Abstract
The prevalence of eight respiratory viruses detected in patients with acute respiratory infections (ARIs) in Korea was investigated through analysis of data recorded by the Korea Influenza and Respiratory Viruses Surveillance System (KINRESS) from 2013 to 2015. Nasal aspirate and throat swabs specimens were collected from 36 915 patients with ARIs, and viral nucleic acids were detected by real‐time (reverse‐transcription) polymerase chain reaction for eight respiratory viruses, including human respiratory syncytial viruses (HRSVs), influenza viruses (IFVs), human parainfluenza viruses (HPIVs), human coronaviruses (HCoVs), human rhinovirus (HRV), human adenovirus (HAdV), human bocavirus (HBoV), and human metapneumovirus (HMPV). The overall positive rate of patient specimens was 49.4% (18 236/36 915), 5% of which carried two or more viruses simultaneously. HRV (15.6%) was the most predominantly detected virus, followed by IFVs (14.6%), HAdV (7.5%), HPIVs (5.8%), HCoVs (4.2%), HRSVs (3.6%), HBoV (1.9%), and HMPV (1.6%). Most of the ARIs were significantly correlated with clinical symptoms of fever, cough, and runny nose. Although HRV and HAdV were frequently detected throughout the year in patients, other respiratory viruses showed apparent seasonality. HRSVs and IFVs were the major causative agents of acute respiratory diseases in infants and young children. Overall, this study demonstrates a meaningful relationship between viral infection and typical manifestations of known clinical features as well as seasonality, age distribution, and co‐infection among respiratory viruses. Therefore, these data could provide useful information for public health management and to enhance patient care for primary clinicians.
Keywords: acute respiratory infections, respiratory viruses, surveillance
1. INTRODUCTION
Acute respiratory infections (ARIs) cause significant morbidity and mortality, posing an enormous economic burden to society as the most common infectious diseases of humans worldwide.1 The main causal viruses of respiratory illnesses, ranging from a mild cold to severe pneumonia, include human respiratory syncytial viruses (HRSVs), influenza viruses (IFVs), human parainfluenza viruses (HPIVs), human coronaviruses (HCoVs), human adenovirus (HAdV), human bocavirus (HBoV), and human metapneumovirus (HMPV).2, 3, 4 Several novel respiratory viruses have also been identified, such as human bocavirus (HBoV) and human metapneumovirus (HMPV), and novel strains of coronaviruses such as severe acute respiratory syndrome coronavirus (SARS‐CoV), Middle East respiratory syndrome coronavirus (MERS‐CoV), HCoV‐NL63, and HCoV‐HKU1.5, 6, 7, 8, 9 There is currently no effective vaccine or therapeutic reagent against most of these respiratory viruses, except for the influenza viruses. Therefore, continuous laboratory monitoring is required to predict future epidemics and prevent a public health threat.
The Korea Centers for Disease Control and Prevention (KCDC) has been running a surveillance system to detect the major respiratory viruses causing ARIs since December 2005. Respiratory viruses and influenza laboratory surveillance were initially operated separately with different patient inclusion criteria until May 2009, and were then merged as the Korea Influenza and Respiratory Viruses Surveillance System (KINRESS). In this study, we investigated the prevalence of eight respiratory viruses (including 15 subtype viruses) and determined the associations with characteristics such as age distribution, seasonality, and clinical features of patients with ARIs for samples collected between 2013 and 2015 in Korea.
2. MATERIALS AND METHODS
2.1. Specimen and clinical data collection
A total of 36 915 throat swab specimens were collected from 36 sentinel hospitals located nation‐wide through the KINRESS between 2013 and 2015. The specimens were collected in viral transport medium (BD, Franklin Lakes, NJ, USA) from patients with upper respiratory symptoms in all age groups and transported to 17 regional public health and environmental research institutes to establish a diagnosis of infections with respiratory viruses with real‐time reverse transcription‐polymerase chain reaction (RT‐PCR) or PCR promptly. This study was approved by the Korea National Institute of Health institutional review board (Approval number 2013‐08EXP‐03‐5C and 2014‐08EXP‐6C‐A) and written informed consent was obtained from patients or their parents/guardians.
