Abstract
Human rhinovirus (RV) is commonly associated with severe acute lower respiratory infections (ALRI) in children. We aimed to describe the distribution of RV species and associations between RV species and clinical features in children hospitalized with clinically severe pneumonia (CSP) in Morocco. Nasopharyngeal aspirates (NPAs) were collected from 700 children, 2–59 months of age, admitted with CSP to the Hôpital d'Enfants de Rabat in Morocco. At least one respiratory virus was identified in 92% of children, of which RV was the most common (53%). PCR assays, sequencing, and phylogenetic tree analyses were carried out on 183 RV‐positive NPAs to determine RV species and genotypes. Of 157 successfully genotyped NPAs, 60 (38.2%) were RV‐A, 8 (5.1%) were RV‐B, and 89 (56.7%) were RV‐C. Wheezing and cyanosis were more common in RV‐C‐positive than RV‐A‐positive children (80.9% vs. 56.7%; P = 0.001 for wheezing and 10.1% vs. 0%; P = 0.011 for cyanosis). Physician's discharge diagnosis of pneumonia was more frequent among RV‐A‐positive (40.0%) than RV‐C‐positive children (20.2%; P = 0.009). RV‐A and RV‐C showed distinct seasonal patterns. Our findings suggest that RV‐C is associated with wheezing illness while RV‐A is associated with pneumonia. J. Med. Virol. 89:582–588, 2017 . © 2016 Wiley Periodicals, Inc.
Keywords: rhinovirus, respiratory infections, pneumonia, children, Morocco
INTRODUCTION
Acute lower respiratory infections (ALRI) are the leading cause of childhood mortality worldwide and are particularly burdensome in low‐ and middle‐income countries [Liu et al., 2012; Rudan et al., 2013]. Respiratory viruses are the most common cause of ALRI, of which human rhinovirus (RV) is often the most commonly identified [Hayden, 2004]. Advances in molecular diagnosis techniques led to the identification of a third, previously unidentified species, RV‐C, in 2006 [Lamson et al., 2006; McErlean et al., 2007]. Although several studies have since investigated the prevalence of RV‐C in children with upper and lower respiratory tract infections, these studies have largely been limited to developed countries. The majority of studies investigating all three RV species in children hospitalized with ALRI found that RV‐C is the most prevalent RV species and is often associated with more severe illness [Arden et al., 2006; Lamson et al., 2006; Renwick et al., 2007; Lau et al., 2009; Linsuwanon et al., 2009; Miller et al., 2009a, b; Bizzintino et al., 2011].
Only one study has reported on the overall prevalence of RV in children with ALRI in Morocco [Jroundi et al., 2014] and none have investigated RV species. Very few studies have investigated the prevalence of RV species in children in sub‐Saharan Africa [Smuts et al., 2011; Esposito et al., 2012a, b; Onyango et al., 2012] or the Middle East [Miller et al., 2009a, b]. While these studies confirmed the importance of RV in African children, they were inconclusive with respect to the role of RV species in ALRI. The aim of this study was to describe the distribution of RV species in RV‐positive children hospitalized with clinically severe pneumonia (CSP) in Rabat, Morocco and to compare demographic, clinical, and laboratory features between the RV species.
MATERIALS AND METHODS
Study Setting and Design
This study, conducted from the Hôpital d'Enfants de Rabat (HER) in Rabat, Morocco is part of a larger project investigating the epidemiology and etiology of CSP in Moroccan children [Jroundi et al., 2014]. From November 2010 to December 2011, children 2–59 months of age admitted to HER with CSP, according to the WHO definition [Mulholland et al., 1992; WHO, 2005] (history of cough or reported breathing difficulty and increased respiratory rate according to age and chest indrawing), were approached for recruitment. Seven hundred children who fulfilled the inclusion criteria and provided parent/guardian consent were included in the study. Recruited children underwent standardized procedures including anteroposterior chest X‐ray, pulse oximetry (Bionics palm care), and nasopharyngeal aspirates (NPAs) for identification of respiratory viruses. Radiologically confirmed end‐point pneumonia was defined as episodes with evidence of consolidation and/or pleural effusion.
