Abstract
Aim
Species distributions are one of the most important ways to understand how communities interact through macroecological relationships. The functional abilities of a species, such as its plasticity in various environments, can determine its distribution, species richness and beta diversity patterns. In this study, we evaluate how functional traits influence the distribution of amphibians, and hypothesize which functional traits explain the current pattern of amphibian species composition.
Location
Atlantic Forest, Brazil.
Taxon
Amphibia (Anura and Gymnophiona)
Methods
Using potential distributions of Brazilian amphibians from Atlantic Forest based on their functional traits, we analysed the influence of biotic and abiotic factors on species richness, endemism (with permutation multivariate analysis) and beta diversity components (i.e. total, turnover and nestedness dissimilarities).
Results
Environmental variables explained 59.5% of species richness, whereas functional traits explained 15.8% of species distribution (geographical species range) for Anuran and 88.8% for Gymnophiona. Body size had the strongest correlation with species distribution. Results showed that species with medium to large body size, and species that are adapted to living in open areas tended to disperse from west to east direction. Current forest changes directly affected beta diversity patterns (i.e. most species adapted to novel environments increase their ranges). Beta diversity partitioning between humid and dry forests showed decreased nestedness and increased turnover by increasing altitude in the south‐eastern region of the Atlantic Forest.
Main Conclusions
Our study shows that functional traits directly influence the ability of the species to disperse. With the alterations of the natural environment, species more apt to these alterations have dispersed or increased their distribution, which consequently changes community structure. As a result, there are nested species distribution patterns and homogenization of amphibian species composition throughout the Brazilian Atlantic Forest.
Keywords: Anura, beta diversity partitioning, conservation, functional abilities, Gymnophiona, spatial distribution
1. INTRODUCTION
Distribution patterns, dispersion processes and permanence of species are some of the most studied topics by ecologists and biogeographers. The distribution of organisms is the basis of ecological studies and can be determined by biotic and abiotic factors (Hutchinson, 1957; Soberón, 2007). For example, current patterns of species distributions are linked to historical and contemporary dispersals influenced by species characteristics and their specific functional traits (Baselga, Lobo, Svenning, Aragón, & Araújo, 2012; Carnaval, Hickerson, Haddad, Rodrigues, & Moritz, 2009; Carnaval & Moritz, 2008; Oberdorff, Hugueny, & Guegan, 1997; Ricklefs, 1987; Silva, Almeida‐Neto, & Arena, 2014; Svenning & Skov, 2007). On the other hand, habitat characteristics influence the spatial and temporal distributions of species (Campos, Trindade‐Filho, Brito, Llorente, & Solé, 2014; Ferreira, Beard, & Crump, 2016; Figueiredo, Storti, Lourenço‐de‐Moraes, Shibatta, & Anjos, 2019; Hawkins, 2001). Thus, historical dispersal can be understood using environmental data of the localities at which species have been recorded and the geographical boundaries that restrict them (Gaston, 1991).
Because ectothermic species are largely limited by climatic zones (Pfrender, Bradshaw, & Kleckner, 1998), both dispersal limitation and climate variation can be critical determinants of species ranges (Baselga et al., 2012; Lourenço‐de‐Moraes, Lansac‐Toha, et al., 2019). Furthermore, the distribution of a species is often related to species characteristics, such as body size and local abundance (Brown & Maurer, 1989; Gaston, 1990; Lawton, 1993). For example, small ectothermic species can dehydrate faster than large species (MacLean, 1985), and many are prey to vertebrates and invertebrates (Toledo, Ribeiro, & Haddad, 2007; Wells, 2007). Thus, ectotherms rely upon their morphological and physiological adaptations to succeed in surviving and dispersing. In this sense, understanding functional traits (Jiménez‐Valverde et al., 2015) may be key to understanding the potential distribution of ectotherms (Díaz et al., 2007).
Amphibian species richness is non‐random process and is often related to temperature and precipitation (Casemiro, Souza, Rangel, & Diniz‐Filho, 2007; Vasconcelos, Santos, Haddad, & Rossa‐Feres, 2010). The sites of high species richness can provide species for sites of low species richness (e.g. Baselga, 2010; Leibold & Mikkelson, 2002; Lourenço‐de‐Moraes, Malagoli, et al., 2018; Ulrich & Gotelli, 2007). Geographical distributions of amphibians are strongly affected by type of habit, as terrestrial and aquatic preferences of juveniles and adults, and their ability to disperse across the landscape (Patrick, Harper, Hunter, & Calhoun, 2008). Microclimate characteristics of habitat as forests and open areas can provide physiological and ecological constraints for many species because they influence foraging, reproduction, and survival (Huey, 1991). Such constraints strongly affect the causes and consequences of dispersal abilities as well as the nature of species interactions (McGill, Enquist, Weiher, & Westoby, 2006), including reproductive modes, activity time and antipredator mechanisms (Fahrig, 2001; Ferreira et al., 2019; Monkkonen & Reunanen, 1999). Given that short‐term impacts of habitat loss increase and with dispersal ability of amphibians (Homan, Windmiller, & Reed, 2004), there is a critical need to investigate the spatial mismatches between the distribution of species and environmental changes under functional traits approaches (Berg et al., 2010; Cushman, 2006). Forest isolation is a critical factor in biological community structure and fundamentally important in a habitat fragmentation context (Dixo, Metzger, Morgante, & Zamudio, 2009). Understanding beta diversity patterns and evaluating their different compositions (i.e. turnover or nestedness components) along a latitudinal and longitudinal gradient can be an important tool for understanding the dispersal processes of these species (Baselga, 2008, 2010).
Knowing that amphibians are dispersal limited due to their morphological, physiological and ecological characteristics (Richter‐Boix, Llorente, & Montori, 2007), we evaluated the species richness (correlated with environmental variables) and beta diversity (turnover and nestedness) of amphibians in the Atlantic Forest, while assessing their potential dispersal based on functional traits. In this context, species typical of open areas can benefit from the alteration of forests due to the increase in their habitat area; and smaller species should be more associated with areas with milder temperatures (e.g. areas of high altitude) due to lower water loss rate to the environment. In this study, we tested the hypothesis that functional traits explain the current pattern of amphibian species composition in the Atlantic Forest. Depending on the functional trait (e.g. body size and ecological specializations) the species may have more ability to disperse and increase its distribution.
2. MATERIALS AND METHODS
2.1. Study region
The Brazilian Atlantic Forest has a latitudinal range extending into both tropical and subtropical regions (Myers, Mittermeier, Mittermeier, Fonseca, & Kent, 2000). The longitudinal range extends from the coast to 1,000 km inland, and the altitudinal range extends from 0 to 2000 m a.s.l. (Cavarzere & Silveira, 2012). Originally, this biome covered around 150 million ha with a wide range of climatic belts and vegetation formations (Ribeiro, Metzger, Martensen, Ponzoni, & Hirota, 2009; Tabarelli, Pinto, Silva, Hirota, & Bede, 2005). Currently only about 12% of the original biome remains (Ribeiro et al., 2009). This biome occurs across 14 states from the south to the northeast of Brazil (Figure 1). To test the hypothesis that functional traits explain the current pattern of amphibian species composition (see Appendix S1, Figure 1.1) and understand the pattern of beta diversity in each study site, we analysed differences in species compositions (richness and endemism) and mapped out potential dispersal routes. We delimitated the study sites in relation to: (a) geomorphological barriers (see Bittencourt, Dominguez, Martin, Silva, & Medeiros, 2007; Dominguez, Bittencourt, & Martin, 1981); (b) abiotic barriers (Worldclim database; see below); (c) forest composition barriers (see Olson et al., 2001); (d) names based in political divisions; and (e) size of area.
Figure 1.
