Abstract
Human bocavirus (HBOV) has been reported as a worldwide distributed respiratory pathogen. It has also been associated with encephalitis recently by detection of the virus in cerebrospinal fluid (CSF) of patients presented with encephalitis. This retrospective study aimed to present clinical features of HBOV infections in children with respiratory symptoms and describe unexplained encephalopathy in a subgroup of these patients. Results of 1,143 pediatric nasal samples from mid‐December 2013 to July 2014 were reviewed for detection of HBOV. A multiplex real time polymerase chain reaction assay was used for viral detection. Medical records of the patients were retrospectively analyzed. HBOV was detected in 30 patients (2.6%). Median age was 14 months (5–80). Clinical diagnoses were upper respiratory tract infection (n = 10), bronchopneumonia (n = 9), acute bronchiolitis (n = 5), pneumonia (n = 4), acute bronchitis (n = 1), and asthma execarbation (n = 1). Hospitalization was required in 16 (53.3%) patients and 10 (62.5%) of them admitted to pediatric intensive care unit (PICU). Noninvasive mechanical ventilation modalities was applied to four patients and mechanical ventilation to four patients. Intractable seizures developed in four patients while mechanically ventilated on the 2nd–3rd days of PICU admission. No specific reason for encephalopathy was found after a thorough investigation. No mortality was observed, but two patients were discharged with neurological sequela. HBOV may lead to respiratory infections in a wide spectrum of severity. This report indicates its potential to cause severe respiratory infections requiring PICU admission and highlights possible clinical association of HBOV and encephalopathy, which developed during severe respiratory infection. J. Med. Virol. 87:1831–1838, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: human bocavirus, respiratory tract infections, encephalopathy, pediatric intensive care unit
INTRODUCTION
Human bocavirus (HBOV) was first described in 2005 by Allender et al. [2005] in the nasopharyngeal aspirates of children with respiratory infection. So far, it has been frequently associated with upper and lower respiratory tract infections in many reports worldwide with a frequency ranging from 2.2% to 19% [Allander et al., 2007; Canducci et al., 2008; Zhou et al., 2014]. Its role as an etiologic agent in respiratory infections has been supported by significant associations between HBOV detection and concurrent otherwise unexplained diseases and also significantly higher detection of the virus in symptomatic patients compared to asymptomatic ones [Kesebir et al., 2006; Brieu et al., 2008; Ghietto et al., 2015]. On the other hand, HBOV has also been associated with encephalitis recently [Mitui et al., 2012; Mori et al., 2013; Yu et al., 2013]. It has been postulated that if HBOV is able to cause viremia it can also disseminate to other parts of body [Allander et al., 2007]. Therefore, researchers investigated the presence of HBOV in cerebrospinal fluid (CSF) of the children presented with a clinical picture of encephalitis and detected HBOV in 3–15% of these patients [Mitui et al., 2012; Mori et al., 2013; Yu et al., 2013]. In this retrospective study, clinical features of HBOV infected children admitted to pediatric emergency room of a tertiary referral center with signs and symptoms of respiratory tract infection are presented, with a special interest on a subgroup of patients complicated with unexplained encephalopathy during their disease period.
PATIENTS AND METHODS
From mid‐December 2013 to July 2014, a total of 1,143 pediatric nasal samples from patients with signs and symptoms suggestive of respiratory tract infection (presence of fever, rhinorrhea, nasal congestion, sore throat, cough, positive chest X ray finding) were analyzed by multiplex real time–reverse transcriptase polymerase chain reaction (rRT‐PCR) in National Influenza Reference Laboratory located in Virology Department which accepts nasal samples from outpatient clinics, emergency room, and other wards of Istanbul Medical Faculty. Medical records of the all patients with HBOV detection were retrospectively analyzed. They were all admitted to pediatric emergency room of Istanbul Medical Faculty, a tertiary university referral center in Turkey. Patients with comorbidities were also included in the analysis. We retrieved following clinical and laboratory data from patient records: age, gender, presenting symptoms, underlying disease, current diagnosis, duration, and characteristics of the illness, findings on physical examination, laboratory parameters on admission (hemogram, CRP, procalcitonin, chest X ray), need for pediatric intensive care unit, duration of hospitalization, and prognosis.
Blood cultures were obtained from the febrile patients, who were decided to be hospitalized. The blood culture bottles were incubated and monitored for the presence of microorganisms for 7 days in a BACTEC 9120 (Becton Dickinson Diagnostic Systems, Sparks, MD) automated system. Sputum specimens could not be collected from the patients due to small age. However, tracheal aspirate cultures were obtained from the entubated patients to rule out possible bacterial agents responsible for the lower respiratory tract infection.