A minimum of 2 ml of venous blood was collected for blood culture, full blood cell count, and biochemical determinations. The epidemiology and etiology of respiratory infections in this population has been published previously [Jroundi et al., 2014, 2016].
Clinical discharge diagnoses, as determined by the physician (pneumonia, bronchiolitis, bronchitis/asthma, laryngitis, or other diagnosis) were coded using the International Classification of Diseases, 10th Revision [WHO, 2012], after a thorough evaluation of all the supporting clinical and laboratory data. Presumed bacterial pneumonia was determined by the physician based on clinical, analytical, or radiological features. Respiratory viruses were identified in nasopharyngeal aspirates (NPAs) using TrueScience® RespiFinder Pathogen Identification Panel (Applied Biosystems, Waltham, MA). As previously reported, a respiratory virus was identified in 628/684 children (92%), of which RV was the most commonly identified virus (360/685, 53%), followed by respiratory syncytial virus (RSV) 18% and adenovirus 17% [Jroundi et al., 2014]. A subset of 183 children with a positive RV result was randomly selected for RV genotyping.
This study was approved by the University of Western Australia Human Research Ethics Committee, the Ethics Committee of the Hospital Clinic (Barcelona, Spain), and by the Comité d’Éthique de la Recherche Biomédicale (Départ N° 1252—December 16, 2009) of the Faculty of Medicine in Rabat.
Laboratory Methods
Virus detection
NPAs were stored at −80 °C in Rabat until sent to the research laboratory in Perth, Western Australia, on dry ice. RV species identification and genotyping was based on a published molecular method [Lee et al., 2007]. Viral RNA was first extracted from a 240 μl volume of NPAs using the QIAGEN QIAamp Viral RNA Mini Kit (Spin protocol). This was used for the PCR amplification of a 260‐bp variable sequence in the 5′ non‐coding region of the RV genome using specifically designed primers. PCR products were then sequenced by the Australian Genome Research Facility using commercially available methods. Genotypes were assigned based on comparisons of the 5′ non‐coding region sequences with those of 101 classical serotypes as well as 52 newly identified genotypes using ClustalX software (Conway Institute, University College Dublin, Dublin, Ireland). Representative samples of each genotype were sequenced at the VP4‐VP2 coding region to confirm the species assignment [Kiang et al., 2008; Lee et al., 2012].
Statistical Analyses
Demographic, clinical, and laboratory features (categorical variables) associated with RV species (RV‐A and RV‐C) were examined using Chi‐squared (χ2) or Fisher's exact tests. Continuous variables were analyzed using variance (ANOVA) models and presented as means with SD or medians with IQR. Variables that were not normally distributed were logarithm‐transformed and presented as means. RV‐B was excluded from demographic, clinical, and laboratory analyses due to small numbers. Statistical analyses were performed using SPSS version 22.0 (SPSS, Inc., Chicago, IL) and a P‐value <0.05 was considered statistically significant.
RESULTS
Population Demographics
RV‐positive NPAs from 183 children (66.1% males) were available for RV species identification and genotyping. The median age of the study population was 2.00 years (SD 1.38). One hundred fifty‐seven of 183 RV‐positive NPAs (85.8%) were successfully genotyped and classified into one of the three RV species. Clinical diagnoses upon discharge, as provided by the physician included, among the 157 genotyped specimens, 45 (28.7%) episodes of presumed bacterial pneumonia, 15 (9.6%) of bronchiolitis, 88 (56.1%) of bronchitis/asthma, and 3 (1.9%) cases of laryngitis.
RV Species and Genotypes
Of the 157 RV‐positive samples that were successfully genotyped, 60 (38.2%) were RV‐A, 8 (5.1%) were RV‐B, and 89 (56.7%) were RV‐C. Of the 60 RV‐A‐positive specimens, 58 were assigned to 1 of 26 known genotypes while two specimens were equally related to two genotypes. The eight RV‐B‐positive specimens were assigned to one of five known genotypes and the 89 RV‐C‐positive specimens were assigned to one of 27 known genotypes. The two most commonly identified RV‐C genotypes, W24 and W15, were identified 13 (14.6%) and 11 (12.4%) times, respectively. The most commonly identified RV‐A genotypes, R12 and R2, were identified 6 (10.0%) times each.
