubMed] [Google Scholar]
- 219. Singh S, Malhotra AG, Jha M, et al. Development of 3D QSAR based pharmacophore model for neuraminidase in Influenza A Virus. Biosci Biotech Res Comm. 2017;10(1):63‐71. [Google Scholar]
- 220. Perilla JR, Schulten K. Physical properties of the HIV‐1 capsid from all‐atom molecular dynamics simulations. Nature. Communications. 2017;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Andoh Y, Yoshii N, Yamada A, et al. All‐atom molecular dynamics calculation study of entire poliovirus empty capsids in solution. J Chem Phys. 2014;141:165101 10.1063/1.4897557 [DOI] [PubMed] [Google Scholar]
- 222. Le L, Lee E, Schulten K, Truong TN. Molecular modeling of swine influenza A/H1N1, Spanish H1N1, and avian H5N1 flu N1 neuraminidases bound to Tamiflu and Relenza. PLOS Curr. 2009;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Wong CF. Conformational transition paths harbor structures useful for aiding drug discovery and understanding enzymatic mechanisms in protein kinases. Protein Sci. 2016;25:192‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Perilla JR, Beckstein O, Denning EJ, Woolf TB. Computing ensembles of transitions from stable states: Dynamic importance sampling. J Comput Chem. 2011;32:196‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Asamitsu K, Hirokawa T, Hibi Y, Okamoto T. Molecular dynamics simulation and experimental verification of the interaction between cyclin T1 and HIV‐1 Tat proteins. PLOS One. 2015;10:e0119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Bhakat S, Martin AJM, Soliman MES. An integrated molecular dynamics, principal component analysis and residue interaction network approach reveals the impact of M184V mutation on HIV reverse transcriptase resistance to lamivudine. Mol BioSys. 2014;10:2215‐2228. [DOI] [PubMed] [Google Scholar]
- 227. Hou T, Yu R. Molecular dynamics and free energy studies on the wild‐type and double mutant HIV‐1 protease complexed with amprenavir and two amprenavir‐related inhibitors: mechanism for binding and drug resistance. J Med Chem. 2007;50:1177‐1188. [DOI] [PubMed] [Google Scholar]
- 228. Hoffmann A, Richter M, von Grafenstein S, et al. Discovery and characterization of diazenylaryl sulfonic acids as inhibitors of viral and bacterial neuraminidases. Front Microbiol. 2017;8:205 10.3389/fmicb.2017.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Anusuya S, Gromiha MM. Quercetin derivatives as non‐nucleoside inhibitors for dengue polymerase: molecular docking, molecular dynamics simulation, and binding free energy calculation. J Biomol Struct Dyn. 2017;35:2895‐2909. [DOI] [PubMed] [Google Scholar]
- 230. Guan S, Wang T, Kuai Z, et al. Exploration of binding and inhibition mechanism of a small molecule inhibitor of influenza virus H1N1 hemagglutinin by molecular dynamics simulation. Sci Rep. 2017;7 10.1038/s41598-017-03719-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Speelman B, Brooks BR, Post CB. Molecular dynamics simulations of human rhinovirus and an antiviral compound. Biophys J. 2001;80:121‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Luzhkov V, Decroly E, Canard B, Selisko B, Åqvist J. Evaluation of adamantane derivatives as inhibitors of dengue virus mRNA cap methyltransferase by docking and molecular dynamics simulations. Mol Inform. 2013;32:155‐164. [DOI] [PubMed] [Google Scholar]
- 233. Zhang C, Feng T, Cheng J, et al. Structure of the NS5 methyltransferase from Zika virus and implications in inhibitor design. Biochem Biophys Res Commun. 2017;492:624‐630. [DOI] [PubMed] [Google Scholar]
- 234. Pan D, Niu Y, Xue W, Bai Q, Liu H, Yao X. Computational study on the drug resistance mechanism of hepatitis C virus NS5B RNA‐dependent RNA polymerase mutants to BMS‐791325 by molecular dynamics simulation and binding free energy calculations. Chemom Inteli Lab Sys. 2016;154:185‐193. [Google Scholar]
- 235. Leonis G, Steinbrecher T, Papadopoulos MG. A contribution to the drug resistance mechanism of Darunavir, Amprenavir, Indinavir, and Saquinavir complexes with HIV‐1 protease due to flap mutation I50V: A systematic MM–PBSA and thermodynamic integration study. J Chem Inform Model. 2013;53:2141‐2153. [DOI] [PubMed] [Google Scholar]
- 236. Pan P, Li L, Li Y, Li D, Hou T. Insights into susceptibility of antiviral drugs against the E119G mutant of 2009 influenza A (H1N1) neuraminidase by molecular dynamics simulations and free energy calculations. Antiviral Res. 2013;100:356‐364. [DOI] [PubMed] [Google Scholar]
- 237. Wang J, Ma C, Fiorin G, et al. Molecular dynamics simulation directed rational design of inhibitors targeting drug‐resistant mutants of influenza A virus M2. J Am Chem Soc. 2011;133:12834‐12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Lee DJ, Robinson WE. Preliminary mapping of a putative inhibitor‐binding pocket for human immunodeficiency virus type 1 integrase inhibitors. Antimicrob Agents Chemother. 2006;50:134‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Borhani DW, Shaw DE. The future of molecular dynamics simulations in drug discovery. J Comput Aided Mol Des. 2012;26:15‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Gershon E. New qubit control bodes well for future of quantum computing. Phys Org. 2014;7(41):83‐89. [Google Scholar]
- 241. Piana S, Lindorff‐Larsen K, Shaw DE. How robust are protein folding simulations with respect to force field parameterization? Biophys J. 2011;100:L47‐L49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Smietana K, Siatkowski M, Møller M. Trends in clinical success rates. Nat Rev Drug Discov. 2016;15:379‐381. [DOI] [PubMed] [Google Scholar]
- 243. Maggiora GM. The reductionist paradox: are the laws of chemistry and physics sufficient for the discovery of new drugs? J Comput Aided Mol Des. 2011;25:699‐708. [DOI] [PubMed] [Google Scholar]
- 244. Hoenen T, Safronetz D, Groseth A, et al. Mutation rate and genotype variation of Ebola virus from Mali case sequences. Science. 2015;348:117‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Cortez KJ, Maldarelli F. Clinical management of HIV drug resistance. Viruses. 2011;3:347‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. van der Vries E, Schutten M, Boucher CAB. The potential for multidrug‐resistant influenza. Curr Opin Infect Dis. 2011;24:599‐604. [DOI] [PubMed] [Google Scholar]
- 247. Spurney CF, Gordish‐Dressman H, Guerron AD, et al. Preclinical drug trials in the mdx mouse: assessment of reliable and sensitive outcome measures. Muscle Nerve. 2009;39:591‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Martic‐Kehl, MI, Schibli, R, Schubiger, PA. Can animal data predict human outcome? Problems and pitfalls of translational animal research. Eur. J. Nucl. Med. Mol. Imaging. 2012;39:1492‐1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Houser J. How many are enough? Statistical power analysis and sample size estimation in clinical research. J Clin Res Best Pract. 2007;3:1‐5. [Google Scholar]
- 250. Kimmelman J, Mogil JS, Dirnagl U. Distinguishing between exploratory and confirmatory preclinical research will improve translation. PLOS biology. 2014;12:e1001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3:711‐716. [DOI] [PubMed] [Google Scholar]


