Abstract
Aim This paper reports the diversity and endemism patterns of African ferns, and explores the potential role of diversity refuges and environmental and historical factors in the shaping of these patterns.
Material and locations The extant fern taxa occupying Africa south of the Sahara, Madagascar and some islands of the South Atlantic.
Methods The number of taxa in each area or operational geographical unit (OGU) was scored, and the correlation between this number and physical and climatic variables analysed by standard pairwise and stepwise multiple regression analysis (SPR and SMR). The effects of biological factors such as dispersal capacity, reproductive biology, genetic features and certain physiological adaptations were evaluated by comparing the number of species in each OGU. Floral affinities among OGUs were analysed using non‐metric multi‐dimensional scaling (NMS) and parsimonic analysis of dispersion (PAD), and compared with β‐turnover and inter‐OGU distances.
Results OGU area, elevation and the distance between refuges determined the composition of local floras, but only greater OGU area and the existence of higher maximum elevations increased species richness. The distance between refuges also affected the number of endemic species, especially on islands. The biological features studied only slightly influenced fern distribution. The main climatic predictor of species number was humidity. SPR and SMR revealed three main groups of ferns with different ecological trends. NMS and PAD analyses separated the four areas of highest diversity in Africa, three of which are inhabited by ferns with distinct ecological requirements. The fourth area was Madagascar, which shows an accumulation of endemic and relict diversity that is not easy to explain.
Main conclusions The distribution of ferns in Africa has been influenced by refuges. These probably allowed many species to recolonize the neighbouring areas after the extinctions of the Pleistocene. Three major components were detected in the African flora: Guinea‐Congolian thermophilous, cold‐tolerant Afro‐montane, and Southern drought‐tolerant elements. These are related to the three main refuge areas, i.e. the Gulf of Guinea area, the eastern tropical region, and the Cape region. Endemicity in ferns was found to be lower than that of seed plants due to the higher dispersability of fern spores. The distance between OGUs seems to be the main predictor of the number of endemic fern species these areas contain.
Keywords: Pteridophytes, biogeography, habitat diversity, centres of diversity, extinction, climatic variables, dispersal capability, isolation, γ‐diversity, δ‐diversity
Introduction
Africa is quite poor in pteridophytes compared with other tropical areas (Tryon, 1986). It is even poor compared with Madagascar, which is 40 times smaller: of a global pteridophyte flora of c. 12,000 species, c. 627 grow in Africa and c. 557 in Madagascar (Kornaś, 1993).
According to some authors, the low pteridophyte diversity of Africa is mainly the result of the paucity of the continent's rain forest flora, which is frequently explained by extinction due to Tertiary droughts (Kornaś, 1993). Other authors indicate the absence of extensive mountain ranges in Africa as the main reason for low fern diversity (Moran, 1995). In contrast, the Malgache region is extraordinarily rich – a discrepancy still unexplained.
Pteridophytes are generally distributed along a latitudinal gradient, with the highest diversity in the tropics (mainly in mountainous areas) (Jacobsen & Jacobsen, 1989; Kornaś, 1993; Linder, 2001). However, in Africa, ferns are highly diversified in the eastern mountain arc, and somewhat less so in the western mountains (Schelpe, 1983). Three main latitude groupings – tropical, Mediterranean and Cape – are usually used to describe the continental pteridophyte flora of Africa (Kornaś, 1993). Island floras include the Macaronesian flora, the flora of the Atlantic islands, and the flora of the Malgache region. The Macaronesian flora is mainly related to Mediterranean or Palaeomediterranean floras. Madagascar has an extraordinarily rich tropical flora with a very high number of endemic species, probably influenced by a very conservative climate. The flora of the South Atlantic islands is most closely related to that of South America, the sub‐Antarctic islands, and to a lesser degree to the Cape flora. A feature of this flora is their adaptation for long‐distance dispersal (Smith, 1972).
Habitat diversity, elevation, cloud cover, rainfall, temperature, seasonality, exposure and soils have all been suggested to explain pteridophyte richness and distribution (Moran, 2002), but biological factors such as dispersal capacity, reproductive biology, genetic features and certain physiological adaptations have also been proposed (Barrington, 1993; Kornaś, 1993). The role of these features, however, has not been examined in Africa and its related islands.
Refuges, places that have shown climatic stability over geological time (Fjedså & Lovett, 1997), are areas from which species spread out when climatic conditions became more favourable. Diversity and local endemism should therefore increase towards refuges because of their isolation and the maintenance of favourable climatic conditions. The existence of refuges has been questioned by several authors. The main arguments against them are Neotropical (Colinvaux et al., 2000), while those in favour are based on evidence from Africa (Linder, 2001). Based on the distribution of several groups of plants (Linder, 2001) and animals (Mayr & O'Hara, 1986; Harcourt, 2000), Africa is thought to harbour several of these refuges. There is remarkable congruence between centres of endemism and refuges, suggesting that the latter are not artefacts and that the pattern of African flora is well defined (Linder, 2001).
The main aim of this work was to document the patterns of diversity and endemism of African ferns and to analyse the potential role of environmental and historical factors in shaping them. Seven complementary analyses were undertaken that together provide the most complete picture yet of the biogeography of African fern flora:
-
1
The number of taxa in 29 operational geographical units (OGUs) was determined in order to evaluate γ‐diversity, and the ratios between species and genera richness (and other taxa) compared to determine whether any OGUs do not follow the general pattern.
-
2
The correlation between species number and latitude or longitude was analysed to study diversity gradients. Standard pairwise and stepwise multiple regression analyses (SPR and SMR) were used to determine the explanatory power of climatic and physical variables.
-
3
Independent SMR analyses were performed for large genera to show their climatic preferences, and in order to explain their distribution patterns. These analyses were performed using the relative contributions of species to each OGU and environmental variables.
-
4
The influence of dispersal and reproduction methods on the colonization of isolated marine or ‘habitat’ islands and on the evolution of endemism was assessed, relating the proportion of species with trilete spores and gemmiferous reproduction, etc., to environmental factors.
-
5
The floristic affinities among OGUs were analysed using non‐metric multi‐dimensional scaling (NMS) and compared with β‐turnover and inter‐OGU distances.
-
6
Patterns of endemicity were documented by performing several tests: (i) the determination of correlations between the number of endemic species and latitude to look for gradients in endemicity; (ii) the determination of correlations between the number of endemic species and the distances between OGUs, in order to study their isolation; and (iii) SMR analyses of the relative contribution of endemic species and climatic and physical variables, in order to determine these variables’ explanatory power.
-
7
The consistency of floral affinities deduced from NMS analyses was tested. To phenetically classify biotas and to search for possible historical patterns, parsimonic analysis of dispersion (PAD) was performed.
Methods
Choice of taxa
Data on ferns south of the Saharan desert, Madagascar and some islands of the South Atlantic (1001 species) were obtained from published floral surveys and taxonomic monographs. The main floral studies used were the following: Cape Verde (Hansen & Sunding, 1993), Mt Loma, the Kwahu Plateau and Togo Ranges (Alston, 1959), Mt Cameroon (Tardieu‐Blot, 1964a; Edwards, 1998), Mt Oku (Edwards et al., 2000), Annobón, Bioko and Río Muni (Velayos et al., 2002), Porto Principe and S. Tomé (Figueiredo, 1998, 2001), Mt Moukandé (Tardieu‐Blot, 1964b), Angola (Schelpe, 1977), St Helena (Ashmole & Ashmole, 2000), Ascension Island (Ashmole & Ashmole, 2000), Tristan da Cunha and Gough Island (Groves, 1981), Marion Island (Alston & Schelpe, 1957), Jebel Marra (Wickens, 1976), Ethiopia (Pichi Sermolli, 1978; Thulin, 1993), the Rukiya, Aberdare and Kipengere Ranges (Johns, 1991; Schippers, 1993a, 1993b), Inyanga (Schelpe, 1970), Drakensberg, Mt Knysna, Table Mountain and Namibia (Schelpe & Anthony, 1986; Glen, 1993; Burrows & Crouch, 1995; Roux, 2001b), Madagascar (Tardieu‐Blot, 1951, 1952, 1958, 1960, 1971), and Tropical Africa (Tardieu‐Blot, 1953; Moran & Smith, 2001). The main monographs used were: Blechnum L. (Schelpe, 1952), Cyathea Sm. (Holttum, 1981), Davallia Sm. (Nooteboom, 1994), Lindsaea Dryand. ex Sm. and related genera (Kramer, 1972), Lastreopsis Ching (Tindale, 1965), Triplophyllum Holttum (Holttum, 1986), Polystichum Roth (Roux, 2000, 2001a), Grammitidaceae (Schelpe, 1969; Parris, 1981), Hymenophyllum Sm. (Kornaś, 1984), Lomariopsidaceae (Schelpe, 1969; Pichi Sermolli, 1975; Hennipman, 1977; Roux, 1993), Lycopodiaceae (Øllgaard, 1989), Marattia Sw. (Rolleri et al., 2001), Arthropteris J. Smith ex Hook. f. (Lawalrée, 1990, 1991), Ophioglossum L. (Burrows & Edwards, 1995; Burrows, 1996), Lepisorus (Sm.) Ching (Zink, 1993), Microsorum Link (Bosman, 1991), Pyrrosia Mirb. (Hovenkamp, 1986), Belvisia Mirb. (Hovenkamp & Franken, 1993), Platycerium Desv. (Sandbrink et al., 1992), Actiniopteris Link (Kornaśet al., 1982), Ceratopteris Brongn. (Lloyd, 1974), Pellaea Link and Cheilanthes Sw. (Anthony, 1984; Jacobsen & Jacobsen, 1986, 1988), Pteris L. (Schelpe, 1969), Mohria Sw. (Roux, 1995), Selaginella P. Beauv. (Bizzarri, 1985; Stefanović & Rakotondrainibe, 1996, 1997), Amphineuron Holttum (Holttum, 1977), Christella H. Lév. (Holttum, 1976), Pseudocyclosorus Ching (Holttum & Grimes, 1980), Pneumatopteris Nakai (Holttum, 1973), Cyclosorus Link and Thelypteris Schmidel (Viane, 1985) and Vittariaceae (Schelpe, 1969).
Taxa were scored for their absence (0) or presence (1) in each area in a data matrix (see Appendix S1 in Supplementary material). The final compilation was the result of collecting, interpreting and harmonizing the alternative taxonomic treatments of ferns in the literature. Some heterogeneity in the quality of the data was unavoidable. However, due to the large number of taxa involved, its effect was almost certainly of minor importance.
The limits of family and genus are those of Kramer & Green (1990). For the Thelypteridaceae, Holttum's (1974) classification (especially useful for Old World species) and the Index of Thelypteridaceae (Grimes & Parris, 1986) were used. The terminology used for diversity measurements was that of Whittaker (1972).
Choice of areas
Twenty‐nine sites or OGUs were selected from the areas south of the Saharan desert, Madagascar and some of the South Atlantic islands as they have been described as refuges for animal and plant species (Fig. 1, Table 1). These are reported to be particularly rich in ferns and flowering plants, including endemic species. Except for some of the islands, most have high mountains. Continental mountain sites were understood to be quite similar to islands; their borders were defined by elevational contours (Brown, 1971; Lomolino et al., 1989). Subjectivity with respect to the delimitation of refuges was unavoidable.
Figure 1.
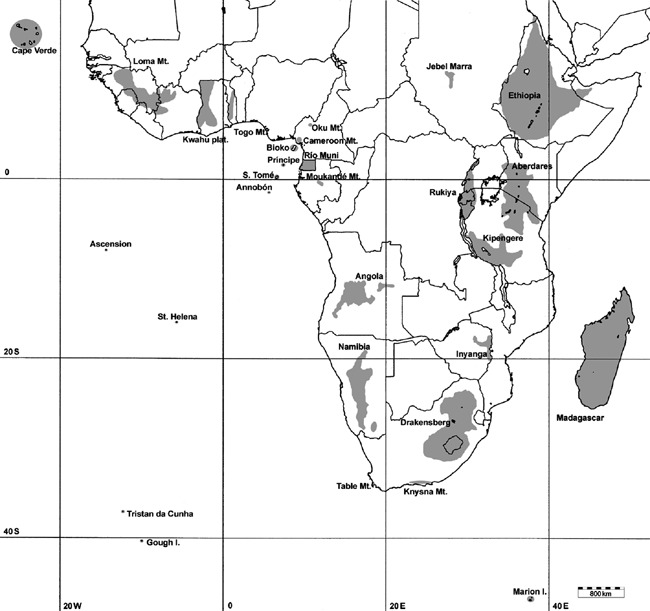
Operational geographical unities.
Table 1.
Areas, number of taxa, taxa ratios, residuals of species‐area regression and number of endemics in Africa, Madagascar, different sites of Africa and two flora refuges, one of Asia and the other of South America. Data of Africa are from this study, while data of P. Carrasco and Mt Kinabalu are from Kessler et al. (2001) (identified with *)
| Area (km2) | Elevation (m) | Species (S) | Genera (G) | Families (F) | G/F | S/G | S/F | Residuals of species‐area regression | Endemic species total | Endemic species % | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mt Kinabalu* | – | – | 613 | 139 | 29 | 4.793 | 4.41 | 21.14 | – | – | – |
| P. Carrasco* | – | – | 497 | 99 | 26 | 3.808 | 5.02 | 19.11 | – | – | – |
| Madagascar and associated islands | 601,197 | – | 569 | 104 | 28 | 3.714 | 5.471 | 20.32 | 0.348 | 298 | 53. 5 |
| Madagascar | 594,180 | 2876 | 557 | 102 | 28 | 3.642 | 5.46 | 19.89 | 0.350 | 237 | 41.7 |
| Africa (incl. close islands, excl. Madagascar) | 29,710,000 | – | 627 | 95 | 27 | 3.52 | 6.6 | 23.2 | −0.019 | – | – |
| Kipengere | 178,200 | 2829 | 337 | 86 | 27 | 3.185 | 3.919 | 12.481 | 0.260 | 8 | 2.4 |
| Aberdares | 285,120 | 5895 | 313 | 85 | 28 | 3.036 | 3.682 | 11.18 | 0.132 | 7 | 2.2 |
| Rukiya | 90,234 | 4507 | 242 | 75 | 26 | 2.885 | 3.227 | 9.308 | 0.196 | 6 | 2.5 |
| Drakensberg | 311,040 | 3375 | 206 | 71 | 28 | 2.536 | 2.915 | 7.357 | −0.002 | 10 | 4.9 |
| Bioko | 2017 | 3007 | 204 | 68 | 23 | 2.957 | 3 | 8.87 | 0.548 | 3 | 1.5 |
| Inyanga | 17,820 | 2596 | 188 | 70 | 27 | 2.593 | 2.686 | 7 | 0.269 | 1 | 0.5 |
| Cameroon Mt | 2700 | 4095 | 184 | 59 | 19 | 3.105 | 3.119 | 9.684 | 0.471 | 1 | 0.5 |
| Loma Mt | 186,300 | 1948 | 167 | 53 | 23 | 2.304 | 3.151 | 7.260 | −0.038 | 1 | 0.6 |
| Angola | 133,000 | 1829 | 163 | 66 | 26 | 2.538 | 2.47 | 6.308 | 0.012 | 3 | 1.8 |
| S. Tomé | 860 | 2025 | 139 | 57 | 20 | 2.85 | 2.439 | 6.95 | 0.476 | 7 | 5.0 |
| Kwahu pl. | 92,644 | 884 | 123 | 45 | 22 | 2.045 | 2.733 | 5.591 | −0.094 | 1 | 0.8 |
| Río Muni | 26,000 | 1200 | 117 | 45 | 20 | 2.25 | 2.6 | 5.9 | 0.024 | 1 | 0.9 |
| Moukandé Mt | 2670 | 1575 | 113 | 43 | 22 | 1.955 | 2.628 | 5.182 | 0.260 | 1 | 0.9 |
| Knysna mts | 12,000 | 915 | 101 | 45 | 23 | 1.957 | 2.244 | 4.391 | 0.045 | 3 | 2.9 |
| Table mts | 820 | 1086 | 85 | 37 | 21 | 1.762 | 2.297 | 4.048 | 0.267 | 0 | 0 |
| Porto Principe | 104 | 995 | 80 | 36 | 16 | 2.25 | 2.222 | 5 | 0.480 | 1 | 1.3 |
| Ethiopia | 680,000 | 2400 | 76 | 40 | 23 | 1.739 | 1.9 | 3.304 | −0.558 | 1 | 1.3 |
| Oku Mt | 1550 | 3011 | 51 | 32 | 17 | 1.882 | 1.594 | 3 | −0.024 | 0 | 0 |
| Namibia | 149,202 | 1800 | 48 | 13 | 7 | 1.857 | 3.692 | 6.857 | −0.555 | 1 | 2.0 |
| Annobón | 17 | 613 | 42 | 26 | 18 | 1.444 | 1.615 | 5 | 0.350 | 1 | 2.4 |
| Togo Mt | 20,736 | 986 | 39 | 20 | 13 | 1.538 | 1.95 | 3 | −0.427 | 0 | 0 |
| Tristan da Cunha I. | 159 | 2058 | 36 | 16 | 12 | 1.333 | 2.25 | 3 | 0.106 | 5 | 16.6 |
| Helena I. | 121 | 823 | 31 | 19 | 10 | 1.9 | 1.632 | 2.9 | 0.041 | 12 | 38.7 |
| Gough I. | 75 | 910 | 30 | 15 | 12 | 1.25 | 2 | 2.5 | 0.065 | 1 | 3.4 |
| Cape Verde | 4033 | 2829 | 29 | 21 | 11 | 1.909 | 1.381 | 2.636 | −0.633 | 2 | 3.4 |
| Jebel Marra | 10,141 | 3071 | 28 | 15 | 7 | 2.143 | 1.867 | 4 | −0.492 | 0 | 0 |
| Ascension I. | 90 | 860 | 10 | 9 | 8 | 1.125 | 1.111 | 1.375 | −0.417 | 3 | 30.0 |
| Marion I. | 334 | 1230 | 9 | 7 | 6 | 1.167 | 1.286 | 1.5 | −0.608 | 2 | 22.2 |
Data analysis
Both SPR and SMR (Sneath & Sokal, 1973) were performed using the Statistica software package. These methods show whether recognized independent variables are significantly related to dependent variables. For these tests, a raw matrix of variables was produced, and the data then transformed to decimal logarithms (for inter‐OGU distances, OGU areas, latitude and number of species, etc.) or transformed to arc‐sines (for ratios). Mantel tests to compare Jaccard indices or β‐turnover with logarithms of distances were performed using triangular matrices and employing the matrix comparison option of the NTSYS‐pc 1.7 software package (Rohlf, 1992).
