Abstract
The emergence of the Middle East Respiratory Syndrome (MERS) in Saudi Arabia has intensified focus on Acute Respiratory Infections [ARIs]. This study sought to identify respiratory viruses (RVs) associated with ARIs in children presenting at a tertiary hospital. Children (aged ≤13) presenting with ARI between January 2012 and December 2013 tested for 15 RVs using the SeeplexR RV15 kit were retrospectively included. Epidemiological data was retrieved from patient records. Of the 2235 children tested, 61.5% were ≤1 year with a male: female ratio of 3:2. Viruses were detected in 1364 (61.02%) children, 233 (10.4%) having dual infections: these viruses include respiratory syncytial virus (RSV) (24%), human rhinovirus (hRV) (19.7%), adenovirus (5.7%), influenza virus (5.3%), and parainfluenzavirus‐3 (4.6%). Children, aged 9–11 months, were most infected (60.9%). Lower respiratory tract infections (55.4%) were significantly more than upper respiratory tract infection (45.3%) (P < 0.001). Seasonal variation of RV was directly and inversely proportional to relative humidity and temperature, respectively, for non MERS coronaviruses (NL63, 229E, and OC43). The study confirms community‐acquired RV associated with ARI in children and suggests modulating roles for abiotic factors in RV epidemiology. However, community‐based studies are needed to elucidate how these factors locally influence RV epidemiology. J. Med. Virol. 89:195–201, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: respiratory viruses, acute respiratory illness, multiplex molecular testing platform, pediatrics, MERS‐CoV, season
INTRODUCTION
Acute respiratory illnesses (ARIs) remain a global burden causing nearly 4 million deaths annually. In developing countries, it is the primary cause of death in children <5 years [Liu et al., 2012]. In Saudi Arabia, over 5.4 million cases of ARI presented to emergency departments in 2013 [Ministry of Health, 2015]. However, much remains unknown of the proportion of these ARI aetiologically linked to respiratory viruses. This situation is further complicated by the emergence of the Middle East Respiratory Syndrome Coronavirus (MERS CoV) in Saudi Arabia in 2012 [Zaki et al., 2012]. The global threat posed by MERS CoV had led to efforts disproportionately focused on defining MERS‐CoV burden while the overall burden (denominator) of respiratory viruses in the country remains largely uncharacterized. A broadened understanding of respiratory viruses having overlapping ecologies and transmission modes should provide more modalities for integrated public health intervention and prevention.
Previous studies on ARI in Saudi Arabia have largely focused on local and foreign pilgrims visiting Makkah and Madeenah during the annual Hajj pilgrimage [El‐Sheikh et al., 1998; Balkhy et al., 2004; Alborzi et al., 2009; Mandourah et al., 2012]. Respiratory infections constitute a recognized health burden during hajj [Alzeer, 2009] with pneumonia in pilgrims being a major reason for hospitalization [Bukhari and Elhazmi, 2013]. During the 2013 Hajj season, almost 10% of documented deaths of pilgrims was due to respiratory illnesses including pneumonia [Ministry of Health, 2015]. As the overwhelming majority of the pilgrims are adults and elderly, children are virtually excluded from these studies. Giving that foreign pilgrims originate from all over the world, a major limitation with most of these Hajj‐centered studies is that they are not adequately powered to accurately discriminate between imported and locally acquired respiratory viruses. In 2013, almost 2 million pilgrims performed the hajj; of these, 70% were from outside the Kingdom [Ministry of Health, 2015]. Additionally, these studies often lacked seasonal or climatic data in their analyses. There have been previous studies conducted on children with ARI in the Kingdom; most of these studies [al‐Hajjar et al., 1998; Ghazal et al., 1998; Akhter et al., 2009; Al Hajjar et al., 2011; Alanazi et al., 2013; Amer et al., 2015] have been conducted in Riyadh, the country's capital. The city of Riyadh is also part of the Riyadh province that reported a population of 7.5 million in 2013 [Ministry of Health, 2015]. The few studies reported from elsewhere in the Kingdom include those done in Abha, Taif, and Najran [Al‐Shehri et al., 2005; Abdel‐Moneim et al., 2013; Al‐Ayed et al., 2014]. Although, most of these studies have been plagued with small sample sizes, one large sized study of children with ARI used a qualitative direct immunofluorescence test [Alanazi et al., 2013]; such tests are insensitive often giving false negative results [Uyeki, 2003]. Serological assays have diagnostic limitations in the clinical management of children with ARI [Henrickson, 2004]. The requirement of convalescent phase sera to detect a fourfold rise and thus confirm acute infections makes it irrelevant in the management of acutely ill children. However, it is a good tool in retrospective, epidemiologic studies [Anderson et al., 1985; Falsey et al., 2002]. Additionally, it has been shown that infants with ARI due to respiratory syncytial virus (RSV) usually lack detectable serologic responses [Murphy et al., 1986; Hall et al., 1991]. Similarly, it has been the insensitivity of colorimetric or immunofluorescence based antigen assays for RSV, in comparison with molecular diagnostics, has been reported [Falsey et al., 2002; Casiano‐Colon et al., 2003]. The advent of rapid and highly sensitive multiplex molecular testing platforms overcome the drawbacks of serology and antigenic tests while permitting simultaneous detection of multiple respiratory viruses. This platform was used to detect respiratory viruses in children presenting with ARI under climatic conditions prevailing in the Riyadh province.