Demographic Characteristics, Clinical Features, and Laboratory Findings
Demographic characteristics, past morbidity and co‐morbidity, and characteristics during hospitalization in children with RV‐A and RV‐C are described in Table I. The age‐specific distribution of each of RV‐A, RV‐B, RV‐C, and RSV is shown in Figure 1. RV‐C accounted for a higher proportion of RVs in the 2 to <3 age group than in the 4 to <6 age group (82.1% vs. 17.9%; P = 0.021).
Table I.
Demographic Characteristics, Patient History, and Clinical Features of Children With RV‐A and RV‐C Hospitalized With Clinically Severe Pneumonia From Rabat
| RV‐A (n = 60) | RV‐C (n = 89) | P‐value | |
|---|---|---|---|
| Demographic | |||
| Age in months: median (SD) | 16.5 (18.6) | 24.0 (15.0) | 0.737 |
| Age group <12 months: n (%) | 22 (36.7%) | 24 (27.0%) | 0.209 |
| Gender, male: n (%) | 37 (61.7%) | 59 (66.3%) | 0.563 |
| Patient history | |||
| Prematurity: n (%) | 3 (5.0%) | 5 (5.6%) | 0.870 |
| Breastfeeding ≥6 months: n (%) | 36 (60%) | 57 (64.8%) | 0.555 |
| Previous admission for ALRI: n (%) | 26 (43.3%) | 35 (39.3%) | 0.626 |
| Diagnosed chronic condition: n (%) | 7 (11.7%) | 4 (4.5%) | 0.101 |
| Known asthmatic patient: n (%) | 14 (23.3%) | 25 (28.1%) | 0.517 |
| Smokers at home: n (%) | 31 (51.7%) | 42 (47.2%) | 0.592 |
| History of current disease | |||
| Symptoms >1 week: n (%) | 7 (11.7%) | 5 (5.6%) | 0.183 |
| History of fever: n (%) | 38 (63.3%) | 49 (55.1%) | 0.315 |
| History of vomiting: n (%) | 28 (46.7%) | 44 (49.4%) | 0.740 |
| History of diarrhea: n (%) | 16 (26.7%) | 12 (13.5%) | 0.043 |
| Respiratory signs and symptoms | |||
| Axillary temperature (°C): mean (SD) | 37.8 (1.0) | 37.5 (1.0) | 0.007 |
| Fever on admission | 35 (58.3%) | 40 (44.9%) | 0.109 |
| Hyperpyrexia (temp > 39 °C) | 9 (15.0%) | 4 (4.5%) | 0.026 |
| Oxygen saturation: mean (SD) | 96.1 (3.37) | 95.0 (4.36) | 0.113 |
| Hypoxemia (Sa02 < 90%) | 2 (3.5%) | 5 (5.9%) | 0.522 |
| Cyanosis | 0 (0.0%) | 9 (10.1%) | 0.011 |
| Wheeze | 34 (56.7%) | 72 (80.9%) | 0.001 |
| Crackles | 9 (15.0%) | 10 (11.2%) | 0.499 |
| Cough | 58 (96.7%) | 88 (98.9%) | 0.346 |
| Rhonchi | 32 (53.3%) | 40 (44.9%) | 0.315 |
| Nutritional status | |||
| Weight for age Z score (WAZ): mean (SD) | −0.71 (1.45) | −0.28 (1.35) | 0.157 |
| Under nutrition | 26 (44.1%) | 27 (31.0%) | 0.108 |
RV‐B excluded from analyses due to small numbers (n = 8).
Figure 1.

Age‐specific prevalence of RV‐A, RV‐B, and RV‐C in children hospitalized with clinically severe pneumonia from Rabat.