The following climatic parameters were selected because previous studies have shown them to be relevant for ferns (Kornaś, 1993; Moran, 2002) and because such data is readily obtained (see Appendix S2): the Kira warmth index (Oshawa, 1991), the difference between maximum and minimum monthly mean temperatures (which indicates thermal seasonality), the number of hours of sunshine per year, mean annual rainfall, driest month rainfall, percentage cloudiness and mean relative humidity. Most data were obtained from the IPCC and WMO web pages (http://ipcc-ddc.cru.uea.ac.uk/ and http://www.wmo.ch/indexflash.html) and from CLINO 1931–1960 (Anonymous, 1971). No climatic data were available for Annobón and Porto Principe. When one of selected areas has more than one place with climatic information we calculated the mean. We refrained from including local sea currents, winds, microclimatic influences, proximity to the sea, vegetation, soil and bedrock type as such data were not available for many sites.
The non‐climatic variables studied were elevation (measured as maximal elevation above sea), inter‐OGU distance and OGU area. The pairs of OGUs to be compared were not the nearest but those in which the minimal floral differences occur, as nearest sites sometimes have dispersal barriers between them, and therefore have a small number of common species. A more practical approach is to identify the closest sites by the minimum spanning tree (MST) matrix obtained from the Jaccard indices among sites. The inter‐OGU distances were calculated using their geographical centres.
In pairwise regression analysis of the number of species and the independent variables, the log‐log model was preferred as it always produced a better R 2 and a lower standard error. Some variables used in the regression analyses were significantly correlated with one another (see Appendix S3). This was largely inevitable as some climatic variables are related to solar energy, which varies gradually with latitude. To avoid redundancy, those variables that showed a tolerance of less than 0.3 were excluded from taxa and predictor SMR analyses. The number of hours of sunshine per year, mean annual rainfall, driest month rainfall and cloudiness were deleted from these analyses. The final results were compared using forward and backward stepwise regression procedures. As all the results were similar, multicollinearity was not a serious problem.
To determine the distribution areas that were most similar (based on species concurrence) OGUs were compared pairwise with respect to the presence or absence of different species (Holloway & Jardine, 1968; Hengeveld, 1990). The regions × taxa matrix was analysed to extract the Jaccard indices. This is appropriate for the examination of general biotic similarity based on the number of common taxa (Sneath & Sokal, 1973). The matrix was then used to perform an NMS to assess the goodness of fit of the resulting spatial configurations with stress values (Kruskal & Wish, 1978). To interpret the plot, an MST was superimposed on the vectors to detect any undue distortion of the multidimensional configuration of the different OGUs (Dunn & Everitt, 1982). NMS analysis was performed with NTSYS‐pc 1.7 software package (Rohlf, 1992).
For PAD, cladistic analyses were performed using the PAUP software package (Swofford, 1993). Sites were used as characters and the presence or absence of species as states of these characters. Endemic species were deleted. All characters were unweighted and unordered. Fitch parsimony was used for all the assays. Data were analysed using the exhaustive option. Polarization of characters into plesiomorphic and apomorphic states was assessed using the standard comparison procedure with an outgroup composed entirely of zeros (Lundgberg rooting; Rosen, 1988). MacClade version 3.04 software was used to edit the data set analysed with PAUP (Maddison & Maddison, 1992), and also to map the distribution of particular character‐state changes. A recommended prerequisite for PAD analyses is to select areas with a comparable number of taxa (Rosen, 1988; Crisci et al., 2000). If areas with a very dissimilar number of species are studied, the sites with a lower number usually end up at the base of the respective clade. This apparent artefact is one of the main problems of PAD or parsimonic analyses of endemicity (PAE). Thus, sites with a low number of taxa should be excluded. In this analysis, OGUs with less than 45 species were excluded. Even if PAD analyses unveil hierarchical patterns among OGUs, they should not be used to search for historical patterns between areas, but only to phonetically classify biotas (Rosen, 1988; Crisci et al., 2000).
Results and discussion
Taxa richness
Table 1 shows the number of fern species, genera, families, species/genus, genus/family and species/family of the different OGUs. The absolute number of species, genera and families in continental Africa (with its greatly superior area), Madagascar (Table 1, Fig. 2a), and two small mountain refuges in South America (Parque Carrasco) and Asia (Mt Kinabalu) (2002, 2001) are comparable in magnitude. Thus, the African fern flora appears to be quite impoverished (Tryon, 1986; Kornaś, 1993). The sites of the East African mountain arc showed the highest number of species of continental Africa, with the Kipengere, Aberdare and Rukiya Ranges, and Drakensberg in first position, and Bioko in fifth place. The large areas covered by these sites, as well as their elevation, allow a large variety of habitats to exist. These factors, plus the stability of the Agulhas current, increase the possibilities of a species surviving there during times of drought.
Figure 2.
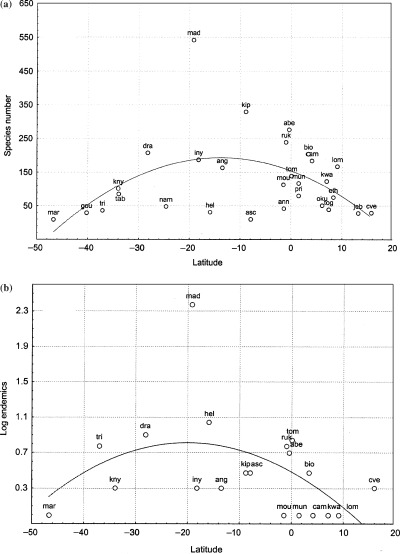
(a) Plot of species number against latitude. (b) Plot of logarithm of number of endemic species against latitude. Regression between absolute value of latitude and logarithm of endemics R = 0.07, P = 0.71. Acronyms are: lom, Loma Mt; kwa, Kwahu plateau; oku, Oku Mt; cam, Cameroon Mt; mou, Moukandé Mt; bio, Bioko; tom, S. Tomé; mun, R. Muni; eth, Ethiopia; ruk, Rukiya; abe, Aberdares; kip, Kipengere; iny, Inyanga; dra, Drakensberg; kny, Knysna Mt; tab, Table Mt; nam, Namibia; ang, Angola; and mad, Madagascar.
A general relationship is found between species richness and higher taxon richness (Gaston & Blackburn, 1995; Williams et al., 1997), and this includes ferns (Williams & Gaston, 1994) (Fig. 3). The species/genus, genera/family and species/family ratios showed quite comparable patterns and were related to species richness.
Figure 3.
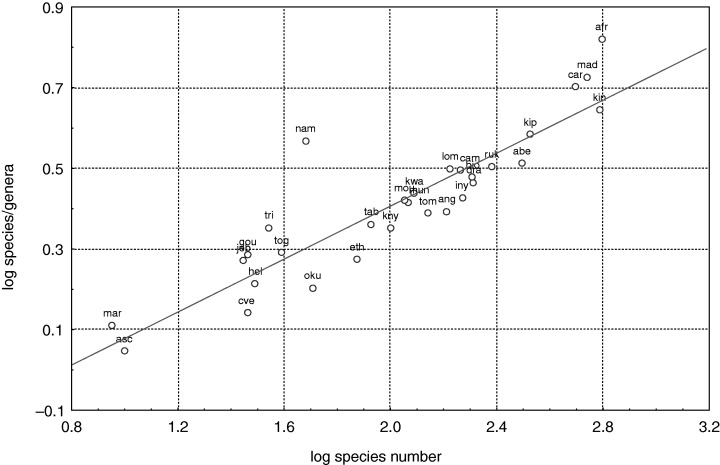
Regression plot of logarithm of ratio species/genera and the logarithm of species number. Acronyms are according to Fig. 2.
Namibia appears considerably separated from the regression lines for species–species/genus and species–species/family (Fig. 3). The Namibian species/genus ratio is considerably higher because this site has 48 fern species in only 13 genera (the lowest total number of genera of any area studied with the exception of Ascension Island and Marion Island). This might be related to the climatic conditions of Namibia, which only allow drought‐tolerant ferns to become established. These and other peculiarities of Namibia, as well as those of other arid sites, are discussed below.
Diversity gradients, species–area relationships and predictors of taxa richness
As with flowering plants (Linder, 2001), African ferns are distributed along a latitudinal gradient, but with considerable heterogeneity (Fig. 2a, Table 1). The correlation between species number and latitude (absolute value) was not significant; neither was that between the ratio species/area and latitude. The correlation between the logarithm of the species number and latitude was low (R = −0.41, P = 0.035) due to climatic heterogeneity and differences in area and elevation. The main climatic variable that changes with latitude is usually temperature, with lower latitudes showing high overall temperatures and weaker seasonal variations (see Appendix S2). But other climatic variables also depend on latitude, e.g. rainfall, moisture, the number of hours of sunshine per year and cloud cover. However, temperature can be also influenced by other factors. Moisture varies with longitude due to the effects of sea currents, but inversely on either side of Africa (Iriondo, 1999; Gasse, 2000). As expected, species number (logarithm transformed) is weakly correlated with longitude (R = 0.478, P = 0.011).
To determine the explanatory power of climatic and physical variables on species number, SPR and SMR analyses were performed. Table 2 shows the correlations between species number and predictors. In SPR analyses, the variables most related to species richness were area and elevation, while warmth index was weakly correlated. Area and elevation (both indicators of habitat diversity) were correlated with species number (R = 0.653, P < 0.0001 for area and R = 0.51, P = 0.007 for elevation).
Table 2.
Correlation coefficients and signification in standard pairwise regression analysis (SPR) of the environmental factors and the species number
| Log area (log m2) | Log Kira warmth index (log) | Log difference between max. and min. of monthly mean of temperatures (log °C) | Log mean sun hours/year (log h) | Arc‐sin of mean relative humidity (arc‐sin of proportion) | Log mean year rainfall (log mm) | Log driest month rainfall (log mm) | Arc‐sin of cloudiness (arc‐sin of proportion) | Log elevation | Log distance to the nearest site (log km) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Log of species number | 0.6534 (P = 0.000) | 0.4031 (P = 0.037) | −0.131 (P = 0.514) | 0.383 (P = 0.048) | 0.129 (P = 0.519) | 0.013 (P = 0.948) | −0.181 (P = 0.365) | 0.084 (P = 0.675) | 0.510 (P = 0.007) | −0.243 (P = 0.222) |
In SMR analyses using variables with low redundancy, relative humidity, area and elevation were the most explanatory variables, accounting for 82.6% of variation in species number and 87.7% of variation in genera number (logarithm transformed). Table 3 shows the results obtained with species, endemic species, genera and families. The elimination of oceanic islands (to consider Africa and its nearby islands alone), led to a similar model and comparable results.
Table 3.
Values of partial correlation coefficients and signification (in brackets) in stepwise multiple regression (SMR) analysis of the endemics number and the independent variables in the log–log model
| Variables | Log species number | Log endemic species number | Log genera number | Log family number |
|---|---|---|---|---|
| Log area (log m2) | 0.776 (0.0001) | n.s. | 0.71 (<0.0001) | 0.755 (<0.0001) |
| Log elevation (log m) | 0.350 (0.016) | n.s. | 0.377 (0.008) | 0.626 (0.0002) |
| Arc‐sin of mean relative humidity (arc‐sin of proportion) | 0.649 (0.001) | 0.82 (0.021) | 0.547 (0.0003) | n.s. |
| Log distance to the nearest site (log km) | n.m. | n.s. | n.m. | n.m. |
| Log difference between max. and min. of monthly mean of temperatures (log °C) | n.m. | 0.596 (0.034) | n.m. | n.m. |
| Standard error of estimate | 0.264 | 0.47 | 0.19 | 0.136 |
| Total adjusted R 2 | 0.676 | 0.272 | 0.654 | 0.584 |
n.m., not in model; n.s., not significant.
Species, genera and family richness were significantly correlated with area in SMR analyses (Table 3). The regression slopes for the species number were z = 0.24 for all OGUs, z = 0.11 for all OGUs excluding the oceanic islands (St Helena, Ascension Island, Tristan da Cunha, Gough Island and Marion Island), z = 0.58 for these oceanic islands alone, and z = 0.18 for all OGUs excluding islands and extratropical areas (Namibia, Table Mountain, Mt Knysna, Drakensberg, Ethiopia and Jebel Marra). As expected, the inclusion of the oceanic islands increased the z‐value. These values are lower than those (z = 0.20) given by Malyshev (1991) for vascular plants of tropical Africa, and lower than the z = 0.66 reported by Hobohm (2000) for vascular plants of the West African inselbergs. The isolation of these inselbergs is considerable, and their floral differences are comparable with those of some very isolated islands, with species facing similar emigration difficulties.
The residuals of the regression equations between species and area (as well as genera–area and families–area) show the differences between the number of species by site, and the number that might be expected if the site were on the regression line. More positive residuals indicate that species richness is greater than that of comparable sites of similar size. The residuals of the species–area regression can therefore be used to test the potential of a site as a refuge. Several islands and archipelagos have already been compared by Hobohm (2000) (Table 4). The values of residuals were highly negative for islands with impoverished floras, while they were highly positive for islands considered to be refuges, e.g. Madagascar and New Guinea, (Hobohm, 2000 and the present results: see 1, 4).
Table 4.
