Abstract
Background A severe upper respiratory tract infection occurred in a breeding group of rhesus monkeys housed together in one of six indoor/outdoor corals of the German Primate Center. The clinical signs of the disease included severe purulent conjunctivitis, rhinitis, pharyngitis, respiratory distress and lethargy. Six of 45 animals died within a few days after developing signs of infection.
Methods and results Histopathologic and microbiologic examinations of the dead animals were consistent with a severe fibrinopurulent bronchopneumonia. Microbiology revealed a Lancefield group C streptococcus identified as Streptococcus equi subsp. zooepidemicus as the causative agent of infection.
Conclusions The infection was passed on from animal to animal but did not spread to the other five breeding groups nearby. Extensive diagnostic testing failed to reveal the consisting presence of copathogens in individual cases. A visitor with upper respiratory disease was suspected as source of infection.
Keywords: non‐human primate, purulent bronchopneumonia, respiratory disease, Streptococcus equi zooepidemicus
Introduction
Streptococcus (S.) equi subspecies zooepidemicus belongs to the β‐hemolytic group C streptococci. It is able to cause disease both in animals and humans. Streptococcus equi subsp. zooepidemicus primarily causes equine infections. The agent may be found in the nasopharynx, on the tonsils, in the respiratory tract and on the genital mucous membranes of healthy horses and cattle. It is an important cause of respiratory tract infections in foals and young horses and it is involved in uterine infections in mares. The agent has also been associated with a wide variety of infections including mastitis in pigs, sheep, cows, goats and several other mammalian species [8]. These hosts can be a reservoir for human infections. The clinical manifestation includes pharyngitis, septicemia, meningitis, purulent arthritis and endocarditis. The source of human infection is often traced back to contact with domestic animals, especially horses, or ingestion of unpasteurized milk or milk products. Streptococci are colonizers of mucous membranes and are transmitted through droplets or direct contact.
Streptococcus equi subsp. zooepidemicus has sporadically been described in non‐human primates. One outbreak occurred in the National Zoological Park, Washington D.C., leading to the death of several callithrichids after contact to infected horse meat fed to armadillos kept in the same exhibition area [4, 7]. Another outbreak of group C streptococcal infection with a high mortality rate occurred in a group of wanderoos (Macaca silenus) of the Zoological Garden of Rheine/Germany. The source was suspected to be a human being [1]. The Russian Primate Center in Sochi reported an outbreak of septicaemia caused by S. equi subsp. zooepidemicus in representatives of five species of lower monkeys [2]. In 1994, an outbreak among the pig and monkey population was reported from the island of Bali, Indonesia. The infection spread from pigs to monkeys and infected animals died within a few days with signs of bronchopneumonia, pleuritis, epicarditis, endocarditis and meningitis [6]. However, most of the reported disease outbreaks were characterized by symptoms of an enteric infection. The present case report describes an outbreak of respiratory diseases among rhesus monkeys induced by S. equi subsp. zooepidemicus.
Materials and methods
The outbreak involved one of six breeding colonies housed in an indoor–outdoor facility. Each unit was composed of an indoor area, a heated and roofed outdoor room and a large henced outdoor enclosure. Contact to any other animal species except wild birds was not possible. The animals could move freely between the different compartments of the unit. They were fed twice a day with a primate specific diet, composed of standard commercial monkey pellets (ssniff, Spezialdiäten GmbH, Soest, Germany), fresh fruits and vegetables. Water was available ad libitum. The animals are kept in accordance with the guidelines of the European Union for the accommodation and care of animals used for experimental and other scientific purposes (2007/526/EG). The primate husbandry is controlled by local and regional veterinary authorities in accordance with the German Animal Protection Law. All procedures are supervised by an animal welfare officer and the ethical committee for experiments using animals in the federal state of Lower Saxony.
A total of 12 animals died in one of these breeding colonies in a short time period. At the moment of the outbreak, the group was composed of 49 animals; most of them were adult females with their offspring and one adult male. Necropsies were performed on all carcasses and samples for histology, parasitology, microbiology and molecular diagnostics were taken. For histopathology, samples were taken from all thoracic, abdominal and pelvic organs, fixed in 10% neutrally buffered formalin, embedded in paraffin and stained with hematoxylin and eosin. For parasitological diagnosis fresh samples were examined microscopically.