Comparing clinical features (Table I) between RV‐A and RV‐C‐positive cases, wheezing and cyanosis was more common among RV‐C‐positive than RV‐A‐positive children (80.9% vs. 56.7%; P = 0.001 and 10.1% vs. 0%; P = 0.011, respectively). Hyperpyrexia and axillary temperature was higher in RV‐A than in RV‐C‐positive children. Comparing physician's attributed discharge diagnoses between RV‐A‐ and RV‐C‐positive children (Table II), pneumonia was more common among RV‐A‐positive (40.0%) than RV‐C‐positive children (20.2%; P = 0.009). However, there was no difference in radiological end‐point pneumonia (Table II) between RV‐A‐ and RV‐C‐positive children (19.6% vs.15.7%; P = 0.577). Although not statistically significant, bronchiolitis and bronchitis were more common among RV‐C‐positive than RV‐A‐positive children. There were no other significant differences in clinical features or laboratory findings (Tables I and II) between children with different RV species.
Table II.
Discharge diagnoses, radiology endpoints, laboratory findings and outcomes in children with RV‐A and RV‐C hospitalized with clinically severe pneumonia from Rabat
| RV‐A (n = 60) | RV‐C (n = 89) | P‐value | |
|---|---|---|---|
| Physician discharge diagnoses | |||
| Pneumonia of presumed bacterial origin: n (%) | 24 (40.0%) | 18 (20.2%) | 0.009 |
| Bronchiolitis: n (%) | 3 (5.0%) | 12 (13.5%) | 0.091 |
| Bronchitis: n (%) | 29 (48.3%) | 56 (62.9%) | 0.078 |
| Laryngitis: n (%) | 3 (5.0%) | 0 (0.0%) | 0.063 |
| Radiology endpoints | |||
| Normal chest X‐ray: n (%) | 37 (72.5%) | 51 (72.9%) | 0.970 |
| Other infiltrates: n (%) | 4 (7.8%) | 8 (11.4%) | 0.515 |
| End‐point pneumonia (condensation/pleural effusion): n (%) | 10 (19.6%) | 11 (15.7%) | 0.577 |
| Laboratory findings | |||
| All cause bacteremia: n (%) | 4 (6.7%) | 3 (3.4%) | 0.359 |
| Pneumococcus carriage in the nasopharynx: n (%) | 21 (35.0%) | 22 (24.7%) | 0.174 |
| White blood cell (WBC) count (103/µl): mean (SD) | 16.7 (7.75) | 16.9 (7.35) | 0.870 |
| C‐reactive protein (mg/dl): mean (SD) | 3.67 (4.50) | 2.15 (3.01) | 0.015 |
| Procalcitonine (PCT) (ng/ml): mean (SD) | 3.57 (14.1) | 2.41 (7.93) | 0.527 |
| Outcome | |||
| Required oxygen during admission: n (%) | 43 (71.7%) | 76 (85.4%) | 0.040 |
| Required bronchodilators during admission: n (%) | 36 (60.0%) | 69 (77.5%) | 0.021 |
| Required corticosteroids during admission: n (%) | 35 (58.3%) | 65 (73.0%) | 0.061 |
| Received antibiotics during admission: n (%) | 24 (40.0%) | 26 (29.2%) | 0.171 |
| Length of admission (days): mean (SD) | 6.35 (7.73) | 5.20 (3.64) | 0.226 |
| RISC score: mean (SD) | 1.45 (1.35) | 0.98 (1.12) | 0.021 |
RV‐B excluded from analyses due to small numbers (n = 8).
RV Species and Viral Co‐Infections
This study investigated co‐infections between RV species and other respiratory viruses as previously described (Table III) [Jroundi et al., 2014]. Of the 183 RV‐positive cases analyzed, 59 (32.2%) were co‐infected with one other virus, 17 (9.3%) were co‐infected with two other viruses, 1 (0.5%) was co‐infected with three, and 1 (0.5%) with four other viruses. Children with RV‐A were more likely to have co‐infection with metapneumovirus than children with RV‐C, although numbers were very small (Table III). There were no epidemiological, clinical, or laboratory differences between children with RV only and those with co‐infections (data not shown).
Table III.