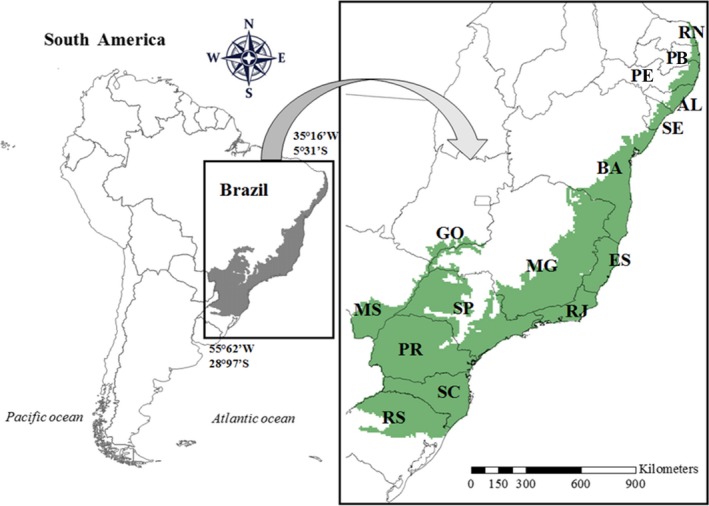
Original distribution of Brazilian Atlantic Forest hotspot (in grey) in South America. Brazilian states: AL, Alagoas; BA, Bahia; ES, Espirito Santo; GO, Goiás; MG, Minas Gerais; MS, Mato Grosso do Sul; PB, Paraíba; PE, Pernambuco; PR, Paraná; RJ, Rio de Janeiro; RN, Rio Grande do Norte; RS, Rio Grande do Sul; SC, Santa Catarina; SE, Sergipe; SP, São Paulo [Colour figure can be viewed at http://wileyonlinelibrary.com]
Given that each state has different environmental laws (e.g. IAP—Instituto Ambiental do Paraná—Paraná state, COTEC—ComissãoTécnico‐Científica do Instituto Florestal, São Paulo state, INEMA—Instituto do Meio Ambiente e Recursos Hídricos, Bahia state), we used spatial data that allow different conservation strategies at local scales (i.e. environmental state policies). Two states have all their territory included in determining the composition of species, RJ (Rio de Janeiro) and ES (Espírito Santo), due to their smaller sizes, similar forest composition and abiotic features. Four states have all their territory separated into eastern and western sections, because they are large and have different forest composition (eastern rain forest, western seasonal forest): EPR (eastern Paraná),WPR (western Paraná), ESC (eastern Santa Catarina), WSC (western Santa Catarina), ERS (eastern Rio Grande do Sul), WRS (western Rio Grande do Sul); and the ‘SMGM’ refers to four connected states in seasonal forests (includes western of São Paulo—S, north Mato Grosso do Sul—M, south Goiás—G and extreme south Minas Gerais—M); MS refers to the south‐eastern Mato Grosso do Sul. The Pernambuco, Sergipe, Ceará, Paraíba and Rio Grande do Norte states were included in region N (Northeast), due to their smaller territories inside this biome, and similar forest composition and abiotic features. We also separated two states in regions north and south, due to their large territory, and different forest composition and abiotic features—SBA (south Bahia), NBA (north Bahia), SMG (south Minas Gerais) and NMG (north Minas Gerais). In total, we assessed 16 study sites (see Figure 2).
Figure 2.
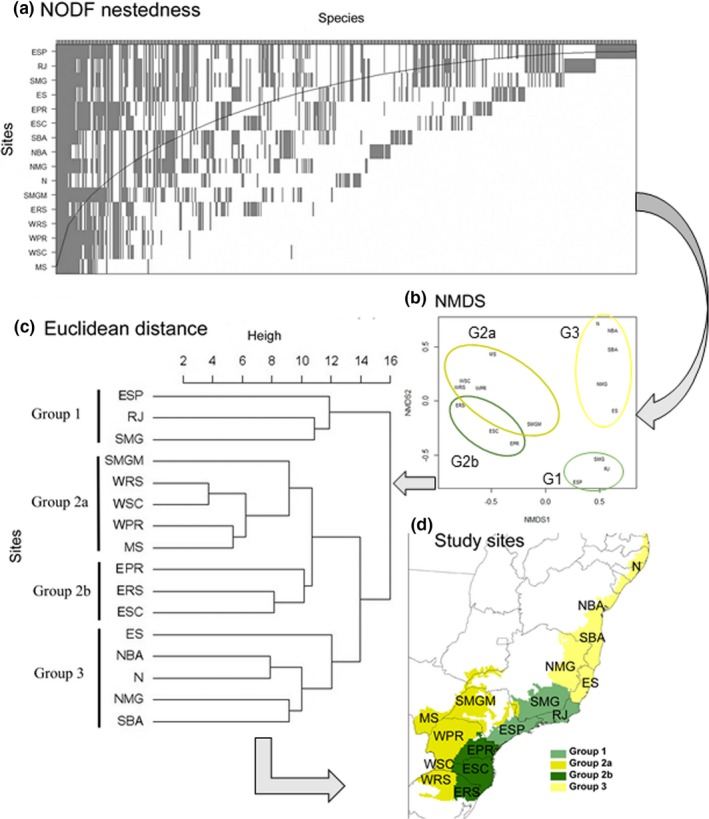
Steps designed to determine the amphibian beta diversity patterns in the Brazilian Atlantic Forest. (a) NODF for the study sites with greater species richness and subset, (b) NMDS for the species composition across the study sites, (c) NMDS clusters and (d) map showing the groups similarities among the study sites. Acronyms: RJ (Rio de Janeiro state), ES (Espírito Santo state), EPR (eastern Paraná state), WPR (western Paraná state), ESC (eastern Santa Catarina), WSC (western Santa Catarina), ERS (eastern Rio Grande do Sul state), WRS (western Rio Grande do Sul state), SMGM (western of São Paulo‐S, north Mato Grosso do Sul‐M, south Goiás‐G and extreme south Minas Gerais‐M), MS (Mato Grosso do Sul state), N (Northeast), SBA (south Bahia state), NBA (north Bahia state), SMG (south Minas Gerais) and NMG (north Minas Gerais). NMDS, nonparametric multidimensional scaling analysis [Colour figure can be viewed at http://wileyonlinelibrary.com]
2.2. Species distributional data
We included species occurrence records available through the Global Biodiversity Information Facility (GBIF: http://www.gbif.org), and added range maps of each species from the IUCN Red List of Threatened Species (IUCN, 2017: http://www.iucnredlist.org/technical-documents/spatial-data). In addition, we conducted acoustic and visual nocturnal/diurnal amphibian survey (Crump & Scott Jr., 1994; Zimmerman, 1994) in 11 Protected Areas (PAs), from southern to the northeastern Brazil (see Appendix S1, Figure 1.2).We followed Frost (2019) for the amphibian nomenclature with exception of the species synonymized as Allobates olfersioides which we consider to be distinct species (A. olfersioides, A. alagoanus and A. capixaba see Forti, Silva, & Toledo, 2017).
We used ArcGIS 10.1 software (ESRI, 2011) to build presence/absence matrices from the species distributional data by superimposing a grid system with cells of 0.1 latitude/longitude degrees, creating a network with 10,359 grid cells. We used the ‘Spatial Join’ ArcGIS toolbox to transform species' spatial occurrences in matrices, matching rows from the join features to the target features based on their relative spatial locations. Then, we combined vector files based on expert knowledge of the species' ranges and forest remnant polygons into an overall coverage for species distribution modelling. We only considered spatial occurrences by those species where the distributional data intersected at least a grid cell. We used forest remnant data to meet the habitat patch requirements based on visual interpretation at a scale of 1:50,000, delimiting more than 260,000 forest remnants with a minimum mapping area of 0.3 km2. Therefore, we considered a species present in a cell if its spatial range intersected more than 0.3 km2. We also used the ‘Count Overlapping Polygons’ ArcGIS toolbox to obtain the species richness at the spatial resolution assessed, removing all duplicate records from the analyses (i.e. repeated records of a species at a single locality).
2.3. Environmental variables
We took the mean of six environmental variables for each grid cell, which included one topographic (altitude), one biotic (tree cover [TC]) and four climatic variables (annual precipitation, mean annual temperature [MAT], annual evapotranspiration and net primary productivity [NPP]). We obtained altitude, annual precipitation and MAT from the WorldClim database at 0.05° spatial resolution (http://www.worldclim.org/). We obtained annual evapotranspiration (AET) from the Geonetwork database (http://www.fao.org/geonetwork/srv/); NPP from the Numeral Terra dynamic Simulation Group (http://www.ntsg.umt.edu/data) and TC from Global Forest Change 2000–2014 database (http://earthenginepartners.appspot.com/science-2013-global-forest/download_v1.2.html). All these variables are known to represent either potential physiological limits for amphibians or barriers to dispersal (Silva, Almeida‐Neto, Prado, Haddad, & Rossa‐Feres, 2012; Vasconcelos et al., 2010). We drew maps using the ArcGis10.1 software (ESRI, 2011).
2.4. Functional traits
We characterized the functional traits of 531 amphibian species using Haddad et al. (2013), ecological information from the IUCN database, scientific literature on the original descriptions of the species, and data from our fieldwork (see Appendix S1, Table 1.1). We set out the following functional traits: body size, reproductive mode (subtraits: direct or indirect), habitat (subtraits: forested areas, open areas and both), activity time (subtraits: nocturnal, diurnal and both), toxicity (subtraits: toxic, unpalatable or bad odour and non‐toxic) and habit (subtraits: arboreal, phytotelmata, terrestrial, cryptic, fossorial, rheophilic, semi‐aquatic and aquatic). The subtraits terrestrial (on the ground or in the leaf litter on the forest floor) and cryptic (hidden in galleries, crevices, natural or excavated holes in the ground or under the leaf litter) were included because they are important characteristics which generates different patterns of geographical dispersion. All these functional traits interact (directly or indirectly) in the ecosystem functioning (Duellman & Trueb, 1994; Haddad et al., 2013; Hocking & Babbitt, 2014; Toledo et al., 2007; Wells, 2007). See Appendix S1, Table 1.2 for more details about the specific functions and the ecosystem supporting services of each one of these traits.
2.5. Data analyses
2.5.1. Effect of environmental variables on species richness and endemic species
We evaluated the response of species richness and endemic species separately to the predictor variables: altitude (minimum/maximum elevation), mean annual precipitation (MAP), MAT, mean annual evapotranspiration, NPP and TC. We used the term endemic species for species that occur in only one of the 16 study sites (see Figure 2), and calculated endemic and richness species for each grid cell of 0.1° (10,359 cells). For these analyses, we used permutation multivariate analysis of variance (PERMANOVA), with 1,000 permutations based on a Euclidean distance matrix through the 'adonis' function of the package 'vegan' (Oksanen et al., 2013), in the R software (R Development Core Team, 2017).