For microbiological, investigations swabs from liver, spleen, kidney, heart and lung were streaked immediately onto Columbia blood agar plates. Swabs recovered from the intestine were swabbed onto Columbia agar, EMB, McConkey and Skirrows campylobacter agar plates and inoculated into Rapaport and Skirrow’s enrichment broth. Biochemical characterization of the isolated bacterial colonies was achieved using the Crystel‐system from Becton Dickinson. Bacterial isolates were sent to the German National Reference Center for Streptococci for further characterization.
Pulsed field gel electophoresis (PFGE) of bacterial DNA was carried out on a BIO‐RAD CHEF‐DR III system using the BIO‐RAD (BIO‐RAD Laboratories GmbH, Munich, Germany) Gene Path Gel Kit and the restriction enzyme SmaI.
Results
The outbreak started at the end of October 2007. Two adult females were found seriously ill with signs of severe upper respiratory tract infection and severe purulent conjunctivitis (Fig. 1). Antibiotic therapy with Baytril® (dosage 2.5 mg/kg) and Aviapen® (dosage 40.000–80.000 IE/kg) was started but both animals died within 2 days after onset of the first symptoms (animals No. 1, 2). One month later, four more female animals developed severe upper respiratory tract infection. Two died suddenly without obvious clinical findings (animals No. 3, 4), one of these was a pregnant one (animal No. 3). The other two died after 10 days on antibiotic therapy with the antibiotics mentioned above (animals No. 5, 6).
Figure 1.
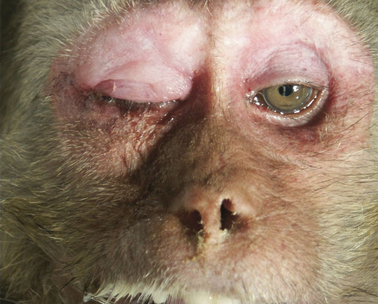
Rhesus monkey, case No. 1. Severe fibrinopurulent conjunctivitis and rhinitis.
Necropsy findings were similar for all six animals. The main findings were severe inflammatory alterations of the upper respiratory tract and the lung (Table 1). All six animals showed a severe subacute tonsillitis and pharyngitis and a severe subacute fibrinopurulent pleuritis and pneumonia. The inflammation spread to the heart and developed into a fibrinopurulent epi‐ and pericarditis with exception of animal No. 3 (2, 3). Variably sized and poorly demarcated atelectatic areas were present in all lung lobes.
Table 1.
Signalment, clinical signs of disease, and pathohistologic findings in rhesus monkeys with streptococcosis due to Streptococcus equi subsp. zooepidemicus infection
| Monkey No. | Sex/age (year) | Clinical observation | Main pathohistologic findings | Culture‐positive organs |
|---|---|---|---|---|
| 1 (7488) | Female/10 | Upper respiratory tract infection severe fibrinopurulent conjunctivitis | Severe purulent tonsillitis and pharyngitis severe purulent lymphadenitis severe subacute fibrinopurulent pleuropneumonia, epi‐ and pericarditis mild diffuse purulent hepatitis and splenitis severe cardiomegaly | Lung, heart, liver, spleen, kidney, CNS, eye, tonsil |
| 2 (7490) | Female/20 | Upper respiratory tract infection severe fibrinopurulent conjunctivitis | Severe purulent tonsillitis and pharyngitis severe purulent lymphadenitis severe subacute fibrinopurulent pleuropneumonia,epi‐ and pericarditis mild diffuse purulent hepatitis and splenitis severe cardiomegaly | Lung, heart, liver, spleen, kidney, CNS, eye, tonsil |
| 3 (7502) | Female/9 | Sudden death without obvious findings | Severe acute purulent endometritis severe fibrinopurulent serositis severe purulent lymphadenitis moderate subacute fibrinopurulent pleuropneumonia mild diffuse purulent hepatitis and splenitis | Lung, heart, liver, spleen, kidney, CNS, tonsil, uterus, placenta |