METHODS
The King Fahad Medial City (KFMC) is an advanced 1,200‐bed tertiary health facility located in Riyadh, in the central province of Saudi Arabia. Typically, the country usually experiences high temperatures and low precipitation. In Riyadh, average winter temperatures dips as low as 8°C; average summer temperatures peak in July at well over 40°C. Rainfall often occur between January and May with an annual rainfall of 100 mm [UNFCCC, 2011]. Single respiratory samples (nasopharyngeal swab, bronchoalveolar lavage, sputum, tracheal aspirate) were collected from Children aged ≤13 years presenting to the emergency department with ARI symptoms between January 2012 and December 2013 and extracted nucleic acids tested simultaneously for 15 viruses (adenovirus [ADV], enterovirus [hEV], human bocavirus [hBoV], human coronaviruses OC43 and 229E/NL63, human rhinovirus [hRV], influenza A and B viruses [IFLA & IFLB], parainfluenza viruses [PIV]1–4, RSV A and B using Seeplex RV15 ACE multiplex respiratory viral assay (Seegene Inc., Seoul, South Korea) as previously described [Zhang et al., 2012]. Extracted cDNAs were tested in a 3‐tube reaction according to manufacturer's protocol. Primer set A in tube1 was directed at 229E/NL63, AdV and PIV1‐3; primer set B in tube 2 OC43/HKU1, hRV, RSV A and B, and IFLA; and contained primer set C Tube‐3 at hBoV, IFLB, hMPV, PIV4, and hEV. A reaction mix consisting of 4 μl of each 5 × RV15 multiplex primer sets (A, B, and C), 3 μl 8‐methoxypsoralen (8‐MOP) solution, 10 μl 2 × multiplex master mix (HotstartR Taq DNA polymerase and dNTPs included), and 3 μl cDNA template with a final volume of 20 μl. Mixtures were subjected to a first round of denaturing at 94°C for 15 min and a second round by 40 cycles at 94°C for 30 sec. This was followed by annealing at 60°C for 90 sec and extension at 72°C for 90 sec. Lastly, the mixtures were extended at 72°C for 10 min. Internal controls included a mixture of all 15 virus clones (positive) and ddH2O (negative) [Roh et al 2008; Bruijnesteijn van Coppenraet et al., 2010]. The end products were visualized by electrophoresis on a 2% agarose gels with ethidium bromide staining. The Seeplex RV15 has a limit of detection of 100 copies/10 μl. Samples positive for IAV were tested for H1N1 using a commercial kit (artus® Infl./H1 LC/RG RT‐PCR Kit; Qiagen, Germany). Children with symptoms such as runny nose, nasal or throat congestion, or itchiness of the throat were described as having upper respiratory infections while those with productive cough, shortness of breath, weakness, and fatigue were classified as having lower respiratory infections.