Viral polymerase chain reaction (PCR) examination of CSF was performed by in‐house PCR method using Seeplex® Meningitis‐V1/V2 ACE Detection (V2.0), Seegene (Seoul, Korea), which is able to detect Herpes simplex virus type 1‐2, cytomegalovirus, Epstein–Barr virus, varicella‐zoster virus, human herpesvirus‐6, and enterovirus in CSF.
HBOV Detection
Nasopharyngeal swabs were collected using Virocult (Medical Wire & Equipment, UK). EZ1 Virus mini kit V2.0 (Catalog number: 955134, Qiagen, Germany) was used for total nucleic acid extraction. Real‐time PCR based, multiplex FTD® Respiratory Pathogens 21 kit (Fast‐track diagnostics Ltd. Malta) was used for detection of respiratory pathogens on RotorGene Q platform (Qiagen, Germany). The kit which has been validated by CDC, Atlanta has been compared with in‐house singleplex real‐time RT‐PCR assays and showed good concordance (87.7% and 86% positivity for one or more viruses, respectively; K = 0.812, CI: 0.786–0.838) [Sakthivel et al., 2012]. Furthermore, the current kit have shown very similar performance to that of other three different multiplex PCR assays in a recent study, with 93–100% agreement for all comparisons [Anderson et al., 2013]. It is able to detect following 21 respiratory pathogens: Human bocavirus, influenza A, (including H3N2 and H1N1), influenza B, human rhinovirus, coronavirus NL63, 229E, OC43, HKU1, parainfluenza 1, 2, 3, 4, human metapneumovirus (HMPV) A/B, Mycoplasma pneumonia, respiratory syncytial virus A/B, adenovirus, enterovirus, parechovirus and has an internal control.
Statistical Analysis
Normality was assessed by Shapiro Wilk test and histogram graphics. Data are presented as median, minimum, maximum, frequency, and percentage. Categorical variables between groups were compared with the χ2 test or Fisher exact test when the expected cell size was below 5. Continuous variables were compared by the Kruskal–Wallis one‐way analysis of variance. Two‐group comparisons were made by using Mann–Whitney U test with Bonferroni correction (Bonferroni‐adjusted significance level was P < 0.0167). All P values are based on two‐tailed statistical analyses and P values less than 0.05 were considered statistically significant. All statistical analysis was performed with SPSS for Windows version 21.0.
RESULTS
HBOV Detection and Demography
From mid‐December 2013 to July 2014, a total of 1,143 nasal secretion samples from pediatric patients with respiratory symptoms were investigated and 615 (53.8%) were positive for at least one virus or M. pneumonia. HBOV was detected in 30 specimens from 30 patients (2.6% among all patients with respiratory symptoms, 3.7% among only virus positive patients). The male/female ratio of the 30 children with positive HBOV was 1.72 (19/11). Median age was 14 months with a range of 5–80 months. Co‐infection with other viruses was found in 7 (23.3%) samples: HBOV + respiratory syncytial virus (RSV) in 4, HBOV + adenovirus in 2, and HBOV + human parainfluenza virus type 3 in 1 case. HBOV was the 9th common respiratory agent detected in the study period. The frequency distribution of other respiratory pathogens among all patients with respiratory symptoms was presented in Table I. Monthly distribution of the most commonly detected respiratory viruses, HBOV and the viruses co‐detected with HBOV is depicted in Figure 1, which shows the most frequent HBOV detection in February.
Table I.
The Frequency Distribution of Respiratory Pathogens Among All Patients With Respiratory Symptoms Between Mid‐ December 2013 to July 2014
| Respiratory pathogens | Frequency distribution in percentage (n) | Frequency distribution in percentage a (n) |
|---|---|---|
| Rhinovirus | 9.0 (103) | 14.6 (168) |
| Respiratory syncytial virus | 8.7 (100) | 16.2 (136) |
| Influenza A | 4.7 (54) | 5.4 (62) |
| Adenovirus | 4.3 (50) | 7.1 (82) |
| Coronaviruses | 4.1 (48) | 6.1 (70) |
| Parainfluenza viruses | 4.1 (47) | 5.1 (59) |
| Human metapneumovirus | 3.1 (36) | 4.1 (48) |
| Influenza B | 2.6 (30) | 2.7 (32) |
| Human bocavirus | 2.0 (23) | 2.6 (30) |
| Enterovirus | 1.2 (14) | 2.0 (24) |
| Mycoplasma pneumonia | 0.3 (4) | 0.4 (5) |
| Coinfections | 9.2 (106) | – |
When the pathogens detected in co‐infections were distributed to every individual pathogen group.