Viral Co‐Infections in Children With RV‐A and RV‐C Hospitalized With Clinically Severe Pneumonia From Rabat
| Variables | RV‐A n = 60 | RV‐C n = 89 | P‐value |
|---|---|---|---|
| Adenovirus | 9 (15.0%) | 13 (14.6%) | 0.947 |
| RSV | 4 (6.7%) | 9 (10.1%) | 0.465 |
| Parainfluenza virus | 3 (5.0%) | 12 (13.5%) | 0.091 |
| Influenza virus | 0 (0.0%) | 1 (1.1%) | 0.410 |
| Metapneumovirus | 3 (5.0%) | 0 (0.0%) | 0.033 |
| Coronavirus | 5 (8.3%) | 7 (7.9%) | 0.918 |
Seasonal Distribution of RV Species
RV circulated throughout the year and overall was highest in autumn and lowest in winter (42.7% vs. 13.4%). Different seasonal patterns were observed for RV‐A and RV‐C (P < 0.001 for both, Fig. 2). RV‐A was higher in spring (43.3%) and autumn (31.7%) than in summer (15.0%) and winter (10.0%; P < 0.001) while RV‐C was highest in autumn (49.4%) and lowest in spring (11.2%).
Figure 2.
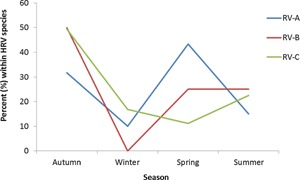
Season‐specific prevalence of RV‐A, RV‐B, and RV‐C in children hospitalized with clinically severe pneumonia from Rabat.
DISCUSSION
A previous study on this cohort reported that over half the children admitted to hospital with an illness fulfilling the WHO diagnostic criteria of clinically severe pneumonia had RV infection [Jroundi et al., 2014]. The current study shows that of the children infected with RV, those with RV‐A compared with RV‐C infections were twice as likely to have a discharge diagnosis of pneumonia, half as likely to have a wheeze‐related discharge diagnosis and less likely to have wheezed. The current findings are important as they show that the two RV species produce distinctly different clinical patterns in children with an acute respiratory illness that fulfills the WHO diagnostic criteria of CSP. RV‐A is associated with an illness that more closely resembles bacterial pneumonia and RV‐C is more likely to be associated with airway symptoms. These findings are consistent with previous reports showing that RV‐A is be more likely to be associated with fever and less likely to be associate with wheeze than RV‐C [Miller et al., 2009a, b]. However, in the setting of the WHO diagnosis of CSP, clearly further studies are needed to determine the prevalence and severity of bacterial co‐infections and the presence of viral co‐infections.
Our findings should help in the interpretation of other RV studies from Africa. The three existing such studies have shown varying RV‐A versus RV‐C prevalences; one reporting equal prevalence [Onyango et al., 2012], one with RV‐A as the most common [Esposito et al., 2012a] and one with RV‐C predominating [Smuts et al., 2011]. However, it is important to note that the populations in the three studies differed in age and clinical presentation. The finding that RV‐C is more likely than RV‐A to be associated with wheeze has been supported by many studies around the world [Arden et al., 2006; Lamson et al., 2006; Renwick et al., 2007; Lau et al., 2009; Linsuwanon et al., 2009; Miller et al., 2009a, b; Bizzintino et al., 2011], with only a few studies reporting no differences in wheeze between RV‐A and RV‐C [Wildenbeest et al., 2016].
This study was part of a series of comprehensive epidemiological, clinical, and laboratory analyses describing Moroccan children admitted to hospital with CSP. Hence, associations between RV species and epidemiological, clinical, and laboratory findings were investigated in Moroccan children for the first time. Physician's attributed discharge diagnosis of pneumonia was more common among RV‐A‐positive than RV‐C‐positive children while wheeze was less common among RV‐A‐positive than RV‐C‐positive children. RV‐C was the most common RV species and wheeze was more common among RV‐C than RV‐A cases, and these findings have been supported by studies from around the world [McErlean et al., 2007; Miller et al., 2009a, b; Bizzintino et al., 2011] including a study from the Middle East [Miller et al., 2009a, b]. Although Miller et al. found RV‐A to be the most common identified species overall in children with respiratory symptoms and/or fever, only RV‐C was associated with wheeze. In contrast, in two studies from China [Xiang et al., 2010] and Italy [Esposito et al., 2012b] of childhood pneumonia, RV‐A was more common than RV‐C, identified in about half the children compared with RV‐C, only being identified in 38% and 31% of children, respectively. Hence, RV‐C is likely to play a more important role in asthma and wheeze type illness while RV‐A plays a more important role in pneumonia. However, this conclusion may also be challenged by findings from this study that radiologically confirmed end‐point pneumonia was equally common between RV‐A‐ and RV‐C‐positive children.
