Abstract
The 2009 pandemic H1N1 influenza A virus spread quickly worldwide in 2009. Since most of the fatal cases were reported in developing countries, rapid and accurate diagnosis methods that are usable in poorly equipped laboratories are necessary. In this study, a mobile detection system for the 2009 H1N1 influenza A virus was developed using a reverse‐transcriptase loop‐mediated isothermal amplification (RT‐LAMP) kit with a disposable pocket‐warmer as a heating device (designated as pwRT‐LAMP). The pwRT‐LAMP can detect as few as 100 copies of the virus—which is nearly as sensitive as real‐time reverse‐transcription polymerase chain reaction (RT‐PCR)—and does not cross‐react with RNA of seasonal influenza viruses. To evaluate the usefulness of the pwRT‐LAMP system, nasal swab samples were collected from 56 patients with flu‐like symptoms and were tested. Real‐time RT‐PCR confirmed that the 2009 H1N1 influenza A virus was present in 27 of the 56 samples. Of these 27 positive samples, QuickVue Influenza A + B immunochromatography detected the virus in only 11 samples (11/27; 40.7%), whereas the pwRT‐LAMP system detected the virus in 26 of the 56 samples (26/27 of the positive samples; 96.3%). These findings indicate that the mobile pwRT‐LAMP system is an accurate diagnostic system for the 2009 H1N1 influenza A virus, and has great potential utility in diagnosing future influenza pandemics. J. Med. Virol. 83:568–573, 2011. © 2011 Wiley‐Liss, Inc.
Keywords: 2009 pandemic H1N1influenza A virus, pocket‐warmer RT‐LAMP, real‐time RT‐PCR
INTRODUCTION
The 2009 pandemic H1N1 influenza A virus spread quickly worldwide in 2009. According to a World Health Organization report in April 2010, more than 155 countries reported laboratory‐confirmed cases of this virus, including over 17,900 deaths [WHO, 2010]. Of note, most of these fatal cases were reported in developing countries. The 2009 H1N1 influenza A virus infection can be diagnosed reliably with real‐time reverse‐transcription polymerase chain reaction (RT‐PCR) [Carr et al., 2009; Poon et al., 2009; Wang et al., 2009; Whiley et al., 2009]. However, real‐time RT‐PCR equipment can be too expensive or unwieldy to have onsite for many small and front‐line laboratories, which requires personnel to ship the isolated clinical samples to outside laboratories equipped for the procedure. Even if the real‐time RT‐PCR equipment is available onsite, processing of samples using this technique takes more than 2 hr. In contrast, immunochromatography assays can be performed typically in just 10–15 min with a convenient diagnosis kit, but this method is not sensitive enough for accurate diagnosis of the 2009 H1N1 influenza A virus infection [Chan et al., 2009; Faix et al., 2009]. Because the H1N1 influenza A virus is especially impactful in developing countries, which often have limited facilities, rapid and accurate diagnostic methods that can be used in poorly equipped laboratories should be developed.
The loop‐mediated isothermal amplification (LAMP) system is a nucleic acid amplification system that involves self‐recurring strand‐displacement DNA synthesis primed by a specially designed set of target‐specific primers under isothermal conditions [Notomi et al., 2000; Nagamine et al., 2002]. LAMP can be completed within 30–60 min at 60°C. A reverse transcriptase (RT) reaction was available before LAMP (RT‐LAMP); RT‐LAMP has been used successfully to detect human pathogenic RNA viruses [Hong et al., 2004; Poon et al., 2004, 2005; Fujino et al., 2005; Okafuji et al., 2005; Ushio et al., 2005; Ito et al., 2006], and has already been developed to detect the 2009 H1N1 influenza A virus [Kubo et al., 2010]. Specifically, RT‐LAMP targets the 2009 H1N1 influenza A virus hemagglutinin (HA) gene, with 97.8% sensitivity and 100% specificity in clinical samples compared with the corresponding measurements for real‐time RT‐PCR specific to 2009 influenza A H1N1 virus. However, the traditional RT‐LAMP method requires a heat block, and a freezer in which the necessary reagents are stocked. A mobile LAMP system for anthrax diagnosis was developed recently using a disposable pocket‐warmer as a heating device [Hatano et al., 2010]. This advancement is particularly convenient because disposable pocket‐warmers are cheap, and are well‐known winter commodities in Japan. Thus, the pocket‐warmer LAMP method requires no expensive or specialized equipment, such as heat blocks or thermocyclers. The aforementioned anthrax study showed that the sensitivity and specificity of the pocket‐warmer LAMP method were similar to those of the conventional LAMP method that uses a heat block [Hatano et al., 2010], making it a potentially powerful diagnostic tool for poorly equipped laboratories in areas of pandemic infection. In the present study, the 2009 H1N1 influenza A virus‐specific pocket‐warmer RT‐LAMP (pwRT‐LAMP) system was developed, and its sensitivity and specificity were evaluated using nasal swab samples from individuals with flu‐like symptoms. A dried form of the LAMP reagent was also examined to develop a mobile diagnosis system that does not require heat blocks or freezers.