2.5.2. Effects of functional trait body size in the species richness
We used simple linear models to test the relationship between body size (original body size of each species) on species richness across Atlantic Forest. For this, we calculated mean body size for each grid cell. Then, we evaluated the relationship between mean body size, and latitude, longitude and altitude. We performed these analyses using the package 'vegan' (Oksanen et al., 2013) in the R software (R Development Core Team, 2017).
2.5.3. Effects of functional traits in geographical distribution of species
First, we calculated the geographical range of each species to the predicted body size, toxicity, reproductive mode, habit, habitat and activity time for Anura and body size, reproductive mode, habitat and habit for Gymnophiona. For this, we used PERMANOVA, with 1,000 permutations.
Second, we used Boxplots (categorized—miniature <2 cm, small ≥2 and <3 cm, medium ≥3 and <8 cm, large ≥8 cm) and percentage histograms to visualize the traits that better explained species distribution. We analysed the differences between the traits separately for anurans (ANOVA) and gymnophionas (Kruskal–Wallis), depending on normality using the Shapiro‐Wilk test (parametric and nonparametric data). We performed these analyses separately due to lack of information on Gymnophiona and their different characteristics of ecological traits and body size (Haddad et al., 2013). We calculated these analyses for each grid cell, using the R packages 'vegan' (Oksanen et al., 2013), 'car' (Fox & Weisberg, 2011), 'FSA' (Ogle, 2016) and 'lattice' (Sarkar, 2008) in the R software (R Development Core Team, 2017).
2.6. Similar geographical species groups
First, we examined whether there was independence of spatial correlation of species composition among the 16 study sites (matrix of spatial data vs. matrix of species composition, Euclidean distance matrix). For this, we used Pearson correlation tests using the Mantel with 1,000 permutation (Legendre & Legendre, 1998). Second, we used a similarity measure (i.e. Euclidean distance matrix) of the 16 study sites to rank the groups of similar species composition. Ordination of the 16 study sites was based on this faunal dissimilarity matrix, which was submitted to a nonparametric multidimensional scaling analysis (NMDS, Legendre & Legendre, 1998) and the most likely solution was evaluated by Pearson correlation. The calculation of the variance is captured by a regression matrix from the original distances (Bray–Curtis) and the final array distances (Euclidean) (Figure 2b). Third, we draw a dendrogram by taking Euclidean distance as the measure of resemblance and average linkage procedure as the linkage rule (Figure 2c). We performed these tests using the package 'vegan' (Oksanen et al., 2013) in the R software (R Development Core Team, 2017).
2.7. Beta diversity measures
First, we used two complementary metrics for beta diversity analysis by considering species presence and absence. To determine if the pattern among anuran communities is nested, we calculated a nestedness metric based on overlap and decreasing fill (index NODF) of Almeida‐Neto, Guimarães, Guimarães, Loyola, and Ulrich (2008) and Ulrich, Almeida‐Neto, and Gotelli (2009) across the 16 study sites (Figure 2a). The matrix is decreasing for both columns and rows; columns ranked the areas according to their species richness and rows ranked the species from, the most frequent to the rarest. With this approach, we created and ordered study sites as major biota and its subsets biota. To perform this analysis, original matrices were submitted to 1,000 simulations. We performed this analysis for all localities separately using the package 'vegan' (Oksanen et al., 2013) in the R software (R Development Core Team, 2017).
Second, we conducted beta diversity partitioning and computed distance matrices using pairwise dissimilarities βsor (i.e. measure total beta diversity), βsim (i.e. measure spatial turnover) and βnes (i.e. measure nestedness) (Baselga, 2010). We computed these analyses among the 16 study sites to show the directions of the species distributions. We considered larger nesting values means more species similar to the area of major species richness, and larger numbers of turnover means less similar species composition. We follow the sequence provided by NODF for analysis of beta diversity partitioning. This method partitions the pairwise Sørensen dissimilarity between two communities (βsor) into two additive components accounting for species spatial turnover (βsim) and nestedness‐resultant dissimilarities (βsne). Since βsor and βsim are equal in the absence of nestedness, their difference is a net measure of the nestedness‐resultant component of beta diversity, so βsne = βsor − βsim (Baselga, 2010).
Third, to evaluate the effects of topographic variation among the 16 study sites and species composition, we ran the analyses using four approaches: (a) entire altitudinal range (0–2000 m a.s.l.); (b) 0–300 m; (c) 300–700 m and (d) 700–2000 m. We performed these tests using the package 'betapart' (Baselga & Orme, 2012) in the R software (R Development Core Team, 2017).
3. RESULTS
We found that the environmental variables explained 59.5% of the amphibian species richness across Atlantic Forest. Temperature‐MAT was the main variable (39.3%), followed by precipitation‐MAP (11.4%) and NPP (8.6%) (Appendix S2, Table 2.1). For study sites with endemic amphibian species, the environmental values explained 26% of the endemism, of which temperature was the main variable (22%), followed by altitude (2%) (Appendix S2, Table 2.2).
For Anura, changes in functional traits explained 15.8% of the species distribution (geographical species range). Habitat was responsible for about 9%, body size 3%, habit 1% and toxicity 0.8% of the variation in the species distribution. Reproductive mode and activity time traits did not show any significant relationship with species distribution (Appendix S2, Table 2.3).
For Gymnophiona, two main traits explained 88.8% of the species distribution. Habitat was responsible for about 67.4% and reproductive mode 15.7% of the variation in the species distribution patterns. The body size and habit traits did not show any relationship with species distribution (Appendix S2, Table 2.4).
For Anura and Gymnophiona, the trait body size showed low but significant correlations with species richness (r 2 = .398; p < .001), longitude (r 2 = .103; p < .001), latitude (r 2 = .025; p < .001) and altitude (r 2 = .006; p < .001). Medium to larger species are more abundant in the west region (lower longitudes) and extreme north (higher latitudes) in the Atlantic Forest (Figure 3). Medium to smaller species are more abundant in the east region (lower latitudes and higher altitudes) in the Atlantic Forest (Figure 3).
Figure 3.
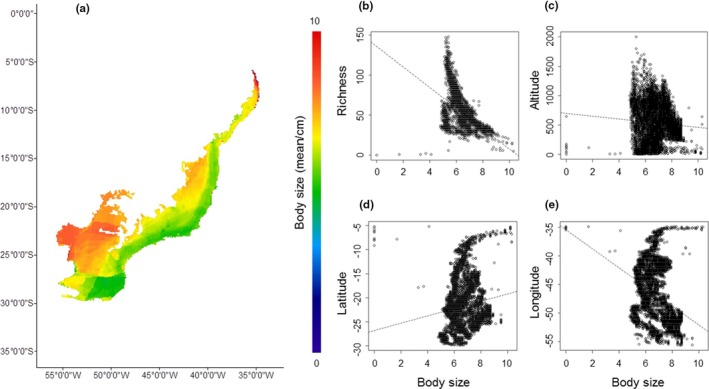
Distribution of amphibian species based in the mean of body size by grid cells (a). Linear regression of species richness (b), altitude (c), latitude (d) and longitude (e) in relation to body size [Colour figure can be viewed at http://wileyonlinelibrary.com]
For the functional subtraits, the largest distributional distances in Anura (p < .05) was found between species of open areas and species of open/forested areas, species of medium body size, terrestrial and toxic and non‐toxic species (Appendix S2, Table 2.5, Figure 2.1a and Figure 4a). The subtraits in Gymnophiona are non‐significant (p > .05) (Appendix S2, Table 2.6 and Figure 4b), but 40% of fossorial species, 35% of species of open/forested areas are distributed in more than 2,000 range cells, and 100% of viviparous species are distributed in more than 6,000 range cells (Appendix S2, Figures 2.1b and 2.2).
Figure 4.