Number of flowering plants and number of ferns, showing the residuals of log species–log area regression
| Number of flowering plants | Number flowering plant endemics (% of species) | Residuals of species–area in flowering plants | Number of ferns | Number of fern endemics (% of species) | Residuals of species–area in ferns | |
|---|---|---|---|---|---|---|
| Madagascar | 12,000 | 8160 (68) | 0.452 | 552 | 237 (41.7) | 0.348 |
| Cape Verde | 659 | 92 (14) | −0.212 | 29 | 2 (6.89) | −0.633 |
| St Helena | 320 | 256 (80) | −0.107 | 31 | 12 (38.7) | 0.041 |
| Tristan da Cunha | 157 | 40 (26) | −0.449 | 35 | 6 (17.14) | 0.106 |
All data about vascular plants are from Hobohm (2000).
Ferns and flowering plants show similar residuals in Madagascar, and there is a considerably higher number of endemic species of both (Table 4). These data suggest the island has had a role as a refuge for both groups, although biogeographical isolation is greater for the flowering plants. St Helena and Tristan da Cunha showed distinctive patterns: there are fewer endemic ferns than endemic flowering plants, while the residual of the species number was positive for ferns and negative for flowering plants (Hobohm, 2000). These data indicate that these islands maintained a comparatively richer flora of ferns than other islands of similar area. Thus, both islands may have suffered intense extinctions of flowering plants or lower colonization. Conversely, in Cape Verde, both ferns and flowering plants show negative residuals, probably because of droughts causing a general impoverishment of the flora.
In Africa, the highest positive residuals were those of Bioko and Mt Cameroon, while the most negative were those of Cape Verde and Ethiopia (1, 4). As expected, relative humidity was the variable most strongly correlated with the residuals of species, genera and families (Table 5). Consequently, droughts are one of the most plausible causes of extinction and the reduction of residuals. Other climatic variables which correlated well with taxa–area residuals were mean annual rainfall and cloudiness.
Table 5.
Values of correlation coefficients and signification (in brackets) in standard pairwise regression analysis between residuals of species–area, genera–area and families–area regressions and some environmental variables
| Species–area residuals | Genera–area residuals | Families–area residuals | |
|---|---|---|---|
| Log mean relative humidity (log %) | 0.531 (0.004) | 0.571 (0.002) | 0.657 (0.0001) |
| Log mean year rainfall (log mm) | 0.402 (0.038) | 0.361 (0.064) | 0.357 (0.067) |
| Log cloudiness (log %) | 0.477 (0.012) | 0.489 (0.010) | 0.450 (0.018) |
Generic distribution patterns
Stepwise multiple regression analyses showed humidity to be the main climatic variable related to fern richness. But they also suggested that different species or genera can have opposite climatic preferences. This can lead to low regression values for many climatic variables. Some genera have a sufficiently large number of species to be able to encompass these types of preference. Consequently, independent SMR analyses can be performed to reveal them. SMR analyses conducted with the relative contributions of species to each OGU were tested for significance (Table 6). Five variables with low redundancies were used, two non‐climatic (elevation and distance to closest refuge) and three climatic (difference between maximum and minimum mean temperatures, i.e. thermal seasonality, warmth index and relative humidity). Four major ecological groups of genera showing different preferences appeared.
Table 6.
Relative contribution of ferns of group 1 (termophilous ferns) in stepwise multiple regression analyses negatively correlated with seasonality and/or positively with warmth index. The only variable which was not significantly correlated or out of the model was the arc‐sin of relative humidity
| Genus | Regression summary | Kira Warmth Index (log) | Difference between max. and min. monthly mean temperatures (log °C) | Elevation (log m) | Distance to closest site (log km) |
|---|---|---|---|---|---|
| Arthropteris (Oleandraceae) | R 2 = 0.490, F = 4.04, P < 0.009, SE = 0.005 | n.m. | B = −0.875, P = 0.0001, pc = 0.683 | n.s. | n.s. |
| Pneumatopteris (Thelypteridaceae) | R 2 = 0.532, F = 8.72, P < 0.0004, SE = 0.005 | n.m. | B = −0.522, P = 0.0022, pc = −0.582 | B = 0.402, P = 0.009, pc = 0.506 | n.s. |
| Lastreopsis (Dryopteridaceae) | R 2 = 0.37, F = 7.06, P < 0.003, SE = 0.008 | n.s. | B = −0.508, P = 0.009, pc = −0.501 | n.m. | n.m. |
| Triplophyllum (Dryopteridaceae) | R 2 = 0.385 F = 7.53, P < 0.002, SE = 0.017 | n.s. | B = −0.474, P = 0.013, pc = −0.48 | n.s. | n.m. |
| Blotiella (Dennstaedtiaceae) | R 2 = 0.314, F = 5.5, P < 0.01, SE = 0.008 | n.m. | B = −0.437, P = 0.016, pc = −0.466 | B = 0.362, P = 0.042, pc = 0.4 | n.s. |
| Selaginella (Selaginellaceae) | R 2 = 0.373, F = 3.27, P < 0.029, SE = 0.036 | n.m. | B = −0.687, P = 0.008, pc = −0.523 | n.s. | n.s. |
| Lepisorus (Polypodiaceae) | R 2 = 0.490, F = 4.04, P < 0.009, SE = 0.005 | B = 0.448, P = 0.035, pc = 0.44 | B = −0.67, P = 0.021, pc = −0.44 | B = 0.473, P = 0.01, pc = 0.522 | n.s. |
| Pteris (Pteridaceae) | R 2 = 0.465, F = 10.44, P < 0.0005, SE = 0.029 | B = 0.661, P = 0.0002, pc = 0.665 | n.m. | B = −0.313, P = 0.049, pc = −0.388 | n.s. |
| Christella (Thelypteridaceae) | R 2 = 0.357, F = 3.05, P < 0.038, SE = 0.023 | B = 0.541, P = 0.01, pc = 0.511 | n.m. | n.m. | n.m. |
| Xiphopteris (Grammitidaceae) | R 2 = 0.269, F = 6.25, P < 0.001, SE = 0.021 | B = 0.411, P = 0.036, pc = 0.429 | n.m. | n.m. | n.m. |
| Trichomanes (Hymenophyllaceae) | R 2 = 0.531, F = 4.42, P < 0.02, SE = 0.017 | B = 0.409, P = 0.027, pc = 0.431 | n.s. | n.s. | n.s. |
| Bolbitis (Lomariopsidaceae) | R 2 = 0.486, F = 5.2, P < 0.004, SE = 0.015 | B = 0.429, P = 0.021, pc = 0.467 | n.s. | n.s. | n.s. |
| Lomariopsis (Lomariopsidaceae) | R 2 = 0.625, F = 9.17, P < 0.0001, SE = 0.01 | B = 0.432, P = 0.016, pc = 0.483 | n.s. | n.s. | n.m. |
| Tectaria (Dryopteridaceae) | R 2 = 0.492, F = 4.45, P < 0.001, SE = 0.006 | B = 0.554, P = 0.0014, pc = 0.602 | n.m. | n.m. | n.s. |
| Diplazium (Dryopteridaceae) | R 2 = 0.441, F = 6.05, P < 0.003, SE = 0.009 | B = 0.652, P = 0.0004, pc = 0.645 | n.m. | n.m. | B = 0.319, P = 0.071, pc = 0.366 |
| Total | R 2 = 0.865, F = 49.2, P < 0.0001, SE = 0.06 | B = 0.77, P = 0.0001, pc = 0.708 | B = −0.482, P < 0.0001, pc = 0.763 | n.s. | n.s. |
| Total plus associated genera (see text) | R 2 = 0.841, F = 63.9, P < 0.0001, SE = 0.07 | B = 0.590, P = 0.0001, pc = 0.80 | B = −0.50, P < 0.0001, pc = 0.756 | n.s. | n.s. |
n.m., not in model; n.s., not significant.
Group 1
Ferns whose contribution is negatively correlated with seasonality and/or positively correlated with the warmth index (Table 6) such as: Blotiella R.M. Tryon (Dennstaedtiaceae), Tectaria Cav., Diplazium Sw., Lastreopsis Ching and Triplophyllum Holttum (Dryopteridaceae), Xiphopteris Kaulf. (Grammitidaceae), Trichomanes L. (Hymenophyllaceae), Bolbitis Schott, Lomariopsis Fée (Lomariopsidaceae), Arthropteris J. Smith ex Hook. f. (Oleandraceae), Lepisorus (Sm.) Ching (Polypodiaceae), Pteris L. (Pteridaceae), Selaginella P. Beauv. (Selaginellaceae), Pneumatopteris Nakai, and Christella H. Lév. (Thelypteridaceae). Most of their species grow in tropical or subtropical environments of central Africa. The number of species in these genera decreases as latitude increases. The above ferns are rare in arid sites, the Cape area, and on oceanic islands, where they generally grow at below 2000 m (Table 6, and see Appendix S4; Jacobsen & Jacobsen, 1989). The relative contribution of this group shows a negative correlation with the absolute value of latitude (Fig. 4, r = −0.803, P < 0.0001) and an even poorer correlation with longitude (r = −0.43, P = 0.024). The relationship with longitude suggests that tropical West Africa is a centre of diversity for these species, particularly the humid sites of Lower and Upper Guinea (White, 1979). Many are forest species, and many of these are epiphytes (Boyer, 1964; Dowsett‐Lemaire, 1988, 1989).
Figure 4.
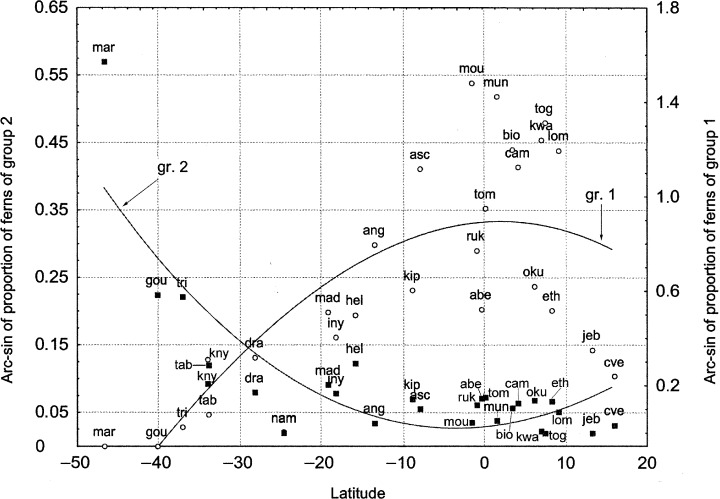
Plot of arc‐sin of proportion of ferns of groups 1 (right) and 2 (left) against latitude. Acronyms are according to Fig. 2.
Many genera with few species could not be studied in this way. However, some did seem to show comparable trends (i.e. they are more frequent in low latitude sites), e.g. Dicksonia L'Hér. (Dicksoniaceae), Nephrolepis Schott (Nephrolepidaceae), Oleandra Cav. (Oleandraceae), Loxogramme (Blume) C. Presl, Microsorum Link, Pyrrosia Mirb. and Platycerium Desv. (Polypodiaceae), Afropteris Alston, Coniogramme Fée (Pteridaceae), Metathelypteris (H. Itô) Ching, Pseudophegopteris Ching (Thelypteridaceae) and Antrophyum Kaulf. (Vittariaceae). These were included in the analyses and tested for significance, and quite similar results were obtained (Table 6, last row).
Group 2
Ferns negatively correlated with the warmth index and/or positively correlated with seasonality (Table 7, and see Appendix S5): Blechnum L. (Blechnaceae), Polystichum Roth (Dryopteridaceae), Grammitis Sw. (Grammitidaceae), Hymenophyllum Sm. (Hymenophyllaceae), Elaphoglossum Schott ex J. Sm. (Lomariopsidaceae), Huperzia Bernh. and Lycopodium L. (Lycopodiaceae) (Fig. 4). These are mainly found in southern and south‐eastern Africa and on the islands of the South Atlantic. They seem to tolerate low temperatures, marked seasonality, high humidity and cloudiness. Some genera of this group include mountain ferns such as Elaphoglossum (E. conforme (Sw.) Schott, E. deckenii (Kuhn) C. Chr., E. hybridum (Bory) Brack., E. subcinnamomeum Hieron., E. tanganjicense Krajina ex Pic. Serm.), Huperzia (H. saururus (Lam.) Trevis., and H. bampsiana Pic. Serm.), Hymenophyllum (H. peltatum (Poir.) Desv.), Lycopodium (L. aberdaricum Chiov., L. clavatum L.), Polystichum (P. magnificum Ballard, P. volkensii (Hier.) C. Chr., P. dracomontanum Schelpe, and P. fuscopaleaceum Alston). These can grow at elevations above 2500 m (Jacobsen & Jacobsen, 1989). An appreciable number of species of this group are also found on Tristan da Cunha and Gough Island. Here an effect similar to the ‘Massenerhebung effect’ can be argued, as genera which usually grow at c. 2000 m in the central and southern African ranges live at considerably lower elevations on these islands.
Table 7.
Negative correlation of relative contribution of ferns of group 2 (cold‐tolerant ferns), in stepwise multiple regression analysis. The variables not significantly correlated or out of the model were log of elevation (in m) and log of distance to closest site (in km)
| Genus | Regression summary | Mean relative humidity (arc‐sin of proportion) | Kira Warmth Index (log) | Difference between max. and min. monthly mean temperatures (log °C) |
|---|---|---|---|---|
| Polystichum (Dryopteridaceae) | R 2 = 0.371, F = 7.09, P < 0.0037, SE = 0.035 | n.s. | B = −0.672, P = 0.0009, pc = −0.608 | n.s. |
| Blechnum (Blechnaceae) | R 2 = 0.783, F = 27.76, P < 0.00001, SE = 0.016 | B = 0.418, P = 0.010, pc = 0.504 | B = −0.708, P < 0.00001, pc = −0.769 | n.s. |
| Grammitis (Grammitidaceae) | R 2 = 0.632, F = 13.16, P < 0.00003, SE = 0.016 | B = 0.364, P = 0.012, pc = 0.494 | B = −0.645, P < 0.00005, pc = −0.717 | n.m. |
| Lycopodium (Lycopodiaceae) | R 2 = 0.713, F = 10.43, P < 0.00004, SE = 0.015 | n.s. | B = −0.782, P = 0.00003, pc = −0.751 | n.s. |
| Huperzia (Lycopodiaceae) | R 2 = 0.339, F = 3.94, P < 0.020, SE = 0.036 | n.m. | B = −0.56, P = 0.006, pc = −0.528 | B = −0.42, P = 0.039, pc = −0.414 |
| Hymenophyllum (Hymenophyllaceae) | R 2 = 0.744, F = 22.29, P < 0.00001, SE = 0.014 | B = 0.465, P = 0.0003, pc = 0.656 | B = −0.686, P < 0.00001, pc = −0.796 | n.m. |
| Elaphoglossum (Lomariopsidaceae) | R 2 = 0.612, F = 12.12, P < 0.00006, SE = 0.033 | B = 0.360, P = 0.014, pc = 0.483 | B = −0.712, P < 0.00001, pc = −0.748 | n.m. |
| Total | R 2 = 0.573, F = 10.3, P < 0.0001, SE = 0.21 | B = 0.362, P = 0.019, pc = 0.465 | B = −0.598, P = 0.0002, pc = −0.579 | |
| Total plus associated genera (see text) | R 2 = 0.649, F = 14.2, P < 0.0001, SE = 0.87 | B = 0.367, P = 0.009, pc = 0.50 | B = −0.653, P = 0.0003, pc = −0.632 |
n.m., not in model; n.s., not significant.
