Abstract
Background: Diarrhea is common in foals but there are no studies investigating the relative prevalence of common infectious agents in a population of hospitalized diarrheic foals.
Objectives: To determine the frequency of detection of infectious agents in a population of hospitalized foals with diarrhea and to determine if detection of specific pathogens is associated with age, outcome, or clinicopathologic data.
Animals: Two hundred and thirty‐three foals ≤ 10 months of age with diarrhea examined at a referral institution.
Methods: Retrospective case series. Each foal was examined for Salmonella spp., viruses, Clostridium difficile toxins, Clostridium perfringens culture, C. perfringens enterotoxin, Cryptosporidium spp., and metazoan parasites in feces collected at admission or at the onset of diarrhea.
Results: At least 1 infectious agent was detected in 122 foals (55%). Rotavirus was most frequently detected (20%) followed by C. perfringens (18%), Salmonella spp. (12%), and C. difficile (5%). Foals < 1 month of age were significantly more likely to be positive for C. perfringens (odds ratio [OR] = 15, 95% confidence interval [CI] = 3.5–66) or to have negative fecal diagnostic results (OR = 3.0, 95% CI = 1.7–5.2) than older foals. Foals > 1 month of age were significantly more likely to have Salmonella spp. (OR = 2.6, 95% CI = 1.2–6.0), rotavirus (OR = 13.3, 95% CI = 5.3–33), and parasites (OR = 23, 95% CI = 3.1–185) detected compared with younger foals. Overall 191 of the 223 foals (87%) survived. The type of infectious agent identified in the feces or bacteremia was not significantly associated with survival.
Conclusions and Clinical Importance: In the population studied, foals with diarrhea had a good prognosis regardless of which infectious agent was identified in the feces.
Keywords: Clostridium, Diarrhea, Foal, Rotavirus, Salmonella
Diarrhea is one of the most common conditions requiring veterinary intervention in foals and as many as 80% of foals will have at least 1 episode of diarrhea in the 1st 6 months of life. 1 Diarrhea in foals may be the result of noninfectious causes such as foal heat diarrhea, dietary intolerances, ingestion of sand, asphyxia‐associated gastroenteropathies, and possibly gastroduodenal ulceration. 2 Many infectious agents have been implicated as causes of diarrhea in foals. Infectious agents most commonly listed as causes of acute diarrhea in foals include Clostridium perfringens type A and type C, Clostridium difficile, Salmonella enterica, and rotavirus. 2 , 3 , 4 Other infectious agents less commonly associated with acute diarrhea in foals include Cryptosporidium parvum, 5 Strongyloides westeri, 6 coronavirus, 7 Aeromonas hydrophilia, 8 Rhodococcus equi, 9 Streptococcus durans, 10 and Bacteroides fragilis. 11 However, the clinical importance of many of these pathogens in causing disease is poorly understood.
In a case control study of foal diarrhea carried out over a 3‐year period, C. perfringens, rotavirus, Cryptosporidium spp., and S. westeri, were significantly associated with diarrhea, whereas Salmonella spp. and C. perfringens were associated with fatal illness. 6 In the same study, Campylobacter spp., Yersinia enterocolitica, Escherichia coli, and other parasites were not associated with diarrhea. 6 Multiple studies have evaluated the prevalence of a single infectious agent in hospitalized diarrheic foals. 4 , 12 , 13 However, the relative prevalence of common infectious agents in a large population of foals hospitalized for the treatment of diarrhea is uncertain. A better understanding of age, physical examination findings, and clinicopathologic parameters associated with detection of each infectious agent as well as identification of factors associated with survival may affect the clinical management of foals with diarrhea.
Thus, the objectives of this study were to determine the frequency of detection of common infectious agents in a population of hospitalized foals with diarrhea; to determine if detection of specific pathogens was associated with age, outcome, or clinicopathologic data; to determine the factors associated with outcome; and to determine the agreement between 2 different diagnostic tests for rotavirus and C. perfringens.
Materials and Methods
Animals
The medical records of foals ≤ 10 months of age presented to the Veterinary Medical Center of the University of Florida between May 2003 and June 2008 were reviewed. Foals were included in the study if: (1) they were examined for diarrhea or developed diarrhea within the 1st 48 hours of admission, and (2) they had a single fecal screening panel performed on feces collected at admission or at the onset of diarrhea. In all cases, this panel included culture for Salmonella spp. by selective enrichment in selenite cystine broth, 14 detection of viruses by electron microscopy and rotavirus ELISA, a detection of C. difficile toxin A and B by ELISA, b anaerobic culture for C. perfringens, detection of Cryptosporidium spp. by modified acid‐fast stain, c and detection of gastrointestinal parasite ova by flotation. Most C. perfringens isolates cultured were submitted to an outside laboratory for amplification of alpha, beta, beta‐2, epsilon, iota, and enterotoxin genes by multiplex PCR. d For all foals admitted after 2004, C. perfringens enterotoxin (CPE) ELISA e was also performed. When a given infectious agent was tested with 2 different methods (eg, rotavirus and C. perfringens), a foal was considered positive if at least 1 of the tests was determined to be positive.
