Abstract
A group of presumed drug‐like molecules that possess high in silico affinity for angiotensin‐converting enzyme 2 were computationally designed. This enzyme is a promising new target in both cardiorenal disease and some coronavirus infections. A set of substrate analogous molecules were optimized by means of the LeapFrog module of the SYBYL package. Later, Molinspiration and Molsoft were used for screening out the compounds with low oral bioavailability. Similarly, OSIRIS was used for screening out the compounds having serious side effects. At the end of several stages of screening, seven candidates to anti‐viral drugs fulfiling all the evaluated criteria were obtained. They are amenable for future studies in vitro and in vivo. These designed ligands were finally evaluated by Quantitative Structure Activity Relationship studies. 21 molecules were used to carry out the qsar models. Fom these four molecules were taken as external sets yielding models with q 2 = 0.652 and r 2 = 0.962 values.
Keywords: Angiotensin, SYBYL, QSAR, Cheminformatics
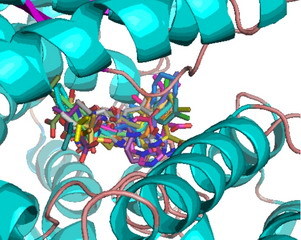
Blind Docking of ACE2 with the Dales Ligands Carried Out by eHiTS and Visualized with Pymol.
REFERENCES
- 1. Lambert, D. ; Hooper, N. ; Turner, A. ; Biochem. Pharmacol., 2008, 75, 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guy, J. ; Jackson, R. ; Acharya, K. ; Sturrock, E. ; Hooper, N. ; Turner, A. ; Biochemistry, 2003, 42, 13185. [DOI] [PubMed] [Google Scholar]
- 3. Burrell, L. M. ; Burchill, L. ; Dean, R. G. ; Griggs, K. ; Patel, S. K. ; Velkoska, E. ; Exp. Physiol., 2012, 97 (4), 477–485. [DOI] [PubMed] [Google Scholar]
- 4. Byrnes, J. ; Gross, S. ; Ellard, C. ; Connolly, K. ; Donahue, S. ; Picarella, D. ; Inflamm Res., 2009, 58 (11), 819–27. [DOI] [PubMed] [Google Scholar]
- 5. Feng, Y. ; Gao, G. ; Comp. Immun. Microbiol. Infect. Dis., 2007, 30 (5‐6), 309–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li, W. ; Moore, M. ; Vasilieva, N. ; Sui, J. ; Wong, S. ; Berne, M. ; Somasundaran, M. ; Sullivan, J. ; Luzuriaga, K. ; Greenough, T. ; Choe, H. ; Farzan, M. ; Nature., 2003, 426, 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hofmann, H. ; Pyrc, K. ; van der Hoek, L. ; Geier, M. ; Berkhout, B. ; Pohlmann, S. ; Proc. Natl. Acad. Sci. USA., 2005, 102, 7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuba, K. ; Imai, Y. ; Rao, S. ; Gao, H. ; Guo, F. ; Guan, B. ; Huan, Y. ; Yang, P. ; Zhang, Y. ; Deng, W. ; Bao, L. ; Zhang, B. ; Liu, G. ; Wang, Z. ; Chappell, M. ; Liu, Y. ; Zheng, D. ; Leibbrandt, A. ; Wada, T. ; Slutsky, A. ; Liu, D. ; Qin, C. ; Jiang, C. ; Penninger, J. ; Nat Med., 2005, 11, 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han, D. ; Penn‐Nicholson, A. ; Cho, M. ; Virology, 2006, 350, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li, W. ; Sui, J. ; Huang, I. ; Kuhn, J. ; Radoshitzky, S. ; Marasco, W. ; Choe, H. ; Farzan, M. ; Virology, 2007, 367, 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pyrc, K. ; Bosch, B. ; Berkhout, B. ; Jebbink, M. ; Dijkman, R. ; Rottier, P. ; van der Hoek, L. ; Antimicrob. Agents Chemother., 2006, 50, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Der Sarkissian, S. ; Huentelman, M. ; Stewart, J. ; Katovich, M. ; Raizada, M. ; Prog. Biophys. Mol. Biol., 2006, 91, 163. [DOI] [PubMed] [Google Scholar]
- 13. Towler, P. ; Staker, B. ; Prasad, S. ; Menon, S. ; Tang, J. ; Parsons, T. ; Ryan, D. ; Fisher, M. ; Williams, D. ; Dales, N. ; Patane, M. ; Pantoliano, M. ; J. Biol. Chem., 2004, 279, 17996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berman, H. ; Westbrook, J. ; Feng, Z. ; Gilliland, G. ; Bhat, T. ; Weissig, H. ; Shindyalov, I. ; Bourne, P. ; Nucleic Acids Res., 2000, 28, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dales, N. ; Gould, A. ; Brown, J. ; Calderwood, E. ; Guan, B. ; Minor, C. ; Gavin, J. ; Hales, P. ; Kaushik, V. ; Stewart, M. ; Tummino, P. ; Vickers, C. ; Ocain, T. ; Patane, M. ; J. Am. Chem. Soc., 2002, 124, 11852. [DOI] [PubMed] [Google Scholar]
- 16. Tripos Inc. 1699 South Hanley Rd.: St. Louis, Missouri, 63144, USA.