2.2. RT‐PCR
Total nucleic acids were extracted from 140 μL of the patient samples using the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Five commercial respiratory virus detection real‐time one‐step RT‐PCR kits (Kogene Bio, Seoul, South Korea) were used to detect the viral nucleic acids of the 15 subtypes for eight respiratory viruses, including HRSVs (type A, B), IFVs (type A/H1N1pdm09, A/H3N2, B), HPIVs (type 1, 2, 3), HCoVs (type OC43, 229E, NL63), HRV, HAdV, HBoV, and HMPV. Viral cDNAs, except those from HAdV and HBoV, were synthesized from 5 μL of extracted nucleic acids by reverse transcriptase at 50°C for 30 min, followed by inactivation of the reverse transcriptase at 95°C for 10 min. PCR amplification was performed with 40 cycles of 95°C for 15 s and 60°C for 1 min in the ABI 7500 Fast instrument (Thermo Fisher Scientific, Waltham, MA).
2.3. Statistical analysis
The analysis was performed through multivariate logistic regression analysis to identify the association between each virus and clinical symptoms or age groups. Odds ratios (ORs) and their 95% confidence intervals (CIs) were estimated using maximum‐likelihood methods. All statistical analyses were conducted using SPSS ver. 23. 0 (SPSS Inc., Chicago, IL).
3. RESULTS
3.1. Prevalence of respiratory viruses
Of the 36 915 nasal aspirate and throat swab samples 18 236 (49.4%) were positive for at least one respiratory virus (Figure 1A). The monthly positive rates ranged from 28.2% to 76.5% (Figure 1B), with no significant difference between males (27.2%) and females (27.6%) overall or for each type of respiratory virus detection. Overall, HRV and IFVs detection rates were higher than those of the other viruses (Table 1).
Figure 1.
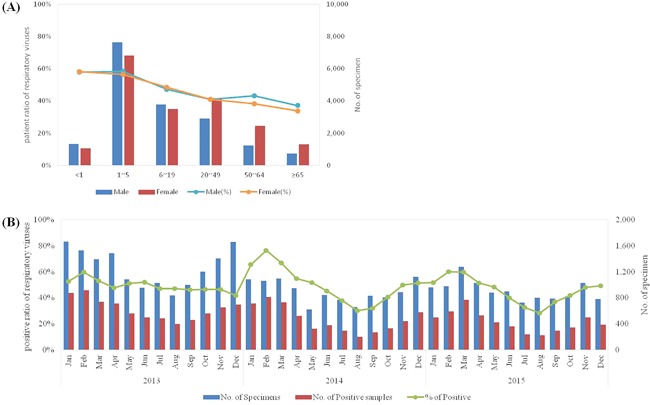
Distribution of total specimens and numbers of samples positive for respiratory viruses every month. (A) Male:female patient ratio according to six age groups. (B) Numbers of specimens, and numbers and detection rates of positive samples for each month from 2013 to 2015
Table 1.
Characteristics of patients (n = 18 236) detected with one or more respiratory viruses
| HAdY | HPIYs | HRSVs | IFYs | HCoYs | HRV | HBoV | HMPV | |
|---|---|---|---|---|---|---|---|---|
| Characteristics | (n = 2,764, 7.5%) | (n = 2,125, 5.8%) | (n = 1,32K, 3.6%) | (n = 5,396, 14.6%) | (n = 1,537, 4.2%) | (n = 5,777, 15.6%) | (n = 718, 1.9%) | (n = 576, 1.6%) |
| No. (%) of patients with co‐detection a | 833 (30.1) b | 432 (20.3) | 246 (18.6) | 375 (6.9) | 313 (20.4) | 1.136 (19.7) | 383 (53.3)b | 106 (18.4) |
| Sex | ||||||||
| Male:female | 1,507:1,257 | 1,050:1,075 | 676:645 | 2,506:2,890 | 722:815 | 2,891:2,886 | 389:329 | 285:291 |
| Clinical symptoms (OR c , 95%Cl) | ||||||||
| Fever | 2,422 | 1,653 | 1,043 | 4,455 | 948 | 3,430 | 559 | 473 |