Data Collection
Data extracted from the records included age, breed, sex, vital signs at admission (temperature, heart rate, and respiratory rate), results of the fecal screening tests, initial clinicopathologic data (PCV, plasma protein, white blood cell count [WBC], segmented neutrophil count, band neutrophil count, neutrophil toxicity score, platelet count, lymphocyte count, monocyte count, fibrinogen, alkaline phosphatase, aspartate amino transferase, creatine kinase, γ glutamyl transferase, total bilirubin, albumin, globulin, creatinine, blood urea nitrogen [BUN], triglyceride, calcium, phosphorous, magnesium, sodium, potassium, chloride, pH, base excess, bicarbonate, venous Po 2, venous Pco 2, anion gap, glucose, total CO2, and lactate), blood culture results, use of metronidazole, use of systemic antimicrobial agents other than metronidazole, use of gastric acid suppressants (omeprazole or ranitidine), use of probiotics, f length of hospital stay, and outcome (survival to discharge versus death or euthanasia). In foals ≤30 days of age, sepsis score was calculated as previously reported from information available in the medical records. 15
Data Analysis
Normality and equality of variance of the data were assessed with the Kolmogorov‐Smirnov and Levene's tests, respectively. Continuous variables were summarized as medians (10th–90th percentile) and categorical data were summarized as proportions. First, foals were grouped on basis of outcome (survivors versus nonsurvivors [death and euthanasia]). The Student t‐test or the Mann‐Whitney U‐test was used to test for differences between survivors and nonsurvivors with regard to continuous variables. The χ2 test or Fisher's exact test was used to test for differences between outcome and dichotomous variables such as blood culture results, use of metronidazole, use of systemic antimicrobial agents, use of gastric acid suppressant, and use of probiotics. Initial screening of potential factors associated with survival was performed by use of univariate logistic regression. Variables for which the screening P value was < .10 were included in the multivariate model. The multivariate model was a backward stepwise model, whereby variables were removed sequentially starting with that having the largest P value. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. An OR > 1 corresponds to a positive association with survival, whereas a ratio < 1 corresponds to a negative association.
Foals were grouped according to their ages (≤1 month versus >1 month) and the odds of detecting each infectious agent were calculated using logistic regression. Foals were then grouped based on the infectious agent detected in the feces (or lack thereof). Differences in the median ages between foals with various infectious agents were compared with a Kruskal‐Wallis 1 way ANOVA on ranks and multiple pairwise comparisons were performed with Dunn's method. Finally, foals were divided in 4 groups based on the infectious agents most commonly detected in the feces (C. perfringens, C. difficile, Salmonella spp., and rotavirus). Virtually all coinfections involved detection of a parasite (Parascaris equorum, Strongylus spp., Giardia spp., Trichostrongylus spp.) along with Salmonella, C. difficile, or rotavirus. Because these parasites are not typically recognized as causes of diarrhea in foals, they were grouped based on the bacterial or viral agent detected. In preliminary analyses, age was found to be significantly different between groups. Because many clinical examination parameters and laboratory variables vary according to age, a general linear model with age as a covariate was used to compare continuous variables between the 4 groups. When necessary, the data were log‐transformed to meet the assumptions for parametric testing. Multiple pairwise comparisons adjusted for age were done with the Holm‐Sidak test. Dichotomous variables were compared between the 4 groups using multinomial logistic regression with age as a covariate. Sensitivity, specificity, and accuracy of the rotavirus ELISA were calculated using electron microscopy as the gold standard. Agreement between rotavirus ELISA and electron microscopy as well as agreement between C. perfringens culture and CPE ELISA were assessed using the κ statistic. κ differentiates between poor (κ < 20), fair (0.21–0.40), moderate (0.41–0.60), good (0.61–0.80), and very good (0.81–1.00) agreement between tests without the need to identify a gold standard. 16 For all analyses, significance was set at P≤ .05.
Results
Study Population and Fecal Diagnostic Testing Results
Two hundred and twenty‐three foals met the inclusion criteria. There were 98 fillies and 125 colts with a median age at presentation of 16 days (2–125 days). There were 171 Thoroughbreds, 17 Quarter horses, 12 Warmbloods, and 23 foals of other breeds.
