EXECUTIVE SUMMARY
The WSAVA Vaccination Guidelines Group (VGG) was convened in order to develop guidelines for the vaccination of dogs and cats that have global application. The first version of these guidelines was published in 2007 and they were updated in 2010. The present document provides an updated and expanded version of these international guidelines for the vaccination of small companion animals and indicates the scientific evidence base on which the recommendations are made. The VGG recognises that the keeping of pet small animals is subject to significant variation in practice and associated economics throughout the world and that vaccination recommendations that might apply to a developed country may not be appropriate for a developing country. These guidelines are not a mandatory edict, but rather should be used by national associations and individual veterinary practices to develop vaccination schedules relevant to the local situation. However, the VGG strongly recommends that wherever possible ALL dogs and cats receive the benefit of vaccination. This not only protects the individual animal, but provides optimum ‘herd immunity’ that minimizes the likelihood of infectious disease outbreaks.
With this background in mind, the VGG has defined core vaccines as those which ALL dogs and cats, regardless of circumstances or geographical location, should receive. Core vaccines protect animals from severe, life‐threatening diseases that have global distribution. Core vaccines for dogs are those that protect against canine distemper virus (CDV), canine adenovirus (CAV) and the variants of canine parvovirus type 2 (CPV‐2). Core vaccines for cats are those that protect against feline parvovirus (FPV), feline calicivirus (FCV) and feline herpesvirus‐1 (FHV‐1). In areas of the world where rabies virus infection is endemic, vaccination against this agent should be considered core for both species, even if there is no legal requirement for routine vaccination.
The VGG recognizes that maternally derived antibody (MDA) significantly interferes with the efficacy of most current core vaccines administered to pups and kittens in early life. As the level of MDA varies significantly among litters, the VGG recommends the administration of multiple core vaccine doses to pups and kittens, with the final dose of these being delivered at 16 weeks or older or above and then followed by a booster at 6‐ or 12‐months of age. In cultural or financial situations where a pet animal may only be permitted the benefit of a single vaccination, that vaccination should be with core vaccines at 16 weeks of age or older.
The VGG supports the use of simple in‐practice tests for determination of seroconversion to the core vaccine components (CDV, CAV, CPV‐2 and FPV) following vaccination, for determination of seroprotection in adult dogs and for management of infectious disease outbreaks in shelters.
Vaccines should not be given needlessly. Core vaccines should not be given any more frequently than every three years after the 6‐ or 12‐month booster injection following the puppy/kitten series, because the duration of immunity (DOI) is many years and may be up to the lifetime of the pet.
The VGG has defined non‐core vaccines as those that are required by only those animals whose geographical location, local environment or lifestyle places them at risk of contracting specific infections. The VGG has also classified some vaccines as not recommended (where there is insufficient scientific evidence to justify their use) and has not considered a number of minority products which have restricted geographical availability or application.
The VGG strongly supports the concept of regular (usually annual) health checks which removes the emphasis from, and client expectation of, annual revaccination. The annual health check may still encompass administration of selected non‐core vaccines which should be administered annually, as the DOI for these products is generally 1 year.
The VGG has considered the use of vaccines in the shelter environment, again recognizing the particular circumstances of such establishments and the financial constraints under which they sometimes operate. The VGG minimum shelter guidelines are simple: that all dogs and cats entering such an establishment should be vaccinated before, or at the time of entry, with core vaccines. Where finances permit, repeated core vaccines should be administered as per the schedules defined in the guidelines and non‐core vaccines against respiratory disease may be included.
The VGG recognizes the importance of adverse reaction reporting schemes, but understands that these are variably developed in different countries. Wherever possible, veterinarians should be actively encouraged to report all possible adverse events to the manufacturer and/or regulatory authority to expand the knowledge base that drives development of improved vaccine safety.
These fundamental concepts proposed by the VGG may be encapsulated in the following statement:
We should aim to vaccinate every animal with core vaccines.
Non‐core vaccines should be given no more frequently than is deemed necessary.
INTRODUCTION
The WSAVA Vaccination Guidelines Group (VGG) was convened in 2006 with the aim of producing global vaccination guidelines for dogs and cats that would consider international differences in economic and societal factors that impact on the keeping of these small companion animals. The WSAVA guidelines are therefore intended to be much broader in scope than those produced for North America by the American Academy of Feline Practitioners (Scherk et al. 2013) and the American Animal Hospital Association (Welborn et al. 2011) or for Europe by the Advisory Board on Cat Diseases (Hosie et al. 2013). The first WSAVA guidelines were published in 2007 (Day et al. 2007) and these were updated in 2010 (Day et al. 2010) with an accompanying document written for the owners and breeders of pet dogs and cats. Between 2011 and 2013, the VGG focused on dog and cat infectious disease and vaccinology on the Asian continent and produced regional recommendations on aspects of vaccination for Asian practitioners (Day et al. 2014). In 2014 and 2015, the VGG has worked on updating the global canine and feline vaccination guidelines as now presented in this document.
The format and much of the content of this 2015 revision remain similar to the guidelines published in 2010; however, specific changes in the current document include:
More explicit attention to demonstrating an evidence‐based approach to the WSAVA recommendations, with development of a new classification scheme for evidence related to vaccinology and more complete referencing of pertinent scientific literature.
Changes to recommendations made for the timing of core vaccination of puppies and kittens to take into account new data on persistence of maternally‐derived antibody (MDA) in these animals. Specifically, timing of the final vaccine in the puppy or kitten series has been extended to 16 weeks of age or older.
Changes to the recommendation for a 12‐month booster vaccine for puppies and kittens to provide the option of reducing this interval to 6 months (26 weeks) of age.
Clarification and further discussion of the revaccination intervals for adult cats receiving modified live virus (MLV) vaccines against feline herpesvirus (FHV‐1) and feline calicivirus (FCV).
Inclusion of information concerning newly available vaccines (e.g. oral Bordetella bronchiseptica vaccine for dogs, FCV vaccine containing two strains of virus and multiple‐serogroup Leptospira vaccines).
Reclassification of the feline immunodeficiency virus (FIV) vaccine to non‐core.
Modification of the timing of core vaccinations for puppies and kittens in the shelter setting.
An extended discussion on the use of in‐house serological testing for antibodies specific for core vaccine antigens, including the application of these tests to the management of shelter outbreaks of infectious disease.
Further consideration of the optimum anatomical site for vaccination of cats.
Update of the VGG disease fact sheets and expansion of the list of frequently asked questions.
EVIDENCE‐BASED VETERINARY MEDICINE
The concept of evidence‐based veterinary medicine (EBVM) has become increasingly prominent since the WSAVA vaccination guidelines were first published in 2007. Categories defining the weight of evidence underlying any procedure in veterinary practice (e.g. medical, surgical or diagnostic procedures or the administration of pharmaceuticals) have been defined and applied previously to European recommendations for feline vaccination (Lloret, 2009). The VGG aimed for the current update of the WSAVA global vaccination guidelines to adopt a more explicitly evidence‐based approach, so that practitioners could be made aware of the nature of evidence that underpins the recommendations made. Accordingly, this document is more fully referenced than previous iterations of the guidelines. Additionally, the VGG wished to apply a ranking of supportive evidence, but found that the currently used schemes were poorly applicable to the specialist area of vaccinology. For this reason, the VGG has developed its own EBVM classification, proposing four levels of evidence related to investigations of small companion animal vaccination. These are:
Category 1 evidence: a recommendation supported by peer‐reviewed scientific publication of either experimental or field data. Evidence within this category might still be of variable scientific quality despite peer review, as the peer review process does not conform to a universal standard.
Category 2 evidence: a recommendation supported by unpublished commercially sensitive studies submitted as part of a regulatory package for licensed veterinary vaccines. The assumption for this level of evidence is that information appearing on the datasheets of licensed products has been through competent peer review by regulatory authorities.
Category 3 evidence: a recommendation supported by commercial or independent experimental or field data that have not been published in the peer reviewed scientific literature or were not included in a formal regulatory package and subjected to scrutiny by regulators.
Category 4 evidence: a recommendation unsupported by experimental or field data, but assumed from knowledge of the ‘first principles’ of microbiology and immunology or supported by widely‐held expert opinion.
Throughout this document, statements may be followed by a qualifier [EB1], [EB2], [EB3] or [EB4] reflecting an ‘evidence base’ of category 1, 2, 3 or 4, respectively. For each occasion of use only the most rigorous level of evidence available will be given.
THE PURPOSE OF GUIDELINES
These WSAVA vaccination guidelines do NOT serve as a set of globally‐applicable rules for the administration of vaccines to dogs and cats. It is simply not possible to produce a set of guidelines that applies equally to each of the 80 WSAVA member nations as there are vast differences between countries and geographical regions with respect to infectious disease presence/absence or prevalence, vaccine product availability, owned versus free‐roaming dog and cat populations, practice and client economics and societal attitudes.
Instead, these guidelines are intended to provide national small animal veterinary associations and WSAVA members with current scientific advice and best practice vaccination concepts. It is up to national associations or individual practices to read, discuss and adapt these guidelines for their own particular practice situations. These guidelines are not proscriptive; for example, it is entirely possible that what might be considered a non‐core vaccine in many countries, or particular geographical regions, might be used as a core vaccine elsewhere.
Practitioners are sometimes alarmed that guidelines recommendations appear contrary to those given on the product datasheet (or ‘summary of product characteristics’ [SPC] in Europe), and therefore feel that if they adopt guidelines recommendations, they are leaving themselves open to litigation. The distinct difference between a datasheet and a guidelines document has been clearly discussed by Thiry and Horzinek (2007).
The data sheet or SPC is a document that forms part of the registration process for a specific vaccine. A datasheet will give details of the quality, safety and efficacy of a product and in the case of vaccines will describe the minimum duration of immunity (DOI) of the product. The DOI is based on experimental evidence (i.e. how long after vaccination is an animal protected from infection or disease as determined by challenge with virulent infectious agent), represents a minimum value and need not reflect the true DOI of a vaccine. Most companion animal core vaccines, until relatively recently, had a 1‐year minimum DOI and carried a recommendation for annual revaccination. In more recent years many of the same products have been licensed with a minimum DOI of 3 (or sometimes 4) years. In fact, in many countries the majority of core MLV vaccines are now licensed for triennial revaccination of adult animals. However, there are many other countries in which the identical products still carry a 1‐year minimum DOI; simply because the manufacturer has not applied for a change in its product label recommendations or because the national licensing authority has not permitted the change to be made. This unfortunate situation does lead to confusion amongst practitioners in those countries. Above all, it must be remembered that even a 3‐year license is a minimum DOI for core vaccines and for most core vaccines the true DOI is likely to be considerably longer, if not lifelong, for the majority of vaccine recipients.
Therefore, there will remain instances where the guidelines may recommend triennial or less frequent revaccination, but all available products in a particular country still carry a 1‐year licensed DOI. In this instance, the veterinarian may use a vaccine according to guidelines (and therefore current scientific thinking) by obtaining informed (and documented) owner consent for this deviation from manufacturer's recommendations (‘off‐label use’). Veterinarians should also be aware that company technical representatives will continue to advise that the veterinarian must adhere to the recommendations given in their datasheets, as they are obliged to do since these documents have been through the licensing procedure.
Further confusion may arise when veterinarians compare the recommendations given in different sets of guidelines. There are, for example, subtle differences in recommendations made in different countries that reflect differences in the opinions of local expert groups, the prevalence of particular infectious diseases and in the typical lifestyles of pet animals that may make them more or less exposed to infections. The VGG faces the difficult challenge of setting a middle‐course through various national or regional guidelines. Its recommendations attempt to provide a balanced perspective to account for global differences in the keeping of small companion animals.
In summary, veterinarians should feel comfortable about vaccinating according to the schedules given in these guidelines, but should cross‐reference these with local recommendations where available. Where the VGG recommendations differ from current product label recommendations the practitioner needs to be sure to obtain informed client consent in order to use the vaccine in accordance with the VGG recommendations.
CURRENT ISSUES IN SMALL ANIMAL VACCINOLOGY
If vaccination has been so successful, then why is it necessary to continually re‐evaluate vaccination practice? There is little doubt that, in most developed countries, some of the major infectious diseases of dogs and cats are considered at most uncommon in the pet population. However, even in those countries there remain geographical pockets of infection and sporadic outbreaks of disease may occur, and the situation regarding free‐roaming or shelter populations is distinctly different from that in owned pet animals. In many developing countries these key infectious diseases remain as common as they once were in developed nations and a major cause of mortality in small animals. Although it is difficult to obtain accurate figures, even in developed countries it is estimated that only 30–50% of the pet animal population is vaccinated, and this is significantly less in developing nations. The global economic recession post‐2008 has had further impact on the uptake of preventive healthcare by pet owners in developed countries and survey data suggests a recent decline in vaccination (Anon 2013a).
In small animal medicine, we have been slow to grasp the concept of ‘herd immunity’ – that vaccination of individual pet animals is important, not only to protect the individual, but to reduce the number of susceptible animals in the regional population, and thus the prevalence of disease. Herd immunity related to use of core vaccines that provide a long (many years) DOI is highly dependent on the percentage of animals in the population vaccinated and not the number of vaccinations that occur annually. Therefore, every effort should be made to vaccinate a higher percentage of cats and dogs with the core vaccines. It is simply not possible to induce ‘better’ immunity in an individual animal by giving repeated vaccinations, i.e. a dog receiving a core MLV vaccine every 3 years will be equally well protected compared with one receiving the same vaccine annually (Bohm et al. 2004, Mouzin et al. 2004, Mitchell et al. 2012) [EB1], but this may not necessarily be the case for feline core vaccines (see below).
In recent years the re‐emerging concept of ‘One Health’ has also impacted on the field of vaccinology. The management of infectious diseases through the collaborative interaction of human medical, animal and environmental healthcare professionals provides a rational and cost‐effective goal at a time when the majority of newly emergent human infectious diseases is proposed to derive from wild or domestic animal sources (Gibbs 2014). The WSAVA has embraced the One Health concept with establishment of a One Health Committee in 2010 (Day 2010), the work of which overlaps with that of the VGG when tackling the major small companion animal zoonoses of canine rabies and leishmaniosis.
A second major concept regarding vaccination of dogs and cats has been the recognition that we should aim to reduce the ‘vaccine load’ on individual animals in order to minimize the potential for adverse reactions to vaccine products and reduce the time and financial burden on clients and veterinarians of unjustified veterinary medical procedures. For these reasons we have seen the development of vaccination guidelines based on a rational analysis of the vaccine requirements for each pet, and the proposal that vaccines be considered ‘core’ and ‘non‐core’ in nature. To an extent this categorization of products has been based on available scientific evidence and personal experience – but concerted effort to introduce effective companion animal disease surveillance on a global scale would provide a more definitive basis on which to recommend vaccine usage (Day et al. 2012). In parallel with the categorization of vaccines has been the push towards marketing products with extended DOI, to reduce the unnecessary administration of vaccines and thereby further improve vaccine safety. Both of these changes have necessitated a frame‐shift in the mind‐set of veterinary practitioners, which is now becoming the accepted norm in many countries.
The following VGG guidelines are prepared when considering the optimum model of committed pet owners, willing and able to bring their animals to the veterinarian, for the full recommended course of vaccination. The VGG is aware that there are less committed or able pet owners in every country and there are countries where severe financial or societal constraints often determine the nature of the vaccine course that can be administered. In situations where, for example, a decision must be made that an individual pet may have to receive only a single core vaccination during its lifetime, the VGG would emphasise that this should optimally be given at a time when that animal is most capable of responding immunologically, i.e. at greater than 16 weeks of age.
The VGG has additionally considered vaccination in the shelter situation. The guidelines that we have proposed are those that we consider provide the optimum level of protection for these highly susceptible animals. The VGG also recognises that many shelters run with limited financial support which may constrain the extent of vaccination used. The minimum vaccination protocol in this situation would be a single administration of core vaccines at or before the time of admission to the shelter.
This document seeks to address these current issues in canine and feline vaccinology, and to suggest practical measures by which the veterinary profession may move further towards more rational use of vaccines in these species. The most important message of the VGG is therefore encapsulated in the following statement:
We should aim to vaccinate every animal with core vaccines.
Non‐core vaccines should be given no more frequently than is deemed necessary.
TYPES OF VACCINE
Before discussing specific vaccination guidelines, a brief review of the types of small companion animal vaccine available is justified. Vaccines may be considered simply as either ‘infectious’ or ‘non‐infectious’ in nature.
Most infectious vaccines used in dogs and cats contain organisms that are attenuated to reduce virulence (i.e. ‘modified live virus’ [MLV] or attenuated vaccines), but the organisms are intact and viable and induce immunity by inducing low‐level infection and replicating within the animal, without producing significant tissue pathology or clinical signs of infectious disease. Infectious vaccines have the advantage of more effectively inducing immunity at relevant anatomical sites when administered parenterally and are more likely to induce robust cell‐mediated and humoral (antibody‐mediated) immunity. Some infectious vaccines are administered directly to mucosal sites (i.e. intranasal or oral vaccines) where they are even more effective at inducing relevant protective mucosal immunity. Some recombinant vectored vaccines (i.e. a live vector organism carrying genetic material encoding an antigen from the target pathogen) may also be considered ‘infectious’; however, the vector organism is not relevant to, or pathogenic in, the dog or cat. When administered to an animal that lacks maternally‐derived antibody (MDA) an infectious vaccine will generally induce protection with a single dose.
Non‐infectious vaccines (also known as killed or inactivated vaccines and including subunit and naked DNA vaccines) contain an inactivated but antigenically intact virus or organism, or a natural or synthetic antigen derived from that virus or organism, or the DNA that can encode such an antigen. Non‐infectious agents are unable to infect, replicate or induce pathology or clinical signs of infectious disease. They generally require an adjuvant to increase their potency and usually require multiple doses (even in an adult animal) to induce protection. Non‐infectious vaccines are administered parenterally and may be less likely to induce both cell‐mediated and humoral immunity and generally have a shorter DOI compared with infectious vaccines.
CANINE VACCINATION GUIDELINES
VACCINATION OF INDIVIDUAL DOGS
The Basic Immunization Schedule
Guidelines and recommendations for core (recommended), non‐core (optional) and not recommended vaccines for the general veterinary practice are given in Table 1. The VGG considers that a core vaccine is one that all dogs throughout the world must receive, at recommended intervals, in order to provide life‐long protection against infectious diseases of global significance. The core vaccines for the dog are those that confer protection against infection by canine distemper virus (CDV), canine adenovirus (CAV; types 1 and 2) and canine parvovirus type 2 (CPV‐2) and its variants. The VGG recognizes that particular countries will identify additional vaccines that they consider core. A particular example of a vaccine that may be considered core in only some countries is that against rabies virus. In a geographical area in which this infection is endemic, all dogs should be vaccinated routinely for the protection of both the pet and human populations. The VGG strongly endorses the joint statement of the WSAVA One Health Committee and the International Organisation for Animal Health (OIE) which sets a target for global elimination of canine rabies by 2030 (Anon 2013b). In many countries, rabies vaccination is a legal requirement, and is generally also required for international pet travel.
Table 1.
WSAVA Canine Vaccination Guidelines
| Vaccine | Initial Puppy Vaccination | Initial Adult Vaccination | Revaccination Recommendation | Comments and Recommendations |
|---|---|---|---|---|
|
Canine Parvovirus‐2 (CPV‐2; MLV, parenteral) Canine Distemper Virus (CDV; MLV, parenteral) Recombinant Canine Distemper Virus (rCDV, parenteral) Canine Adenovirus‐2 (CAV‐2; MLV, parenteral) |
Administer at 6–8 weeks of age, then every 2–4 weeks until 16 weeks of age or older [EB1]. | Two doses 2–4 weeks apart are generally recommended by manufacturers, but one dose of MLV vaccine or rCDV is considered protective [EB4]. | Revaccination (booster) at either 6 months or 1 year of age, then not more often than every 3 years. | Core |
|
CPV‐2 (killed, parenteral) |
Not recommended where MLV available. | |||
| Canine Adenovirus‐1 (CAV‐1; MLV and killed parenteral) | Not Recommended where CAV‐2 MLV available. | |||
| Rabies (killed parenteral) |
Administer one dose at 12 weeks of age. If vaccination is performed earlier than 12 weeks of age, the puppy should be revaccinated at 12 weeks of age. In high risk areas a second dose may be given 2–4 weeks after the first. |
Administer a single dose. | Revaccination (booster) at 1 year of age. Canine rabies vaccines with either a 1‐ or 3‐year DOI are available. Timing of boosters is determined by this licensed DOI, but in some areas may be dictated by statute. | Core where required by statue or in areas where the disease is endemic. |
|
Parainfluenza Virus (CPiV; MLV, parenteral) |
Administer at 6–8 weeks of age, then every 2–4 weeks until 16 weeks of age or older [EB4]. | Two doses 2–4 weeks apart are generally recommended by manufacturers, but one dose is considered protective [EB4]. | Revaccination (booster) at either 6 months or 1 year of age, then annually. | Non‐core. Use of CPiV (MLV‐intranasal) is preferred to the parenteral product as the primary site of infection is the upper respiratory tract. |
|
Bordetella bronchiseptica (live avirulent bacteria, intranasal) B. bronchiseptica + CPiV (MLV) intranasal B. bronchiseptica + CPiV (MLV) + CAV‐2 (MLV) intranasal |
Administer a single dose as early as 3 weeks of age. |
A single dose. | Annually or more often in very high‐risk animals not protected by annual booster. | Non‐core. B. bronchiseptica is available as a single product or in combination with CPiV or with both CPiV and CAV2. Transient (3–10 days) coughing, sneezing, or nasal discharge may occur in a small percentage of vaccinates. Intranasal or oral vaccines MUST NOT be delivered by parenteral injection as this may lead to severe adverse reactions, including death. |
| B. bronchiseptica (live avirulent bacteria, oral) | The current manufacturer's recommendation is for use of this vaccine from 8 weeks of age. | |||
|
Bordetella bronchiseptica (killed bacterin, parenteral Bordetella bronchiseptica (cell wall antigen extract, parenteral) |
Administer one dose at 6–8 weeks and one dose at 10–12 weeks of age. | Two doses 2–4 weeks apart. | Annually or more often in very high‐risk animals not protected by annual booster. | Non‐core. Intranasal or oral products are preferred to the killed parenteral to provide local protection [EB4]; however, a review published at the time of compilation questions this advantage (Ellis 2015). |
|
Borrelia burgdorferi (Lyme borreliosis; killed whole bacterin, parenteral) Borrelia burgdorferi (rLyme borreliosis) (recombinant‐Outer surface protein A [OspA], parenteral) |
Recommendation is for initial dose at 12 weeks of age or older. A second dose is given 2–4 weeks later. Borrelia vaccines may be given as early as 9 weeks of age if there is a high risk of exposure. For some vaccines, this will constitute off‐label use. | Two doses, 2–4 weeks apart. | Annually. Revaccinate just prior to start of tick season as determined regionally. | Non‐core. Generally recommended only for use in dogs with a known high risk of exposure, living in or visiting regions where the risk of vector tick exposure is considered to be high, or where disease is known to be endemic. |
|
Leptospira interrogans (with serogroups canicola and icterohaemorrhagiae; killed bacterin, parenteral) Also available in the USA and some other countries with serogroups grippotyphosa and pomona, in Europe with serogroups grippotyphosa and australis, and in Europe with serogroup grippotyphosa. In Australia there is a monovalent vaccine containing serogroup australis and in New Zealand monovalent serogroup icterohaemorrhagiae vaccines are available. |
Initial dose at 8 weeks of age or older. A second dose is given 2–4 weeks later. | Two doses 2–4 weeks apart. | Annually. |
Non‐core. Leptospira vaccines have been developed to account for the known circulating pathogenic serogroups in different geographical areas. Note that Leptospira serogroups may include multiple serovars. There is often confusion with the use of the terms ‘serogroup’ and ‘serovar’. Vaccination should be restricted to use in geographical areas where a risk of exposure has been established or for dogs whose lifestyle places them at risk. This vaccine is known to provide protection that is less robust and may be of shorter duration, and therefore these products must be administered annually [EB1]. In the past, Leptospira bacterin vaccines have been suggested to be linked to a higher prevalence of allergic adverse events – particularly in small breed dogs. The evidence base for this is low [EB4] and one published study indicates no greater risk from Leptospira bacterins (Moore et al. 2005) [EB1]. The European Consensus Statement on Leptospirosis (Schuller et al. 2015) also takes this view. |
| Canine influenza virus (CIV; H3N8; killed adjuvanted, parenteral) | Two doses 2–4 weeks apart with initial dose at >6 weeks of age. | Two doses 2–4 weeks apart | Annually | Non‐core. Licensed only in USA. Consider for at‐risk groups of co‐housed dogs such as those in kennels, dog shows or day care [EB1]. |
| Canine Coronavirus (CCV; killed and MLV, parenteral) | Not Recommended. CCV infections are usually subclinical or cause mild clinical signs. Prevalence of confirmed CCV disease does not justify use of currently‐available vaccines. There is no evidence that existing vaccines would protect against pathogenic variants of CCV (Buonavoglia et al. 2009, Decaro et al. 2009) [EB1]. Although CCV can be isolated commonly, the VGG remains unconvinced that CCV is a significant primary enteric pathogen in the adult dog. No studies have satisfied Koch's postulates for this infectious agent. | |||
Where the recommendations in this table are not consistent with those on datasheets [EB2] the level of evidence supporting the recommendation is given.