| 3a (7503) | Fetus from animal No. 3 | – | Mild diffuse purulent hepatitis and splenitis | Lung, heart, liver, spleen, kidney, CNS |
| 4 (7508) | Female/8 | Sudden death without obvious findings | Moderate purulent tonsillitis and pharyngitis severe subacute fibrinopurulent pleuropneumonia, epi‐ and pericarditis mild diffuse purulent hepatitis and splenitis severe fibrinopurulent serositis | Lung, heart, liver, spleen, kidney, CNS, tonsil |
| 5 (7515) | Female/23 | Severe upper respiratory tract infection cardiac murmur antibiotic treatment of two weeks | Severe purulent lymphadenitis severe subacute fibrinopurulent pleuropneumonia severe fibrinopurulent epi‐ and pericarditis severe cardiomegaly | Lung, heart, liver, spleen, kidney, CNS |
| 6 (7516) | Female/8 | Severe upper respiratory tract infection antibiotic treatment of two weeks | Severe purulent lymphadenitis severe fibrinopurulent pleuropneumonia severe subacute fibrinopurulent epi‐ and pericarditis severe cardiomegaly moderate multifocal purulent encephalitis | Lung, heart, liver, spleen, kidney, CNS, tonsil |
| 7 (7519) | Female/14 | Sudden death without obvious findings after fighting | Severe acute purulent tonsillitis severe acute purulent lymphadenitis | Tonsil |
| 8 (7520) | Female/4 | Sudden death without obvious findings after fighting | Moderate acute purulent tonsillitis | Tonsil |
| 9 (7522) | Female/7 | Sudden death without obvious findings after fighting | Moderate acute purulent tonsillitismoderate acute purulent lymphadenitis | Tonsil |
| 10 (7523) | Female/2 | Sudden death without obvious findings after fighting | – | – |
| 11 (7524) | Female/4 | Sudden death without obvious findings after fighting | – | – |
| 12 (7525) | Male/2 | Sudden death without obvious findings after fighting | – | – |
IHC, immunohistochemistry; PCR, polymerase chain reaction; CNS, central nervous system.
Figure 2.
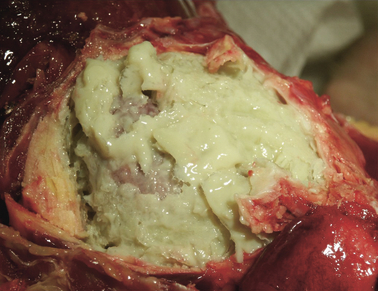
Heart; rhesus monkey, case No. 6. Severe fibrinopurulent epi‐ and pericarditis.
Figure 3.
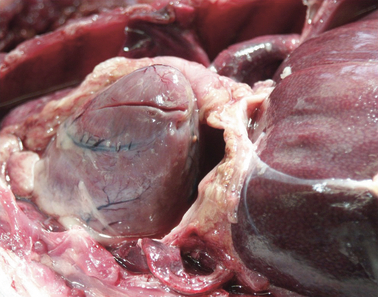
Heart; rhesus monkey, case No. 4. Severe fibrinopurulent epi‐ and pericarditis.
Histologically, there was a fibrinosuppurative pneumonia with extensive to lobular obliteration of the airways and alveolar spaces with neutrophils (Fig. 5A). Large and medium sized airways were unaffected but terminal airways were intensely altered and showed signs of a purulent bronchopneumonia (Fig. 5B). The inflammatory process extended from the pleura to the diaphragm leading to severe purulent myositis (Fig. 5C). One pregnant animal additionally developed a severe purulent endometritis. The aborted fetus was nearly fully developed and showed signs of a purulent hepatitis and splenitis (animal No. 3a). Splenitis and hepatitis were mild side effects in four more animals. Only one animal developed a purulent encephalomeningitis (animal No. 6).
Figure 5.
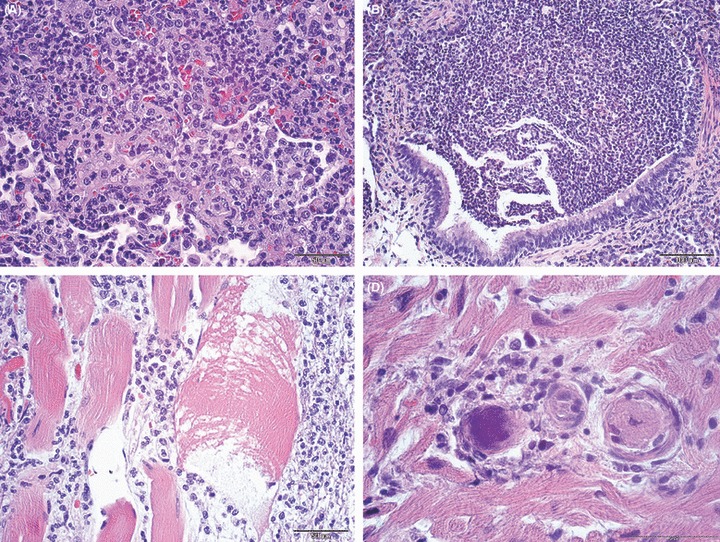
(A) Lung; rhesus monkey, case No. 1. Severe fibrinopurulent pneumonia. H&E stain. Scale bar = 50 μm. (B) Lung; rhesus monkey, case No. 1. Severe fibrinopurulent bronchopneumonia. H&E stain. Scale bar = 100 μm. (C) Muscle; rhesus monkey, case No. 1. Severe purulent myositis of the diaphragma. H&E stain. Scale bar = 50 μm. (D) Heart; rhesus monkey, case No. 1. mild mycarditis with septic thromboemboli. H&E stain. Scale bar = 50 μm.