Figure 1.
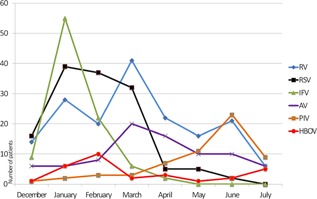
Monthly distribution of the most commonly detected respiratory viruses, HBOV and the viruses co‐detected with HBOV from mid‐December 2013 to July 2014. (RV: rhinovirus, RSV: respiratory syncytial virus, IFV: influenza viruses (A + B), AV: adenovirus, PIV: parainfluenza viruses, HBOV: human bocavirus).
Clinical Features
Clinical characteristics of patients infected with HBOV are shown in Table II. Fourteen patients (46.6%) were followed in the outpatient setting. Of the 16 hospitalized patients, 10 (62.5% of the hospitalized patients) children required pediatric intensive care unit (PICU) admission. The remaining six patients were treated in pediatric wards. Eighteen patients (60%) had a preexisting medical condition. The most common one, being in nine patients, was recurrent wheezing, defined as history of at least two wheezy attacks. It was followed by neurological disease in three patients (cerebral palsy in two, epileptic disease in one), metabolic diseases in two patients (one methylmalonic aciduria, one phenylketonuria), malignity in two patients (one eye tumor, one non‐neutropenic acute lymphoblastic leukemia), and asthma in one patient. When the patients were grouped in terms of presence of comorbidity and then compared, clinical diagnoses and need for hospitalization were found to be significantly different between the groups: Eight patients without comorbiditiy (66.6%) was diagnosed as upper respiratory tract infection, whereas only one patient with comorbidity had upper respiratory tract infection (5.5%; P < 0.001). Hospitalization were required in 77.7% (14 out of 18) of patients with underlying illness, while only 25% (3 out of 12) of patients without underlying illness were admitted to hospital (P = 0.004). Eight out of nine patients with a history of recurrent wheeze required PICU admission.
Table II.
Clinical and laboratorial features of children infected with HBOV
| HBOV positive children | |||||
|---|---|---|---|---|---|
| Inpatients (n:16) | |||||
| All (n:30) | Outpatients (n:14) | Pediatric wards (n:6) | PICU (n:10) | P a | |
| Median age, months (range) | 14 (5–80) | 14 (5–64) | 11.5 (6–80) | 17.5 (12–69) | 0.40 |
| Boys /girls (n/n) | (19/11) (63.3%/36.7%) | (8/6) (57.1%/42.9%) | (5/1) (83.3%/16.7%) | (6/4) (60%/40%) | 0.51 |
| Clinical findings, n (%) | |||||
| Fever | 23 (76.7) | 10 (71.4) | 6 (100) | 7 (70) | 0.46 |
| Cough | 26 (86.7) | 14 (100) | 4 (66.7) | 8 (80) | 0.05 |
| Rhinorrhea | 14 (46.7) | 11 (78.6) | 1 (16.7) | 2 (20) | 0.004 |
| Dyspnea | 16 (53.3) | 1 (7.1) | 5 (83.3) | 10 (100) | <0.001 |
| Wheezing | 19 (63.3) | 4 (28.6) | 5 (83.3) | 10 (100) | <0.001 |
| Rales | 13 (43.3) | 1 (7.1) | 3 (50) | 9 (90) | <0.001 |
| Pharyngitis | 10 (33.3) | 6 (42.9) | 1 (16.7) | 3 (30) | 0.61 |
| Abdominal pain | 4 (13.3) | 3 (21.4) | 0 (0) | 1 (10) | 0.51 |
| Laboratory findings, median (range) | |||||
| WBC | 12,195 (7,700–29,300) | 12,127 (8,900–16,400) | 12,183 (7,700–15,500) | 14,722 (8,800–29,300) | 0.48 |
| Neutrophil count | 7,450 (770–24,900) | 6,026 b (770–11,100) | 7,716 (3,900–10,400) | 11,722 (4,800–24,900) | 0.021 |
| Lymphocyte count | 3,250 (800–7,800) | 4,169c (1,830–7,800) | 3,466 (2,500–4,600) | 2,217 (800–5,300) | 0.012 |
| Neutrophil/lymphocyte | 2.53 (0.31–11.88) | 1.26d (0.31–4.33) | 2.33 (1.26–3.14) | 6.76 (0.91–11.88) | 0.002 |
| Eosinophil count | 100 (0–1,000) | 202e (30–1,000) | 56 (0–150) | 57 (0–200) | 0.006 |
| CRP | 13.5 (1.5–86) | 16.4 (2–86) | 10.26 (1.5–28) | 20.2(2.5–35) | 0.136 |
| Procalcitonin (n = 14) | 1.05 (0.01–13) | 1.4 (n = 1) | 0.56 (n = 3) (0.01–1.5) | 4.01 (n = 10) (0.01–13) | ‐ |
| Chest x ray findings, n (%) | ‐ | ||||
| Normal | 10 (33.3) | 10 (71.4) | 0 | 0 | |
| Peribronchitis | 2 (6.7) | 1 (7.1) | 1 (16.7) | 0 | |
| Hyperinflation | 5 (16.7) | 2 (14.3) | 3 (50) | 0 | |