| (3.40, 3.03‐3.82) | (1.61, 1.45‐1.79) | (1.71, 1.50‐1.96) | (2.35, 2.18‐2.53) | (0.71, 0.64‐0.79) | (0.61, 0.57‐0.64) | (1.59, 1.33‐1.90) | (2.08, 1.68‐2.58) | |
| Cough | 1.528 | 1.523 | 1.085 | 4.064 | 1.037 | 3.922 | 516 | 498 |
| (0.68, 0.63‐0.74) | (1.46, 1.33‐1.61) | (2.67, 2.32‐3.08) | (1.87, 1.75‐2.00) | (1.18, 1.06‐1.32) | (1.23, 1.16‐1.31) | (1.46, 1.24‐1.71) | (3.67, 2.89‐4.66) | |
| Runny nose | 1.500 | 1.224 | 912 | 3.088 | 1.019 | 3.867 | 486 | 363 |
| (0.97, 0.90‐1.05) | (1.12, 1.03‐1.23) | (1.87, 1.66‐2.11) | (1.12, 1.05‐1.18) | (1.65, 1.48‐1.84) | (1.81, 1.71‐1.92) | (1.74, 1.49‐2.04) | (1.41, 1.19‐1.67) | |
| Sore tliroat | 832 | 706 | 290 | 2.755 | 595 | 2.253 | 148 | 208 |
| (0.57, 0.53‐0.62) | (0.67, 0.61‐0.74) | (0.38, 0.33‐0.43) | (1.53, 1.44‐1.62) | (0.87, 0.78‐0.96) | (0.86, 0.81‐0.91) | (0.35, 0.29‐0.42) | (0.78, 0.65‐0.92) | |
| Chills | 269 | 244 | 107 | 1.644 | 213 | 681 | 34 | 82 |
| (0.48, 0.42‐0.54) | (0.58, 0.51‐0.67) | (0.40, 0.33‐0.48) | (2.33, 2.19‐2.49) | (0.73, 0.63‐0.85) | (0.57, 0.53‐0.62) | (0.23, 0.16‐0.32) | (0.76, 0.60‐0.96) | |
| Headache | 411 | 384 | 157 | 2.301 | 383 | 1.288 | 63 | 114 |
| (0.42, 0.37‐0.46) | (0.54, 0.48‐0.60) | (0.33, 0.28‐0.39) | (2.09, 1.97‐2.22) | (0.83, 0.74‐0.93) | (0.68, 0.64‐0.73) | (0.24, 0.18‐0.31) | (0.62, 0.50‐0.76) | |
| Myalgia | 245 | 275 | 122 | 2.156 | 336 | 959 | 29 | 95 |
| (0.31, 0.27‐0.35) | (0.48, 0.42‐0.55) | (0.33, 0.27‐0.40) | (2.60, 2.45‐2.76) | (0.93, 0.82‐1.05) | (0.62, 0.58‐0.67) | (0.14, 0.10‐0.20) | (0.65, 0.52‐0.82) | |
| Sputum | 838 | 955 | 755 | 1.841 | 546 | 2.293 | 316 | 311 |
| (0.78, 0.71‐0.84) | (1.52, 1.39‐1.66) | (2.50, 2.24‐2.80) | (0.93, 0.88‐0.99) | (1.00, 0.90‐1.11) | (1.23, 1.17‐1.31) | (1.44, 1.24‐1.67) | (2.16, 1.83‐2.54) | |
| Diarrhea | 11 | 6 | 12 | 14 | 6 | 20 | 1 | 2 |
| (1.06, 0.57‐1.96) | (0.74, 0.33‐1.67) | (2.54, 1.41‐4.60) | (0.65, 0.38‐1.13) | (1.03, 0.46‐2.35) | (0.90, 0.56‐1.45) | (0.36, 0.05‐2.60) | (0.92, 0.23‐3.71) |
Episodes of coinfection with >1 virus were excluded from the analysis of demographic and clinical feamres.
Co‐detection rates of HBoV and HAdV were higher than other viruses.
Odds ratios were calculated based on the multivariate logistic regression for correlation of each viruses and clinical symptoms.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.2. Co‐detection of respiratory viruses
Among the positive cases, more than one respiratory virus infection was identified in 1846 patients. HRV was the most frequently co‐detected virus with other viruses, followed by HAdV. HBoV showed co‐detection (53.3%) with various respiratory viruses, mostly HRV (38.5%, 175), HPIVs (24.4%, 111), HAdV (22.2%, 101), HRSVs (4.8%, 22), IFVs (4.0%, 18), HCoVs (3.1%, 14), and HMPV (3.1%, 14). However, HMPV had the lowest co‐detection rate. Co‐detection were significantly more frequent in children and adolescents (1‐19 years old) than in the <1 and ≥20‐year‐old age groups (data not shown).