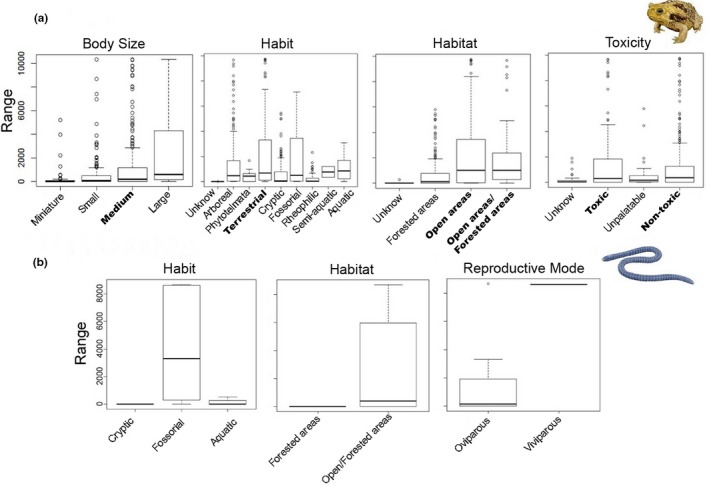
Boxplots showing the functional traits that better explain the spatial ranges (measure number cells of 10 km2) for (a) Anura and (b) Gymnophiona in the Brazilian Atlantic Forest. Bold indicate the functional subtraits that better explained the spatial ranges of species [Colour figure can be viewed at http://wileyonlinelibrary.com]
The Mantel tests indicated spatial correlation of species composition and geographical distance among the 16 study sites (r 2 = .30, p = .019). The dendrogram used the scores of the NMDS axis (7 dimensions, r 2 = .99, p > .001) and showed the presence of three groups of similar composition in the Atlantic Forest (Figure 2b). The major species richness was in group 1 (ESP, RJ and SMG), characterized by humid forests with high species richness and endemism. Group 2 was divided into two subgroups: one formed by MS, WPR, WSC, WRS and SMGM (subgroup 2a), through seasonally dry forests with low species richness and endemism, and another one formed by EPR, ESC and ERS (subgroup 2b), through humid forests with presence of Araucarias forest. Group 3 was formed by ES, NMG, SBA, NBA and N and has high temperature and low or no seasonality (Appendix S2, Figure 2.3).
Our results of the NODF values indicated significant nestedness (NODF = 38.7, p < .001), showing the sites ESP, RJ and SMG as the major biota (Figure 2a). The beta diversity partitioning revealed that the highest values were between groups 1 and 3 (βsor mean 0.684 ± 0.093) followed by the group 2a (βsor mean 0.675 ± 0.126). Among group 3, the values increase by increasing the difference in species composition according to altitude, decreasing βsim and increasing βnes; wherein the site N has highest βnes (0.281) among the species that occur 700–2000 m a.s.l., and the site SBA has highest values of βsim (0.582) among the species composition that occur in 300 m a.s.l. (Appendix S2, Table 2.7 and Figure 5).
Figure 5.
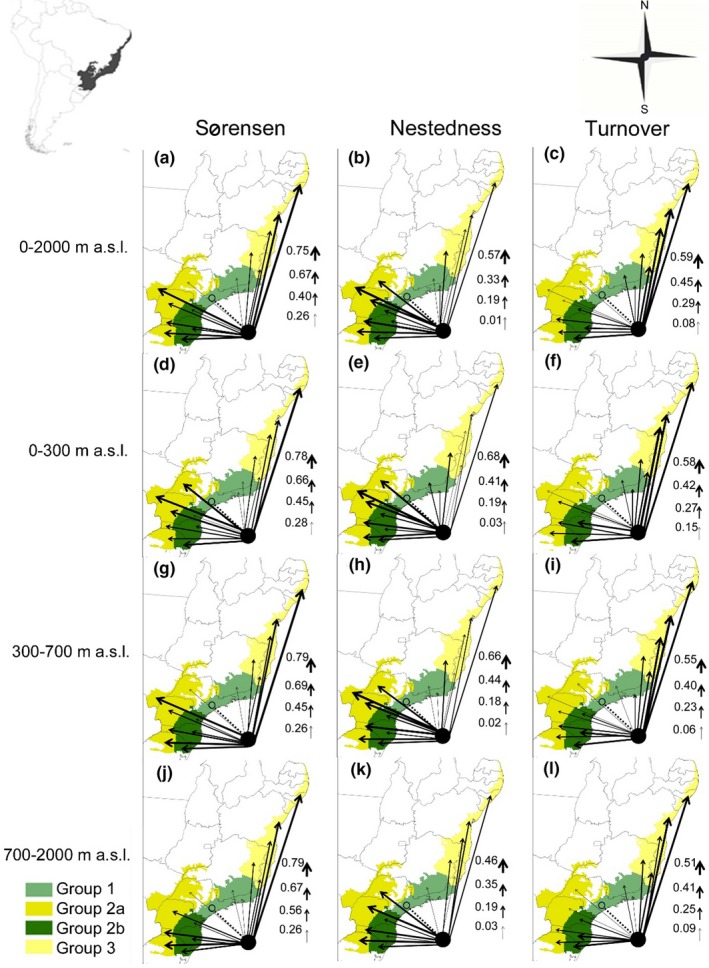
Relative partitioning of amphibian beta diversity across the groups of study sites assessed in the Brazilian Atlantic Forest. Thinner arrows indicate smaller values, thicker arrows larger values. Beta diversity values are shown from the large biota ESP (circle) to the subsets. (a–c) beta diversity values with all altitude variations (0–2000 m), (d–e) beta diversity values with 0–300 m altitude variations, (g–i) beta diversity values with 300–700 m altitude variations, and (j–l) beta diversity values with 700–2000 m altitude variations [Colour figure can be viewed at http://wileyonlinelibrary.com]
There is a similar pattern among species from the group 2a. However, the βnes decreases and βsim decreases then increases slightly at higher altitudes, wherein the site MS has highest values of βnes (0.669), whereas that site WRS has highest values of βsim (0.397) both among species which occur between 300–700 m a.s.l.. Among the groups 1 and 2b the values of βsor increase abruptly with increasing altitude (βsor mean 0.215–0.609). The main difference in this value of βsim (0.353–0.395) increases according to increasing the differences in the species composition between altitudes. The site ERS had the highest value of βnes (0.281) among the species that occur from 700–2000 m a. s. l. and the highest value of βsim (0.480) among the species that occur 300–700m a.s.l. (Appendix S2, Table 2.7 and Figure 5).
The differences between the compositions were among the species that occur in the lowland (0–300 m) and hilltops (700–2000 m). Between the groups 1 and 2a, nestedness decrease with increasing altitude and increasing turnover between 700–2000 m and 0–300 m altitude. Between the groups 1 and 2b, nestedness decrease and increased composition turnover with increasing elevation, which has a slight increase in beta diversity pattern between species that occur in 300–700 m altitude. Between groups 1 and 3 nestedness increases and then decreases gradually by increasing altitude, increasing turnover pattern. Group 2b indicates higher endemism rate on the mountainous region, whereas group 3 in the higher endemism rate on lowlands (Figure 5).
4. DISCUSSION
4.1. Effect of environmental variables on species richness and endemic species
Our results showed that MAT has the greatest influence on amphibian richness and endemic species in the Atlantic Forest. Due to their physiological characteristics, amphibians are dependent on humidity and mild temperatures (Crump, 2010; Wells, 2007). We also found correlations between species richness and precipitation, corroborating other related studies (Casemiro et al., 2007; Ortiz‐Yusty, Paez, & Zapata, 2013; Vasconcelos, Prado, Silva, & Haddad, 2014). The correlation of species richness with NPP and natural forest formations were also strongly correlated with rainfall (Rueda, Rodrıguez, & Hawkins, 2010). High altitudes have a higher number of endemic species due to lower temperatures and higher humidity (Cruz & Feio, 2007). However, lowland areas with the same features (i.e. milder temperatures and higher humidity) also have endemic species, and this may be related to historical events (Carnaval et al., 2009).
Due to high humidity and diversity of microhabitats, many Neotropical amphibians evolved to be small (Rittmeyer, Allison, Gründler, Thompson, & Austin, 2012). These species are more susceptible to humidity loss (Ashton, 2001; MacLean, 1985) and many are restricted to conserved forests to maintain their environmental favourability (Ferreira et al., 2016; Lourenço‐de‐Moraes, Ferreira, Fouquet, & Bastos, 2014); such as areas of milder temperatures, higher rainfall and higher vegetation cover. Our results showed that amphibians in areas with lower latitudes, intermediary longitudes and high altitudes tend to have smaller body sizes. Therefore, we highlight relationships between species richness and body size that directly relate to environmental conditions. This may lead to restricted gene flow between populations and accelerate genetic differentiation (Pabijan, Wollenberg, & Vences, 2012). Consequently, it may have limited these species to rain forests where such genetic differentiation is most commonly observed (Rodríguez et al., 2015), and these factors may have contributed to the large number of small species (Lourenço‐de‐Moraes, Dias, et al., 2018).
4.2. Effects of functional traits in geographical distribution of species
We showed that amphibian species (especially anurans) with the widest distribution are adapted to live in open areas, have medium body size, and are terrestrial and toxic. In our study, species with a medium to large body size have better dispersal abilities, such as Odontophrynus americanus (family Odontophrynidae), Leptodactylus mystacinus (family Leptodactylidae) and Rhinella diptycha (family Bufonidae) (Haddad et al., 2013). Species as L. mystacinus, with the same characteristics except toxicity also have wide distributional ranges due to distinct factors that may improve their range expansion (e.g. physiological, behavioural and morphological traits). For example species with a variety of antipredator mechanisms may be more likely to avoid a wider range of predators (Lourenço‐de‐Moraes et al., 2016), that allows successful dispersal. In addition, species from open areas with larger ranges also occur in drier biomes, such as the Cerrado and the Caatinga.