The VGG did not consider the following products that have restricted geographical availability:
Crotalus atrox (western rattlesnake vaccine) and Crotalux adamanteus (eastern rattlesnake vaccine) – Conditional USDA License
Babesia vaccine (soluble parasite antigen from B. canis in saponin) – EU Licensed
Canine herpesvirus vaccine – EU Licensed
Leishmania vaccines – licensed in Brazil and the EU
Non‐core vaccines are those for which use is determined on the basis of the geographical and lifestyle exposure risks of the individual and an assessment of risk–benefit ratios (i.e. the risk of being unvaccinated and susceptible or the risk of being vaccinated and developing an adverse reaction versus the benefit of being protected against the infection in question). Not recommended vaccines are those for which there is little scientific justification (insufficient evidence base) for their use.
Puppy Vaccination and the 6‐ or 12‐Month Booster
Most puppies are protected by MDA in the first weeks of life. In most puppies, passive immunity will have waned by 8–12 weeks of age to a level that allows active immunization. Puppies with poor MDA may be vulnerable (and capable of responding to vaccination) at an earlier age, while others may possess MDA at such high titres that they are incapable of responding to vaccination until ≥12 weeks of age (Friedrich & Truyen 2000) [EB1]. No single primary vaccination policy will therefore cover all possible situations. The recommendation of the VGG is for initial core vaccination at 6–8 weeks of age, then every 2–4 weeks until 16 weeks of age or older. Therefore the number of puppy primary core vaccinations will be determined by the age at which vaccination is started and the selected interval between vaccinations. Possible schedules are outlined in Table 5. By this recommendation, when vaccination is started at 6 or 7 weeks of age, a course of four primary core vaccines would be administered with a 4‐week interval, but only three would be required with an 8‐ or 9‐week start and a similar 4‐week interval.
Table 5.
Core Vaccination Schedules for Puppies and Kittens First Presented Between 6–9 Weeks of Age and Revaccinated Every 3 or 4 Weeks
| Age at first presentation | Core vaccination schedule |
|---|---|
| 6 weeks |
6 weeks, 9 weeks, 12 weeks, 16 weeks then 26 or 52 weeks or 6 weeks, 10 weeks, 14 weeks, 18 weeks then 26 or 52 weeks |
| 7 weeks |
7 weeks, 10 weeks, 13 weeks, 16 weeks then 26 or 52 weeks or 7 weeks, 11 weeks, 15 weeks, 19 weeks then 26 or 52 weeks |
| 8 weeks |
8 weeks, 11 weeks, 14 weeks, 17 weeks then 26 or 52 weeks or 8 weeks, 12 weeks, 16 weeks then 26 or 52 weeks |
| 9 weeks |
9 weeks, 12 weeks, 15 weeks, 18 weeks then 26 or 52 weeks or 9 weeks, 13 weeks, 17 weeks then 26 or 52 weeks |
This table provides examples of possible vaccination schedules for puppies and kittens where vaccines are given either every 3 or 4 weeks, as would normally be done in veterinary practice for owned pet animals. Although revaccination every 2 weeks might be used in areas of high infectious disease pressure in some geographical areas, such a protocol is not shown for simplicity of presentation.
After the 26 or 52 week booster vaccine; vaccinate with core products no more frequently than every 3 years (with the exception of feline respiratory virus vaccines for higher risk cats).
In contrast, many vaccine datasheets continue to recommend an initial course of two injections of core vaccine. Some products are also licensed with a ‘10 week finish’ designed such that the second of two core vaccinations is given at 10 weeks of age. The rationale behind this protocol is to permit ‘early socialization’ of puppies while diminishing the risk of infectious diseases. The VGG recognizes that early socialization is essential to the behavioural development of dogs (Korbelik et al. 2011, AVSAB 2008) [EB1]. Where such protocols (i.e. ‘puppy classes’) are adopted, vigilance should still be maintained by the owner – allowing restricted exposure of their puppy to controlled areas and only to other puppies and adults that appear healthy and are fully vaccinated. In particular ‘puppy classes’ should be held in venues away from the veterinary practice. Alternatively, if it is decided that veterinary premises must be used, the floors should be cleaned and disinfected before each class and the classes held in an area not highly trafficked by dogs of unknown vaccination or disease status. A recent US study has shown the minimal risk for CPV‐2 amongst vaccinated puppies attending socialization classes (Stepita et al. 2013). The VGG recommends that whenever possible the last of the puppy primary series of core vaccines be given at 16 weeks of age or older [EB1].
An integral part of core vaccination of puppies is the ‘booster’ vaccine that has traditionally been given either at 12 months of age or 12 months after the last of the primary series of puppy vaccines. The main aim of this vaccine is to ensure that a protective immune response develops in any dog that may have failed to respond to any of the vaccines in the primary core series, rather than necessarily ‘boosting’ the immune response. The delivery of this vaccine at 12 months of age is likely to have been chosen historically as a convenient time to request the owner to attend the practice for a first annual health check. This therefore implies that should an individual puppy fail to respond to any of the primary core vaccinations, that puppy may be unprotected until it receives this 12‐month vaccine. This might account for occurrences of infectious disease (e.g. canine parvoviral enteritis) in a proportion of vaccinated puppies at less than 12 months of age. The VGG has re‐evaluated this practice and now suggests that veterinarians might wish to reduce this possible window of susceptibility by bringing forward this vaccine from 52 weeks to 26 weeks of age (or indeed at any time point between 26 and 52 weeks of age; however, 26 weeks of age provides a convenient timing). This practice will require that pet owners clearly understand why this is recommended, because as indicated in Table 5, adopting such a protocol will mean that vaccination started in a 6 or 7 week old puppy, might now entail up to five vaccine visits in the first 6 months of life. For core vaccines, after a 26 week ‘booster’, another core vaccine would not be required for at least another 3 years. This new recommendation for vaccination at 6 months of age as an alternative to vaccination at about 1 year of age is certainly not mutually exclusive to, and does not preclude, a 1‐year or 16‐month ‘first annual health check’. Many veterinarians are understandably keen to check the animals under their care at around the time they reach skeletal maturity.
Revaccination of Adult Dogs
Dogs that have responded to vaccination with MLV core vaccines maintain a solid immunity (immunological memory) for many years in the absence of any repeat vaccination (Bohm et al. 2004, Mouzin et al. 2004, Schultz 2006, Mitchell et al. 2012) [EB1]. Following the 26 or 52 week booster, subsequent revaccinations are given at intervals of 3 years or longer. It should be emphasized that triennial adult revaccination does not generally apply to killed core vaccines (except for rabies) nor to the non‐core vaccines, and particularly not to vaccines containing bacterial antigens. Thus Leptospira, Bordetella and Borrelia (Lyme disease) products, but also parainfluenza virus components, require more frequent boosters for reliable protection (Ellis & Krakowka 2012, Klaasen et al. 2014, Ellis 2015, Schuller et al. 2015) [EB1].
Therefore an adult dog may, according to these guidelines, still be revaccinated annually, but the components of these vaccinations may differ each year. Typically, core vaccines are currently administered triennially, with chosen non‐core products being given annually. The VGG is aware that in some countries only multi‐component products containing core and non‐core combinations are available. The VGG would encourage manufacturers to make a full range of reduced‐component vaccines (or at least separate core and non‐core vaccines (Mitchell et al. 2012) available wherever possible.
An adult dog that had received a complete course of core vaccinations as a puppy, including a 26 or 52 week booster, but that may not have been vaccinated regularly as an adult, requires only a single dose of MLV core vaccine to boost immunity (Mouzin et al. 2004, Mitchell et al. 2012) [EB1]. Similarly, an adopted adult dog (or puppy over 16 weeks of age) of unknown vaccination history requires only a single dose of MLV core vaccine to engender a protective immune response. Many vaccine datasheets will advise in these circumstances that the dog requires two vaccinations (as for a puppy), but this practice is unjustified and contrary to fundamental immunological principles [EB4]. Note again, that this does not apply to non‐core vaccines, many of which will require two doses in an adult dog.
Particular mention should be made of canine rabies vaccines. The VGG recommends that in any country in which canine rabies is endemic, vaccination of dogs should be strongly recommended to clients by veterinarians, even if not required by law. Revaccination intervals for canine rabies are often mandated by law. Internationally available killed rabies vaccines were initially produced with a licensed 1‐year DOI and so statutes required annual revaccination. These same products now carry a 3‐year DOI in many countries, where laws have been modified to incorporate this change. However, in some countries the legal requirement is at odds with the vaccine license and in others neither the vaccine license, nor the law, has been changed. Finally, some countries also have locally‐manufactured rabies vaccines with a 1‐year DOI that most likely cannot safely be extended to 3 years. Veterinarians should be mindful of the law, but where they have access to a product that confers a minimum of 3‐years immunity, national associations might lobby to have the laws changed to match the current scientific evidence.
Serological Testing to Monitor Immunity to Canine Vaccines
Since publication of the 2010 guidelines there have been advances in the availability of rapid and simple in‐practice serological test kits that can detect the presence of protective antibody specific for CDV, CAV and CPV‐2 in individual dogs. These test kits complement the traditional laboratory‐based modalities (i.e. virus neutralization and haemagglutination inhibition test) that remain the ‘gold standards’ for serological testing. Two commercially produced test kits are available and have been applied and validated in the practice and shelter setting (Gray et al. 2012, Litster et al. 2012) [EB1]. These test kits have proven popular with veterinarians who wish to be able to offer their clients an alternative to routine core revaccination at 3‐yearly intervals, but the kits remain relatively expensive and unfortunately, at present, testing costs more than a dose of vaccine.
A negative test result indicates that the dog has little or no antibody, and that revaccination is recommended. Some seronegative dogs are in fact immune (false‐negative) and their revaccination would be unnecessary because they would make a rapid and substantial anamnestic response to vaccination (Mouzin et al. 2004). However, such dogs cannot be detected readily and an animal with a negative result, regardless of the test used, should be considered as having no antibody and potentially susceptible to infection. In contrast, a positive test result would lead to the conclusion that revaccination is not required.
Monitoring serum antibody specific for canine rabies is not generally used in the same manner for determining revaccination requirements as these are mandated by law. Laboratory testing for a protective rabies antibody titre (considered as more than 0 · 5 IU/ml) is required for international pet travel. Rabies serology is only performed by recognized reference laboratories.
Serological testing for CDV, CAV and CPV‐2 has application for determining protective immunity in the puppy, for informing revaccination intervals in adult dogs and in management of infectious disease outbreaks in shelters.
A dedicated owner may wish to confirm that a puppy is protected after the course of primary vaccinations when these are completed at 16 weeks or older (Figure 1). A serum sample taken at least 4 weeks after the final vaccination may be tested. This interval will ensure that MDA is no longer present and that even ‘slow responder’ puppies have seroconverted. A seropositive puppy would not require a 26 or 52 week booster and could next receive core vaccine 3 years later. Seronegative puppies should be revaccinated and retested. If the pup again tests negative, it should be considered a non‐responder that is possibly incapable of developing protective immunity.
Figure 1.
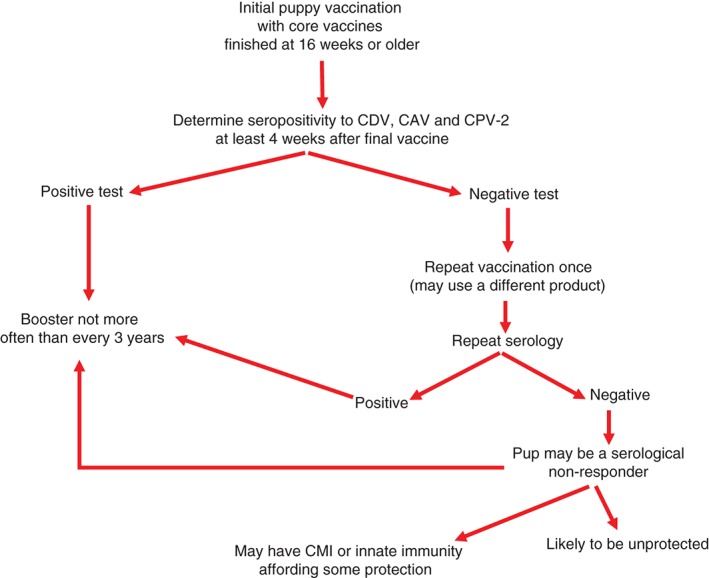
Flow chart for serological testing of puppies
Testing for antibody is presently the only practical way to ensure that a puppy's immune system has recognized the vaccinal antigen. Vaccines may fail to induce protective immunity in a puppy for various reasons:
(1) MDA neutralizes the vaccine virus
This is the most common reason for vaccination failure. However, when the last vaccine dose is given 16 weeks of age or older, MDA should have decreased to a low level (Friedrich & Truyen 2000) [EB1], and active immunization will succeed in most puppies.
(2) The vaccine is poorly immunogenic
Poor immunogenicity may reflect a range of factors from the stage of vaccine design and manufacture to administration to the animal. For example, the virus strain, its passage history or production errors in the manufacture of a particular batch of product may be a cause of vaccine failure. In reality, such effects rarely affect vaccines produced by large, well‐established manufacturers that market their vaccines internationally. These manufacturers have strict requirements from government regulatory agencies for batch potency testing before release. Post‐manufacture factors such as incorrect storage or transportation (interrupted cold chain) and handling (disinfectant use) of the vaccine in the veterinary practice, may result in inactivation of an MLV product. The VGG has recognized that such ‘vaccine husbandry’ remains an issue in many countries and has included some simple guidelines in Table 6.
Table 6.
Vaccine Husbandry: Key Points for Veterinary Practitioners
|
[From Day & Schultz, 2014].
(3) The animal is a poor responder (its immune system intrinsically fails to recognize the vaccinal antigens)
If an animal fails to develop an antibody response after repeated revaccination, it should be considered a genetic non‐responder. Because immunological non‐responsiveness is genetically controlled in other species, certain breeds of dogs have been suspected to be poor‐responders. It is believed (but unproven) that the high susceptibility to CPV‐2 recognized in certain Rottweilers and Dobermanns during the 1980s (regardless of their vaccination history) relates in part to a high prevalence of non‐responders (Houston et al. 1994) [EB4]. In the USA today, these two breeds seem to have no greater numbers of non‐responders to CPV‐2 than other breeds, possibly because carriers of the genetic trait may have died from CPV‐2 infection. Some dogs of these breeds may be low or non‐responders to other antigens. For example, in the UK and Germany, the non‐responder phenotype remains prevalent amongst Rottweilers [EB3] for CPV‐2 and recent studies have shown this breed to have a higher proportion of animals failing to achieve the titre of rabies antibody required for pet travel (Kennedy et al. 2007) [EB1]. Some broad estimates have been made of the proportion of genetic non‐responders in the canine population, these being: 1 in every 5,000 dogs for CDV, 1 in every 100,000 dogs for CAV and 1 in every 1,000 dogs for CPV‐2 [EB4].
Serological Testing to Determine the Duration of Immunity (DOI)
Antibody tests can be used to demonstrate the DOI after vaccination with core vaccines. It is known that a large majority of dogs maintain protective antibody against CDV, CPV‐2, CAV‐1 and CAV‐2 for many years and numerous experimental studies support this observation (Bohm et al. 2004, Mouzin et al. 2004, Schultz 2006, Mitchell et al. 2012) [EB1]. Therefore, when antibody is absent (irrespective of the serological test used) the dog should be revaccinated unless there is a medical reason for not so doing, even though some will be protected by immunological memory.
Antibody determinations to other vaccine components are of limited or no value because of the short time period these antibodies persist (e.g. Leptospira products) or the lack of correlation between serum antibody and protection (e.g. Leptospira and canine parainfluenza) (Hartman et al. 1984, Klaasen et al. 2003, Ellis & Krakowka 2012, Martin et al. 2014) [EB1].
The VGG recognizes that at present such serological testing might be relatively expensive. However, the principles of ‘evidence‐based veterinary medicine’ suggest that testing for antibody status (for either puppies or adult dogs) should be better practice than simply administering a vaccine booster on the basis that this would be ‘safe and cost less’.
Passive Immunization
While vaccination (i.e. active immunization) dominates infectious disease prevention, passive immunization continues to be used in the treatment of infectious disease in many countries.
Although virus infections trigger both cellular and humoral immunity, it is mainly the antibody response that contributes to the reduction of viral load and recovery. In many virus infections, antibody levels are therefore taken as correlates of protection. During viraemia, pre‐existing or injected antibodies directed against surface structures of virions bind to the particles, neutralize their infectivity and prepare them for removal. Therapeutically, most serum or immunoglobulin preparations used in passive immunization are injected subcutaneously (because they are from a different animal species) and quickly reach the circulation. Not unexpectedly, intravenous infusions of plasma or serum (from the same species) have been found to work as well. In local infections, such as those initiated by the bite of a rabid carnivore, post‐exposure antibody prophylaxis has also proven invaluable in human medicine. Human rabies immune globulin provides rapid protection when given on the first day of the post‐exposure prophylaxis regimen. As much as possible of the preparation is infiltrated into and around the wound, and may be given intramuscularly at a site distant from the rabies vaccine, which is applied simultaneously.
In companion animal practice, preventive active immunization is so commonplace that serum prophylaxis/therapy is considered only under exceptional circumstances (e.g. when a dog is presented with distemper or a cat is presented with panleukopenia, or during a disease outbreak in a kennel/cattery). There is still a market for serum and immunoglobulin products, and companies producing them exist in the USA, Germany, the Czech Republic, Slovakia, Russia and Brazil. The preparations are either of homologous or heterologous (e.g. horse) origin, are polyvalent (directed against several viruses) and consist of sera or their immunoglobulin fraction.
Despite the availability of such products, the VGG recommends that they be used conservatively, and only after careful consideration. In the case of an outbreak of CDV infection in a kennel, it is much safer and more effective to vaccinate all dogs with CDV vaccine rather than give immune serum (see below and Table 7) (Larson & Schultz 2006) [EB1]. In such a situation it has previously been recommended that MLV vaccines be administered intravenously (off‐label) rather than subcutaneously or intramuscularly, but there is little evidence that this practice provides more effective or rapid protection than subcutaneous or intramuscular injection. Administration of CDV vaccines by any of those routes will provide protection from severe disease and death immediately or very shortly after vaccination. In this instance the vaccine does not prevent infection, but instead it protects from severe disease (especially from neurological disease) so the animal will survive and will subsequently be immune for life.
Table 7.
Use of Serological Testing in a Shelter Infectious Disease Outbreak
| Situation | Serological Status | Recommendation for Animals |
|---|---|---|
| Disease outbreak within a shelter: all animals within the shelter should be tested serologically (i.e. for CDV, CPV2 and FPV outbreaks) | Seropositive animals |
These are protected and will not become infected or die. These should be separated from the non‐ or low‐responder animals. |
| Seronegative animals |
These should be separated from the seropositive animals. These animals are susceptible and should not be adopted out of the shelter until after the incubation period for the infection (i.e. at least 2 weeks for CPV, at least 6 weeks for CDV). These animals should be vaccinated and retested to confirm seropositivity after the incubation periods above. |
|
| Animals outside of a shelter needing to be admitted in the face of a disease outbreak in the shelter | Seropositive animals | These may safely enter the shelter as they are protected from disease. |
| Seronegative animals | These animals should be vaccinated and sent to foster homes until after they have seroconverted. They should not be allowed to enter the shelter until they are seropositive. |
In the case of a cattery outbreak of FPV infection, or a kennel outbreak of CPV‐2 infection, a recent study has shown that if immune plasma is given after clinical signs appear, there is no benefit in reduction of morbidity or mortality (Bragg et al. 2012) [EB1]. However, this work has been criticised because only a small volume (12 ml) of immune plasma was given to each puppy in this study. Much larger volumes (6 · 6–11 ml/kg) are routinely used by researchers and practitioners and these large doses are believed by some experienced clinicians and investigators to have efficacy (Dodds 2012) [EB4]. In order to have a maximal beneficial effect, immune serum or plasma must be given after infection, but prior to the onset of clinical signs. In this case administration of immune serum or plasma is best provided within 24–48 hours after infection and a large amount of high titred serum or plasma is required. The serum or plasma must be given parenterally (e.g. subcutaneously, intravenously or intraperitoneally) and not orally. There is no benefit from oral administration even when treatment is started prior to infection.
An important consideration in a shelter situation is the relative cost of these commercial products. An alternative practice that is sometimes used in a shelter situation is to collect serum or plasma from animals in the shelter that have survived disease or have been recently vaccinated. However, this practice carries risk as the serum will not necessarily have been screened for transmissible pathogens (e.g. haemoparasites or feline retroviruses). Serological testing provides a more effective approach to controlling disease outbreaks in a shelter situation (see below and Table 7).
An Update on New Canine Vaccines
Since publication of the 2010 WSAVA guidelines, newly introduced vaccines include a Bordetella bronchiseptica vaccine for oral administration (Hess et al. 2011, Ellis 2015) and, globally, an increased range of Leptospira vaccines containing multiple, geographically relevant serogroups (Klaasen et al. 2012, 2014, Wilson et al. 2013, Schuller et al. 2015). These products are described in Table 1.
A vaccine against canine influenza virus (CIV) infection is licensed only in the USA (Deshpande et al. 2009, Larson et al. 2011). The influenza A subtype H3N8 has been well recognized as a cause of respiratory disease in North American dogs that are housed together (Crawford et al. 2005, Payungporn et al. 2008, Castleman et al. 2010), but to date only sporadic outbreaks have been recognized and reported elsewhere (Crawford et al. 2005, Daly et al. 2008, Kirkland et al. 2010, Pratelli & Colao 2014, Schulz et al. 2014). The CIV vaccine contains inactivated virus and is administered to pups from 6 weeks of age with a second dose 2–4 weeks later and then annual revaccination. Immunity develops approximately 7 days after the second dose. The vaccine is considered non‐core and is recommended only for at‐risk dogs in North America that are likely to be exposed as part of their lifestyle (Anderson et al. 2013) [EB1]. At the time of writing, a local outbreak of canine influenza attributed to virus of the H3N2 subtype was reported from the Chicago and Wisconsin region of the USA and a conditionally licensed vaccine against this subtype has been released.
The first canine immunotherapeutic vaccine for malignant melanoma was licensed in 2010. This product comprises the human tyrosinase gene incorporated into a plasmid (a ‘naked DNA’ vaccine) that is repeatedly delivered by use of a high‐pressure transdermal injection device. The vaccine is used as an adjunctive treatment in dogs with oral melanomas and induces an immune response to this melanoma target antigen. Initial studies showed that the median survival time of dogs with grade II–IV melanoma increased to 389 days (from an expected survival of 90 days) (Bergman et al. 2006), but more recent studies have shown a lesser effect (Grosenbaugh et al. 2011, Ottnod et al. 2013) [EB1]. The vaccine is also available in Europe and, as in the USA, is limited to use by recognized veterinary oncology specialists.
Two licensed vaccines for canine leishmaniosis were until recently available in Brazil, where leishmaniosis is a disease of major importance to the canine and human populations. The first of these is a subunit product containing GP63 of Leishmania donovani (also known as the ‘fucose mannose ligand’; FML) in saponin adjuvant. It is considered to induce antibody that blocks the transmission of the organism from the dog to the sandfly vector by preventing binding of Leishmania to the midgut of the sand fly and has been extensively evaluated in immunological and epidemiological field studies (Palatnik de Sousa et al. 2009; Palatnik de Sousa & Day 2011) [EB1]. However, this product has been recently withdrawn from the Brazilian market. The second Brazilian vaccine contains the A2 antigen from L. donovani in saponin adjuvant. This vaccine is reported to induce similar protective effects in vaccinated dogs (i.e. with respect to seroconversion, prevention of infection and clinical signs and transmission to the vector) to the FML vaccine, when both were compared in a natural exposure field trial in an endemic area over an 11 month period. Dogs vaccinated with the A2 vaccine developed a lesser humoral immune response but showed a greater frequency of adverse events post vaccination (Fernandes et al. 2014).
