Abstract
Background
Schistosomiasis causes liver and intestinal damage and can be very debilitating. The pairing of a male worm with a female worm residing in the gynaecophoral canal of male plays a critical role in the development of female parasite. Because the male specific gynaecophoral canal protein of Schistosoma japonicum (SjGCP) is found in significant quantities in the adult female worm after pairing, it could play an important role in parasite pairing.
Methods
In the present study, three small interfering (si)RNA duplexes targeting the SjGCP gene were designed, synthesized and the silencing effects were evaluated in vitro as well as in mice infected with S. japonicum in vivo.
Results
In vitro studies using semi‐quantitative reverse transcriptase‐polymerase chain reaction (RT‐PCR) and real‐time RT‐PCR revealed the reduction of SjGCP at the transcript level. Similarly, western blotting and immunofluorescence studies showed its reduction at the protein level after treatment of parasites with siRNAs. At a concentration of 200 nm, two siRNAs totally abolished the parasite pairing. To evaluate such a pairing inhibitory effect in vivo, mice infected with S. japonicum were treated with siRNA and both parasite pairing and burden were evaluated. In vivo tests confirmed the in vitro silencing effect of SjGCP siRNA and revealed that the systemic delivery of siRNA significantly inhibited early parasite pairing and the associated burden.
Conclusions
Our preliminary results demonstrated that the SjGCP plays an important role in pairing and subsequent development in S. japonicum, and its silencing might have potential as a therapeutic approach for controlling schistosomiasis. Copyright © 2009 John Wiley & Sons, Ltd.
Keywords: gynaecophoral canal protein, pairing, RNA interference, Schistosoma japonicum, schistosomiasis
Introduction
In schistosomiasis, a large number of schistosome eggs is retained in the final host liver, eliciting inflammatory immune responses and the subsequent formation of granuloma and fibrosis 1. After transmission of these parasites in the human host, male–female pairing is the key event for the development of female parasites. Female schistosomes from single sex infection are stunted in size and are sexually immature, and their successful development depends on their pairing with a male worm inside the gynaecophoral canal of the male worm 2, 3. When paired female worms are separated from male worms, they stop laying eggs and regress to an immature state. However, when such immature females are allowed to couple, they maturate again 2, 3, 4, 5, 6, suggesting a specific interaction between male and female worms during such pairing, leading to the development of mature female worms. Different factors have been proposed for such sexual maturation. They include physical contact, nutrition 5, 7, 8, chemical stimuli 9, 10, 11 and some polypeptides and proteins 8, 12, 13, 14. In addition, studies on differently expressed genes and proteins between male and female worm 3, 15, 16, 17, 18, 19 also suggested the presence of a development signaling from males leading to the direct or indirect activation of female‐specific gene expression 4, 12, 20, 21, 22, 23, 24, 25, 26, 27.
The gynaecophoral canal protein, a 80‐kDa cell surface glycoprotein in the gynaecophoral canal of male schistosomes, is found in a low amount in unmated male worms 28. However, this amount is significantly increased post male–female pairing 29. A wide distribution of SjGCP is also found on the surface of adult female worms after pairing. Additionally, the maturation of schistosome vitelline glands and the ovary is limited to the regions of the female worm elapsed within the gynaecophoral canal of the adult male 24. Therefore, a strong maturational signal is likely to be associated with the male–female pairing.
RNA interference (RNAi), a mechanism by which gene‐specific double strand RNA triggers degradation of a homologous mRNA transcript 30, has been described in organisms of diverse phylogeny. Within the platyhelminthes, RNAi has been described in human parasitic worms, including schistosoma 31, 32, 33, 34, and a particularly important gene in RNAi, the Dicer, has been characterized by bioinformatics and the real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR) 32. In the present study, we designed small interfering (si)RNA duplexes targeting the SjGCP gene to evaluate the role of SjGCP on male–female pairing in Schistosoma japonicum in vitro culture as well as in mice infected with S. japonicum.
Materials and methods
Parasites
All animal care and procedures were conducted according to the guidelines for animal use in toxicology (Society of Toxicology USP, 1989). The study protocol was approved by the Animal Care and Use Committee of the Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences. The life cycle of S. japonicum (Anhui isolation) was maintained in New Zealand rabbits and Oncomelania hapensis in the Shanghai Veterinary Research Institute of China. Rabbits were infected with approximately 6000 cercariaes and the parasites were aseptically perfused at 12 days post‐infection. Isolated parasites were cultured in RPMI 1640 medium with 10% rabbit serum (Invitrogen, Carlsbad, CA, USA) as well as siRNA at 37 °C in an atmosphere of 5% CO2, 95% air for 3 and 7 days, respectively. Next, silencing effects were determined using PCR assay, western blotting and immunofluorescence.
siRNAs
The gynaecophoral canal protein gene of S. japonicum (GenBank accession number: AF519183) was selected for silencing. One unmodified siRNA targeting severe acute respiratory syndrome (SARS) virus and three unmodified siRNAs (s1, s2 and s3) targeting SjGCP were chemically synthesized at the Shanghai Institute of Biochemistry and Cell Biology (Table 1). Upon in vitro anneal, those siRNAs were used for in vitro studies. 2′‐O‐methyl sugar modified and unmodified s1 siRNA and SARS siRNA were chemically synthesized in Genepharma (Shanghai, China) and used for the administration of mice infected with S. japonicum after in vitro annealing.
Table 1.