Our findings revealed that the distributions of Atlantic Forest amphibians are related to their functional traits, of which the habitats comprising open areas can favour larger species better adapted to high temperatures and low humidity rates. Most of the species that occur in the Atlantic Forest have body size less than 30 mm, losing water more quickly to the environment (MacLean, 1985). In addition, the type of habitat preference may be determinant for the species distribution (Gouveia & Correia, 2016). Because of this, even small arboreal species, such as Dendropsophus nanus and D. minutus, have a greater ability to dispersal due to their ability to occur in open areas (Haddad et al., 2013). However, small and miniature species with wide distribution, such as D. minutus and Pseudopaludicola falcipes are species complexes; and thus their current distribution may be overestimated (Gehara et al., 2014; Langone, Camargo, & Sá, 2016).
Many open area species are expanding or expanded their ranges due to forest destruction (e.g. Leptodactylus fuscus). Habitat is an important trait for dispersal in an environment transformed into open areas. However, species of open areas are not found in forests, or they are found in low abundance (Campos & Lourenço‐de‐Moraes, 2017; author's pers. obs.), therefore, we suggest that these species are not generalists, but specialists of open areas and opportunistic.
4.3. Beta diversity patterns
We revealed a homogenized pattern of species in the Atlantic Forest, which can be explained by beta diversity (NODF nestedness). Species of open areas tend to disperse from west to east due to deforestation. The mountains of Serra do Mar and Mantiqueira can be limiting geographical barriers for small amphibians restricted to forest habits (Haddad, 1998; Morellato & Haddad, 2000). Moreover, the geographical barrier Rio Doce divides the region ES and part of NMG (Bates, Hackett, & Cracraft, 1998; Costa, Leite, Fonseca, & Fonseca, 2000) and influences dispersal and composition of species from group 3. ES has southern (group 1) and northern (group 3) species of the Atlantic Forest, whereas NMG is more related to northern (group 3) species. Few strictly forest species have large ranges in the Atlantic Forest. Haddadus binotatus is a forest species that is widely distributed in the Atlantic Forest. This species may have dispersed through drier forests in the western region or during the Glacial period due to ocean regression (Atlantic Forest hypothesis, see Leite et al., 2016). However, it is possible that this is a species complex (Dias, Lourenço‐de‐Moraes, & Solé, 2012) and the current distribution of this species may be overestimated.
Our results indicate changes in beta diversity pattern between hilltops and lowland, the nestedness pattern decreasing with increasing altitude and increasing the turnover. This biogeographical difference in species compositions according to topographic location is a non‐random pattern; temporal and environmental processes influenced this pattern (Carvalho, 2010). Different altitudes (0–2000 m) have different degrees of environmental variations that determine amphibian composition. Mountainous areas and geological events create important biogeographical barriers (Haddad, 1998; Costa et al., 2000), and generate vicariance processes (Almeida & Santos, 2010). Consequently, high‐altitude areas (i.e. Serra do Mar, Cruz & Feio, 2007) and natural lowland forests (i.e. south Bahia, Mira‐Mendes et al., 2018) have high species diversity and endemism reflecting the turnover pattern. The nestedness pattern is generated by species with functional traits that allow dispersal between sites.
According to our findings, it is possible to separate the Atlantic Forest into three major regions of endemism. Our results point to groups 1, 2b and 3 as the areas with the highest rates of endemic and rare species. The beta diversity values corroborate the hypothesis of endemism during the Pleistocene glacial (Carnaval et al., 2009, 2014) and Anthropocene (Lourenço‐de‐Moraes, Campos, et al., 2019). The two extremes of this biome (i.e. southern and northern most) have higher turnover rates compared to group 1. Group 3 has shared genera with the Amazon forest, such as Pristimantis (Frost, 2019), and Allophryne (Caramaschi, Orrico, Faivovich, Dias, & Solé, 2013)—genera that do not occur in other groups of the Atlantic Forest. These data support the hypothesized connection between the north of the Atlantic Forest and the eastern Amazon Rainforest (Batalha‐Filho, Fjeldsa, Fabre, & Miyaki, 2013; Sobral‐Souza, Lima‐Ribeiro, & Solferini, 2015). On the other hand, group 3 also received species from the Atlantic Forest, such as Boana faber. Group 2a and 2b also have higher turnover rates at some sites. In group 2a, site WRS and in group 2b the sites ESC and ERS.
The south of the Atlantic Forest region had a strong influence of the western Amazon composition and the Andean forests (Batalha Filho et al., 2013; Sobral‐Souza et al., 2015). Most Melanophryniscus, Scythrophrys and Lymnomedusa species of the genera occur in group 2. These genera tolerate colder areas of the Atlantic Forest (Frost, 2019). Moreover, the nestedness sites of greatest values were in group 1 and group 2a. This group has sites of warmer forests and more pronounced seasonality due to these species that occur at these points, especially in MS and WPR are mostly species of open areas. In the SMGM site, species that occur at this point are directly related to the Cerrado biome; many species of open areas and forest edges occur at this site.
Late Pleistocene glaciations may be the main driver of the current species richness of amphibians in the Atlantic Forest (Carnaval & Moritz, 2008). The species richness provided by these events reflects direct speciation and specialization with several functional traits, which depending on the specialization, will direct the dispersal of species in a current historical panorama. This process is a cycle that has been repeated for thousands of years, but the current change on the planet by human actions can be directly affected by mass extinction processes (Barnosky et al., 2011; Dirzo et al., 2014).
The current spatial composition and distribution of species are also related to anthropogenic actions and reflects the species most suitable for the new environment, whereas forest and restricted species of the Atlantic Forest are more endemic and endangered. Furthermore, anthropogenic actions can accelerate global warming causing total losses of functional traits (e.g. phytotelmata species, Lourenço‐de‐Moraes, Campos, et al., 2019). The strength of this study is its innovative approach to incorporating functional traits into species dispersal assumptions; we need to consider that deforestation has limited most amphibians to small and scattered fragments. In addition, the proximity of the fragments to urban environments affects the permanence of the species in the long term, even the species often found in open area (Lourenço‐de‐Moraes, Malagoli, et al., 2018). The maintenance of functional processes as a justification for amphibian conservation actions can be an effective strategy to reduce extinction risk and avoid species loss (Campos, Lourenço‐de‐Moraes, Llorente, & Solé, 2017). In this context, our research highlights the importance of maintaining forest cover remnants in the Atlantic Forest, and may help to move forward the usefulness of functional traits approaches for other biodiversity hotspots.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
BIOSKETCH
Ricardo Lourenço‐de‐Moraes is a postdoctoral researcher at the Postgraduate Program in the Ecology and Environmental Monitoring, in the Federal University of Paraíba and linked at the Laboratory of Herpetology and Animal Behaviour, in the Federal University of Goiás, Brazil. The presented work was a part of his Doctoral thesis in Environmental Sciences at the Postgraduate Program in the Ecology of Continental Aquatic Environments, State University of Maringá, Brazil. His research focuses on understanding the potential roles of amphibian and reptile species on the evolution and function inside of ecosystems.
Author contributions: R.L.M. conceived the study and wrote the manuscript with contributions from all co‐authors. R.L.M. and F.S.C. designed the analyses, collected the data and created the figures. All authors discussed the results and edited the manuscript.
Supporting information
ACKNOWLEDGEMENTS
We thank Camila Ribas and two anonymous referees for their careful reading of the manuscript and suggestions. RLM, RPB and RBF thank Conselho Nacional de Desenvolvimento Científico e Tecnológico for scholarships (140710/2013‐2; 152303/2016‐2; 430195/2018‐4) and research productivity. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001 (FSC 99999.001180/2013‐04). We thank the ComissãoTécnico‐Científica do Instituto Florestal, Estado de São Paulo (COTEC), Instituto Ambiental do Paraná—Estado do Paraná (IAP) and the Instituto Chico Mendes de Conservação da Biodiversidade for logistical support and collection licenses (ICMBio/SISBIO 30344 and 44755).