MATERIALS AND METHODS
Clinical Samples
This study was approved by Institutional Review Boards of Japan Self‐Defense Force Central Hospital (approval no. 21‐012) and the National Institute of Infectious Diseases (approval no. 230). Written informed consent was obtained from all participants. All clinical specimens were collected at the Japan Self‐Defense Force Central Hospital in January and February of 2010. Fifty‐six total patients including 43 males and 13 females were enrolled. The mean age of the patients was 31.6 years (range: 20–51 years). All patients who presented with histories of fever or feverishness, and coughs and/or sore throats within 5 days of symptom onset were included in this study. A specimen was collected from the nares of each patient with a dry swab tip; tip of the collection swab was inserted approximately 3 cm into the nares and rolled several times in each nostril. Subsequently, the tips of the swabs were placed in the solutions of QuickVue tests Influenza A + B (Quidel Corporation, San Diego, CA). In addition to the clinical samples, supernatants of 2009 H1N1 influenza A virus (strain A/Tokyo/NIID1/2009(H1N1)), seasonal influenza A (H1 [strain New Caledonia 20/99], H3 [strain Hiroshima], H5 [strain A/HK/Z13/03]), influenza B, para‐influenza virus types 1 and 3, respiratory syncytial (RS) virus (type A), human metapneumovirus and severe acute respiratory syndrome (SARS) virus‐infected cells were used as positive or negative controls.
Immunochromatography Test for Influenza Virus
Nasal swab samples were examined with the QuickVue Influenza A + B rapid immunochromatography test, according to the manufacturer's instructions.
RNA Extraction
RNA was extracted from remnant solutions of QuickVue tests using QIAamp viral RNA mini kits (Qiagen, Hilden, Germany). The RNA was eluted in a final volume of 60 µl of elution buffer.
Design of pwRT‐LAMP Primers
Primers specific for the 2009 H1N1 influenza A virus HA gene were designed for the RT‐LAMP assay. Briefly, nucleotide sequences of human isolates of the 2009 H1N1 influenza A virus HA genes were retrieved from the GenBank database and aligned to identify potential target regions using a Genetyx analyzer (Genetyx, Tokyo, Japan). Sequences of 20 strains of the 2009 H1N1 influenza A virus HA genes were aligned and compared to those of 20 randomly selected strains of seasonal H1N1 influenza A viruses. Finally, a set of six primers, which was comprised of two outer primers (forward primer F3 5′‐AAGCTCAGCAAATCCTACA‐3′ and backward primer B3 5′‐TCCCTCACTTTGGGTCTT‐3′); two inner primers (forward inner primer [FIP] 5′‐GACTTTGTTGGTCAGCACTAGTAGAAAAGGGAAAGAAGTCCTCG‐3′ and backward inner primer [BIP] 5′‐TCTATCAGAATGCAGATGCATATGTTGCTATTTCCGGCTTGAA‐3′); and two loop primers (forward loop primer [LF] 5′‐GATGGTGAATGCCCCATAGC‐3′ and backward loop primer [LB] 5′‐TTTTGTGGGGTCATCAAGATACAG‐3′) that recognize eight distinct regions on the target sequence, was designed by using the LAMP primer design software Primer Explorer (version 4; Eiken Chemical, Tokyo, Japan; http://primerexplorer.jp/elamp4.0.0/index.html). The target for the RT‐LAMP assay was based on 529–716 nt in the HA gene sequence of GenBank GQ165814, from the strain A/Narita/1/2009(H1N1). All primers used in this study were synthesized by Sigma‐Genosys Japan (Tokyo, Japan).
