Abstract
Background: The clinical efficacy of IV administered hypertonic saline solution and hypertonic bicarbonate solution (HBS) in the treatment of inappetent diarrheic calves has not been compared yet.
Hypothesis: HBS is more advantageous than hypertonic saline in the treatment of calves with severe metabolic acidosis.
Animals: Twenty‐eight dehydrated, inappetent calves with neonatal diarrhea.
Methods: In 2 consecutive clinical studies, calves were initially treated with saline (5.85%; 5 mL/kg body weight [BW] over 4 minutes; study I: N = 16) or bicarbonate solution (8.4%; 10 mL/kg BW over 8 minutes; study II: N = 12), respectively, followed by oral administration of 3 L isotonic electrolyte solution 5 minutes after injection. Clinical and laboratory variables were monitored for 72 hours.
Results: Treatment failed in 6 calves of study I and in 1 calf of study II as indicated by a deterioration of the general condition. All treatment failures had more severe metabolic acidosis compared with successfully treated calves before treatment. In the latter, rehydration was completed within 18 hours after injection; metabolic acidosis was corrected within 24 hours (study I) and 6 hours (study II) after injection.
Conclusions and Clinical Importance: Diarrheic calves with slight metabolic acidosis (base excess [BE] >−10 mM) can be treated successfully with hypertonic saline. HBS is appropriate in calves without respiratory problems with more severe metabolic acidosis (BE up to −20 mM). Intensive care of the calves is required to ensure a sufficient oral fluid intake after the initial IV treatment.
Keywords: Dehydration, Hypertonic rehydration, Metabolic acidosis, Suckling reflex
Nonspecific neonatal diarrhea represents the most important cause of fatalities and economic losses in calf husbandry. The clinical course of neonatal diarrhea is characterized by severe dehydration and imbalances in the acid‐base and electrolyte status. Objectives in the treatment of diarrhea are rehydration and correction of the metabolic acidosis. 1 , 2
In diarrheic calves with a failing suckling reflex, the continuous IV infusion of isotonic solution is recommended as the treatment of choice. 1 However, this therapeutic approach is laborious and expensive. As an alternative, the rapid IV injection of hypertonic saline solution (HSS) followed by application of oral rehydration solution (ORS) has been suggested. 2 In veterinary medicine, HSS has been established in the treatment of endotoxic shock in cattle 3 and dogs 4 as well as in the treatment of hemorrhagic shock in sheep, 5 swine, 6 horses, 7 dogs, 8 and cats. 9 HSS was also found to be successful for the rehydration of hypovolemic calves, 10 , 11 which were dehydrated experimentally by the administration of sucrose and diuretics. Although severely dehydrated, these calves exhibited only a mild acidemia and metabolic acidosis. However, a pronounced metabolic acidosis is typical, in particular for older calves (>8 days) suffering from neonatal diarrhea. 12 The general condition, the suckling reflex, and the ability to stand are influenced by the extent of the acidosis. 13 , 14 In particular, increases in serum d‐lactate concentrations commonly found in diarrheic calves 15 because of extensive microbial fermentation of carbohydrates in the rumen and large intestine 16 were found to have a stronger influence on behavior and posture than metabolic acidosis per se. 15
Because parenteral saline induces a mild acidifying effect, 2 , 9 , 17 , 18 the correction of the acidemia in diarrheic calves depends significantly on the consecutive consumption of buffers in ORS. The initial application of hypertonic bicarbonate solution (HBS) may be more advantageous in the treatment of calves with metabolic acidosis. 19 However, HBS has been suggested to induce a paradoxical intracellular and cerebrospinal acidosis (PIA); the relevance of paradoxical intracellular acidosis in calves after administration of HBS is discussed controversially. 17 , 19
It was the objective of the 2 clinical studies presented here to investigate in inappetent calves suffering from neonatal diarrhea the clinical efficacy of IV administered HSS and HBS, respectively.
Materials and Methods
Two consecutive studies were performed using an identical experimental protocol. The only differences between them were the solutions for hypertonic treatment and the number of calves. In study I, 16 Holstein Friesian calves (11 females, 5 males) received hypertonic saline as initial treatment. In study II, HBS was applied to 12 Holstein Friesian calves (7 females, 5 males). All clinical assessments were carried out following a standardized protocol by the same person.
Animals
Both studies were carried out with calves admitted to the Clinic for Cattle. Calves were chosen based on the following selection criteria: age <15 days, neonatal diarrhea (defined as soupy or watery consistency of the feces), dehydration (distance between the medial canthus and the eyeball at least 2 mm), no suckling reflex, and no clinical symptoms indicating moderate to severe secondary organ diseases.
Initial Clinical Examination and Collection of Samples
Immediately after admission, each calf was weighed. The general condition was assessed using a score system (1 = agile, physiologic; 2 = slight depression, able to stand; 3 = moderate depression, sternal recumbency; 4 = severe depression, lateral recumbency). A nipple bottle filled with milk was offered to the animal to assess the suckling reflex. The extent of enophthalmus was quantified by estimating the distance between the medial canthus and the eyeball (mm). 20 Respiratory rate, heart rate, and rectal temperature were assessed. Lung auscultation and navel as well as joints' examination were carried out to exclude secondary diseases.
After checking by clinical investigation that all selection criteria were fulfilled, blood was collected from the jugular vein to assess PCV, serum variables, and blood gases. Centrifugation of the blood (3,000 ×g, 4 °C, 12 minutes) took place within 60 minutes after withdrawal. Finally, a grab sample of feces was taken from the Ampulla recti, which was tested for rotavirus, coronavirus, Cryptosporidium parvum, and E. coli F 5 a , and for salmonellae by fecal culture. b
Initial Treatment
The initial treatment was conducted after introducing a venous catheter c into the jugular vein. In study I, each calf received 5 mL/kg body weight (BW) saline solution d (5.85%) over 4 minutes (study I: NaCl) corresponding to 5 mmol NaCl/kg BW. In study II, each calf received 10 mL/kg BW bicarbonate solution e (8.4%) over 8 minutes (study II: NaHCO3) corresponding to 10 mmol NaHCO3/kg BW.
Immediately after injection, calves were allowed to suckle 3 L ORS. If calves refused to drink, they were administered fluid with a drencher f 10 minutes after injection. ORS contained per liter 4 g NaCl, 0.5 g KCl, 2.5 g NaHCO3, and 20 g glucose (calculated osmolarity 321 mOsmol/L). Procain‐penicillin g (20,000 IU/kg BW SC q24h) was administered for 5 days.
Additional blood samples were withdrawn from 9 randomly chosen calves of each study 0, 10, and 60 minutes after injection for assessing the serum concentration of sodium, potassium, and chloride (studies I and II), and for determination of acid‐base status (study II).
Clinical examination was carried out 6, 12, 18, 24, 30, 36, 48, 60, and 72 hours after the initial treatment. After each of these clinical examinations, blood was collected from the jugular vein to assess PCV, serum variables, and acid‐base status. Fecal grab samples (approximately 10 mL) were taken after each clinical investigation and kept frozen at −20 °C for subsequent dry matter (DM) determination.
Feeding
Milk replacer h (MR; 125 g/L) corresponding to 10% of BW per day was offered divided into 4 meals (7:00 am, 12:00 am, 3:00 pm, 9:00 pm). ORS was available in nipple buckets from 9:00 to 11:00 am, 1:00 to 2:00 pm, 5:00 to 9:00 pm, and 11:00 pm to 6:00 am, respectively. Recumbent calves were encouraged to drink ORS at least 10 minutes at each time. Intake of MR and ORS was recorded.