The species of the islands of the South Atlantic and southern Africa are frequently common to South America and the sub‐Antarctic islands (from 31% on Tristan da Cunha and Gough Island to 67% in Marion Island, and from 5.8% in Drakensberg to 11% on Table Mountains; 11, 12). The relative contribution of the second group to the flora of these areas shows a significant correlation with the absolute value of latitude (Fig. 4, r = 0.64, P < 0.0001). This type of distribution seems to be related to long‐distance dispersal by the westerly winds (Scott & Van Zinderen Bakker, 1985; Parris, 2001). The main genera which show this type of distribution are Blechnum L. (B. australe L., B. palmiforme (Thouars) C.Chr., B. penna‐marina (Poir.) Kuhn), Grammitis Sw. (G. poeppigiana (Mett.) Pic. Serm., G. magellanica Desv.), Huperzia Bernh. (H. insularis (Carmich.) Rothm., H. saururus (Lam.) Trevis.), Hymenophyllum Sm. (H. peltatum (Poir.) Desv.), Lycopodium L. (L. magellanicum (P. Beauv.) Sw., L. diaphanum (P. Beauv.) Sw.), and Polystichum Roth (P. mohrioides (Bory ex Willd.) C. Presl, P. marionense Alston & Schelpe) (7, 12, and see Appendix S5). However, many species of Elaphoglossum Schott ex J. Sm. can be found on the Atlantic islands that cannot be found in South America (Parris, 2001).
Table 11.
Number and percentage of species shared by Africa, Asia and America (subcosmopolitan species), by Africa, Asia and/or Polynesia or by Africa, South America and/or sub‐Antarctic islands
| OGU | Number of species of ferns shared by Africa, Asia and America (percentage) | Number of species of ferns shared by Africa, Asia, and/or Polynesia (percentage) | Number of ferns shared by Africa, South America and/or sub‐Antarctic islands (percentage) |
|---|---|---|---|
| Madagascar | 17 (3) | 49 (8.9) | 18 (3.3) |
| Kipengere | 18 (5) | 39 (12) | 10 (3) |
| Aberdares | 19 (6) | 40 (13) | 9 (3) |
| Rukiya | 12 (5) | 28 (12) | 7 (3) |
| Drakensberg | 14 (7) | 28 (14) | 12 (5.8) |
| Bioko | 14 (7) | 18 (8.8) | 8 (4) |
| Inyanga | 13 (7) | 28 (15) | 9 (4.8) |
| Cameroon Mt | 14 (8) | 17 (9.2) | 7 (4) |
| Loma Mt | 10 (5.9) | 13 (7.8) | 4 (2.4) |
| Angola | 14 (9) | 21 (13) | 4 (2.5) |
| S. Tomé | 10 (7) | 19 (14) | 6 (4) |
| Kwahu pl. | 7 (5.7) | 17 (14) | 2 (1.6) |
| Río Muni | 7 (6) | 12 (10) | 3 (3) |
| Moukandé Mt | 6 (5) | 8 (7.1) | 2 (2) |
| Knysna mts. | 9 (9) | 7 (6.9) | 10 (9.9) |
| Table mts. | 7 (8) | 5 (5.9) | 9 (11) |
| Ethiopia | 8 (11) | 15 (20) | 2 (3) |
| Oku Mt | 7 (14) | 6 (12) | 1 (2) |
| Namibia | 3 (6) | 6 (13) | 0 |
| Togo Mt | 3 (8) | 2 (5.1) | 1 (3) |
| P. Principe | 6 (8) | 10 (13) | 2 (3) |
| Tristan da Cunha I. | 2 (6) | 0 | 11 (31) |
| Helena I. | 2 (6.5) | 2 (6.5) | 2 (6.5) |
| Gough I. | 2 (7) | 0 | 9 (31) |
| Annobón | 2 (5) | 7 (17) | 2 (5) |
| Cape Verde | 8 (28) | 6 (21) | 0 |
| Jebel Marra | 5 (18) | 4 (14) | 0 |
| Ascension I. | 3 (30) | 2 (20) | 0 |
| Marion I. | 0 | 0 | 6 (67) |
Table 12.
Taxonomic composition of species 1‐ shared by Africa and Asia, 2‐by Asia, America and Africa, 3‐by Africa, South America and/or sub‐Antarctic islands, 4‐growing on mountains in South Africa, 5‐ growing on mountains in Rwanda‐Burundi‐Kivu and 6‐ in Kenya
| Family | 1‐Percentage of species shared by Africa and Asia | 2‐Percentage of species shared by Africa, Asia and America | 3‐Percentage of species shared by Africa, South America and/or Australia and sub‐Antarctic islands | 4‐Number and percentage of species growing above 2500 m in S. Africa* | 5‐Number and percentage of species growing above 2500 m in Rwanda‐ Burundi‐Kivu* | 6‐Number and percentage of species growing above 2500 m in Kenya* |
|---|---|---|---|---|---|---|
| Aspleniaceae | 9.37 | 31.6 | 20.7 | 17 (21.2) | 18 (29.5) | 16 (20.7) |
| Blechnaceae | 0 | 0 | 3.45 | 1 (2.5) | 0 | 1 (2.5) |
| Cyatheaceae | 0 | 0 | 0 | 1 (2.5) | 0 | 0 |
| Davalliaceae | 1.56 | 0 | 0 | 0 | 0 | 0 |
| Dennstaedtiaceae | 4.6 | 10.5 | 0 | 1 (1.2) | 4 (6.5) | 5 (6.4) |
| Dryopteridaceae | 10.9 | 10.5 | 10.3 | 13 (16.2) | 7 (11.4) | 10 (12.9) |
| Gleicheniaceae | 1.56 | 0 | 0 | 2 (2.5) | 0 | 0 |
| Grammitidaceae | 0 | 0 | 24.1 | 2 (2.5) | 2 (3.2) | 2 (2.5) |
| Hymenophyllaceae | 3.12 | 0 | 10.3 | 4 (5) | 2 (3.2) | 9 (11.6) |
| Lomariopsidaceae | 3.12 | 0 | 0 | 6 (7.5) | 7 (11.4) | 9 (11.6) |
| Lycopodiaceae | 3.12 | 5.2 | 10.3 | 4 (5) | 6 (9.8) | 3 (3.8) |
| Marattiaceae | 3.12 | 0 | 0 | 0 | 0 | 0 |
| Marsileaceae | 3.12 | 0 | 0 | 0 | 0 | 0 |
| Nephroplepidaceae | 1.56 | 10.5 | 0 | 0 | 0 | 0 |
| Oleandraceae | 1.56 | 0 | 0 | 0 | 0 | 0 |
| Ophioglossaceae | 7.81 | 0 | 3.45 | 1 (1.2) | 0 | 1 (2.5) |
| Polypodiaceae | 3.12 | 5.2 | 3.45 | 3 (3.7) | 6 (9.8) | 2 (2.5) |
| Psilotaceae | 1.56 | 0 | 0 | 0 | 0 | 0 |
| Pteridaceae | 20.3 | 21.1 | 13.8 | 12 (15) | 8 (13.1) | 14 (18.1) |
| Schizeaceae | 4.6 | 0.59 | 1.56 | 3 (3.7) | 0 | 0 |
| Selaginellaceae | 4.6 | 0 | 3.45 | 5 (21.2) | 0 | 1 (1.2) |
| Thelypteridaceae | 12.5 | 10.5 | 0 | 2 (2.5) | 0 | 1 (1.2) |
| Vittariaceae | 3.12 | 0 | 0 | 0 | 0 | 0 |
*According to Jacobsen & Jacobsen (1989).
Some genera show comparable trends to group 2: Histiopteris (J. Agardh) J. Sm. and Hypolepis Bernh. (Dennstaedtiaceae), Anemia Sw., Mohria Sw. and Schizaea Sm. (Schizeaceae), Eriosorus Fée (Pteridaceae), and Ampelopteris Kunze and Amauropelta Kunze (Thelypteridaceae). These were included in analyses, tested for significance, and gave quite similar results (Table 7, last row).
Finally, Dryopteris Adans. might be included in this group, but it gave no significant result in SMR analysis. This is probably explained by the heterogeneous preferences of African Dryopteris species.
Group 3
Ferns negatively correlated with humidity: Isoetes L. (Isoetaceae), Marsilea L. (Marsileaceae), Ophioglossum L. (Ophioglossaceae), Adiantum L., Actiniopteris Link, Cheilanthes Sw., Pellaea Link, and Pityrogramma Link (Pteridaceae) (Table 8, and see Appendix S6). These drought‐tolerant pteridophyte genera are more diversified in southern and sub‐Saharan African OGUs (Kornaś, 1977; Dzwonko & Kornaś, 1978).
Table 8.
Negative correlation of relative contribution of ferns of group 3 (drought‐tolerant ferns) in stepwise multiple regression analysis. The variables which were not significantly correlated or out of the model were Kira warmth index, difference between max. and min. monthly mean temperatures, elevation (in m), and distance to closest site (in km)
| Genus | Regression summary | Mean relative humidity (arc‐sin of proportion) |
|---|---|---|
| Cheilanthes (Pteridaceae) | R 2 = 0.636, F = 13.44, P < 0. 00003, SE = 0.048 | B = −0.570, P = 0.0027, pc = −0.573 |
| Actiniopteris (Pteridaceae) | R 2 = 0.646, F = 21.98, P < 0. 0001, SE = 0.010 | B = −0.745, P = 0.0001, pc = −0.767 |
| Pellaea (Pteridaceae) | R 2 = 0.219, F = 2.15, P < 0. 121, SE = 0.014 | n.s. |
| Pityrogramma (Pteridaceae) | R 2 = 0.182, F = 2.68, P < 0. 008, SE = 0.005 | B = −0.427, P = 0.037, pc = −0.767 |
| Adiantum (Pteridaceae) | R 2 = 0.616, F = 19.26, P < 0.0001, SE = 0.0187 | B = −0.816, P < 0.0001, pc = 0.782 |
| Ophioglossum (Ophioglossaceae) | R 2 = 0.684, F = 16.63, P < 0.0001, SE = 0.0167 | B = −0.551, P = 0.0020, pc = −0.587 |
| Marsilea (Marsileaceae) | R 2 = 0.517, F = 8.23, P < 0.0006, SE = 0.033 | B = −0.747, P = 0.00006, pc = −0.713 |
| Isoetes (Isoetaceae) | R 2 = 0.382, F = 4.75, P < 0.010, SE = 0.010 | B = −0.617, P = 0.0478, pc = −0.596 |
| Total | R 2 = 0.724, F = 14.4, P < 0.0001, SE = 0.54 | B = −0.77, P = 0.0001, pc = −0.708 |
| Total plus associated genera (see text) | R 2 = 0.714, F = 19.2, P < 0.0001, SE = 0.12 | B = −0.707, P = 0.0001, pc = −0.699 |
n.s., not significant.
Two of the most studied drought‐tolerant genera are Cheilanthes Sw. and Pellaea Link (Pteridaceae). These are represented by a comparatively high number of species in Namibia, on Jebel Marra and on Table Mountain. They also grow in the tropical zone – at least the species adapted to inselbergs – on dry slopes and in other arid habitats (Reitsma et al., 1992). Some genera of this group grow in mountainous areas, where they reach high elevations. They are sometimes found in forests but never live as epiphytes (Table 9). Species such as Adiantum thalictroides Willd. ex Schltdl., Cheilanthes hyaloglandulosa W. Jacobsen & N. Jacobsen, C. eckloniana (Kunze) Mett., C. hirta Sw., C. multifida (Sw.) Sw., Pellaea calomelanos (Sw.) Link, P. boivinii Hook., P. quadripinnata (Forssk.) Prantl, Pityrogramma aurantiaca (Hieron.) C. Chr., P. elongata (C. Chr.) Pichi‐Serm., P. rupicola Pichi‐Serm., grow above 2000 m (Jacobsen & Jacobsen, 1989). Thus, in some cases, these Cheilanthoid ferns grow at high elevations in the microthermal region (Jacobsen & Jacobsen, 1989). They can be found in cloud forests, which rarely suffer water shortages (Bruijnzeel & Veneklaas, 1998; Foster, 2001), and in ericaceous brush. The abundance of ferns with xerophytic morphology in cloud forests is comparable with that of other plants (Flenley, 1992a, 1995). Bruijnzeel & Proctor (1995) and Flenley (1992b) suggest this morphology can be explained by exposure to harmful UV‐B radiation at these elevation. Reduced leaf transpiration, root respiration and nutrient uptake in these poor soils have also been proposed as compatible with the development of xeromorphic features (Hafkenscheid, 2000; Foster, 2001). The drought‐tolerant taxa are characterized by particular adaptations such as curling, and by being poikilohydrous or winter‐ or summer‐dormant. A revision of these life styles was published by Kornaś (1977). Some peculiar features include long dormancy with shedding of fronds, the protection of buds with scales and the bases of the stipes, resurrection after extreme desiccation, a geophytic life style, a very fast life cycle with a shortening of the gametophytic phase, and large increases in spore production and survival time, etc. (Given, 1993).
Table 9.
Distribution in the fern groups of heterosporous ferns, and those with chlorophyllous spores, trilete spores or gemmiferous gametophytes
| Chlorophyllous spores | Trilete spores | Heterosporous | Gemmiferous gametophytes | Gemmiferous sporophytes | Epiphytes | |
|---|---|---|---|---|---|---|
| Thermophilous genera (group 1) | Xiphopteris Trichomanes | Pteris Xiphopteris Trichomanes | Selaginella | Xiphopteris Trichomanes | Some Bolbitis | Trichomanes Platycerium Microsorum Xiphopteris Some Pyrrosia Some Lepisorus Some Loxogramme |
| Cold‐tolerant genera (group2) | Grammitis Hymenophyllum | Grammitis Lycopodium Huperzia Hymenophyllum | – | Grammitis Hymenophyllum | Some Huperzia | Grammitis Hymenophyllum Some Elaphoglossum Some Lycopodium |
| Drought‐tolerant genera (group 3) | – | Cheilanthes Actiniopteris Pellaea Pityrogramma Adiantum Ophioglossum Marsilea | Isoetes Marsilea | – | ||
| Madagascar endemics and related species (group 4) | Ctenopteris | Cyathea Gymnosphaera Most Lindsaea Doriopteris Ctenopteris | Ctenopteris | Ctenopteris Some Rumohra | ||
| Aspleniaceae | Some Asplenium | Some Asplenium | ||||
| Others | Vittaria | Marattia | Belvisia Phymatosorus Drynaria Some Polypodium Some Vittaria Some Davallia |
Ophioglossum L. shows high species richness mainly in Namibia, Cape Verde and on Jebel Marra. It can remain dormant for long periods, withstanding drought and growing during favourable periods.
Namibia is a centre of diversity for Marsilea L. and Isoetes L., ferns that live in environments with fluctuating water availability. This can be explained in that the Namib and Kalahari deserts are older than the Sahara or other African deserts. The Benguela current originated some time after the upper Eocene (Maley, 1996), causing the drying of the south‐western coast of Africa (forming the Namib and Kalahari Deserts), while the Sahara appeared as late as the lower Pliocene (Maley, 1980). The pronounced glacial Antarctic cooling that occurred during the late Quaternary was accompanied by intensification of the south‐east trade winds which caused stronger aridification along the east African coast (Shi et al., 2001). There are several other cases of arid‐adapted genera with their centre of distribution in Namibia which reach the Saharo‐Sindian deserts or the dry habitats of the Mediterranean (De Winter, 1971). These distributions are most easily explained if these taxa were dispersed from south‐eastern Africa to the North via an arid tract of territory (Van Zinderen Bakker, 1975; Coetzee, 1983). Until the late Miocene, the woodland savanna of the Sahara would have prevented the spread of many of these genera (Axelrod & Raven, 1978; Maley, 1996).
Group 4
Ferns endemic or nearly‐endemic to the Malgache region showing no conspicuous climatic trend: (1) Cyathea Sm. (Cyatheaceae), with 33 endemic species in Madagascar and eight in the other OGUs, and Gymnosphaera Blume (Cyatheaceae) with all its African species growing in Madagascar. The tree ferns are present in most tropical mountain zones of the world, where they show considerable diversity and endemism. In New Guinea and in the Andes, the optimum elevation is between 1000 and 2500 m; only a few species reach 3000–5000 m (Tryon & Gastony, 1975); (2) Lindsaea Dryand. ex Sm. (Dennstaedtiaceae) with 19 species in Madagascar, three of which are also found in other OGUs; (3) Cornopteris Nakai, Pseudotectaria Tardieu‐Blot and Stenosemia C. Presl (Dryopteridaceae), all African species endemic to Madagascar; (4) Ctenitis (C. Chr.) C. Chr. (Dryopteridaceae), with one species endemic to the sub‐Antarctic islands and 16 species growing in Madagascar, three of which are also found in other OGUs; (5) Rumohra Raddi (Dryopteridaceae), with six species in Madagascar, only one of which is shared with other OGUs; (6) Ctenopteris Blume ex Kunze (Grammitaceae) with seven species, all in Madagascar; (7) Trachypteris André ex H. Christ (Pteridaceae), with one species endemic to Madagascar; (8) Doryopteris Sm. (Pteridaceae), with nine species in Madagascar, two of which are shared with other OGUs; (9) Thelypteris Schmidel (Thelypteridaceae) with eight species in Madagascar, only one of which is shared with other OGUs.