pwRT‐LAMP and Conventional RT‐LAMP
The pw RT‐LAMP assays were carried out using the Loopamp RNA Amplification Reagent for RT‐LAMP (LMP244‐246; Eiken Chemical) with LAMP primers specific for 2009 H1N1 virus as described in the section above. For each pwRT‐LAMP assay, 9 µl of extracted RNA was added to 41 µl of master mix containing 25 µl of 2× reaction mix, 1 µl of enzyme mix, 2 µl of fluorescence detection reagent (LMP‐221; Eiken Chemical), 40 pmol FIP and BIP primers, 5 pmol outer primers (F3 and B3 primers) and 20 pmol loop primers (LF and LB primers). The mixtures were incubated using a disposable pocket‐warmer (Hokaron Haru‐type, Lotte Health Products, Tokyo, Japan) and a Styrofoam box for 50 min as described previously [Hatano et al., 2010]. After the RT‐LAMP reaction was carried out, the related fluorescence was detected directly in the reaction tubes using an ultraviolet (UV) transilluminator (365 nm wave length; LAS‐3000; Fujifilm, Tokyo, Japan). A dried form of RNA Amplification Reagent (LMP‐271; Eiken Chemical) was also examined as a RT‐LAMP reagent for use in the pwRT‐LAMP system. For the assays with the dried form of the reagent, 15 µl of the 2009 H1N1 influenza A virus primer reagent (PM0021; Eiken Chemical) and 10 µl of RNA sample were added to a dried‐form RT‐LAMP tube, and the tube was incubated with a pocket‐warmer in a Styrofoam box for 50 min.
Conventional RT‐LAMP with Loopamp RNA Amplification Reagent was also carried out with a heat block. For the conventional RT‐LAMP method, 50 µl of RT‐LAMP mixture including 9 µl of RNA sample was incubated in a Loopamp Real‐Time Turbidimeter (LA‐320C; Eiken Chemical) for 60 min at 60°C and then for 5 min at 80°C to terminate the reaction.
Real‐Time RT‐PCR for the 2009 H1N1 Influenza A Virus
TaqMan real‐time RT‐PCR was performed to measure the copy numbers of the 2009 H1N1 influenza A virus with a Mx3005P real‐time PCR system (Stratagene, La Jolla, CA) and a QuantiTect Probe RT‐PCR kit (Qiagen) as described previously [Nakajima et al., 2010], using 1 µl of extracted RNA as the template in each reaction mixture. Each sample whose amplification plot crossed the threshold line within 40 cycles (i.e., the threshold cycle was <40) was considered positive. A recombinant plasmid containing a fragment of the HA gene was used as a positive control in the standard curve.
RESULTS
Establishment and Evaluation of the 2009 H1N1 Influenza A Virus‐Specific pwRT‐LAMP
The RT‐LAMP method requires a constant temperature of 60–65°C for more than 60 min for reverse‐transcription and amplification of cDNA. A previous study showed that the temperature of a disposable pocket‐warmer in a Styrofoam box reached 58°C in 30 min and remained around 60°C for more than 60 min [Hatano et al., 2010]. In addition, this study revealed that the temperature inside a Styrofoam box was consistent both under cold (4°C) and warm (37°C) conditions [Hatano et al., 2010]. Thus, the pwRT‐LAMP was used in the present study to detect the 2009 H1N1 influenza A virus. Observation under UV or ambient light showed that the pwRT‐LAMP system coupled with the HA primer set detected 100 copies of target RNA per reaction volume (both in 25 and 50 µl) after incubation for 60 min (Fig. 1A). To confirm the specificity of pwRT‐LAMP, RNA samples extracted from seasonal influenza viruses and other RNA viruses were also examined. These results clearly showed that the pwRT‐LAMP system detected the 2009 H1N1 influenza A virus, but not the other viruses, including the seasonal influenza A (H1, H3, H5) viruses (Fig. 1B). The sensitivity and specificity of pwRT‐LAMP corresponded with the results from conventional RT‐LAMP that used a heat block at 60°C for 30 min (data not shown).
Figure 1.