- 17. Wang, R. ; Lu, Y. ; Wang, S. ; J. Med. Chem., 2003, 46, 2287. [DOI] [PubMed] [Google Scholar]
- 18. Jones, G. ; Willett, P. ; Glen, R. ; Leach, A. ; Taylor, R. ; J. Mol. Biol., 1997, 267, 727. [DOI] [PubMed] [Google Scholar]
- 19. Muegge, I. ; Martin, Y. ; J. Med. Chem., 1999, 42, 791. [DOI] [PubMed] [Google Scholar]
- 20. Kuntz, I. ; Blaney, J. ; Oatley, S. ; Langridge, R. ; Ferrin, T. ; J. Mol. Biol., 1982, 161, 269. [DOI] [PubMed] [Google Scholar]
- 21. Eldridge, M. ; Murray, C. ; Auton, T. ; Paolini, G. ; Mee, R. ; J. Comput.‐Aided Mol. Des., 1997, 11, 425. [DOI] [PubMed] [Google Scholar]
- 22. Cramer, R. ; Patterson, D. ; Bunce, J. ; J. Am. Chem. Soc., 1988, 110, 5959–5967. [DOI] [PubMed] [Google Scholar]
- 23. Kapetanovic, I. ; Chem. Biol. Interact., 2008, 171, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sander, T. Actelion Pharmaceuticals Ltd. Gewerbestrasse 16, 4123 Allschwil, Switzerland.
- 25. Lipinski, C. ; Drug Discovery Today: Technol., 2004, 1, 337. [DOI] [PubMed] [Google Scholar]
- 26. Van de Waterbeemd, H. ; Gifford, E. ; Nat. Rev. Drug Disc., 2003, 2, 192. [DOI] [PubMed] [Google Scholar]
- 27. Fülöp, V. ; Böcskei, Z. ; Polgár, L. ; Cell, 1998, 94, 161. [DOI] [PubMed] [Google Scholar]
- 28. Zsoldos, Z. ; Reid, D. ; Simon, A. ; Sadjad, S. ; Johnson, A. ; J. Mol. Graph Model., 2006, 7, 421. [DOI] [PubMed] [Google Scholar]
- 29. Guy, L. ; Lambert, D. ; Warner, F. ; Hooper, N. ; Turner, A. ; Biochim. et Biophys. Acta, 2005, 1751, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schechter, I. ; Berger, A. ; Biophys. Res. Com., 1967, 27, 157. [DOI] [PubMed] [Google Scholar]
- 31. Asif, M. ; Curr. Med. Chem., 2012, 19, 2984–2991. [DOI] [PubMed] [Google Scholar]
- 32. Aronov, A. M. ; Drug Discov. Today, 2005, 10, 149. [DOI] [PubMed] [Google Scholar]
- 33. Nair, P. ; Sobhia, E. ; J. Mol. Graph. Model., 2007, 26, 117. [DOI] [PubMed] [Google Scholar]
- 34. Dong, X. ; Zhang, Z. ; Wen, R. ; Shen, J. ; Shen, X. ; Jiang, H. ; Bioorg. Med. Chem. Lett., 2006, 16 (22), 5913–5916. [DOI] [PubMed] [Google Scholar]