Sequences of siRNAs and primers
| Name | Sequence | Targeting regions of SjGCP/proposed | ||
|---|---|---|---|---|
| s1siRNA | Sense | 5′‐GUGGUGGUCAACAUAUUCAdTdT‐3′ | 1309–1327 | |
| Antisense | 5′‐UGAAUAUGUUGACCACCACdTdT‐3′ | |||
| S2 siRNA | Sense | 5′‐GCAUUAGAAACGCCGAGAAdTdT‐3′ | 447–465 | |
| Antisense | 5′‐UUCUCGGCGUUUCUAGUACdTdT‐3′ | |||
| S3 siRNA | Sense | 5′‐UCUACAUGCACGUGACGGUdTdT‐3′ | 2038–2056 | |
| Antisense | 5′‐ACCGUCACGUGCAUGUAGAdTdT‐3′ | |||
| Irrelevant siRNA | Sense | 5′‐UUGCGAAUGGCCGGACACUCCdTdT‐3′ | No | |
| Antisense | 5′‐GGAGUGUCCGGCCAUUCGCAAdTdT‐3′ | |||
| Primers of SjGCP | Forward | 5′‐GGATCCAAGAGCTACACAGACAACAATT‐3′ | Semi‐Q reactio | RT‐PCR |
| Reverse | 5′‐GACTCAATAAGTGTAACCGTTGTTTCAC‐3′ | |||
| Primers of β tubulin | Forward | 5′‐AGGCGGGACAGTGTGGTAAT‐3′ | Semi‐Q reactio | RT‐PCR |
| Reverse | 5′‐TTGGAGAAGGAACTACTGAA‐3′ | |||
| Primers of SjGCP | Forward | 5′‐TAACTCGGGCATCATACATAC‐3′ | Real‐time reactio | RT‐PCR |
| Reverse | 5′‐TTGACTTGGTACAATCACAGTGT‐3′ | |||
| Primers of α tubulin | Forward | 5′‐CTGATT TTCCATTCGTTTG‐3′ | Real‐time reactio | RT‐PCR |
| Reverse | 5′‐GTTGTCTACCATGAAGGCA‐3′ | |||
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Treatment of parasites with siRNAs in vitro culture
Parasites obtained from rabbit at 12 days post‐infection were treated with siRNAs (SARS, s1, s2, and s3; final concentration 50 nm or 200 nm) in RPMI 1640 medium (Invitrogen) containing 10% rabbit serum (Invitrogen) at 37 °C in an atmosphere of 5% CO2, 95% air for 3 or 7 days in 12‐well culture clusters (100 ± 10 parasites/well) and then we proceeded to observe the silencing effect on male–female pairing in vitro and examine SjGCP silencing by semi‐quantitative real‐time RT‐PCR, western blotting and immunofluorescence studies.
Evaluation of siRNA transfection efficiency in vitro cultured parasites
Fluorescently‐labeled SjGCP siRNA was chemically synthesized in Genepharma (Shanghai, China) and used for treatment of parasites at final concentration of 200 nm after in vitro anneal. At 3 h post‐treatment, parasites were collected and then washed thrice with phosphate‐buffered saline (PBS, pH = 7.4). Whole‐mount parasites were examined using a Zeiss confocal microscope (Zeiss, New York, NY, USA). Autofluorescences in the parasites examined were removed by using the parasites treated with unlabeled siRNA as control to set the parameters.
Semi‐Q RT‐PCR analysis
At 3 or 7 days post‐siRNA treatment, total RNA from parasites was isolated using Trizol (Sangon, Shanghai, China) following the manufacturer's instruction and semi‐Q RT‐PCR was performed using the access RT‐PCR system (Promega, Madison, WI, USA). The total reaction volume of 50 µl containing total RNA (1 µg), 10 µl of 5 × reaction buffer, 1 µl of dNTP (10 mm), 2 µl MgSO4 (25 mm) and primers of SjGCP (10 µM) were processed at 37 °C for 5 min and 48 °C for 45 min, followed by 32 cycles at 94 °C for 1 min, 58 °C for 1 min and 68 °C for 1 min 30 s. Beta tubulin was used as an internal control for normalization. Primers used are listed in Table 1. Thirty‐two cycles were performed to remain in the linear range of PCR. PCR products were size‐separated on a 1.5% agarose gel, treated with ethidium bromide staining and visualized using electrophoresis image analysis system (Furi, Shanghai, China). Bands in the gel were also quantified using Smartview analysis software (Furi, Shanghai, China) and their volume/intensity integration was computed using Microsoft Excel 2000 (Microsoft Corp., Redmond, CA, USA).
Real‐time RT‐PCR analysis
At 7 days post‐SjGCP s1 siRNA treatment, total RNA from parasites was isolated using Trizol (Sangon) following the manufacturer's instruction and real‐time RT‐PCR was performed. Briefly, SjGCP cDNA was synthesized using 1 µg of total RNA. RNA was added to a reverse transcript transcription reaction containing multiScribe reverse transcriptase reagent and random hexamers (Applied Biosystems, Foster City, CA, USA), incubated at 25 °C for 10 min, 48 °C for 30 min and 95 °C for 5 min. Real‐time PCR was performed using 1 µl of cDNA in a final volume of 25 µl containing 5 µl of 5 × reaction buffer (TaKaRa, Dalian, China), 0.75 µl of dNTP (10 mm) (TaKaRa), 0.3 µl of MgSO4 (250 mm), 1 µl of 25 × SYBR Green I (TaKaRa), 0.25 µl of HS‐Ex‐taq (2.5 U) (TaKaRa) and 0.5 µl of primers specific for SjGCP (10 µ m) in Light cycler 480 (Roche, Basel, Switzerland) using the thermal cycling profile: 95 °C for 1 min 30 s, followed by 40 cycles of amplification (95 °C for 5 s, 56 °C for 30 s, 75 °C for 6 s). Alpha tubulin was used as an internal control for normalization. The primers used are listed in Table 1.