A European Leishmania vaccine for dogs was introduced in 2011 (Bongiorno et al. 2013; Moreno et al. 2013). This vaccine contains excretory–secretory antigens of Leishmania infantum in adjuvant. The vaccine is used in seronegative dogs from 6 months of age as three primary doses administered 3 weeks apart with an annual booster. Vaccinated dogs will seroconvert, but the product datasheet describes a discriminatory serological test. Evidence for a cell‐mediated immune response is also suggested. The vaccine claims to reduce the likelihood of infection and reduce the severity of clinical signs in infected dogs, but makes no public health claim for an effect on human disease prevalence [EB2].
FELINE VACCINATION GUIDELINES
VACCINATION OF INDIVIDUAL CATS
The Basic Immunization Schedule
Guidelines and recommendations for core (recommended), non‐core (optional) and not recommended vaccines for cats visiting the general veterinary practice are given in Table 3. The core vaccines for the cat are those that protect against feline panleukopenia (FPV), FHV‐1 and FCV. A particular example of a vaccine that may be considered core in only some countries is that against rabies virus. In a geographical area in which this infection is endemic, the VGG recommends that all cats should be routinely vaccinated for the protection of both the pet and human populations. In some countries, mandatory rabies vaccination is a legal requirement (although this does not always include cats) and rabies vaccination is also required for international pet travel.
Table 3.
WSAVA Feline Vaccination Guidelines
| Vaccine | Initial kitten vaccination | Initial adult vaccination | Revaccination recommendation | Comments |
|---|---|---|---|---|
|
Feline Parvovirus (FPV; MLV, parenteral) FPV (killed, adjuvanted or killed, non‐adjuvanted, parenteral) FPV (MLV, non‐adjuvanted, intranasal) |
Begin at 6–8 weeks of age, then every 2–4 weeks until 16 weeks of age or older [EB1]. | Two doses 2–4 weeks apart are generally recommended by manufacturers, but one dose of MLV vaccine is considered protective [EB4]. | Revaccination (booster) at either 6 months or 1 year of age, then not more often than every 3 years. | Core. Vaccination of queens should occur before and not during pregnancy. Should vaccination during pregnancy be essential, only killed core vaccines should be used. MLV vaccines must not be used in pregnant animals. MLV vaccines should not be used in FeLV‐ and/or FIV‐infected cats [EB4]. |
|
Feline Herpesvirus‐1 (FHV‐1; MLV, non‐adjuvanted, parenteral and intranasal products are available) FHV‐1 (killed, adjuvanted, parenteral) |
Begin at 6–8 weeks of age, then every 2–4 weeks until 16 weeks of age or older [EB1]. | Two doses 2–4 weeks apart are generally recommended. | Revaccination (booster) at either 6 months or 1 year of age, then not more often than every 3 years for a low‐risk cat [EB1]. Annual revaccination should be provided for a higher risk cat. |
Core. MLV FHV‐1/FCV vaccines are invariably combined with each other, either as bivalent products or in combination with additional vaccine antigens (e.g. FPV). Mild upper respiratory disease signs are occasionally seen following intranasal vaccination or aerosolization or leakage from the injection site of parenteral MLV vaccine. Note: for definition of low and higher risk cat refer to text. |
|
Feline Calicivirus (FCV; MLV, non‐adjuvanted, parenteral and intranasal products are available) FCV (killed, non‐adjuvanted parenteral; containing two strains of calicivirus) FCV (killed, adjuvanted, parenteral) |
Begin at 6–8 weeks of age, then every 2–4 weeks until 16 weeks of age or older [EB1]. | Two doses 2–4 weeks apart are generally recommended. | Revaccination (booster) at either 6 months or 1 year of age, then not more often than every 3 years for a low‐risk cat [EB1]. Annual revaccination should be provided for a higher‐risk cat. |
Core. MLV FHV‐1/FCV vaccines are invariably combined with each other, either as bivalent products or in combination with additional vaccine antigens (e.g. FPV). Mild upper respiratory disease signs are occasionally seen following intranasal vaccination or aerosolization or leakage from the injection site of parenteral MLV vaccine. Transient polyarthritis is occasionally reported after FCV vaccination. Note: for definition of low and higher‐risk cat refer to text. |
|
Rabies (canary pox virus‐vectored recombinant, non‐adjuvanted, parenteral) |
Administer a single dose as early as 12 weeks of age, with revaccination 1 year later. | Administer a single dose with revaccination at 1 year of age. | Revaccination (booster) as per licensed DOI or as required by local regulations. | Core in areas where the disease is endemic. |
|
Rabies (1‐ and 3‐year killed, adjuvanted products are available, parenteral) |
Administer a single dose as early as 12 weeks of age, with revaccination 1 year later. | Administer a single dose with revaccination 1 year later. | Revaccination (booster) as per licensed DOI or as required by local regulations. | Core in areas where the disease is endemic. |
| Feline Leukemia Virus (FeLV; canary pox virus‐vectored recombinant, non‐adjuvanted, injectable) | Administer an initial dose as early as 8 weeks of age; a second dose must be administered 3–4 weeks later. | Two doses, 3–4 weeks apart | A single dose 1 year following the last dose of the initial series, then not more often than every 2–3 years in cats determined to have sustained risk of exposure [EB4]. | Non‐Core. Only FeLV‐negative cats should be vaccinated. FeLV testing must be performed prior to vaccine administration to avoid unnecessary administration of vaccine. |
|
FeLV (killed, adjuvanted, parenteral) FeLV (recombinant protein subunit, adjuvanted, parenteral) |
Administer an initial dose as early as 8 weeks of age; a second dose must be administered 3–4 weeks later. | Two doses, 3–4 weeks apart | A single dose 1 year following the last dose of the initial series, then not more often than every 2–3 years in cats determined to have sustained risk of exposure [EB4]. | Non‐Core. Only FeLV‐negative cats should be vaccinated. FeLV testing must be performed prior to vaccine administration |
| Feline Immunodeficiency Virus (FIV; killed, adjuvanted, parenteral) |
Three doses are required. The initial dose is administered as early as 8 weeks of age; two subsequent doses should be administered at an interval of 2–3 weeks. |
Three doses are required. Each dose is administered 2–3 weeks apart. |
A single dose 1 year following the last dose of the initial series, then annually in cats determined to have sustained risk of exposure. | Non‐core. Vaccination induces production of antibodies indistinguishable from those developed in response to FIV infection as detected by in‐practice test kits. Some discriminatory serological tests have been reported. Validated PCR diagnostics are becoming more widely available and are recommended by the VGG. |
|
Chlamydia felis (avirulent live, non‐adjuvanted, parenteral) Chlamydia felis (killed, adjuvanted, parenteral) |
Administer the initial dose as early as 9 weeks of age; a second dose is administered 2–4 weeks later. | Administer two doses, 2–4 weeks apart. | Annual booster is indicated for cats with sustained exposure risk. | Non‐Core. Vaccination is most appropriately used as part of a control regime for animals in multicat environments where infections associated with clinical disease have been confirmed. Inadvertent conjunctival inoculation of vaccine has been reported to cause clinical signs of infection. |
|
Bordetella bronchiseptica (avirulent live, non‐adjuvanted, intranasal) |
Administer a single dose intranasally as early as 4 weeks of age. | Administer a single dose intranasally. | Annual booster is indicated for cats with sustained risk. | Non‐Core. Vaccination may be considered in cases where cats are likely to be at specific risk of infection; for example, cats that are kept in large colonies. |
|
Feline Infectious Peritonitis (FIP; MLV, non‐adjuvanted, intranasal) |
Administer a single dose as early as 16 weeks of age and a second dose 3–4 weeks later. | Two doses 3–4 weeks apart. | Annual booster is recommended by the manufacturer. | Not Recommended. According to the limited studies available, only cats known to be feline coronavirus antibody‐negative at the time of vaccination are likely to develop some level of protection. It is rare that a cat will be coronavirus antibody negative at 16 weeks of age or older. |
Where the recommendations in this table are not consistent with those on datasheets [EB2] the level of evidence supporting the recommendation is given.
In terms of feline core vaccines it is important to realize that the protection afforded by the FCV and FHV‐1 vaccines will not match the immunity provided by FPV vaccines. Thus the feline core respiratory disease vaccines should not be expected to give the same robust protection, nor the duration of immunity, that is seen with canine core vaccines. FCV vaccines have been designed to produce cross‐protective immunity against multiple strains of FCV; however, it is still possible for infection and disease to occur in vaccinated adult animals (Pedersen et al. 2000, Schorr‐Evans et al. 2003) [EB1]. There is no FHV‐1 vaccine that can protect against infection with virulent virus and infection may lead to the virulent virus becoming latent with the possibility of reactivation during periods of severe stress (Richter et al. 2009, Maes 2012) [EB1]. The reactivated virus may cause clinical signs in the vaccinated animal or the virus can be shed to susceptible animals and cause disease in them. The VGG recommends triennial revaccination of low‐risk cats for FHV‐1 and FCV on the basis of a published study showing a minimum duration of partial, but clinically significant, immunity of 7.5 years for these core vaccines (Scott & Geissinger 1999). A more recent study of a MLV FHV‐1/FCV vaccine seemed to show much less substantial, partial protection against FHV‐1 at 3 years post vaccination; although the FCV partial protection was comparable to that shown by Scott and Geissinger in1999 (Jas et al. 2015). [EB1]. The VGG recommends that annual revaccination of cats against FHV‐1/FCV be carried out in higher‐risk situations. A low‐risk cat might be defined as a solitary, indoor animal that does not visit a boarding cattery. A higher‐risk cat might be defined as an animal that regularly visits a boarding cattery or that lives in a multicat, indoor–outdoor household. Moreover, the VGG encourages practitioners to consider the timing of administration of FHV‐1/FCV vaccines to higher‐risk, regularly boarding cats. The most robust immunity conferred by these vaccines occurs within the 3‐month period after vaccination (Gaskell et al. 2007) [EB1], and so administration of these vaccines might best be timed for immediately before a regularly boarded cat is due to make an annual visit to the cattery.
Vaccination against feline leukaemia virus (FeLV) is also often a point of debate amongst experts. The VGG regards FeLV as a non‐core vaccine (Table 3), but fully appreciates that use of this product must be determined by the lifestyle and perceived exposure risk of individual cats and the prevalence of infection in the local environment. Many feline experts believe that even though the prevalence of FeLV infection is now markedly reduced in many parts of the world due to successful control programmes (Weijer and Daams 1976, Weijer et al. 1986,1989, Meichner et al. 2012) [EB1], in geographical areas in which FeLV infection remains prevalent, any cat less than 1 year old with an element of outdoor lifestyle (e.g. even living with a cat that goes outdoors) should receive the benefit of protection by routine vaccination with two doses of vaccine given 2–4 weeks apart starting not earlier than 8 weeks of age. This ‘risk‐benefit’ analysis for FeLV should form a routine part of the feline vaccination interview and only FeLV‐negative cats should be vaccinated.
The VGG has also reconsidered the FIV vaccine, which in previous iterations of these guidelines has been categorized as ‘not recommended’. The basis for this categorization was: (1) questions over the cross‐protection between subtypes of virus included in the vaccine and those subtypes and recombinants in the field in different geographical areas (Hosie et al. 1995, Dunham et al. 2006, Yamamoto et al. 2007, Coleman et al. 2014, Beczkowski et al. 2015a) [EB1], (2) the interference of the vaccine with antibody testing used for diagnosis of FIV infection (Hosie & Beatty 2007) [EB1], and (3) the fact that this is an adjuvanted vaccine that must be given repeatedly (a primary course of three injections and annual revaccination) to a species susceptible to injection site sarcoma. The VGG is aware that in some parts of the world, there remains a significant prevalence of FIV seropositivity and/or infection (Bennett et al. 1989, Hosie et al. 1989, Friend et al. 1990, Glennon et al. 1991, Bandecchi et al. 1992, Hitt et al. 1992, Ueland and Lutz 1992, Jones et al. 1995, Hofmann‐Lehmann et al. 1996, Yilmaz et al. 2000, Lee et al. 2002, Muirden 2002, Norris et al. 2007, Gleich et al. 2009, Ravi et al. 2010, Bande et al. 2012, Chang Fung Martel et al. 2013, Rypula et al. 2014) [EB1]. There are now discriminatory serological tests (Kusuhara et al. 2007, Levy et al. 2008, Westman et al. 2015) and more robust polymerase chain reaction (PCR) testing for the diagnosis of FIV infection (Arjona et al. 2007, Wang et al. 2010, Morton et al. 2012) [EB1]. In many countries, it is most unlikely that cat owners will be persuaded to keep their cats indoors, away from the major risk of FIV transmission (bites by infected cats). Disease progression in FIV‐infected cats has recently been shown to be impacted by housing conditions and number of cats in the household (Beczkowski et al. 2015b). Given that this vaccine has been shown to have efficacy in some studies, but not in others, and might benefit some at‐risk populations of cats, the VGG has reclassified the product as a non‐core vaccine.
Kitten Vaccination and the 6‐ or 12‐Month Booster
As discussed for puppies, most kittens are protected by MDA in the first weeks of life. However, without serological testing, the level of protection and the point at which the kitten will become susceptible to infection and can respond immunologically to vaccination are unknown. This is related to the level of maternal antibody and variation in uptake of MDA between litters and individuals. In general, MDA will have waned by 8–12 weeks of age to a level that allows an active immunological response; however, kittens with poor MDA may be vulnerable (and capable of responding to vaccination) at an earlier age, while others may possess MDA at such high titres that they are incapable of responding to vaccination until sometime after 12 weeks of age. The VGG has reviewed recent studies suggesting that up to one third of kittens may fail to respond to a final core vaccine given at 16 weeks of age and that a proportion of kittens may still have blocking MDA at 20 weeks of age (DiGangi et al. 2012, Jakel et al. 2012). The VGG notes that one of these studies was of a relatively low number of animals, dominated by one breed, within a cattery setting, and suggests that the data may not be fully applicable to the wider feline population. Nevertheless, the VGG has increased the recommended age for the final vaccination in the series of primary core vaccinations from 14–16 weeks of age to 16 weeks or older [EB1].
The VGG recommendation for the core vaccination of kittens is therefore in line with the schedules proposed for puppies above: beginning at 6–8 weeks of age and then repeating vaccination every 2–4 weeks until 16 weeks of age or older. Therefore the number of kitten primary core vaccinations will be determined by the age at which vaccination is started and the chosen revaccination interval. Possible schedules are outlined in Table 5. By this recommendation, when vaccination is started at 6 or 7 weeks of age, a course of four primary core vaccines would be administered, but only three would be required with an 8‐ or 9‐week start.
An integral part of core vaccination of kittens is the ‘booster’ vaccine that has traditionally been given either at 12 months of age or 12 months after the last of the primary series of kitten vaccines. The main aim of this vaccine is to ensure that a protective immune response develops in any cat that may have failed to respond to any of the three vaccines in the primary core series, rather than necessarily ‘boosting’ the immune response. The delivery of this vaccine at 12 months of age is likely to have been chosen historically as a convenient time to request the owner to attend the practice for a first annual health check. This therefore implies that should an individual kitten fail to respond to any of the three primary core vaccinations, that kitten may be unprotected until it receives this 12‐month vaccine. This might account for occurrences of infectious disease in a proportion of vaccinated kittens at less than 12 months of age. The VGG has re‐evaluated this practice and now suggests that veterinarians might wish to reduce this possible window of susceptibility by bringing forward this vaccine from 52 weeks to 26 weeks of age (or indeed at any time point between 26 and 52 weeks of age; however, 26 weeks of age provides a convenient timing). This practice will require that pet owners clearly understand why this is recommended, because as indicated in Table 5, adopting such a protocol will mean that vaccination started in a 6 or 7 week old kitten, might now entail up to five vaccine visits in the first 6 months of life. For core vaccines, after a 26 week ‘booster’, another core vaccine would not be required for at least another 3 years (for a low‐risk cat). As for puppies, adoption of the 26 week vaccination approach would not preclude a first annual health check at 12 or 16 months of age.
Revaccination of Adult Cats
Cats that have responded to vaccination with MLV core vaccines maintain a solid immunity (immunological memory) against FPV for many years in the absence of any repeat vaccination. Immunity against FCV and FHV‐1 is only partial (Scott and Geissinger 1999, Jas et al. 2015). The VGG recommendation for adult ‘low‐risk’ cats is for revaccination with MLV core vaccines at intervals of 3 years or longer. For ‘higher‐risk’ cats (see definitions above) the veterinarian might consider administering FPV vaccine no more frequently than every 3 years, but giving FCV and FHV‐1 vaccines annually, with these latter products timed for administration shortly before any regular annual visit to a boarding cattery [EB1]. These recommendations do not generally apply to killed core vaccines (except for rabies) nor to the non‐core vaccines, and particularly not to vaccines containing bacterial antigens. Thus Chlamydia (formerly Chlamydophila; Sachse et al. 2015) and Bordetella products, if their use is deemed necessary, require annual boosters for the limited protection afforded by these products [EB2].
Therefore, according to these guidelines, an adult cat may still receive an annual vaccination; however, the components of that vaccination may differ from year to year. Typically, core vaccines (especially FPV) are currently administered triennially with respiratory virus vaccines given according to risk and chosen non‐core products being given annually. The VGG is aware that in some countries only multi‐component products containing core and non‐core combinations are available. The VGG would encourage manufacturers to make a full range of vaccines available wherever possible or, at the very least, make a core‐only combination for those not wanting to give any of the non‐core vaccines.
An adult cat that received a complete course of vaccination for FPV, FHV‐1 and FCV as a kitten (including the 6‐ or 12‐month booster), but may not have been regularly vaccinated as an adult requires only a single dose of MLV core vaccine to boost immunity [EB4]. An adopted adult cat (or kitten over 16 weeks of age) of unknown vaccination history requires only a single dose of MLV FPV core vaccine to engender a protective immune response to that virus. In contrast, an adopted adult cat of unknown vaccination history should receive two doses of MLV FHV‐1/FCV vaccine (2–4 weeks apart) to establish an adequate immune response [EB2].
Sites of Vaccination for Cats
Vaccines (of any type) are one class of injectable product that has been linked to the pathogenesis of the feline injection site sarcoma (FISS) and particular attention has focused on the administration of adjuvanted FeLV and rabies vaccines (Kass et al. 1993). FISS has been the subject of much research and there are a number of recent reviews on the subject (Martano et al. 2011, Srivastav et al. 2012, Ladlow 2013, Hartmann et al. 2015). Although the pathogenesis of FISS remains unproven, current belief is that a localized chronic inflammatory reaction initiates malignant transformation of mesenchymal cells and that this process has some genetic basis. Most subcutaneous injections (including of vaccines) have traditionally been given into the interscapular region of the cat, which remains a common site for formation of a FISS. The infiltrative nature of these tumours means that radical surgical resection is often necessary to attempt removal of these lesions although adjunctive treatment modalities are also used (Martano et al. 2011, Ladlow 2013).
In North America the response to this issue was the recommendation of a protocol whereby the two perceived high‐risk adjuvanted vaccines would be administered into distinct anatomical sites that would be more amenable to surgical removal of any FISS that might develop. Accordingly the recommendation ‘left leg leukaemia, right leg rabies’ suggested that FeLV vaccine should be given as far distal as possible into the left hindlimb, whilst rabies vaccine should be given as far distal as possible into the right hindlimb. This recommendation remains in the current AAFP guidelines (Scherk et al. 2013), which also specify administration of the three feline core vaccines into a distal forelimb. One study evaluated the effect of this practice by comparing the anatomical distribution of FISS in cats before the recommendation was made (1990–1996) and after the practice was adopted (1997–2006) (Shaw et al. 2009). The data showed a significant decrease in the prevalence of interscapular FISS and an increase in prevalence of tumours in the right (but not left) forelimb. More notably, there was also an increase in the number of tumours reported arising in the combined regions of the right hindlimb with right lateral aspect of the abdomen (12.5% to 25.0%) and the left hindlimb with left lateral aspect of the abdomen (11.4% to 13.8%). This was attributed to the difficultly of injecting into the distal hindlimb and these abdominal sites being accidentally injected. This practice has not been widely adopted outside of North America.
Recently, one publication has shown the efficacy of administering FPV and rabies vaccines into the tail of cats (Hendricks et al. 2014). Adult cats from a community trap–neuter–return programme were given trivalent MLV core vaccine (FPV, FHV‐1, FCV) into the distal third of the dorsal tail with inactivated rabies vaccine administered 2 cm distal to the site of the trivalent vaccination. Seroconversion occurred in all cats to FPV and all but one cat for rabies virus. Tail vaccination was reported to be well tolerated by the cats in this small study. In the 2010 WSAVA vaccination guidelines, the VGG proposed the alternative of delivering vaccine into the skin of the lateral thorax, or better, the lateral abdomen (Day et al. 2010). Tail injection may prove to be a safer alternative than distal limb injections or lateral body wall injections, but further studies of tail vaccination will be required.
This remains a confusing and contentious area and individual practitioners must decide for themselves which approach is practical for their own practice setting. However, the following principles should still be applied:
Any risk of FISS is outweighed by the benefit of protective immunity conferred by vaccines. Current estimates of the prevalence of FISS are 1 in every 5,000 to 12,500 cats vaccinated (Gobar and Kass 2002, Dean et al. 2013).
Non‐adjuvanted vaccines should be administered to cats wherever possible.
Vaccines (particularly adjuvanted products) or other injectables should not be administered into the interscapular region.
Vaccines (particularly adjuvanted products) should be administered into other subcutaneous (and not intramuscular) sites. The choice of these sites should be based on a balance between the ease of surgical resection of any FISS that might develop and acceptable safety for the vaccinator (i.e. to avoid accidental self‐injection during difficult restraint of the animal).
Vaccines should be administered into a different site on each occasion. This site should be recorded in the patient's record or on the vaccination card by use of a diagram indicating which products were administered on any one occasion. The sites should be ‘rotated’ on each occasion. Alternatively, a practice might develop a group policy that all feline vaccinations are administered to a specific site during one calendar year and this site is then rotated during the following year.
The VGG encourages all cases of suspected FISS to be notified via the appropriate national reporting route for suspected adverse reactions or to the vaccine manufacturer.
Serological Testing
Since the publication of the 2010 guidelines, one commercial in‐practice rapid test for determination of serum antibody to FPV, FCV and FHV‐1 has become available. This test has now been validated and applied in a series of published investigations (DiGangi et al. 2011, Mende et al. 2014) [EB1]. This test kit may be used for the determination of the presence of protective antibody against FPV as there is excellent correlation between the presence of such antibody and resistance to infection (Lappin et al. 2002) [EB1]. The FPV test kit is reported to have 89% specificity and 79% sensitivity (Mende et al. 2014) or 99% specificity and 49% sensitivity (DiGangi et al. 2011) when compared with a haemagglutination inhibition test. A negative test result indicates that a cat has little or no antibody, and that revaccination is recommended. However, some seronegative cats are in fact immune (false‐negative) and their revaccination would be unnecessary. In contrast, a positive test result would lead to the conclusion that revaccination is not required.
The correlation between circulating serum antibody and protection against FCV and FHV‐1 infection is less robust than the presence of adequate local mucosal immunity and cell‐mediated immunity, respectively. For that reason, a negative test result for FCV or FHV‐1 antibody would not necessarily indicate lack of protection in a particular cat (Lappin et al. 2002) [EB1]. These tests can be applied in practice as described above for the dog: for determination of protection of kittens following FPV vaccination, for determination of protection against FPV in adult cats (in order to inform decisions about revaccination) and for use in the shelter situation in the control of outbreaks of FPV infection. It should be emphasized that antibody testing for FIV is used to diagnose disease and is of no value in determining immunity to FIV, but as discussed above, where FIV vaccine is used and FIV infection is suspected, diagnosis should be made using a discriminatory serological test or, preferably, a validated PCR test.
VACCINATION OF DOGS AND CATS IN THE SHELTER ENVIRONMENT
An animal shelter is a holding facility for animals usually awaiting adoption, rescue or reclamation by owners. In general, animal shelters are characterized by a random source population with a mostly unknown vaccination history, high population turnover and high infectious disease risk. The term ‘shelter’ encompasses situations ranging from sanctuaries that possess a stable population, to facilities that admit hundreds of animals per day, to rescue and foster homes that care for multiple individuals or litters at any given time. Just as vaccination strategy varies with each individual pet, there is no one‐size‐fits‐all strategy for vaccinating shelter animals. The likelihood of exposure and the potentially devastating consequences of infection necessitate a clearly defined shelter vaccination program.
Shelter medicine differs from individual care in that clinicians have to practice in an environment where eradication of infectious disease cannot be attained. It is possible, however, to minimize the spread of infections within a high‐density, high‐risk population and maintain the health of not‐yet‐infected individuals. When the overall purpose is to place healthy pets into welcoming homes, the time and effort dedicated to controlling infectious disease is only one of many variables in the complex shelter medicine and husbandry equation. The recommendations provided here attempt to address some shelter‐unique issues as they pertain to vaccination and disease control.