3.3. Clinical symptoms
Most of the respiratory virus infections were significantly associated with fever, cough, and runny nose, except for HAdV. However, HCoV and HRV infections were less commonly correlated with fever and a few of the HRSVs‐infected patients showed diarrhea. As expected, IFVs infection resulted in influenza‐like illness (ILI) such as sore throat, chills, headache, and myalgia (Table 1) in most of the patients.
3.4. Seasonality of respiratory virus
The seasonal distribution of the eight respiratory viruses is shown in Figure 2. HRV and HAdV were the most frequently detected viral pathogens, and were prevalent year‐round with peaks occurring during fall. These findings suggest that the major viral agents of the ARI patients were HRV and HAdV during this period (Figure 2F).
Figure 2.
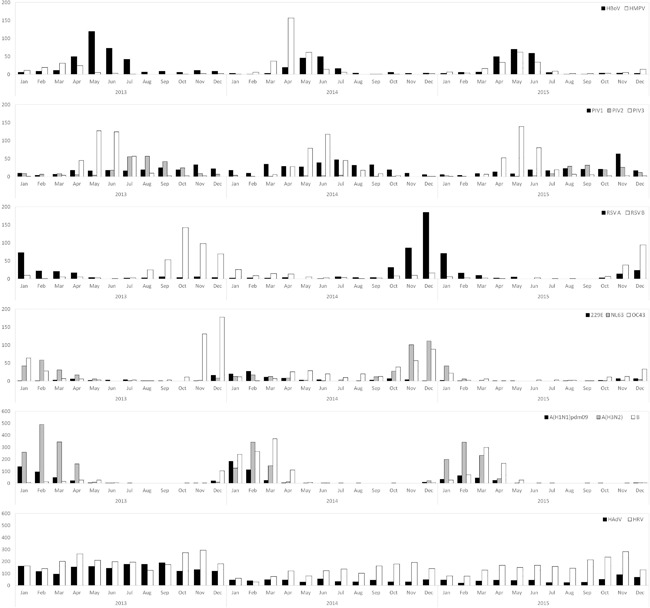
Seasonal distribution of eight respiratory viruses from January 2013 to December 2015. (A) human metapneumovirus (HMPV) and human bocavirus (HBoV), (B) human parainfluenza viruses (HPIVs), (C) human respiratory syncytial viruses (HRSVs), (D) human coronaviruses (HCoVs), (E) influenza viruses (IFVs), (F) human rhinovirus (HRV) and human adenovirus (HAdV)
The other respiratory viruses exhibited seasonality. HMPV was mainly detected during spring with a peak in April 2014, and HBoV was prevalent from spring to early summer with a peak in May 2013 (Figure 2A). Various subtypes of HPIVs were detected during the sampling period (Figure 2B). HPIV type 2 was not identified in February 2014 and June 2015, and a peak of type 3 HPIV was observed in early summer (May and June). The incidence of HRSVs showed alternations between type A and B in each year. (Figure 2C).
Among the HCoVs identified, HCoV‐OC43 and HCoV‐NL63 were the most frequent, with a few cases of infections with HCoV‐229E. HCoV‐OC43 and HCoV‐NL63 were prevalent in winter of 2013 and 2014, respectively (Figure 2D).
The prevalence of IFV infections began to increase in early winter, and peaked between January and March. Among IFV A, H3N2 virus was more predominantly detected compared to H1N1pdm09 virus. Moreover, the incidence of IFV B increased, followed by a peak of IFV A in 2014 and 2105 (Figure 2E).
3.5. Distribution of respiratory viruses according to age groups
The patients ranged from <1 to 95 years of age and were classified into six age groups. Most of the respiratory viruses identified mainly occurred in infants and children under 5 years of age. Although IFVs and HRV were detected most frequently for all age groups, IFV infections were particularly more prevalent in school children and adolescents between 6 and 19 years of age. Moreover, HCoV infections were evenly distributed across all age groups, unlike the other viruses (Table 2).
Table 2.