Western blotting
At 3 days post‐siRNA treatment, soluble proteins of parasites were extracted by Tris buffer (pH = 7.8), quantified by the Bradford method. Next, proteins were run in 10% sodium dodecyl‐polyacrylamide gel electrophoresis and electrotransferred onto polyvinylidene difluoride membranes (Sino‐American Biotechnology, Shanghai, China). Nonspecific protein–protein interactions were blocked using 5% nonfat dry milk in PBS (pH = 7.4) containing 0.1% Tween 20 (Sigma, St Louis, MO, USA). The membrane was incubated for 1 h at room temperature in primary antibodies: rabbit anti‐SjGCP (Shanghai Veterinary Research Institute, Shanghai, China) and anti‐alpha tubulin (Beijing Zhongshan Biotechnology, Beijing, China) diluted 1 : 100 in blocking buffer, and washed five times for 5 min each in 0.1% Tween‐20 PBS. The secondary antibodies (Kexin Bioscience, Beijing, China) were horseradish peroxidase conjugated goat anti‐[rabbit immunoglobulin (Ig)G]. They were diluted to 1 : 1000 in PBS, and incubated with the membrane for 1 h. The membrane was developed with 0.05% diaminobenzoic acid.
Immunofluorescence patterns in gynaecophoral canal
At 7 days post‐siRNA treatment, male schistosomes were analysed for immunofluorescence. Briefly, parasites were first fixed in ice‐cold acetone for 5 min, dried, washed thrice with PBS (pH = 7.4) and incubated for 30 min in PBS containing 1% goat serum for 30 min. Parasites were then incubated in rabbit anti‐SjGCP antibodies at 1 : 10 dilution for 1 h at room temperature and washed thrice in PBS for 10 min. A 1 : 300 dilution of fluorescence‐conjugated goat anti‐rabbit IgG was then added and incubated for 30 min. After multiple washings as described above, the parasites were mounted on slides that were covered with 90% glycerol, PBS and 2% 1,4‐ diazabicyclo (2,2,2) octane and examined by fluorescence microscopy.
Effect of SjGCP silencing on male–female pairing
At 7 days post‐siRNA treatment, schistosomes were analysed for the RNAi effect on pairing by microscopical observation of the paired parasites. To eliminate other factors that have influenced on pairing, pairing was retested in three independent experiments.
Development of schistosomiasis disease model
Four‐ to 6‐week‐old male Balb/c mice (mean weight 25 ± 2 g) were purchased from the Shanghai Center for Experimental Animals and randomly divided into three groups: 19 days, 28 days and 32 days groups. Each group was subdivided into control, irrelevant siRNA treatment and SjGCP s1 siRNA treatment groups. Each mouse was challenged with 100 ± 10 normal Schistosoma japonicum cercarie obtained from snails by abdominal skin penetration.
SjGCP s1 siRNA treatment in infected mice with S. japonicum
Starting at 11 days post‐infection, each mouse in the control, irrelevant siRNA and SjGCP s1 siRNA subgroups of three groups (19, 28 and 32 days) was injected via the tail vein with 0.1 ml of PBS, 400 µg/ml of irreverent siRNA in PBS and 400 µg/ml of SjGCP s1 siRNA in PBS, respectively. Mice in the 19 days group were injected at 11, 12, 13, 14, 15, 16, 17 and 18 days post‐infection. At 14 and 15 days, the doses were doubled. Similarly, mice in the 28 and 32 days groups were injected the same dose at 11, 14, 17, 20, 23 and 26 days post‐infection.
Evaluation of SjGCP silencing in infected mice with S. japonicum
At 19, 28 or 32 days post‐infection, the parasites in each mouse of three groups were gently perfused using sterile PBS and the number of single and paired parasites was microscopically counted. Parasites isolated from the 19 days group were processed for semi‐Q RT‐PCR and immunofluorescence analysis. However, parasites from the 32 days group were processed for real‐time RT‐PCR and western blotting using the methods described above. The percentages of reduction or pairing inhibition were calculated by the formula:
Where Y is percentages of reduction (pairing inhibition); a is average number of worms (percent pairing) in siRNA treated groups; b is average number of worms (percent pairing) in untreated groups; c is average number of worms (percent pairing) in irrelevant siRNA treated groups.
Statistical analysis
Statistical analysis of data was performed to compare the differences among each group (SjGCP siRNA versus untreated and SjGCP siRNA versus irrelevant) using Student's t‐test.
Results
siRNAs transfection efficiency
siRNA transfection efficiency in parasites was determined by soaking the schistosomes with fluorescent siRNAs. As shown in Figure 1, at 3 h post‐treatment, there were strong fluorescence signals in the parasites, particularly in oral and ventral acetabular glands (Figure 1c).
Figure 1.