This study investigated associations between RV species and clinical features. RV‐A‐positive children had a higher axillary temperature than RV‐C‐positive children. In addition to wheeze, cyanosis was more common among RV‐C‐positive than RV‐A‐positive children. Miller et al. reported that children in Jordan with RV‐C were more likely to require supplemental oxygen and less likely to report otalgia (ear pain) and suggested that RV‐C was more virulent than RV‐A [Miller et al., 2009a, b]. The association between RV‐C and supplemental oxygen reported by Miller et al. concurs with the finding in this study that RV‐C was associated with cyanosis. This study reported relatively low prevalence of hypoxemia (3–6%) in both RV‐A‐ and RV‐C‐positive children, despite high oxygen provision during admission (72–85%). Such a contrast may arise from the fact that oxygen administration at the hospital is very common and proactive, generally starting upon admission, while performing most of the procedures related to canalizing a vein and/or administering the first doses of inhaled bronchodilators. With the exception of wheeze, cyanosis, and axillary temperature, clinical features between children with different RV species were similar, suggesting that many clinical features overlap between the species. Similarly, other studies have also reported no differences in clinical features or severity of illness between RV species [Luchsinger et al., 2014].
Seasonal differences were observed between RV‐A and RV‐C, with RV‐A peaking in spring and RV‐C peaking in autumn. Other studies from around the world have supported these findings of RV‐C to be most prevalent during autumn [Lau et al., 2007, 2009; Miller et al., 2009a, b; Richter et al., 2015] and RV‐A to be most prevalent in spring [Miller et al., 2009a, b]. Viral interference or cross‐serological protection (as seen with other viruses such as human parainfluenza virus [Lau et al., 2009, 2010] have been suggested as contributing to the alternating activity between RV‐A and RV‐C.
This study has a few limitations. Firstly, contemporaneous healthy controls were not included in the analysis to compare the distribution of RV species between sick and healthy children. Previous studies from developing countries have reported RV identification rates of up to 40% in non‐respiratory controls [Chidlow et al., 2012]. Secondly, using NPAs, viruses identified in the upper airway may not be entirely representative of the lower airway. However, the use of NPAs for studying lower respiratory disease is widely accepted given the difficulties of obtaining lower respiratory tract samples. Furthermore, utilizing molecular methods to detect RV in NPAs does not allow differentiation to be made between pathogenic and non‐pathogenic infection. Even in the absence of other viruses, identification of RV suggests but does not prove RV was the causative agent. Further investigations of viruses present in lower respiratory samples as well as identification of more bacteria in the respiratory tract could contribute to better understanding of the pathogenesis of RV. Finally, the study population comprised a group of WHO‐defined CSP cases admitted to a hospital and, hence, our findings may not be representative of respiratory illness overall. Nonetheless, this study provides data on the distribution of RV species in Morocco for the first time, with important findings on the associations between RV‐A and pneumonia and RV‐C and wheeze.
ACKNOWLEDGMENTS
We thank all the children and families who agreed to take part in the study. This study resulted from the collaborative work of groups from the School of Paediatrics and Child Health, Faculty of Medicine, Dentistry and Health Sciences, University of Western Australia, and the Barcelona Institute for Global Health (ISGlobal). This study was funded by the Spanish Agency of International Cooperation for Development through the grant 07‐CO1‐021 awarded to Fundació Clínic per a la Recerca Biomèdica (Convenio de Fortalecimiento del sistema nacional de salud, con especial atención a la salud materno‐infantil, Marruecos, 2008–2012). This research study also benefits from contributions from the Ministry of Health of the Kingdom of Morocco, in particular the Children's Hospital of Rabat, and from the Centre de Recerca en Salut Internacional de Barcelona (CRESIB). Additional funding was provided by an NHMRC grant (PI Peter Le Souëf).