Finally, the Aspleniaceae fell into none of the resulting groups due to its large size and heterogeneity. Their contribution to OGU floras was inversely correlated with the absolute value of latitude (r = 0.63, P = 0.0004, Fig. 5), with more diversity seen in tropical or subtropical sites near the equator, especially in mountainous areas. This family includes species that grow on islands (Asplenium alvarecense Rudm. Brown, A. obtusatum G. Forst., A. platybasis Kze.), in high mountains (A. kassneri Hieron., A. majus (Hieron.) Pichi‐Serm., A. actinopteroides A. Peter, A. abyssinicum Fée, A. sertularioides Bak., A. volkensii Hieron. and A. adamsii Alston), and also in dry sites (A. abyssinicum Fée, A. pumilum Sw., A. suppositum Hieron.).
Figure 5.
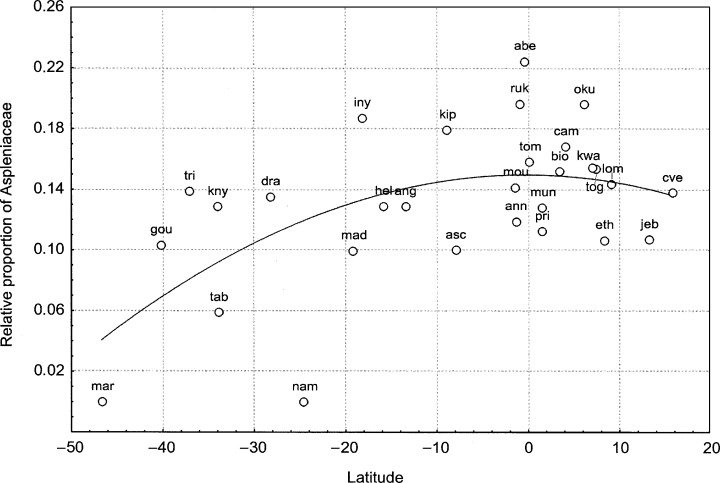
Plot of relative contribution of Aspleniaceae against latitude. Acronyms are according to Fig. 2.
Biological types and fern distribution
From features related to reproduction such as the possession of a homosporic or heterosporic cycle, whether spores are chlorophyllous and monolete or trilete, whether the gametophytes have gemmiferous propagules, and whether the sporophytes have adventitious buds, conclusions can be drawn about biogeographical influences on ferns (Kornaś, 1977, 1993; Schelpe, 1983; Pausas & Sáez, 2000; Parris, 2001) (Table 9). The differences between OGUs with respect to plants with these traits are shown in Table 10.
Table 10.
Percentage of species with gemmiferous gametophytes, chlorophyllous spores, trilete spores or heterosporic in studied operational geographical units
| Percentage of species with gemmiferous gametophytes | Percentage of species with chlorophyllous spores | Percentage of heterosporic species | Percentage of species with trilete spores | |
|---|---|---|---|---|
| Madagascar | 15.28 | 13.84 | 2.34 | 46.4 |
| Kipengere | 10.71 | 9.22 | 6.85 | 44.64 |
| Aberdares | 8.65 | 7.69 | 7.37 | 44.5 |
| Rukiya | 8.29 | 7.46 | 8.3 | 44.4 |
| Drakensberg | 5.82 | 6.79 | 7.28 | 48.06 |
| Bioko | 12.25 | 11.27 | 4.9 | 35.78 |
| Inyanga | 6.95 | 7.48 | 4.81 | 42.78 |
| Cameroon Mt | 12.5 | 10.32 | 4.89 | 36.96 |
| Loma Mt | 13.17 | 11.3 | 10.2 | 48.5 |
| Angola | 3.68 | 4.29 | 12.9 | 89.58 |
| S. Tomé | 16.54 | 12.94 | 3.6 | 38.85 |
| Kwahu pl. | 11.38 | 9.75 | 9.75 | 47.15 |
| Río Muni | 15.38 | 11.96 | 7.69 | 42.7 |
| Moukandé Mt | 11.5 | 9.73 | 7.96 | 39.82 |
| Knysna mts. | 6.93 | 8.91 | 5.94 | 51.49 |
| Table mts. | 8.23 | 9.41 | 7.06 | 58.82 |
| Ethiopia | 5.33 | 5.33 | 13.3 | 52 |
| Oku Mt | 5.88 | 7.84 | 1.96 | 33.3 |
| Namibia | 0 | 0 | 22.9 | 89.59 |
| Togo Mt | 5.12 | 2.56 | 20.5 | 43.5 |
| P. Principe | 13.75 | 8.75 | 5 | 37.5 |
| Tristan da Cunha I. | 17.14 | 17.1 | 0 | 34.29 |
| Helena I. | 6.45 | 6.45 | 0 | 35.4 |
| Gough I. | 13.79 | 13.79 | 0 | 34.82 |
| Annobón | 28.57 | 23.8 | 0 | 47.62 |
| Cape Verde | 0 | 6.89 | 0 | 51.72 |
| Jebel Marra | 0 | 0 | 3.57 | 60.71 |
| Ascension I. | 10 | 10 | 0 | 40 |
| Marion I. | 22.22 | 22.22 | 0 | 55.72 |
A higher ratio between gemmiferous and non‐gemmiferous ferns was found for some islands (Annobón, Gough Island, Marion Island, Porto Principe and Tristan da Cunha), as reported by Dassler & Farrar (2001). Conversely, on Cape Verde and St Helena, the ratio was lower than on the continent, while on Ascension Island it was similar to the African mainland (Table 10).
A higher ratio between chlorophyllous and non‐chlorophyllous spores was also recorded for some islands (Annobón, Gough Island, Marion Island, Porto Principe and Tristan da Cunha) (Table 10). According to both Lloyd & Klekowski (1970) and Tryon (1986), chlorophyllic spores constrain long‐distance dispersal as they have a shorter viability period. However, the evidence indicates that this potential constraint is ineffective (Tryon, 1986). Dassler & Farrar (2001) supported this opinion because they found a higher ratio of chlorophyllous ferns on islands. There is a strong coincidence between fern species with gemmiferous gametophytes and chlorophyllous spores (Table 9). Species with the latter characteristics were somewhat related to high humidity. The arc‐sines of the relative proportions of ferns with chlorophyllous spores, and those of gemmiferous gametophytes, correlated with the arc‐sines of humidity (R = 0.721, P < 0.0001, and R = 0.781, P < 0.0001, respectively).
Heterosporous ferns were absent from Annobón, Ascension Island, Cape Verde, Gough Island, Marion Island, St Helena, and Tristan da Cunha, and the ratio between heterosporous and non‐heterosporous ferns was low in Bioko, Porto Principe and S. Tomé (Table 10). On the contrary, in Namibia, Ethiopia and Angola, and on Mt Togo and Mt Loma, there were more heterosporous ferns. The arc‐sines of the relative proportions of heterosporous ferns were negatively correlated with the arc‐sines of humidity (R = −0.47, P = 0.012). Consequently, drought‐tolerant ferns are frequently heterosporic. Marsilea L. and Isoetes L. persist throughout the dry period as dormant ‘corms’ beneath the surface of the soil.
Finally, the four ecological groups mentioned above included sporophytes with adventitious buds (except group 3) or trilete spores. A higher proportion of ferns with trilete spores was found in some dry OGUs (Angola, Namibia), and a negative correlation seen with the arc‐sines of humidity (R = −0.53, P = 0.004). However, trilete spores do not seem to provide any adaptive advantage to growth in dry habitats (Lugardon, 1996). The trends associating monolete spores with high latitudes and elevations reported in some studies (Ito, 1972, 1978; Pausas & Sáez, 2000) were not observed.
Floral affinities, inter‐OGU distances and dispersion among OGUs
The NMS analysis produced a plot for the floral affinities of all sites, the centre of which was the Great Lakes area of Africa, and from which arms extend to the other sites (Fig. 6). One arm goes from Rukiya to the Gulf of Guinea and the West African OGUs. The second reaches Ethiopia, Jebel Marra and Cape Verde. The third reaches Angola and the fourth goes out to the Kipengere Range, Madagascar, Inyanga and the southern African OGUs. Two branches extend from Table Mountain: one to St Helena and Ascension, the other to Tristan da Cunha and Gough and Marion Islands. The similarity of the central and southern Atlantic island flora with that of Africa is scant, and only a few species are shared with the Cape area (mainly Table Mountain) (Jaccard indices, I J = 0.101 with Tristan da Cunha, and I J = 0.123 with St Helena). As previously commented, Tristan da Cunha, Gough Island and Marion Island have more species in common with South America and other sub‐Antarctic islands than with South Africa (11, 12; Schelpe, 1983).
Figure 6.
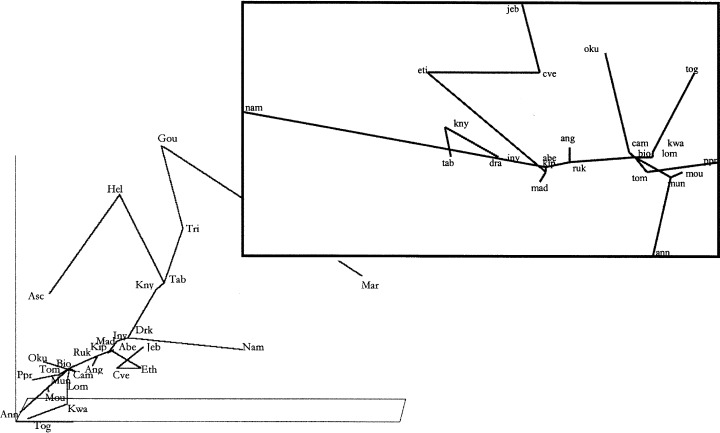
Plot of two first axes of non‐metric multidimensional scaling of matrix of Jaccard indexes obtained with all sites. In the inset there is the plot of all sites but mid‐Atlantic islands. The presence or absence of fern species was used to extract the matrix of Jaccard indexes. Line is minimum spanning tree of these distances. Acronyms are according to Fig. 2.
The centre of this network is shown in the inset of Fig. 6. The sites of Upper and Lower Guinea form a homogeneous group related to the East African mountain arc, possibly as a consequence of migrations between eastern and western refuges during cold periods (Maley, 1990, 1991). The β‐turnover between these OGUs is relatively low. In addition, a line links the East African mountain arc sites to Angola, Madagascar, Namibia, and the rest of southern Africa. The flora of Bioko, Mt Cameroon and S. Tomé are therefore similar. The Jaccard indexes are I J = 0.678 between Bioko and Mt Cameroon, and I J = 0.513 between Bioko and S. Tomé. Mt Oku and Mt Cameroon, however, are less similar (I J = 0.22), as is Annobón to Río Muni (I J = 0.27). The distribution of animal groups in these areas show comparable patterns, e.g. the fauna of Mt Cameroon and Bioko are quite similar while that of Mt Oku is somewhat different (Smith et al., 2000). These differences might be caused by the climatic characteristics of Mt Oku and Annobón, which receive considerably less rain (3000 mm year−1 on Mt Oku and 1000 mm year−1 on Annobón, compared with 10,000 mm year−1 on Mt Cameroon and 5000 mm year−1 on S. Tomé).
The fern floras of Ethiopia, Jebel Marra and Cape Verde differ from those of the remaining OGUs. Only weak similarities were seen between these three cited areas and those of the East African mountain arc. Cape Verde, Ethiopia, Ascension Island and Annobón showed the greatest similarities to Asia, while Ascension Island, Cape Verde, and Jebel Marra had the highest proportion of subcosmopolitan species (12, 13). Many of their ferns are drought‐tolerant, and may be the remains of an ancient Mediterranean or Palaeoafrican xerophytic flora (Schnell, 1977).
Table 13.
Comparison of distances, floral similitude using Jaccard indices and β‐turnover between operational geographical units (OGUs). The pairs of OGUs compared were not the nearest but those in which the minimal floral differences occur (calculated by Jaccard index)
| Pairs of sites (OGUs) | Distance (km) | Logarithm of distance | Jaccard index | β‐turnover |
|---|---|---|---|---|
| Togo ranges‐Kwahu plateau | 280 | 2.447 | 0.2778 | 0.57 |
| Loma Mt‐Kwahu plateau | 1120 | 3.049 | 0.565 | 0.31 |
| Loma Mt‐Bioko | 2280 | 3.358 | 0.4467 | 0.41 |
| Río Muni‐Annobón | 645 | 2.81 | 0.2727 | 0.59 |
| Cameroon Mt‐Oku Mt | 265 | 2.423 | 0.2251 | 0.63 |
| Aberdares‐Ethiopia | 990 | 2.996 | 0.219 | 0.66 |
| Ethiopia‐Cape Verde | 6790 | 3.832 | 0.2125 | 0.68 |
| Cape Verde‐Jebel Marra | 1600 | 3.204 | 0.225 | 0.69 |
| Bioko‐Cameroon Mt | 95 | 1.978 | 0.6784 | 0.21 |
| Bioko‐S. Tomé | 430 | 2.633 | 0.5138 | 0.35 |
| Bioko‐Río Muni | 290 | 2.462 | 0.4601 | 0.38 |
| Río Muni‐Moukandé Mt | 400 | 2.602 | 0.5135 | 0.34 |
| Sao Tomé‐Príncipe | 175 | 2.243 | 0.4354 | 0.42 |
| Bioko‐Rukiya | 2420 | 3.384 | 0.3686 | 0.48 |
| Rukiya‐Aberdares | 750 | 2.875 | 0.5455 | 0.33 |
| Aberdares‐Kipengere | 1010 | 3.004 | 0.7119 | 0.21 |
| Kipengere‐Madagascar | 1855 | 3.268 | 0.4933 | 0.58 |
| Kipengere‐Inyanga | 1045 | 3.019 | 0.4696 | 0.38 |
| Inyanga‐Drakensberg | 1180 | 3.072 | 0.6579 | 0.24 |
| Drakensberg‐Knysna Mt | 865 | 2.937 | 0.4236 | 0.44 |
| Knysna Mt‐Table Mt | 430 | 2.633 | 0.6762 | 0.24 |
| Rukiya‐Angola | 2080 | 3.318 | 0.3601 | 0.49 |
| Drakensberg‐Namibia | 1340 | 3.127 | 0.1675 | 0.74 |
| Table Mt‐St Helena | 3140 | 3.497 | 0.1279 | 0.82 |
| St Helena‐Ascension | 1295 | 3.112 | 0.1818 | 0.81 |
| Table Mt‐Tristan da Cunha | 2800 | 3.447 | 0.101 | 0.84 |
| Tristan da Cunha‐Gough I. | 405 | 2.607 | 0.875 | 0.15 |
| Gough I.‐Marion I. | 3860 | 3.587 | 0.2 | 0.73 |
The OGUs of the East African mountain arc show high floral similarity. Kipengere is related to Inyanga and Madagascar (I J = 0.469 and 0.493, respectively). Inyanga is related to Drakensberg (I J = 0.657). Finally, Drakensberg is also related to the Knysna Mountains (I J = 0.423), and the latter related to Table Mountain (I J = 0.676) and Namibia (I J = 0.167). Thus, the floras of eastern and southern Africa show high similarity along the mountain ranges, from the Great Lakes area to the Cape.