Riyadh weather data (King Khalid International Airport) was electronically retrieved from https://weatherspark.com/history/32777/2012/Riyadh-Saudi-Arabia. For statistical analysis, SPSS (version 22.0, SPSS Inc., Chicago, IL) was used. Specific prevalence for positive cases have been derived from total positive cases in the respective groups and the one‐to‐one inferences have been drawn at 95% CI using Chi‐square analysis and Fisher's exact tests as appropriate. All tests were two‐tailed and P < 0.05 was considered statistically significant. The study was approved by the KFMC institutional review board (IRB).
RESULTS
A total of 2,235 children (mean age—19.6 months; range 1 day to 13 years) were tested, 1374 (61.5%) were <1 year with a male to female ratio of 3:2. Nasopharyngeal samples represented 99% of samples analyzed. Respiratory viruses were detected in 1364 (61%) children, significantly higher in children (55.4%) with acute lower respiratory infection (ALRI) than acute upper respiratory infection (AURI) (45.3%) (P < 0.001). As shown in Table I, more than a third of the children were diagnosed with bronchiolitis and pneumonia; of these, over 70% had respiratory viruses detected in their tested respiratory specimens. As shown in Table II, reveal that RSVs were the most detected viruses (23%); this was followed by hRV found in almost 20% of positive samples. Most respiratory viruses were detected in children 9–11 months of age. More boys had RSV and hEV infections. Single infections with RSV, hRV, hMPV, ADV, and IFLB showed a significant association with age group. Half of the children had single infections, while multiple infections occurred in over 10%, mostly dual infections (233/1364; 10.4%). Co‐infections were significant amongst those aged 9–11 months (13.5%), 2 months (13.9%), and 6–8 months (15.0%) (P< 0.001). Co‐infection rates were similar in children with both ALRI and AURI: 11.5% and 9.5% for dual infections, and 0.7% and 0.5% for triple infections, respectively. Viral co‐infections in ARI cases were predominantly associated with, in descending order, hBoV, hEV, and ADV (P < 0.001) (Fig. 1). More than one virus was detected in 18.1% (247 out of 2235) of patients. Children with RSV and hRV co‐infection required more oxygen therapy and longer length of stay than those RSV mono‐infections. The detection of co‐infections with more than two viruses was not significant. Children with ALRI had significant infections with RSVs. Human coronaviruses‐229E/NL63 and OC43 were detected in <4% of the children. A 45‐day‐old male infant had quadruple infection with ADV, PIV3, hRV, and RSV. The pandemic H1N1 virus was only detected in an 8‐month‐old infant. Post MERS CoV emergence in late 2012, children were also screened, according to prevailing Ministry of Health guidelines, for MER‐CoV infection in the regional Ministry of Health lab: all were negative.
Table I.
Virus Detection Rate Per Age‐Group, Respiratory Tract Site, and Diseases in Children With ARIs (2012 and 2013)
| Patients | Total | Positive (N/%) | P‐value |
|---|---|---|---|
| Female | 931 (41.7) | 567 (60.9) | 0.917 |
| Male | 1304 (58.3) | 797 (61.1) | |
| Upper respiratory tract | 1202 (53.8) | 666 (55.4) | <0.001 |
| Lower respiratory tract | 1033 (46.2) | 698 (67.6) | |
| 0 month | 288 (12.9) | 157 (54.5) | 0.015 |
| 1 month | 208 (9.3) | 126 (60.6) | 0.888 |
| 2 months | 202 (9.0) | 140 (69.3) | 0.011 |
| 3–5 months | 336 (15.0) | 219 (65.2) | 0.091 |
| 6–8 months | 207 (9.3) | 134 (64.7) | 0.251 |
| 9–11 months | 133 (6.0) | 100 (75.2) | 0.001 |
| 1–5 years | 673 (30.1) | 396 (58.8) | 0.164 |
| 6–12 years | 188 (8.4) | 92 (48.9) | <0.001 |
| Bronchiolitis and pneumonia | 841 (37.6) | 590 (70.2) | <0.001 |
Table II.