| Bronchopneumonia | 10 (33.3) | 1 (7,1) | 2 (33.3) | 7 (70) | |
| Pneumonia | 3 (10) | 0 | 0 | 3 (30) | |
| Patients with coinfection, n(%) | 7 (23.3) | 2 (14.3) | 3 (50) | 2 (20) | 0.24 |
| HBOV only | 23 (76.7) | 12 (85.7) | 3 (50) | 8 (80) | – |
| HBOV + RSV | 4 (13.3) | 1 (7.1) | 2 (33.3) | 1 (10) | |
| HBOV + Adenovirus | 2 (6.7) | 0 | 1 (16.7) | 1 (10) | |
| HBOV + HPIV3 | 1 (3.3) | 1 (7.1) | 0 | 0 | |
WBC, white blood cell; CRP, C reactive protein; HBOV, human bocavirus; PICU, pediatric intensive care unit; HPIV3, human parainfluenza virus type 3; RSV, respiratory syncytial virus.
P values represent the comparison of “outpatient”, “pediatric wards”, and “PICU” groups.
P = 0.007, c P = 0.005, d P = 0.001, and e P = 0.005 represent the comparison of “outpatients” and “inpatients” groups.
Fever, cough, dyspnea, and rhinorrhea were the most common complaints. Only one child had stridor and was diagnosed as croup syndrome. One of the patients had edema and erythema of the left eye together with fever on admission. There were no accompanying gastrointestinal symptoms like vomiting or diarrhea except abdominal pain, which was described by four patients. No signs and symptoms that may be relevant to encephalitis existed in the patients on admission.
The median duration of symptoms before admission to the hospital was 2 days (range:1–6 days); with no statistical difference among outpatients, inpatients, and patients admitted to PICU. The most common clinical diagnosis was upper respiratory tract infection (n = 10), followed by bronchopneumonia (n = 9), acute bronchiolitis (n = 5), pneumonia (n = 4), acute bronchitis (n = 1), and asthma execarbation (n = 1). The median duration of hospitalization for the patients admitted to pediatric wards was 7 days (3–10 days). Patients admitted to PICU had a significantly longer hospitalization time (median:14 days; range:4–63 days; P = 0.033). 70% of the patients were prescribed antibiotics: all of the hospitalized patients and 35.7% of outpatients. Nebulized bronchodilators and systemic steroid use were significantly higher in hospitalized patients (P< 0.001). Nebulized adrenalin was used in three patients in PICU.
Laboratory findings of the patients are presented in Table II. Neutrophil counts were higher in hospitalized patients (P = 0.021), while lymphocyte counts were lower (P = 0.012). Neutrophil/lymphocyte ratio was significantly higher in patients admitted to PICU compared to outpatients (P = 0.002). In addition, the patients admitted to hospital had significantly low eosinophil counts than outpatients (P = 0.006). CRP (normal: 0–5 mg/dl) was found to be moderately increased in all patients with no significant difference in between the groups. Procalcitonin was measured in 14 patients, 10 of them were in PICU group and had higher levels.
Pediatric Intensive Care Unit Admissions
One‐third of HBOV detected patients required pediatric intensive care due to a very severe acute lower respiratory tract infection on admission. Of these, eight patients (80%) had a medical history of recurrent wheeze and were otherwise healthy. One patient had metabolic disease and another one had neurological disease. Dual infections were detected in two patients: one with adenovirus and one with RSV. The most common clinical diagnosis on PICU admission was bronchopneumonia. No bacteria was recovered in cultures of tracheal aspirate and blood specimens. Fever, cough, dyspnea, and rhinorrhea were the most common complaints. Six (60%) patients needed mechanical ventilation with a median duration of 102 hr (range 36–480). Noninvasive mechanical ventilation modalities were applied to 4 (40%) patients with a median duration of 42 hr (range 4–96 hr). Median duration of PICU admissions were 4 days with a range of 0–32 days. No mortality was observed.