Treatment Failure
A treatment failure was defined as a deterioration of the general condition between 2 consecutive clinical investigations during the 72‐hour monitoring period. After the exclusion from the study, calves were treated by an established protocol, ie, a continuous infusion of an isotonic solution in the ear vein (8 L of 0.9% saline solution, 2 L of 5% glucose solution, 0.25 L of 8.4% bicarbonate solution).
Analyses
PCV was measured with a hemogram analyzer. i Venous blood pH, base excess (BE), partial pressure of carbon dioxide (PCO2), and standard bicarbonate (sHCO3 −) were determined with a blood gas analyzer. j Serum concentrations of total protein (TP), urea, sodium, chloride, and potassium were determined with an automatic analyzing system. k The concentration of l‐lactate in plasma was measured by a quantitative enzymatic test. l After thawing of the fecal samples, their DM content was assessed by weighing the sample twice before and after the drying process (95 °C, 36 hours).
Calculations
Anion gap (AG) was calculated as
Strong ion gap (SIG) was calculated according to Constable et al 21 :
Statistics
Sigmastat 2.0 m was used for statistical analysis of the results. Results not differing significantly from a normal distribution as indicated by the Kolmogorov‐Smirnov test are presented as means and standard deviations; otherwise, they are depicted as medians with 25/75 quartiles. The significance of differences between the treatment studies I and II was tested by a t‐test and a rank‐sum test, respectively. Differences between points of time within a study group were checked by one‐way repeated‐measures analysis of variance (ANOVA) and one‐way repeated‐measures ANOVA on ranks, respectively. The significance of differences between proportion of treatment failures in studies I and II was tested by Fishers exact test. Differences were classified as significant if P < .05.
Results
Infectious Agents
Infectious agents were found in the fecal smear of all calves investigated. E. coli (F 5) were detected in 6 calves (21%); mixed infections with either rotavirus and C. parvum or coronavirus and C. parvum were evident in 8 calves (29%) and in 3 calves (11%), respectively. Exclusively, C. parvum were found in 11 calves (39%). Salmonellae were found in the feces of none of the calves. Patients infected with E. coli (F 5) were younger and the metabolic acidosis was not as severe as compared with the calves suffering from neonatal diarrhea caused by viral or cryptosporidium infections, or both (Fig 1).
Figure 1.
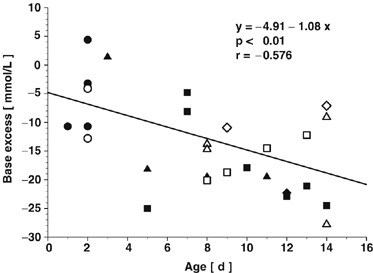
Age, base excess, and infectious agents of calves before hypertonic IV treatment with saline (5.85%; 5 mL/kg body weight [BW] over 4 minutes; study I; filled symbols) and sodium bicarbonate (8.4%; 10 mL/kg BW over 8 minutes; study II; open symbols), respectively. ○, E. coli (F 5); ▵, rotavirus and Cryptosporidium parvum; ◊, coronavirus and C. parvum; □, C. parvum.
Basal Conditions and Treatment Failures
The status of the calves before hypertonic rehydration indicated profound diarrhea, moderate to severe dehydration, moderate to severe depression, and a pronounced metabolic acidosis.
Hypertonic rehydration using saline (study I) was successful in 10 of 16 calves (63%). Six calves had to be removed between 6 and 30 hours after injection. These treatment failures were on average 5 days older and exhibited before treatment a more severe acidemia (pH: 6.96 ± 0.10; sHCO3 −: 8 ± 1 mM; BE: −22.6 mM [−24.5/−19.5]) compared with successfully treated calves (pH: 7.17 ± 0.12; sHCO3 −: 17 ± 6 mM; BE: −9.4 mM [−17.9/−3.2]); no further differences between successfully treated calves and treatment failures were found (Table 1).
Table 1.
Age, body weight (BW), clinical variables, and results of blood analysis before initial IV treatment of diarrheic calves with saline (5.85%; 5 mL/kg BW over 4 minutes; study I) and sodium bicarbonate (8.4%; 10 mL/kg BW over 8 minutes; study II), respectively, followed by administration of 3 L of oral rehydration solution.
| Study I (NaCl) | ||
|---|---|---|
| Successfully Treated (N = 10) | Therapy Failures (N = 6) | |
| Age (days) | 5.2 a± 4.0 | 10.3 b± 3.3 |
| BW (kg) | 37.2 ± 6.1 | 36.8 ± 6.5 |
| General condition (score 1–4) | 3.0 (3.0 / 3.5) | 3.0 (2.5 / 3.5) |
| Heart rate (1/min) | 120 ± 32 | 117 ± 15 |
| Respiratory rate (1/min) | 46 ± 16 | 34 ± 9 |
| Enophthalmus (mm) | 4.0 (3.0 / 5.5) | 3.3 (2.0 / 4.0) |
| Dehydration (% of BW) | 7.0 (6.0 / 10.0) | 6.0 (4.0 / 7.0) |
| PCV (L/L) | 0.38 ± 0.07 | 0.35 ± 0.09 |
| TP (g/L) | 59.0 ± 10.7 | 61.5 ± 12.5 |
| Urea (mM) | 14.6 (8.8 / 6.0) | 14.5 (10.3 / 18.4) |
| l‐Lactate (mM) | 4.0 ± 2.3 | 3.2 ± 2.4 |
| Sodium (mM) | 135 ± 10 | 134 ± 9 |
| Potassium (mM) | 7.6 ± 2.0 | 7.8 ± 2.6 |
| Chloride (mM) | 92 ± 8 | 98 ± 10 |
| pH | 7.17 a± 0.12 | 6.96 b± 0.10 |
| PCO2 (mmHg) | 56 ± 11 | 42 ± 17 |
| PO2 (mmHg) | 22 a± 4 | 34 b± 8 |
| BE (mM) | −9.4 a (−17.9 /−3.2) | −22.6 b (−24.5 /−19.5) |
| sHCO3 − (mM) | 17 a± 6 | 8 b± 1 |
| AG (mM) | 34.6 ± 5.7 | 36.5 ± 8.4 |
| SIG (mM) | −23.5 ± 6.1 | −27.5 ± 9.3 |
| Glucose (mM) | 5.8 ± 1.8 | 5.0 ± 1.7 |
| Fecal dry matter (%) | 7.7 ± 3.9 | 8.3 ± 0.5 |
Means and standard deviations or medians and 25‐/75‐quartiles are depicted, respectively, for calves successfully treated and those for calves excluded from the study because of treatment failure. Within a row, different superscripts indicate significant differences (P<.05) between the basal values of successfully treated calves and treatment failures in study I (NaCl).
TP, total protein; PCO2, partial pressure of carbon dioxide; PO2, partial pressure of oxygen; BE, base excess; sHCO3, standard bicarbonate; AG, anion gap.
Hypertonic rehydration using bicarbonate solution (study II) was successful in 11 of 12 calves (92%). The sole calf that was not successfully resuscitated with hypertonic sodium bicarbonate was removed 60 hours after application of the hypertonic solution. This calf had severe metabolic acidosis (pH: 6.88; BE: −28 mM) before treatment despite only slight dehydration (4% of BW, PCV 0.29 L/L; urea 7.2 mM).
The proportion of treatment failures was 36, 48, and 60 hours after injection significantly higher in study I (NaCl) compared with study II (NaHCO3) (P= .024) after injection.