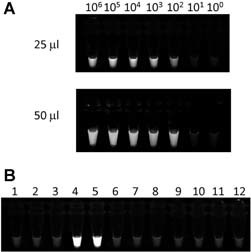
Sensitivity and specificity of pocket‐warmer reverse‐transcriptase loop‐mediated isothermal amplification (pwRT‐LAMP) for the 2009 H1N1 influenza A virus. A: Sensitivity of pwRT‐LAMP for the 2009 H1N1 influenza A virus in volumes of 25 µl (upper panel) and 50 µl (lower panel). Culture supernatants containing the 2009 H1N1 influenza A virus whose copy number (106–100) was measured by real‐time RT‐PCR were used as controls. B: Specificity of pwRT‐LAMP for the 2009 H1N1 influenza A virus. 1, Influenza virus H1; 2, H3; 3, H5; 4, 2009 H1N1 virus (strain A/Tokyo/NIID1/2009(H1N1)); 5, H1N1pdm (strain A/Nagano/RC1/2009(H1N1)); 6, Influenza virus B; 7, PIV1; 8, PIV3; 9, RS virus type A; 10, metapneumovirus; 11, SARS corona virus; 12, cellular RNA of human lymphocytes.
The pwRT‐LAMP with the dried RNA Amplification Reagent was also performed, which showed that 100 copies per tube was the lower detection limit and involved a specific reaction to the 2009 H1N1 influenza A virus (data not shown). These results indicate that the pwRT‐LAMP system specifically detects the HA gene of the 2009 H1N1 influenza A virus without cross‐reacting with other RNA viruses.
Diagnostic Evaluation of pwRT‐LAMP With Clinical Samples
To evaluate the diagnostic accuracy of pwRT‐LAMP, 56 nasal swab samples from individuals with flu‐like symptoms were examined. All samples were examined with immunochromatography tests, conventional RT‐LAMP, pwRT‐LAMP, pwRT‐LAMP with the dried RNA Amplification Reagent, and real‐time RT‐PCR (Table I and Fig. 2). Of the 56 samples, immunochromatography tests detected influenza virus type A or B in 11 samples (19.6%), whereas real‐time RT‐PCR detected the 2009 H1N1 influenza A virus in 27 samples (48.2%). Of the 27 samples identified as positive for 2009 H1N1 by real‐time RT‐PCR, pwRT‐LAMP and pwRT‐LAMP with dried RNA Amplification Reagent positively identified 26 (96.3%) and 27 samples (100%), respectively. In contrast, only 11 of the 27 samples (40.7%) were positively identified by the immunochromatography tests. All pwRT‐LAMP reaction tubes were visualized by both a UV transilluminator and under ambient light, and determined the results of reactions were identical under both types of light, which suggests that ambient light is sufficient for identification of positive signals. In addition, the samples were subjected to conventional RT‐LAMP with a turbidimeter. The threshold times, defined as the time (in seconds) needed to reach the threshold turbidity level (0.1), significantly correlated with virus copy number as determined by real‐time RT‐PCR (R2 = 0.7858, Fig. 3). Conventional RT‐LAMP detected the virus in all 27 samples that were found positive by real‐time RT‐PCR (Table I). These findings indicate that the pwRT‐LAMP technique detects the 2009 H1N1 influenza A virus in clinical samples with sensitivity and specificity similar to real‐time RT‐PCR and conventional RT‐LAMP.
Table I.
Assay Results and Virus Copy Numbers
| No. | Immunochromatography | RT‐LAMP | pwRT‐LAMP | pwRT‐LAMP/dried reagent | Real‐time RT‐PCR (copies) |
|---|---|---|---|---|---|
| 1 | + | + | + | + | 3.73 × 107 |
| 2 | + | + | + | + | 1.12 × 107 |
| 3 | + | + | + | + | 8.41 × 106 |
| 4 | + | + | + | + | 7.24 × 106 |
| 5 | + | + | + | + | 2.02 × 106 |
| 6 | + | + | + | + | 1.70 × 106 |
| 7 | + | + | + | + | 6.77 × 105 |
| 8 | + | + | + | + | 5.33 × 105 |
| 9 | − | + | + | + | 5.27 × 105 |
| 10 | + | + | + | + | 2.73 × 105 |
| 11 | + | + | + | + | 1.74 × 105 |
| 12 | − | + | + | + | 7.37 × 104 |
| 13 | − | + | + | + | 7.04 × 104 |
| 14 | + | + | + | + | 5.04 × 104 |
| 15 | − | + | + | + | 4.08 × 104 |
| 16 | − | + | + | + | 1.70 × 104 |
| 17 | − | + | + | + | 1.39 × 104 |
| 18 | − | + | + | + | 8.39 × 103 |
| 19 | − | + | + | + | 7.03 × 103 |
| 20 | − | + | + | + | 6.51 × 103 |
| 21 | − | + | + | + | 3.82 × 103 |
| 22 | − | + | + | + | 3.02 × 103 |
| 23 | − | + | + | + | 1.01 × 103 |
| 24 | − | + | + | + | 8.65 × 102 |
| 25 | − | + | + | + | 5.70 × 102 |
| 26 | − | + | + | + | 4.89 × 102 |
| 27 | − | + | − | + | 4.75 × 102 |
| 28–56 | − | − | − | − | 0 |
| Positivity rate in 27 samples found positive through real‐time RT‐PCR | |||||
| 40.7% (11/27) | 100% (27/27) | 96.3% (26/27) | 100% (27/27) | ||
Figure 2.