Respiratory Viruses Detected, According to Age Group Between 2012 and 2013
| Virus | <1 m, no (%) | 1–<2 m, no (%) | 2–<3 m, no (%) | 3–<6 m, no (%) | 6–<9 m, no (%) | 9–<12 m, no (%) | 1–<6 y, no (%) | 6–<13 y, no (%) | Total | P‐value |
|---|---|---|---|---|---|---|---|---|---|---|
| ADV | 2 (0.7) | 2 (1.0) | 6 (3.0) | 28 (8.3) | 19 (9.2) | 10 (7.5) | 53 (7.9) | 7 (3.7) | 127 (5.7) | <0.001 |
| hBoV | 0 (0.0) | 1 (0.5) | 1 (0.5) | 6 (1.8) | 7 (3.4) | 3 (2.3) | 15 (2.2) | 1 (0.5) | 34 (1.5) | 0.023 |
| NL63/229E/OC43 | 13 (4.5) | 5 (2.4) | 11 (5.5) | 11 (3.3) | 7 (3.4) | 7 (5.3) | 16 (2.4) | 7 (3.7) | 77 (3.4) | 0.35 |
| hEV | 3 (1.0) | 4 (1.9) | 5 (2.5) | 4 (1.2) | 1 (0.5) | 1 (0.8) | 17 (2.5) | 1 (0.5) | 36 (1.6) | 0.234 |
| IFLA | 4 (1.4) | 3 (1.4) | 4 (2.0) | 11 (3.3) | 7 (3.4) | 6 (4.5) | 29 (4.3) | 9 (4.8) | 73 (3.3) | 0.147 |
| IFLB | 2 (0.7) | 3 (1.4) | 1 (0.5) | 4 (1.2) | 3 (1.4) | 2 (1.5) | 20 (3.0) | 10 (5.3) | 45 (2) | 0.005 |
| hMPV | 6 (2.1) | 2 (1.0) | 10 (5.0) | 17 (5.1) | 13 (6.3) | 10 (7.5) | 32 (4.8) | 4 (2.1) | 94 (4.2) | 0.012 |
| PIV1 | 0 (0.0) | 3 (1.4) | 3 (1.5) | 5 (1.5) | 3 (1.4) | 5 (3.8) | 16 (2.4) | 2 (1.1) | 37 (1.7) | 0.129 |
| PIV2 | 1 (0.3) | 0 (0.0) | 2 (1.0) | 5 (1.5) | 1 (0.5) | 2 (1.5) | 9 (1.3) | 7 (3.7) | 27 (1.2) | 0.028 |
| PIV3 | 8 (2.8) | 11 (5.3) | 11 (5.4) | 18 (5.4) | 9 (4.3) | 3 (2.3) | 37 (5.5) | 6 (3.2) | 103 (4.6) | 0.435 |
| PIV4 | 5 (1.7) | 0 (0.0) | 3 (1.5) | 1 (0.3) | 2 (1.0) | 2 (1.5) | 5 (0.7) | 2 (1.1) | 20 (0.9) | 0.416 |
| hRV | 50 (17.4) | 44 (21.2) | 61 (30.2) | 63 (18.8) | 44 (21.3) | 30 (22.6) | 116 (17.2) | 32 (17.0) | 440 (19.7) | 0.006 |
| RSV A | 55 (19.1) | 51 (24.5) | 46 (22.8) | 74 (22.0) | 36 (17.4) | 25 (18.8) | 83 (12.3) | 11 (5.9) | 381 (17) | <0.001 |
| RSV B | 29 (10.1) | 14 (6.7) | 9 (4.5) | 15 (4.5) | 13 (6.3) | 14 (10.5) | 35 (5.2) | 2 (1.1) | 131 (5.9) | <0.001 |
m, month; y, year.
Figure 1.
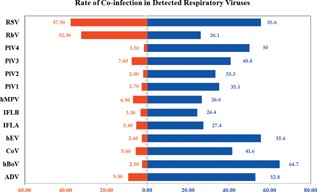
Co‐infection pattern in respiratory viruses detected in children tested.