At least 1 infectious agent was detected in 122 foals (55%). Of these 122 foals, a single infectious agent was identified from 95 foals (78%), 2 agents were identified from 25 foals (21%), and 3 agents were identified from 2 foals (2%). Rotavirus was the infectious agent most frequently detected (n = 47 foals; 20%) followed by C. perfringens (n = 42 foals; 18%), Salmonella spp. (n = 28 foals; 12%), parasites (n = 16 foals; 7%), C. difficile (n = 11 foals; 5%), coronavirus (n = 3 foals; 1%), and Cryptosporidium spp. (n = 3; 1%). Toxin genotyping was performed for 24 C. perfringens isolates. All 24 isolates were of type A and 3 of these isolates were positive for the enterotoxin gene. Serogrouping was performed on all 28 Salmonella isolates with serogroup B being most common (n = 13), followed by groups C (n = 5), D (n = 5), E (n = 3), and non‐A–E (n = 3). Parasites identified included P. equorum (n = 10), Strongylus spp. (n = 6), Giardia spp. (n = 3), Trichostrongylus spp. (n = 1), and S. westeri (n = 1). Infectious agents were not identified in the feces of 101 foals (45%). It must be emphasized that detection of 1 or multiple infectious agents in the feces of diarrheic foals does not necessarily indicate that the agents(s) was the cause of disease.
The median age of foals from which C. perfringens was isolated was significantly lower than that of foals from which C. difficile, parasites, rotavirus, or Salmonella spp. were detected (Fig 1). Foals < 1 month of age were more likely to have C. perfringens detected (OR = 15, 95% CI = 3.5–66; P= .0002) or to have negative fecal diagnostic test results (OR = 3.0, 95% CI = 1.7–5.2; P= .0002) than foals > 1 month of age. Foals > 1 month of age were significantly more likely to have Salmonella spp. (OR = 2.6, 95% CI = 1.2–6.0; P= .022), rotavirus (OR = 13.3, 95% CI = 5.3–33; P < .00001), and parasites (OR = 23, 95% CI = 3.1–185; P= .002) detected than those <1 month of age. These same associations remained significant when foals were grouped as ≤2 weeks and >2 weeks of age.
Figure 1.
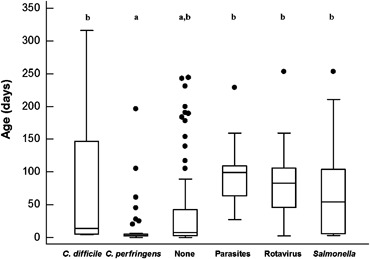
Age of foals with diarrhea according to the results of fecal diagnostic testing. None indicates that all testing was negative. The central box represents the values from the lower to upper quartile (25th–75th percentile). The middle line represents the median. The vertical line extends from the minimum to the maximum value, excluding outliers (lower or upper quartile ± 1.5 times the interquartile range), which are displayed as separate points. a,bDifferent letters between groups indicate a significant difference in age (P < .05). When at least 1 letter is common between groups, the difference is not statistically significant.
Factors Associated with Survival
Overall, 191 of the 223 foals (87%) survived. Of 32 nonsurvivors, 6 died and 26 were euthanized. Reasons for euthanasia included continued diarrhea despite prolonged therapy (n = 6 foals), development of septic arthritis (n = 5 foals), pneumonia (n = 5 foals), gastrointestinal rupture (n = 1 foal), mesenteric rent (n = 1 foal), and acute renal failure (n = 1 foal). Reasons for euthanasia were not recorded in 7 foals. The proportion of survivors was not significantly (P > .10) affected by the type of infectious agent identified in the feces. The proportion of survivors for each infectious agent was rotavirus 44/47 (94%), Salmonella spp. 23/28 (82%), C. perfringens 37/42 (88%), and C. difficile 9/11 (81%). The proportion of survivors in foals with a negative enterotoxin ELISA and positive C. perfringens culture (14/15; 93%) was not significantly different (P= 1.00) to that of foals positive for CPE by ELISA (8/9; 89%). The proportion of survivors in foals from which infectious agents were not identified in the feces was 82 of 101 (82%). Heart rate, PCV, BUN, creatinine, total calcium, and magnesium were all significantly higher in the nonsurvivors compared with survivors (Table 1). The proportion of survivors that were treated with metronidazole (121/175) after the onset of diarrhea was not significantly different (P= .256) from that of nonsurvivors (22/27). The proportion of survivors that were treated with gastric acid suppressant (118/170) was not significantly different (P= .185) from that of nonsurvivors (15/27). Similarly, the proportion of survivors (41/43; 95%) in foals that received probiotics did not differ significantly (P= .075) to that of foals that did not receive probiotics (133/158; 84%). The final multivariate logistic regression model (overall significance of the model P= .0004) for survival included heart rate, segmented neutrophils, total calcium, and glucose (Table 2). Addition of other variables did not significantly contribute to the ability of the model to predict survival.
Table 1.