Distribution of eight respiratory viruses according to the age groups patients with acute respiratory infections
| Age group (OR a , 95% Cl) | |||||||
|---|---|---|---|---|---|---|---|
| <1 | l‐5 b | 6‐19 | 20‐49 | 50‐64 | >65 | ||
| (n = 2,386) | (n = 14,456) | (n = 7,274) | (n = 7,084) | (n = 3,665) | (n = 2,050) | Total | |
| HAdV | 192 | 2.013 | 376 | 136 | 39 | 8 | 2,764 |
| (1.09, 0.93‐1.27) | (4.67, 4.28‐5.09) | (0.62, 0.56‐0.70) | (0.20, 0.17‐0.24) | (0.12, 0.09‐0.17) | (0.05, 0.02‐0.09) | ||
| HPIVs | 237 | 1.285 | 223 | 178 | 137 | 65 | 2,125 |
| (1.90, 1.65‐2.19) | (2.52, 2.31‐2.76) | (0.46, 0.40‐0.53) | (0.37, 0.32‐0.43) | (0.61, 0.51‐0.72) | (0.52, 0.41‐0.67) | ||
| HRS Vs | 241 | 875 | 60 | 52 | 60 | 33 | 1,321 |
| (3.48, 3.01‐4.03) | (3.18, 2.83‐3.57) | (0.19, 0.14‐0.24) | (0.17, 0.13‐0.22) | (0.42, 0.33‐0.55) | (0.43, 0.30‐0.60) | ||
| IF Vs | 115 | 1.298 | 1.828 | 1.241 | 634 | 280 | 5,396 |
| (0.28, 0.23‐0.34) | (0.44, 0.41‐0.47) | (2.45, 2.30‐2.62) | (1.31, 1.22‐1.41) | (2.25, 1.15‐1.37) | (0.92, 0.81‐1.05) | ||
| HCoVs | 137 | 652 | 201 | 272 | 162 | 113 | 1,537 |
| (1.44, 1.20‐1.73) | (1.15, 1.04‐1.28) | (0.61, 0.52‐0.70) | (0.90, 0.79‐1.03) | (1.07, 0.91‐1.27) | (1.37, 1.12‐1.67) | ||
| HRV | 534 | 2.662 | 899 | 1.039 | 429 | 214 | 5,777 |
| (1.61, 1.46‐1.78) | (1.40, 1.33‐1.49) | (0.72, 0.66‐0.77) | (0.91, 0.85‐0.98) | (0.69, 0.62‐0.77) | (0.61, 0.53‐0.71) | ||
| HBoV | 141 | 536 | 28 | 10 | 3 | 0 | 718 |
| (3.69, 3.05‐4.46) | (4.72, 3.99‐5.59) | (0.16, 0.11‐0.24) | (0.06, 0.03‐0.11) | (0.04, 0.01‐0.12) | |||
| HMPV | 50 | 357 | 57 | 56 | 38 | 18 | 576 |
| (1.38, 1.03‐1.84) | (2.59, 2.19‐3.07) | (0.44, 0.34‐0.58) | (0.45, 0.34‐0.59) | (0.63, 0.46‐0.88) | (0.54, 0.34‐0.87) | ||
| Total | 1,647 (69.0) | 9,678 (67.0) | 3,672 (50.5) | 2,984 (42.1) | 1,502 (41.0) | 731 (35.7) | 20,214 |
Odds ratios were calculated based on the multivariate logistic regression for correlation of each virus and age groups.
The most frequently detected age group of each respiratory viruses.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
4. DISCUSSION
ARI is one of the most ubiquitous human diseases and the major etiology is respiratory virus infection. The KINRESS was established in 2009 immediately following a pandemic to investigate the community‐based prevalence of respiratory viruses in Korea. In this study, we used three years of surveillance data to determine the current status of ARIs in Korea with respect to the prevalence patterns, clinical characteristics, seasonality, age group distribution, and cases of multiple infections for 15 types of respiratory viruses detected from 2013 to 2015. Of the 36 915 specimens, 18 236 were positive for at least one respiratory virus based on real‐time RT‐PCR detection, which has been verified to show higher sensitivity and specificity than direct fluorescent antibody detection and viral isolation.10, 11 However, these results do not represent all of the known respiratory viruses responsible for ARI, moreover currently unknown or undetected viruses may also be circulating in Korea to cause ARI.