Patients Complicated With Encephalopathy
Four of the HBOV detected patients admitted to PICU due to severe lower respiratory tract infection had convulsions while mechanically ventilated on the 2nd–3rd days of PICU admission (Table III). Nasal and tracheal samples revealed only HBOV. When sedatives were reduced in dosage, it was realized that consciousness of the patients was also deteriorated. No biochemical disarrangement like electrolyte imbalance or hypoglycemia was detected. There were no remarkable preexisting medical conditions in these patients except recurrent wheezing. Sampling of CSF revealed no abnormality in glucose and protein levels. No pleocytosis was found and no bacteria was recovered on the CSF culture. Viral PCR examination of CSF for Herpes simplex virus type 1–2, cytomegalovirus, Epstein–Barr virus, varicella–zoster virus, human herpesvirus‐6, and enterovirus were negative. The presence of HBOV could not be investigated in CSF of these patients. Cranial MRI of the patients were normal. Electroencephalogram (EEG) displayed status epilepticus in two patients and generalized slow spike activity in other two. Patients with status epilepticus required multi‐drug anticonvulsive therapy while convulsions of other two patients were controlled with phenobarbital only. Two patients with more severe course of encephalopathy had much longer PICU and hospital stay and discharged with cognitive and auditory sequela. There were no differences in demographic characteristics and clinical presentations between the patients complicated with encephalopathy and those without.
Table III.
Clinical Features of HBOV Infected Patients Complicated With Encephalopathy
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Age (months)/sex | 13/F | 33/F | 18/M | 27/M |
| Date of admission | 09.01.2014 | 15.04.2014 | 24.04.2014 | 07.05.2014 |
| Clinical diagnosis on admission | Pneumonia | Pneumonia | Pneumonia | Pneumonia |
| Underlying disease | Recurrent wheezing | Recurrent wheezing | Recurrent wheezing | Recurrent wheezing |
| Need for PICU and MV | Yes | Yes | Yes | Yes |
| Beginning time of seizures | 2nd day of PICU admission | 2nd day of PICU admission | 2nd day of PICU admission | 3rd day of PICU admission |
| Type of seizure | Generalized tonic clonic | Generalized tonic clonic | Myoclonic | Myoclonic |
| Biochemical and microbiological evaluation of CSF | Normal | Normal | Normal | Normal |
| Cranial MRI | Normal | Normal | Normal | Normal |
| EEG | Generalized slow spike acivity | Status epilepticus | Generalized slow spike acivity | Status epilepticus |
| Anticonvulsive therapy | Phenobarbital | Phenobarbital, Phenytoin, Levetirasetam, Thiopental | Phenobarbital | Phenobarbital, Valproic acid, Topiramate, Midazolam, Thiopental |
| Duration of MV (hours) | 36 | 192 | 48 | 480 |
| Duration of hospitalization (days) | 12 | 26 | 17 | 55 |
| Prognosis | No sequela | Mild cognitive sequela | No sequela | Severe cognitive and auditory sequela |
PICU, pediatric intensive care unit; MV, mechanical ventilation; EEG, electroencephalography; CSF, cerebrospinal fluid; MRI, magnetic resonance imaging.
DISCUSSION
HBOV prevalance rate in respiratory tract infections has been reported in a range of 1.5–19%. In this study, HBOV was detected in 2.6% of 1,143 children with respiratory symptoms aged 0–18 years from mid‐December 2013 to July 2014. In terms of frequency, it was the 9th most common virus in the studied period. However, it should be considered while interpreting this result that our data did not cover the whole year and did include all pediatric ages from 0 to 18 years. It is known that HBOV mainly infects children ≤2 years of age, which our study also supports [Allander et al., 2005; Kesebir et al., 2006; Bharaj et al., 2010; Ahn et al., 2014]. In a study, HBOV presence was found to be 10.8% in children aged <5 years [Brieu et al., 2008]. Regional differences may also contribute to the distribution of HBOV infection.
Literature reveals different findings about seasonality of HBOV infection. There are studies indicating a preference of winter and spring months [Allander et al., 2005; Chuang et al., 2011], or a peak prevalence in summer [Zhou et al., 2014; Lu et al., 2015]. On the other hand, no apparent seasonal prevalence was determined in some other studies [Bastien et al., 2007; Bharaj et al., 2010]. Because the current study did not cover the whole year, it cannot contribute to evaluation of HBOV incidence over seasons. However, it is worthy to note that in this study the third most common HBOV detection was in summer (July), following February and January.