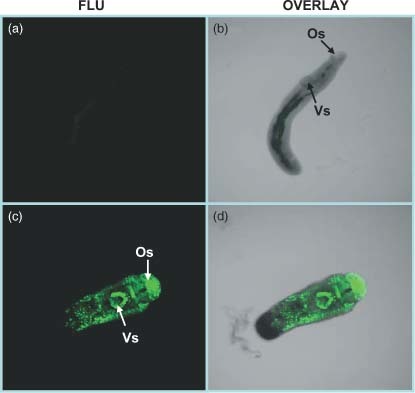
Soaking the parasite with fluorescent siRNA. Parasites were cultured for 3 h in culture medium containing unlabeled siRNA and fluoresceine‐labeled siRNA at a final concentration of 200 nM. Whole‐mount parasites treated with unlabeled siRNA (a, b) and fluoresceine‐labeled (c, d) were examined using a Zeiss confocal microscope. The parasite treated with unlabeled siRNA (a) was first used to adjust the parameters to remove autofluorescence. Os, oral sucker; Vs, ventral sucker
Effect of in vitro RNAi on SjGCP at the transcript level
As shown in Figure 2, SjGCP siRNA duplexes showed varying degrees of SjGCP silencing efficiency, in the range 5–84% (Figure 2). s1 siRNA produced a 20% and 84% reduction and s3 siRNA produced a 34% and 84% reduction at 50 nm and 200 nm, respectively, at 3 days post‐treatment. By contrast, the effect of s2 siRNA was dose‐independent and produced a 74% reduction at the transcript level (Figures 2a and 2b). Similarly, SjGCP reduction at 7 days post‐siRNA treatment was in the range 5–67% at 50 nm and 200 nm siRNA concentrations, respectively (Figure 2c and 2d). However, the irrelevant siRNA (SARS siRNA) treatment did not significantly alter the levels of SjGCP transcript. Real‐time RT‐PCR further confirmed that s1 siRNA led to a 38–72% SjGCP reduction at the transcript level (Figure 2e). The best silencing effect was obtained with s1 siRNA duplex at 7 days post‐treatment.
Figure 2.
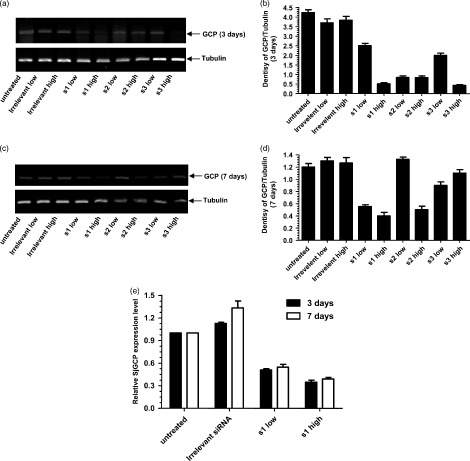
Effect of in vitro RNAi on SjGCP at the transcript level. (a) Semi‐Q RT‐PCR analysis at 3 days post‐treatment. (b) Image from (a) analyzed by Smartview software. Each value in the column is the ratio of the optical density of SjGCP and beta tubulin. (c) Semi‐Q RT‐PCR analysis of at 7 days post‐treatment. (d) Image from (c) analyzed by Smartview software. (e) Real‐time RT‐PCR analysis of SjGCP s1 siRNA at 3 and 7 days post‐treatment. Data are expressed as the mean ± SD of triplicate experiments
Effect of in vitro siRNA silencing on SjGCP at the protein level
Because siRNA is presumed to act directly on the transcript of targeted gene, the effect of siRNA silencing on SjGCP at the protein level was also examined by western blotting. As shown in Figure 3a, SjGCP protein was significantly inhibited in parasites at 3 days post‐siRNA treatment. We also evaluated SjGCP siRNA silencing at 7 days post‐siRNA treatment at the protein level, and, unlike SjGCP inhibition at the transcript level, it was not significantly different between different SjGCP siRNA treatments (data not shown).
Figure 3.
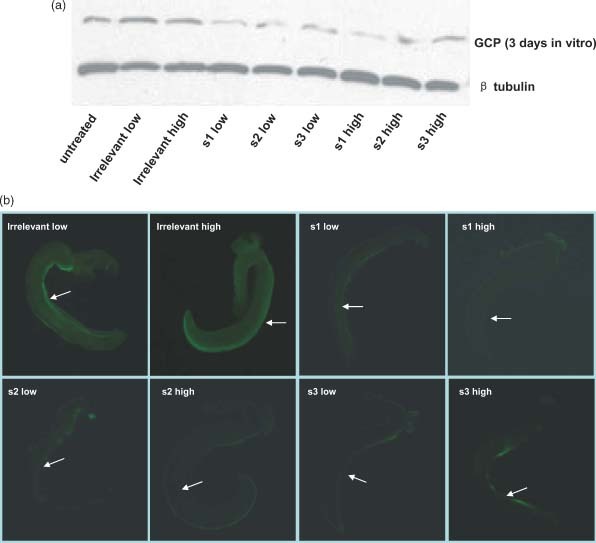
Effect of RNAi on SjGCP at the protein level. (a) Western blot analysis at 3 days post‐treatment. (b) Immunofluorescence patterns in the male gynaecophoral canal at 7 days post‐treatment. The arrow shows the side of the gynaecophoral canal in schistosomes; data are the representative results of 20 male worms examined in each treatment
Immunofluorescence patterns in gynaecophoral canal in vitro culture parasites
Immunofluorescence, which is a sensitive technique for detecting protein expression, was used to examine the level of SjGCP expression in SjGCP siRNA‐treated and control parasites at 7 days post‐treatment. As shown in Figure 3b, compared to control or parasites treated with irrelevant siRNA, SjGCP siRNA treatment resulted in a decrease of the fluorescence signal in the segment of the gynaecophoral canal. Overall, s1 siRNA demonstrated the best silencing effect among three SjGCP siRNAs.