The frequency of detection of viruses is shown in Figure 2, with RSV, hRV, and ADV being the most common in descending order. Seasonal variation in the detection of respiratory viruses was observed. For both years, the detection rates of hRV, RSVs, and hMPV were lowest in June, July, and August (Fig. 3A). For hRV, increase in detection began in September for both years. As shown in Figure 3B, of the two RSV genotypes, RSV A was more frequently detected throughout the 2 year study period with both peaking during the winter months of December and January. No temporal genotype shift in RSV detection pattern was recorded. Though hMPV detection was low (4.2%; 94/2235) when compared to RSVs and hRV, it was mostly detected between January and March. July, the hottest month during the study period [average temperature of 44°C and 43°C for 2012 and 2013, respectively; Fig. 4B], recorded the least incidence for most respiratory viruses. June, the least humid month in 2012 and 2013 (Fig. 4A), was the period when RSV and hRV were least detected (Fig. 1). Cumulatively, respiratory virus detection was highest in December 2013 and lowest in June 2012 (Fig. 4A and B).
Figure 2.
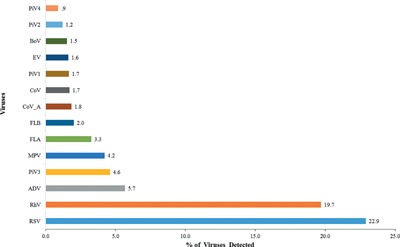
Frequency of detection of respiratory viruses detected in children tested.
Figure 3.
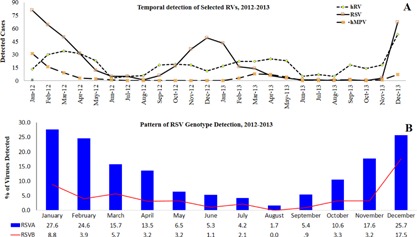
A: Seasonal variation of hRV, RSV, and hMPV viruses. B: Pattern of RSV A and B detection.
Figure 4.
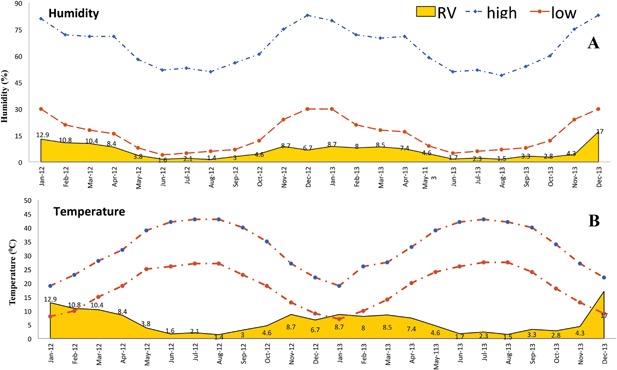
Temporal correlation of respiratory virus detection with weather station documented average humidity (A) and temperature, (B) 2012–2013.
DISCUSSION
This large study population determined the burden of respiratory viruses associated with ARI in children over a 2‐year period a multiplex molecular testing platform. With over 60% of 2235 children testing positive for respiratory viruses, more infections were detected than previous studies [Akhter et al., 2009; Al Hajjar et al., 2011; Alanazi et al., 2013; Bukhari and Elhazmi, 2013]. Researchers from a similarly large tertiary care facility based in Riyadh undertook a year‐long (between 2006 and 2007) study of 10,617 children; using direct antigen testing and shell vial assay as testing platforms, they found respiratory viruses in only 8.3% [Akhter et al., 2009] of them. Most (88%, 733/883) of these children has RSV infection. This high rate was significantly different from that obtained in the present 2‐year study (23% detection for RSV in children positive for respiratory viruses), as well as others [Alanazi et al., 2013; Amer et al., 2015]. Similarly, higher detection rates were observed for influenza and parainfluenza viruses when compared to the results from this this study. It was observed that the 4.2% hMPV detection rate in the present study was lower than the 8.3% rate obtained from another study earlier conducted in a similar tertiary health care facility in Riyadh [Al Hajjar et al., 2011]. Although, the earlier study used a lesser advanced version of the Seeplex kit, the seemingly increased rate may be due to most of the children tested in the earlier study being immunocompromised.