In this study, co‐detection rate of other viruses and HBOV was 23.3%, being mostly with RSV. It was similar to the finding of a study from China, which have a high number of patients with HBOV detection (n = 502) and a co‐infection rate of 29% [Chen et al., 2014]. They found no difference between HBOV single infection and co‐infection in terms of clinical and laboratory findings, similar to the current study. Another similar finding was reported by Brieu et al. [2008] who showed lack of difference in severity between children co‐infected with HBOV and RSV and those infected with RSV alone. A much higher co‐detection rate of HBOV (78.1%) was reported in a recent study from Argentina [Ghietto et al., 2015]. They also stated that clinical features of patients with HBoV‐single infection were not significantly different from those with co‐infection. In the current study, the majority of co‐infections of HBOV occurred with RSV, similar to some other reports in the literature [Wang et al., 2010; Ghietto et al., 2015]. They co‐circulated in winter and spring in our study period, but not in summer. It may be speculated that there may be any other interaction between these two viruses. However, it is noteworthy that there are also studies reporting other viruses like rhinovirus and parainfluenza viruses as the most commonly co‐detected viruses with HBOV [Ahn et al., 2014; Zhou et al., 2014; Lu et al., 2015]. The interaction of other viruses and HBOV is a complicated issue which should be further investigated in a different manner other than clinical features alone.
Most reports include only hospitalized patients with respiratory infection [Völz et al., 2007; Brieu et al., 2008; Chen et al., 2014]. Patients admitted to emergency room or pediatric outpatient clinics were included in this study, which would have enabled a more precise picture of clinical severity of HBOV infections. In the studies with a similar setting, very low hospitalization rates have been reported [Chuang et al., 2011;]. However, severity of illness was very remarkable in the current study: 43.3% of patients had radiologically confirmed bronchopneumonia/pneumonia, 33.3% of patients required PICU admission, and 20% of the patients were mechanically ventilated. A study conducted by Völz et al. [2007] indicated pneumonia and need for oxygen supplementation in the majority of the study group, but no administration of mechanical ventilation. On the contrary, in another study, only 2 patients were hospitalized among 22 patients with HBOV infection [Bharaj et al., 2010].
Major clinical symptoms were cough, fever, and wheezing in the HBOV infected patients. On the other hand, rhinorrhea, if present, was almost exclusively a sign/symptom of outpatients. The most common clinical diagnoses included upper respiratory tract infection, bronchopneumonia, and bronchiolitis. This spectrum of symptoms and diagnoses were in accordance with other reports defining HBOV and other viral respiratory tract infections [Brieu et al., 2008; Ghietto et al., 2015]. All patients were ordered a chest X ray and only one‐third of chest X rays were normal. High rate of radiological investigation may be explained by relatively young age of the patients and presence of an underlying disease in most of them. Comorbidity was detected in 60% of our study group. It was associated with increased severity of the clinical picture caused by HBOV. However, in a recent study, comorbidity was found to be present in 31% of HBOV infected patients and no difference other than age of patients with comorbidities, which were older than previously healthy ones, was reported [Ghietto et al., 2015]. Recurrent wheezing was the most common preexisting medical condition in our patients (30%), and one patient had a history of asthma. This finding supports some studies in the literature, one of which reported a significantly high rate of preexisting wheezing and asthma episode in patients infected with HBOV, compared to HMPV and RSV [Brieu et al., 2008; Ghietto et al., 2015].
There was no detailed information about the laboratory findings of the patients infected with HBOV. Generally, a mild to moderate leucocytosis and increase in CRP were reported in several studies, as in this study [Völz et al., 2007; Chen et al., 2014]. When differential blood counts were analyzed, it was noticed that neutrophilia and lymphopenia was significantly increasing as disease severity increases. Thus, neutrophil to lymphocyte ratio, a convenient marker of systemic inflammation, was significantly higher in patients admitted to pediatric wards and PICU. On the other hand, eosinophil counts were significantly decreased in hospitalized patients compared to outpatients. These findings are noteworthy, but we do not think that they are specific to HBOV infection, since their relation to increased disease severity was established for other diseases as well [Zahorec, 2001; Abidi et al., 2008].