REFERENCES
- Arden KE, McErlean P, Nissen MD, Sloots TP, Mackay IM. 2006. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol 78:1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzintino JA, Lee WM, Laing IA, Vang F, Pappas T, Zhang G, Martin AC, Khoo SK, Cox DW, Geelhoed GC, McMinn PC, Goldblatt J, Gern JE, Le Souëf PN. 2011. Association between human rhinovirus C and severity of acute asthma in children. Eur Respir J 37:1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidlow GR, Laing IA, Harnett GB, Greenhill AR, Phuanukoonnon S, Siba PM, Pomat WS, Shellam GR, Smith DW, Lehmann D. 2012. Respiratory viral pathogens associated with lower respiratory tract disease among young children in the highlands of Papua New Guinea. J Clin Virol 54:235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito S, Daleno C, Baggi E, Ciarmoli E, Lavizzari A, Pierro M, Semino M, Groppo M, Scala A, Terranova L, Galeone C, Principi N. 2012a. Circulation of different rhinovirus groups among children with lower respiratory tract infection in Kiremba, Burundi. Euro J Clin Microbiol 31:3251–3256. [DOI] [PubMed] [Google Scholar]
- Esposito S, Daleno C, Tagliabue C, Scala A, Tenconi R, Borzani I, Fossali E, Pelucchi C, Piralla A. Principi N. 2012b. Impact of rhinoviruses on pediatric community‐acquired pneumonia. Eur J Clin Microbiol Infect Dis 31:1637–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden FG. 2004. Rhinovirus and the lower respiratory tract. Rev Med Virol 14:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jroundi I, Mahraoui C, Benmessaoud R, Moraleda C, Tliqui H, Seffar M, Kettani SC, Benjelloun BS, Chaacho S, Maaroufi A, Hayes EB, Álvarez‐Martínez MJ, Muñoz‐Almagro C, Ruiz J, Alonso PL, Bassat Q. 2014. The epidemiology and aetiology of infections in children admitted with clinical severe pneumonia to a university hospital in Rabat, Morocco. J Trop Pediatr 60:270–278. [DOI] [PubMed] [Google Scholar]
- Jroundi I, Mahraoui C, Benmessaoud R, Moraleda C, Tligui H, Seffar M, El Kettani SE, Benjelloun BS, Chaacho S, Muñoz‐Almagro C, Ruiz J, Alonso PL, Bassat Q. 2016. A comparison of human metapneumovirus and respiratory syncytial virus WHO‐defined severe pneumonia in Moroccan children. Epidemiol Infect 144:516 –526. [DOI] [PubMed] [Google Scholar]
- Kiang D, Kalra I, Yagi S, Louie JK, Boushey H, Boothby J, Schnurr D P. 2008. Assay for 5' noncoding region analysis of all human rhinovirus prototype strains. J Clin Microbiol 46:3736–3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamson D, Renwick N, Kapoor V, Liu Z, Palacios G, Ju J, Dean A, St George K, Briese T, Lipkin WI. 2006. MassTag polymerase‐chain‐reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza‐like illness in New York State during 2004–2005. J Infect Dis 194:1398–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau SK, Yip CC, Tsoi HW, Lee RA, So LY, Lau YL, Chan KH, Woo PC, Yuen KY. 2007. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV‐C, associated with acute respiratory illness in children. J Clin Microbiol 45:3655–3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau SK, Yip CC, Lin AW, Lee RA, So LY, Lau YL, Chan KH, Woo PC, Yuen KY. 2009. Cinical and molecular epidemiology of human rhinovirus C in children and adults in Hong Kong reveals a possible distinct human rhinovirus C subgroup. J Infect Dis 200:1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau SK, Yip CC, Woo PC, Yuen KY. 2010. Human rhinovirus C: A newly discovered human rhinovirus species. Emerg Health Threats J 3:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WM, Kiesner C, Pappas T, Lee I, Grindle K, Jartti T, Jakiela B, Lemanske RF Jr, Shult PA, Gern JE. 2007. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illness in infants. PLoS ONE 2:e966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WM, Lemanske RF Jr, Evans MD, Vang F, Pappas T, Gangnon R, Jackson DJ, Gern JE. 2012. Human rhinovirus species and season of infection determine illness severity. Am J Respir Crit Care Med 186:886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsuwanon P, Payungporn S, Samransamruajkit R, Posuwan N, Makkoch J, Theanboonlers A, Poovorawan Y. 2009. High prevalence of human rhinovirus C infection in Thai children with acute lower respiratory tract disease. J Infect 59:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, Mathers C, Black RE, Child Health Epidemiology Reference Group of WHO and UNICEF. 2012. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 379:2151–2161. [DOI] [PubMed] [Google Scholar]