Species richness was not significantly influenced by inter‐OGU distance but this did affect endemicity and species composition (Table 13). The three main factors affecting the Jaccard indices were inter‐OGU distance, elevation and ecological particularities. The Jaccard index was negatively correlated with the logarithm of the distance between the OGUs, as shown by the results of a Mantel test between Jaccard indices and logarithms of inter‐OGU distances (R = −0.553, t = −6.91 and P = 0). Inter‐OGU distance significantly hinders dispersion from centres of diversity, reducing the number of species shared with potentially associated sites (Table 13). These data underscore another point discussed later: the relationship between the relative number of endemic species in a site and its distance to other centres of endemism.
The β‐turnovers between OGUs were also calculated to evaluate the δ‐diversity (Shmida & Wilson, 1985, Table 13; Fig. 7a). If the most similar pairs of sites are compared, β‐turnover is significantly correlated with the logarithm of the distance between them (R = 0.537, P = 0.003). If all sites are compared using a Mantel test, the correlation between both variables is also significant (R = 0.636, t = 7.47 and P = 1). β‐turnover is more useful for comparing sites with a similar degree of diversity as it is sensitive to differences in the total number of species. With respect to the β‐turnovers of the sites of Upper and Lower Guinea, the largest differences in fern flora were seen between Mt Oku, Annobón, and Mt Togo; all other sites showed quite similar values. These three sites have a considerably impoverished flora with only 51, 42 and 39 species, respectively. This might be due to the lower elevation of their mountains (which also occupy a reduced area) and the drier climate (they lie beyond the equatorial forest zone). The largest differences between central and south‐eastern Africa were those of Drakensberg and Madagascar respect to the Kipengere Range (β‐turnover Drakensberg‐Kipengere = 0.656 and Madagascar‐Kipengere = 0.580; the number of species is 207 in Drakensberg, 336 in Kipengere and 542 in Madagascar). These differences are possibly related to the γ‐diversity and endemism rates, which are extraordinarily high in Madagascar and Drakensberg.
Figure 7.
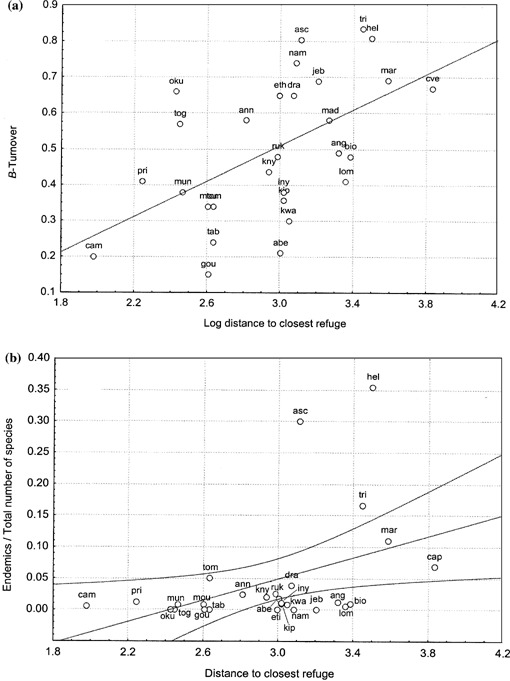
(a) Regression plot of distance between related sites and their β‐turnover. (b) Regression plot of the distance between related sites and the endemism ratio. Acronyms are according to Fig. 2.
Distribution of endemic species in Africa
The number of endemic species is irregular along the latitude gradient, with large differences between OGUs (Fig. 2b). SMR analyses were performed with the same variables and transformations as before (see SMR of species number). The arc‐sines of the relative contributions of endemics were used as the dependent variable. The variables showing the greatest beta and partial correlations were inter‐OGU distance (B = 0.45, pc = 0.46, P = 0.020) and humidity (B = 0.54, pc = 0.45, P = 0.021) (R 2 = 0.25, F = 3.89 and SE = 0.1). These data indicate a weak correlation. However, several endemic species were shared by the closest sites (i.e. the Aberdare, Kipengere Ranges, and Rukiya Ranges, or Mt Cameroon and Bioko). The number of endemic species by ensemble of sites was, therefore, also plotted. These ensembles were Ethiopia and Jebel Marra, Upper Guinea (Loma, Togo and Kwahu), Lower Guinea (Bioko, Río Muni and Mt Cameroon), the islands of the Gulf of Guinea (Annobón, S. Tomé and Porto Principe), Mt Moukandé, Angola, Namibia and the Cape sites (Table Mountain and Mt Knysna), the mountains of south‐eastern Africa (Drakensberg and Inyanga), the Great Lakes mountain ranges (the Aberdare, Kipengere and Rukiya Ranges), Madagascar (including the associated islands of Reunion, The Seychelles, The Comoros and Mauritius), Cape Verde, the islands of the Central Atlantic (St Helena and Ascension Island), and the sub‐Antarctic islands (Tristan da Cunha, Gough Island and Marion Island). The distribution of the number of endemic species in these groups with respect to latitude underlines the high relative endemicity seen in Madagascar, the islands of the central Atlantic and the sub‐Antarctic islands.
If the data for ferns are compared with those for flowering plants in Madagascar (Hobohm, 2000; Myers et al., 2000), a considerably higher number of the latter is seen (Table 4). This suggests the island was a refuge for both groups, but that the flowering plants suffered greater isolation leading to higher endemicity (41.7% of ferns and 80.6% of plants are endemic, Myers et al., 2000). These results are not surprising as endemicity in ferns is usually less than that observed in flowering plants. Myers et al. (2000) reported a higher ratio of plant endemicity than Hobohm (2000). They also recorded data for birds, mammals, reptiles and amphibians, most of which showed degrees of endemicity of around 80–90%. The exception was birds (55.4%) which is similar to the figure for ferns. These results might be explained by the superior dispersal capabilities of ferns and birds. They also confirm the important role of Madagascar as a refuge for most taxonomic groups. The extraordinary number of endemic species in Madagascar is not explainable by isolation, climatic stability or low competition alone; only a combination of these can explain the problem satisfactorily. This island seems to be a ‘museum of endemic diversity’ for many taxonomic groups including primates (Harcourt, 2000), amphibians (Bossuyt & Milinkovitch, 2000), butterflies (Torres et al., 2001), and plants such as Winteraceae, Canellaceae, Monimiaceae, Diegodendraceae, Sphaerosepalaceae, Sarcolenaceae, Asteropeiaceae and Medusagynaceae. (Leroy, 1978). Comparable patterns of high endemicity on islands close to a relatively depauperate continent have been reported for flowering plants, e.g. on the Canary Islands, Ceylon, New Guinea, New Caledonia, New Zealand, the Greater Antilles, or even the Californian coastal islands (Cronk, 1992; Medail & Quézel, 1997; Mueller‐Dombois & Fosberg, 1998; Baldwin, pers. comm.). In some cases, species currently extinct on the continent are preserved, have related taxa, or have even undergone adaptive radiation on these islands. Cronk (1987, 1990) suggested that Madagascar is a centre of ‘reliction’, meaning that groups of plants now extinct in all the remaining areas of their initial distribution continue to survive here. Such ‘reliction’ has also been reported on St Helena (Cronk, 1987, 1990).
A related matter is the relationship between endemicity and isolation. If the ratio between the number of endemic species and the total number of species is plotted against the logarithm of the inter‐OGU distances, the correlation is weak but significant (Fig. 7b; R = 0.404, P = 0.029). The sites were ordered according to the MST obtained from the Jaccard indices matrix used for multidimensional scaling (see previous section). If the observed values are plotted against those predicted, four sites are recorded outside the 95% confidence limits (CI): Madagascar, St Helena, Ascension Island and Tristan da Cunha. Below the 95% CI are Jebel Marra, Namibia, Angola and Mt Loma (Fig. 7b). The latter OGUs now have either a dry climate or a small habitable area. The isolation of the central Atlantic and sub‐Antarctic Islands is sufficient to explain their impoverished flora and high endemic ratios. But this cannot be the case of Madagascar, which is too close to the continent to seriously impair the windblown arrival of spores; even large vertebrates have made the crossing (Mc Call, 1997). There are also large differences in endemicity in continental Africa, e.g. between the Eastern African mountain arc and Upper and Lower Guinea. These differences are at their greatest, however, between Drakensberg (4.5% endemicity) and Bioko (1.5% endemicity) (Table 1). Myers et al. (2000) indicated the eastern mountains and coastal forests of Tanzania and Kenya to have the highest endemism ratios in the world for flowering plants.
Parsimonic analysis of dispersion
An analysis of 20 selected OGUs produced 10 equally parsimonious trees. The majority rule consensus tree showed three major groups of OGUs: (1) those of Upper and Lower Guinea (Mt Loma, Kwahu, Mt Cameroon, Bioko, S. Tomé, Mt Moukandé, Río Muni) and Angola; (2) those of the Eastern African mountain arc (the Rukiya, Aberdare and Kipengere Ranges, Inyanga and Drakensberg) and Madagascar; and (3) those of the Cape area (Mt Knysna, Table Mountain and Namibia). Ethiopia and Mt Oku fell at the base of the two first clades. However, these sites have a rather impoverished flora, making it difficult to interpret their holding this position (Fig. 8). Three major components were also detected in numerical analyses of Zambian ferns by Dzwonko & Kornaś (1978)– the Guineo‐Congolian, Afro‐montane and Southern xerophilic elements – which essentially correspond to groups 1, 2 and 3, respectively. These three large biogeographical areas seem to show good floral and climatic coherence. A consistent clade is that of tropical eastern Africa: Inyanga plus Drakensberg appears as a small clade sister of the main clade containing the Aberdare, Kipengere and Rukiya Ranges. The crown of the tree has Madagascar as its sister. The close relationships between Madagascar and Kipengere were also shown by NMS, as were those between Drakensberg, Inyanga and the Great Lakes sites. In the tropical western Africa clade, Angola appears as the sister of Upper and Lower Guinea. Mt Cameroon is closely related to the islands of the African aseismic dorsal (Bioko and S. Tomé) while the two continental areas of Río Muni and Mt Moukandé (Gabon) are beside one another. These area relationships are also quite similar to those revealed by NMS analysis.
Figure 8.
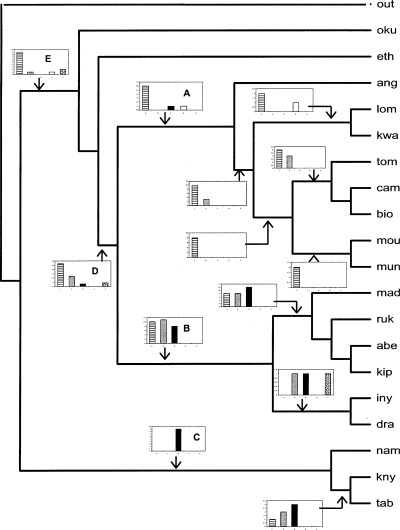
Consensus tree of floristic affinities of main African OGUs. This tree is a majority rule consensus of 10 trees with a CI = 0.368, HI = 0.633, RI = 0.567 and RC = 0.209. The insets are histograms of relative contribution of four groups of genera studied in this work plus the Aspleniaceae: 1‐lined, warm preferring species; 2‐grey, cold‐tolerant species; 3‐black, drought‐tolerant species; 4‐white, species related to Madagascar; and 5‐spoted, Aspleniaceae. Acronyms are according to Fig. 2.
The species shared by the subclades are classified into five groups and their contribution to African fern flora are shown by the histograms in the insets (the true synapomorphies and synapomorphies with less than two reversals were taken as common species) (Fig. 8). The large subclades of the Guinea and Angola areas (histogram A) share a large number of group 1 species (thermophilous ferns) in the basal nodes. The subclade of the Eastern African mountain arc and Madagascar (histogram B) have similar proportions of species of thermophilous ferns, cold‐tolerant ferns and drought‐tolerant ferns. Finally the southern African clade (histogram C) has many species of drought‐tolerant ferns. The basal clades of the entire tree are characterized by having many species of thermophilous ferns (histograms D and E). These data suggest a possible historical pattern for African mountain refuges: an older flora mainly formed by thermophilous ferns (group 1) could have given rise to several other floras diverse in their ecological preferences, some richer in cold‐tolerant ferns or in drought‐tolerant ferns. This hypothesis seems to agree with data concerning the recent evolution of the African climate (Maley, 1990, 1991).
Supplementary material
The following material is available from http://www.blackwellpublishing.com/products/journals/suppmat/JBI/JBI1106/JBI1106sm.htm
Appendix S1 Data matrix of fern presence in each OGU.
Appendix S2 Climatic variables in the OGUs.
Appendix S3 Correlation coefficients and signification in standard pairwise regression analysis of the environmental factors.
Appendix S4 Number of species and endemics included in the genera which resulted in group 1 of termophilous ferns.
Appendix S5 Number of species and endemics included in the genera which resulted in group 2 (cold‐tolerant termophilous ferns).
Appendix S6 Number total of species and endemics included in group 3 (drought‐tolerant ferns).
Biosketches
Carlos Aedo obtained his PhD in the University of Salamanca in 1994. From 1992 until the present, Carlos worked in the Real Jardín Botánico de Madrid, carrying out studies involved in the project Flora Iberica. His main area of interest is systematics, centred in the taxonomy of Mediterranean and Euro‐Siberian plants. He has worked on Maloideae (Sorbus, Pyrus and Crataegus), Geraniaceae (Geranium, Erodium and Monsonia) and ferns (Aspleniaceae), studying taxonomy, phylogeny and distribution of these groups. Now he is preparing a monograph of genus Geranium. Recently, he has also became interested in the paleotropical flora (mainly in equatorial Guinea).
Francisco Cabezas obtained his degree in Biology at the Complutense University of Madrid in 2000. He is currently studying for his PhD at the Real Jardín Botánico de Madrid. He has worked in the herbarium MA, where he is involved in a research project on the flora of equatorial Guinea. He completed his MSc thesis on Cyperaceae in this region and he has also worked on other taxa (e.g. Marantaceae and Piperaceae) in the Gulf of Guinea.
Juan José Aldasoro obtained his biology degree and PhD in the University of Salamanca. From 1992 to the present he has been a researcher in the Real Jardín Botánico de Madrid, developing studies in several projects about the flora and ecology of Spanish lakes, Spanish vascular flora (Flora Iberica), and the taxonomy and phylogeny of the family Geraniaceae. Current research work includes the systematics of vascular plants (mainly Geraniaceae, Campanulaceae and Rosaceae), and the ecology of several groups of plants, mainly those growing in aquatic environments.
Supporting information
Appendix S1. Data matrix of fern presence in each operational geographic unit.
Appendix S2. Climatic variables in the operational geographic units.
Appendix S3. Correlation coefficients and signification in standard pairwise regression analysis of the environmental factors.
Appendix S4. Number of species and endemics included in the genera which resulted in group 1 of termophilous ferns.
Appendix S5. Number of species and endemics included in the genera which resulted in group 2 (cold‐tolerant termophilous ferns).
Appendix S6. Number total of species and endemics included in group 3 (drought‐tolerant ferns).
Supporting info item
Acknowledgments
The authors wish to thank C. Peña, P. Perret and J. Velkdam for help with literature; G. Moreno and J. Muñoz for help with climatic data; P. de Hoyos for help on maps and A. Burton for the English correction. R. Moran for accurate review of the manuscript. This work was partly financed by the Spanish Dirección General de Investigación Científica y Técnica (DGICYT) through the research project REN2000‐0818.