Effect of SjGCP silencing at transcript and protein levels in parasites collected from mice infected with S. japonicum and then treated with SjGCP s1 siRNA
As shown in Figure 4, at 8 days post‐s1 siRNA injection (19 days group) there was an approximately 90% reduction in the transcript level as determined by semi‐Q RT‐PCR analysis (Figure 4a). Similarly, immunofluorescence analysis also showed significant suppression of SjGCP protein in this group (Figure 4b). The SjGCP silencing effect of s1 siRNA on parasites isolated from the 32 days group mice is shown in Figures 4c and 4d, as determined by real‐time RT‐PCR and western blotting, respectively. The injection of 2′‐O‐methyl sugar modified SjGCP s1 siRNA led a significant reduction in both SjGCP transcript (64%) and protein levels.
Figure 4.
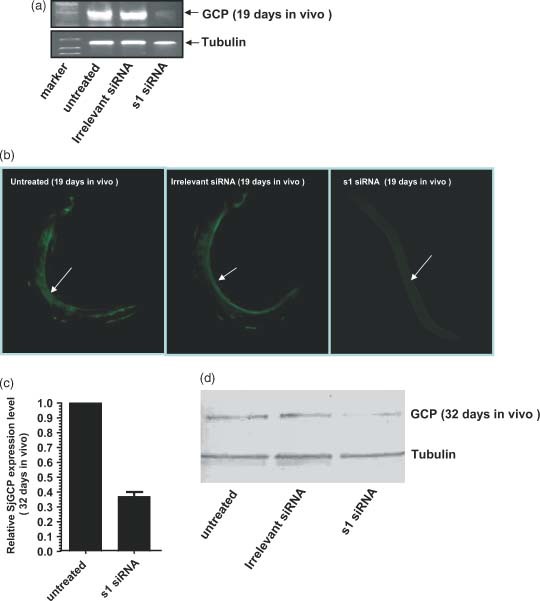
RNAi effect of SjGCP s1 siRNA at the transcript and protein levels in parasites isolated from mice infected with S. japonicum that were administered s1 siRNA. (a) Semi‐Q RT‐PCR analysis at 19 days post‐infection. (b) Immunofluorescence patterns in the male gynaecophoral canal at 19 days post‐infection, where the arrow shows the side of the gynaecophoral canal of schistosomes; data are the representative results obtained from ten male worms examined in each treatment. (c) Real‐time RT‐PCR analysis at 32 days post‐infection; data are expressed as the mean ± SD of triplicate experiments. (d) Western blot analysis at 32 days post‐infection
Effect of SjGCP silencing on pairing in vitro culture and on parasite burden and pairing in infected mice
The effect of SjGCP silencing on parasite paring in vitro is shown in Table 2. A significant reduction in male–female pairing was observed by SjGCP siRNA treatment. However, such inhibition was variable for different siRNAs or their concentration (Table 2). A complete abolition of pairing was observed in parasites treated with a high concentration (200 nm) of s1 and s2 siRNA treatment at 7 days post‐treatment. Moreover, there was no other clear phenotypic alternation in cultured parasites at 7 days post‐treatment. Comparable results were also obtained in two additional independent experiments.
Table 2.
Effect of in vitro SjGCP inhibition on parasite pairing at 7 days post‐siRNA treatment
| Group | Subgroup | Number of parasites (mean ± SD) | Number of pairs (mean ± SD) | Percent inhibitiona |
|---|---|---|---|---|
| Control* | Untreated | 108 ± 10 | 12 ± 4 | 0 |
| Irrelevant, 50 nm | 99 ± 15 | 10 ± 3 | 0 | |
| Irrelevant, 200 nm | 94 ± 18 | 8 ± 3 | 0 | |
| Test | SjGCP s1, 50 nm | 105 ± 11 | 4 ± 2 | 60 |
| SjGCP s1, 200 nm | 86 ± 17 | 0 | 100 | |
| SjGCP s2, 50 nm | 101 ± 8 | 6 ± 4 | 40 | |
| SjGCP s2, 200 nm | 79 ± 20 | 0 | 100 | |
| SjGCP s3, 50 nm | 110 ± 5 | 8 ± 4 | 20 | |
| SjGCP s3, 200 nm | 104 ± 9 | 4 ± 2 | 60 |
For statistical average of the worm number/number of pairing in untreated, irrelevant 50 nm and irrelevant 200 nm was used as the control values (consideration as 100% uninhibition).
Calculated by multiplying (×100) the ratio of the average number of pairing in SjGCP siRNA‐treated groups to the control (statistical mean of the number of pairing in untreated, irrelevant 50 nm and irrelevant 200 nm siRNA‐treated) groups. The value obtained was subtracted from 100. Data are expressed as the mean ± SD of three independent experiments.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The effect on pairing and parasite burden of the systemic administration of SjGCP s1 siRNA in mice infected with S. japonicum is shown in Table 3. Consistent with the SjGCP siRNA‐induced reduction in parasite SjGCP at transcript and protein levels, s1 siRNA treatment of the infected mice resulted in a 52% and 27% reduction in pairing and parasite burden, respectively, in the 19 days groups. The use of 2′‐O‐methyl sugar‐modified s1 siRNA led to a 74%, 38% and 7.2% reduction in pairing at 19, 28 and 32 days post‐infection, respectively. By contrast, the parasite burden in the mice was reduced by 20%, 35% and 36%, respectively, at 19, 28 and 32 days post‐infection by this siRNA treatment. Moreover, the mice survival rates were unaffected (data not shown).
Table 3.