Given its effectiveness as demonstrated in this study, multiplex testing platforms such as the Seeplex RV15 ACE can be modified to include MERS‐CoV consensus probes. Consequently, the augmented ability to simultaneously test a single specimen for MERS‐CoV and other endemic respiratory viruses should diagnostic turnaround time and optimize utilization of limited specimen volume scenarios as may be envisaged in pediatric settings. Analyzing limited specimens using monoplex assays may not only preclude detection of untested respiratory viruses, aetiologically unresolved ARI quagmires will abound. Additionally, such modified platforms have the potential to elicit more epidemiological data on respiratory viruses including MERS‐CoV. In the backdrop of the incessant multidirectional human traffic of residents between countries within the Arabian peninsula and as more recently demonstrated by MERS‐CoV transmission events, it may not be impossible that the epidemiology of respiratory viruses in these countries are somewhat inter‐related. More detailed research will, however, be needed to elucidate this. Comparatively, a study from neighboring Oman reported ADV being the second most detected virus in children with ARI, aged 5 years or younger [Khamis et al., 2012]; in the present Riyadh study, ADV infections was associated with age groups 6–8 months, 3–5 months, 1–5 years, and 9–11 months in a decreasing order. Extending the findings of previous studies that linked seasonal patterns of ARI in children with fewer detection rates of respiratory viruses [Alanazi et al., 2013; Bukhari and Elhazmi, 2013] or none [Ghazal et al., 1998], this study seasonally correlated the large RV detection rate which showed an inverse and direct relationship with average temperature and humidity, respectively (Fig. 2). However, the depicted graphical correlation was obtained while analyzing our data with externally acquired meteorological data.
This study has limitations. Firstly, its retrospective design may not permit capture of all ARI presenting to the facility. Secondly, the results obtained may not represent the true burden of ARI due to respiratory viruses in children in the community, a limitation shared by other studies. Usually, most mild cases of respiratory viral infections are unlikely to seek care at health care facilities with some patients engaging in self‐medication. Consequently, these cases remain uncaptured by hospital‐based studies or surveillance systems. To address this limitation, community based respiratory virus epidemiologic studies are suggested. Though such studies are presently lacking in Saudi Arabia, similar work done elsewhere [Lambert et al., 2008b; van der Zalm et al., 2011; Chu et al., 2013; Alsaleh et al., 2014] have yielded valuable epidemiological data. In Australia, workers using community centered studies have shown the strong association between child care attendance and the detection of human metapneumovirus and human coronavirus NL63 in children [Lambert et al., 2007]. They also documented evidence of significant economic impact of ARI managed outside health care settings on families; such costs were greater in healthy preschool aged children with influenza infection than those infected with RSV and other respiratory viruses [Lambert et al., 2008a] . In the Netherlands, researchers showed that respiratory viruses regularly occur in asymptomatic children sampled in the community [van der Zalm et al., 2009]. By including appropriate temporal and climatic data, such studies will further elucidate the transmission dynamics of respiratory viruses in children in community settings in Riyadh. Additionally, it is possible that the results of such studies may provide collateral data to help understand possible community transmission of MERS CoV suggested in a recent study [Fagbo et al., 2015].
ACKNOWLEDGMENTS
The technical assistance of Nura Al Ragi, Hanan Al Qudairy, Johara Al Mutairy, and Eman Al Karafi is acknowledged. We also appreciate the assistance of Abdul Basir Pula and Roderick Mallete with the figures.