Lourenço‐de‐Moraes R, Campos FS, Ferreira RB, et al. Functional traits explain amphibian distribution in the Brazilian Atlantic Forest. J Biogeogr. 2020;47:275–287. 10.1111/jbi.13727
Handling Editor: Camila Ribas
REFERENCES
- Almeida, E. A. B. , & Santos, C. M. D. (2010). Lógica da biogeografia de vicariância In Carvalho C. J. B., & Almeida E. A. B. (Eds.), Biogeografia da América do Sul: Padrões e Processos (pp. 52–62). São Paulo, Brazil: Editora Rocca. [Google Scholar]
- Almeida‐Neto, M. , Guimarães, P. , Guimarães, P. R. Jr , Loyola, R. D. , & Ulrich, W. (2008). A consistent metric for nestedness analysis in ecological systems: Reconciling concept and measurement. Oikos, 117, 1227–1239. 10.1111/j.0030-1299.2008.16644.x [DOI] [Google Scholar]
- Ashton, K. G. (2001). Are ecological and evolutionary rules being dismissed prematurely? Diversity and Distributions, 7, 289–295. 10.1046/j.1366-9516.2001.00115.x [DOI] [Google Scholar]
- Barnosky, A. D. , Matzke, N. , Tomiya, S. , Wogan, G. O. U. , Swartz, B. , Quental, T. B. , … Ferrer, E. A. (2011). Has the earth’s sixth mass extinction already arrived? Nature, 471, 51–57. 10.1038/nature09678 [DOI] [PubMed] [Google Scholar]
- Baselga, A. (2008). Determinants of species richness, endemism and turnover in European longhorn beetles. Ecography, 31, 263–271. 10.1111/j.0906-7590.2008.5335.x [DOI] [Google Scholar]
- Baselga, A. (2010). Partitioning the turnover and nestedness components of beta diversity. Global Ecology and Biogeography, 19, 134–143. 10.1111/j.1466-8238.2009.00490.x [DOI] [Google Scholar]
- Baselga, A. , Lobo, J. M. , Svenning, J. C. , Aragón, P. , & Araújo, M. B. (2012). Dispersal ability modulates the strength of the latitudinal richness gradient in European beetles. Global Ecology and Biogeography, 21, 1106–1113. 10.1111/j.1466-8238.2011.00753.x [DOI] [Google Scholar]
- Baselga, A. , & Orme, C. D. L. (2012). Betapart: An R package for the study of beta diversity. Methods in Ecology and Evolution, 3, 808–812. [Google Scholar]
- Batalha‐Filho, H. , Fjeldsa, J. , Fabre, P.‐H. , & Miyaki, C. Y. (2013). Connections between the Atlantic and the Amazonian forest avifaunas represent distinct historical events. Jounal Ornithological, 154, 41–50. 10.1007/s10336-012-0866-7 [DOI] [Google Scholar]
- Bates, J. M. , Hackett, S. J. , & Cracraft, J. (1998). Area‐relationships in the Neotropical lowlands: A hypothesis based on raw distributions of passerine birds. Journal of Biogeography, 25, 783–793. 10.1046/j.1365-2699.1998.2540783.x) [DOI] [Google Scholar]
- Berg, M. P. , Kiers, E. T. , Driessen, G. , Van der Heijden, M. , Kooi, B. W. , Kuenen, F. , … Ellers, J. (2010). Adapt or disperse: Understanding species persistence in a changing world. Global Change Biology, 16(2), 587–598. 10.1111/j.1365-2486.2009.02014.x [DOI] [Google Scholar]
- Bittencourt, A. C. , Dominguez, J. M. , Martin, L. , Silva, I. R. , & Medeiros, K. O. (2007). Past and current sediment dispersion pattern estimates through numerical modeling of wave climate: An example of the Holocene delta of the Doce River, Espírito Santo, Brazil. Anais da Academia Brasileira de Ciências, 79(2), 333–341. 10.1590/S0001-37652007000200014 [DOI] [PubMed] [Google Scholar]
- Brown, J. H. , & Maurer, B. A. (1989). Macroecology: The division of food and space among species on continents. Science, 243, 1145–1150. 10.1126/science.243.4895.1145 [DOI] [PubMed] [Google Scholar]
- Campos, F. S. , & Lourenço‐de‐Moraes, R. (2017). Amphibians from the mountains of the Serra do Mar Coastal Forest, Brazil. Herpetology Notes, 10, 547–560. [Google Scholar]
- Campos, F. S. , Lourenço‐de‐Moraes, R. , Llorente, G. A. , & Solé, M. (2017). Cost‐effective conservation of amphibian ecology and evolution. Science Advances, 3(6), e1602929 10.1126/sciadv.1602929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos, F. S. , Trindade‐Filho, J. , Brito, D. , Llorente, G. A., & Solé, M. (2014). The efficiency of indicator groups for the conservation of amphibians in the Brazilian Atlantic Forest. Ecology and Evolution, 4, 2505–2514. 10.1002/ece3.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramaschi, U. , Orrico, V. G. D. , Faivovich, J. , Dias, I. R. , & Solé, M. (2013). A new species of Allophryne (Anura: Allophrynidae) from the Atlantic Rain Forest Biome of eastern Brazil. Herpetologica, 69, 480–491. [Google Scholar]
- Carnaval, A. C. , Hickerson, M. J. , Haddad, C. F. B. , Rodrigues, M. T. , & Moritz, C. (2009). Stability predicts genetic diversity in the Brazilian Atlantic Forest hotspot. Science, 323, 785–789. 10.1126/science.1166955 [DOI] [PubMed] [Google Scholar]
- Carvalho, C. J. B. (2010). Áreas de Endemismo In Carvalho C. J. B., & Almeida E. A. B. (Eds.), Biogeografia da América do Sul: Padrões e Processos (pp. 41–51). São Paulo, Brazil: Editora Rocca. [Google Scholar]
- Carnaval, A. C. , & Moritz, C. (2008). Historical climate modelling predicts patters of current biodiversity in the Brazilian Atlantic Forest. Journal of Biogeography, 35, 1187–1201. [Google Scholar]
- Carnaval, A. C. , Waltari, E. , Rodrigues, M. T. , Rosauer, D. , VanDerWal, J. , Damasceno, R. , … Moritz, C. (2014). Prediction of phylogeographic endemism in an environmentally complex biome. Proceedings of Royal Society Biological Science, 281, 20141461 10.1098/rspb.2014.1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casemiro, F. A. S. , Souza, B. , Rangel, T. F. L. V. B. , & Diniz‐Filho, J. A. F. (2007). Non‐stationarity, diversity gradients and the metabolic theory of ecology. Global Ecology and Biogeography, 16, 820–822. 10.1111/j.1466-8238.2007.00332.x [DOI] [Google Scholar]
- Cavarzere, V. , & Silveira, L. F. (2012). Bird species diversity in the Atlantic Forest of Brazil is not explained by the Mid‐domain Effect. Zoologia, 29, 285–292. 10.1590/S1984-46702012000400001 [DOI] [Google Scholar]
- Costa, L. P. , Leite, Y. L. R. , Fonseca, G. A. B. , & Fonseca, M. T. (2000). Biogeography of South American forest mammals: Endemism and diversity in the Atlantic forest. Biotropica, 32, 872–881. 10.1111/j.1744-7429.2000.tb00625.x [DOI] [Google Scholar]
- Crump, L. M. (2010). Amphibian diversity and life history In Dodd C. K. (Ed.), Amphibian ecology and conservation a handbook of techniques (pp. 1–19). Oxford, UK: Oxford University Press. [Google Scholar]
- Crump, M. L. , & Scott, N. J. Jr (1994). Visual encounter surveys In Heyer W. R., Donnelly M. A., McDiarmid R. W., Hayek L. A. C., & Foster M. S. (Eds.), Measuring and monitoring biological diversity: Standard methods for amphibians (pp. 92–97). Washington DC: Smithsonian Institution Press. [Google Scholar]
- Cruz, C. A. G. , & Feio, R. N. (2007). Endemismos em anfíbios em áreas de altitude na Mata Atlântica no sudeste do Brasil In Nascimento L. B., & Oliveira M. E. (Eds.), Herpetologia Do Brasil II (pp. 117–126). Belo Horizonte, Brazil: Sociedade Brasileira de Herpetologia. [Google Scholar]
- Cushman, S. A. (2006). Effects of habitat loss and fragmentation on amphibians: A review and prospectus. Biological Conservation, 128(2), 231–240. 10.1016/j.biocon.2005.09.031 [DOI] [Google Scholar]
- Dias, I. R. , Lourenço‐de‐Moraes, R. , & Solé, M. (2012). Description of the advertisement call and morphometry of Haddadus binotatus (Spix, 1824) from a population from southern Bahia, Brazil. North‐western Journal of Zoology, 8(1), 107–111. [Google Scholar]
- Díaz, S. , Lavorel, S. , Bello, F. , Quetier, F. , Grigulis, K. , & Robson, M. (2007). Incorporating plant functional diversity effects in ecosystem service assessments. Proceedings of National Academy of Science USA, 104, 20684–20689. 10.1073/pnas.0704716104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirzo, R. , Young, H. S. , Galetti, M. , Ceballos, G. , Isaac, N. J. B. , & Collen, B. (2014). Defaunation in the Anthropocene. Science, 345, 401–406. 10.1126/science.1251817 [DOI] [PubMed] [Google Scholar]
- Dixo, M. , Metzger, J. P. , Morgante, J. S. , & Zamudio, K. R. (2009). Habitat fragmentation reduces genetic diversity and connectivity among toad populations in the Brazilian Atlantic Coastal Forest. Biological Conservation, 142, 1560–1569. 10.1016/j.biocon.2008.11.016 [DOI] [Google Scholar]