Effect of SjGCP s1 siRNA‐induced SjGCP inhibition on burden parasites and parasite pairing at 19, 28 and 32 days post‐infection in mice challenged with S. japonicum, followed by injection of siRNAs
| Group | Subgroup | Worm number | Pairing | |||
|---|---|---|---|---|---|---|
| Number of parasites (mean ± SD) | % Reduction | Number of pairs (mean ± SD) | % Pairing | % Inhibition | ||
| 19 days | Untreated (n = 7) | 37 ± 20 | 0 | 14 ± 6.8 | 76.8 ± 9.4 | 0 |
| Irrelevant (n = 7) | 34 ± 15 | 0 | 12 ± 4 | 74.3 ± 14.3 | 0 | |
| SjGCP s1 (n = 7) | 30 ± 6 | 27 | 5 ± 2 | 36.3 ± 11.4 | 52 p < 0.05 | |
| SjGCP cm s1 (n = 5) | 29 ± 5 | 20 | 3 ± 1 | 19.6 ± 5.3 | 74 p < 0.05 | |
| 28 days | Untreated (n = 8) | 46 ± 3 | 0 | 20 ± 5 | 89.4 ± 6.9 | 0 |
| Irrelevant (n = 8) | 63 ± 5 | 0 | 28 ± 5 | 88.9 ± 6.9 | 0 | |
| SjGCP cm s1 (n = 8) | 38 ± 6 | 35 p < 0.05 | 13 ± 3 | 55.47 ± 15.6 | 38 p < 0.05 | |
| 32 days | Untreated (n = 8) | 50 ± 7 | 0 | 24 ± 6 | 98.5 ± 3.3 | 0 |
| Irrelevant (n = 8) | 53 ± 11 | 0 | 26 ± 3 | 98.3 ± 2.4 | 0 | |
| SjGCP cm s1 (n = 8) | 34 ± 9 | 36 p < 0.05 | 16 ± 4 | 91.6 ± 7.7 | 7.2 p > 0.05 | |
n, number of mice per group; s1, unmodified s1 siRNA; cm s1, chemically modified s1 siRNA.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Discussion
In the present study, we selected three SjGCP siRNAs (s1, s2 and s3) based on the position of the SjGCP transcript. They silenced SjGCP expression at the transcript and protein levels in schistosomes. Pairing of s1 siRNA in the middle of SjGCP mRNA away from potential mRNA binding proteins sites could represent a possible reason for its relatively superior silencing efficacy.
We used a simple soaking method for siRNA transfection in the present study because it does not cause much stress and physical damage to the parasites. We also attempted to transfect siRNA into the parasites after complex formation with Lipofectamine (Invitrogen). However, the reagent was toxic to the cultured parasites and subsequently led to parasite death (data not shown), which was also noted by Krautz‐Peterson et al. 34. Soaking the parasites with fluorescein‐labeled siRNA for 3 h indicated that there were strong fluorescence signals in the whole parasites, particularly in oral and ventral acetabular glands (Figure 1). This observation is consistent with the results obtained by Krautz‐Peterson et al. 34, who demonstrated that siRNA in young schistosomes appeared to enter primarily into the pre‐ and post‐acetabular glands.
Although a significant SjGCP suppression was observed, the silencing effect was better for short‐term transfection (3 days versus 7 days). This may be due to: (i) the unstability of naked siRNA as noted by Krautz‐Peterson et al. 34 and (ii) the fact that target gene mRNA levels are higher to begin with 35. The appearance of some SjGCP protein in immunofluorescence and western blotting studies probably suggests incomplete SjGCP mRNA silencing, such that the unsilenced portion translated into protein. Because the SjGCP antibodies obtained from rabbit by immunizing the full length recombinant SjGCP are polyantibodies, it was not surprising to observe some weak noise in the fluorescence signal in an area other than the gynaecophoral canal (e.g. the protruding part of the schistosomes). This could also be due to direct exposure of this part to fluorescent light.
It has been reported that pairing between male and female worm in S. japonicum usually taked place after 14 days post‐infection in the final host 36. Thus, there might be incomplete pairing at 7 days post‐treatment in vitro. This could one of the possible reasons why less pair numbers were obtained than expected theoretically. This might also be due to the difference in the habitat for parasite in culture and in the live host. Furthermore, the type and concentration of nutrients in media, culturing environment and the parasite density might have had some influence in pairing in vitro. To eliminate the influence of such intangible factors and to corroborate the in vitro result, siRNAs were administered in the mice infected with S. japonicum. Pereira et al. 37 also used a similar method to deliver siRNA in the infected mice with Schistosoma mansoni to silence hypoxanthine‐guanine phosphoribosyltransferase. At 6 days post‐treatment, a significant reduction in target mRNA was also observed and the total number of parasites was reduced by approximately 27% 37. In the present study, upon systemic administration of siRNA in the infected mice with S. japonicum, reductions in parasite pairing and burden were observed. Moreover, relatively better inhibition was observed in the 19 days group by injecting 2′‐O‐methyl modified siRNAs compared to unmodified siRNAs. This could be due to 2′‐O‐methyl modified siRNAs being more stable and resistant to nuclear degradation 38. We administered a double dose at 14 and 15 days post‐infection in the 19 days groups because parasites usually initiate male–female pairing at that period of time. After applying a smaller total dose of 2′‐O‐methyl modified siRNAs, considerable reductions were also observed in the 28 days and 32 days groups. It remains to be determined in future studies how much siRNA was taken up in the parasite. We also plan to determine the effect of SjGCP silencing and siRNA dosage for achieving the best inhibition of male–female pairing in infected mice.