Initial treatment
No clinically important adverse effects occurred during the administration of hypertonic solutions. Roughly 75% of the calves exhibited signs of discomfort and moderate to severe restlessness during the injection of the hypertonic solutions as indicated by twitching of the limbs, general shivering, and occasionally groaning. No obvious differences with respect to the extent of these clinical signs were observed between calves of study I (NaCl) and study II (NaHCO3); however, during application of HBS, the respiratory rate increased more profoundly up to roughly 60 breaths per minute compared with the calves treated with hypertonic saline. These signs disappeared gradually within 5 minutes after the injection in all calves. Nine calves (56%) of study I (NaCl) and 6 calves (50%) of study II (NaHCO3) drank voluntarily 3 L ORS within 10 minutes after the injection of hypertonic solutions; the remaining calves received the ORS by drenching.
Changes after Initial Hypertonic Treatment
The forthcoming comparisons of various variables obtained from the calves of study I (NaCl) and study II (NaHCO3) are carried out exclusively for only those calves that still remained in the study. Thus, the higher percentage of treatment failures in study I (NaCl) compared with study II (NaHCO3) is not taken into account.
Clinical Variables
The general condition of calves of both studies improved significantly within 6 hours after injection from a mean clinical score of 3 (moderate depression, sternal recumbency) to a mean clinical score of 2 (slight depression, able to stand) (Table 2). For successfully treated calves of both studies, the clinical status of the calves improved furthermore, resulting in an almost undisturbed general behavior in the majority of these calves 18–24 hours after injection. No significant changes of rectal temperature, heart rate, and respiratory rate were found during the investigation period of 72 hours for calves of both studies (Table 2).
Table 2.
General condition, rectal temperature, enophthalmus, heart rate, respiratory rate, and fecal dry matter before and after initial IV treatment of diarrheic calves with saline (5.85%; 5 mL/kg BW over 4 minutes; study I) and sodium bicarbonate (8.4%; 10 mL/kg BW over 8 minutes; study II), respectively, followed by administration of 3 L of oral rehydration solution.
| Number of calves in the study | Study | 0 h a. inj. | 6 h a. inj. | 12 h a. inj. | 18 h a. inj. | 24 h a. inj. | 30 h a. inj. | 36 h a. inj. | 48 h a. inj. | 60 h a. inj. | 72 h a. inj. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NaCl | 16 | 16 | 14 | 14 | 12 | 12 | 10 | 10 | 10 | 10 | |
| NaHCO3 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 11 | |
| General condition (score 1–4) | NaCl | 3.0 (2.8 / 3.5) | 2.0 (2.0 / 3.0) | 2.3 (1.5 / 2.5) | 2.0 (1.0 / 2.0) | 1.5 (1.0 / 2.0) | 1.0 (1.0 / 2.3) | 1.0 (1.0 / 1.5) | 1.0 (1.0 / 1.0) | 1.0 (1.0 / 1.0) | 1.0 (1.0 / 1.0) |
| NaHCO3 | 3.0 (2.5 / 3.3) | 2.0 (1.3 / 3.0) | 1.8 (1.0 / 2.5) | 1.5 (1.0 / 2.3) | 1.3 (1.3 / 2.3) | 1.3 (1.0 / 2.3) | 1.5 (1.0 / 1.5) | 1.5 (1.0 / 2.0) | 1.0 (1.0 / 1.8) | 2.0 (1.0 / 2.0) | |
| Rectal temperature (°C) | NaCl | 38.6 ± 1.1 | 39.0 ± 0.9 | 39.4 ± 0.5 | 39.4 ± 0.5 | 39.3 ± 0.4 | 39.3 ± 0.4 | 39.5 ± 0.4 | 39.5 ± 0.3 | 39.5 ± 0.3 | 39.5 ± 0.5 |
| NaHCO3 | 38.8 ± 1.4 | 39.3 ± 0.6 | 39.3 ± 0.4 | 39.1 ± 0.4 | 39.4 ± 0.5 | 39.6 ± 0.4 | 39.5 ± 0.4 | 39.4 ± 0.3 | 39.5 ± 0.3 | 39.6 ± 0.5 | |
| Enophthalmus (mm) | NaCl | 3.8 (3.0 / 5.5) | 2.0 (0.8 / 3.5) | 0.3 (0 / 2.0) | 0 (0 / 0.5) | 0.3 (0 / 2.0) | 0 (0 / 1.0) | 0.5 (0 / 1.0) | 0* (0 / 0) | 0 (0 / 0) | 0 (0 / 0) |
| NaHCO3 | 4.8 (3.0 / 6.5) | 2.5 (1.5 / 3.5) | 1.5 (0.5 / 2.3) | 0.8 (0 / 1.5) | 0 (0 / 0.8) | 0 (0 / 1.0) | 0.3 (0 / 1.0) | 1.0* (0 / 1.5) | 1.0 (0 / 2.0) | 1.5 (0 / 2.5) | |
| Heart rate (1/min) | NaCl | 120 (100 / 138) | 124 (106 / 139) | 108 (96 / 128) | 116 (104 / 128) | 110 (94 / 122) | 112 (108 / 120) | 108 (98 / 112) | 106 (96 / 116) | 112 (100 / 116) | 112 (99 / 124) |
| NaHCO3 | 122 (86 / 134) | 120 (106 / 146) | 100 (100 / 128) | 104 (104 / 120) | 114 (98 / 130) | 114 (104 / 128) | 108 (100 / 122) | 112 (97 / 120) | 116 (94 / 130) | 108 (104 / 124) | |
| Respiratory rate (1/min) | NaCl | 41 ± 15 | 40 ± 22 | 34 ± 15 | 33 ± 15 | 32 ± 10 | 27 *± 5 | 32 ± 10 | 37 ± 13 | 30 ± 6 | 34 ± 10 |
| NaHCO3 | 43 ± 20 | 39 ± 22 | 34 ± 13 | 33 ± 11 | 37 ± 19 | 39 *± 18 | 39 ± 19 | 40 ± 19 | 36 ± 22 | 41 ± 26 | |
| Fecal dry matter [%] | NaCl | 7.9 ± 3.1 | 6.0 ± 3.2 | 5.1 ± 3.1 | 4.8 ± 3.1 | 5.2 ± 3.7 | 5.8 ± 2.9 | 6.8 ± 4.9 | 4.8*± 2.7 | 6.5 *± 4.4 | 8.5 ± .6.8 |
| NaHCO3 | 7.3 ± 3.0 | 3.9 ± 2.0 | 6.9 ± 4.9 | 7.9 ± 7.2 | 6.0 ± 4.3 | 6.8 ± 5.2 | 11.0 ± 7.0 | 10.5*± 8.0 | 13.6 *± 8.1 | 9.9 ± 8.1 |
Means and standard deviations or medians and 25‐/75‐quartiles are depicted, respectively. Asterisks indicate significant differences (P < .05) between the results in studies I and II.
a. inj., after injection; BW, body weight; h, hours.
Hydration Status
In both studies, concomitant with the improvement in the general condition, dehydration of the patients could be corrected within 18–24 hours after injection. The enophthalmus was significantly reduced already within 6–12 hours; a further, but slower improvement was obvious within the subsequent 18 hours (Table 2). The PCV decreased in calves of both studies within 6 hours by 16% from 0.38 L/L to roughly 0.31 L/L. Constant values were reached in both studies 24 hours after injection (Table 3). The serum concentration of TP decreased almost linearly in both studies within 18 hours after injection from initially 60 g/L by 25% to roughly 45 g/L for the remaining investigation period. In both studies, urea concentration decreased from 15 mM (studies I and II) before administration of hypertonic solutions significantly until 48 hours after injection to 4–5 mM (Table 3).
Table 3.