Guidelines and recommendations for vaccines to be used in shelters are given in Tables 2 and 4. In these updated guidelines, we have standardized the recommendations for puppies and kittens entering a shelter to indicate that core vaccination may be started as early as 4–6 weeks of age, and (where funding permits) revaccination should be every 2 weeks until the animal reaches 20 weeks of age, if it remains in the shelter until that time [EB4]. Recent US studies have shown that cats entering shelters may be seropositive for vaccine‐preventable infectious disease agents. DiGangi et al. (2012) reported seropositivity for FPV (60.2%), FHV‐1 (89%) and FCV (63.4%) and Fischer et al. (2007) reported seropositivity for FPV (33%), FHV‐1 (21%), FCV (64%) and rabies virus (3%). Seropositivity to CDV (41.2%) was less than for CPV (84.3%) in dogs entering one US shelter (Litster et al. 2012) and in another study 35.5% of dogs were seropositive to both CDV and CPV, 7.7% to CDV only, 31.5% to CPV only and 25.3% to neither virus (Lechner et al. 2010). If unambiguous documentation of vaccination is provided for an adult animal at the time of admission to a shelter, there is no reason to revaccinate with canine core vaccines, but feline core vaccines, specifically FCV and FHV‐1, may be of value in boosting immunity.
Table 2.
WSAVA Guidelines on Canine Vaccination for the Shelter Environment
| Recommended Vaccines in Various Combinations (also refer to Table 1) | Initial Vaccine Series for Puppies | Initial Vaccine Series for Adults | Comments |
|---|---|---|---|
|
CDV + CAV‐2 + CPV‐2 (MLV) with or without CPiV rCDV + CAV‐2 + CPV‐2 with or without CPiV Parenteral |
Administer one dose prior to or immediately on admission, as early as 4 weeks of age. Repeat at 2 week intervals until 20 weeks of age if animal is still in the facility. | Administer one dose prior to or immediately on admission. Repeat in 2 weeks. |
Ideally puppies should be vaccinated beginning at 6 weeks of age. In the face of an outbreak, vaccination as early as 4 weeks of age (for CDV and/or CPV‐2) may be indicated. MDA, if present, can interfere with immunization, but nursing history is often not available. |
|
Bordetella bronchiseptica (live avirulent bacteria, intranasal) B. bronchiseptica + CPiV (MLV) intranasal B. bronchiseptica + CPiV (MLV) + CAV‐2 (MLV) intranasal B. bronchiseptica (live avirulent bacteria, oral) |
Administer a single dose as early as 3 weeks of age. For best results, if administered prior to 6 weeks of age, an additional dose should be given after 6 weeks of age [EB4]. | Two doses 2 weeks apart are recommended. A single dose may be protective, but in this high‐risk situation a second dose might provide greater protection [EB4]. | Intranasal or oral vaccine is strongly recommended in the shelter situation. Intranasal or oral vaccines MUST NOT be administered parenterally as this may lead to severe adverse reactions or death. |
| Bordetella bronchiseptica (bacterin or antigen extract for parenteral administration only) | Administer one dose at time of admission (from 6–8 weeks of age) and a second dose 2 weeks later. | Two doses 2 weeks apart are recommended. |
Parenteral vaccination is recommended only when it is not possible to administer an intranasal or oral vaccine. Canine respiratory disease complex (‘kennel cough’) is not a vaccine‐preventable disease and the vaccine should only be used to help manage the disease. |
| Rabies | A single dose should be administered at the time of discharge from the facility. | A single dose should be administered at the time of discharge from the facility. | The administration of rabies vaccine will be determined by whether the shelter is in a country in which the disease is endemic, and by local statute. |
In the USA, the CIV vaccine is often used in the shelter situation when two doses can be given 2 weeks apart.
Many of the recommendations for shelters are different for use of the same vaccines for owned pet dogs in the practice situation. These recommendations take into account the potential for high infectious disease pressure in the shelter environment.
Table 4.
WSAVA Guidelines on Feline Vaccination for the Shelter Environment
| Vaccine | Kittens | Adult | Comments |
|---|---|---|---|
|
FPV FHV‐1 FCV |
Administer a single dose prior to or at the time of admission as early as 4–6 weeks of age; then, every 2 weeks until 20 weeks of age if still in the facility. | Administer a single dose at the time of admission; repeat in 2 weeks if the animal remains in the shelter. |
MLV preparations are preferable. Use of intranasal FPV vaccines is not recommended in the shelter environment (Schultz 2009). Use of intranasal FCV/FHV‐1 MLV vaccines may be preferable when rapid onset (48 hrs) of immunity is important. Post‐vaccinal sneezing, more commonly seen following administration of intranasal FCV/FHV‐1 vaccine is impossible to distinguish from active infection. |
| Rabies | A single dose should be administered at the time of discharge from the facility. | A single dose should be administered at the time of discharge from the facility. | The administration of rabies vaccine will be determined by whether the shelter is in a country in which the disease is endemic and vaccination is required by law. |
The VGG does not recommend the use of other feline vaccines in the shelter situation.
The VGG discriminates between a shelter and a boarding kennel/cattery. The latter are facilities where fully vaccinated animals may be temporarily boarded for relatively short periods of time (e.g. when owners are on vacation). It should be a requirement of entry to any such facility that the individual dog or cat is fully vaccinated with core products given according to the guidelines presented herein. In dogs, the use of non‐core vaccines against respiratory infections is also appropriate under these circumstances. The VGG is aware that in some countries vaccination protocols for animals entering a boarding kennel/cattery are formulated by local authorities and may be contrary to current guidelines (e.g. insistence on annual revaccination). The VGG encourages such authorities to reconsider these recommendations in light of current scientific thinking and product availability and encourages the veterinary profession and national associations to lobby for such change.
Since publication of the 2010 guidelines, the availability of rapid in‐house serological test kits has had major impact on the management of outbreaks of CDV, CPV or FPV in animal shelters [EB3]. The approach to use of these kits in such situations is outlined in Table 7.
GENERAL CONSIDERATIONS
Comprehensive Individual Care beyond Vaccination
In the past, veterinary practice has benefited from the annual administration of vaccines. By encouraging owners to bring their pets yearly for vaccination, veterinarians were able to recognize and treat disease earlier than might otherwise have been the case. In addition, the annual visit provided an opportunity to inform clients of important aspects of canine and feline health care.
Unfortunately, many clients have come to believe that vaccination is the most important reason for annual veterinary visits. Veterinarians have been concerned that a reduction in vaccination frequency will cause clients to forgo the annual visits and that the quality of care will diminish. It is therefore essential that veterinarians stress the importance of all aspects of a comprehensive individualized health care program. Emphasis should be placed on detailed history taking; thorough physical examination performed in the presence of the client, and individualized patient care. The importance of dental care, proper nutrition, appropriate diagnostic testing and the control of parasites and of zoonotic diseases should be addressed during evaluation of each pet. Behavioural concerns should be discussed, as well as the necessity for more frequent, tailored examination of young and geriatric animals and animals of particular breeds with well characterized disease predispositions. Discussion of vaccination is simply one part of the annual health check visit.
During regular (usually annual) health checks, clinicians should assess the need for core and non‐core vaccines for that particular year. The practitioner should explain to the client the types of vaccines available, their potential benefits and risks, and their applicability to the particular animal, given its lifestyle and risk of exposure. While an animal might not receive core vaccination every year, most non‐core vaccines require annual administration – so owners will continue to see their animal vaccinated annually. The regional incidence and risk factors for various infectious diseases should also be discussed. Ways to reduce the impact of acquired disease (e.g. avoiding overcrowding, improving nutrition, and restricting access to infected animals) should also be reviewed.
Vaccinations should be considered as only one component of a comprehensive preventive health care plan individualized based on the age, breed, health status, environment (potential exposure to harmful agents), lifestyle (contact with other animals) and travel habits of the pet.
Age has a significant effect on the preventive health care needs of any given individual. Puppy/kitten programs have traditionally focused on vaccinations, parasite control and neutering. Today, opportunity exists to incorporate behaviour counselling and zoonotic disease management. For the ageing pet, senior care programs are becoming increasingly popular. Nutritional, dental disease and parasite control assessment and counselling should take place on an individualized basis throughout the life of the pet. There is no evidence that older dogs and cats, which have been fully vaccinated as pups or kittens, require a specialized programme of core vaccination (Day 2010, Horzinek 2010, Schultz et al. 2010). Experimental evidence shows that older dogs and cats have persisting immunological memory to core vaccines, as detected by measurement of serum antibody, and that this may be readily boosted by administration of a single vaccine dose (Day 2010) [EB1]. In adult animals, decisions about revaccination with most core products (CDV, CAV and CPV and FPV) may be made via serological testing. Practitioners who offer this alternative to vaccination report that it is greatly appreciated by owners who may have concerns about vaccination frequency and offering this alternative acts as a ‘practice builder’. By contrast, aged animals may not be as efficient at mounting primary immune responses to novel antigens that they have not previously encountered (Day 2010) [EB1]. Studies of UK dogs and cats vaccinated for the first time against rabies for pet travel have clearly shown that more aged animals fail to achieve the legally required antibody titre (Kennedy et al. 2007) [EB1].
The environment in which a pet resides can profoundly affect its health status and should be assessed during annual health care visits in order to define risk factors and develop appropriate preventive measures.
By estimating the extent to which dogs and cats come into contact with other animals in unobserved circumstances, veterinarians can assess the need for non‐core vaccinations. Dogs that visit kennels, grooming salons, common areas and wooded, tick‐infested areas are potentially at greater risk from certain infectious diseases than dogs that do not frequent these areas.
Just as the human population has become more mobile, so has the pet population, resulting in potential exposure to infectious agents, parasites and environmental hazards not found where the animal normally lives. Determining past and anticipated future travel during each visit allows for greater individualization of preventive care and diagnostic testing plans.
Medical Record Documentation
At the time of vaccine administration, the following information should be recorded in the patient's permanent medical record:
date of vaccine administration
identity (name, initials or code) of the person administering the vaccine
vaccine name, lot or serial number, expiry date and manufacturer
site and route of vaccine administration.
The use of peel‐off vaccine labels and stamps that imprint the medical record with the outline of a pet facilitates this type of record keeping which is mandatory in some countries. Adverse events should be recorded in a manner that will alert all staff members during future visits. Informed consent should be documented in the medical record in order to demonstrate that relevant information was provided to the client and that the client authorized the procedure (e.g. ‘off‐label’ use of products as discussed above). At the very least, this notation should indicate that a discussion of risks and benefits took place prior to vaccination.
VGG recommends that vaccination certificates be designed to include not just the dates on which vaccines were administered, but also a field for the veterinarian to state the date on which vaccination is next recommended. This will help diminish confusion in the minds of pet owners and kennel/cattery proprietors.
Adverse Events
Adverse events are defined as any side effects or unintended consequences (including lack of protection) associated with the administration of a vaccine product. They include any injury, toxicity or hypersensitivity reaction associated with vaccination, whether or not the event can be directly attributed to the vaccine. Adverse events should be reported, whether their association with vaccination is recognized or only suspected. A vaccine adverse event report should identify the product(s) and animal(s) involved in the event(s) and the individual submitting the report.
Reporting field observations of unexpected vaccine performance is the most important means by which the manufacturer and the regulatory agency are alerted to potential vaccine safety or efficacy problems that may warrant further investigation. The purpose of pre‐licensure safety studies is to detect relatively common adverse events. Rare or delayed adverse events will be detected only by post‐marketing surveillance through analysis of reported adverse events. Adverse events should be reported to the manufacturer and/or the local regulatory authority. In many countries governmental surveillance schemes are not available and reactions should therefore be notified to the manufacturer. The VGG recognizes that there is gross under‐reporting of vaccine‐associated adverse events, because of the passive nature of reporting schemes, which impedes knowledge of the ongoing safety of these products [EB4]. The VGG would actively encourage all veterinarians to participate in such surveillance schemes.
If a particular adverse event is well documented, reporting serves to provide a baseline against which future reports can be compared. In addition, reported adverse events can lead to detection of previously unrecognized reactions, detection of increases in known reactions, recognition of risk factors associated with reactions, identification of vaccine lots with unusual events or higher numbers of adverse events, and can further stimulate clinical, epidemiological or laboratory studies. Therefore, veterinarians are encouraged to report any clinically significant adverse event occurring during or after administration of any licensed vaccine. Reporting a vaccine adverse event is not an indictment against a particular vaccine; it facilitates review of temporally associated conditions and adds to the safety database of the product.
ACKNOWLEDGMENTS
The work of the Vaccination Guidelines Group has been generously sponsored by MSD Animal Health and the WSAVA. The VGG is an independent group of academic experts who have formulated these guidelines without consultation with industry. Representatives of the sponsoring company do not attend VGG meetings and the company does not have the right of veto over VGG recommendations.
The VGG again acknowledges the important work undertaken by the American Animal Hospital Association (AAHA) Canine Vaccine Task Force, the American Association of Feline Practitioners (AAFP) Feline Vaccine Advisory Panel in developing recommendations for the vaccination of dogs and cats (respectively) in North America. The VGG also acknowledges the work of the European Advisory Board on Cat Diseases (ABCD) in formulating recommendations for feline vaccination from the European perspective.
FACT SHEET: CANINE PARVOVIRUS TYPE 2 (CPV‐2) VACCINES
Types of Vaccines Available
Modified Live Virus (MLV) Vaccines: There are three contemporary variants of CPV‐2, which are referred to as CPV‐2a, CPV‐2b and CPV‐2c. The original CPV‐2 variant is rarely isolated nowadays, although it is still present in some modified live vaccines and can be shed from vaccine recipients. The most recent variant to emerge is CPV‐2c and this genotype is recognized in North and South America, Europe, Africa and Asia (Ohneiser et al. 2015). All genotypes are antigenically related; challenge studies have shown that vaccination of dogs with current CPV vaccines containing either CPV‐2 or CPV‐2b will provide protective immunity against all the other variants, including CPV‐2c (Spibey et al. 2008, Decaro & Buonavoglia 2012, Wilson et al. 2013). Conversely, there is one report of an outbreak of CPV‐2c infection in vaccinated adult dogs (Decaro et al. 2008). These dogs had been vaccinated at 42, 57 and 90 days of age and the adults had received annual boosters.
Inactivated (Killed) Vaccines: Only a few killed CPV‐2 vaccines are available; they are less effective and take much longer to induce an immune response when compared with the MLV vaccines (Pollock & Carmichael 1982b). They are not recommended for routine use. Killed vaccines may provide some benefit in wild and exotic species or pregnant bitches, where some MLV vaccines are not recommended. However, killed CPV‐2 vaccines have not been tested for safety or efficacy in these situations.
Mechanisms and Duration of Immunity (DOI)
DOI after natural infection/disease is thought to be life‐long in the majority of dogs.
DOI after vaccination with MLV vaccines is 9 years or longer, based on challenge and serological studies (Schultz et al. 2010).
DOI after vaccination with killed vaccines is 3 years or longer.
MDA interferes with active immunization for varying periods of time in the puppy, depending on the titre of colostral antibody and the amount of antibody absorbed after birth, as well as the specific vaccine (Pollock & Carmichael 1982a).
The ‘window of susceptibility’ is defined as the period of time during which a pup can be infected by field virus, but vaccines cannot immunize. For highly effective MLV vaccines (i.e. high titre, low passage) the ‘window of susceptibility’ is as short as 2 weeks or less, while for less effective MLV vaccines, the window of susceptibility is as long as 10–12 weeks (Schultz & Larson, 1996, Hoare et al. 1997).
After completing the puppy series at 16 weeks or older and vaccinating again at 26 or 52 months of age, revaccination need not be done more often than every 3 years.
In the absence of MDA, MLV vaccines provide immunity as early as 3 days after vaccination (Schultz & Larson 1996).
The presence of serum antibody, regardless of titre, in an actively immunized dog over the age of 20 weeks is correlated with protection.
Precautions
MLV vaccines should not be used in wildlife species.
MLV vaccines should not be used in pregnant bitches unless specifically indicated.
Puppies younger than 4–6 weeks of age should not be vaccinated with MLV products.
Disease Facts
After infection, it takes 3–7 days for signs of disease to appear.
CPV‐2 faecal shedding rarely persists for >2 weeks.
Dogs persistently infected for >4 weeks have not been reported and one can expect the animal to die or clear the virus in that period of time.
In the environment, the virus can remain infectious for 1 year or more. Therefore, all facilities where infected animals have been present must be considered infected.
A positive faecal antigen detection test result in a puppy with clinical signs suggestive of canine parvoviral enteritis will not have been caused by any recent CPV vaccine the animal may have received (DeCaro et al. 2014).
FACT SHEET: CANINE ADENOVIRUS (CAV)‐2 VACCINES
Types of Vaccines Available
Modified Live Virus (MLV) Vaccines: CAV‐2 containing vaccines are the most commonly available products. They are the only vaccines recommended for the prevention of infectious canine hepatitis (ICH) caused by CAV‐1 and for reducing the signs of respiratory disease associated with CAV‐2 infection. They are exceptionally effective and will not cause the adverse reaction commonly seen with CAV‐1 vaccines known as allergic uveitis or ‘blue eye’ (Curtis & Barnett, 1983). In addition to parenteral MLV CAV‐2 vaccine preparations there are combination or monovalent products to protect against the canine infectious respiratory disease complex (CIRDC), which includes Bordetella bronchiseptica and canine parainfluenza virus (CPiV) and CAV‐2. The intranasal product that contains CAV‐2, CPiV and Bordetella can be used to decrease the severity of CIRDC, but should not be used as the only vaccine to prevent ICH; for this purpose, the parenteral MLV‐CAV‐2 should also be given.
Inactivated (Killed) Vaccines: Inactivated (killed) CAV‐1 and CAV‐2 vaccines are sold in some countries, but they are not recommended when MLV products are available, as they are less effective.
Mechanisms and Duration of Immunity (DOI)
DOI after naturally‐acquired canine infectious hepatitis is thought to be life‐long in the majority of dogs.
DOI after vaccination with MLV vaccines is 9 years or longer in the majority of dogs, based on challenge and serological studies (Schultz et al. 2010).
DOI for protection from ICH with killed CAV‐1 or CAV‐2 vaccines is likely to be shorter than for MLV products.
MDA will block immunization after vaccination with the parenteral product and so the last dose should be given along with the other core viral vaccines (e.g. CDV, CPV‐2) when the puppy is 16 weeks of age or older.
After completing the puppy series at 16 weeks or older and vaccinating again at 26 or 52 weeks of age, revaccination need not be done more often than every 3 years.
In the absence of MDA, MLV vaccines protect against ICH as early as 5 days after vaccination.
The presence of serum antibody, regardless of titre, in an actively immunized dog over the age of 20 weeks is correlated with protection.
Precautions
Intranasal CAV‐2 vaccine is intended as an aid in the prevention of upper respiratory disease caused by CAV‐2 and is not intended to protect against CAV‐1 infection.
Disease Facts
CAV‐1 is transmitted primarily through contaminated secretions/excretions such as saliva and urine.
CAV‐1 and CAV‐2 are moderately stable, surviving for several days to weeks in the environment.
After experimental infection with CAV‐1, it takes 5 days or longer for signs of ICH to appear.
The ‘window of susceptibility’ is defined as the period of time during which a puppy can be infected by field virus, but vaccines cannot immunize. Unlike CPV‐2 vaccines, there generally is not a prolonged ‘window’ for CAV‐2 vaccines (i.e. <2 weeks).
CAV‐2 is transmitted primarily through the air.
CIRDC has complex pathogenesis involving stress, poor ventilation, dust, ammonia gas in unsanitary facilities and infections with Streptococcus spp., Bordetella bronchiseptica, Mycoplasma spp., CAV‐2, CPiV, CIV, canine pneumovirus and canine respiratory coronavirus.
CAV‐2 combined with other agents associated with CIRDC can cause respiratory disease in 3–4 days.
As a multifactorial disease CIRDC is not vaccine‐preventable. The current vaccines only help in reducing disease severity.
FACT SHEET: CANINE DISTEMPER VIRUS (CDV) VACCINES
Types of Vaccines Available
Modified Live Virus (MLV) Vaccines: These are the most common products. They generally contain the CDV strains Rockborn, Snyder Hill, Onderstepoort, Lederle or others at various titres. There are many pathotypes of CDV (Kapil et al. 2008, Espinal et al. 2014) which can cause varying clinical signs in a wide variety of species. However, serological differences among the many isolates are insignificant, and vaccination with any one of the current vaccines should provide protective immunity against any pathotype. MLV vaccines must not be used in wildlife species unless there is specific evidence that shows them to be safe.
Vectored Recombinant (rCDV) Vaccines: A canarypox virus recombinant product is available in the USA and a few other countries. A specific canarypox vectored recombinant product has also been used in wildlife and exotic species (Connolly et al. 2013).
Inactivated (Killed) Vaccines: Killed vaccines, not readily available, are not as effective and therefore should not be used for immunization against distemper (with the possible exception of wildlife species).
Mechanisms and Duration of Immunity (DOI)
DOI after natural infection/disease is thought to be life‐long in a majority of dogs.
DOI after vaccination with MLV vaccines is 9 years or longer, based on challenge and serological studies (Schultz et al. 2010).
DOI after vaccination with rCDV vaccine is ≥5 years, based on challenge and ≥ 6 years based on serology.
DOI after vaccination with killed vaccines is likely to be shorter than for MLV or recombinant vaccines.
MDA interferes with active immunization for varying periods of time in the puppy, depending on the titre of colostral antibody and the amount of antibody absorbed after birth.
The ‘window of susceptibility’ is defined as the period of time during which a pup can be infected by field virus, but vaccines cannot immunize. Unlike CPV‐2 vaccines, there generally is not a prolonged ‘window of susceptibility’ for CDV vaccines (<2 weeks).
Puppy vaccination using MLV products should not start earlier than 6 weeks of age unless the product has a specific license (some products may be used from 4 weeks of age); after completing the series at 16 weeks or older and vaccinating again at 26 or 52 weeks of age, revaccination need not be done more often than every 3 years.
In the absence of MDA, MLV and recombinant vaccines provide immunity rapidly after vaccination.
The presence of serum antibody, regardless of titre, in an actively immunized dog over the age of 20 weeks is correlated with protection.
Precautions
The MLV preparations are attenuated (modified and safe) for use in the domestic dog, not for use in wild and exotic species. These vaccines are highly virulent (e.g. in the black‐footed ferret and grey fox), causing disease and death (Carpenter et al. 1976, Pearson 1977, Durchfeld et al. 1990). Vaccination of these species with MLV vaccines should not be performed unless there is evidence to support the safety of a specific product.
Puppies younger than 4–6 weeks of age should not be vaccinated with MLV vaccines.
Disease Facts
Signs of disease appear between 2–6 weeks after infection.
During the incubation period, CDV causes immunosuppression, making the animal more susceptible to microbial infections. These secondary infections may lead to respiratory disease, pneumonia and death, before the more typical signs of distemper virus infection appear.
In the environment, the virus quickly loses infectivity.
FACT SHEET: FELINE PARVOVIRUS (FPV) VACCINES
Types of Vaccines Available
Modified Live Virus (MLV) Vaccines: These preparations contain attenuated (avirulent) feline parvovirus (feline panleukopenia virus) at various titres, without adjuvant. There are injectable preparations and others for intranasal application, in combination with other vaccinal antigens (e.g. FCV and FHV‐1). MLV vaccines are advantageous for their faster onset of action, greater efficacy at overcoming maternal antibody and greater likelihood of conferring sufficient immunity (DiGangi et al. 2011, Lappin 2012). Intranasal FPV combination vaccines should not be used in the shelter environment or if used for FCV/FHV‐1 immunity, they should be given simultaneously with an MLV‐FPV parenteral product (Schultz, 2009).
Inactivated (Killed) Vaccines: Killed adjuvanted FPV vaccines are available; a single injected dose of some products may induce good antibody responses in naïve cats within a relatively short time span. However, all killed FPV products require two doses 2–4 weeks apart and immunity is present only after the second dose. Killed vaccines may be beneficial in wild and exotic species, pregnant queens or retrovirally‐infected cats, where MLV vaccines are not recommended.
Mechanisms and Duration of Immunity (DOI)
DOI after natural infection/disease is life‐long.
DOI after vaccination with MLV vaccines is 7 years or longer, based on challenge and serological studies.
DOI after vaccination with a killed panleukopenia vaccine was demonstrated to last for a minimum of 7.5 years (Scott & Geissinger 1999).
While most cases of feline panleukopenia are caused by infection with FPV, variants of canine parvovirus (CPV‐2a, CPV‐2b and CPV‐2c) have emerged that infect cats and may cause disease (Decaro & Buonavoglia 2012). Certain current FPV vaccines afford some protection against these CPV variants.