Clusters and chains of gram‐positive cocci were present within the altered organs. They were found within the cytoplasm of macrophages and free within the extracellular spaces laying in pairs or chains (Fig. 4). Septic thromboemboli were less consistently observed (Fig. 5D). Septicemia was attributed as cause of death in all cases.
Figure 4.
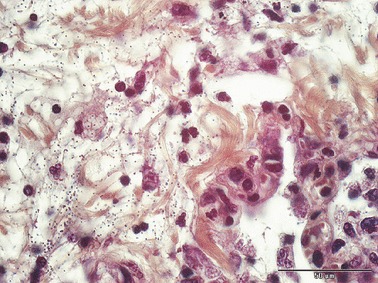
Lung; rhesus monkey, case No. 1. Purulent pneumonia with clusters and chains of gram‐positive cocci Gram‐stain; Scale bar = 50 μm.
Septicemia and bacteriemia were confirmed by microbiological investigations on samples taken during necropsy. Pure cultures of large colonies of β‐hemolytic streptococci were grown not only from the respiratory tract, respectively tonsils and lung, but also from nearly all internal organs including the central nervous system. Bacterial characterization was based on the observation of gram‐positive, catalase‐negative chain forming cocci which fermented lactose and trehalose. Agglutination tests showed that they belong to Lancefield group C.
Pulsed field gel electophoresis patterns were identical for all seven isolates analyzed, showing that the infections were caused by the same strain in all cases (Fig. 6).
Figure 6.
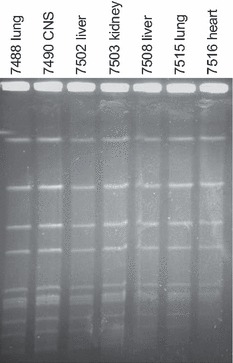
Pulsed field gel electrophoresis of seven different Streptococcus equi subsp. zooepidemicus isolates from a Macaca mulatta colony in the German primate center in Göttingen.
Polymerase chain reaction based on amplification of common primate respiratory pathogens was performed on the lung tissue of the necropsied animals to exclude the presence of other important viral pathogens. The methods used are summarized in Köndgen et al. 2008 [3]. All samples were negative for influenza A and B, parainfluenza, rhinovirus, coronavirus, adenovirus, human metapneumovirus, respiratory syncytial virus and enterovirus.
The six dead animals were high ranked closely related animals. Their loss induced severe ranking fights, a problem which is well known in rhesus monkey colonies. As a consequence of these heavy fights six more animals (animal No. 7–12) died due to severe injuries, stress and shock‐symptomatic. Streptococcus equi subsp. zooepidemicus was isolated from the tonsils of three of these animals (animal No. 7–9).
Discussion
A fatal outbreak of streptococcus infection occurred in one of six breeding colonies housed in an indoor/outdoor facility.
The source of infection with S. equi subsp. zooepidemicus in the present outbreak remains unknown. Contact to horsemeat, equines or domestic animals could reliably be excluded. Instead a human to animal transmission was suspected. Generally S. equi subsp. zooepidemicus can be transmitted by aerosols, via the oral route or through wound contamination. Aerosol transmission is most likely in the present case. It was assumed that contact to a visitor with upper respiratory disease led to the initial infection of two elderly and closely related animals.
Streptococci of Lancefield group C can be recovered from the pharynx of 1.5% of normal humans. Infections of the upper respiratory tract in humans are rare but may occur after contact with infected horses [5].
