Abstract
A 2‐year‐old male ferret was presented with central nervous system signs. Computed tomography (CT) of the brain revealed a well‐defined contrast‐enhancing lesion on the rostral forebrain that appeared extraparenchymal. Surgical excision of the mass was performed and the ferret was euthanised during the procedure. Histopathology of the excised mass showed multiple meningeal nodular lesions with infiltrates of epithelioid macrophages, occasionally centred on degenerated neutrophils and surrounded by a broad rim of plasma cells, features consistent with pyogranulomatous meningitis. The histopathological features in this ferret were similar to those in cats with feline infectious peritonitis. Definitive diagnosis was assessed by immunohistochemistry, confirming a ferret systemic coronavirus (FSCV) associated disease. This is the first case of coronavirus granuloma described on CT‐scan in the central nervous system of a ferret.
INTRODUCTION
Over the past 10 years, several cases of systemic disease characterised by pyogranulomatous perivasculitis and peritonitis in ferrets, pathologically similar to feline infectious peritonitis (FIP) in cats, have been reported (Martinez et al. 2006, Garner et al. 2008, Martinez et al. 2008, Perpinan et al. 2008, Michimae et al. 2010, Murray et al. 2010, Wise et al. 2010, Dominguez et al. 2011, Graham et al. 2012). In ferrets, clinical signs are generally non‐specific and include diarrhoea, weight loss, lethargy, hyporexia and vomiting (Garner et al. 2008, Murray et al. 2010, Dominguez et al. 2011). Central nervous system and respiratory signs are uncommonly reported (Garner et al. 2008, Murray et al. 2010). This case report describes the tomodensitometry and histopathological features of a ferret with a focal meningeal pyogranulomatous lesion associated with coronavirus infection.
CASE HISTORY
A 2‐year‐old male, 900 g ferret was evaluated following an acute onset of cluster seizures, pacing and disorientation over the previous week. A 3‐month history of mild lethargy with intermittent diarrhoea was also reported. Abdominal ultrasonography performed 2 months earlier by the referring veterinarian revealed abnormalities interpreted as intestinal inflammation and pancreatitis without peritoneal effusion. The ferret was treated symptomatically for diarrhoea and with glucocorticoids (Prednisolone 1 mg/day) for the suspected inflammation. After a transient improvement, the ferret showed an acute deterioration of body condition and neurological signs. On clinical presentation, the ferret was cachectic and was presented in lateral recumbency. Neurological status examination revealed postural reaction deficits in all four limbs. Spinal reflexes were intact. Pain sensation was present. The ferret had a depressed mental status and responded to noxious and auditory stimuli. There was no seizure activity. Cranial nerves were not altered: palpebral reflexes and pupillary light reflexes were both normal. These findings were consistent with a forebrain lesion.
Haematology and serum biochemistry were performed and revealed hyperproteinaemia (103 g/L; reference interval 52–73 g/L), hyperglobulinaemia (86 g/L; reference interval 18–31 g/L) and a slight thrombocytopenia (162 × 3/mL; reference interval 300–600 × 3/mL). The globulin/albumin ratio was markedly elevated (86/17 = 5) which was suggestive of an inflammatory/infectious disease process or neoplasia.
Computed tomography (CT) of the brain was performed under general anaesthesia. Transverse CT images were obtained using a Toshiba helical scanner (Aquilion) (Fig 1). The ferret was placed in sternal recumbency and 0 · 5‐mm slices were acquired (120 kV, 100 mA) then reconstruction in a low spatial resolution (soft tissue) algorithm and high spatial resolution (bone) algorithm, before and after IV administration of iodine (Telebrix 35, Sodium Ioxithalamate, corresponding to 700 mg Iodine/kg). Post‐contrast CT revealed a well‐delimited ovoid plaque‐like mass, with homogeneous contrast enhancement, extending from the rostral aspect of the olfactory bulb to the caudal aspect of the frontal lobe. The lesion appeared to be extraparenchymal, with a broad base against the bone, though an intraparenchymal location could not be excluded. The differential diagnosis included: extraparenchymal neoplasms (meningioma, lymphoma) and, less likely, intraparenchymal neoplasms (gliomas), inflammatory or infectious granuloma. Cisternal cerebrospinal fluid analysis was performed, although the ferret was given glucocorticoids for 2 months; it revealed no pleocytosis (266 RBC/mm3, 2 lymphocytes/mm3). This result was more consistent with a neoplastic process, although an infectious disease (such as cryptococcosis) or an abscess could not be ruled out.