PCV, total protein (TP), urea, l‐lactate, sodium, potassium, chloride, pH, partial pressure of carbon dioxide (PCO2), partial pressure of oxygen (PO2), base excess (BE), standard bicarbonate (sHCO3 −), anion gap (G), and strong ion gap (SIG) before and after initial IV treatment of diarrheic calves with saline (5.85%; 5 mL/kg body weight [BW] over 4 minutes; study I) and sodium bicarbonate (8.4%; 10 mL/kg BW over 8 minutes; study II), respectively, followed by administration of 3 L of oral rehydration solution.
| Number of calves | Study | 0 h a. inj. | 6 h a. inj. | 12 h a. inj. | 18 h a. inj. | 24 h a. inj. | 30 h a. inj. | 36 h a. inj. | 48 h a. inj. | 60 h a. inj. | 72 h a. inj. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NaCl | 16 | 16 | 14 | 14 | 12 | 12 | 10 | 10 | 10 | 10 | |
| NaHCO3 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 11 | |
| PCV (L/L) | NaCl | 0.37 ± 0.08 | 0.31 ± 0.06 | 0.31 ± 0.06 | 0.30 ± 0.07 | 0.29 ± 0.06 | 0.29 ± 0.07 | 0.28 ± 0.06 | 0.27 ± 0.05 | 0.27 ± 0.06 | 0.26 ± 0.05 |
| NaHCO3 | 0.38 ± 0.07 | 0.32 ± 0.05 | 0.31 ± 0.05 | 0.29 ± 0.06 | 0.28 ± 0.05 | 0.28 ± 0.05 | 0.27 ± 0.05 | 0.27 ± 0.05 | 0.26 ± 0.04 | 0.27 ± 0.05 | |
| TP (g/L) | NaCl | 59.9 ± 11.0 | 50.8 ± 7.8 | 49.2 ± 8.7 | 46.4 ± 7.5 | 47.6 ± 7.2 | 46.8 ± 7.8 | 45.9 ± 6.6 | 44.5 ± 6.3 | 45.1 ± 6.8 | 43.9 ± 8.6 |
| NaHCO3 | 59.4 ± 11.2 | 51.4 ± 7.4 | 50.8 ± 7.0 | 45.5 ± 6.1 | 45.9 ± 6.4 | 45.8 ± 4.8 | 45.5 ± 4.9 | 46.1 ± 5.3 | 47.3 ± 5.8 | 46.2 ± 6.0 | |
| Urea (mM) | NaCl | 14.5 (9.5 / 16.2) | 13.1 (6.8 / 17.8) | 8.3 (5.8 / 16.2) | 7.1 (5.8 / 12.3) | 6.3 (5.4 / 12.9) | 6.6 (4.7 / 11.9) | 5.2 (3.7 / 7.0) | 4.8 (3.3 / 8.4) | 3.8 (2.1 / 4.9) | 2.9 (1.4 / 4.2) |
| NaHCO3 | 14.8 (8.6 / 24.2) | 14.6 (8.6 / 22.6) | 14.7 (7.9 / 20.5) | 11.7 (7.8 / 15.6) | 9.2 (6.0 / 11.5) | 6.7 (5.0 / 9.3) | 4.9 (4.0 / 8.2) | 3.9 (3.4 / 5.2) | 3.6 (2.9 / 5.4) | 3.7 (2.5 / 7.1) | |
| l‐Lactate (mM) | NaCl | 3.4 ± 2.2 | 2.6 ± 2.4 | 1.8 ± 1.3 | 1.4 ± 0.9 | 1.3 ± 0.8 | 1.1 ± 0.5 | 1.1 ± 0.5 | 1.3 ± 0.3 | 1.4 ± 0.9 | 1.3 ± 0.6 |
| NaHCO3 | 3.9 ± 3.1 | 3.1 ± 1.5 | 2.5 ± 1.6 | 1.8 ± 0.9 | 1.7 ± 0.9 | 1.4 ± 0.6 | 1.2 ± 0.6 | 1.2 ± 0.5 | 1.1 ± 0.6 | 1.2 ± 0.6 | |
| Sodium (mM) | NaCl | 134 ± 8 | 139 ± 6 | 137*± 7 | 136 ± 7 | 134*± 7 | 134 ± 7 | 133*± 7 | 132*± 7 | 135 ± 5 | 135 ± 7 |
| NaHCO3 | 139 ± 12 | 144 ± 9 | 143*± 8 | 142 ± 8 | 140*± 7 | 139 ± 7 | 139*± 7 | 138*± 7 | 138 ± 7 | 137 ± 8 | |
| Potassium (mM) | NaCl | 7.6 ± 2.2 | 6.5 ± 1.7 | 6.0 ± 1.5 | 5.5 ± 1.4 | 6.0 ± 1.3 | 5.8 ± 0.9 | 5.4*± 1.0 | 5.6 ± 1.0 | 5.3 ± 0.8 | 5.4 ± 0.9 |
| NaHCO3 | 7.1 ± 2.0 | 5.5 ± 1.5 | 5.3 ± 1.5 | 4.7 ± 1.3 | 5.2 ± 1.1 | 5.2 ± 0.8 | 4.6*± 0.8 | 5.0 ± 1.1 | 4.7 ± 0.9 | 5.1 ± 0.7 | |
| Chloride (mM) | NaCl | 94 ± 9 | 99 ± 8 | 99 ± 9 | 98 ± 8 | 95 ± 8 | 95 ± 8 | 94 ± 7 | 93 ± 7 | 95 ± 6 | 95 ± 6 |
| NaHCO3 | 94 ± 9 | 94 ± 10 | 89 ± 27 | 97 ± 11 | 96 ± 10 | 97 ± 9 | 98 ± 9 | 98 ± 9 | 100 ± 10 | 99 ± 11 | |
| pH | NaCl | 7.09 ± 0.15 | 7.18*± 0.15 | 7.24 ± 0.12 | 7.27 ± 0.14 | 7.28 ± 0.15 | 7.28 ± 0.14 | 7.33 ± 0.09 | 7.34 ± 0.08 | 7.31 ± 0.10 | 7.35 ± 0.09 |
| NaHCO3 | 7.12 ± 0.11 | 7.32*± 0.10 | 7.31 ± 0.10 | 7.31 ± 0.12 | 7.31 ± 0.11 | 7.32 ± 0.10 | 7.30 ± 0.10 | 7.30 ± 0.09 | 7.27 ± 0.09 | 7.26 ± 0.11 | |
| PCO2 (mmHg) | NaCl | 50 ± 15 | 46 ± 11 | 43 ± 12 | 44 ± 9 | 45 ± 8 | 46 ± 10 | 50 ± 6 | 50 ± 10 | 52 ± 9 | 50 ± 7 |
| NaHCO3 | 45 ± 12 | 51 ± 8 | 50 ± 10 | 49 ± 9 | 50 ± 9 | 48 ± 9 | 49 ± 9 | 48 ± 7 | 47 ± 8 | 48 ± 8 | |
| PO2 (mmHg) | NaCl | 27 ± 8 | 30 ± 7 | 29 ± 7 | 28 ± 8 | 30 ± 9 | 27*± 8 | 24*± 5 | 27*± 8 | 22*± 5 | 28 ± 10 |
| NaHCO3 | 30 ± 14 | 28 ± 5 | 30 ± 7 | 30 ± 8 | 31 ± 6 | 33*± 6 | 33*± 5 | 35*± 5 | 34*± 5 | 34 ± 4 | |
| BE (mM) | NaCl | −18.1(−21.7/−6.5) | −12.4* (−19.5/−2.7) | −3.2(−16.2/0.7) | 0.4(−15.6/2.4) | 1.6(−14.4/4.0) | 2.3(−13.6/4.5) | 3.4(−1.7/5.9) | 5.9(−5.8/7.3) | 4.0(−7.0/6.7) | 6.5(−7.7/8.9) |
| NaHCO3 | −13.3(−16.7/−10.0) | −0.5* (−2.8/8.3) | −1.6(−5.4/7.0) | −2.6 (−6.6/5.4) | −0.6(−7.4/7.3) | 0.2 (−8.3/4.6) | −1.4(−7.9/3.0) | −2.0(−8.1/2.9) | −4.5(−9.6/0.6) | −3.6(−10.0/0.9) | |
| sCHO3 − (mM) | NaCl | 13 ± 7 | 16*± 8 | 18 ± 7 | 20 ± 8 | 20 ± 7 | 21 ± 8 | 24 ± 6 | 25 ± 6 | 24 ± 6 | 25 ± 9 |
| NaHCO3 | 13 ± 4 | 24*± 8 | 23 ± 8 | 23 ± 8 | 23 ± 8 | 23 ± 8 | 23 ± 8 | 22 ± 6 | 20 ± 6 | 21 ± 7 | |
| AG (mM) | NaCl | 35 ± 7 | 29 ± 7 | 26 ± 3 | 24 ± 4 | 24 ± 5 | 23 ± 5 | 21 ± 4 | 20 ± 5 | 21 ± 4 | 21 ± 8 |
| NaHCO3 | 39 ± 7 | 31 ± 6 | 29 ± 6 | 26 ± 5 | 26 ± 5 | 24 ± 6 | 22 ± 4 | 23 ± 2 | 22 ± 3 | 23 ± 3 | |
| SIG (mM) | NaCl | −25 ± 7 | −20 ± 8 | −16 ± 4 | −15 ± 5 | −14 ± 6 | −13 ± 6 | −11 ± 5 | −10 ± 5 | −12 ± 4 | −11 ± 7 |
| NaHCO3 | −28 ± 8 | −20 ± 6 | −18 ± 6 | −16 ± 5 | −16 ± 5 | −14 ± 7 | −13 ± 5 | −13 ± 3 | −13 ± 3 | −13 ± 4 |
Means and standard deviations or medians and 25‐/75‐quartiles are depicted, respectively. Asterisks indicate significant differences (P < .05) between results in studies I and II.