Comparison of admission variables between diarrheic foals that survived (n = 192) and those that died (n = 31).
| Variable | Survivors (n = 192) | Nonsurvivors (n = 31) | P Value |
|---|---|---|---|
| Age (days) | 19 (2–145) | 5 (1–102) | .071 |
| Temperature (°F) | 101.6 (100.0–103.0) | 101.1 (98.1–103.4) | .25 |
| Heart rate (bpm) | 90 (60–120) | 100 (73–140) | .018 |
| Respiratory rate (bpm) | 40 (23–74) | 40 (24–60) | .87 |
| WBC (× 103/μL) | 8.05 (3.39–16.28) | 8.85 (2.84–21.09) | .28 |
| Lymphocytes (× 103/μL) | 1.70 (0.53–3.60) | 1.67 (0.33–3.20) | .58 |
| Neutrophils (× 103/μL) | 4.26 (0.13–28.07) | 5.7 (0.02–30.00) | .41 |
| Monocytes (× 103/μL) | 0.540 (0.18–1.34) | 0.55 (0.08–1.20) | .48 |
| Total protein (g/dL) | 5.79 (4.4–6.9) | 5.0 (4.0–6.8) | .051 |
| Fibrinogen (mg/dL) | 500 (200–700) | 500 (210–890) | .34 |
| Toxicity | 0 (0–3) | 0 (0–3) | .83 |
| PCV (%) | 35 (27–45) | 40 (27.2–48.9) | .019 |
| Platelets (× 103/μL) | 238 (162–520) | 245 (117–478) | .77 |
| ALP (IU/L) | 664 (330–1783) | 588 (235–2908) | .93 |
| AST (IU/L) | 259 (173–414) | 228 (154–546) | .19 |
| CK (IU/L) | 302 (114–908) | 638 (107–3509) | .22 |
| GGT (IU/L) | 27 (15–58) | 21 (6–51) | .11 |
| Total bilirubin (mg/dL) | 2.5 (1.2–5.1) | 2.3 (0.9–7.4) | .71 |
| Albumin (g/dL) | 3.0 (2.4–3.4) | 2.8 (1.9–3.6) | .27 |
| Globulin (g/dL) | 2.8 (1.9–3.7) | 2.3 (1.0–4.3) | .12 |
| Creatinine (mg/dL) | 1.5 (1–3.6) | 2.2 (1.2–6.3) | .007 |
| BUN (mg/dL) | 19 (8–55) | 29 (10–58) | .041 |
| Total calcium (mg/dL) | 11.0 (9.5–12.6) | 11.9 (7.9–14.0) | .025 |
| Phosphorous (mg/dL) | 6.7 (5.0–8.7) | 6.5 (4.7–12.6) | .75 |
| Magnesium (mg/dL) | 1.6 (1.2–2.3) | 1.9 (1.0–3.3) | .048 |
| Lactate (mmol/L) | 2.0 (0.9–9.0) | 3.0 (1.4–10.1) | .055 |
| Glucose (mg/dL) | 133 (97–203) | 123 (52–191) | .129 |
| Sodium (mEq/L) | 136 (125–144) | 136 (117–144) | .720 |
| Potassium (mEq/L) | 3.5 (2.6–4.4) | 3.7 (2.6–5.8) | .409 |
| Chloride (mEq/L) | 98 (85–110) | 96 (80–104) | .051 |
| PH (mEq/L) | 7.35 (7.16–7.42) | 7.34 (7.03–7.45) | .64 |
| Base excess (mEq/L) | −2.1 (−14.8 to 5.4) | −2.6 (−20.6 to 5.1) | .35 |
| HCO3 − (mEq/L) | 22.9 (11.6–30.3) | 23.2 (9.3–32.8) | .98 |
| Hospital stay (days) | 5 (2–12) | 3 (0–17) | .015 |
Data are presented as median (10th and 90th percentile).
ALP, alkaline phosphatase; AST, aspartate amino transferase; BUN, blood urea nitrogen; CK, creatine kinase; GGT, γ glutamyl transferase; WBC, white blood cell count.
Table 2.
Result of univariate and multivariate logistic regression analysis of factors potentially associated with survival in foals with diarrhea.
| Variable a | Survival | P |
|---|---|---|
| OR (95% CI) | ||
| Univariate analysis | ||
| Heart rate (bpm; n = 201) | 0.979 (0.963–0.996) | .015 |
| Temperature (°F; n = 201) | 1.28 (0.986–1.67) | .064 |
| Age (days; n = 217) | 1.01 (0.998–1.01) | .167 |
| Total protein (g/dL; n = 167) | 1.49 (0.930–2.39) | .097 |
| Neutrophils (× 1000/μL; n = 194) | 0.946 (0.889–1.01) | .080 |
| PCV (%; n = 199) | 0.942 (0.894–0.992) | .024 |
| Calcium (mg/dL; n = 190) | 0.778 (0.597–1.01) | .063 |
| Glucose (mg/dL; n = 195) | 1.01 (0.999–1.02) | .063 |
| Chloride (mEq/L; n = 195) | 1.04 (1.00–1.09) | .049 |
| Sepsis score (n = 57) | 0.866 (0.745–1.01) | .060 |
| Use of probiotics (n = 201) | 3.85 (0.875–17.0) | .074 |
| Multivariate model (n = 184) b | ||
| Heart rate (bpm) | 0.979 (0.873–0.995) | .030 |
| Neutrophils (× 1000/μL) | 0.932 (0.999–1.00) | .034 |
| Calcium (mg/dL) | 0.668 (0.483–0.922) | .014 |
| Glucose (mg/dL) | 1.01 (1.00–1.02) | .012 |
Laboratory and Clinical Data
To determine if detection of a given infectious agents in the feces is associated with specific abnormalities in clinical parameters or clinicopathologic data, vital signs as well as CBC and plasma chemistry results were compared between 4 groups (C. perfringens, C. difficile, Salmonella spp., and rotavirus) based on the infectious agents most commonly detected in the feces. There was a significant effect of the infectious agent detected in the feces on WBC, lymphocyte, and monocyte counts as well as cell toxicity, sepsis score, and fibrinogen, phosphorus and total calcium concentrations (Table 3).