Figure 1.
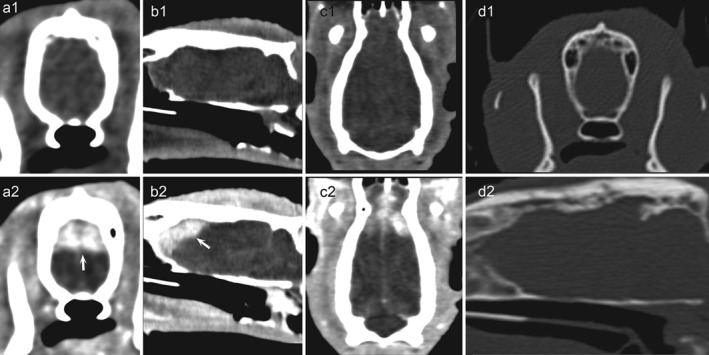
CT‐examination of the head (120 kVp, 100 mA, slice thickness 0 · 5 mm, FOV 180). Transverse (a1, a2), sagittal (b1, b2) and dorsal (c1, c2) reconstructions displayed with a soft tissue window show an isodense pre‐contrast area with a strong post‐contrast enhancement (arrows) in the dorsal part of the right and left forebrain. The lesion shows a wide basis with a large osseous contact. No underlying bone lysis or sclerosis is observed (d1, d2: transverse and sagittal reconstructions displayed with a bone window)
Surgical excision of the mass was proposed and approved by the owner. The ferret was pre‐medicated intramuscularly with midazolam (0 · 5 mg/kg) and methadone (0 · 2 mg/kg). Anaesthesia was induced intravenously with propofol (1 · 5 mg/kg). The ferret was orally intubated and anaesthesia was maintained with isoflurane in oxygen diluted. A transfrontal craniectomy was performed, following the same procedure reported for dogs (Greco et al. 2006). This allowed an incomplete excision of an ill‐defined granular mass that appeared infiltrative within the cerebral parenchyma. Because the mass was not macroscopically consistent with a well‐delimited tumour as expected, and because the remainder of the cerebral parenchyma appeared abnormal and the ferret had stopped responding to medical treatment, the owners were informed of the poor prognosis and elected for euthanasia during the procedure. Complete post‐mortem examination was not permitted.
The mass was fixed in 10% neutered formalin and then embedded in paraffin‐wax for neuropathological evaluations. Paraffin‐embedded sections were submitted to routine histology stains (haematoxylin and eosin).
Microscopically, the meninges were diffusely and severely thickened by a densely cellular inflammatory infiltrate rich in mononuclear cells (Fig 2) and multiple nodular foci. These foci were usually centred on small necrotic areas containing degenerated neutrophils, surrounded by a large rim of epithelioid macrophages, plasma cells and a lesser number of lymphocytes (Fig 3), consistent with severe pyogranulomatous meningitis. The encephalic parenchyma was globally spared excepted for rare mononuclear perivascular cuffs. Special stains (Periodic Acid‐Schiff and Fite‐Faraco) were negative.
Figure 2.
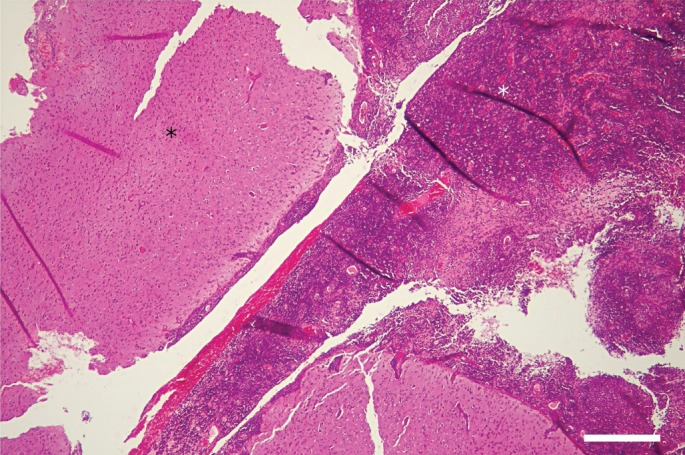
Photomicrograph of the meningeal biopsy. The meninges are diffusely and severely thickened by a densely cellular inflammatory infiltrate. H&E stain; bar = 500 µ
Figure 3.