Maternally derived antibody (MDA) interferes with active immunization for varying periods of time in the kitten, depending on the titre of colostral antibody and the amount of antibody absorbed during the first hours after birth.
The ‘window of susceptibility’ is defined as the period of time during which a kitten can be infected by field virus, but vaccines cannot immunize. By analogy with canine parvovirus, an immunity gap is assumed to exist, when antibody levels are too low to protect against natural infection, but still high enough to interfere with vaccination.
After completing the kitten series at 16 weeks or older and vaccinating again at 26 or 52 weeks of age, revaccination need not be done more often than every 3 years.
The presence of serum antibody, regardless of titre, in an actively immunized cat over the age of 20 weeks is correlated with protection.
When vaccination is being used to control disease in the face of an outbreak in a shelter situation, the more rapid induction of immunity induced by MLV vaccines is of clinical advantage.
There is a very early onset of protection after vaccination with MLV products (Brun & Chappuis 1979).
Precautions
MLV FPV vaccines should not be used in wild animal species unless there is evidence to show that they are safe.
MLV FPV vaccines should never be used in pregnant queens because of the risk of transfer of virus to the fetus and fetal damage. In some countries, inactivated FPV vaccines are licensed for use in pregnant queens, but in general, unnecessary administration of products to pregnant queens should be avoided.
MLV FPV vaccines should never be administered to kittens less than 4–6 weeks of age, to avoid damage to the cerebellum which is still developing in neonates.
MLV FPV vaccines should not be used in severely immunosuppressed individuals – although the risk appears small, with severe immunosuppression (for example with clinical FIV or FeLV infection or with the use of highly immunosuppressive drugs) failure to control viral replication could potentially lead to clinical signs after vaccination.
Disease Facts
After infection, it takes 2–7 days for signs of disease to appear.
Vomiting usually develops 1–2 days after the onset of fever. Diarrhoea may begin later, but is not always present. Dehydration develops rapidly, and an affected cat may sit at a water bowl, obviously thirsty, but without drinking. Terminal cases are hypothermic and may develop septic shock and disseminated intravascular coagulation.
In the environment, the virus can remain infectious for 1 year or more (Gordon & Angrick 1986) so all facilities where infected animals have been present must be considered contaminated.
FACT SHEET: FELINE HERPESVIRUS (FHV)‐1 VACCINES
Types of Vaccines Available
Modified Live Virus (MLV) Vaccines: These preparations contain attenuated FHV‐1 (feline rhinotracheitis virus, occurring as a single serotype) at various titres, without adjuvant. There are injectable preparations and others for intranasal application, alone or in combination with other vaccinal antigens (always with FCV).
Inactivated (Killed) Vaccines: Adjuvanted killed vaccines are available.
Mechanisms and Duration of Immunity (DOI)
Protection afforded by FHV‐1 (as well as FCV) vaccines is not as complete as that seen with the FPV vaccines. The other two feline core vaccines (FHV‐1 and FCV) should not be expected to provide the same robust degree and duration of immunity as seen with the canine core vaccines or FPV.
Assessment of the DOI is difficult. Complete clinical protection is seen only shortly after vaccination, and the degree of protection decreases with time (Gaskell et al. 2007).
Immunity is far from solid after natural infection/disease and is of variable duration.
Persistence of antibody titre after vaccination with a killed FHV‐1 vaccine was demonstrated for 3 years (Scott & Geissinger 1997), but antibody titre for FHV‐1 does not correlate well with protection (Gaskell et al. 2007).
Protection from challenge with virulent FHV‐1 7.5 years after vaccination with two doses of killed vaccines was not complete, but was similar to protection after 1 year with the killed product (Scott & Geissinger 1999).
After completing the kitten series at 16 weeks or older and vaccinating again at 26 or 52 weeks of age, revaccination need not be done more often than every 3 years in cats at low risk; however, cats at higher risk (e.g. those that regularly attend boarding catteries) should be revaccinated more frequently.
If booster vaccinations have lapsed in a previously well‐vaccinated cat, a single injection is considered adequate to boost immunological memory.
No herpesvirus vaccine can protect against infection with virulent virus; FHV‐1 will become latent and may be reactivated during periods of severe stress. The reactivated virus may cause clinical signs in vaccinated animals (Gaskell et al. 2007); the virus may be shed, transmitted to susceptible animals and cause disease in susceptible kittens and cats (Gaskell et al. 2007).
Cell‐mediated immunity plays an important role in protection, since the absence of detectable serum antibody levels in vaccinated cats does not necessarily indicate that cats are susceptible to disease.
MDA interferes with active immunization for varying periods of time in the kitten, depending on the titre of colostral antibody and the amount of antibody absorbed after birth. The primary course of vaccination is usually started at around 6–8 weeks of age. MDA interferes less with MLV intranasal (IN) vaccines than parenterally administered MLV products. It would be expected that the IN vaccines will immunize earlier than the parenteral vaccines in kittens with MDA.
In breeding catteries, infections mostly appear in kittens prior to weaning, typically between 4–8 weeks of age, as MDA wanes. In most cases, the source of infection is the queen, whose latent virus is reactivated due to the stress of parturition and lactation.
Precautions
Modified live parenteral FHV‐1 and FCV vaccines retain some pathogenic potential and may induce disease if administered incorrectly (i.e. when accidentally aerosolized or ingested or inhaled from vaccine deposited on the skin/hair).
Upper respiratory disease signs are sometimes seen following intranasal vaccination.
Disease Facts
Viral excretion starts as soon as 24 hours after infection and lasts for 1–3 weeks.
Acute disease appears after 2–6 days and resolves within 10–14 days.
The virus spreads along the sensory nerves and reaches neuronal cell bodies, particularly in the trigeminal ganglia, which are the main sites of latency. Most cats become lifelong latent carriers, shedding the virus periodically, upon stressful events (Gaskell et al. 2007). In contrast, shedding of FCV is continuous for a period of months after infection. Herpesvirus genomic DNA persists in the nucleus of infected neurons without replication.
In the environment, the virus is labile and inactivated by commonly used disinfectants.
FACT SHEET: FELINE CALICIVIRUS (FCV) VACCINES
Types of Vaccines Available
Modified Live Virus (MLV) Vaccines: These preparations most often contain feline caliciviruses of the F9 strain without an adjuvant. There are injectable preparations and others for intranasal application, alone or in combination with other vaccinal antigens (always with feline herpesvirus).
Inactivated (Killed) Vaccines: Killed adjuvanted vaccines are also available. One killed vaccine (non‐adjuvanted) contains two calicivirus strains (G1 and 431 strains; Poulet et al. 2005).
Mechanisms and Duration of Immunity (DOI)
There is considerable antigenic variability amongst FCV strains. Prior infection with one strain can significantly reduce the acute clinical signs upon exposure to a heterologous strain, and also oral virus shedding. In general, the level of heterologous protection depends on the pair of virus strains examined.
Virus neutralizing antibodies first appear approximately 7 days after infection; their titre correlates well with protection against homologous challenge. Cats may also be protected in the absence of serum antibody, since local secretory IgA antibody and cellular responses have been demonstrated to provide protection in vaccinated cats.
After vaccination with a killed and adjuvanted FCV vaccine, antibody was shown to persist for at least 4 years (Scott & Geissinger 1997).
Protection from challenge with virulent FCV 7.5 years after vaccination with two doses of killed adjuvanted vaccine was incomplete, but was similar to protection after 1 year with the killed product (Scott & Geissinger 1999).
Protection afforded by FCV (as well as by FHV‐1) vaccines is not as complete as that seen with the FPV vaccines. The two core respiratory vaccines should not be expected to provide the same robust degree and duration of immunity as seen with FPV or the canine core vaccines. Reinfection with FCV of differing strains is possible in vaccinated cats.
After completing the kitten series at 16 weeks or older and vaccinating again at 26 or 52 weeks of age, revaccination need not be done more often than every 3 years in cats at low risk; however, cats at higher risk (e.g. those that regularly attend boarding catteries) may be revaccinated more frequently.
It is recommended that vaccines that contain the same virus strains be used in the kitten series.
MDA is important for protection during the first weeks of life and may interfere with vaccination. The average half‐life of MDA was determined to be 15 days with persistence for 10–14 weeks (Johnson & Povey 1983). In a field study, about 20% of kittens at 6 weeks of age had no detectable antibodies against a widely used vaccine strain (Dawson et al. 2001). MDA interferes less with MLV given intranasally than with parenterally administered MLV products. It would be expected that the IN vaccines will immunize earlier than the parenteral vaccines in kittens with MDA.
Precautions
Upper respiratory disease signs may be seen occasionally as a complication of intranasal vaccination (Lappin et al. 2006, 2009).
Because of the multitude of antigenically differing viruses circulating in the field, vaccine strain combinations have been chosen to cross‐protect against severe clinical disease, but mild disease may still occur in vaccinated cats.
In contrast to FHV‐1, which is shed intermittently after stressful events, shedding of FCV is continuous, but usually ceases after several months (Coyne et al. 2006a). The impact of vaccination on shedding is controversial, with observations ranging from moderate reduction to extension of the period of virus shedding post infection. Live parenteral FCV vaccine strains can be shed, although infrequently.
Disease Facts
FCV infection can cause acute oral and upper respiratory signs, but has also been associated with chronic gingivostomatitis, which may be immune‐mediated.
The incubation period is 2–10 days. Oral ulceration (particularly of the margins of the tongue), sneezing and serous nasal discharge are the main signs. Acute oral and upper respiratory disease signs are mainly seen in kittens.
A distinct syndrome, the ‘virulent systemic feline calicivirus (VS‐FCV) disease’ is occasionally described (Coyne et al. 2006b). The incubation period for this infection in cats exposed in shelters and hospitals is 1–5 days; in the home environment it may be up to 12 days. This disease appears to be more severe in adults than kittens. Vaccination with current vaccines does not protect cats against field infections, but some protection has been shown experimentally (Poulet & Lemeter 2008, Huang et al. 2010). This might be due to the inherent characteristics of the hypervirulent strains. There is a killed VS‐FCV strain in a vaccine available in the USA that contains both ‘traditional’ and VS‐FCV isolates and is reported to provide protection against homologous VS‐FCV (Huang et al. 2010). It is not known if this strain of VS‐FCV will provide protection against heterologous VS‐FCV strains.
FACT SHEET: RABIES VACCINES
Types of Vaccines Available
Modified Live Virus (MLV) Vaccines: In addition to their use in pet dogs and cats, these have been used widely for oral immunization of wildlife (e.g. foxes in Canada and Europe, raccoon dogs in Finland). All are all safe derivatives of the SAD (Street alabama Dufferin) virus strain.
Vectored Recombinant Rabies Vaccines: Recombinant vaccine viruses are particularly safe because they contain only the rabies virus glycoprotein G gene that is relevant for protection. Poxvirus (vaccinia and canary pox) and adenovirus vectors expressing the rabies virus glycoprotein are used routinely in North America for the control of rabies in wildlife (vaccinia and adenovirus vectors) by the oral route, and in cats (canary pox vector) by the parenteral route. These vaccines are avirulent in all avian and mammalian species tested.
Inactivated (Killed) Vaccines: The use of killed vaccines is the rule for individual dog and cat protection and mass canine vaccination programmes. The killed vaccines are easier to manage than live preparations because of their stability at ambient temperatures, and accidents of self‐inoculation do not represent a risk, as would be the case for MLV vaccines.
Mechanisms and Duration of Immunity (DOI)
Canine and feline rabies is controlled mostly by the use of inactivated vaccines. However, in the USA and Europe recombinant canary pox vectored rabies vaccine is licensed and widely used in cats because it is not associated with the inflammation at the injection site caused by adjuvanted rabies vaccines (Day et al. 2007). All initial rabies vaccinations must be followed 1 year later by revaccination. Only after that second vaccination can the interval for revaccination be extended legally to 3 years with a product that has a 3‐year DOI label.
DOI after natural infection cannot be assessed, because disease following street virus infection is fatal in the dog and cat.
DOI after vaccination with commercially available inactivated and recombinant products is 3 years, based on challenge and serological studies (Jas et al. 2012).
First vaccination is not earlier than 12 weeks of age with revaccination 1 year later; antibody titres generally reach protective levels 4 weeks after vaccination. Where serological testing is required for legal purposes the interval between vaccination and testing is crucial and may be product‐dependent. The product datasheet and legal requirements should be consulted.
Some vaccines are proven to protect against virulent rabies virus challenge for 3 years, but national or local legislation may call for annual boosters. The VGG encourages all legislators to consider scientific advances when formulating policy. Some vaccines (e.g. nationally produced products) may not reliably protect for more than 1 year.
The presence of serum antibody of ≥0.5 IU/ml in an actively immunized dog over the age of 16 weeks is correlated with protection. Achieving this concentration (≥0.5 IU/ml) is also considered a legal requirement for pet travel to some countries which include serological testing post‐vaccination in their protocol for movement of pets.
Disease Facts
Signs of disease appear between 2 weeks and several months after infection, depending upon the site of infection (transmission is generally by bite or scratch). Any unexplained aggressive behaviour or sudden behavioural change must be considered suspicious.
The disease manifests itself as a ‘furious’ or a ‘dumb’ form. Signs of the classical ‘furious’ form of rabies include reduced palpebral, corneal and pupillary reflexes, strabismus, dropped jaw, salivation, pica, seizures, twitching, tremors, disorientation, aimless pacing, aimless snapping and biting, exaggerated emotional responses (irritability, rage, fear), photophobia, as well as ataxia and paralysis, ultimately followed by coma and death from respiratory arrest. The ‘dumb’ form of rabies is more common in dogs than cats and presents as lower motor neuron paralysis that progresses from the site of the bite injury to involve the entire central nervous system. The paralysis rapidly leads to coma and death from respiratory failure.
In the environment, the virus quickly loses infectivity, and is readily inactivated using detergent‐based disinfectants.
FREQUENTLY ASKED QUESTIONS (FAQS)
QUESTIONS RELATED TO VACCINE PRODUCTS
1. May I give a MLV product to a wild, exotic species or to a domestic species other than to the ones which the vaccine was licensed to protect?
No, never give MLV vaccines unless they have been shown to be safe in that species. Many MLV vaccines have caused disease in animal species other than those for which they had been licensed. Even worse, the vaccine could be shed from the wild animals, regain virulence through multiple passages and cause disease even in the target species for which it had been developed.
A safe and effective vaccine for species that are susceptible to CDV is the canarypox virus‐vectored recombinant CDV vaccine that is available as a monovalent product for ferrets or a combination product for dogs. The monovalent vaccine is used in many wild and exotic species susceptible to CDV, but is only available in certain countries.
2. May I vaccinate a puppy that is at high risk of getting CDV with a human measles vaccine?
No. Due to an insufficient amount of virus, the human MV vaccine is not immunogenic in the puppy. Measles virus vaccines made specifically for the dog (sometimes combined with CDV and additional viral components) may give temporary protection at an earlier age than a CDV vaccine. At 16 weeks or older, the puppy must be vaccinated with a CDV vaccine, to achieve permanent immunity.
3. Can certain vaccines immunize pups having maternally derived antibody (MDA) against CDV at an earlier age?
Yes. The heterotypic measles vaccine for dogs will immunize pups about 4 weeks earlier than the MLV‐CDV vaccines. Similarly, the canarypox vectored recombinant CDV vaccine will immunize approximately 4 weeks earlier than some MLV vaccines and there are some high titre MLV vaccines (i.e. vaccines containing a greater mass of virus in the vaccine ampoule) which also immunize at an earlier age in puppies with MDA.
4. I know that maternally derived antibodies (MDA) can prevent active immunization with MLV vaccines ‐ but can they also block immunity to killed vaccines?
Yes. MDA can block certain killed vaccines. If the killed product requires two doses, as is often the case, and the first dose is blocked by MDA, then the second dose will not immunize. In this circumstance, the second dose will prime (if not blocked), and a third dose is required to immunize and boost.
This is not true for MLV vaccines, where in the absence of MDA it only takes a single dose to prime, immunize, and boost. Nevertheless two doses are often recommended, particularly in young animals, to be sure one is given when MDA has waned and cannot block. That is why in the puppy or kitten series, the last dose should be given at 16 weeks of age or older.
5. I have been told that certain canine MLV combination core products need only be given twice, with the last dose at an age as young as 10 weeks. Is that accurate?
The VGG is aware that certain canine vaccines are licensed for such an ‘early finish’ in order to allow pups the benefit of early socialization. The VGG accepts the importance of puppy socialization, but has reservations about the immunological validity of this approach to vaccination. No combination core product currently available will immunize an acceptable percentage of puppies (particularly not against CPV‐2) when the last dose is given at 10 weeks of age. The VGG advises that wherever possible the last dose should be given at 16 weeks of age or older, regardless of the number of doses given earlier. The VGG recommends that owners of pups that have not completed a full puppy vaccination series carefully control the exposure of their pup to environments outside of the home and only permit contact with healthy and fully vaccinated dogs.
6. Are there parenteral and intranasal vaccines that protect against the same disease?
Yes, particularly canine vaccines against the canine infectious respiratory disease complex (CIRDC) and feline vaccines against upper respiratory disease caused by FCV and FHV‐1.
You should be careful to give the product by the route for which it is intended. If you use the parenteral (i.e. subcutaneous) MLV vaccines containing FCV and FHV‐1 locally (i.e. intranasally or orally), you could cause serious disease in the cat. If you use the killed FCV and FHV‐1 vaccines locally, you would not get any immunity and might cause significant adverse reactions. If you gave the intranasal live CIRDC vaccine parenterally, you could cause a severe necrotizing local reaction and even kill the dog, while giving the parenteral killed Bordetella vaccine intranasally will not immunize and may cause a hypersensitivity reaction.
However, both types of products can be given at the same time or at various times in the life of the animal. Vaccinating both parenterally and intranasally may actually provide better immunity than vaccinating at only one site (Reagan et al. 2014, Ellis 2015). Thus parenteral vaccination provides protection in the lung, but little or no immunity in the upper respiratory tract (especially local secretory IgA and CMI), while intranasal vaccination will engender good secretory IgA and local CMI and non‐specific immunity (e.g. type‐I interferons), but will not always provide immunity in the lung.
7. How long after vaccination does it take for the dog to develop immunity that will prevent severe disease when the core vaccines are used?
This is dependent on the animal, the vaccine and the disease.
The fastest immunity is provided by MLV and recombinant canarypox virus vectored CDV vaccines. The immune response starts within minutes to hours and provides protection within a day to animals without interfering levels of MDA and in dogs that are not severely immunosuppressed.
Immunity to CPV‐2 and FPV develops after as few as 3 days and is usually present by 5 days when an effective MLV vaccine is used. In contrast, the killed CPV‐2 and FPV vaccines often take 2 to 3 weeks or longer to provide protective immunity.
CAV‐2 MLV given parenterally would provide immunity against CAV‐1 in 5–7 days. However, when given intranasally, the same level of immunity to CAV‐1 is not present until after 2 or more weeks and in some dogs it doesn't develop. Thus parenteral CAV‐2 is recommended for immunity to CAV‐1.
Time from vaccination to immunity is difficult to determine for FCV and FHV‐1 because some animals will not develop protective immunity. However, when it does develop, it takes 7–14 days (Lappin 2012).
8. What can I expect from the core vaccines in terms of efficacy in the properly vaccinated puppy/dog and kitten/cat?
Dogs properly vaccinated with MLV or recombinant CDV, CPV‐2 and CAV‐2 would have ≥98% protection from disease. Similarly we would expect a very high protection from infection.
For the properly vaccinated cat that had received MLV vaccines, we would estimate that ≥98% would be protected from disease and infection with FPV. In contrast, we can expect FCV and FHV‐1 vaccines, at best, to protect from disease, not infection, especially in a highly contaminated environment (e.g. shelter) and protection would be seen in 60 to 70% of recipients in a high risk environment. Protection would appear to be much higher in the household pet cat isolated from other cats or with cats that have been vaccinated and in the household for a long time because the risk for infection with the viruses is so much lower, as is the stress level.
9. Are there mutants (biotypes or variants) of CDV or CPV‐2 in the field that the current vaccines cannot provide protective immunity against?
Not as far as we know. This is not controversial for CDV. All of the current CDV and CPV‐2 vaccines provide protection from all the known isolates of CDV or CPV‐2, respectively, when tested experimentally as well as in the field. However, there is one report of an outbreak of CPV‐2c in Italian dogs that were vaccinated with a MLV vaccine (Decaro et al. 2008). There is another report from the same group of an aged vaccinated dog developing CPV‐2c‐related disease (Decaro et al. 2009).
10. Do the current CPV‐2 vaccines provide protection from disease caused by the new variant CPV‐2c? How long does the protection last?
Yes. The CPV‐2 vaccines, regardless of what variant they contain, stimulate an active immune response (e.g. antibody response), that provides long term (4 or more years) protection from all current CPV‐2 variants (2a, 2b, and 2c) when the dogs are challenged.
11. Can parvovirus vaccines (e.g. canine parvovirus‐2 and feline parvovirus [panleukopenia]) be administered orally?
No. CPV‐2 and FPV vaccines, when given orally, will not immunize. They will immunize when given intranasally, however the most effective route is parenteral (subcutaneous or intramuscular) vaccination using the appropriate vaccines.
12. Can certain CPV‐2 vaccines immunize pups with MDA at an earlier age than other CPV‐2 vaccines?
Yes. Certain CPV‐2 vaccines with higher viral titres (i.e. mass of virus in the vaccine ampoule) and/or with more immunogenic isolates (regardless of variant) will immunize quite a few weeks earlier than other standard CPV‐2 vaccines.
13. When a Leptospira vaccine (bacterin) is used, should it be a product with two serogroups or one with more than two serogroups (e.g. three or four component products available in some countries)?
When a Leptospira vaccine is used in high risk dogs, the commercial vaccine that contains all of the serogroups that cause disease in the dog in that region, if available, should be used. In many countries there is insufficient knowledge of which serogroups are circulating in the canine population. The VGG would encourage collection of such data.
14. Do Leptospira vaccines give long term (e.g. years) immunity and are they highly effective, like the core viral vaccines?
No. Leptospira vaccines provide relatively short‐term immunity. Also, some Leptospira products prevent clinical disease, but fail to protect against infection and shedding of the bacteria, especially when infection occurs more than 6 months after vaccination. Persistence of antibody after vaccination will often be only for a few months and immunological memory for protective immunity is relatively short (e.g. 1 year).
15. Do any feline leukaemia virus vaccines (e.g. killed adjuvanted, subunit, recombinant) provide protection with only one dose of vaccine?
No. All feline leukaemia virus vaccines require a minimum of two doses of vaccine. The two doses should preferably be given 2–4 weeks apart, starting at 8 weeks of age or older. Only after that initial series of two vaccines can you then give a single dose to boost the response. When the interval between the initial two doses exceeds 6 weeks or more, it is recommended that the cat be revaccinated, making certain that two doses be given at an interval of 2–4 weeks.
16. Do cats need to be revaccinated with FeLV vaccines every year after they have received the kitten vaccine and a booster at one year?
No. Revaccination should be every 2–3 years. Annual revaccination with adjuvanted vaccines might increase the risk of development of injection site sarcoma.
17. Why don't I have the FIV vaccine in my country?
The availability of vaccines is generally determined by the manufacturer and the local or regional licensing authority on the basis of scientific knowledge pertaining to the local disease situation (and marketing considerations). The current FIV vaccine contains examples of two clades (subtypes) of FIV (A and D) and although cross‐protection against other subtypes is claimed there are geographical differences in the viruses circulating in different countries. Cats given FIV vaccine should be tested for serum antibody before vaccination and identified with a microchip.
18. Can a cat vaccinated with FIV vaccine be infected with FIV?
Yes. The vaccine will not prevent infection and latency for all subtypes of FIV, thus FIV vaccinated cats can also be infected and act as a source of virus for susceptible cats.
19. Will the current CIRDC vaccines provide any protection from disease caused by canine influenza virus (CIV)?
No. The racing greyhounds that have been found infected and that developed CIV disease had been routinely vaccinated 3 or more times a year with commercial CIRDC vaccines. CIV is antigenically unrelated to any other virus of dogs, but related to Equine Influenza Virus (H3N8). A CIV vaccine is available in the USA and is recommended for at‐risk dogs. A vaccine against the newly emerged (2015) H3N2 virus in the United States has just been conditionally licensed.
20. Is there a vaccine available to aid in the prevention of disease caused by canine influenza virus (CIV)?
Yes. There is a vaccine available in the USA that is designed to aid in the prevention of influenza in dogs caused by the H3N8 virus. The product is an adjuvanted killed vaccine that, like many killed vaccines, requires two initial doses given 2–4 weeks apart. The efficacy and duration of immunity of this CIV vaccine or others that may be developed in the future will be determined in the next few years as information accumulates in the field.