Table 3.
Comparison of selected variables between foals with diarrhea from which Clostridium perfringens, Clostridium difficile, Salmonella spp., or rotavirus were identified in the feces.
| Variable a | C. perfringens (n= 42) | C. difficile (n= 11) | Salmonella (n= 28) | Rotavirus (n= 47) | P Value d |
|---|---|---|---|---|---|
| WBC (/μL) | 6,650 (1,802–15,166) | 5,540 (716–14,826) | 10,100 (2,704–32,032) | 8,450 (4,059–14,335) | .049 |
| Lymphocytes (/μL) | 840 (260–2,360) b | 1,240 (651–3,770) b,c | 2,035 (580–3,580) b,c | 2,300 (1,380–3,998) c | .013 |
| Monocytes (/μL) | 520 (102–1,596) | 364 (232–950) | 825 (183–1,435) | 400 (160–774) | .049 |
| Fibrinogen (mg/dL) | 400 (200–600) b | 500 (340–720) b,c | 500 (210–900) c | 500 (300–710) b,c | .050 |
| Toxicity | 1 (0–2.8) b,c | 1 (0–3.0) b,c | 1 (0–3.0) b | 0 (0–2.0) c | .036 |
| Phosphorus (mg/dL) | 6.35 (5.23–9.04) | 5.70 (4.94–8.76) | 6.70 (5.04–9.72) | 7.40 (6.26–10.08) | .048 |
| Calcium (mg/dL) | 10.7 (8.1–12.6) b | 10.9 (8.6–12.4) b,c | 11.3 (10.5–12.6) c | 10.7 (8.2–12.2) b,c | .036 |
| Sepsis score | 11 (2.8–14.0) b,c | 12.5 (11.0–14.0) b,c | 11 (8.9–21.8) b | 2.0 (1.0–5.0) c | .028 |
Different letters within a row indicate a statistically significant age‐adjusted differences between groups (P < .05).
P value adjusted for the effect of age.
Data are presented as median (10th and 90th percentile).
Blood Culture Results and Sepsis Score
Blood culture results were available from 102 foals and 50 were positive (49%) for at least 1 microorganism resulting in a total of 64 isolates. A single microorganism was isolated from 36 samples (72%) and multiple microorganisms were isolated from 14 samples (28%). Forty‐eight isolates were Gram negatives (75%) and 16 were Gram positives (25%). The most common Gram‐negative isolates were E. coli (23 isolates, 36%), Actinobacillus spp. (5 isolates, 8%), Salmonella spp. (4 isolates, 6%), Enterobacter/Pantoea spp. (4 isolates, 6%), Klebsiella spp., (3 isolates, 5%). The most common Gram‐positive isolates were Enterococcus spp. (7 isolates, 11%), Streptococcus spp. (4 isolates, 6%), and Clostridium spp. (2 isolates, 3%).
There was no significant association between a positive blood culture and survival (P= .77). Similarly, isolation of Gram‐positive or Gram‐negative bacteria was not significantly associated with outcome (P= .698 and .650, respectively). There was no association between a positive blood culture result and the type of infectious agent identified in the feces (P= .739). The odds of obtaining a positive blood culture result were 3.0 times higher (95% CI = 1.1–10; P= .034) in foals < 2 weeks of age than in foals > 2 weeks. There was no significant association between blood culture results and sepsis score (P= .420). Using the reported cut‐off of 11, the sensitivity and specificity of sepsis score to predict a positive blood culture were 41 and 64%, respectively. The odds of survival were 6.1 times lower (95% CI = 1.2–32; P= .031) in foals with a sepsis score ≥ 14 than in foals with a lower sepsis score on admission.
Agreement between Diagnostic Tests
There was very good agreement between detection of rotavirus by ELISA and by electron microscopy (κ= 0.88, 95% CI = 0.81–0.96). Using electron microscopy as the gold standard, rotavirus ELISA had a sensitivity of 91%, specificity of 98%, and accuracy of 96% (Table 4). Agreement between culture and enterotoxin ELISA for detection of C. perfringens in the feces was poor (κ= 0.085; 95% CI =−0.275 to 0.444) (Table 5).
Table 4.