a. inj., after injection; h, hours.
Acid‐Base Status
The metabolic acidosis of calves in study I was equalized gradually within roughly 36 hours as indicated by an increase of blood pH from 7.09 ± 0.15 before saline application up to 7.33 ± 0.09 at 36 hours after injection; the BE was corrected within 12 hours (−18 mM before saline application versus −3 mM 12 hours after injection) (Table 3).
A faster and more pronounced increase of plasma pH was observed in calves of study II (NaHCO3). The effect of hypertonic bicarbonate application was assessed more in detail by evaluating the short‐term effects in a subgroup of the patients (Table 4). The results revealed that the calves became seriously alkalemic during the injection period; a pH 7.57 ± 0.04 and a BE of 29 ± 7 mM were measured directly after the 8‐minute injection period. However, this was a transient effect; already 10 minutes later the plasma pH reached the upper limit of the reference range (pH: 7.43 ± 0.04) and decreased furthermore to 7.37 ± 0.07 60 minutes after injection. Six hours after injection, plasma pH averaged 7.32 ± 0.10; the mean BE was −0.5 mM (−2.8/8.3) (Table 3). Although the plasma pH tended to decrease during the subsequent 66 hours, no serious acidemia developed, as indicated by a pH of 7.26 ± 0.11 at the end of the investigation period (72 hours after injection). The basal serum concentration of l‐lactate decreased linearly in both studies within 18 hours after injection by about 50% from 3.7 mM to approximately 1.6 ± 1.5 mM and remained at this level for the further investigation period (Table 3).
Table 4.
Serum concentrations of electrolytes and pH, partial pressure of carbon dioxide (PCO2), base excess (BE), and standard bicarbonate (sHCO3) in venous blood and within 60 minutes after initial IV treatment of diarrheic calves with saline (5.85%; 5 mL/kg BW over 4 minutes; study I) and sodium bicarbonate (8.4%; 10 mL/kg BW over 8 minutes; study II), respectively, followed by administration of 3 L of oral rehydration solution.
| Study | Before Injection | 0 Minute after Injection | 10 Minutes after Injection | 60 Minutes after Injection | |
|---|---|---|---|---|---|
| Sodium (mM) | NaCl | 134a± 8 | ND | ND | 140b± 9 |
| NaHCO3 | 139a± 11 | 162b± 7 | 151bc± 13 | 147ac± 11 | |
| Potassium (mM) | NaCl | 7.5a± 2.2 | ND | ND | 7.7a± 2.0 |
| NaHCO3 | 6.9a± 2.1 | 5.4a± 1.9 | 5.9a± 1.9 | 5.6a± 1.7 | |
| Chloride (mM) | NaCl | 92a± 7 | ND | ND | 102b± 11 |
| NaHCO3 | 96a± 9 | 93a± 7 | 92a± 11 | 92a± 10 | |
| pH | NaCl | 7.16 ± 0.13 | ND | ND | ND |
| NaHCO3 | 7.08a± 0.11 | 7.57b± 0.04 | 7.43c± 0.04 | 7.37c± 0.07 | |
| PCO2 (mmHg) | NaCl | 51 ± 12 | ND | ND | ND |
| NaHCO3 | 46a± 16 | 63b± 13 | 58b± 15 | 55ab± 13 | |
| PO2 (mmHg) | NaCl | 24 ± 6 | ND | ND | ND |
| NaHCO3 | 31 ± 17 | 38 ± 6 | 34 ± 9 | 33 ± 6 | |
| BE (mM) | NaCl | −10.6 ± 8.9 | ND | ND | ND |
| NaHCO3 | −15.2a± 7.2 | 29.3b± 6.1 | 11.6c± 7.6 | 5.2d± 9.6 | |
| sHCO3 − (mM) | NaCl | 16 ± 7 | ND | ND | ND |
| NaHCO3 | 12a± 5 | 52b± 5 | 35c± 8 | 29d± 9 | |
| AG (mM) | NaCl | 34 ± 5 * | ND | ND | ND |
| NaHCO3 | 40a± 4 * | 25b± 10 | 32ab± 8 | 36a± 7 |
Means and standard deviations or medians and 25‐/75‐quartiles are depicted, respectively. Within a row, different superscripts indicate significant differences (P < .05) between different points of time in each study.
BW, body weight; PO2, partial pressure of oxygen; AG, anion gap; SIG, strong ion gap; ND, not determined.
Electrolyte Status
For calves of study I (NaCl), serum sodium concentrations increased significantly from mean basal values of 134 ± 8 mM before saline application to 140 ± 9 mM measured 1 hour after injection. In calves of study II (NaHCO3), a massive transient hypernatremia was evident from the results of short‐interval collection of samples in 9 calves (Table 4). However, 6 hours after application of HBS, the mean sodium concentrations were again in the physiologic range (144 ± 9 mM). For the subsequent 72 hours, mean concentrations between 139 and 132 mM were found (Table 3).
Serum chloride concentration increased in calves receiving hypertonic saline (study I) within 60 minutes after injection significantly up to 102 ± 11 mM. Thereafter, values within the reference range were found. In study II (NaHCO3), no significant changes of serum chloride concentration were observed after hypertonic rehydration (3, 4).
Before hypertonic rehydration, hyperkalemia was found in calves of both studies (>7 mM). A distinct bradyarrhythmia because of hyperkalemia, 17 however, was not detected (Table 2). A gradual decrease to a level of about 5 mM was observed within 18 hours in both studies; during the subsequent 54 hours, the serum concentrations remained in this range without significant differences between the calves of both studies (Table 3).