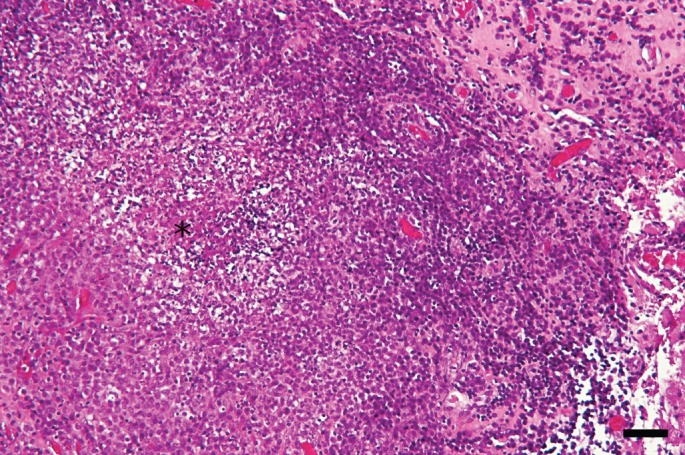
Photomicrograph of the meningeal biopsy. Pyogranulomatous foci with a necrotic centre (asterisk) containing a few neutrophils and surrounded by macrophages. H&E stain; bar = 100 µ
Immunohistochemistry was performed using the monoclonal antibody FIPV3‐70 (Thermo Scientific) previously described for coronaviral diseases in ferrets (Garner et al. 2008). Positive staining was observed in the cytoplasm of few macrophages within the pyogranulomatous lesions (Fig 4). Findings were therefore consistent with a diagnosis of coronavirus pyogranulomatous meningitis.
Figure 4.
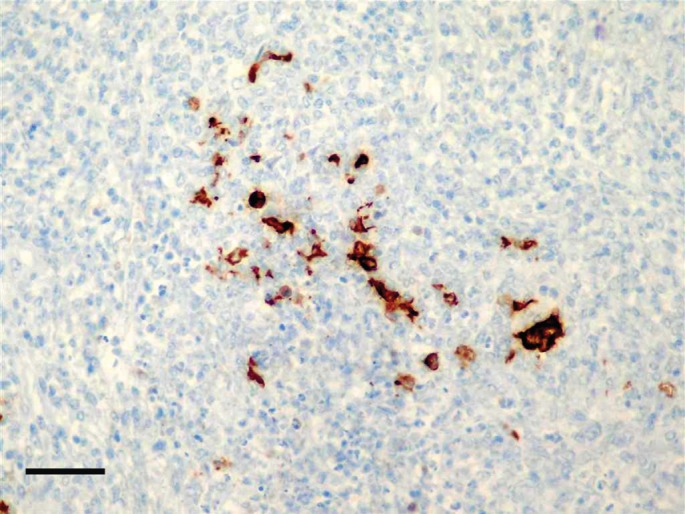
Immunohistochemistry showing intracytoplasmic staining for coronavirus antigen within granulomatous lesions. Streptavidin‐biotin stain; bar = 100 µ
DISCUSSION
Ferret systemic coronavirus (FSCV)‐associated disease (FSCD) is an emerging fatal disease of ferrets, which was first recognised in 2004 (Martinez et al. 2006). This disease characterised by FIP‐like lesions (Garner et al. 2008, Martinez et al. 2008) has been reported in Europe (Spain, UK) (Martınez et al. 2008, Perpinan et al. 2008, Dominguez et al. 2011, Graham et al. 2012), Japan (Michimae et al. 2010) and the United States (Garner et al. 2008, Murray et al. 2010). It is not clear whether the FSCV is derived from the enteric counterpart (FRECV) by in vivo mutation, whether it is a distinct strain or whether the FSCD has originated as a result of recombination between the enteric coronavirus and another coronavirus (Wise et al. 2010).
The clinicopathological characteristics of FSCD are remarkably similar to FIP in cats. The mean age at the time of diagnosis is 11 months (range 2–36 months) with most younger than 18 months (Garner et al. 2008). No sex predilection is observed. All affected ferrets are from indoor environments with or without exposure to dogs and cats or other ferrets. Clinical signs reported in FSCD are non‐specific, including diarrhoea, weight loss, lethargy, hyporexia (Garner et al. 2008, Murray et al. 2010, Dominguez et al. 2011). Less frequent findings included respiratory signs (dyspnoea). Only Murray (2010) and Garner (2008) described 12 ferrets with neurological signs including hind limb paresis, ataxia, tremors and seizures. The clinical signs and physical examination of the ferret in this report were consistent with the clinical data of the previously described ferrets infected by FSCV (Garner et al. 2008).