Comparison of results of rotavirus ELISA and electron microscopy.
| Rotavirus ELISA | Rotavirus Electron Microscopy | |
|---|---|---|
| Negative | Positive | |
| Negative | 170 | 4 |
| Positive | 4 | 39 |
Table 5.
Comparison of results of Clostridium perfringens enterotoxin ELISA and culture results.
| C. perfringens culture | C. perfringens Enterotoxin ELISA | |
|---|---|---|
| Negative | Positive | |
| Negative | 109 | 6 |
| Positive | 15 | 2 |
Discussion
This study determined the prevalence of a broad range of infectious agents believed to be associated with diarrhea in a large population of foals hospitalized for the treatment of diarrhea. The identification of infectious organisms was common in this population of foals with diarrhea, with over 54% of samples being positive for 1 or more organism. However, the main limitation of this study is the lack of a control population of hospitalized foals without diarrhea subjected to the same fecal diagnostic testing. As a result, the present study cannot establish whether or not there is an association between detection of a given infectious agent in the feces and presence of diarrhea. Detection of 1 or multiple infectious agents in the feces of diarrheic foals does not necessarily indicate that these agents are the cause of disease. Nevertheless, in clinical situations, detection of infectious agents in the feces is the method used to establish a diagnosis and formulate a therapeutic plan. The fecal screening panel in the present study was only performed at the time of admission or as soon as possible after the onset of diarrhea within 48 hours of admission. Repeated testing would have likely enhanced our ability to identify infectious agents in this population. Including the foals with diarrhea on admission only would have introduced a bias by excluding many foals with enterocolitis that present with nonspecific signs such as fever, anorexia, colic, or lethargy. Many of these foals have enterocolitis on admission but they do not develop diarrhea until later. On the other hand, inclusion of foals that developed diarrhea after several days of hospitalization may have been a confounding factor by including cases associated with antimicrobial therapy or nosocomial infection. As a compromise, we included foals that developed diarrhea within 48 hours of admission. Finally, the sensitivities and specificities of diagnostic tests likely vary for different agents, which may affect the relative prevalence of detection of some infectious agents.
Consistent with the results of previous field investigations, rotavirus was the most common agent identified from foals referred for diarrhea in the current study. 6 , 8 , 17 The mean age of foals positive for rotavirus in the present study (81 days; range 2–253 days) is similar to that previously reported on farms in Kentucky (77 days; range 4–155 days). 13 , 17 These results are in contrast to another study in which most diarrheic foals from which rotavirus was identified were between 5 and 35 days of age with the majority of the foals at the younger end of this range. 18 The lymphocyte count was significantly higher in foals with rotavirus compared with foals with C. perfringens and neutrophil toxicity and sepsis scores were significantly lower in foals with rotavirus compared with those with Salmonella. These results suggest that rotavirus is less likely to induce a systemic inflammatory response than those other pathogens. Rotavirus is a suspected cause of disease in many species and has been positively correlated with diarrhea in foals. 6 Electron microscopy is usually considered the gold standard test for detection of rotavirus. Consistent with previous reports, the current study found very good sensitivity, specificity, accuracy, and agreement between rotavirus ELISA when compared with electron microscopy. 8
C. perfringens was cultured frequently in this population of foals with a median age of 3 days. In a case‐control study of foal diarrhea, culture of C. perfringens was significantly associated with both diarrhea and death from diarrhea. 6 This is in direct contrast to another report in which C. perfringens was isolated from 90% of 3‐day‐old foals on 6 farms and no association with diarrhea was found. 19 In that 2nd report, genotyping revealed that 85% of the isolates were of type A and only 2% of these isolates were positive for the enterotoxin gene by PCR. 19 In the present study, only 2 of 17 foals with positive C. perfringens fecal culture were also positive for the enterotoxin (CPE) by ELISA and only 3 of 24 isolates tested were positive for the enterotoxin gene by PCR amplification. In the present study, 6 of 8 foals positive by CPE ELISA had a negative C. perfringens culture. This indicates either a falsely positive CPE ELISA or a falsely negative culture. In adult horses, the proportion of CPE positive is significantly greater in animals with diarrhea than in healthy controls. 20 In the aforementioned study, there was no association between C. perfringens spore count and CPE in the feces. 20 Consistent with these findings, there was poor agreement between C. perfringens culture and toxin detection in the present study. Detection of CPE in the feces of foals was not associated with a worse prognosis in the present study when compared with foals with a positive C. perfringens culture and negative CPE test results. The prevalence of C. difficile A and/or B toxin detection in the feces of diarrheic foals in the present study (11/233; 5%) was considerably lower than previously reported (5/30; 17%) in another study. 20 In contrast, C. difficile toxins or toxin genes are rarely detected in the feces of normal foals. 20
Salmonella is known to cause enteritis and colitis in many species including adult horses. 21 In 1 case‐control study of foal diarrhea, there was no significant association between culture of Salmonella from the feces and presence of diarrhea. 6 However, presence of Salmonella in the feces was significantly associated with fatal illness. 6 In the present study, the survival proportion of foals with a positive Salmonella culture (23/28; 82%) was not significantly different from any other group including foals with rotavirus (44/47; 94%). However, the lack of statistical significance may be the result of the small sample size; hence, low statistical power of the comparison. In the present study, 7 of 12 (58%) foals with a positive Salmonella culture had a blood culture positive for at least 1 microorganism. Two of 4 foals, which grew Salmonella from a blood culture, were also positive for Salmonella in the feces. These results differ slightly from a previous report in which 64% of 22 Salmonella shedders were blood culture positive and all 8 foals who had Salmonella bacteremia were also fecal shedders. 22 This can be explained by the fact that Salmonella was more likely to be cultured from the feces of foals > 1 month of age in the present study and older foals were significantly less likely to be bacteremic.