References
- Alston, A.H.G. (1959) The ferns and fern‐allies of West Tropical Africa. The Crown Agents for Overseas Governments and Administrations, London. [Google Scholar]
- Alston, A.H.G. & Schelpe, E.A.C.L.E. (1957) The Pteridophyta of Marion island. The Journal of South African Botany, 23, 105–109. [Google Scholar]
- Anonymous (1971) Climatological Normals (CLINO) for climat and climat ship stations for the period 1931–1960. World Meteorological Organization, Geneva. [Google Scholar]
- Anthony, N.C. (1984) A revision of the southern African species of Cheilanthes Swartz and Pellaea Link (Pteridaceae). Contribution from the Bolus Herbarium, 11, 1–293. [Google Scholar]
- Ashmole, M. & Ashmole, P. (2000) Pteridophyta: ferns, club‐mosses and relatives St Helena and Ascension Island: a natural history (ed. by Nelson A.), pp. 456–465. Oswestry, Shropshire. [Google Scholar]
- Axelrod, D.I. & Raven, P.H. (1978) Late Cretaceous and Tertiary vegetation history of Africa Biogeography and ecology of Southern Africa (ed. by Werger M.J.A.), pp. 79–130. W. Junk, The Hague. [Google Scholar]
- Barrington, D.S. (1993) Ecological and historical factors in fern biogeography. Journal of Biogeography, 20, 275–280. [Google Scholar]
- Bizzarri, M.P. (1985) Selaginellaceae Flore d'Afrique Centrale (Zaire‐Rwanda‐Burundi) (ed. by Bamps P.), pp. 1–55. Jardin Botanique National du Belgique, Meise. [Google Scholar]
- Bosman, M.T.M. (1991) A monograph of the fern genus Microsorum (Polypodiaceae). Leiden Botanical Series, 14, 1–161. [Google Scholar]
- Bossuyt, F. & Milinkovitch, M.C. (2000) Convergent adaptative radiations in Madagascan and Asian ranid frogs reveal covariation between larval and adult traits. Proceedings of the National Academy of Sciences of the United States of America, 97, 6585–6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer, Y. (1964) Contribution a l'etude de l'ecophysiologie de deux épiphytes: Platycerium stemaria (Beauv.) Desv. et P. angolense Welch. Annales des Sciences Naturelles, Botanique, 5, 87–228. [Google Scholar]
- Brown, J.H. (1971) Mammals on mountaintops: nonequilibrium insular biogeography. American Naturalist, 105, 467–468. [Google Scholar]
- Bruijnzeel, L.A. & Proctor, J. (1995) Hydrology and biogeochemistry of tropical montane cloud forests: what do we really know? Tropical montane cloud forests: proceedings of an international symposium (ed. by Hamilton L.S., Juvik J.O. and Scatena F.N.), pp. 38–78. Springer‐Verlag, New York. [Google Scholar]
- Bruijnzeel, L.A. & Veneklaas, E.J. (1998) Climatic conditions and tropical montane forest productivity: the fog has not lifted yet. Ecology, 79, 3–9. [Google Scholar]
- Burrows, J.E. (1996) The genus Ophioglossum L. in south‐central Africa. Pteridology in perspective: pteridophyte symposium ’95 Proceedings of the Holttum memorial pteridophyte symposium (ed. by Camus J.M., Johns R.J. and Gibby M.), pp. 329–336. Royal Botanic Gardens, Kew. [Google Scholar]
- Burrows, J.E. & Crouch, N.R. (1995) Pteridophyta: new distribution records of South African pteridophytes. Bothalia, 25, 236–238. [Google Scholar]
- Burrows, J.E. & Edwards, T.J. (1995) A new species and a change of status in Ophioglossum (Ophioglossaceae: Pteridophyta) in Africa. Bothalia, 25, 61–63. [Google Scholar]
- Coetzee, J. (1983) Evidence for a considerable depression of the vegetation belts during upper pleistocene on the East African mountains. Nature, 204, 564–566. [Google Scholar]
- Colinvaux, P.A. , De Oliveira, P.E. & Bush, M.B. (2000) Amazonian and neotropical plant communities on glacial time‐scales: the failure of the aridity and refuge hypotheses. Quaternary Science Reviews, 19, 141–169. [Google Scholar]
- Crisci, J.V. , Katinas, L. & Posadas, P. (2000) Introducción a la teoría y práctica de la biogeografía histórica. Sociedad Argentina de Botánica, Buenos Aires. [Google Scholar]
- Cronk, Q.C.B. (1987) History of the endemic flora of St Helena: a relictual series. New Phytologist, 105, 509–520. [DOI] [PubMed] [Google Scholar]
- Cronk, Q.C.B. (1990) The history of the endemic flora of St Helena: late Miocene ‘Trochetiopsis‐like’ pollen from St Helena and the origin of Trochetiopsis. New Phytologist, 114, 159–165. [DOI] [PubMed] [Google Scholar]
- Cronk, Q.C.B. (1992) Relict floras of Atlantic islands: patterns assessed. Biological Journal of the Linnean Society, 46, 91–103. [Google Scholar]
- Dassler, C. & Farrar, D. (2001) Significance of gametophyte form in long‐distance colonization by tropical, epiphytic ferns. Brittonia, 53, 352–369. [Google Scholar]
- De Winter, B. (1971) Floristic relationships between the northern and southern arid areas of Africa. Mitteilungen der Botanischen Staatssammlung, München, 10, 424–437. [Google Scholar]
- Dowsett‐Lemaire, F. (1988) The forest vegetation of t Mulanje (Malawi). A floristic and chorological study along an altitudinal gradient (650–1950 m). Bulletin du Jardin Botanique National de Belgique, 58, 77–107. [Google Scholar]
- Dowsett‐Lemaire, F. (1989) The flora and phytogeography of the evergreen forests of Malawi I: Afromontane and mid‐elevation forests. Bulletin du Jardin Botanique National de Belgique, 59, 3–131. [Google Scholar]
- Dunn, G. & Everitt, B.S. (1982) An introduction to mathematical taxonomy. Cambridge University Press, Cambridge. [Google Scholar]
- Dzwonko, Z. & Kornaś, J. (1978) A numerical analysis of the distribution of pteridophytes in Zambia. Zeszyty Naukowe Uniwersytetu Jagiellońskiego, Prace Botaniczne, 6, 39–49. [Google Scholar]
- Edwards, P.J. (1998) Ferns The plants of Mount Cameroon. A conservation checklist (ed. by Cable S. and Cheek M.), pp. 182–198. Royal Botanic Garden, Kew. [Google Scholar]
- Edwards, P.J. , Onana, J.M. & Pollard, B.J. (2000) Filicopsida The plants of Mount Oku and the Ijim Ridge, Cameroon. A conservation checklist (ed. by Cheek M., Onana J.M. and Pollard B.J.), pp. 94–199. Royal Botanic Garden, Kew. [Google Scholar]
- Figueiredo, E. (1998) The pteridophytes of São Tomé and Príncipe (Gulf of Guinea). Bulletin of the Natural History Museum London, Botany, 28, 41–66. [Google Scholar]
- Figueiredo, E. (2001) New findings of pteridophytes from the mountain rainforests of São Tomé and Príncipe. Fern Gazette, 16, 191–193. [Google Scholar]
- Fjedså, J. & Lovett, J.C. (1997) Geographical patterns of old and young species in African forest biota: the significance of specific montane areas as evolutionary centres. Biodiversity and Conservation, 6, 325–346. [Google Scholar]
- Flenley, J.R. (1992a) Palynological evidence relating to disturbance and other ecological phenomena in rain forests Tropical forests in transition (ed. by Goldammer J.G.), pp. 17–24. Birkhauser, Verlag, Basel. [Google Scholar]
- Flenley, J.R. (1992b) Ultraviolet‐B insulation and the altitudinal forest limit Nature and dynamics of forest savanna boundaries (ed. by Furley P.A., Proctor J. and Ratter J.A.), pp. 273–282. Chapman & Hall, London. [Google Scholar]
- Flenley, J.R. (1995) Cloud forest, the Massenerhebung effect, and ultraviolet insolation Tropical montane cloud forests: proceedings of an international symposium (ed. by Hamilton L.S., Juvik J.O. and Scatena F.N.), pp. 38–78. Springer‐Verlag, New York. [Google Scholar]
- Foster, P. (2001) The potential negative impacts of global climate change on tropical montane cloud forests. Earth-Science Reviews, 55, 73–106. [Google Scholar]
- Gasse, F. (2000) Hydrological changes in the African tropics since the last glacial maximum. Quaternary Science Reviews, 19, 189–211. [Google Scholar]
- Gaston, K.J. & Blackburn, T.M. (1995) Mapping biodiversity using surrogates for species richness: macro‐scales and New World birds. Proccedings of the Royal Society of London, Series B, Botanical Sciences, 263, 1571–1575. [Google Scholar]
- Given, B.R. (1993) Changing aspects of endemism and endargement in Pteridophyta. Journal of Biogeography, 20, 293–302. [Google Scholar]
- Glen, R. (1993) Pteridophyta Plants of Southern Africa: names and distribution (ed. by Arnold T.H. and De Wet B.C.), pp. 47–60. National Botanical Institute, Pretoria. [Google Scholar]
- Grimes, J.W. & Parris, B.S. (1986) Index of Thelypteridaceae. Royal Botanic Gardens, Kew. [Google Scholar]
- Groves, E.W. (1981) Vascular plant collections from the Tristan da Cunha group of islands. Bulletin of the British Museum (Natural History) Botany, 8, 333–420. [Google Scholar]
- Hafkenscheid, R.L. (2000) Hydrology and biogeochemistry of tropical montane rainforests of contrasting stature in the blue mountains, Jamaica. PhD Thesis. Vrije Universiteit, Print Partners Ipskamp; Enschede, The Netherlands. [Google Scholar]
- Hansen, A. & Sunding, P. (1993) Flora of Macaronesia. Checklist of vascular plants, fourth revised edition. Sommerfeltia, 17, 1–295. [Google Scholar]
- Harcourt, A.H. (2000) Coincidence and mismatch of biodiversity hotspots: a global survey for the order, primates. Biological Conservation, 93, 163–175. [Google Scholar]
- Hengeveld, H.G. (1990) Dynamic biogeography. Cambridge University Press, Cambridge. [Google Scholar]
- Hennipman, E. (1977) A monograph of the fern genus Bolbitis (Lomariopsidaceae). Leiden Botanical Series, 12, 1–331. [Google Scholar]
- Hobohm, C. (2000) Plant species diversity and endemism on islands and archipelagos, with special reference to the Macaronesian Islands. Flora, 195, 9–24. [Google Scholar]
- Holloway, J.D. & Jardine, N. (1968) Two approaches to zoogeography: a study based on the distributions of butterflies, birds and bats in the Indo‐Australian area. Proceedings of the Linnean Society of London, 179, 153–188. [Google Scholar]
- Holttum, R.E. (1973) Studies in the family Thelypteridaceae: 5. The genus Pneumatopteris Nakai. Blumea, 21, 293–325. [Google Scholar]
- Holttum, R.E. (1974) Thelypteridaceae of Africa and adjacent islands. South African Journal of Botany, 40, 123–168. [Google Scholar]
- Holttum, R.E. (1976) The genus Christella Leveille, sect. Christella, studies in the family Thelypteridaceae: 11. Kew Bulletin, 31, 293–339. [Google Scholar]
- Holttum, R.E. (1977) Studies in the family Thelypteridaceae: 12. The genus Amphineuron Holttum. Blumea, 23, 205–218. [Google Scholar]
- Holttum, R.E. (1981) The tree‐ferns of Africa. Kew Bulletin, 36, 463–482. [Google Scholar]
- Holttum, R.E. (1986) Studies in the fern‐genera allied to Tectaria: V. Triplophyllum, a new genus of Africa and America. Kew Bulletin, 41, 237–260. [Google Scholar]
- Holttum, R.E. & Grimes, J.W. (1980) The genus Pseudocyclosorus Ching (Thelypteridaceae). Kew Bulletin, 34, 499–516. [Google Scholar]
- Hovenkamp, P.H. (1986) A monograph of the fern genus Pyrrosia . Leiden Botanical Series, 9, 1–280. [Google Scholar]
- Hovenkamp, P.H. & Franken N.A.P. (1993) An account of the fern genus Belvisia Mirbel (Polypodiaceae). Blumea, 37, 511–527. [Google Scholar]
- Iriondo, M.H. (1999) Last glacial maximum and hypsithermal in the Southern hemisphere. Quaternary International, 62, 11–19. [Google Scholar]
- Ito, H. (1972) Distribution of monolete and trilete ferns in eastern Asia and northern Oceania. Journal of Japanese Botany, 47, 321–325. [Google Scholar]
- Ito, H. (1978) Distribution of two spore patterns in the fern floras of the world (a preliminary survey). Journal of Japanese Botany, 53, 164–171. [Google Scholar]
- Jacobsen, W.B.G. & Jacobsen, N.H.G. (1986) Adiantaceae: Cheilanthes deltoidea Kunze in the Waterberg, Transvaal. Bothalia, 16, 41–42. [Google Scholar]
- Jacobsen, W.B.G. & Jacobsen, N.H.G. (1988) The Cheilanthes hirta complex and allied species (Adiantaceae‐Pteridaceae) in Southern Africa. Bothalia, 18, 57–77. [Google Scholar]
- Jacobsen, W.B.G. & Jacobsen, N.H.G. (1989) Comparison of the pteridophyte floras of Southern and Eastern Africa, with special reference to high‐elevation species. Bulletin du Jardin National de Belgique, 59, 261–317. [Google Scholar]
- Johns, R.J. (1991) Pteridophytes of tropical East Africa. Royal Botanic Gardens, Kew. [Google Scholar]
- Kessler, M. (2002) Range size and its ecological correlates among the pteridophytes of Carrasco National Park, Bolivia. Global Ecology and Biogeography, 11, 89–102. [Google Scholar]
- Kessler, M. , Parris, B.S. , & Kessler, E. (2001) A comparison of the tropical montane pteridophyte floras of Mount Kinabalu, Borneo, and Parque Nacional Carrasco, Bolivia. Journal of Biogeography, 28, 611–622. [Google Scholar]
- Kornaś, J. (1977) Life‐forms and seasonal patterns in the pteridophytes in Zambia. Acta Societatis Botanicorum Poloniae, 46, 669–690. [Google Scholar]
- Kornaś, J. (1984) Notes on African Hymenophyllaceae: 3. Hymenophyllum inaequale (Poir.) Desv. new to East Tropical Africa. Bulletin du Jardin Botanique Nationale de Belgique, 54, 15–21. [Google Scholar]
- Kornaś, J. (1993) The significance of historical factors and ecological preference in the distribution of African pteridophytes. Journal of Biogeography, 20, 281–286. [Google Scholar]
- Kornaś, J. , Dwzonko, Z. , Harmata, K. & Pacyna, A. (1982) Biometrics and numerical taxonomy of the genus Actiniopteris (Adiantaceae, Filicopsida) in Zambia. Bulletin du Jardin Botanique Nationale de Belgique, 52, 265–309. [Google Scholar]
- Kramer, K.U. (1972) The Lindsaeoid ferns of the Old World‐IX Africa and its islands. Bulletin du Jardin Botanique National du Belgique, 42, 305–345. [Google Scholar]
- Kramer, K.U. & Green, P.S. (1990) Pteridophytes and Gymnosperms The families and genera of vascular plants (ed. by Kubitzki K.), pp. 1–404. Springer‐Verlag, Berlin. [Google Scholar]
- Kruskal, J.B. & Wish, M. (1978) Multidimensional scaling. California Sage, Beverly Hills. [Google Scholar]
- Lawalrée, A. (1990) Deux espèces africaines nouvelles d’ Arthropteris (Nephrolepidaceae). Bulletin du Jardin Botanique National de Belgique, 60, 317–324. [Google Scholar]
- Lawalrée, A. (1991) Arthropteris charletiana (Nephrolepidaceae), espèce nouvelle du Kivu (Zaire). Bulletin du Jardin Botanique National de Belgique, 61, 356–357. [Google Scholar]
- Leroy, J.F. (1978) Composition, origin and affinities of the Madagascar vascular flora. Annals of the Missouri Botanical Garden, 65, 535–589. [Google Scholar]