Laboratory results in this ferret were also consistent with previously published studies (Garner et al. 2008, Perpinan et al. 2008). Typical haemotologic signs include non‐regenerative mild anaemia, hyperglobulinaemia due to polyclonal hypergammaglobulinaemia, and thrombocytopenia (Garner et al. 2008, Murray et al. 2010). The globulin/albumin ratio was markedly elevated, which is also reported in FIP (Sparkes 1991).
Only a minimal amount of information about imaging features in FSCD has been published (abdominal radiographs and ultrasonography) (Lewis et al. 2010, Dominguez et al. 2011). No ante‐mortem cerebral images have been reported in ferrets. In a study evaluating MR images of inflammatory diseases of the CNS in cats, four out of eight cats with FIP showed abnormalities on MRI evaluation (Negrin et al. 2007). MRI abnormalities included ventricular dilation with obstructive hydrocephalus (Kitagawa et al. 2007, Negrin et al. 2007), periventricular contrast enhancement (Foley et al. 1998) and syringomyelia (Kitagawa et al. 2007). A well‐delineated enhancing cerebral mass has never been reported in the cat. Gross lesions of FIP in the CNS of cats (Foley et al. 1998) and ferrets (Murray et al. 2010) are generally subtle, with moderate meningeal opacity and thickening.
In the dry form of FIP, microscopic pyogranulomas may become larger after replication of coronavirus in tissues and attraction of neutrophils and macrophages (Gunn‐Moore et al. 2011). Microscopic pyogranulomatous lesions were detected in five ferrets in one study (Garner et al. 2008). Tumour‐like lesions, as described in this case, have often been reported in the abdominal cavity in cats and ferrets (kidney, liver, spleen, mesenteric lymph nodes) (Kipar et al. 1999, Murray et al. 2010, Dominguez et al. 2011) but not in the brain, of cats nor ferrets. These images of a space‐occupying mass would be more suggestive of a cryptococcal granuloma, as previously reported in the central nervous system of a ferret (Ropstad et al. 2011) or cats (Foster et al. 2000).
Serological testing for FIP using ELISA and PCR techniques were negative in all cases previously described in ferrets (Garner et al. 2008) and were not performed in this ferret, on blood, or CSF. The significance and value of serological tests for FIP in ferrets with FSCD is still questionable. A new sensitive RT‐PCR method was recently established by Terada (2014) to detect ferret coronaviruses in ferrets in Japan. The authors characterised genetically two genotypes of coronaviruses: type 1 causing FIP‐like disease and type 2 causing epizootic catarrhal enteritis. Recently, the use of immunohistochemistry for detection of feline coronavirus within CSF macrophages permitted diagnosis of FIP in a cat (Ives et al. 2013). These new diagnostic techniques could be helpful to diagnose ante‐mortem FSCD infection in ferrets.
In the case reported here, the definitive diagnosis was based on immunohistochemical results. Coronavirus antigens were detected in the cytoplasm of macrophages within the granulomas, using FCV3‐70 monoclonal antibody as described in earlier confirmed outbreaks (Lewis et al. 2010, Murray et al. 2010, Dominguez et al. 2011).
As FSCD leads to a pathological immune‐mediated response, immunosuppressive treatments such as glucocorticoids are likely to provide the best clinical response. Symptomatic treatment may provide short‐term clinical improvement. The course of the disease is usually progressive and duration of the clinical course is highly variable but the disease is invariably fatal (Garner et al. 2008).
In conclusion, FSCD should be considered in the differential diagnosis of young ferrets with neurological signs presenting cerebral masses, associated with non‐specific clinical signs. Although definitive diagnosis of systemic coronavirus infection is based on histological and immunohistochemical findings, imaging techniques – CT or MRI – provide additional information that is helpful for ante‐mortem diagnosis of FSCD.