REFERENCES
- Abdel‐Moneim AS, Kamel MM, Al‐Ghamdi AS, Al‐Malky MI. 2013. Detection of bocavirus in children suffering from acute respiratory tract infections in Saudi Arabia. PLoS ONE 8:e55500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter J, Al Johani S, Dugaishm F, Hfdi R, Al Hassan I. 2009. Etiology of respiratory viral infections using rapid virus isolation methods at a tertiary care center in Riyadh, Saudi Arabia. Saudi Pharm J 17:177–181. [Google Scholar]
- Al‐Ayed MS, Asaad AM, Qureshi MA, Ameen MS. 2014. Viral etiology of respiratory infections in children in southwestern Saudi Arabia using multiplex reverse‐transcriptase polymerase chain reaction. Saudi Med J 35:1348–1353. [PMC free article] [PubMed] [Google Scholar]
- al‐Hajjar S, Akhter J, al Jumaah S, Hussain Qadri SM. 1998. Respiratory viruses in children attending a major referral centre in Saudi Arabia. Ann Trop Paediatr 18:87–92. [DOI] [PubMed] [Google Scholar]
- Al‐Shehri MA, Sadeq A, Quli K. 2005. Bronchiolitis in Abha, Southwest Saudi Arabia: Viral etiology and predictors for hospital admission. West Afr J Med 24:299–304. [DOI] [PubMed] [Google Scholar]
- Al Hajjar S, Al Thawadi S, Al Seraihi A, Al Muhsen S, Imambaccus H. 2011. Human metapneumovirus and human coronavirus infection and pathogenicity in Saudi children hospitalized with acute respiratory illness. Ann Saudi Med 31:523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanazi A, Alzahrani N, Almutairi M, Motasim B, Aqel H. 2013. Viruses associated with respiratory tract infections in children attending to the emergency room, king abdulaziz medical city, riyadh, Saudi Arabia. World J Med Sci 8:103–106. [Google Scholar]
- Alborzi A, Aelami MH, Ziyaeyan M, Jamalidoust M, Moeini M, Pourabbas B, Abbasian A. 2009. Viral etiology of acute respiratory infections among Iranian Hajj pilgrims, 2006. J Travel Med 16:239–242. [DOI] [PubMed] [Google Scholar]
- Alsaleh AN, Whiley DM, Bialasiewicz S, Lambert SB, Ware RS, Nissen MD, Sloots TP, Grimwood K. 2014. Nasal swab samples and real‐time polymerase chain reaction assays in community‐based, longitudinal studies of respiratory viruses: The importance of sample integrity and quality control. BMC Infect Dis 14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzeer AH. 2009. Respiratory tract infection during Hajj. Ann Thorac Med 4:50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer HM, Alshaman MS, Farrag MA, Hamad ME, Alsaadi MM, Almajhdi FN. 2015. Epidemiology of 11 respiratory RNA viruses in a cohort of hospitalized children in Riyadh, Saudi Arabia. J Med Virol 88:1086–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LJ, Hierholzer JC, Bingham PG, Stone YO. 1985. Microneutralization test for respiratory syncytial virus based on an enzyme immunoassay. J Clin Microbiol 22:1050–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkhy HH, Memish ZA, Bafaqeer S, Almuneef MA. 2004. Influenza a common viral infection among Hajj pilgrims: Time for routine surveillance and vaccination. J Travel Med 11:82–86. [DOI] [PubMed] [Google Scholar]
- Bruijnesteijn van Coppenraet LE, Swanink CM, van Zwet AA, Nijhuis RH, Schirm J, Wallinga JA, Ruijs GJ. 2010. Comparison of two commercial molecular assays for simultaneous detection of respiratory viruses in clinical samples using two automatic electrophoresis detection systems. J Virol Methods 169:188–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari EE, Elhazmi MM. 2013. Viral agents causing acute lower respiratory tract infections in hospitalized children at a tertiary care center in Saudi Arabia. S Med J 34:1151–1155. [PubMed] [Google Scholar]
- Casiano‐Colon AE, Hulbert BB, Mayer TK, Walsh EE, Falsey AR. 2003. Lack of sensitivity of rapid antigen tests for the diagnosis of respiratory syncytial virus infection in adults. J Clin Virol 28:169–174. [DOI] [PubMed] [Google Scholar]
- Chu HY, Kuypers J, Renaud C, Wald A, Martin E, Fairchok M, Magaret A, Sarancino M, Englund JA. 2013. Molecular epidemiology of respiratory syncytial virus transmission in childcare. J Clin Virol 57:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Sheikh SM, El‐Assouli SM, Mohammed KA, Albar M. 1998. Bacteria and viruses that cause respiratory tract infections during the pilgrimage (Haj) season in Makkah, Saudi Arabia. Trop Med Int Health 3:205–209. [PubMed] [Google Scholar]