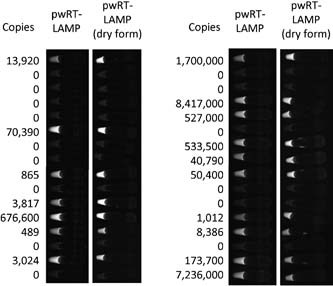
Example copy numbers and UV images of pocket‐warmer reverse‐transcriptase loop‐mediated isothermal amplification (pwRT‐LAMP) in clinical samples. Copy numbers were measured with real‐time RT‐PCR. Images of the tubes used in pwRT‐LAMP and pwRT‐LAMP with dried reagent were taken under UV light with an image analyzer.
Figure 3.
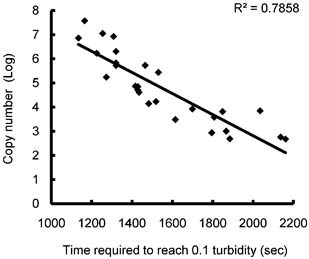
Relationship of turbidity with copy number of the 2009 H1N1 influenza A virus. Real‐time turbidity was detected with a Loopamp real‐time turbidimeter. The time required to reach the threshold turbidity level (0.1) (threshold time) were plotted against the copy numbers of the samples determined by real‐time RT‐PCR.
DISCUSSION
In the present study, the pwRT‐LAMP method was developed to detect the 2009 H1N1 influenza A virus. The pwRT‐LAMP technique has similar sensitivity and specificity to real‐time RT‐PCR and requires only 50 min for HA gene amplification without the use of a heat block or thermal cycler. The positive signals of pwRT‐LAMP can be visualized directly by placing a reaction tube either on a UV transilluminator (254–366 nm wave‐length), or in ambient light. These characteristics make this detection system particularly useful for poorly equipped laboratories, or for any front‐line station that treats or investigates pandemic influenza.
The biggest advantage of this system is that it does not require a heat block or a PCR machine. Currently, hospitals and clinics that lack PCR equipment must ship samples to equipped laboratories for final diagnoses of influenza infection, which is time‐consuming and inefficient. The results in the present study indicate that the pwRT‐LAMP system could be a time‐saving and convenient method in comparison, by allowing analysis to be performed onsite. Although hot water is more available than disposable pocket‐warmers in laboratories, it is difficult to maintain an optimal water temperature for analysis outside of laboratories. In that sense, a pocket warmer is much more convenient and mobile than hot water. Furthermore, a disposable pocket‐warmer that contains iron powder, water, vermiculite, activated carbon, and salt is easily obtainable via the internet (i.e., if outside of Japan), and costs less than US$1, suggesting that this system can be established quite easily with low cost.
In the present study, pwRT‐LAMP using a dried form of the RNA Amplification Reagent had the same sensitivity and specificity as real‐time RT‐PCR. The dried form of the RNA Amplification Reagent and primer mix for the 2009 H1N1 influenza A virus can be stored at 4°C for 6 months (LMP462; Eiken Chemical). Therefore, inclusion of this dried reagent in pwRT‐LAMP negates the need for a freezer. Thus, the pwRT‐LAMP method with the dried RNA Amplification Reagent may be a particularly powerful tool for accurately detecting influenza virus in clinics and hospitals with unequipped laboratories or at the site of influenza virus pandemics.