HRV and IFVs were the most frequently identified viruses in the three years, and the detection rates of other viruses ranged from 3.6% to 7.5%, except for HBoV and HMPV, which showed positive rates below 2%. Our results are similar to previous studies.12, 13 Most of the identified respiratory viruses in this study caused the typical clinical symptoms of ARI. In contrast to other viral infections, IFVs infection resulted in apparent ILI symptoms, including sore throat, chills, headache, and myalgia, as described previously.14
Analysis of the seasonal prevalence patterns of each respiratory virus is useful for improving diagnosis and developing effective prevention and quarantine strategies. Similar to previous reports, most of the viruses showed seasonality except for HRV and HAdV, which occurred throughout the year.2 IFV type A, including the strains H1N1 pdm09 and H3N2, and IFV type B were most prevalent between December and April, and the incidence of type B increased followed by type A. Previous studies have suggested that HBoV infections occur during the winter and increase in the spring and summer.15 However, HBoV mainly detected from April to July, similar to another study reported in Korea.11 This suggests that HBoV may show a regionally specific prevalence pattern. During the study period, cases of HMPV detection were relatively rare throughout the year, but were mainly identified in the spring with a high peak in April 2014. Which is similar to other reports and HRSVs were mainly detected in the fall and early winter before the IFVs epidemic.16, 17, 18 HPIVs showed different prevalence and seasonality patterns depending on the subtype. HPIV 1 circulated throughout the year and HPIV 3 was prevalent between spring and summer. However, HPIV 2 was rarely detected throughout the year and did not show an annual seasonal pattern, consistent with reports from China and Japan.19, 20 Although the incidence of HCoV‐OC43 and HCoV‐229E is higher in the winter in temperate regions, the trend for HCoV‐NL63 prevalence also shows geographical variation; for example, it mainly occurs in the winter in the Netherlands but is predominant in the spring and summer in Hong Kong.21, 22 In this study, HCoV‐NL63 was mainly detected in the winter with a maximum peak in 2014 and the highest HCoV‐OC43 incidence was showed tended to be higher in winter in 2013. However, the seasonality of HCoV‐229E could not be determined because it was rarely detected during the observation period. Therefore, these findings suggest that continuous surveillance is needed to further investigate the seasonal trends in the relative occurrence of various coronaviruses.
Most of surveillance studies of infections with respiratory viruses, including HRSVs, HPIVs, HAdV, HBoV, and HMPV, have shown a predominance in infants and young children,15, 17, 19 which was confirmed in the present study. In line with previous studies, the rate of detection for HAdV was highest in young children under the age of five and peaked between the 1 and 5‐year age group; HCoVs, HRV, and IFVs were detected across all age groups and HCoVs were frequently detected in elderly adults.4, 8, 16, 23 IFV infection was predominant detected in children over 6 years old and peaked in the 6‐19‐year‐old group, indicating a higher risk of infection in schools.The prevalence of respiratory virus co‐detection in infants and young children, in whom viral shedding in the respiratory tract is usually high, has been reported to be higher than that in adults,12, 24, 25 although the clinical symptoms associated with cases of co‐detection are currently unclear. Consistently, co‐detection was more frequent in young children and adolescents in this study. Despite the low HBoV detection rate, it showed a high (53.3%) co‐infection rate compared to that of the other viral agents, which is also in accordance with other studies.11, 26 This result is probably attributed to its long shedding period of HBoV containing a DNA genome. In some studies, co‐infections of HRSV and other viruses were found at a relatively higher incidence of nearly 40%, and multi‐infection cases with or without clinical differences were associated with a longer duration of hospitalization compared to cases of single infection.24, 25 However, the co‐detection rate for HRSV was approximately 20% in this study, its clinical relevance is uncertain. IFVs showed a relatively low co‐detection rate, which might be associated with its distinct seasonal prevalence and age distribution from those of the other viruses.
Taken together, these results should be useful for predicting future trends and could help to prevent outbreaks. However, long‐term surveillance studies are needed to conduct a more in‐depth analysis of the relationship between the epidemiology of respiratory virus infections and typical manifestations of known clinical outcomes, which would be helpful for the management of patients with ARIs.
CONFLICTS OF INTEREST
All authors declare no conflict of interest relevant to this study.
ACKNOWLEDGMENTS
This study was supported by a Korea Centers for Disease Control and Prevention fund (4851‐304‐210‐13).