- Fagbo SF, Skakni L, Chu DK, Garbati MA, Joseph M, Peiris M, Hakawi AM. 2015. Molecular epidemiology of hospital outbreak of middle east respiratory syndrome, Riyadh, Saudi Arabia, 2014. Emerg Infect Dis 21:1981–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey AR, Formica MA, Walsh EE. 2002. Diagnosis of respiratory syncytial virus infection: Comparison of reverse transcription‐PCR to viral culture and serology in adults with respiratory illness. J Clin Microbiol 40:817–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazal SS, Al Howasi M, Chowdhury D. 1998. Acute respiratory tract infections: Epidemiological data, guided case management and outcome in a pediatric hospital in Riyadh. Ann Saudi Med 18:75–78. [DOI] [PubMed] [Google Scholar]
- Hall CB, Walsh EE, Long CE, Schnabel KC. 1991. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis 163:693–698. [DOI] [PubMed] [Google Scholar]
- Henrickson KJ. 2004. Advances in the laboratory diagnosis of viral respiratory disease. Pediatr Infect Dis J 23:S6–10. [DOI] [PubMed] [Google Scholar]
- Khamis FA, Al‐Kobaisi MF, Al‐Areimi WS, Al‐Kindi H, Al‐Zakwani I. 2012. Epidemiology of respiratory virus infections among infants and young children admitted to hospital in Oman. J Med Virol 84:1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert SB, Allen KM, Carter RC, Nolan TM. 2008a. The cost of community‐managed viral respiratory illnesses in a cohort of healthy preschool‐aged children. Respir Res 9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert SB, Allen KM, Druce JD, Birch CJ, Mackay IM, Carlin JB, Carapetis JR, Sloots TP, Nissen MD, Nolan TM. 2007. Community epidemiology of human metapneumovirus, human coronavirus NL63, and other respiratory viruses in healthy preschool‐aged children using parent‐collected specimens. Pediatrics 120:e929–e937. [DOI] [PubMed] [Google Scholar]
- Lambert SB, Allen KM, Nolan TM. 2008b. Parent‐collected respiratory specimens—A novel method for respiratory virus and vaccine efficacy research. Vaccine 26:1826–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, Mathers C, Black RE. 2012. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet (London, England) 379:2151–2161. [DOI] [PubMed] [Google Scholar]
- Mandourah Y, Al‐Radi A, Ocheltree AH, Ocheltree SR, Fowler RA. 2012. Clinical and temporal patterns of severe pneumonia causing critical illness during Hajj. BMC Infec Dis 12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health. 2015. Health Statistics Annual Book 2013 G/1434 H. Riyadh. Saudi Arabia: Ministry of Health. [Google Scholar]
- Murphy BR, Alling DW, Snyder MH, Walsh EE, Prince GA, Chanock RM, Hemming VG, Rodriguez WJ, Kim HW, Graham BS, Wright PF. 1986. Effect of age and preexisting antibody on serum antibody response of infants and children to the F and G glycoproteins during respiratory syncytial virus infection. J Clin Microbiol 24:894–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh KH, Kim J, Nam MH, Yoon S, Lee CK, Lee K, Yoo Y, Kim MJ, Cho Y. 2008. Comparison of the Seeplex reverse transcription PCR assay with the R‐mix viral culture and immunofluorescence techniques for detection of eight respiratory viruses. Ann Clin Lab Sci 38:41–46. [PubMed] [Google Scholar]
- UNFCCC. 2011. Second National Communication Kingdom of Saudi Arabia.
- Uyeki TM. 2003. Influenza diagnosis and treatment in children: A review of studies on clinically useful tests and antiviral treatment for influenza. Pediatr Infect Dis J 22:164–177. [DOI] [PubMed] [Google Scholar]
- van der Zalm MM, van Ewijk BE, Wilbrink B, Uiterwaal CS, Wolfs TF, van der Ent CK. 2009. Respiratory pathogens in children with and without respiratory symptoms. J Pediatr 154:396–400, 400 e391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zalm MM, Wilbrink B, van Ewijk BE, Overduin P, Wolfs TF, van der Ent CK. 2011. Highly frequent infections with human rhinovirus in healthy young children: A longitudinal cohort study. J Clin Virol 52:317–320. [DOI] [PubMed] [Google Scholar]
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. 2012. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 367:1814–1820. [DOI] [PubMed] [Google Scholar]
- Zhang G, Hu Y, Wang H, Zhang L, Bao Y, Zhou X. 2012. High incidence of multiple viral infections identified in upper respiratory tract infected children under 3 years of age in Shanghai, China. PLoS ONE 7:e44568. [DOI] [PMC free article] [PubMed] [Google Scholar]