However, to establish an entirely mobile pwRT‐LAMP system, RNA extraction methods must be improved. In this study, the QIAamp viral RNA mini kit that takes approximately 60 min to complete the whole RNA extraction process was used, but this protocol requires the use of a microcentrifuge. Several RNA extraction kits that do not require the use of a centrifuge or a heat incubator were then tried; however, the RNA samples extracted with these kits showed low sensitivity in both pwRT‐LAMP and real‐time RT‐PCR (data not shown). Further studies are necessary to establish RNA extraction methods suitable for pwRT‐LAMP, ultimately to enhance the mobility of the method.
Both the pwRT‐LAMP and real‐time RT‐PCR revealed more 2009 H1N1 influenza A virus‐positive results than did immunochromatography assays, confirming the low sensitivity of the latter method for influenza virus [Chan et al., 2009; Faix et al., 2009]. Since real‐time RT‐PCR and RT‐LAMP amplify target genes, the sensitivity of these assays is much higher than immunochromatography, which detects viral antigens immunologically. It is possible that the virus copies in some nasal swab samples were not sufficient for detection by immunochromatography. In addition, since immunochromatography can detect influenza A and B, there may be certain mutations in the amino acid sequences of the 2009 H1N1 influenza A virus HA that cause the virus to go unrecognized by the antibody. However, immunochromatography for 2009 H1N1 virus was recently developed [Miyoshi‐Akiyama et al., 2010]. Although this method works as a means of quick diagnosis for the 2009 H1N1 influenza A virus, its sensitivity still may not be high enough for accurate diagnosis, because it is an immunological method and the amount of virus titer in nasal swabs may be insufficient for detection. Thus, the pwRT‐LAMP method is more sensitive than immunochromatography, and offers diagnoses that are more accurate.
In summary, pwRT‐LAMP was developed as a mobile and accurate diagnostic system for the 2009 H1N1 influenza A virus. This technique does not require the use of a heat block or a PCR machine, and its high sensitivity and specificity are sufficient for diagnosis of 2009 H1N1 influenza A virus infection. The rapid worldwide spread of the 2009 H1N1 influenza A virus in 2009 resulted in international demands for a speedy and accurate diagnostic system for the pandemic virus. The pwRT‐LAMP method described herein can facilitate diagnosis efforts in the event of a subsequent pandemic influenza virus.
Acknowledgements
We greatly thank Setsuko Ohtsuka, Kyoko Saiki, Tomoko Nishimoto, Tamami Kudo, and Hiromi Nagata (Japan Self‐Defense Force Central Hospital, Tokyo, Japan) for their excellent support in collecting clinical samples. We also thank Tatsumi Shimizu (Administration Officer of Military Medicine Research Unit) for his special administrative assistance. We declare that we have no potential conflicts of interest relevant to this article.
REFERENCES
- Carr MJ, Gunson R, Maclean A, Coughlan S, Fitzgerald M, Scully M, O'Herlihy B, Ryan J, O'Flanagan D, Connell J, Carman WF, Hall WW. 2009. Development of a real‐time RT‐PCR for the detection of swine‐lineage influenza A (H1N1) virus infections. J Clin Virol 45: 196–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KH, Lai ST, Poon LL, Guan Y, Yuen KY, Peiris JS. 2009. Analytical sensitivity of rapid influenza antigen detection tests for swine‐origin influenza virus (H1N1). J Clin Virol 45: 205–207. [DOI] [PubMed] [Google Scholar]
- Faix DJ, Sherman SS, Waterman SH. 2009. Rapid‐test sensitivity for novel swine‐origin influenza A (H1N1) virus in humans. N Engl J Med 361: 728–729. [DOI] [PubMed] [Google Scholar]
- Fujino M, Yoshida N, Yamaguchi S, Hosaka N, Ota Y, Notomi T, Nakayama T. 2005. A simple method for the detection of measles virus genome by loop‐mediated isothermal amplification (LAMP). J Med Virol 76: 406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano B, Maki T, Obara T, Fukumoto H, Hagisawa K, Matsushita Y, Okutani A, Bazartseren B, Inoue S, Sata T, Katano H. 2010. LAMP using a disposable pocket warmer for anthrax detection, a highly mobile and reliable method for anti‐bioterrorism. Jpn J Infect Dis 63: 36–40. [PubMed] [Google Scholar]