HBOV is associated with respiratory disease in vast majority of reports. Its association with encephalitis has been shown only by three reports [Mitui et al., 2012; Mori et al., 2013; Yu et al., 2013]. Researchers investigated the presence of HBOV genome in CSF samples of children with signs and symptoms of encephalitis. They found HBOV in 3%, 5.8% and 15% (number of patients: 191, 69, and 67, respectively) of patients with encephalopathy. HBOV viremia have been documented previously [Allander et al., 2007]. Mitui et al. [2012] detected HBOV genome also in the serum of one study patient. They found that the sequence of the HBOV detected in CSF and the corresponding serum were similar. This finding may indicate that HBOV encephalitis was caused by hematogenous route of the virus [Mitui et al., 2012]. However, the disruption mechanism of blood–brain barrier is to be determined. It is currently not known that whether HBOV dissemination to the central nervous system is related to host immunological factors, or associated with viral mutations leading to altered cell tropism or pathogenesis [Mitui et al., 2012]. CSF pleocytosis and abnormal protein/glucose levels are not common findings in all three studies evaluating HBOV encephalitis [Mitui et al., 2012; Mori et al., 2013; Yu et al., 2013]. Although normal or near‐normal CSF findings may be expected in viral encephalitis, it may be argued that an impaired inflammatory response related to host may exist. Mitui et al. [2012] linked this issue to the malnourished condition of their study group. However, other studies have also reported HBOV encephalitis in well nourished children [Mori et al., 2013]. So, there may be other host related immunological impairments, unknown currently. In our study group, patients were admitted to hospital with respiratory sypmtoms. On admission, no signs and symptoms related to encephalopathy were present. However, in four patients, who were admitted to PICU because of severe respiratory disease, a clinical picture of encephalopathy characterized by seizures and decreased consciousness developed within first 2–3 days of PICU admission. We described this clinical picture as encephalopathy rather than encephalitis due to negative findings on CSF investigations and cranial imaging. They had pneumonia and HBOV was determined in their tracheal and nasal specimens by PCR method. They had no preexisting neurological or metabolic disease. They neither required resuscitation, nor exposed to ischemia. No specific reason for encephalopathy was found after a thorough investigation. HBOV genome could not be investigated in CSF samples of these patients. Based on these findings, it could be postulated that HBOV viremia developed in patients with pneumonia and hematogenous spread of HBOV to the central nervous system caused the encephalopathy in these patients. However, why these four patients suffered from such a severe pneumonia followed by encephalopathy and others not is remained to be elucidated.
In conclusion, this study indicates that HBOV is a circulating respiratory viral pathogen in children of our region and especially important for younger children and children with comorbidities. It may cause severe respiratory infection requiring hospitalization and PICU admission. Although not confirmed microbiogically, its association with encephalopathy in four of our patients with pneumonia is the first report on this issue. Future studies with the use of quantitative PCR and serology of CSF are needed to determine the exact role of HBOV infection in encephalopathy.
ACKNOWLEDGMENT
We thank Hayriye Vehid, PhD, Department of Family Health, Institute of Pediatrics, Istanbul University, Istanbul, for her support in the statistical evaluation of this report.
The institution at which the work was performed: Istanbul Faculty of Medicine, Istanbul University, Istanbul, Turkey.
REFERENCES
- Abidi K, Khoudri I, Belayachi J, Madani N, Zekraoui A, Zeggwagh AA, Abouqal R. 2008. Eosinopenia is a reliable marker of sepsis on admission to medical intensive care units. Crit Care 12:R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JG, Choi SY, Kim DS, Kim KH. 2014. Human bocavirus isolated from children with acute respiratory tract infections in Korea, 2010–2011. J Med Virol 86:2011–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung‐Lindell A, Andersson B. 2005. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA 36:12891–12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allander T, Jartti T, Gupta S, Niesters HG, Lehtinen P, Osterback R, Vuorinen T, Waris M, Bjerkner A, Tiveljung‐Lindell A, van den Hoogen BG, Hyypiä T, Ruuskanen O. 2007. Human bocavirus and acute wheezing in children. Clin Infect Dis 44:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson TP, Werno AM, Barratt K, Mahagamasekera P, Murdoch DR, Jennings LC. 2013. Comparison of four multiplex PCR assays for the detection of viral pathogens in respiratory specimens. J Virol Methods 191:118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien N, Chui N, Robinson JL, Lee BE, Dust K, Hart L, Li Y. 2007. Detection of human bocavirus in Canadian children in a 1‐year study. J Clin Microbiol 45:610–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharaj P, Sullender WM, Kabra SK, Broor S. 2010. Human bocavirus infection in children with acute respiratory tract infection in India. J Med Virol 82:812–816. [DOI] [PubMed] [Google Scholar]