The importance of many parasites as a cause of diarrhea in foals is uncertain. One previous study identified a significant association between the identification of S. westeri and the presence of diarrhea in foals. 6 In the current study, P. equorum was the most commonly identified parasite. P. equorum is a documented cause of colic, mild respiratory disease, and overall poor doing in foals. Diarrhea has not been shown to be related to infection with this pathogen. In 8 of 10 foals from which P. equorum was identified, a 2nd infectious agent was also detected in the feces. This suggests that, although Parascaris ova were present, they were probably not the cause of diarrhea. It is not surprising that foals >1 month were significantly more likely to have parasite ova identified in their feces. Parascaris and Strongylus ova were most commonly identified in the present population. With the exception of S. westerii, these parasites have prepatent periods ranging from 4 weeks to 6 months. Giardia was the only infectious agent identified in 2 foals. The role of Giardia in equine diarrhea is poorly understood.
The bacterial isolates obtained from blood cultures in this population contrasts with that of a previous study examining a population of foals with positive blood culture and diarrhea. 22 The characteristics of both populations were similar with a median age of 4 days at the time of blood culture collection. In both studies, approximately 50% of the blood cultures were positive for at least 1 microorganism. However, the frequency of isolation or microorganisms was considerably different between the 2 studies. Gram‐negative bacteria (75 versus 57% of isolates) and E. coli (36 versus 11% of isolates) were isolated more frequently in our population, whereas Enterococcus spp. (11 versus 29% of isolates) was identified more frequently at the University of Pennsylvania. 22 This could simply reflect differences in geographic area, ambient temperature, or climate.
The survival rate of foals in this population was high regardless of the infectious agent identified. In contrast, there was a significant effect of the infectious agent detected in the feces on WBC, lymphocyte, and monocyte counts as well as cell toxicity, sepsis score, and fibrinogen, phosphorus and total calcium concentrations. Because of the small number of animals in each category, a lack of significant difference in a given laboratory variable between groups must be taken cautiously due to the low statistical power of such comparison. There was no relationship between blood culture status and survival as also reported by Hollis et al. 22 In a recent retrospective study of 423 bacteremic foals, presence of diarrhea was associated with higher odds of survival. 23 In the present study, sepsis score was not associated with blood culture results. Using the reported cut‐off of 11, the sensitivity and specificity of sepsis score to predict a positive blood culture were 41 and 64%, respectively. The low sensitivity and specificity of the sepsis score in predicting blood culture results has also been recognized in prior studies. 24 , 25 However, sepsis score was a good indicator of outcome in the current study with odds of survival 6.1 times lower in foals with a sepsis score ≥ 14 than in foals with a lower sepsis score on admission. It is likely that sepsis score is a better indicator of severe sepsis and subsequently survival rather than just sepsis or bacteremia.
Footnotes
aSure‐Vue Rota Test. SA Scientific Ltd, San Antonio, TX
b Clostridium difficile Tox A/B II, Techlab Inc, Blacksburg, VA
cTB kinyoun carbolfuchsin, Remel, Lenexa, KS
dWA Animal Disease Diagnostic Lab, Pullman, WA
e Clostridium perfringens enterotoxin test. Techlab Inc
fCulturelle Lactobacillus GG, Amerifit Brands Inc, Cromwell, CT
S. Giguère is presently affiliated with Department of Large Animal Medicine, College of Veterinary Medicine, University of Georgia, Athens, GA.