21. Are there vaccines available for dogs and/or cats that are not designed to prevent infectious diseases caused by viruses, bacteria, fungi/yeasts and/or parasites?
Yes. There are vaccines that aid in the prevention of death from snakebites with certain species of snakes, and to aid in the treatment of oral melanomas in dogs.
22. Can nosodes (holistic preparations) be used to immunize pets?
No. Nosodes cannot be used for the prevention of any disease. They do not immunize because they do not contain antigen.
23. What does the VGG think of the use of canine enteric coronavirus vaccines?
The VGG does not recommend the use of canine coronavirus vaccines as there is insufficient evidence that this vaccine is protective, or indeed that enteric coronavirus is a significant canine pathogen. Variant strains of this virus have been reported to cause severe systemic disease in adult dogs and puppies in various parts of the world, but it is unclear whether the available vaccines would protect against these variants. The identification of coronavirus with a test kit does not necessarily mean it is the cause of disease.
24. Is monovalent vaccine better than multivalent vaccine?
Vaccines with the fewest components possible enable practitioners to adhere to the WSAVA guidelines. Multicomponent core MLV vaccines (e.g. for CDV, CAV‐2 and CPV‐2) are ideal for delivery of core vaccinations, but it is best to have individual vaccines for non‐core antigens (e.g. Leptospira, CIRDC) so that these may be given only when risk:benefit analysis suggests that they will be of benefit. For Leptospira vaccines, multicomponent products may provide the best protection if their formulation is based on scientific evidence that justifies the inclusion of multiple serogroups in the vaccine.
25. Will the number of different antigens in multivalent vaccines adversely affect the efficacy of the vaccine?
No. For a multivalent vaccine to be licensed, the manufacturer must prove that each component of the vaccine can induce protective immunity, generally in challenge studies.
26. Can you give all vaccinations at once to an adult dog presented with no previous history of vaccination?
This is a similar question to that above. Yes, a dog should be able to respond to multiple antigens delivered simultaneously. However, you should never mix different vaccines in the same syringe unless specifically indicated by the datasheet. From first principles, it would be good practice to deliver the different vaccines to different anatomical sites so that different lymph nodes are involved in generating the adaptive immune response, but no studies have formally proven this.
27. What are the differences between MLV vaccines and ‘genetically modified’ vaccines?
Genetically modified vaccines include virus vectored vaccines, genetically mutated (gene deleted) vaccines and naked DNA vaccines. These vaccines may theoretically be safer than certain MLV vaccines as there is no chance of ‘reversion to virulence’. These vaccines are also designed to produce an optimum immune response.
28. Can infectious (MLV) vaccines ‘break through’ MDA better than non‐infectious (killed or subunit) vaccines?
Yes, some MLV vaccines and some genetically modified vaccines appear to be able to generate immunity in the presence of MDA earlier than non‐infectious vaccines.
29. Why don't we have suitable combinations of core vaccines available to allow them to be used in accordance with the guidelines?
Suitable products are not available in all countries. If you do not have them, then you and your national small animal veterinary association should lobby the manufacturers and government regulators to bring the suitable products to your marketplace. In many cases, industry would like to make new products available, but the block lies with the licensing authority.
30. Is it better to use vaccines containing local strains rather than international vaccines?
There is no evidence that international core vaccines are unable to provide good protection against CDV, CAV‐1, CAV‐2, CPV‐2, FPV, FCV, FHV‐1 and rabies virus, worldwide. In most instances strain variation does not change the key protective antigens of the organism that are conserved between strains. In the case of Leptospira, inclusion of additional, locally‐important serogroups in a vaccine may lead to enhanced protection.
31. How do practices know that vaccines delivered to them have been stored correctly and that they are still potent?
International manufacturers utilize temperature indication systems during the bulk delivery stages to ensure continuation of the cold chain from importation to practice delivery.
32. How common is tetanus in dogs? Should we vaccinate against it?
In many parts of the world, tetanus is uncommon in dogs. There are no licensed vaccines for dogs, but in some areas deemed as being at high risk, veterinarians do use equine tetanus vaccine in dogs (off‐label use). Given that tetanus is nowadays considerably more frequently observed than canine infectious hepatitis and canine distemper in many parts of the world, development of a licensed canine tetanus vaccine may be justifiable and commercially viable.
33. Does the VGG recommend which vaccine brand should be used?
No. The VGG is an independent academic group that does not make product‐specific recommendations. However, in the case of international vaccines, the VGG knows that all of these products have undergone rigorous assessment of quality, safety and efficacy that has permitted their licensing in many countries. The VGG does not recommend the use of some vaccines – but this is based on a lack of adequate scientific evidence (i.e. peer‐reviewed scientific literature) that the vaccine is necessary or efficacious. Recommendations are reviewed and adjusted as needed periodically.
34. If one wants to use just the DHPPi without the Leptospira component of a vaccine what should be used to reconstitute the DHPPi?
You should ask this question of the manufacturer or supplier of the particular vaccine, but a suitable diluent may be sterile normal saline or sterile water for injection. If not, the manufacturer should be able to provide you with the specific diluent required.
35. Can rabies vaccine be used in small mammals (e.g. rabbits, guinea pigs etc.)?
The VGG does not recommend routine rabies vaccination of small mammals, except for ferrets; however some rabies vaccines are licensed for use in all mammalian species.
36. Should Leptospira vaccine be used 6‐monthly in high‐risk areas?
There is no clear evidence that 6‐monthly revaccination confers greater protection that annual revaccination with Leptospira vaccine; even in high‐risk areas.
37. What happens if a dog is bitten by a free‐roaming dog after receiving the initial puppy rabies vaccine; should it receive post‐exposure prophylaxis (PEP)? What if that dog receives PEP and is then bitten again some weeks later, should it receive another course of PEP?
If the bitten puppy has been vaccinated properly it should be protected against rabies. The VGG is aware that in some countries, PEP is used in this situation for the benefit of the puppy, and more importantly for the benefit of the human family. Repeated PEP is not justified. By that time the puppy will have received multiple vaccinations and further injections will provide no added benefit.
QUESTIONS RELATED TO VACCINATION PROCEDURE
38. May I mix different types of vaccines in the syringe?
No. One should never mix different vaccine preparations in the syringe unless specified by the data sheet.
39. May I co‐inject different vaccines (not part of a single commercial product) into the same animal?
Yes. However, different vaccines should be injected into separate sites that are drained by different lymph nodes.
40. Can you give rabies and DHPPi vaccine at the same time (concurrently)?
Yes, but unless the vaccines have a specific concurrent use claim on the product label, then this may be considered ‘off‐label’ use. Ideally the two vaccines used concurrently in this way should be given at different anatomical sites in order that vaccine antigens are carried to different lymph nodes in order to stimulate adaptive immunity at two distinct locations.
41. May I use smaller vaccine doses in small breeds to reduce the risk of adverse reactions?
No. The volume (e.g. 1.0 ml) as recommended by the manufacturer generally represents the minimum immunizing dose, therefore the total amount must be given. In the USA a new product has been released that is designed for small dogs. This is formulated as a 0.5 ml dose, but contains much the same amount of antigen and adjuvant as a conventional 1.0 ml vaccine. A 0.5 ml dose feline vaccine is also available and again it is only the volume (and not antigen or adjuvant content) that has been reduced.
42. Should the large dog (Great Dane) be injected with the same volume of vaccine as the small dog (Chihuahua)?
Yes. Unlike pharmaceuticals that are dose‐dependent, vaccines are not based on volume per body mass (size), but rather on the minimum immunizing dose.
43. May I vaccinate the anaesthetized patient?
It is best not to do this if possible as the patient may develop a hypersensitivity reaction and vomit, leading to an increased risk of aspiration. Also, anaesthetic agents may be immunomodulatory.
44. May I vaccinate pregnant pets?
Vaccines should not be given during pregnancy unless specifically indicated in the datasheet. The best approach is to ensure that breeding bitches are vaccinated (with core vaccines), but it is unnecessary to give additional core vaccines to breeding bitches immediately before pregnancy – their standard vaccination schedule (e.g. triennial core revaccination) will provide adequate protective immunity and colostral antibody for the puppies. Vaccination with MLV and killed products during pregnancy should be avoided, if at all possible. There are exceptions, especially in shelters, where vaccination would be advised if the pregnant animal has never been vaccinated and there is an outbreak of disease (e.g. CDV or FPV).
45. Does immunosuppressive glucocorticoid treatment in the cat or dog interfere with vaccine immunity?
Studies of both species suggest that immunosuppressive glucocorticoid treatment prior to or concurrently with vaccination does not have a significant suppressive effect on antibody production in response to vaccines. However, revaccination is recommended several weeks (2 or more) after glucocorticoid therapy has ended, especially when treatment occurred during administration of the initial series of core vaccines.
46. May I vaccinate pets that are on immunosuppressive or cytotoxic therapy (other than glucocorticoids) (e.g. for cancer or autoimmune diseases)?
No. Vaccination especially with MLV products should be avoided as they may cause disease; vaccination with killed products may not be effective or may aggravate the immune‐mediated disease. A study of cats treated with high‐dose ciclosporin demonstrated that there was no effect on the serological response to booster FPV and FCV vaccines given during treatment, but that protective antibody responses to FHV‐1, FeLV and rabies were delayed. In contrast, treated cats failed to develop antibody after a primary course of FIV vaccine, suggesting that ciclosporin treatment impairs the primary, but not memory, vaccinal immune response (Roberts et al. 2015).
47. How long after stopping immunosuppressive therapy do I wait before revaccinating a pet?
A minimum of 2 weeks.
48. Should you vaccinate Ehrlichia canis‐infected dogs since these dogs can be immunosuppressed?
There is no evidence that a dog with monocytic ehrlichiosis cannot respond adequately to vaccination, or that protective antibody titres against core vaccine components diminish in E. canis‐infected dogs. Ideally, the dog would be treated and any essential vaccination performed after the cessation of therapy. It may be a legal requirement to give rabies vaccine to such cases in any event.
49. Should one vaccinate an animal which is diseased, hyperthermic or stressed?
No. This is contrary to good practice and the advice on most vaccine datasheets.
50. May I vaccinate every week if an animal is at high risk of disease?
No. Vaccines should not be given more often than every other week, even when different vaccines are being given.
51. If a puppy has no MDA when should you start vaccination?
In a practical setting it would be difficult to prove that a pup had no MDA. This would necessitate knowing definitively that the pup did not take in colostrum. However, if this was known then core vaccination may be given from 4–6 weeks of age. Certain MLV vaccines must not be given any earlier than 4 weeks of age as they may cause pathology in the pup. If this pup definitively had no MDA, it may respond adequately to a single dose of vaccine at 6 weeks of age; however, it may be pragmatic to give a second dose at 16 weeks of age.
52. Can we vaccinate puppies at less than 4 weeks of age?
No. Puppies at this age will have MDA that blocks the ability of MLV vaccines to prime the immune system. Moreover, vaccine datasheets do not support this practice and there may be safety issues with giving MLV vaccine to such young animals. One exception is the use of intranasal vaccines against CIRDC. These can be used safely from 3 weeks of age.
53. When should the last vaccine dose be given in the puppy and kitten vaccine series?
The last dose of vaccine should be given at 16 weeks of age or older.
54. Why don't the VGG recommend rabies vaccination until 12 weeks of age?
Some rabies vaccines are licensed to be given earlier than 12 weeks of age, but we recommend that where this is done the animal receives another vaccine at 12 weeks of age. In the context of mass vaccination campaigns against rabies, it is important to vaccinate as many dogs in the area as possible, including puppies less than 12 weeks of age.
55. May I inject a killed vaccine, followed a short time later with a MLV for the same disease?
No. The killed vaccine may induce an effective antibody response that will neutralize the MLV in the vaccine, thereby preventing immunization. It would be preferable to give the MLV vaccine first and if/when needed, revaccinate with the killed vaccine preparation.
56. May I inject parenterally a modified live intranasal Bordetella vaccine?
No. The vaccine can cause a severe local reaction and may even kill the pet by causing systemic disease (e.g. liver failure)
57. May I give a killed Bordetella vaccine destined for parenteral use intranasally?
No. This will not stimulate a protective response to the Bordetella, but may cause a hypersensitivity response; you should give a live intranasal vaccine via the intranasal route, as specified by the data sheet.
58. If the puppy sneezes after intranasal vaccination is it necessary to vaccinate again?
Sneezing, with loss of some of the vaccine, is commonly observed after the use of intranasal products. These vaccines have been designed to allow for partial loss of the product and so it should not be necessary to revaccinate, unless it is clear that none or very little of the product was delivered successfully.
59. Are precautions necessary when using MLV FHV‐1/FCV parenteral vaccines in cats?
Yes. Mucosal (e.g. conjunctival and nasal) contact with the preparation must be avoided, because the vaccine virus can cause disease. Such contact might come via inappropriate aerosolization of the vaccine or by the cat grooming off vaccine that leaks from an injection site.
60. May I use different vaccine brands (manufacturers) during the vaccination program?
Yes. It may even be desirable to use vaccines from different manufacturers during the life of an animal, because different products may contain different strains (e.g. of feline calicivirus). However, it is not recommended to mix vaccines that contain different strains (e.g. FCV or Leptospira serogroups) during a primary vaccination programme.
61. Is it OK to mix different manufacturer's products during the primary course?
Core MLV vaccines from the different international suppliers are similar in composition and may be mixed during the primary course (e.g. if a puppy has an 8–9 week vaccine from one veterinarian and then moves to another veterinarian who uses a different product range). Manufacturers will not support this practice (and will advise against it) because they have not undertaken studies to prove compatibility of their products with those of other manufacturers. It may also be acceptable to use non‐core vaccines from different manufacturers, with the exception of Leptospira vaccines where a first dose with a two‐serogroup product, and a second dose with a four‐serogroup product, would not induce immunity to the additional two serogroups contained in the four‐way vaccine. The same principle applies to FCV vaccines (see FAQ 60 above).
62. Should I use a disinfectant (e.g. alcohol) on the injection site?
No. The disinfectant might potentially inactivate an MLV product and it is not known to provide a benefit.
63. May I split vaccines in combination products?
Yes. For example, Leptospira bacterins are often used as the diluent for the viral antigen combination. The ‘viral cake’ may be resuspended in sterile water or buffered saline, and the Leptospira bacterin be given separately at another site or time, or discarded.
64. Will a single vaccine dose provide any benefit to the dog or cat? Will it benefit the canine and feline populations?
Yes. One dose of a MLV canine core vaccine (CDV, CPV‐2 CAV‐2) or MLV FPV vaccine should provide long term immunity when given to animals at or after 16 weeks of age. Every puppy and kitten 16 weeks of age or older should receive at least one dose of MLV core vaccines. In the case of feline respiratory core vaccines (FCV and FHV‐1), protection would be maximized by administering two doses of vaccine 2–4 weeks apart.
If that were done, herd (population) immunity would be significantly improved. Even in the USA with its good vaccination record, probably <50% of all puppies and <25% of all kittens ever receive a vaccine. We must vaccinate more animals in the population with core vaccines to achieve better herd immunity (e.g. 75% or higher) and prevent epidemic outbreaks.
65. When an animal first receives a vaccine that requires two doses to immunize (e.g. killed vaccines like Leptospira bacterins or feline leukaemia virus), and it does not return for the second dose within Ä6 weeks, is there any immunity?
No. A single dose of a two‐dose vaccine does not provide immunity. The first dose is for priming the immune system, the second for immunizing. If a second dose is not given within 6 weeks of the first, the regime should start again, making sure the two doses are given within 2–6 weeks. After those two doses, revaccination with a single dose can be done at yearly or greater intervals to boost the response.
66. For how long can a reconstituted MLV vaccine sit at room temperature without losing activity?
At room temperature, some of the more sensitive vaccines (e.g. CDV, FHV‐1) will lose their ability to immunize in 2–3 hours, whereas other components will remain immunogenic for several days (e.g. CPV, FPV). The VGG recommends that MLV vaccines, after reconstitution, should be used within 1 to 2 hrs.
67. If an animal has gone beyond the time that is generally considered to be the minimum DOI for the core vaccine (7 to 9 years for CDV, CPV‐2, CAV‐2; 7 years for FPV), do I have to start the series of vaccinations again (multiple doses 2–4 weeks apart)?
No. For MLV vaccines, multiple doses are only required for puppies or kittens which have MDA. The VGG is aware that many data sheets do advise re‐starting a vaccination series, but does not endorse this practice which is inconsistent with fundamental immune system function and the principles of immunological memory.
68. Should I vaccinate a cat infected with FeLV and/or FIV infection?
A FeLV or FIV positive cat that is clinically well would ideally be housed indoors away from other cats to minimize the risk of exposure to infectious disease. However, if it were deemed necessary to vaccinate with core components (FPV, FCV and FHV‐1) expert groups currently recommend that this should be with killed (not MLV) vaccines. Such cats should not be vaccinated against FeLV or FIV. A FeLV or FIV positive cat with clinical illness should not be vaccinated. In some countries there is a legal requirement for rabies vaccination that would also include retrovirus‐infected cats.
69. Where should I inject vaccine into a cat?
Feline vaccines (particularly adjuvanted products) should not be given into the inter‐scapular region. In the USA the practice of giving separate injections of rabies vaccine into the distal right hind limb, FeLV vaccine into the distal left hind limb and core FPV/FCV/FHV‐1 vaccines into a distal forelimb is practiced. Alternative sites for subcutaneous injection are into the distal tail or over the lateral thoracic or abdominal wall. These options are discussed further in the main text of this document. Whichever site is chosen, the vaccine must be administered subcutaneously and not intramuscularly. Importantly, the anatomical site of feline vaccination should be rotated so that vaccines are not given repeatedly to one location. This may be achieved by recording the site of vaccination for each individual on each occasion and rotating between these, or by adopting a practice policy to use one anatomical location each year.
70. Does severe nutritional deficiency affect the immune response to vaccines?
Yes. It has been shown that certain severe deficiencies of vitamins and trace minerals (e.g. Vitamin E/Selenium) can interfere with the development of a protective immune response in puppies. Known or suspected nutritional deficiencies should be corrected by appropriate nutritional supplementation and the animals should be revaccinated to ensure there is adequate protective immunity.
71. If a puppy or kitten fails to receive colostrum will it have any passive antibody protection from the dam?
Depending on the antibody titre of the dam they will have little or, most likely, no protection as approximately 95% or more of the passive antibody for the newborn puppy and kitten is obtained from the colostrum which is absorbed via the intestine into systemic circulation for up to 24 hours after birth.
72. Should a puppy or kitten that fails to receive colostrum be vaccinated during the first few weeks of life since they will not have maternally derived antibody to block active immunization?
No. Puppies and kittens less than 4–6 weeks of age should not be vaccinated with the MLV core vaccines. Certain of the modified live vaccine viruses when given to puppies/kittens less than 2 weeks of age and without MDA can infect the central nervous system and/or cause disease and possibly death of the animal. This occurs because there is little or no thermoregulatory control of body temperature during the first week or more after birth, thus innate and adaptive immunity is significantly impaired.
73. How can these colostrum‐deprived young animals be protected from the core diseases?
Artificial colostrum can be fed if the puppy or kitten is less than 1 day old. Artificial colostrum is 50% milk replacer (e.g. EsbilacTM or other similar product) and 50% immune serum (preferably from the dam or other well vaccinated animal living in the same environment as the dam). If pups or kittens are older and 1 day of age, serum from a well immunized adult animal (free of infectious disease) can be given subcutaneously or intraperitoneally or citrated plasma can be given intravenously. Depending on size of the animal, approximately 3 to 10 ml of serum or plasma should be administered twice daily for up to 3 days.
74. At what age can one stop vaccinating dogs?
For core vaccines, the current recommendation is for lifelong revaccination no more frequently than every 3 years and if non‐core vaccines are chosen for use, these are generally given annually. One can use serological testing in any adult dog to confirm protection against core diseases (i.e. CDV, CAV and CPV‐2) and elect not to revaccinate that animal. Current advice is that serological assessment is performed every 3 years, but in dogs older than 10 years, this should be done annually. In many countries there is also a legal requirement to vaccinate against rabies at particular intervals.
75. What protocol is recommended for an unvaccinated adult dog?
Core vaccination with a single dose of MLV vaccine (CDV, CAV‐2, CPV‐2) plus rabies in endemic areas. There is no need to give two doses. Revaccination (or serological testing for CDV, CAV and CPV‐2) no more frequently than every 3 years thereafter. Non‐core vaccines should be selected based on a risk:benefit analysis for that individual animal. Non‐core vaccines would require two doses given 2–4 weeks apart and then an annual booster.
76. For an adult dog with an unknown Leptospira vaccination history, what's the recommended vaccination protocol? Is it still two doses 2–4 weeks apart as in puppies?
Yes, this dog would require two doses of vaccine given 2–4 weeks apart and then annual revaccination thereafter.
77. What protocol is recommended for an unvaccinated adult cat?
For an adult cat that has never been vaccinated, the VGG recommends core vaccination with two doses of MLV vaccine (FPV, FCV, FHV‐1) plus one dose of rabies vaccine in endemic areas. Revaccination (or serological testing for FPV) no more frequently than every thereafter for a low‐risk cat, or revaccination no more frequently than every 3 years for FPV and annually for FHV‐1 and FCV for a high‐risk cat. Non‐core vaccines should be selected based on a risk:benefit analysis for that individual animal.
78. Should a cat be vaccinated if it already has signs of upper respiratory disease?
A cat with current clinical disease should not be vaccinated. Once it has recovered, the cat should have some natural immunity to FCV or FHV (or both if both agents were involved in causing the respiratory disease), but such immunity is never sterilizing (even after vaccination). There is no indication NOT to vaccinate a cat that has recovered from a respiratory viral infection. A trivalent vaccine will protect against FPV and also against the respiratory virus (FHV‐1 or FCV) that was not involved in causing the earlier respiratory disease.
79. Power cuts are not uncommon in parts of our country and they can last for 2–3 days. What should one do as regards any vaccine in the fridge at the time – is it OK to use?
MLV vaccine that has not been stored at appropriate temperature for 2–3 days should not be used. Some of the components of these vaccines (e.g. CDV) are temperature sensitive and there may have been inactivation of the virus. If you are in any doubt, you should contact the manufacturer for advice.
QUESTIONS ABOUT THE USE OF SEROLOGICAL TESTING
80. Are serum antibody titres useful in determining vaccine‐induced immunity?
Yes. This is particularly the case for CDV, CPV‐2 and CAV‐1 in the dog, FPV in the cat and (for legal purposes) rabies virus in the cat and dog. Serum antibody titres are of limited or no value for the other vaccines. Assays for CMI are of little or no value for any of the vaccines for various technical and biological reasons. Such factors are less of an issue for serological tests where it is much easier to control many of the variables. However, discrepant results are still obtained, depending on the quality assurance program of the given laboratory.
81. How long after CPV‐2/CDV vaccination should you wait before measuring protective antibody concentrations using in‐clinic tests?
This question is most relevant for puppies, because adult dogs are likely already to have serum antibodies present at the time of booster vaccination, regardless of how long an interval there has been since they were last vaccinated. If a puppy receives its final primary vaccine at 16 weeks of age, then it may be tested from 20 weeks of age onwards. Any antibody present at that stage cannot be of passive, maternal origin and therefore indicates that the puppy is actively protected.
82. Why don't the VGG recommend routine rabies antibody testing?
For many veterinarians, this question may be of little practical consequence, as regular rabies vaccination of dogs and cats is a legal requirement in many countries, irrespective of any titre results. Rabies antibody testing is only required in certain situations related to international pet travel. The international rabies vaccines are highly efficacious and it is generally considered that there is no need to demonstrate immunity post vaccination.
83. Can we use antibody tests (CDV, CPV‐2 and CAV) to test the MDA in order to decide the first vaccination time?
Theoretically this would be possible and years ago a ‘nomogram’ was often used to estimate when pups might best respond to vaccination on the basis of the titre of antibody in the serum of the bitch. In practice, it would be very difficult and expensive to repeatedly sample and test young puppies in order to monitor the decline of MDA.
84. What happens to the antibody titre over the 3‐year period post‐vaccination?
For CDV, CAV‐2, CPV‐2 and FPV the antibody titre will be consistently present at similar titre. This has been shown in numerous field serological surveys of dogs last vaccinated up to 9 years previously and in experimental studies for dogs last vaccinated up to 14 years previously. For Leptospira the titres will decline rapidly after vaccination and in any case are not well correlated with protection. Serum antibody titres are less relevant for FCV and FHV‐1 where the most important type of immunity is mucosal or cell‐mediated, respectively.
85. In an animal that has completed its puppy/kitten shots, is a higher antibody titre required to protect against heavy disease challenge?