- Hong TC, Mai QL, Cuong DV, Parida M, Minekawa H, Notomi T, Hasebe F, Morita K. 2004. Development and evaluation of a novel loop‐mediated isothermal amplification method for rapid detection of severe acute respiratory syndrome coronavirus. J Clin Microbiol 42: 1956–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Watanabe M, Nakagawa N, Ihara T, Okuno Y. 2006. Rapid detection and typing of influenza A and B by loop‐mediated isothermal amplification: Comparison with immunochromatography and virus isolation. J Virol Methods 135: 272–275. [DOI] [PubMed] [Google Scholar]
- Kubo T, Agoh M, Mai le Q, Fukushima K, Nishimura H, Yamaguchi A, Hirano M, Yoshikawa A, Hasebe F, Kohno S, Morita K. 2010. Development of a reverse transcription‐loop‐mediated isothermal amplification assay for detection of pandemic (H1N1) 2009 virus as a novel molecular method for diagnosis of pandemic influenza in resource‐limited settings. J Clin Microbiol 48: 728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi‐Akiyama T, Narahara K, Mori S, Kitajima H, Kase T, Morikawa S, Kirikae T. 2010. Development of an immunochromatographic assay specifically detecting pandemic H1N1 (2009) influenza virus. J Clin Microbiol 48: 703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamine K, Hase T, Notomi T. 2002. Accelerated reaction by loop‐mediated isothermal amplification using loop primers. Mol Cell Probes 16: 223–229. [DOI] [PubMed] [Google Scholar]
- Nakajima N, Hata S, Sato Y, Tobiume M, Katano H, Kaneko K, Nagata N, Kataoka M, Ainai A, Hasegawa H, Tashiro M, Kuroda M, Odai T, Urasawa N, Ogino T, Hanaoka H, Watanabe M, Sata T. 2010. The first autopsy case of pandemic influenza (A/H1N1pdm) virus infection in Japan: Detection of a high copy number of the virus in type II alveolar epithelial cells by pathological and virological examination. Jpn J Infect Dis 63: 67–71. [PubMed] [Google Scholar]
- Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. 2000. Loop‐mediated isothermal amplification of DNA. Nucleic Acids Res 28: E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okafuji T, Yoshida N, Fujino M, Motegi Y, Ihara T, Ota Y, Notomi T, Nakayama T. 2005. Rapid diagnostic method for detection of mumps virus genome by loop‐mediated isothermal amplification. J Clin Microbiol 43: 1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon LL, Leung CS, Tashiro M, Chan KH, Wong BW, Yuen KY, Guan Y, Peiris JS. 2004. Rapid detection of the severe acute respiratory syndrome (SARS) coronavirus by a loop‐mediated isothermal amplification assay. Clin Chem 50: 1050–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon LL, Leung CS, Chan KH, Lee JH, Yuen KY, Guan Y, Peiris JS. 2005. Detection of human influenza A viruses by loop‐mediated isothermal amplification. J Clin Microbiol 43: 427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon LL, Chan KH, Smith GJ, Leung CS, Guan Y, Yuen KY, Peiris JS. 2009. Molecular detection of a novel human influenza (H1N1) of pandemic potential by conventional and real‐time quantitative RT‐PCR assays. Clin Chem 55: 1555–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio M, Yui I, Yoshida N, Fujino M, Yonekawa T, Ota Y, Notomi T, Nakayama T. 2005. Detection of respiratory syncytial virus genome by subgroups‐A, B specific reverse transcription loop‐mediated isothermal amplification (RT‐LAMP). J Med Virol 77: 121–127. [DOI] [PubMed] [Google Scholar]
- Wang R, Sheng ZM, Taubenberger JK. 2009. Detection of novel (swine origin) H1N1 influenza A virus by quantitative real‐time reverse transcription‐PCR. J Clin Microbiol 47: 2675–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiley DM, Bialasiewicz S, Bletchly C, Faux CE, Harrower B, Gould AR, Lambert SB, Nimmo GR, Nissen MD, Sloots TP. 2009. Detection of novel influenza A(H1N1) virus by real‐time RT‐PCR. J Clin Virol 45: 203–204. [DOI] [PubMed] [Google Scholar]
- WHO . 2010. Pandemic (H1N1) 2009—Update 98. Geneva, Switzerland: WHO. [Google Scholar]