- Brieu N, Guyon G, Rodière M, Segondy M, Foulongne V. 2008. Human bocavirus infection in children with respiratory tract disease. Pediatr Infect Dis J 27:969–973. [DOI] [PubMed] [Google Scholar]
- Canducci F, Debiaggi M, Sampaolo M, Marinozzi MC, Berre‘ S, Terulla C, Gargantini G, Cambieri P, Romero E, Clementi M. 2008. Two year prospective study of single infections and co‐ infections by respiratory syncytial virus and viruses identified recently in infants with acute respiratory disease. J Med Virol 80:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZR, Mize M, Wang YQ, Yan YD, Zhu CH, Wang Y, Ji W. 2014. Clinical and epidemiological profiles of lower respiratory tract infection in hospitalized children due to human bocavirus in a subtropical area of China. J Med Virol 86:2154–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang CY, Kao CL, Huang LM, Lu CY, Shao PL, Lee PI, Chang LY. 2011. Human bocavirus as an important cause of respiratory tract infection in Taiwanese children. J Microbiol Immunol Infect 44:323–327. [DOI] [PubMed] [Google Scholar]
- Ghietto LM, Majul D, Ferreyra Soaje P, Baumeister E, Avaro M, Insfrán C, Mosca L, Cámara A, Moreno LB, Adamo MP. 2015. Comorbidity and high viral load linked to clinical presentation of respiratory human bocavirus infection. Arch Virol 160:117–127. [DOI] [PubMed] [Google Scholar]
- Kesebir D, Vazquez M, Weibel C, Shapiro ED, Ferguson D, Landry ML, Kahn JS. 2006. Human bocavirus infection in young children in the United States: Molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. J Infect Dis 194:1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu QB, Wo Y, Wang HY, Huang DD, Zhao J, Zhang XA, Zhang YY, Liu EM, Liu W, Cao WC. 2015. Epidemic and molecular evolution of human bocavirus in hospitalized children with acute respiratory tract infection. Eur J Clin Microbiol Infect Dis 34:75‐ 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitui MT, Tabib SM, Matsumoto T, Khanam W, Ahmed S, Mori D, Akhter N, Yamada K, Kabir L, Nishizono A, Söderlund‐Venermo M, Ahmed K. 2012. Detection of human bocavirus in the cerebrospinal fluid of children with encephalitis. Clin Infect Dis 54:964–967. [DOI] [PubMed] [Google Scholar]
- Mori D, Ranawaka U, Yamada K, Rajindrajith S, Miya K, Perera HK, Matsumoto T, Dassanayake M, Mitui MT, Mori H, Nishizono A, Söderlund‐Venermo M, Ahmed K. 2013. Human bocavirus in patients with encephalitis, Sri Lanka, 2009‐2010. Emerg Infect Dis 19:1859–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakthivel SK, Whitaker B, Lu X, Oliveira DB, Stockman LJ, Kamili S, Oberste MS, Erdman DD. 2012. Comparison of fast‐track diagnostics respiratory pathogens multiplex real‐time RT‐PCR assay with in‐house singleplex assays for comprehensive detection of human respiratory viruses. J Virol Methods 185:259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völz S, Schildgen O, Klinkenberg D, Ditt V, Müller A, Tillmann RL, Kupfer B, Bode U, Lentze MJ, Simon A. 2007. Prospective study of Human Bocavirus (HBoV) infection in a pediatric university hospital in Germany 2005/2006. J Clin Virol 40:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Wang W, Yan H, Ren P, Zhang J, Shen J, Deubel V. 2010. Correlation between bocavirus infection and humoral response, and co‐infection with other respiratory viruses in children with acute respiratory infection. J Clin Virol 47:148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JM, Chen QQ, Hao YX, Yu T, Zeng SZ, Wu XB, Zhang B, Duan ZJ. 2013. Identification of human bocaviruses in the cerebrospinal fluid of children hospitalized with encephalitis in China. J Clin Virol 57:374–377. [DOI] [PubMed] [Google Scholar]
- Zahorec R. 2001. Ratio of neutrophil to lymphocyte counts rapid and simple parameter of systemic inflammation and stress incritically ill. Bratisl Lek Listy 102:5–14. [PubMed] [Google Scholar]
- Zhang LL, Tang LY, Xie ZD, Tan XJ, Li CS, Cui AL, Ji YX, Xu ST, Mao NY, Xu WB, Shen KL. 2008. Human bocavirus in children suffering from acute lower respiratory tract infection in Beijing Children's Hospital. Chin Med J 121:1607–1610. [PubMed] [Google Scholar]
- Zhou L, Zheng S, Xiao Q, Ren L, Xie X, Luo J, Wang L, Huang A, Liu W, Liu E. 2014. Single detection of human bocavirus 1 with a high viral load in severe respiratory tract infections in previously healthy children. BMC Infect Dis 14:424. [DOI] [PMC free article] [PubMed] [Google Scholar]