To date, the most acceptable hypothesis concerning the biological functions of pairing is the separation of labour in sexually dimorphic schistosomes. The male schistosome ensures the survival of females by providing physical transportation and musculature to aid feeding and other maturation factors. SjGCP contains multiple short, conserved repeat regions with a sequence similar to the developmentally regulated neural cell adhesion molecule fisciclin I 28, 35, suggesting that SjGCP may have implications for the development of schistosome. In the present study, we demonstrated that siRNA‐mediated SjGCP silencing has an influence on early male–female pairing and on parasite burden in mice infected with S. japonicum. Our results show that SjGCP silencing led to a significant inhibition of parasite pairing (52% for unmodified siRNA and 74% for 2′‐O‐methyl modified siRNAs) in mice at 19 days post‐infection. Although SjGCP has been significantly suppressed both at transcript and protein levels (Figures 4c and 4d), there was only a 7.2% pairing inhibition at 32 days post‐infection. Furthermore, the reduction of parasite burden increased to 36% instead. The significant reduction in parasite burden was also probably due to the fact that an unpairing parasite could not survive as a single adult parasite in a final host. Our results suggested that SjGCP likely plays multiple roles in the schistosome. SjGCP is likely to be involved in early male–female pairing. SjGCP also likely plays an important role in parasite development. The silencing of SjGCP could postpone parasite pairing and affect subsequent development. It will be interesting to further characterize the effect of SjGCP silencing on the development of the reproductive system, sexual maturation and/or egg production in schistosomes in future studies.
In summary, siRNAs targeting SjGCP silenced SjGCP at the transcript level as well as the protein level, eventually reducing parasite pairing and parasite burden in mice infected with S. japonicum. Our preliminary results demonstrate that the SjGCP plays important roles in parasite pairing and subsequent development in S. japonicum, and that its silencing might have potential as a therapeutic approach for controlling schistosomiasis.
Acknowledgements
We thank Mr Krishna Hari Bhandari and Dr Hong Liu for their valuable comments and help in preparing this manuscript. We also thank Mr Yaojun Shi, Mr Hao Li and Mr Ke Lu of Shanghai Veterinary Research Institute of Chinese Academy Agricultural of Sciences for their contribution to the parasite isolation. This research was supported by National Key Technology R&D Program of China (Grant no. 2006BAD06A09), National Basic Research Program of China (Grant no. 2007CB513108) and 863 High‐tech Project of China (Grant no. 2006AA10A207‐1) for J. Lin, Shanghai Program for Raising Star of China (Grant No.04QMX1462) for Z. Fu and National Natural Science Foundation of China (Grant no. 30270311) for Y. Jin.
Contributor Information
Youxin Jin, Email: yxjin@sibs.ac.cn..
Youmin Cai, Email: cheng_guofeng@yahoo.com.
References
- 1. Smithers SR, Terry RJ. The immunology of schistosomiasis. Adv Parasitol 1969; 7: 41–93. [DOI] [PubMed] [Google Scholar]
- 2. Basch PF. Schistosoma mansoni: nucleic acid synthesis in immature females from single‐sex infections, paired in vitro with intact males and male segments. Comp Biochem Physiol B 1988; 90: 389–392. [DOI] [PubMed] [Google Scholar]
- 3. Cheng GF, Lin JJ, Feng XG, et al. Proteomic analysis of differentially expressed proteins between the male and female worm of Schistosoma japonicum after pairing. Proteomics 2005; 5: 511–521. [DOI] [PubMed] [Google Scholar]
- 4. Den Hollander JE, Erasmus DA. Schistosoma mansoni: male stimulation and DNA synthesis by the female. Parasitology 1985; 91: 449–457. [DOI] [PubMed] [Google Scholar]
- 5. Gupta BC, Basch PF. The role of Schistosoma mansoni males in feeding and development of female worms. J Parasitol 1987; 73: 481–486. [PubMed] [Google Scholar]
- 6. Loverde PT, Chen L. Schistosome female reproductive development. Parasitol Today 1991; 7: 303–308. [DOI] [PubMed] [Google Scholar]
- 7. Basch PF. Why do schistosomes have separate sexes? Parasitol Today 1990; 6: 160–163. [DOI] [PubMed] [Google Scholar]
- 8. Conford EM, Huot ME. Glucose transfer from male to female schistosomes. Science 1981; 213: 1269–1271. [DOI] [PubMed] [Google Scholar]
- 9. Shaw JR, Marshall I, Erasmus DA. Schistosoma mansoni: in vitro stimulation of vitelline cell development by extracts of male worms. Exp Parasitol 1977; 42: 14–20. [DOI] [PubMed] [Google Scholar]
- 10. Silveira AM, Friche AA, Rumjanek FD. Transfer of [14C] cholesterol and its metabolites between adult male and female worms of Schistosoma mansoni . Comp Biochem Physiol B 1986; 85: 851–857. [DOI] [PubMed] [Google Scholar]
- 11. Haseeb MA. Schistosoma mansoni: females enhance [14C]‐tyrosine incorporation in males maintained in vitro. J Helminthol 1998; 72: 123–126. [DOI] [PubMed] [Google Scholar]
- 12. Atkinson KH, Atkinson BG. Biochemical basis for the continuous copulation of female Schistosoma mansoni . Nature 1980; 283: 478–479. [DOI] [PubMed] [Google Scholar]