For CDV, CAV‐2, CPV‐2 and FPV the answer is no. The presence of antibody (no matter what the titre) indicates protective immunity and immunological memory is present in that animal. Giving more frequent vaccines to animals in an attempt to increase antibody titre is a pointless exercise. It is impossible to create ‘greater immunity’ by attempting to increase an antibody titre.
86. Can we test dogs as an alternative to annual vaccination? We are concerned about the advice to only boost every 3 years.
Yes, certainly. There are now well‐validated in‐practice serological test kits that permit determination of the presence of protective serum antibody specific for CDV, CAV, CPV‐2 and FPV. In other countries, these kits are used to confirm protection at 3‐yearly intervals (instead of automatic revaccination for core diseases). You could perform serology annually, but if you were to collect and analyze the data that you generated within your practice, you will quickly find that annual testing is unjustified.
QUESTIONS ABOUT THE ANNUAL HEALTH CHECK
87. In the annual health check, what tests/examinations should you do?
The annual health check should focus on an excellent basic physical examination (including body temperature, cardiac auscultation and palpation). A thorough history should be taken to understand the lifestyle and disease risks (e.g. travel, boarding, indoor versus outdoor exposure). The fundamentals of nutrition and parasite control should be discussed with the owners. In some countries, the health check might also involve routine testing for prevalent infectious diseases.
88. Some owners may be reluctant to come back just for an annual health check. What advice can be provided to promote the health check concept in order to improve owner compliance?
This is all a matter of education. Clients should realize that the health check examines all aspects of the health and wellbeing of their pet and may pick up the early stages of clinical problems. In terms of vaccination, the health check examination might include serology (every 3 years for core vaccine antigens) or the annual administration of non‐core vaccine if such vaccines are required.
89. The costs of an annual health check are far too high for my clients.
The annual health check may be as simple as an excellent clinical history and physical examination – the costs for which are purely the professional time of the veterinarian. Fundamentally, the concept of an ‘annual health check’ is a new way of delivering what most practitioners already offer as a ‘vaccination booster and physical examination’. For more affluent clientele, the annual health check has proven a means of offering other veterinary services and increasing practice profitability. This is also an example of practicing better quality medicine and about redefining the veterinarian – client relationship.
QUESTIONS RELATED TO ADVERSE REACTIONS TO VACCINES
90. Is there a risk of over‐vaccinating a pet (e.g. injecting too often, or using vaccines that are not required for the specific pet)?
Yes. Vaccines should not be given needlessly, as they may cause adverse reactions. Vaccines are medical products that should be tailored to the needs of the individual animal.
91. Are certain vaccines or combinations of vaccines more likely to cause adverse reactions than others?
Although this is often presumed, there is little scientific evidence to support this statement. The development of an adverse reaction is often dependent on the genetics of the animal (e.g. small breed dogs or families of dogs) (Moore et al. 2005, Kennedy et al. 2007). It has been suggested that bacterins (killed bacterial vaccines), such as Leptospira, Bordetella, Borrelia and Chlamydia are more likely to cause type I hypersensitivity adverse reactions than MLV viral vaccines, but evidence to support this is lacking. It has been suggested that adjuvanted FeLV and rabies vaccines are more likely to be associated with feline injection site sarcoma, but again, there is conflicting evidence.
92. Should dogs and cats with a history of adverse reaction or immune‐mediated diseases (e.g. hives, facial oedema, anaphylaxis, injection site sarcoma, autoimmune disease etc.) be vaccinated?
If the vaccine suggested to cause the adverse reaction is a core vaccine, a serological test can be performed and if the animal is found to be seropositive (antibody to CDV, CAV, CPV‐2, FPV) revaccination is not necessary. If the vaccine is an optional non‐core vaccine (e.g. Leptospira or Bordetella bacterin) revaccination is discouraged. For rabies, the local authorities must be consulted to determine whether the rabies vaccine is to be administered by law or whether antibody titre may be determined as an alternative.
If vaccination is absolutely necessary then switching product (manufacturer) may be helpful. However, this strategy may not always be successful since hypersensitivity reactions are known to be related to excipients contained within the vaccine (e.g. traces of bovine serum albumin used in the virus culture process) which are common to many different products. The use of antihistamines or anti‐inflammatory doses of glucocorticoid pre‐revaccination is acceptable and does not interfere with the vaccinal immune response. Revaccinated susceptible animals should be closely monitored for up to 24 hours post‐vaccination although such reactions (Type I hypersensitivity) generally occur within minutes of exposure. Other types of hypersensitivity (II, III or IV) can occur much later (e.g. hours to months).
93. Small breeds of dog commonly suffer from adverse reactions. Is it possible to reduce the dose of vaccine to avoid this?
No. Vaccine doses are not calculated on a mg/kg basis, as are drugs. The entire antigenic load is needed to stimulate immunity effectively. You should not split vaccine doses, nor give reduced volumes to small dogs. In the USA a new product has been released that is designed for small dogs. This is formulated as a 0.5 ml dose, but contains much the same amount of antigen and adjuvant as does a conventional 1.0 ml vaccine and is unlikely to significantly reduce the prevalence of adverse events in small breed dogs. This, and other commercial vaccines now often contain reduced concentrations of excipients (see Q92) and it is the reduction in concentration of extraneous protein that is likely more important in reducing adverse events.
94. Can vaccines cause autoimmune diseases?
Vaccines themselves do not cause autoimmune disease, but in genetically predisposed animals they may trigger autoimmune responses followed by disease – as can any infection, drug, or a variety of other environmental factors.
95. How common are adverse reactions to vaccines?
There is no definitive answer to this question as it is difficult to obtain accurate data. Determining the frequency of adverse reactions relies upon the veterinarian or owner reporting such reactions to the manufacturer or national authority (where such routes exist). It is currently accepted that the vaccines that we use are very safe with a very low incidence of side effects. The benefits of protection from serious infectious disease far outweigh the risks of developing an adverse reaction. Recent analysis of a major US hospital group database has allowed publication of data based on very large numbers of vaccinated dogs and cats. Adverse reactions (of any kind, including very minor reactions) were documented within the first 3 days following vaccination in 38 of 10,000 vaccinated dogs (Moore et al. 2005). Adverse reactions (of any kind, including very minor reactions) were documented within the first 30 days following vaccination in 52 of 10,000 vaccinated cats (Moore et al. 2007). However, some animals may have had reactions that were not reported to the practice, but were reported to other practices or emergency practices where the animal was seen. Some breeds and families of pets may have a higher risk of adverse reactions than the general population of animals.
96. Are there dogs and cats that cannot develop an immune response to vaccines?
Yes. This is a genetic characteristic seen particularly in some breeds, and these animals are called ‘non‐responders’. Genetically related (same family or same breed) animals will often share this non‐responsiveness. If the animal is a non‐responder to a highly pathogenic agent, like canine parvovirus or feline panleukopenia virus, the infected animal may die if infected. If it is a non‐responder to a pathogen that rarely causes death, it may become sick but will survive (e.g. after a Bordetella bronchiseptica infection).
97. Do puppies develop immunosuppression after the initial series of core vaccines?
Yes. If a combination product containing MLV‐CDV and MLV‐CAV‐2 with other components is used, a period of immunosuppression lasting approximately 1 week develops, beginning 3 days after vaccination (Strasser et al. 2003). This immunosuppression is part of the normal vaccine response and rarely, if ever, causes any clinical problem. If the combination vaccine does not contain either MLV‐CDV or MLV‐CAV‐2, then such suppression does not occur.
98. What can be done to avoid the immunosuppression in puppies, as all should receive the core vaccines (CDV, CPV‐2 and CAV‐2)?
The puppies could receive a bivalent vaccine containing CDV and CPV‐2 parenterally and the CAV‐2 could be given later.
99. Is the immune response to Leptospira responsible for causing a hypersensitivity response in certain dogs also short lived (e.g. <1 year), like immunity from infection?
No. Unlike immunity and IgG memory, which are relatively short lived (≤1 year), memory for immediate hypersensitivity, as determined by skin testing, is long lived (≥4 years).
100. Can you use steroids to treat a case of a mild allergic reaction to a vaccine?
Yes; reactions such as facial oedema and pruritus may be treated with anti‐inflammatory (not immunosuppressive) doses of oral glucocorticoid (e.g. prednisolone) and/or with antihistamines.
101. Is there evidence that cutaneous vasculitis can be caused by vaccination?
Yes, this is a very rare, but recognized, adverse reaction following vaccination, particularly rabies vaccination.
102. Do we see signs of cutaneous allergic reactions to vaccines in cats as in dogs?
Yes. Cats may present with the same manifestations of type I hypersensitivity post vaccination as dogs (e.g. facial oedema and cutaneous pruritus).
103. How do we know that a feline sarcoma was caused by a vaccine? How do we deal with this type of sarcoma?
A feline injection site sarcoma (FISS) arises at an anatomical location into which injectable product has been delivered previously. It is suspected that a wide range of injectables, including vaccines, may potentially trigger these tumours. It is important to record the site of vaccination in cats in the medial record of the animal and the WSAVA guidelines give advice on suggested best locations for vaccinating cats. Non‐adjuvanted vaccines should be chosen for cats wherever possible. Unfortunately, these sarcomas are very aggressive. They infiltrate widely and around 20% may metastasize. They require significant surgical resection that is often best performed by a specialist and adjunct radiotherapy and immunotherapy may be used.
104. Why are there more hypersensitivity cases caused by rabies vaccine than before? Why is this more common among toy poodle dogs?
Hypersensitivity reactions may be caused by any type of vaccine. We now know that a dominant antigen that causes these reactions is bovine serum albumin (BSA) that is incorporated into vaccines during their production. Manufacturers are now reducing the concentration of BSA in animal vaccines. Such reactions are more common in many toy breeds and in many countries these breeds are now particularly popular (Miyaji et al. 2012). There is likely to be a genetic susceptibility, but this is poorly understood.
105. Why do some dog breeding kennels continually have problems with dogs dying from CDV and CPV‐2 infections?
The most likely cause for this scenario is that the breeding stock is not adequately vaccinated. Outbreaks might occur amongst puppies that did not obtain sufficient MDA as the bitch was not effectively vaccinated. In contrast, where puppy vaccination is not performed according to WSAVA guidelines (i.e. with a final puppy vaccine at 16 weeks of age or older) there is a risk that some puppies may be unprotected if the bitch does have a high level of MDA. Finally, there are some breeds of dog (e.g. Rottweiler, Dobermann) that have a greater risk of being genetic non‐responders to these vaccines. Good husbandry, hygiene and nutrition all play a role in minimizing disease outbreaks in kennels.
106. Can a modified live virus revert to virulence? Will a dog be infected by a MLV vaccine?
Yes, a MLV vaccine strain can theoretically revert to virulence, but this is exceedingly rare. As part of vaccine licensing manufacturers are required to prove that this cannot occur if the vaccine virus is shed. MLV vaccines are called ‘infectious vaccines’ because they work by inducing a low level of infection (and virus replication) in the dog, sufficient to induce immunity, but not disease. In the case of canine parvovirus, vaccinated dogs might shed the MLV vaccine strain of virus in the faeces for a short period after vaccination. This does not pose a risk to other dogs.
107. Some pups were vaccinated at 6 weeks of age with DHPPi and developed parvovirus infection at 7 weeks of age; why did this happen?
The most common reason for this occurrence (i.e. infection in a vaccinated pup) is that the animal was already incubating infectious virus before it was vaccinated. It is possible that these pups might have been infected during the ‘window of susceptibility’ when they no longer had sufficient MDA to fully protect them against virulent street virus, but the MDA that was present was still sufficient to interfere with their immune response to a recently administered vaccine.
108. Apart from the (very small) risk of adverse reaction, what are the other risks of annual vaccination?
The risks of adverse reaction following vaccination are indeed relatively small. For dogs and cats this is in the order of 30 to 50 reactions for every 10,000 animals vaccinated, respectively, and the vast majority of these are non‐serious reactions (e.g. transient pyrexia and lethargy, allergic reactions). However, if a serious reaction occurs in one of your client's animals – that is a difficult discussion to have. Adoption of new guidelines is not simply about minimizing the risk of adverse reactions – it is about practicing better, evidence‐based veterinary medicine and only performing a medical procedure (i.e. vaccination) when this is required.
109. Some dogs are genetically poor responders (e.g. Rottweilers). How should one vaccinate these breeds?
The WSAVA guidelines contain a useful flow diagram that helps you to identify non‐responder dogs. All puppies should be vaccinated in the same way (with a final vaccination at 16 weeks of age or older) and if you are concerned about the breed and the potential for lack of response, you should serologically test at 20 weeks of age. Most non‐responders will fail to seroconvert to just one of the core vaccine antigens (i.e. CDV, CAV or CPV‐2). You may attempt to revaccinate and retest that dog, but a true non‐responder (or low‐responder) may still not respond to revaccination. Such animals simply lack the immunological ability to make an immune response to that particular antigen and will never respond to that vaccine component. Owners should be made aware that these dogs will be at risk, and ideally they should not be used for breeding.
110. How should we analyze the risk:benefit of vaccinations?
Risk:benefit analysis really only applies to the choice of non‐core vaccines, as it is taken as given that all dogs and cats (no matter where or how they live) should receive core vaccines (including rabies in endemic areas). The risk:benefit analysis is made for the individual animal, taking into consideration what the owner has told you about its housing, indoor‐outdoor access, travel and boarding frequency, exposure to other animals (e.g. part of a multipet household) etc. The risks to consider are: (1) the risk of adverse reaction following vaccination; (2) the risk that you will be performing an unnecessary medical procedure; (3) the risk that the animal will become infected with the infectious agent based on scientific knowledge about the prevalence of disease in your area; and (4) the risk of developing clinical disease following that infection. The possible benefits to consider are: (1) whether the vaccine might protect the animal from infection if its lifestyle or geographical location means it is likely to be exposed to that infectious agent; (2) whether the vaccine might reduce the severity of clinical signs should that animal become infected; and (3) whether the animal being vaccinated contributes to herd immunity amongst the population.
REFERENCES
- Anderson, T. C. , Crawford, P. C. , Dubovi, E. J. , et al. (2013) Prevalence of and exposure factors for seropositivity to H3N8 canine influenza virus in dogs with influenza‐like illness in the United States. Journal of the American Veterinary Medical Association 242, 209‐216 [DOI] [PubMed] [Google Scholar]
- Anon (2013a) Survey suggests many pets do not receive preventive healthcare. Veterinary Record 172, 569 [DOI] [PubMed] [Google Scholar]
- Anon (2013b) WSAVA and OIE call for action on rabies. Veterinary Record 173, 463‐464 [DOI] [PubMed] [Google Scholar]
- Arjona, A. , Barquero, N. , Domenech, A. , et al. (2007) Evaluation of a novel nested PCR for the routine diagnosis of feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV). Journal of Feline Medicine and Surgery 9, 14‐22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AVSAB (American Veterinary Society of Animal Behavior) (2008) Position statement on puppy socialization. http://avsabonline.org/uploads/position_statements/Puppy_Socialization_Position_download_-_10-4-14.pdf [Google Scholar]
- Bande, F. , Arshad, S. S. , Hassan, L. , et al. (2012) Prevalence and risk factors of feline leukaemia virus and feline immunodeficiency virus in peninsular Malaysia. BMC Veterinary Research 8, 33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandecchi, P. , Matteucci, D. , Baldinotti, F. , et al. (1992) Prevalence of feline immunodeficiency virus and other retroviral infections in sick cats in Italy. Veterinary Immunology and Immunopathology 31, 337‐345 [DOI] [PubMed] [Google Scholar]
- Beczkowski, P. M. , Harris, M. , Techakriengkrai, N. , et al. (2015) Neutralising antibody response in domestic cats immunised with a commercial feline immunodeficiency virus (FIV) vaccine. Vaccine 33, 977‐984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beczkowski, P. M. , Litster, A. , Lin, T. L. , et al. (2015) Contrasting clinical outcomes in two cohorts of cats naturally infected with feline immunodeficiency virus (FIV). Veterinary Microbiology 176, 50‐60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, M. , McCracken, C. , Lutz, H. , et al. (1989) Prevalence of antibody to feline immunodeficiency virus in some cat populations. Veterinary Record 124, 397‐398 [DOI] [PubMed] [Google Scholar]
- Bergman, P. J. , Camps‐Palau, M. A. , McKnight, J. A. , et al. (2006) Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the Animal Medical Centre. Vaccine 24, 4582‐4585 [DOI] [PubMed] [Google Scholar]
- Bohm, M. , Thompson, H. , Weir, A. , et al. (2004) Serum antibody titres to canine parvovirus, adenovirus and distemper virus in dogs in the UK which had not been vaccinated for at least three years. Veterinary Record 154, 457‐463 [DOI] [PubMed] [Google Scholar]
- Bongiorno, G. , Paparcone, R. , Foglia Manzillo, V. , et al. (2013) Vaccination with LiESP/QA‐21 (CaniLeish®) reduces the intensity of infection in Phlebotomus perniciosus fed on Leishmania infantum infected dogs ‐ a preliminary xenodiagnosis study. Veterinary Parasitology 197, 691‐695 [DOI] [PubMed] [Google Scholar]
- Bragg, R. F. , Duffy, A. L. , DeCecco, F. A. , et al. (2012) Clinical evaluation of a single dose of immune plasma for treatment of canine parvovirus infection. Journal of the American Veterinary Medical Association 240, 700‐704 [DOI] [PubMed] [Google Scholar]
- Brun, A. , Chappuis, G. , Precausta, P. , et al. (1979) Immunisation against panleukopenia: early development of immunity. Comparative Immunology, Microbiology and Infectious Diseases 1, 335‐339 [DOI] [PubMed] [Google Scholar]
- Buonavoglia, C. , Decaro, N. , Martella, V. , et al. (2006) Canine coronavirus highly pathogenic for dogs. Emerging Infectious Diseases 12, 492‐494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter, J. W. , Appel, M. J. , Erickson, R. C. , et al. (1976) Fatal vaccine‐induced canine distemper virus infection in black‐footed ferrets. Journal of the American Veterinary Medical Association 169, 961‐964 [PubMed] [Google Scholar]
- Castleman, W. L. , Powe, J. R. , Crawford, P. C. , et al. (2010) Canine H3N8 influenza virus infection in dogs and mice. Veterinary Pathology 47, 507‐517 [DOI] [PubMed] [Google Scholar]
- Chang Fung Martel, J. , Gummow, B. , Burgess, G. , et al. (2013) A door‐to‐door prevalence study of feline immunodeficiency virus in Australia. Journal of Feline Medicine and Surgery 15, 1070‐1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, J. K. , Pu, R. , Martin, M. M. , et al. (2014) Feline immunodeficiency virus (FIV) vaccine efficacy and FIV neutralizing antibodies. Vaccine 32, 746‐754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly, M. , Thomas, P. , Woodroffe, R. , et al. (2013) Comparison of oral and intramuscular recombinant canine distemper vaccination in African wild dogs (Lycaon pictus). Journal of Zoo and Wildlife Medicine 44, 882‐888 [DOI] [PubMed] [Google Scholar]
- Coyne, K. P. , Dawson, S. , Radford, A. D. , et al. (2006) Long‐term analysis of feline calicivirus prevalence and viral shedding patterns in naturally infected colonies of domestic cats. Veterinary Microbiology 118, 12‐25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne, K. P. , Jones, B. R. , Kipar, A. , et al. (2006) Lethal outbreak of disease associated with feline calicivirus infection in cats. Veterinary Record 158, 544‐550 [DOI] [PubMed] [Google Scholar]
- Crawford, P. C. , Dubovi, E. J. , Castleman, W. L. , et al. (2005) Transmission of equine influenza virus to dogs. Science 310, 482‐485 [DOI] [PubMed] [Google Scholar]
- Curtis, R. & Barnett, K. C. (1983) The ‘blue eye’ phenomenon. Veterinary Record 112, 347‐353 [DOI] [PubMed] [Google Scholar]
- Daly, J. M. , Blunden, A. S. , Macrae, S. , et al. (2008) Transmission of equine influenza virus to English foxhounds. Emerging Infectious Disease 14, 461‐464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson, S. , Willoughby, K. , Gaskell, R. M. , et al. (2001) A field trial to assess the effect of vaccination against feline herpesvirus, feline calicivirus and feline panleukopenia virus in 6‐week‐old kittens. Journal of Feline Medicine and Surgery 3, 17‐22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, M. J. (2010a) Ageing, immunosenescence and inflammageing in the dog and cat. Journal of Comparative Pathology 142(Suppl. 1), S60‐S69 [DOI] [PubMed] [Google Scholar]
- Day, M. J. (2010b) One health: the small companion animal dimension. Veterinary Record 167, 847‐849 [DOI] [PubMed] [Google Scholar]
- Day, M. J. , Breitschwerdt, E. , Cleaveland, S. , et al. (2012) Surveillance of zoonotic infectious diseases transmitted by small companion animals. Emerging Infectious Diseases. DOI: 10.3201/eid1812.120664 [DOI] [Google Scholar]
- Day, M. J. , Horzinek, M. , Schultz, R. D. (2007) Guidelines for the vaccination of dogs and cats. Journal of Small Animal Practice 48, 528‐541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, M. J. , Horzinek, M. , Schultz, R. D. (2010) Guidelines for the vaccination of dogs and cats. Journal of Small Animal Practice 51, 338‐356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, M. J. , Schoon, H.‐A. , Magnol, J.‐P. , et al. (2007) A kinetic study of histopathological changes in the subcutis of cats injected with non‐adjuvanted and adjuvanted multi‐component vaccines. Vaccine 25, 4073‐4084 [DOI] [PubMed] [Google Scholar]
- Day, M. J. & Schultz, R. D. (2014) Vaccination. In: Veterinary Immunology: Principles and Practice. Taylor and Francis, Boca Raton: p 224 [Google Scholar]
- Dean, R. S. , Pfeiffer, D. U. , Adams, V. J. (2013) The incidence of feline injection site sarcomas in the United Kingdom. BMC Veterinary Research 9, 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro, N. & Buonavoglia, C. (2012) Canine parvovirus ‐ a review of epidemiological and diagnostic aspects with emphasis on type 2c. Veterinary Microbiology 155, 1‐12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro, N. , Crescenzo, G. , Desario, C. , et al. (2014) Long‐term viraemia and fecal shedding in pups after modified‐live canine parvovirus vaccination. Vaccine 32, 3850‐3853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro, N. , Desario, C. , Elia, G. , et al. (2008) Evidence for immunisation failure in vaccinated adult dogs infected with canine parvovirus type 2c. New Microbiology 31, 125‐130 [PubMed] [Google Scholar]