- Dominguez, J. M. L. , Bittencourt, A. C. S. P. , & Martin, L. (1981). Esquema evolutivo da sedimentação quaternária nas feições deltaicas dos rios São Francisco (SE/AL), Jequitinhonha (BA), Doce (ES) e Paraíba do Sul (RJ). Revista Brasileira De Geociências, 11(4), 227–237. 10.25249/0375-7536.1981227237 [DOI] [Google Scholar]
- Duellman, W. E. , & Trueb, L. (1994). Biology of amphibians. Baltimore, MA: The Johns Hopkins University Press. [Google Scholar]
- Environmental Systems Research Institute ESRI . (2011). Arcgis Software: Version10.1. Redlands, CA: ESRI. [Google Scholar]
- Fahrig, L. (2001). How much habitat is enough? Biological Conservation, 100, 65–74. 10.1016/S0006-3207(00)00208-1 [DOI] [Google Scholar]
- Ferreira, R. B. , Beard, K. H. , & Crump, M. L. (2016). Breeding guild determines frog distributions in response to edge effects and habitat conversion in the Brazil’s Atlantic Forest. PLoS ONE, 11(6), e0156781 10.1371/journal.pone.0156781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira, R. B. , Lourenço‐de‐Moraes, R. , Zocca, C. , Duca, C. , Beard, K. H. , & Brodie, E. D. Jr (2019). Antipredator mechanisms of post‐metamorphic anurans: A global database and classification system. Behavioral Ecology and Sociobiology, 73, 69 10.1007/s00265-019-2680-1 [DOI] [Google Scholar]
- Figueiredo, G. T. , Storti, L. F. , Lourenço‐de‐Moraes, R. , Shibatta, O. A. , & Anjos, L. (2019). Influence of microhabitat on the richness of anuran species: A case study of different landscapes in the Atlantic Forest of southern Brazil. Anais da Academia Brasileira de Ciências, 91, e20171023 10.1590/0001-3765201920171023 [DOI] [PubMed] [Google Scholar]
- Forti, L. R. , Silva, T. R. Á. , & Toledo, L. F. (2017). The acoustic repertoire of the Atlantic Forest Rocket Frog and its consequences for taxonomy and conservation (Allobates, Aromobatidae). ZooKeys, 692, 141–153. 10.3897/zookeys.692.12187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, J. , & Weisberg, S. (2011). An R companion to applied regression, second edition, Sage. Thousand Oaks, Califórnia, EUA. R Development Core Team; Retrieved from https://r-forge.r-project.org/projects/car/ [Google Scholar]
- Frost, D. R. (2019). Amphibian species of the world: An online reference. Version 6.0 New York, NY, USA: American Museum of Natural History; Retrieved from http://research.amnh.org/herpetology/amphibia/index.html [Google Scholar]
- Gaston, K. J. (1990). Patterns in the geographical ranges of species. Biological Reviews, 65, 105–129. 10.1111/j.1469-185X.1990.tb01185.x [DOI] [Google Scholar]
- Gaston, K. J. (1991). How large is a species' geographic range? Oikos, 61, 434–438. 10.2307/3545251 [DOI] [Google Scholar]
- Gehara, M. , Crawford, A. J. , Orrico, V. G. D. , Rodríguez, A. , Lötters, S. , Fouquet, A. , … Köhler, J. (2014). High levels of diversity uncovered in a widespread nominal taxon: Continental phylogeography of the neotropical tree frog Dendropsophus minutus . PLoS ONE, 9(9), e103958 10.1371/journal.pone.0103958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia, S. F. , & Correia, I. (2016). Geographical clines of body size in terrestrial amphibians: Water conservation hypothesis revisited. Journal of Biogeography, 43, 2075–2084. 10.1111/jbi.12842 [DOI] [Google Scholar]
- Haddad, C. F. B. (1998). Biodiversidade dos anfíbios no estado de São Paulo In Joly C. A., & Bicudo C. E. M. (Eds.), Biodiversidade do estado de São Paulo (pp. 15–26). São Paulo, Brazil: Editora FAPESP. [Google Scholar]
- Haddad, C. F. B. , Toledo, L. F. , Prado, C. P. A. , Loebmann, D. , Gasparini, J. L. , & Sazima, I. (2013). Guia dos anfíbios da Mata Atlântica: Diversidade e biologia. São Paulo, Brazil: Anolis Books. [Google Scholar]
- Hawkins, B. A. (2001). Ecology’s oldest pattern? Trends in Ecology & Evolution, 16(8), 470 10.1016/S0169-5347(01)02197-8 [DOI] [Google Scholar]
- Hocking, D. J. , & Babbitt, K. J. (2014). Amphibian contributions to ecosystem services. Herpetological Conservation & Biology, 9, 1–17. [Google Scholar]
- Homan, R. N. , Windmiller, B. S. , & Reed, J. M. (2004). Critical thresholds associated with habitat loss for two vernal pool breeding amphibians. Ecological Applications, 14(5), 1547–1553. 10.1890/03-5125 [DOI] [Google Scholar]
- Huey, R. B. (1991). Physiological consequences of habitat selection. American Naturalist Supplement, 137, S90–S115. 10.1086/285141 [DOI] [Google Scholar]
- Hutchinson, G. E. (1957). Population studies: Animal ecology and demography. Concluding remarks. Cold Spring Harbor Symposia on Quantitative Biology, 22, 415–427. 10.1101/SQB.1957.022.01.039 [DOI] [Google Scholar]
- IUCN Red list of threatened species IUCN . (2017). Version 2017.3. Retrieved from http://www.iucnredlist.org
- Jiménez‐Valverde, A. , Gilgado, J. D. , Sendra, A. , Pérez‐Suárez, G. , Herrero‐Borgoñón, J. J. , & Ortuño, V. M. (2015). Exceptional invertebrate diversity in a scree slope in Eastern Spain. Journal of Insect Conservation, 19, 713–728. 10.1007/s10841-015-9794-1 [DOI] [Google Scholar]
- Langone, J. A. , Camargo, A. , & Sá, R. (2016). High genetic diversity but low population structure in the frog Pseudopaludicola falcipes (Hensel, 1867) (Amphibia, Anura) from the Pampas of South America. Molecular Phylogenetics and Evolution, 95, 137–151. 10.1016/j.ympev.2015.11.012 [DOI] [PubMed] [Google Scholar]
- Lawton, J. H. (1993). Range, population abundance and conservation. Trends in Ecology and Evolution, 8, 409–413. 10.1016/0169-5347(93)90043-O [DOI] [PubMed] [Google Scholar]
- Legendre, P. , & Legendre, L. (1998). Numerical ecology, 2 ed. Amsterdam, Netherlands: Elsevier. [Google Scholar]
- Leibold, M. A. , & Mikkelson, G. M. (2002). Coherence, species turnover, and boundary clumping: Elements of meta‐community structure. Oikos, 97, 237–250. 10.1034/j.1600-0706.2002.970210.x [DOI] [Google Scholar]
- Leite, Y. L. R. , Costa, L. P. , Loss, A. C. , Rocha, R. G. , Batalha‐Filho, H. , Bastos, A. C. , … Pardini, R. (2016). Neotropical forest expansion during the last glacial period challenges refuge hypothesis. Proceedings of the National Academy of Sciences, 113, 1008–1013. 10.1073/pnas.1513062113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenço‐de‐Moraes, R. , Campos, S. C. , Ferreira, R. B. , Solé, M. , Beard, K. H. , & Bastos, R. P. (2019). Back to the future: Conserving functional and phylogenetic diversity in amphibian climate‐refuges. Biodiversity and Conservation, 28, 1049–1073. 10.1007/s10531-019-01706-x [DOI] [Google Scholar]
- Lourenço‐de‐Moraes, R. , Dias, I. R. , Mira‐Mendes, C. V. , Oliveira, R. M. D. , Barth, A. , Ruas, D. S. , … Bastos, R. P. (2018). Diversity of miniaturized frogs of the genus Adelophryne (Anura: Eleutherodactylidae): A new species from the Atlantic Forest of northeast Brazil. PlosOne, 13(9), e0201781 10.1371/journal.pone.0201781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenço‐de‐Moraes, R. , Ferreira, R. B. , Fouquet, A. , & Bastos, R. P. (2014). A new diminutive frog species of Adelophryne (Amphibia: Anura: Eleutherodactylidae) from the Atlantic Forest, southeastern Brazil. Zootaxa, 3846, 348–360. 10.11646/zootaxa.3846.3.2 [DOI] [PubMed] [Google Scholar]
- Lourenço‐de‐Moraes, R. , Ferreira, R. B. , Mira‐Mendes, C. C. V. , Zocca, C. Z. , Medeiros, T. , Ruas, D. S. , … Solé, M. (2016). Escalated antipredator mechanisms of two Neotropical marsupial treefrogs. Herpetological Journal, 26, 237–244. [Google Scholar]
- Lourenço‐de‐Moraes, R. , Lansac‐Toha, F. M. , Schwind, L. T. F. , Arrieira, R. L. , Rosa, R. R. , Terribile, L. C. , … Bailly, D. (2019). Climate change will decrease the range size of snake species under negligible protection in the Brazilian Atlantic Forest hotspot. Scientific Reports, 9, 8523 10.1038/s41598-019-44732-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenço‐de‐Moraes, R. , Malagoli, L. R. , Guerra, V. B. , Ferreira, R. B. , Affonso, I. P. , Haddad, C. F. B. , … Bastos, R. P. (2018). Nesting patterns between Neotropical species assemblages: Can reserves in urban areas be failing to protect anurans? Urban Ecosystems, 21(5), 933–942. 10.1007/s11252-018-0767-5 [DOI] [Google Scholar]