References
- 1. Urquhart K. Diarrhoea in foals. In Pract 1981;3:22–23, 25, 27, 29. [DOI] [PubMed] [Google Scholar]
- 2. Magdesian KG. Neonatal foal diarrhea. Vet Clin North Am Equine Pract 2005;21:295–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lester GD, Madigan JE. Diarrhea in neonatal foals In: Smith BP, ed. Large Animal Internal Medicine, 4th ed St Louis, MO: Mosby‐Elsevier; 2009:315–319. [Google Scholar]
- 4. Magdesian KG, Hirsh DC, Jang SS, et al Characterization of Clostridium difficile isolates from foals with diarrhea: 28 cases (1993–1997). J Am Vet Med Assoc 2002;220:67–73. [DOI] [PubMed] [Google Scholar]
- 5. Grinberg A, Learmonth J, Kwan E, et al Genetic diversity and zoonotic potential of Cryptosporidium parvum causing foal diarrhea. J Clin Microbiol 2008;46:2396–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Netherwood T, Wood JL, Townsend HG, et al Foal diarrhoea between 1991 and 1994 in the United Kingdom associated with Clostridium perfringens, rotavirus, Strongyloides westeri and Cryptosporidium spp. Epidemiol Infect 1996;117:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guy JS, Breslin JJ, Breuhaus B, et al Characterization of a coronavirus isolated from a diarrheic foal. J Clin Microbiol 2000;38:4523–4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Browning GF, Chalmers RM, Snodgrass DR, et al The prevalence of enteric pathogens in diarrhoeic thoroughbred foals in Britain and Ireland. Equine Vet J 1991;23:405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zink MC, Yager JA, Smart NL. Corynebacterium equi infections in horses, 1958–1984: A review of 131 cases. Can J Vet Res 1986;27:213–217. [PMC free article] [PubMed] [Google Scholar]
- 10. Tzipori S, Hayes J, Sims L, et al Streptococcus durans : An unexpected enteropathogen of foals. J Infect Dis 1984;150:589–593. [DOI] [PubMed] [Google Scholar]
- 11. Myers LL, Shoop DS, Byars TD. Diarrhea associated with enterotoxigenic Bacteroides fragilis in foals. Am J Vet Res 1987;48:1565–1567. [PubMed] [Google Scholar]
- 12. East LM, Savage CJ, Traub‐Dargatz JL, et al Enterocolitis associated with Clostridium perfringens infection in neonatal foals: 54 cases (1988–1997). J Am Vet Med Assoc 1998;212:1751–1756. [PubMed] [Google Scholar]
- 13. Powell DG, Dwyer RM, Traub‐Dargatz JL, et al Field study of the safety, immunogenicity, and efficacy of an inactivated equine rotavirus vaccine. J Am Vet Med Assoc 1997;211:193–198. [PubMed] [Google Scholar]
- 14. Ekiri AB, MacKay RJ, Gaskin JM, et al Epidemiologic analysis of nosocomial Salmonella infections in hospitalized horses. J Am Vet Med Assoc 2009;234:108–119. [DOI] [PubMed] [Google Scholar]
- 15. Brewer BD, Koterba AM. Development of a scoring system for the early diagnosis of equine neonatal sepsis. Equine Vet J 1988;20:18–22. [DOI] [PubMed] [Google Scholar]
- 16. Altman DG. Preparing to analyse data In: Altman DG, ed. Practical Statistics for Medical Research. London: Chapman and Hall; 1991:132–142. [Google Scholar]
- 17. Dwyer RM, Powell DG, Roberts W, et al A study of the etiology and control of infectious diarrhea among foals in central Kentucky. Proc Am Assoc Equine Pract 1990;36:337–355. [Google Scholar]
- 18. Conner ME, Darlington RW. Rotavirus infection in foals. Am J Vet Res 1980;41:1699–1703. [PubMed] [Google Scholar]
- 19. Tillotson K, Traub‐Dargatz JL, Dickinson CE, et al Population‐based study of fecal shedding of Clostridium perfringens in broodmares and foals. J Am Vet Med Assoc 2002;220:342–348. [DOI] [PubMed] [Google Scholar]
- 20. Weese JS, Staempfli HR, Prescott JF. A prospective study of the roles of Clostridium difficile and enterotoxigenic Clostridium perfringens in equine diarrhoea. Equine Vet J 2001;33:403–409. [DOI] [PubMed] [Google Scholar]
- 21. Smith BP, Reina‐Guerra M, Hardy AJ, et al Equine salmonellosis: Experimental production of four syndromes. Am J Vet Res 1979;40:1072–1077. [PubMed] [Google Scholar]
- 22. Hollis AR, Wilkins PA, Palmer JE, et al Bacteremia in equine neonatal diarrhea: A retrospective study (1990–2007). J Vet Intern Med 2008;22:1203–1209. [DOI] [PubMed] [Google Scholar]
- 23. Sanchez LC, Giguère S, Lester GD. Factors associated with survival of neonatal foals with bacteremia and racing performance of surviving Thoroughbreds: 423 cases (1982–2007). J Am Vet Med Assoc 2008;233:1446–1452. [DOI] [PubMed] [Google Scholar]
- 24. Corley KT, Furr MO. Evaluation of a score designed to predict sepsis in foals. J Vet Emerg Crit Care 2003;13:149–155. [Google Scholar]
- 25. Stewart AJ, Hinchcliff KW, Saville WJ, et al Actinobacillus sp. bacteremia in foals: Clinical signs and prognosis. J Vet Intern Med 2002;16:464–471. [DOI] [PubMed] [Google Scholar]