Kim J‐M, Jung H‐D, Cheong H‐M, et al. Nation‐wide surveillance of human acute respiratory virus infections between 2013 and 2015 in Korea. J Med Virol. 2018;90:1177–1183. 10.1002/jmv.25069
REFERENCES
- 1. Williams BG, Gouws E, Boschi‐Pinto C, Bryce J, Dye C. Estimates of world‐wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002;2:25–32. [DOI] [PubMed] [Google Scholar]
- 2. Druce J, Tran T, Kelly H, et al. Laboratory diagnosis and surveillance of human respiratory viruses by PCR in Victoria, Australia, 2002‐2003. J Med Virol. 2005;75:122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pierangeli A, Gentile M, Di Marco P, et al. Detection and typing by molecular techniques of respiratory viruses in children hospitalized for acute respiratory infection in Rome, Italy. J Med Virol. 2007;79:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ren L, Gonzalez R, Wang Z, et al. Prevalence of human respiratory viruses in adults with acute respiratory tract infections in Beijing, 2005–2007. Clin Microbiol Infect. 2009;15:1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fry AM, Lu X, Chittaganpitch M, et al. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis. 2007;195:1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koetz A, Nilsson P, Linden M, van der Hoek L, Ripa T. Detection of human coronavirus NL63, human metapneumovirus and respiratory syncytial virus in children with respiratory tract infections in south‐west Sweden. Clin Microbiol Infect. 2006;12:1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1978. [DOI] [PubMed] [Google Scholar]
- 8. Lau SK, Woo PC, Yip CC, et al. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol. 2006;44:2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Frouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. [DOI] [PubMed] [Google Scholar]
- 10. Jin Y, Zhang R, Xie Z, et al. Newly identified respiratory viruses associated with acute lower respiratory tract infections in children in Lanzou, China, from 2006 to 2009. Clin Microbiol Infect. 2012;18:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peltola V, Waris M, Kainulainen L, Kero J, Ruuskanen O. Virus shedding after human rhinovirus infection in children, adults and patients with hypogammaglobulinaemia. Clin Microbiol Infect. 2013;19:E322–E327. [DOI] [PubMed] [Google Scholar]
- 12. Bautista E, Chotpitayasunondh T, Gao Z, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–1719. [DOI] [PubMed] [Google Scholar]
- 13. Yu X, Lu R, Wang Z, et al. Etiology and clinical characterization of respiratory virus infections in adult patients attending an emergency department in Beijing. PLoS ONE. 2012;7:e32174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral pneumonia. Lancet. 2011;377:1264–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arnold JC, Singh KK, Spector SA, Sawyer MH. Human bocavirus: prevalence and clinical spectrum at a children's hospital. Clin Infect Dis. 2006;43:283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim YK, Kim JW, Wee YS, Yoo EG, Han MY. Clinical features of human metapneumovirus and respiratory syncytial virus infection in hospitalized children. Pediatr Allergy Respir Dis. 2009;19:12–19. [Google Scholar]
- 17. Manoha C, Espinosa S, Aho SL, Huet F, Pothier P. Epidemiological and clinical features of hMPV, RSV and RVs infections in young children. J Clin Virol. 2007;38:221–226. [DOI] [PubMed] [Google Scholar]
- 18. Bharaj P, Sullender WM, Kabra SK, et al. Respiratory viral infections detected by multiplex PCR among pediatric patients with lower respiratory tract infections seen at an urban hospital in Delhi from 2005 to 2007. Virol J. 2009;6:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu WK, Liu Q, Chen DH, et al. Epidemiology and clinical presentation of the four human parainfluenza virus types. BMC Infect Dis. 2013;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yano T, Fukuta M, Maeda C, et al. Epidemiological investigation and seroprevalence of human parainfluenza virus in Mie prefecture in Japan during 2009‐2013. Jpn J Infect Dis. 2014;67:506–508. [PubMed] [Google Scholar]
- 21. Chiu SS, Chan KH, Chu KW, et al. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin Infect Dis. 2005;40:1721–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Hoek L, Pyrc K, Jebbink MF, et al. Identification of a new human coronavirus. Nat Med. 2004;10:368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Falsey AR, Walsh EE, Hayden FG. Rhinovirus and coronavirus infection‐associated hospitalizations among older adults. J Infect Dis. 2002;185:1338–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Calvo C, Garcia‐Garcia ML, Blanco C, et al. Multiple simultaneous viral infections in infants with acute respiratory tract infections in Spain. J Clin Virol. 2008;42:268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nascimento MS, Souza AV, Ferreira AV, Rodrigues JC, Abramovici S, Silva Filho LV. High rate of viral identification and coinfections in infants with acute bronchiolitis. Clinics. 2010;65:1133–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seo YB, Song JY, Choi MJ, et al. Etiology and clinical outcomes of acute respiratory virus infection in hospitalized adults. Infect Chemother. 2014;46:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]