- MacLean, W. P. (1985). Water‐loss rates of Sphaerodactylus parthenopion (Reptilia: Gekkonidae), the smallest amniote vertebrate. Comparative Biochemistry and Physiology Part A: Physiology, 82(4), 759–761. 10.1016/0300-9629(85)90479-7 [DOI] [Google Scholar]
- McGill, B. J. , Enquist, B. J. , Weiher, E. , & Westoby, M. (2006). Rebuilding community ecology from functional traits. Trends in Ecology & Evolution, 21(4), 178–185. 10.1016/j.tree.2006.02.002 [DOI] [PubMed] [Google Scholar]
- Mira‐Mendes, C. B. , Ruas, D. S. , Oliveira, R. M. , Castro, I. M. , Dias, I. R. , Baumgarten, J. E. , … Solé, M. (2018). Amphibians of the Reserva Ecológica Michelin: A high diversity site in the lowland Atlantic Forest of southern Bahia, Brazil. ZooKeys, 753, 1–21. 10.3897/zookeys.753.21438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monkkonen, M. , & Reunanen, P. (1999). On critical thresholds in landscape connectivity: A management perspective. Oikos, 84, 302–305. 10.2307/3546725 [DOI] [Google Scholar]
- Morellato, L. P. C. , & Haddad, C. F. B. (2000). Introduction: The Brazilian Atlantic Forest. Biotropica, 32, 786–792. [Google Scholar]
- Myers, N. , Mittermeier, R. A. , Mittermeier, C. G. , Fonseca, G. A. , & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403, 853–858. 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Oberdorff, T. , Hugueny, B. , & Guegan, J.‐F. (1997). Is there an influence of historical events on contemporary fish species richness in rivers? Comparisons between Western Europe and North America. Journal of Biogeography, 24, 461–467. 10.1111/j.1365-2699.1997.00113.x [DOI] [Google Scholar]
- Ogle, D. H. (2016). Introductory Fisheries Analyses with R. Chapman & Hall/CRC. Boca Raton, FL: R Development Core Team; Retrieved from https://github.com/droglenc/FSA [Google Scholar]
- Oksanen, J. , Blanchet, F. G. , Kindt, M. F. R. , Legendre, P. , McGlinn, D. , Minchin, P. R. , … Wagner, H. (2013). Vegan: Community ecology package. Helsinki, Finland: R Development Core Team; Retrieved from http://cran.r-project.org/package=vegan [Google Scholar]
- Olson, D. M. , Dinerstein, E. , Wikramanayake, E. D. , Burgess, N. D. , Powell, G. V. N. , Underwood, E. C. , … Kassem, K. R. (2001). Terrestrial ecoregions of the world: A new map of life on Earth. BioScience, 51(11), 933–938. 10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2 [DOI] [Google Scholar]
- Ortiz‐Yusty, C. A. , Paez, V. , & Zapata, F. A. (2013). Temperature and precipitation as predictors of species richness in northern Andean amphibians from Colombia. Caldasia, 35(1), 65–80. [Google Scholar]
- Pabijan, M. , Wollenberg, K. C. , & Vences, M. (2012). Small body size increases the regional differentiation of populations of tropical mantellid frogs (Anura: Mantellidae). Journal of Evolutionary Biology, 25, 2310–2324. 10.1111/j.1420-9101.2012.02613.x [DOI] [PubMed] [Google Scholar]
- Patrick, D. A. , Harper, E. B. , Hunter, M. L. , & Calhoun, A. J. K. (2008). Terrestrial habitat selection and strong density‐dependent mortality in recently metamorphosed amphibians. Ecology, 89, 2563–2574. 10.1890/07-0906.1 [DOI] [PubMed] [Google Scholar]
- Pfrender, M. E. , Bradshaw, W. E. , & Kleckner, C. A. (1998). Patterns in the geographical range sizes of ectotherms in North America. Oecologia, 115, 439–444. 10.1007/s004420050539 [DOI] [PubMed] [Google Scholar]
- R Development Core Team, R . (2017). A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Ribeiro, M. C. , Metzger, J. P. , Martensen, A. C. , Ponzoni, F. J. , & Hirota, M. M. (2009). The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biological Conservation, 142, 1141–1153. 10.1016/j.biocon.2009.02.021 [DOI] [Google Scholar]
- Richter‐Boix, A. L. E. X. , Llorente, G. A. , & Montori, A. (2007). Structure and dynamics of an amphibian metacommunity in two regions. Journal of Animal Ecology, 76(3), 607–618. 10.1111/j.1365-2656.2007.01232.x [DOI] [PubMed] [Google Scholar]
- Ricklefs, R. E. (1987). Community diversity: Relative roles of local and regional processes. Science, 235, 167–171. 10.1126/science.235.4785.167 [DOI] [PubMed] [Google Scholar]
- Rittmeyer, E. N. , Allison, A. , Gründler, M. C. , Thompson, D. K. , & Austin, C. C. (2012). Ecological guild evolution and the discovery of the world's smallest vertebrate. PLoS ONE, 7(1), e29797 10.1371/journal.pone.0029797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez, A. , Börner, M. , Pabijan, M. , Gehara, M. , Haddad, C. F. B. , & Vences, M. (2015). Genetic divergence in tropical anurans: Deeper phylogeographic structure in forest specialists and in topographically complex regions. Evolutionary Ecology, 29, 765–785. 10.1007/s10682-015-9774-7 [DOI] [Google Scholar]
- Rueda, M. , Rodrıguez, M. A. , & Hawkins, B. A. (2010). Towards a biogeographic regionalization of the European biota. Journal of Biogeography, 37, 2067–2076. 10.1111/j.1365-2699.2010.02388.x [DOI] [Google Scholar]
- Sarkar, D. (2008). Lattice: Multivariate Data Visualization with R. Springer. R Development Core Team; Retrieved from http://lattice.r-forge.r-project.org [Google Scholar]
- Silva, F. R. , Almeida‐Neto, M. , & Arena, M. V. N. (2014). Amphibian beta diversity in the Brazilian Atlantic Forest: Contrasting the roles of historical events and contemporary conditions at different spatial scales. PLoS ONE, 9(10), e109642 10.1371/journal.pone.0109642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, F. R. , Almeida‐Neto, M. , Prado, V. H. M. , Haddad, C. F. B. , & Rossa‐Feres, D. C. (2012). Humidity levels drive reproductive modes and phylogenetic diversity of amphibians in the Brazilian Atlantic Forest. Journal of Biogeography, 39, 1720–1732. 10.1111/j.1365-2699.2012.02726.x [DOI] [Google Scholar]
- Soberón, J. (2007). Grinnellian and Eltonian niches and geographic distributions of species. Ecology Letters, 10, 1115–1123. 10.1111/j.1461-0248.2007.01107.x [DOI] [PubMed] [Google Scholar]
- Sobral‐Souza, T. , Lima‐Ribeiro, M. S. , & Solferini, V. N. (2015). Biogeography of neotropical rainforests: Past connections between Amazon and Atlantic Forest detected by ecological niche modeling. Evolutionary Ecology, 29(5), 643–655. 10.1007/s10682-015-9780-9 [DOI] [Google Scholar]
- Svenning, J. C. , & Skov, F. (2007). Could the tree diversity pat‐tern in Europe be generated by postglacial dispersal limitation? Ecology Letters, 10, 453–460. 10.1111/j.1461-0248.2007.01038.x [DOI] [PubMed] [Google Scholar]
- Tabarelli, M. , Pinto, L. P. , Silva, J. M. C. , Hirota, M. , & Bede, L. (2005). Challenges and opportunities for biodiversity conservation in the Brazilian Atlantic Forest. Conservation Biology, 19, 695–700. 10.1111/j.1523-1739.2005.00694.x [DOI] [Google Scholar]
- Toledo, L. F. , Ribeiro, R. S. , & Haddad, C. F. B. (2007). Anurans as prey: An exploratory analysis and size relationships between predators and their prey. Journal of Zoology, 271, 170–177. 10.1111/j.1469-7998.2006.00195.x [DOI] [Google Scholar]
- Ulrich, W. , Almeida‐Neto, M. , & Gotelli, N. J. (2009). A consumer’s guide to nestedness analysis. Oikos, 118, 3–17. 10.1111/j.1600-0706.2008.17053.x [DOI] [Google Scholar]
- Ulrich, W. , & Gotelli, N. J. (2007). Null model analysis of species nestedness patterns. Ecology, 88, 1824–1831. 10.1890/06-1208.1 [DOI] [PubMed] [Google Scholar]
- Vasconcelos, T. S. , Prado, V. H. M. , Silva, F. R. , & Haddad, C. F. B. (2014). Biogeographic distribution patterns and their correlates in the diverse frog fauna of the Atlantic Forest Hotspot. PLoS ONE, 9(8), e104130 10.1371/journal.pone.0104130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos, T. S. , Santos, T. G. , Haddad, C. F. , & Rossa‐Feres, D. C. (2010). Climatic variables and altitude as predictors of anuran species richness and number of reproductive modes in Brazil. Journal of Tropical Ecology, 26, 423–432. 10.1017/S0266467410000167 [DOI] [Google Scholar]
- Wells, K. D. (2007). The ecology and behavior of amphibians. Chicago, IL: University of Chicago Press. [Google Scholar]
- Zimmerman, B. L. (1994). Audio strip transects In Heyer W. R., Donnelly M. A., McDiarmid R. W., Hayek L. A. C., & Foster M. S. (Eds.), Measuring and monitoring biological diversity: Standard methods for amphibians (pp. 92–97). Washington DC: Smithsonian Institution Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