- Linder, H.P. (2001) Plant diversity and endemism in sub‐Saharan tropical Africa. Journal of Biogeography, 28, 169–182. [Google Scholar]
- Lloyd, R.M. (1974) Systematics of the genus Ceratopteris Brongn. (Parkeriaceae): 2. Taxonomy. Brittonia, 26, 139–160. [Google Scholar]
- Lloyd, R.M. & Klekowski, E.J. (1970) Spore germination and viability in Pteridophyta: evolutionary significance of chlorophyllous spores. Biotropica, 2, 129–137. [Google Scholar]
- Lomolino, M.V. , Brown, J.H. , & Davies, R. (1989) Island biogeography on montane forest mammals in the American Southwest. Ecology, 70, 180–194. [Google Scholar]
- Lugardon, B. (1996) Morphologie sporale et ultrastruture du sporoderme chez les Filicinées. Memorie della accademia Lunigianense di Scienze, 65, 15–36. [Google Scholar]
- McCall, R.A. (1997) Implications of recent geological investigations of the Mozambique Channel for the mammalian colonization of Madagascar. Proceedings of the Royal Society of London, Series B, Biological Sciences, 264, 663–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison, W.P. & Maddison, D.R. (1992) MacClade: analysis of phylogeny and character evolution, version 3.0. Sinauer Associates, Sunderland, MA. [Google Scholar]
- Maley, J. (1980) Les changements climatiques de la fin du Tertiaire en Afrique: leus consequence sur l'aparition du Sahara et de sa vegetation The Sahara and the Nile (ed. by Williams M.A. and Faure H.), pp. 63–86. A.A. Balkema, Rotterdam. [Google Scholar]
- Maley, J. (1990) Synthése sur le domaine forestier africain au Quaternarie récent Paysage Quaternaries de l'Afrique Centrale Atlantique (ed. by Lanfranchi R. and Scwartz D.), pp. 383–389. ORSTOM, Paris. [Google Scholar]
- Maley, J. (1991) The African rain forest vegetation and palaeoenvironments during late Quaternary. Climate Change, 19, 79–80. [Google Scholar]
- Maley, J. (1996) The African rain forest – main characteristics of changes in vegetation and climate from the upper Cretaceous to the Quaternary. Proccedings of the Royal Society of Edinburgh, 104B, 31–73. [Google Scholar]
- Malyshev, L.I. (1991) Some quantitative approaches to problems of comparative floristics Quantitative approaches to phytogeography (ed. by Nimis P.L. and Crovello T.J.), pp. 15–33. Kluwer Academic Publishers, Dordrecht. [Google Scholar]
- Mayr, E. & O'Hara, R. (1986) The biogeographic evidence supporting the pleistocene forest refuge hypothesis. Evolution, 49, 55–67. [DOI] [PubMed] [Google Scholar]
- Medail, F. & Quézel, P. (1997) Hot‐spots analysis for conservation of plant biodiversity in the Mediterranean basin. Annals of the Missouri Botanical Garden, 84, 112–127. [Google Scholar]
- Moran, R.C. (1995) The importance of mountains to Pteridophytes, with emphasis on Neotropical montane forests Biodiversity and conservation of neotropical montane forests (ed. by Churchill S.P.), pp. 359–363. New York Botanical Garden, New York. [Google Scholar]
- Moran, R.C. (2002) Tropical diversity. Fiddlehead Forum, 29, 14–15. [Google Scholar]
- Moran, R.C. & Smith, A.R. (2001) Phytogeographic relationships between neotropical and African‐Madagascar pteridophytes. Brittonia, 53, 304–351. [Google Scholar]
- Mueller‐Dombois, D. & Fosberg F.R. (1998) Vegetation of the tropical Pacific Islands. Springer, New York. [Google Scholar]
- Myers, N. , Mittermeier, R.A. , Mittermeier, C.G. , Da Fonseca, G.A.B. & Kent, J. (2000) Biodiversity hotspots for conservation priorities. Nature, 403, 853–858. [DOI] [PubMed] [Google Scholar]
- Nooteboom, H.P. (1994) Notes on Davalliaceae II. A revision of the genus Davallia . Blumea, 39, 151–214. [Google Scholar]
- Øllgaard, B. (1989) Index of the Lycopodiaceae. Biologiske Skrifter, 34, 1–135. [Google Scholar]
- Oshawa, M. (1991) Structural comparison of tropical montane rain forests along latitudinal and altitudinal gradients in south and East Asia. Vegetatio, 97, 1–10. [Google Scholar]
- Parris, B.S. (1981) An analysis of the Grammitis poeppigiana–G. magellanica complex in the South Atlantic and South Indian oceans. Fern Gazette, 12, 165–168. [Google Scholar]
- Parris, B.S. (2001) Circum‐Antarctic continental distribution patterns in pteridophyte species. Brittonia, 53, 270–283. [Google Scholar]
- Pausas, J.G. & Sáez, L. (2000) Pteridophyte richness in the NE Iberian Peninsula: biogeographic patterns. Plant Ecology, 148, 195–200. [Google Scholar]
- Pichi Sermolli, R.E.G. (1975) Taxonomic notes on some African species of Elaphoglossum . Fern Gazette, 11, 95–100. [Google Scholar]
- Pichi Sermolli, R.E.G. (1978) Adumbratio Florae Aethiopicae: 32. Nephrolepidaceae. Webbia, 33, 115–135. [Google Scholar]
- Reitsma, J.M. , Louis, A.M. & Floret, J.J. (1992) Flore et végétation des inselbergs et dalles rocheuses: première étude au Gabon. Bulletin du Muséum d'histoire naturelle, section B, Adansonia, 1, 73–97. [Google Scholar]
- Rohlf, F.J. (1992) NTSYS‐pc, numerical taxonomy and multivariate analysis system, version 1.70. Exeter Software, New York. [Google Scholar]
- Rolleri, C. , Lavalle, M. , Mengascini, A. , & Rodríguez, M. (2001) El género Marattia Sw. (Marattiales, Marattiaceae) en el paleotrópico. Candollea, 56, 97–113. [Google Scholar]
- Rosen, B.R. (1988) From fossils to earth history: applied historical biogeography Analytical biogeography: an integrated approach to the study of animal and plant distributions (ed. by Myers A.A. and Giller P.S.), pp. 437–481. Chapman & Hall, London. [Google Scholar]
- Roux, J.P. (1993) Elaphoglossum Schott ex J. Smith (Lomariopsidaceae: Pteridophyta) in the Tristan da Cunha, Gough and Marion Island groups. Botanical Journal of the Linnean Society, 112, 203–222. [Google Scholar]
- Roux, J.P. (1995) Systematic studies in the genus Mohria (Pteridophyta: Anemiaceae): 6. Taxonomic review. Bothalia, 25, 1–12. [Google Scholar]
- Roux, J.P. (2000) The genus Polystichum (Dryopteridaceae) in Africa. Bulletin of the Natural History Museum (London), Botany, 30, 33–79. [Google Scholar]
- Roux, J.P. (2001a) A review of the fern genus Polystichum (Pteropsida: Dryopteridaceae) in Madagascar and the Mascarene region. Adansonia, 23, 265–287. [Google Scholar]
- Roux, J.P. (2001b) Conspectus of Southern African pteridophyta. Southern African botanical diversityNetwork report no. 13. SABONET, Pretoria. [Google Scholar]
- Sandbrink, J.M. , Van Ham, R.C.H.J. & Van Brederode, J. (1992) Chloroplast DNA and morphological variation in the fern genus Platycerium (Polypodiaceae: Pteridophyta). Fern Gazette, 14, 97–118. [Google Scholar]
- Schelpe, E.A.C.L.E. (1952) A revision of the African species of Blechnum . Journal of the Linnean Society, Botany, 53, 487–510. [Google Scholar]
- Schelpe, E.A.C.L.E. (1969) Reviews of tropical African Pteridophyta 1. Contribution from the Bolus Herbarium, 1, 1–132. [Google Scholar]
- Schelpe, E.A.C.L.E. (1970) Pteridophyta Flora Zambesiaca (ed. by Exell A.W. and Launert E.), pp. 1–254. The Crown Agents for Overseas Governments and Administrations, London. [Google Scholar]
- Schelpe, E.A.C.L.E. (1977) Pteridophyta Conspectus Florae Angolensis (ed. by Fernandez R.B., Launert E. and Mendes E.J.), pp. 1–197. Junta de Investigaçoes Científicas do Ultramar, Lisboa. [Google Scholar]
- Schelpe, E.A.C.L.E. (1983) Aspects of the phytogeography of African Pteridophyta. Bothalia, 14, 417–419. [Google Scholar]
- Schelpe, E.A.C.L.E. & Anthony, N.C. (1986) Pteridophyta Flora of Southern Africa (ed. by Leistner O.A.), pp. 1–292. Department of Agriculture and Water Supply, Pretoria. [Google Scholar]
- Schippers, R.R. (1993a) Pteridophytes of Tanzania with special reference to Pare and Usambara mountains. Fern Gazette, 14, 171–192. [Google Scholar]
- Schippers, R.R. (1993b) Pteridophytes of Tanzania with special reference to Pare and Usambara mountains (part 2). Fern Gazette, 14, 193–214. [Google Scholar]
- Schnell, R. (1977) Introduction à la phytogéographie des pays tropicaux. 4: La flore et la végétation de l'Afrique tropicale. Gauthiers‐Villars, Paris. [Google Scholar]
- Scott, L. & Van Zinderen Bakker, E.M., Sr (1985) Exotic pollen and long‐distance wind dispersal at a sub‐Antarctic Island. Grana, 24, 45–54. [Google Scholar]
- Shi, N. , Schneider, R. , Beug, H.J. & Dupont, L. (2001) Southeast trade wind variations during the last 135 kyr: evidence from pollen spectra in eastern South Atlantic sediments. Earth and Planetary Science Letters, 187, 311–321. [Google Scholar]
- Shmida, A. & Wilson, M.V. (1985) Biological determinants of species diversity. Journal of Biogeography, 12, 1–20. [Google Scholar]
- Smith, A.R. (1972) Comparison of fern and flowering plant distribution with some evolutionary interpretation for ferns. Biotropica, 4, 4–9. [Google Scholar]
- Smith, T.B. , Holder, K. , Girman, D. , O'Keefe, K. , Larison, B. & Chan, Y. (2000) Comparative avian phylogeography of Cameroon and Equatorial Guinea mountains: implications for conservation. Molecular Ecology, 9, 1505–1516. [DOI] [PubMed] [Google Scholar]
- Sneath, P.H. & Sokal, R.R. (1973) Numerical taxonomy. W. H. Freeman, San Francisco. [Google Scholar]
- Stefanović, S. & Rakotondrainibe, F. (1996) New taxa and a new rank of Selaginella (Selaginellaceae) from Madagascar and the Comoros. Novon, 6, 203–209. [Google Scholar]
- Stefanović, S. & Rakotondrainibe, F. (1997) Contribution to the study of the Selaginellaceae of Madagascar. Adansonia, 19, 165–168. [Google Scholar]
- Swofford, D.L. (1993) PAUP: phylogenetic analysis using parsimony (and other methods), version 4.0. Sinauer Associates, Sunderland. [Google Scholar]
- Tardieu‐Blot, M.L. (1951) Marattiacées, Ophioglossacées, Hyménophyllacées, Cyathéacées. Flore de Madagascar et des Comores (ed. by Humbert H.), pp. 1–44. Muséum National d'Historie Naturelle, Paris. [Google Scholar]
- Tardieu‐Blot, M.L. (1952) Parkériacées, Gleichéniacées, Schizéacées, Osmondacées, Marsiléacées, Salviniacées Flore de Madagascar et des Comores (ed. by Humbert H.), pp. 1–2, 1–9, 1–11, 1–2, 1–3, 1–3. Muséum National d'Historie Naturelle, Paris. [Google Scholar]
- Tardieu‐Blot, M.L. (1953) Les pteridophytes de l'Afrique intertropicale française. Mémoires de l'Institut Français d'Afrique Noire, 28, 1–241. [Google Scholar]
- Tardieu‐Blot, M.L. (1958) Polypodiacées I Flore de Madagascar et des Comores (ed. by Humbert H.), pp. 1–361. Muséum National d'Historie Naturelle, Paris. [Google Scholar]
- Tardieu‐Blot, M.L. (1960) Polypodiacées II Flore de Madagascar et des Comores (ed. by Humbert H.), pp. 1–121. Muséum National d'Historie Naturelle, Paris. [Google Scholar]
- Tardieu‐Blot, M.L. (1964a) Pteridophytes Flore du Cameroun, Vol. 3 (ed. by Aubréville A.), pp. 1–372. Muséum National d'Historie Naturelle, Paris. [Google Scholar]
- Tardieu‐Blot, M.L. (1964b) Pteridophytes Flore du Cameroun, Vol. 8 (ed. by Aubréville A.), pp. 1–228. Muséum National d'Historie Naturelle, Paris. [Google Scholar]
- Tardieu‐Blot, M.L. (1971) Lycopodiacées‐Huperziacées Flore de Madagascar et des Comores (ed. by Humbert H.), pp. 1–44. Muséum National d'Historie Naturelle, Paris. [Google Scholar]
- Thulin, M. (1993) Pteridophyta. Flora of Somalia, Vol. 1 (ed. by Thulin M.), pp. 5–14. Royal Botanic Garden, Kew. [Google Scholar]
- Tindale, M.D. (1965) A monograph of the Genus Lastreopsis Ching. Contributions from the New South Wales National Herbarium, 3, 249–339. [Google Scholar]
- Torres, E. , Lees, D.C. , Vane‐Wright, R.I. , Kremen, C. , Leonard, J.A. & Wayne, R.K. (2001) Examining monophyly in a large radiation of Madagascan butterflies (Lepidoptera: Satyrinae: Mycalesina) based on mitochondrial DNA data. Molecular Phylogenetics and Evolution, 20, 460–473. [DOI] [PubMed] [Google Scholar]
- Tryon, R.M. (1986) The biogeography of species, with special reference to ferns. The Botanical Review, 52, 117–156. [Google Scholar]
- Tryon, R.M. & Gastony, G.J. (1975) The biogeography of endemism in the Cyatheaceae. Fern Gazette, 11, 73–79. [Google Scholar]
- Van Zinderen Bakker, E.M. (1975) The origin and palaeoenvironment of the Namib Desert biome. Journal of Biogeography, 2, 65–73. [Google Scholar]
- Velayos, M. , Aedo, C. & Pérez Viso, R. (2002) Check‐list of the pteridophytes of Equatorial Guinea. Belgian Journal of Botany, 134, 145–191. [Google Scholar]
- Viane, R.L.L. (1985) A new species and a new hybrid of Thelypteris (Pteridophyta) from the Ivory Coast. Bulletin de la Société Royale Botanique de Belgique, 118, 41–56. [Google Scholar]
- White, F. (1979) The Guineo‐Congolian Region and its relationships to other phytochoria. Bulletin du Jardin Botanique National de Belgique, 49, 11–55. [Google Scholar]
- Whittaker, R. H. (1972). Evolution and measurement of species diversity. Taxon, 21, 213–251. [Google Scholar]
- Wickens, G.E. (1976) The flora of Jebel Marra (Sudan Republic) and its geographical affinities. Royal Botanic Gardens, Kew. [Google Scholar]
- Williams, P.H. & Gaston, K.J. (1994) Measuring more of biodiversity: can higher‐taxon richness predict wholesale species richness? Biological Conservation, 67, 211–217. [Google Scholar]
- Williams, P.H. , Gaston, K.J. & Humphries, C.J. (1997) Mapping biodiversity value worldwide: combining higher‐taxon richness from different groups. Proceedings of the Royal Society of London, Series B, Biological Sciences, 264, 141–148. [Google Scholar]
- Zink, M.J. (1993) Systematics of the fern genus Lepisorus (J. Smith) Ching (Polypodiaceae: Lepisoreae): with special reference to Africa and including an annotated list to all names published so far. ADAG, Zurich. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Data matrix of fern presence in each operational geographic unit.
Appendix S2. Climatic variables in the operational geographic units.
Appendix S3. Correlation coefficients and signification in standard pairwise regression analysis of the environmental factors.
Appendix S4. Number of species and endemics included in the genera which resulted in group 1 of termophilous ferns.
Appendix S5. Number of species and endemics included in the genera which resulted in group 2 (cold‐tolerant termophilous ferns).
Appendix S6. Number total of species and endemics included in group 3 (drought‐tolerant ferns).
Supporting info item