Intake of MR and ORS
Intake of MR did not differ between the calves in both studies and tended to increase (P= .09) from day 1 to day 3 after hypertonic rehydration (day 1: 2.8 ± 1.7 L; day 2: 3.6 ± 1.5 L; day 3: 3.8 ± 1.4 L [means ± SD]). Also, intake of ORS did not differ between the calves in both studies and tended to decrease (P= .09) from day 1 to day 3 after initial treatment (day 1: 6.2 ± 4.6 L; day 2: 5.3 ± 4.2 L; day 3: 4.5 ± 3.2 L). Thus, the amount of total fluid intake remained constant and averaged 8–10 L/d.
Fecal DM
In study I (NaCl), the mean percentage of fecal DM remained within the investigation period without significant differences between 5 and 8%. In study II (NaHCO3), a significant difference from calves of study I (NaCl) was evident on the 3rd day after initial treatment when fecal DM ranged between mean values of 10 and 13% (Table 2).
Discussion
The objective was to evaluate the clinical efficacy of different hypertonic IV rehydration solutions in inappetent diarrheic calves. To prevent adulteration of positive effects of hypertonic rehydration by an insufficiency of other organs, patients with additional secondary infections were disregarded. Further health problems can be explained partly by a long history of neonatal diarrhea, but also by a high proportion of hypogammaglobulinemia because of insufficient colostral supply among diarrheic calves. 22 This is confirmed by low serum concentrations of TP after successful rehydration in both studies (Table 3); the catabolic state and intestinal protein losses during diarrhea may have had a further impact.
Many authors recommend hypertonic saline (7.2%) with 6% dextran for rehydration. 2 , 10 , 11 In the present study, a different concentration of saline had to be used (5.85%); this solution is licensed and commercially available in Germany, representing prerequisites for application in food animals. Nevertheless, because 5 mL/kg BW of this solution were administered, whereas 4 mL/kg are recommended for higher concentrated saline (7.2%), almost the same amounts of sodium (5.0 versus 4.8 mmol/kg BW) were given to the patients, ensuring comparability of the results. The hypertonic solutions used in the present studies did not contain dextran (not licensed in Germany for food animals). Addition of dextran induces a more pronounced expansion of plasma volume and a sustained effect 23 compared with hypertonic saline without dextran because of the predominantly intravascular distribution of dextran with a water‐binding capacity of roughly 20 mL water per gram dextran 70. 24 Thus, a shorter effect of the hypertonic saline in study I on plasma volume compared with the studies carried out with 7.2% sodium chloride in 6% dextran 70 solution had to be expected. 3 , 10
Continuous IV infusion of isotonic solution is the most effective and gentle approach in the treatment of severely diarrheic calves. Hypertonic rehydration represents an alternative for rehydration in those patients in whom an infusion treatment cannot be carried out for any reason. The primary therapeutic approach is based on a transient replenishment of the intravascular volume from the intracellular and transcellular compartments caused by an increase of plasma osmolarity. 17 Thereby, the cardiovascular system is stabilized for 30–90 minutes. 8 , 17 , 19 A sustained improvement of the clinical status, however, requires a significant oral intake of fluid containing water, electrolytes, buffer, and energy. In the present study, these principles worked successfully in 63% (10/16) of the calves of study I (NaCl) and 93% (11/12) of the calves of study II (NaHCO3). Rehydration, accompanied by a marked improvement of the general condition, and a restoration of the suckling reflex were achieved within 12–24 hours; this period is comparable to successful treatments based on continuous infusion of isotonic solutions. 1
The differences in the proportion of treatment failures between study I (NaCl) and study II (NaHCO3) can be explained predominantly by an advantage of bicarbonate solution in the treatment of diarrheic calves with a severe metabolic acidosis. No differences existed in the status of the calves before hypertonic rehydration between successfully treated calves and treatment failures, except for a more severe acidemia in the latter. Calves that failed to respond were on average older than the successfully treated calves, which indirectly confirms the findings that diarrheic calves older than 1 week of age exhibit a more severe acidemia than younger calves 12 , 13 (Table 1). As an explanation, infections with rotavirus, coronavirus, or C. parvum induce an osmotic diarrhea, accompanied by microbial fermentation of substrates in the large intestine, leading to excessive production of predominantly d‐lactate. 25
Calves treated with HBS (study II) received a double load of sodium compared with calves in study I (NaCl). Sodium is regarded as the major determinant of rehydration success; however, no obvious differences were found with respect to the velocity of rehydration between calves of studies I and II (Table 3). Thus, the concentration of sodium in the hypertonic solution had only a short, transient effect, whereas the final success of the rehydration treatment was determined predominantly by a sufficient intake of ORS.
Unfortunately, the severe metabolic acidosis of recumbent diarrheic calves (BE: −15 to −30 mM) may be slightly aggravated by application of saline—whether administered as a continuous infusion or as a hypertonic injection. Saline creates a strong ion acidosis because the effective SID+ of calf plasma is 42 mEq/L, 21 whereas the effective SID+ of saline is 0 mEq/L. The administration of 4 mL saline (7.2%)/kg BW within 4 minutes decreased the plasma pH in swine with hemorrhagic shock and mixed acidosis by 0.08 pH units. 26 This decrease has been considered to be clinically inconsequential, 17 predominantly because an improvement of the blood pH is achieved by the intake of buffers from ORS. In calves with a pre‐existing severe acidemia, however, a further decrease of plasma pH because of hypertonic saline application may be detrimental. In that respect, the short‐term restoration of the suckling reflex is of crucial importance because intake of ORS represents a prerequisite for successful rehydration treatment. A negative correlation has been suggested between the degree of the acidosis and the vigorousness of the suckling reflex. 13 Also, in our studies, a sustained improvement of oral fluid intake was achieved predominantly in those calves where the acidemia could be corrected quickly. In most calves where hypertonic rehydration failed, the general condition deteriorated, concomitant with a reduction of oral fluid intake and in the face of a prolonged acidemia. The concentrations of d‐lactate were most likely massively increased in the diarrheic calves studied compared with healthy calves as indicated by profoundly increased AG 27 (Table 3). Such increased concentrations of d‐lactate contribute significantly to weakness and disturbed consciousness 27 , 28 but they do not seem to influence the suckling reflex. 15
The administration of HBS represents a therapeutic option to correct the metabolic acidosis faster than by hypertonic saline. Even calves with a BE of −10 to −22 mM were treated successfully and plasma pH increased already within 6 hours up to physiologic values. In calves, 10 mL/kg BW bicarbonate solution (8.4%) provides a buffer capacity sufficient to equalize a BE of about −15 to −20 mM. 2 , 13 Because of the high effective SID+ (1000 mEq/L), a strong ion alkalosis is induced and was measured in our calves (Table 4). Double the amount of sodium compared with 5 mL saline (5.85%)/kg BW was administered, leading to the question of whether the application of 10 mL bicarbonate solution (8.4%)/kg BW within 8 minutes is more hazardous for the patient than hypertonic saline. Three issues have to be taken into account:
-
1
The buffering capacity of bicarbonate requires an effective removal of CO2 by the respiratory system. The tachypnea being more pronounced in calves receiving hypertonic bicarbonate than saline is most likely because of the hypercapnia (Table 4). In study II (NaHCO3), several calves with only slight metabolic acidosis were treated, which did not require buffer capacity at all. Nevertheless, also these patients had had no problem in coping with the application of hypertonic bicarbonate by enhancing ventilation. Even newborn calves with mixed respiratory acidosis because of dystocia (mean blood pH 7.06) were successfully treated by infusion of on average 270 mL sodium bicarbonate solution (5%) over 15 minutes, combined with doxapram IV and intranasal oxygen insufflation 29 ; no negative effects compared with calves treated with carbicarb were found. Also, rapid IV administration of HBS (8.4%; 5 mL/kg over 5 minutes) was found to be effective and safe for treating strong ion acidosis in normovolemic calves with experimentally induced respiratory and strong ion acidosis. 19 In these 2 studies, however, lower amounts of bicarbonate were infused than in study II presented here. Many calves, especially with a longer history of diarrhea, also suffer from respiratory infections. It remains unknown whether removal of excessive CO2 after application of HBS by hyperventilation is possible in such patients. Reports of practitioners about sudden fatalities in calves after hypertonic bicarbonate infusion may be an indication that such incidents could be related to respiratory insufficiencies. At present, it is recommended to avoid the application of hypertonic bicarbonate in calves suffering from severe respiratory disease, whereas hypertonic saline can also be applied in such patients.