- 13. Popiel I, Basch PF. Schistosoma mansoni: cholesterol uptake by paired and unpaired worms. Exp Parasitol 1986; 61: 343–347. [DOI] [PubMed] [Google Scholar]
- 14. Aronstein WS, Strand M. A glycoprotein antigen of Schistosoma mansoni expressed on the gynecophoral canal of mature male worms. Am J Trop Med Hyg 1985; 34: 508–512. [DOI] [PubMed] [Google Scholar]
- 15. Fitzpatrick JM, Hoffmann KF. Dioecious Schistosoma mansoni express divergent gene repertoires regulated by pairing. Int J Parasitol 2006; 36: 1081–1089. [DOI] [PubMed] [Google Scholar]
- 16. Moertel L, McManus DP, Piva TJ, et al. Oligonucleotide microarray analysis of strain‐ and gender‐associated gene expression in the human blood fluke, Schistosoma japonicum . Mol Cell Probes 2006; 20: 280–289. [DOI] [PubMed] [Google Scholar]
- 17. Fitzpatrick JM, Hirai Y, Hirai H, et al. Schistosome egg production is dependent upon the activities of two developmentally regulated tyrosinases. FASEB J 2007; 21: 823–835. [DOI] [PubMed] [Google Scholar]
- 18. Wuhrer M, Koeleman CA, Fitzpatrick JM, et al. Gender‐specific expression of complex‐type N‐glycans in schistosomes. Glycobiology 2006; 16: 991–1006. [DOI] [PubMed] [Google Scholar]
- 19. Waisberg M, Lobo FP, Cerqueira GC, et al. Microarray analysis of gene expression induced by sexual contact in Schistosoma mansoni . BMC Genomics 2007; 8: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Popiel I, Basch PF. Putative polypeptide transfer from male to female Schistosoma mansoni . Mol Biochem Parasitol 1984; 11: 179–188. [DOI] [PubMed] [Google Scholar]
- 21. Chen LL, Rekosh DM, LoVerde PT. Schistosoma mansoni p48 eggshell protein gene: characterization, developmentally regulated expression and comparison to the p14 eggshell protein gene. Mol Biochem Parasitol 1992; 52: 39–52. [DOI] [PubMed] [Google Scholar]
- 22. Gupta BC, Basch PF. Evidence for transfer of a glycoprotein from male to female Schistosoma mansoni during pairing. J Parasitol 1987; 73: 674–675. [PubMed] [Google Scholar]
- 23. Grevelding CG, Sommer G, Kunz W. Female‐specific gene expression in Schistosoma mansoni is regulated by pairing. Parasitology 1997; 115: 635–640. [DOI] [PubMed] [Google Scholar]
- 24. Popiel I, Basch PF. Reproductive development of female Schistosoma mansoni (Digenea: Schistosomatidae) following bisexual pairing of worms and worm segments. J Exp Zool 1984; 232: 141–150. [DOI] [PubMed] [Google Scholar]
- 25. Siegel DA, Tracy JW. Schistosoma mansoni: influence of the female parasite on glutathione biosynthesis in the male. Exp Parasitol 1989; 69: 116–124. [DOI] [PubMed] [Google Scholar]
- 26. Schussler P, Grevelding CG, Kunz W. Identification of Ras, MAP kinases, and a GAP protein in Schistosoma mansoni by immunoblotting and their putative involvement in male–female interaction. Parasitology 1997; 115: 629–634. [DOI] [PubMed] [Google Scholar]
- 27. Knobloch J, Kunz W, Grevelding CG. Herbimycin A suppresses mitotic activity and egg production of female Schistosoma mansoni . Int J Parasitol 2006; 36: 1261–1272. [DOI] [PubMed] [Google Scholar]
- 28. Bostic JR, Strand M. Molecular cloning of a Schistosoma mansoni protein expressed in the gynecophoral canal of male worms. Mol Biochem Parasitol 1996; 79: 79–89. [DOI] [PubMed] [Google Scholar]
- 29. Jin Y, Lin J, Cheng G, et al. Sex‐specific and stage different expression of gynecophoral canal protein gene of Schistosoma japonicum (Chinese strain). Acta Parasitol Med Entomol Sinica 2004; 11: 70–73. [Google Scholar]
- 30. Fire A, Xu S, Montgomery MK, et al. Potent and specific genetic interference by double‐stranded RNA in Caenorhabditis elegans . Nature 1998; 391: 806–811. [DOI] [PubMed] [Google Scholar]
- 31. Ndegwa D, Krautz‐Peterson G, Skelly PJ. Protocols for gene silencing in schistosomes. Exp Parasitol 2007; 117: 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krautz‐Peterson G, Skelly PJ. Schistosoma mansoni: the dicer gene and its expression. Exp Parasitol 2008; 118: 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Correnti JM, Brindley PJ, Pearce EJ. Long‐term suppression of cathepsin B levels by RNA interference retards schistosome growth. Mol Biochem Parasitol 2005; 143: 209–215. [DOI] [PubMed] [Google Scholar]
- 34. Krautz‐Peterson G, Radwanska M, Ndegwa D, et al. Optimizing gene suppression in schistosomes using RNA interference. Mol Biochem Parasitol 2007; 153: 194–202. [DOI] [PubMed] [Google Scholar]
- 35. Osman A, Niles EG, Verjovski‐Almeida S, et al. Schistosoma mansoni TGF‐beta receptor II: role in host ligand‐induced regulation of a schistosome target gene. PLoS Pathog 2006; 2: E54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mao S. The Biology of Schistosomes and the Preventiion of Schistosomasis. People Health Press: Beijing, 1990; 137–218. [Google Scholar]
- 37. Pereira TC, Pascoal VD, Marchesini RB, et al. Schistosoma mansoni: evaluation of an RNAi‐based treatment targeting HGPRTase gene. Exp Parasitol 2008; 118: 619–623. [DOI] [PubMed] [Google Scholar]
- 38. Choung S, Kim YJ, Kim S, et al. Chemical modification of siRNAs to improve serum stability without loss of efficacy. Biochem Biophys Res Commun 2006; 342: 919–927. [DOI] [PubMed] [Google Scholar]