Conflict of interest
None of the authors of this article has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
References
- Dominguez, E. , Novellas, R. , Moya, A. , et al. (2011) Abdominal radiographic and ultrasonographic findings in ferrets (Mustela putorius furo) with systemic coronavirus infection. Veterinary Record 169, 231 [DOI] [PubMed] [Google Scholar]
- Foley, J. E. , Lapointe, J. , Koblik, P. , et al. (1998) Diagnostic features of clinical neurologic feline infectious peritonitis. Journal of Veterinary Internal Medicine 12, 415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, S. F. , Charles, J. A. , Parker, G. , et al. (2000) Cerebral cryptococcal granuloma in a cat. Journal of Feline Medicine and Surgery 2, 201–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner, M. M. , Ramsell, K. , Morera, N. , et al. (2008) Clinicopathologic features of a systemic coronavirus‐associated disease resembling feline infectious peritonitis in the domestic ferret (Mustela putorius). Veterinary Pathology 45, 236–246 [DOI] [PubMed] [Google Scholar]
- Graham, E. , Lamm, C. , Denk, D. , et al. (2012) Systemic coronavirus‐associated disease resembling feline infectious peritonitis in ferrets in the UK. Veterinary Record 171, 200 [DOI] [PubMed] [Google Scholar]
- Greco, J. J. , Aiken, S. A. , Berg, J. M. , et al. (2006) Evaluation of intracranial meningioma resection with a surgical aspirator in dogs: 17 cases (1996–2004). Journal of the American Veterinary Medical Association 229, 394–400 [DOI] [PubMed] [Google Scholar]
- Gunn‐Moore, D. A. & Reed N. (2011) CNS Disease in a cat : current knowledge of infectious causes. Journal of Feline Medicine and Surgery 13, 824–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives, E. J. , Vanhaesebrouck, A. E. , & Cian, F. (2013) Immunocytochemical demonstration of feline infectious peritonitis virus within cerebrospinal fluid macrophages. Journal of Feline Medicine and Surgery 15, 1149–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipar, A. , Koehler, K. , Bellmann, S. , et al. (1999) Feline infectious peritonitis presenting as a tumour in the abdominal cavity. Veterinary Record 144, 118–122 [DOI] [PubMed] [Google Scholar]
- Kitagawa, M. , Okada, M. , Sato, T. , et al. (2007) A feline case of isolated fourth ventricle with syringomyelia suspected to be related with feline infectious peritonitis. Journal of Veterinary Medical Science 69, 759–762 [DOI] [PubMed] [Google Scholar]
- Lewis, K. M. & O'Brien, R. T. (2010) Abdominal ultrasonographic findings associated with feline infectious peritonitis: a retrospective review of 16 cases. Journal of American Animal Hospital Association 46, 152–160 [DOI] [PubMed] [Google Scholar]
- Martinez, J. , Ramis, A. J. , Reinacher, M. , et al. (2006) Detection of feline infectious peritonitis virus‐like antigen in ferrets. Veterinary Record 158, 523 [DOI] [PubMed] [Google Scholar]
- Martinez, J. , Reinacher, M. , Perpinan, D. , et al. (2008) Identification of group 1 coronavirus antigen in multisystemic granulomatous lesions in ferrets (Mustela putorius furo). Journal of Comparative Pathology 138, 54–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michimae, Y. , Mikami, S. , Okimoto, K. , et al. (2010) The first case of feline infectious peritonitis‐like pyogranuloma in a ferret infected by coronavirus in Japan. Journal of Toxicologic Pathology 23, 99–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, J. , Kiupel, M. & Maes, R. K. (2010) Ferret coronavirus‐associated diseases. Veterinary Clinics of North America : Exotic Animal Practice 13, 543–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrin, A. , Lamb, C. , Cappello, R. , et al. (2007) Results of magnetic resonance imaging in 14 cats with meningoencephalitis. Journal of Feline Medicine & Surgery 9, 109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perpinan, D. & Lopez, C. (2008) Clinical aspects of systemic granulomatous inflammatory syndrome in ferrets (Mustela putorius furo). Veterinary Record 162, 180–183 [DOI] [PubMed] [Google Scholar]
- Ropstad, E. O. , Leiva, M. , Peña, T. , et al. (2011) Cryptococcus gattii chorioretinitis in a ferret. Veterinary Ophthalmology 14, 262–266 [DOI] [PubMed] [Google Scholar]
- Sparkes, A. H. (1991) Feline infectious peritonitis: a review of clinicopathological changes in 65 cases, and a critical assessment of their diagnostic value. Veterinary Record 129, 209–212 [DOI] [PubMed] [Google Scholar]
- Terada, Y. , Minami, S. , Noguchi, K. , et al. (2014) Genetic characterization of coronaviruses from domestic ferrets, Japan. Emerging Infectious Diseases Journal 20, 284–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise, A. , Kiupel, M. , Garner, M. M. , et al. (2010) Comparative sequence analysis of the distal one‐third of the genomes of a systemic and an enteric ferret coronavirus. Virus Research 149, 42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]