-
2
Especially in patients with an impaired ability to remove carbon dioxide, a PIA after rapid injection of large quantities of bicarbonate has been discussed as a matter of concern. 30 Accordingly, bicarbonate ions react with H+ ions to form CO2, which readily enters cells and overwhelms the blood‐brain barrier because of its high solubility. Thus, the intracellular PCO2 increases, which induces a local respiratory acidosis. The clinical consequences are unclear; it has been postulated that an acidification of the cerebrospinal fluid (CSF) may exaggerate a depression and increase the respiratory rate. 31 A PIA has been demonstrated in in vitro studies after increasing the extracellular PCO2 by approximately 70 mmHg. 30 , 32 However, in vivo the PCO2 rarely increases more than 15 mmHg. It has been suggested that the increase of PCO2 after bicarbonate application can be aggravated by the concomitant release of protons from extracellular nonbicarbonate buffers in severely acidotic patients. 30 Berchtold et al 19 induced experimentally a mixed respiratory and metabolic acidosis in calves and found, after subsequent injection of bicarbonate solution (8.4%, 5 mL/kg BW over 5 minutes), no pH change in the CSF irrespective of a transient increase of arterial PCO2 from 81 to 93 mmHg. A turnover rate of CO2 into the CSF of 0.01 minute−1 was calculated. In our patients, a statistical trend (P= .08) was found for the relation between plasma pH before injection of hypertonic bicarbonate and the increase of venous PCO2 after injection (Table 4). However, PCO2 increased only by on average 17 mmHg directly after the initial treatment. Thus, PIA seems not to be a reason for profound problems after hypertonic bicarbonate application in calves with a healthy respiratory tract.
-
3
Concerns were expressed with respect to IV treatment of diarrheic calves with bicarbonate solutions because the concomitant hypercapnia and removal of metabolic acidosis may worsen the oxygen extraction in peripheral tissues of such patients. 33 Based on the results presented here, these concerns seem to be inappropriate despite an extensive transient metabolic alkalosis immediately after injection of HBS (Table 4), which can be explained by incomplete distribution of bicarbonate in the extracellular space. The general condition of most calves that received bicarbonate solution improved rapidly—presumably, the improved blood flow because of increased plasma volume overwhelms the negative impact of a left shift of the oxygen equilibrium curve.
Because oral fluid intake is of crucial importance for the success of hypertonic rehydration, an intensive fosterage of the calf after initial treatment represents a prerequisite. The recovery of critically ill patients is undoubtedly facilitated by providing many small meals. The variation of milk as well as ORS intake during each meal after hypertonic rehydration is high. Thus, calves in studies I and II were fed 8 times per day. Moreover, a high feeding frequency reduces the risk of long periods with a low pH in the abomasum, favoring the development of abomasal ulcerations. 34
Hypernatremia (serum sodium >160 mM) is a dreaded syndrome in diarrheic calves 35 ; it is often because of oral application of ORS with an inadequate composition (sodium >160 mM). The results of the present study indicate that a hypernatremia was not a problem in the hypertonic‐rehydrated calves. Hypertonic saline (5.85%, 5 mL/kg) and hypertonic bicarbonate (8.4%, 10 mL/kg) correspond in a 40 kg calf to a total of only 200 and 400 mmol sodium, respectively. Serum sodium increased immediately after completing the injection of HBS by 20 mM, triggering an increase of plasma osmolarity of roughly 20–25 mOsmol/L; an increase in this range has also been reported. 17 The immediate dilution of extracellular compartment by an influx of intracellular and transcellular fluid excludes the risk of a clinically significant hypernatremia. However, hypertonic rehydration is inappropriate in calves that already suffer from hypernatremia before the initial treatment.
In conclusion, hypertonic rehydration represented a safe and reliable method to improve the hydration status of inappetent diarrheic calves. Hypertonic saline seems to be most appropriate for calves within the 1st week of life as most of these patients suffer from a slight to moderate metabolic acidosis (BE >−10 mM). HBS allows successful treatment of diarrheic calves even with severe metabolic acidosis (BE –10 to –20 mM) frequently found in diarrheic calves older than 1 week. Diarrheic patients also suffering from respiratory disease should not be treated with HBS. A high oral fluid intake is a precondition for successful hypertonic treatment requiring an adequate fosterage.
Footnotes
aDigestive Kit: Rota, Corona, E. coli F 5, Cryptosporidium, Bio‐X Diagnostics Sprl., Innovation for veterinary diagnostics, Jemelle, Belgium
bVeterinärinstitut Hannover, Hannover, Germany
cVygonüleT, ∅1.8, 45 mm; Vygon GmbH & Co KG, Aachen, Germany
dKochsalzlösung 1 M, 5.85%, 100 mL; sodium chloride (ingredient): 1 mmol/mL; aqua dest (excipient); Serumwerke Bernburg AG, Bernburg, Germany
eNatriumhydrogencarbonat 8.4% Infusionslösung B. Braun, 250 mL; sodium bicarbonate (ingredient): 1 mmol/mL; aqua dest (excipient); B. Braun Melsungen AG, Melsungen, Germany
fCalf Drencher flexible, 2 L; Jorgen Kruuse, Marslev, Denmark
gProcillin, benzylpenicillin (ingredient): 300,000 IU/mL (excipient), Alvetra GmbH, Neumünster, Germany
hNormi Typ F 10, Nordmilch Zeven, Industriestrasse, Zeven, Germany
iHematology Analyzer Modell MEK‐6108G, Nihon Kohden Europe GmbH, Rosbach v. d. H., Germany
jRapidlab, Bayer Vital GmbH, Fernwald, Germany
kCobas Mira, Hoffmann‐La Roche Ltd, Basel, Switzerland
lSigma Diagnostics Laktat, Nr. 826‐UV, Sigma‐Aldrich Chemie GmbH, Taufkirchen, Germany
mJandel Scientific Corp, Los Angeles, CA
Previously presented in part at the 6th Middle European Buiatrics Congress, Krakow, June 1–4, 2005 (abstract and presentation) and at the 1st Swiss Buiatrics Congress, Bern, October 19–21, 2005 (abstract and presentation). These studies have been published as the thesis of the first author (http://deposit.ddb.de/cgi-bin/dokserv?idn=973952709&dok_var=d1&dok_ext=pdf&filename=973952709.pdf).