- Luchsinger V, Ampuero S, Palomino MA, Chnaiderman J, Levican J, Gaggero A, Larrañaga CE. 2014. Comparison of virological profiles of respiratory syncytial virus and rhinovirus in acute lower tract respiratory infections in very young Chilean infants, according to their clinical outcome. J Clin Virol 61:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McErlean P, Shackelton L, Lambert S, Nissen M, Sloots T, Mackay I. 2007. Characterization of a newly identified human rhinovirus, HRV‐QPM, discovered in infants with bronchiolitis. J Clin Virol 39:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Edwards KM, Weinberg GA, Iwane MK, Griffin MR, Hall CB, Zhu Y, Szilagyi PG, Morin LL, Heil LH, Lu X, Williams JV, New Vaccine Surveillance Network. 2009a. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immun 123:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Khuri‐Bulos N, Williams JV, Shehabi AA, Faouri S, Al Jundi I, Chen Q, Heil L, Mohamed Y, Morin LL, Ali A, Halasa NB. 2009b. Human rhinovirus C associated with wheezing in hospitalised children in the Middle East. J Clin Microbiol 46:85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland EK, Simoes EAF, Costales MO, McGrath EJ, Manalac EM, Gove S. 1992. Standardized diagnosis of pneumonia in developing countries. Pediatr Infect Dis J 11:77–81. [DOI] [PubMed] [Google Scholar]
- Onyango CO, Welch SR, Munywoki PK, Agoti CN, Bett A, Ngama M, Myers R, Cane PA, Nokes DJ. 2012. Molecular epidemiology of human rhinovirus infections in Kilifi, coastal Kenya. J Med Virol 84:823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renwick N, Schweiger B, Kapoor V, Liu Z, Villari J, Bullmann R, Miething R, Briese T, Lipkin WI. 2007. A recently identified rhinovirus genotype is associated with severe respiratory‐tract infection in children in Germany. J Infect Dis 196:1745–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J, Nikolaou E, Panayiotou C, Tryfonos C, Koliou M, Christodoulou C. 2015. Molecular epidemiology of rhinoviruses in Cyprus over three consecutive seasons. Epidemiol Infect 143:1876–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudan I, O'Brien KL, Nair H, Theodoratou E, Qazi S, Luksic I, Fischer Walker CL, Black RE, Cambell H, Child Health Epidemiology Reference Group (CHERG). 2013. Epidemiology and etiology of childhood pneumonia in 2010: Estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Global Health 3:010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smuts H, Workman L, Zar H. 2011. Human rhinovirus infection in young African children with acute wheezing. BMC Infect Dis 11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 2005. Pocket book for hospital care of children: Guidelines for the management of common illness with limited resources. Switzerland, Geneva: World Health Organization. [Google Scholar]
- WHO. 2012. In: Organization W H, editor. International classification of diseases, tenth revision, clinical modification (ICD‐10‐CM). Switzerland, Geneva: World Health Organization. [Google Scholar]
- Wildenbeest JG, van der Schee MP, Hashimoto S, Benschop KS, Minnaar RP, Spirkkelman AB, Haarman EG, van Aalderen WM, Sterk PJ, Pajkrt D, Wolthers KC. 2016. Prevalence of rhinoviruses in young children of an unselected birth cohort from the Netherlands. Clin Microbiol Infect, 22:736.e739–736.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Gonzalez R, Xie Z, Xiao Y, Liu J, Chen L, Liu C, Zhang J, Ren L, Vernet G, Paranhos‐Baccalà G, Shen K, Jin Q, Wang J. 2010. Human rhinovirus C infections mirror those of human rhinovirus A in children with community‐acquired pneumonia. J Clin Virol 49:94–99. 10.1016/j.jcv.2010.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]