It was assumed that the infection was subsequently transmitted from animal to animal because ill and dead animals were closely related to each other. There was a high degree of relationship and close contact among them. Close contact seems to be necessary for the transmission of this agent. None of the animals in adjacent corrals situated at a distance of 2–3 m became infected. None of the staff members was ill before or during the time of the outbreak.
In horses S. equi subsp. zooepidemicus is a commonly isolated mucosal commensal that opportunistically invades after virus infections, transport stress or other immunosuppressive conditions. In the described monkey group the situation seems to be different. Bacteriologic investigation of tonsillar swabs indicates that there was no colonization or asymptomatic infection with S. equi subsp. zooepidemicus among the group members which could have been activated by a stressor. The only exceptions were three animals (animal No. 7–9) that died in the consequence of heavy position fights. In these animals streptococci were found in the tonsils but they developed no clinical findings. The three animals belonged to the same family and had been in close contact to the dead animals. They developed a purulent tonsillitis and it is speculative if these animals may have established a latent infection comparable to horses if they survived the attacks.
The streptococcus outbreak among rhesus monkeys clearly demonstrates the high susceptibility of these animals. This is confirmed by data from the literature describing outbreaks caused by S. equi subsp. zooepidemicus in non‐human primates marked by sudden, explosive appearance and high fatality rate [1, 4, 7]. This observation should lead to a critical discussion of housing primates and other animal species in close vicinity of each other as is often the case in zoological gardens. Particularly equines should not be kept in the vicinity of primate facilities and there should be a strict separation between the different units regarding animal keepers and other staff members.
The problem of the described outbreak for the breeding colony was not only the initial fatal infection but also the death of the first six animals. The loss of these animals caused ranking fights, injuries and death of six more animals. As a consequence the group had to be split up and newly composed. The outbreak ended after the dramatic depopulation of the group and the intense cleaning and disinfection of the facility.
The disease caused heavy losses; not only the loss of the animals, but also a reduced reproductive rate and high costs for therapy. The experience from this outbreak leads to the conclusion that contact of important and valuable breeding colonies to visitors should be strictly avoided.
References
- 1. Brack M, Günther E, Gillhaus H, Salzert W, Meuthen J: An outbreak of Streptococcus equi ssp. zooepidemicus infection of probable human origin in wanderoos (Macaca silenus) – case report. Zentralbl Bakteriol 1997; 286:441–6. [DOI] [PubMed] [Google Scholar]
- 2. Dzhikidze EK, Krylova RI, Voskanyan NA, Kukava GG, Dzhindzhiya PYA: Outbreak of infection caused by Streptococcus zooepidemicus among laboratory primates. Zentralbl Mikrobiol 1996; 1:81–4. [PubMed] [Google Scholar]
- 3. Köndgen S, Kühl H, N’goran PK, Walsh PD, Schenk S, Ernst N, Biek R, Formenty P, Mätz‐Rensing K, Schweiger B, Junglen S, Ellerbrock H, Nitsche A, Briese T, Lipkin WI, Pauli G, Boesch C, Leendertz FH: Pandemic human viruses cause decline of endangered great apes. Curr Biol 2008; 18:1–5. [DOI] [PubMed] [Google Scholar]
- 4. Montali RJ: 1997 Diseases of zoo marmosets, tamarins and Goeldi`s monkeys. Proc Amer Assoc Zoo Vets Ann Meet 1994; Pittsburgh, PA: 237–40. [Google Scholar]
- 5. Rose HD, Allen JR, Witte G: Streptococcus zooepidemicus (Group C) pneumonia in a human. J Clin Microbiol 1980; 11:76–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salasia SIO, Wibawan IWT, Pasaribu FH, Abdulmawjood A, Lämmler C: Persistent occurrence of a single Streptococcus equi subsp. zooepidemicus clone in the pig and monkey population in Indonesia. J Vet Sci 2004; 5:263–5. [PubMed] [Google Scholar]
- 7. Schiller CA, Wolff MJ, Munson L, Montali RJ: Streptococcus zooepidemicus infections of possible horsemeat source in red‐bellied tamarins and Goeldie`s monkeys. J Zoo Wildl Med 1989; 20:322–7. [Google Scholar]
- 8. Soedarmanto I, Pasaribu FH, Wibawan IW, Lammler C: Identification and molecular characterization of serological group C streptococci isolated from diseased pigs and monkeys in Indonesia. J Clin Microbiol 1996; 34:2201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