References
- 1. Berchtold J. Intravenous fluid therapy of calves. Vet Clin North Am Food Anim Pract 1999;15:505–531, vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Constable PD. The treatment of the diarrheic calf: An update. In: Kaske M, Scholz H, Hoeltershinken M, eds. Recent Developments and Perspectives in Bovine Medicine: Keynote Lectures XXII World Buiatrics Congress. Hildesheim, Germany: Hildesheimer Druck‐ und Verlags‐GmbH; 2002:134–142. [Google Scholar]
- 3. Constable PD, Schmall LM, Muir WW III, et al Hemodynamic response of endotoxemic calves to treatment with small‐volume hypertonic saline solution. Am J Vet Res 1991;52:981–989. [PubMed] [Google Scholar]
- 4. Luypaert P, Vincent JL, Domb M, et al Fluid resuscitation with hypertonic saline in endotoxic shock. Circ Shock 1986;20:311–320. [PubMed] [Google Scholar]
- 5. Kramer GC, Perron PR, Lindsey DC, et al Small‐volume resuscitation with hypertonic saline dextran solution. Surgery 1986;100:239–247. [PubMed] [Google Scholar]
- 6. Maningas PA. Resuscitation with 7.5% NaCl in 6% dextran‐70 during hemorrhagic shock in swine: Effects on organ blood flow. Crit Care Med 1987;15:1121–1126. [DOI] [PubMed] [Google Scholar]
- 7. Schmall LM, Muir WW, Robertson JT. Haemodynamic effects of small volume hypertonic saline in experimentally induced haemorrhagic shock. Equine Vet J 1990;22:273–277. [DOI] [PubMed] [Google Scholar]
- 8. Rocha e Silva M, Velasco IT, Nogueira da Silva RI, et al Hyperosmotic sodium salts reverse severe hemorrhagic shock: Other solutes do not. Am J Physiol 1987;253:H751–H762. [DOI] [PubMed] [Google Scholar]
- 9. Muir WW III, Sally J. Small‐volume resuscitation with hypertonic saline solution in hypovolemic cats. Am J Vet Res 1989;50:1883–1888. [PubMed] [Google Scholar]
- 10. Constable PD, Gohar HM, Morin DE, et al Use of hypertonic saline‐dextran solution to resuscitate hypovolemic calves with diarrhea. Am J Vet Res 1996;57:97–104. [PubMed] [Google Scholar]
- 11. Walker PG, Constable PD, Morin DE, et al Comparison of hypertonic saline‐dextran solution and lactated Ringer's solution for resuscitating severely dehydrated calves with diarrhea. J Am Vet Med Assoc 1998;213:113–121. [PubMed] [Google Scholar]
- 12. Naylor JM. Severity and nature of acidosis in diarrheic calves over and under one week of age. Can Vet J 1987;28:599–610. [PMC free article] [PubMed] [Google Scholar]
- 13. Kasari TR. Metabolic acidosis in calves. Vet Clin North Am Food Anim Pract 1999;15:473–486, v. [DOI] [PubMed] [Google Scholar]
- 14. Wendel H, Sobotka R, Rademacher G. Untersuchungen zur klinischen Einschätzung des Azidosegrades von Kälbern mit Neugeborenendurchfall. Tierärztl Umschau 2001;56:569–577. [Google Scholar]
- 15. Lorenz I, Vogt S. Investigations on the association of d‐lactate blood concentrations with the outcome of therapy of acidosis, and with posture and demeanour in young calves with diarrhoea. J Vet Med A Physiol Pathol Clin Med 2006;53:490–494. [DOI] [PubMed] [Google Scholar]
- 16. Ewaschuk JB, Naylor JM, Palmer R, et al d‐lactate production and excretion in diarrheic calves. J Vet Intern Med 2004;18:744–747. [DOI] [PubMed] [Google Scholar]
- 17. Constable PD. Hypertonic saline. Vet Clin North Am Food Anim Pract 1999;15:559–585. [DOI] [PubMed] [Google Scholar]
- 18. Suzuki K, Ajito T, Iwabuchi S. Effect of infusion of hypertonic saline solution of conscious heifers with hypoxemia caused by endotoxin infusion. Am J Vet Res 1998;59:452–457. [PubMed] [Google Scholar]
- 19. Berchtold JF, Constable PD, Smith GW, et al Effects of intravenous hyperosmotic sodium bicarbonate on arterial and cerebrospinal fluid acid‐base status and cardiovascular function in calves with experimentally induced respiratory and strong ion acidosis. J Vet Intern Med 2005;19:240–251. [DOI] [PubMed] [Google Scholar]
- 20. Constable PD, Walker PG, Morin DE, et al Clinical and laboratory assessment of hydration status of neonatal calves with diarrhea. J Am Vet Med Assoc 1998;212:991–996. [PubMed] [Google Scholar]
- 21. Constable PD, Stampfli HR, Navetat H, et al Use of a quantitative strong ion approach to determine the mechanism for acid‐base abnormalities in sick calves with or without diarrhea. J Vet Intern Med 2005;19:581–589. [DOI] [PubMed] [Google Scholar]
- 22. Kaske M, Werner A, Schuberth HJ, et al Colostrum management in calves: Effects of drenching vs. bottle feeding. J Anim Physiol Anim Nutr (Berlin) 2005;89:151–157. [DOI] [PubMed] [Google Scholar]
- 23. Smith GJ, Kramer GC, Perron P, et al A comparison of several hypertonic solutions for resuscitation of bled sheep. J Surg Res 1985;39:517–528. [DOI] [PubMed] [Google Scholar]
- 24. Constable P. Fluid and electrolyte therapy in ruminants. Vet Clin North Am Food Anim Pract 2003;19:557–597. [DOI] [PubMed] [Google Scholar]
- 25. Omole OO, Nappert G, Naylor JM, et al Both l‐ and d‐lactate contribute to metabolic acidosis in diarrheic calves. J Nutr 2001;131:2128–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moon P, Kramer G. Hypertonic saline‐dextran resuscitation from hemorrhagic shock induces transient mixed acidosis. Crit Care Med 1995;23:323–331. [DOI] [PubMed] [Google Scholar]
- 27. Ewaschuk JB, Naylor JM, Zello GA. Anion gap correlates with serum d‐ and dl‐lactate concentration in diarrheic neonatal calves. J Vet Intern Med 2003;17:940–942. [DOI] [PubMed] [Google Scholar]
- 28. Lorenz I, Gentile A, Klee W. Investigations of d‐lactate metabolism and the clinical signs of d‐lactataemia in calves. Vet Rec 2005;156:412–415. [DOI] [PubMed] [Google Scholar]
- 29. Bleul U, Bachofner C, Stocker H, et al Comparison of sodium bicarbonate and carbicarb for the treatment of metabolic acidosis in newborn calves. Vet Rec 2005;156:202–206. [DOI] [PubMed] [Google Scholar]
- 30. Levraut J, Giunti C, Ciebiera JP, et al Initial effect of sodium bicarbonate on intracellular pH depends on the extracellular nonbicarbonate buffering capacity. Crit Care Med 2001;29:1033–1039. [DOI] [PubMed] [Google Scholar]
- 31. Marshall W. Hydrogen ion homeostasis, tissue oxygenation and their disorders In: Marshall JW, Bangert SK, eds. Clinical Biochemistry—Metabolic and Clinical Aspects. New York: Churchill Livingstone; 1995. [Google Scholar]
- 32. Goldsmith DJ, Forni LG, Hilton PJ. Bicarbonate therapy and intracellular acidosis. Clin Sci (London) 1997;93:593–598. [DOI] [PubMed] [Google Scholar]
- 33. Cambier C, Clerbaux T, Detry B, et al Effects of intravenous infusions of sodium bicarbonate on blood oxygen binding in calves with diarrhoea. Vet Rec 2005;156:706–710. [DOI] [PubMed] [Google Scholar]
- 34. Ahmed AF, Constable PD, Misk NA. Effect of feeding frequency and route of administration on abomasal luminal pH in dairy calves fed milk replacer. J Dairy Sci 2002;85:1502–1508. [DOI] [PubMed] [Google Scholar]
- 35. Angelos SM, Van Metre DC. Treatment of sodium balance disorders. Water intoxication and salt toxicity. Vet Clin North Am Food Anim Pract 1999;15:587–607. [DOI] [PubMed] [Google Scholar]