- Decaro, N. , Mari, V. , Campolo, M. , et al. (2009) Recombinant canine coronaviruses related to transmissible gastroenteritis of swine are circulating in dogs. Journal of Virology 83, 1532‐1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande, M. S. , Jirjis, F. F. , Tubbs, A. L. , et al. (2009) Evaluation of the efficacy of a canine influenza virus (H3N8) vaccine in dogs following experimental challenge. Veterinary Therapeutics 10, 103‐112 [PubMed] [Google Scholar]
- DiGangi, B. A. , Gray, L. K. , Levy, J. K. , et al. (2011) Detection of protective antibody titers against feline panleukopenia virus, feline herpesvirus‐1, and feline calicivirus in shelter cats using a point‐of‐care ELISA. Journal of Feline Medicine and Surgery 13, 912‐918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGangi, B. A. , Levy, J. K. , Griffin, B. , et al. (2011) Effects of maternally‐derived antibodies on serologic responses to vaccination in kittens. Journal of Feline Medicine and Surgery 14, 118‐123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGangi, B. A. , Levy, J. K. , Griffin, B. , et al. (2012) Prevalence of serum antibody titers against feline panleukopenia virus, feline herpesvirus 1, and feline calicivirus in cats entering a Florida animal shelter. Journal of the American Veterinary Medical Association 241, 1320‐1325 [DOI] [PubMed] [Google Scholar]
- Dodds, W. J. (2012) Immune plasma for treatment of parvoviral gastroenteritis. Journal of the American Veterinary Medical Association 240, 1056 [PubMed] [Google Scholar]
- Dunham, S. P. , Bruce, J. , MacKay, S. , et al. (2006) Limited efficacy of an inactivated feline immunodeficiency virus vaccine. Veterinary Record 158, 561‐562 [DOI] [PubMed] [Google Scholar]
- Durchfeld, B. , Baumgartner, W. , Herbst, W. , et al. (1990) Vaccine‐associated canine distemper infection in a litter of African hunting dogs (Lycaon pictus). Zentralbl Veterinarmed B 37, 203‐212 [DOI] [PubMed] [Google Scholar]
- Ellis, J. A. (2015) How well do vaccines for Bordetella bronchiseptica work in dogs? A critical review of the literature 1977–2014. Veterinary Journal 204, 5‐16 [DOI] [PubMed] [Google Scholar]
- Ellis, J. A. & Krakowka, G. S. (2012) A review of canine parainfluenza virus infection in dogs. Journal of the American Veterinary Medical Association 240, 273‐284 [DOI] [PubMed] [Google Scholar]
- Espinal, M. A. , Diaz, F. J. , Ruiz‐Saenz, J. (2014) Phylogenetic evidence of a new canine distemper virus lineage among domestic dogs in Colombia, South America. Veterinary Microbiology 172, 168‐176 [DOI] [PubMed] [Google Scholar]
- Fernandes, C. B. , Torres Magalhaes Jr, J. , de Jesus, C. , et al. (2014) Comparison of two commercial vaccines against visceral leishmaniasis in dogs from endemic areas: IgG, and subclasses, parasitism, and parasite transmission by xenodiagnosis. Vaccine 32, 1287‐1295 [DOI] [PubMed] [Google Scholar]
- Fischer, S. M. , Quest, C. M. , Dubovi, E. J. , et al. (2007) Response of feral cats to vaccination at the time of neutering. Journal of the American Veterinary Medical Association 230, 52‐58 [DOI] [PubMed] [Google Scholar]
- Friedrich, K. & Truyen, U. (2000) Untersuchung der wirksamkeit von parvovirussimpfstoffen und der effektivitat zweier impfschemata. Praktischer Tierarzt 81, 988‐994 [Google Scholar]
- Friend, S. C. , Birch, C. J. , Lording, P. M. , et al. (1990) Feline immunodeficiency virus: prevalence, disease associations and isolation. Australian Veterinary Journal 67, 237‐243 [DOI] [PubMed] [Google Scholar]
- Gaskell, R. , Dawson, S. , Radford, A. , et al. (2007) Feline herpesvirus. Veterinary Research 38, 337‐354 [DOI] [PubMed] [Google Scholar]
- Gibbs, E. P. J. (2014) The evolution of One Health: a decade of progress and challenges for the future. Veterinary Record 174, 85‐91 [DOI] [PubMed] [Google Scholar]
- Gleich, S. E. , Krieger, S. , Hartmann, K. (2009) Prevalence of feline immunodeficiency virus and feline leukaemia virus among client‐owned cats and risk factors for infection in Germany. Journal of Feline Medicine and Surgery 11, 985‐992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon, P. J. , Cockburn, T. , Stark, D. M. (1991) Prevalence of feline immunodeficiency virus and feline leukemia virus infections in random‐source cats. Laboratory Animal Science 41, 545‐547 [PubMed] [Google Scholar]
- Gobar, G. M. & Kass, P. H. (2002) World Wide Web‐based survey of vaccination practices, postvaccinal reactions, and vaccine site‐associated sarcomas in cats. Journal of the American Veterinary Medical Association 220, 1477‐1482 [DOI] [PubMed] [Google Scholar]
- Gordon, J. C. & Angrick, E. J. (1986) Canine parvovirus: environmental effects on infectivity. American Journal of Veterinary Research 47, 1464‐1467 [PubMed] [Google Scholar]
- Gray, L. K. , Crawford, P. C. , Levy, J. K. , et al. (2012) Comparison of two assays for detection of antibodies against canine parvovirus and canine distemper virus in dogs admitted to a Florida animal shelter. Journal of the American Veterinary Medical Association 240, 1084‐1087 [DOI] [PubMed] [Google Scholar]
- Grosenbaugh, D. A. , Leard, A. T. , Bergman, P. J. , et al. (2011) Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. American Journal of Veterinary Research 72, 1631‐1638 [DOI] [PubMed] [Google Scholar]
- Hartman, E. G. , van Houten, M. , Frik, J. F. , et al. (1984) Humoral immune response of dogs after vaccination against leptospirosis measured by an IgM‐ and IgG‐specific ELISA. Veterinary Immunology and Immunopathology 7, 245‐254 [DOI] [PubMed] [Google Scholar]
- Hartmann, K. , Day, M. J. , Thiry, E. , et al. (2015) Feline injection site sarcoma: ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery 17, 606‐613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks, C. G. , Levy, J. K. , Tucker, S. J. , et al. (2014) Tail vaccination in cats: a pilot study. Journal of Feline Medicine and Surgery 16, 275‐280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess, T. J. , Parker, D. S. , Hassall, A. J. , et al. (2011) Evaluation of efficacy of oral administration of Bordetella intranasal vaccine when used to protect puppies from tracheobronchitis due to B . Bronchiseptica infection. International Journal of Applied Research in Veterinary Medicine 9, 301‐305 [Google Scholar]
- Hitt, M. E. , Spangler, L. , McCarville, C. (1992) Prevalence of feline immunodeficiency virus in submissions of feline serum to a diagnostic laboratory in Atlantic Canada. Canadian Veterinary Journal 33, 723‐726 [PMC free article] [PubMed] [Google Scholar]
- Hoare, C. M. , DeBouck, P. , Wiseman, A. (1997) Immuonogenicity of a low‐passage, high‐titer modified live canine parvovirus vaccine in pups with maternally derived antibodies. Vaccine 15, 273‐275 [DOI] [PubMed] [Google Scholar]
- Hofmann‐Lehmann, R. , Fehr, D. , Grob, M. , et al. (1996) Prevalence of antibodies to feline parvovirus, calicivirus, herpesvirus, coronavirus, and immunodeficiency virus and of feline leukemia virus antigen and the interrelationship of these viral infections in free‐ranging lions in east Africa. Clinical and Diagnostic Laboratory Immunology 3, 554‐562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horzinek, M. C. (2010) Vaccination protocols for companion animals: the veterinarian's perspective. Journal of Comparative Pathology 142(Suppl. 1), S129‐S132 [DOI] [PubMed] [Google Scholar]
- Hosie, M. J. , Addie, D. , Belak, S. , et al. (2013) Matrix vaccination guidelines: ABCD recommendations for indoor/outdoor cats, rescue shelter cats and breeding catteries. Journal of Feline Medicine and Surgery 15, 540‐544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie, M. J. & Beatty, J. A. (2007) Vaccine protection against feline immunodeficiency virus: setting the challenge. Australian Veterinary Journal 85, 5‐12 [DOI] [PubMed] [Google Scholar]
- Hosie, M. J. , Osborne, R. , Yamamoto, J. K. , et al. (1995) Protection against homologous but not heterologous challenge induced by inactivated feline immunodeficiency virus vaccines. Journal of Virology 69, 1253‐1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie, M. J. , Robertson, C. , Jarrett, O. (1989) Prevalence of feline leukaemia virus and antibodies to feline immunodeficiency virus in cats in the United Kingdom. Veterinary Record 125, 293‐297 [DOI] [PubMed] [Google Scholar]
- Houston, D. M. , Ribble, C. S. , Head, L. L. (1996) Risk factors associated with parvovirus enteritis in dogs: 283 cases (1982–1991). Journal of the American Veterinary Medical Association 208, 542‐546 [PubMed] [Google Scholar]
- Huang, C. , Hess, J. , Gill, M. , et al. (2010) A dual‐strain feline calicivirus vaccine stimulates broader cross‐neutralization antibodies than a single‐strain vaccine and lessens clinical signs in vaccinated cats when challenged with a homologous feline calicivirus strain associated with virulent systemic disease. Journal of Feline Medicine and Surgery 12, 129‐137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakel, V. , Cussler, K. , Hanschmann, K. M. , et al. (2012) Vaccination against feline panleukopenia: implications from a field study in kittens. BMC Veterinary Research 8, 62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jas, D. , Coupier, C. , Edlund Toulemonde, E. , et al. (2012) Three‐year duration of immunity in cats vaccinated with a canarypox‐vectored recombinant rabies virus vaccine. Vaccine 30, 6991‐6996 [DOI] [PubMed] [Google Scholar]
- Jas, D. , Frances‐Duvert, V. , Vernes, D. , et al. (2015) Three‐year duration of immunity for feline herpesvirus and calicivirus evaluated in a controlled vaccination‐challenge laboratory trial. Veterinary Microbiology 177, 123‐131 [DOI] [PubMed] [Google Scholar]
- Johnson, R. P. & Povey, R. C. (1983) Transfer and decline of maternal antibody to feline calicivirus. Canadian Veterinary Journal 24, 6‐9 [PMC free article] [PubMed] [Google Scholar]
- Jones, B. R. , Hodge, H. , Davies, E. (1995) The prevalence of feline immunodeficiency virus infection in hyperthyroid cats. New Zealand Veterinary Journal 43, 23‐24 [DOI] [PubMed] [Google Scholar]
- Kapil, S. , Allison, R. W. , Johnston, L. III , et al. (2008) Canine distemper virus strains circulating among North American dogs. Clinical and Vaccine Immunology 15, 707‐712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass, P. H. , Barnes, W. G. Jr. , Spangler, W. L. , et al. (1993) Epidemiologic evidence for a causal relation between vaccination and fibrosarcoma tumorigenesis in cats. Journal of the American Veterinary Medical Association 203, 396‐405 [PubMed] [Google Scholar]
- Kennedy, L. J. , Lunt, M. , Barnes, A. , et al. (2007) Factors influencing the antibody response of dogs vaccinated against rabies. Vaccine 25, 8500‐8507 [DOI] [PubMed] [Google Scholar]
- Kirkland, P. D. , Finlaison, D. S. , Crispe, E. , et al. (2010) Influenza virus transmission from horses to dogs, Australia. Emerging Infectious Diseases 16, 699‐702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaasen, H. L. , Molkenboer, M. J. , Vrijenhoek, M. P. , et al. (2003) Duration of immunity in dogs vaccinated against leptospirosis with a bivalent inactivated vaccine. Veterinary Microbiology 95, 121‐132 [DOI] [PubMed] [Google Scholar]
- Klassen, H. L. B. M. , van der Veen, M. , Molkenboer, M. J. C. H. , et al. (2012) A novel tetravalent Leptospira bacterin protects against infection and shedding following challenge in dogs. Veterinary Record 172, 181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaasen, H. L. B. M. , van der Veen, M. , Sutton, D. , et al. (2014) A new tetravalent canine leptospirosis vaccine provides at least 12 months immunity against infection. Veterinary Immunology and Immunopathology 158, 26‐29 [DOI] [PubMed] [Google Scholar]
- Korbelik, J. , Rand, J. S. , Morton, J. M. (2011) Comparison of early socialization practices used for litters of small‐scale registered dog breeders and nonregistered dogs breeders. Journal of the American Veterinary Medical Association 239, 1090‐1097 [DOI] [PubMed] [Google Scholar]
- Kusuhara, H. , Hohdatsu, T. , Seta, T. , et al. (2007) Serological differentiation of FIV‐infected cats from dual‐subtype feline immunodeficiency virus vaccine (Fel‐O‐Vax FIV) inoculated cats. Veterinary Microbiology 120, 217‐225 [DOI] [PubMed] [Google Scholar]
- Ladlow, J. (2013) Injection site‐associated sarcoma in the cat: treatment recommendations and results to date. Journal of Feline Medicine and Surgery 15, 409‐418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin, M. R. (2012) Feline panleukopenia virus, feline herpesvirus‐1 and feline calicivirus antibody responses in seronegative specific pathogen‐free kittens after parenteral administration of an inactivated FVRCP vaccine or a modified live FVRCP vaccine. Journal of Feline Medicine and Surgery 14, 161‐164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin, M. R. , Andrews, J. , Simpson, D. , et al. (2002) Use of serologic tests to predict resistance to feline herpesvirus 1, feline calicivirus, and feline parvovirus infection in cats. Journal of the American Veterinary Medical Association 220, 38‐42 [DOI] [PubMed] [Google Scholar]
- Lappin, M. R. , Sebring, R. W. , Porter, M. , et al. (2006) Effects of a single dose of an intranasal feline herpesvirus 1, calicivirus, and panleukopenia vaccine on clinical signs and virus shedding after challenge with virulent feline herpesvirus 1. Journal of Feline Medicine and Surgery 8, 158‐163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin, M. R. , Veir, J. , Hawley, J. (2009) Feline panleukopenia virus, feline herpesvirus‐1, and feline calicivirus antibody responses in seronegative specific pathogen‐free cats after a single administration of two different modified live FVRCP vaccines. Journal of Feline Medicine and Surgery 11, 159‐162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson, L. J. , Henningson, J. , Sharp, P. , et al. (2011) Efficacy of the canine influenza virus H3N8 vaccine to decrease severity of clinical disease after co‐challenge with canine influenza virus and Streptococcus equi subsp. zooepidemicus . Clinical and Vaccine Immunology 18, 559‐564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson, L. J. & Schultz, R. D. (2006) Effect of vaccination with recombinant canine distemper virus vaccine immediately before exposure under shelter‐like conditions. Veterinary Therapeutics 7, 113‐118 [PubMed] [Google Scholar]
- Lee, I. T. , Levy, J. K. , Gorman, S. P. , et al. (2002) Prevalence of feline leukemia virus infection and serum antibodies against feline immunodeficiency virus in unowned free‐roaming cats. Journal of the American Veterinary Medical Association 220, 620‐622 [DOI] [PubMed] [Google Scholar]
- Lechner, E. S. , Crawford, P. C. , Levy, J. K. , et al. (2010) Prevalence of protective antibody titers for canine distemper virus and canine parvovirus in dogs entering a Florida animal shelter. Journal of the American Veterinary Medical Association 236, 1317‐1321 [DOI] [PubMed] [Google Scholar]
- Levy, J. K. , Crawford, P. C. , Kusuhara, H. , et al. (2008) Differentiation of feline immunodeficiency virus vaccination, infection, or vaccination and infection in cats. Journal of Veterinary Internal Medicine 22, 330‐334 [DOI] [PubMed] [Google Scholar]
- Litster, A. , Nichols, J. , Volpe, A. (2012) Prevalence of positive antibody test results for canine parvovirus (CPV) and canine distemper virus (CDV) and response to modified live vaccination against CPV and CDV in dogs entering animal shelters. Veterinary Microbiology 157, 86‐90 [DOI] [PubMed] [Google Scholar]
- Lloret, A. (2009) The process of evidence‐based medicine. Journal of Feline Medicine and Surgery 11, 529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes, R. (2012) Felid herpesvirus type 1 infection in cats: a natural host model for alphaherpesvirus pathogenesis. ISRN Veterinary Science 2012, 495830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martano, M. , Morello, E. , Buracco, P. (2011) Feline injection‐site sarcoma: past, present and future perspectives. Veterinary Journal 188, 136‐141 [DOI] [PubMed] [Google Scholar]
- Martin, L. E. R. , Wiggans, K. T. , Wennogle, S. A. , et al. (2014) Vaccine‐associated Leptospira antibodies in client‐owned dogs. Journal of Veterinary Internal Medicine 28, 789‐792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meichner, K. , Kruse, D. B. , Hirschberger, J. , et al. (2012) Changes in prevalence of progressive feline leukaemia virus infection in cats with lymphoma in Germany. Veterinary Record 171, 348 [DOI] [PubMed] [Google Scholar]
- Mende, K. , Stuetzer, B. , Truyen, U. et al. (2014) Evaluation of an in‐house dot enzyme‐linked immunosorbent assay to detect antibodies against feline panleukopenia virus. Journal of Feline Medicine and Surgery, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, S. A. , Zwijnenberg, R. J. , Huang, J. , et al. (2012) Duration of serological response to canine parvovirus‐type 2, canine distemper virus, canine adenovirus‐type 1 and canine parainfluenza virus in client‐owned dogs in Australia. Australian Veterinary Journal 90, 468‐473 [DOI] [PubMed] [Google Scholar]
- Miyaji, K. , Suzuki, A. , Shimakura, H. , et al. (2012) Large‐scale survey of adverse reactions to canine non‐rabies combined vaccines in Japan. Veterinary Immunology and Immunopathology 145, 447‐452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, G. E. , Guptill, L. F. , Ward, M. P. , et al. (2005) Adverse events diagnosed within three days of vaccine administration in dogs. Journal of the American Veterinary Medical Association 227, 1102‐1108 [DOI] [PubMed] [Google Scholar]
- Moore, G. E. , DeSantis‐Kerr, A. C. , Guptill, L. F. , et al. (2007) Adverse events after vaccine administration in cats: 2560 cases (2002–2005). Journal of the American Veterinary Medical Association 231, 94‐100 [DOI] [PubMed] [Google Scholar]
- Moreno, J. , Vouldoukis, I. , Schreiber, P. , et al. (2014) Primary vaccination with the LiESP/QA‐21 vaccine (CaniLeish®) produces a cell‐mediated immune response which is still present 1 year later. Veterinary Immunology and Immunopathology 158, 199‐207 [DOI] [PubMed] [Google Scholar]
- Morton, J. M. , McCoy, R. J. , Kann, R. K. , et al. (2012) Validation of real‐time polymerase chain reaction tests for diagnosing feline immunodeficiency virus infection in domestic cats using Bayesian latent class models. Preventive Veterinary Medicine 104, 136‐148 [DOI] [PubMed] [Google Scholar]
- Mouzin, D. E. , Lorenzen, M. J. , Haworth, J. D. , et al. (2004) Duration of serologic response to five viral antigens in dogs. Journal of the American Veterinary Medical Association 224, 55‐60 [DOI] [PubMed] [Google Scholar]
- Muirden, A. (2002) Prevalence of feline leukaemia virus and antibodies to feline immunodeficiency virus and feline coronavirus in stray cats sent to an RSPCA hospital. Veterinary Record 150, 621‐625 [DOI] [PubMed] [Google Scholar]
- Norris, J. M. , Bell, E. T. , Hales, L. , et al. (2007) Prevalence of feline immunodeficiency virus infection in domesticated and feral cats in eastern Australia. Journal of Feline Medicine and Surgery 9, 300‐308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohneiser, S. A. , Hills, S. F. , Cave, N. J. et al. (2015) Canine parvoviruses in New Zealand form a monophyletic group distinct from the viruses circulating in other parts of the world. Veterinary Microbiology (in press). [DOI] [PubMed] [Google Scholar]
- Ottnod, J. M. , Smedley, R. C. , Walshaw, R. , et al. (2013) A retrospective analysis of the efficacy of Oncept vaccine for the adjunct treatment of canine oral malignant melanoma. Veterinary and Comparative Oncology 11, 219‐229 [DOI] [PubMed] [Google Scholar]
- Palatnik‐de‐Sousa, C. B. & Day, M. J. (2011) One health: the global challenge of epidemic and endemic leishmaniasis. Parasites and Vectors 4, 197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatnik‐de‐Sousa, C. B. , Silva‐Antunes, I. , de Aguiar Morgado, A. , et al. (2009) Decrease of the incidence of human and canine visceral leishmaniasis after dog vaccination with Leishmune® in Brazilian endemic areas. Vaccine 27, 3505‐3512 [DOI] [PubMed] [Google Scholar]
- Payungporn, S. , Crawford, P. C. , Kouo, T. S. , et al. (2008) Influenza A virus (H3N8) in dogs with respiratory disease, Florida. Emerging Infectious Diseases 14, 902‐908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson, G. L. (1977) Vaccine‐induced canine distemper virus in black‐footed ferrets. Journal of the American Veterinary Medical Association 170, 103‐109 [PubMed] [Google Scholar]
- Pedersen, N. C. , Elliott, J. B. , Glasgow, A. , et al. (2000) An isolated epizootic of hemorrhagic‐like fever in cats caused by a novel and highly virulent strain of feline calicivirus. Veterinary Microbiology 73, 281‐300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock, R. V. & Carmichael, L. E. (1982a) Maternally derived immunity to canine parvovirus infection: transfer, decline, and interference with vaccination. Journal of the American Veterinary Medical Association 180, 37‐42 [PubMed] [Google Scholar]
- Pollock, R. V. & Carmichael, L. E. (1982b) Dog response to inactivated canine parvovirus and feline panleukopenia virus vaccines. Cornell Veterinarian 72, 16‐35 [PubMed] [Google Scholar]
- Poulet, H. , Brunet, S. , Leroy, V. , et al. (2005) Immunisation with a combination of two complementary feline calicivirus strains induces a broad cross‐protection against heterologous challenges. Veterinary Microbiology 106, 17‐31 [DOI] [PubMed] [Google Scholar]
- Poulet, H. , Jas, D. , Lemeter, C. , et al. (2008) Efficacy of a bivalent inactivated non‐adjuvanted feline calicivirus vaccine: relation between in vitro cross‐neutralization and heterologous protection in vivo. Vaccine 26, 3647‐3654 [DOI] [PubMed] [Google Scholar]
- Pratelli, A. & Colao, V. (2014) A population prevalence study on influenza infection in dogs in Southern Italy. New Microbiologica 37, 277‐283 [PubMed] [Google Scholar]
- Ravi, M. , Wobeser, G. A. , Taylor, S. M. , et al. (2010) Naturally acquired feline immunodeficiency virus (FIV) infection in cats from western Canada: prevalence, disease associations, and survival analysis. Canadian Veterinary Journal 51, 271‐276 [PMC free article] [PubMed] [Google Scholar]
- Reagan, K. L. , Hawley, J. R. , Lappin, M. R. (2014) Concurrent administration of an intranasal vaccine containing feline herpesvirus‐1 (FHV‐1) with a parenteral vaccine containing FHV‐1 is superior to parenteral vaccination alone in an acute FHV‐1 challenge model. Veterinary Journal 201, 202‐206 [DOI] [PubMed] [Google Scholar]
- Richter, M. , Schudel, L. , Tobler, K. , et al. (2009) Clinical, virological, and immunological parameters associated with superinfection of latently with FeHV‐1 infected cats. Veterinary Microbiology 138, 205‐216 [DOI] [PubMed] [Google Scholar]
- Roberts, E. S. , VanLare, K. A. , Roycroft, L. M. , et al. (2015) Effect of high‐dose ciclosporin on the immune response to primary and booster vaccination in immunocompetent cats. Journal of Feline Medicine and Surgery 17, 101‐109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypula, K. , Ploneczka‐Janeczko, K. , Bierowiec, K. , et al. (2014) Prevalence of viral infections in cats in southwestern Poland in the years 2006 to 2010. Berl Munch Tierarztl Wochenschr 127, 163‐165 [PubMed] [Google Scholar]
- Sachse, K. , Bavoil, P. M. , Kaltenboeck, B. , et al. (2015) Emendation of the family Chlamydiaceae: proposal of a single genus, Chlamydia to include all currently recognized species. Systemic and Applied Microbiology 38, 99‐103 [DOI] [PubMed] [Google Scholar]
- Schorr‐Evans, E. M. , Poland, A. , Johnson, W. E. , et al. (2003) An epizootic of highly virulent feline calicivirus disease in a hospital setting in New England. Journal of Feline Medicine and Surgery 5, 217‐226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, B. , Klinkenberg, C. , Fux, R. , et al. (2014) Prevalence of canine influenza virus A (H3N8) in dogs in Germany. Veterinary Journal 202, 184‐185 [DOI] [PubMed] [Google Scholar]
- Schultz, R. D. (2006) Duration of immunity for canine and feline vaccines: a review. Veterinary Microbiology 117, 75‐79 [DOI] [PubMed] [Google Scholar]
- Schultz, R. D. (2009) A commentary on parvovirus vaccination. Journal of Feline Medicine and Surgery 11, 163‐164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz, R. D. & Larson, L. J. (1996) The new generation of parvovirus vaccines. A comparison study. Compendium of Continuing Education for the Practicing Veterinarian 18, 640‐641 [Google Scholar]
- Schultz, R. D. , Thiel, B. , Mukhtar, E. , et al. (2010) Age and long‐term protective immunity in dogs and cats. Journal of Comparative Pathology 142(Suppl. 1), S102‐S108 [DOI] [PubMed] [Google Scholar]
- Scott, F. W. & Geissinger, C. M. (1997) Duration of immunity in cats vaccinated with an inactivated feline panleukopenia, herpesvirus and calicivirus vaccine. Feline Practice 25, 12‐19 [Google Scholar]
- Scott, F. W. & Geissinger, C. M. (1999) Long‐term immunity in cats vaccinated with an inactivated trivalent vaccine. American Journal of Veterinary Research 60, 652‐658 [PubMed] [Google Scholar]
- Scherk, M. A. , Ford, R. B. , Gaskell, R. M. , et al. (2013) 2013 AAFP Feline Vaccination Advisory Panel report. Journal of Feline Medicine and Surgery 15, 785‐808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller, S. , Francey, T. , Hartmann, K. , et al. (2015) European consensus statement on leptospirosis in dogs and cats. Journal of Small Animal Practice 56, 159‐179 [DOI] [PubMed] [Google Scholar]
- Shaw, S. C. , Kent, M. S. , Gordon, I. K. , et al. (2009) Temporal changes in characteristics of injection‐site sarcomas in cats: 392 cases (1990–2006). Journal of the American Veterinary Medical Association 234, 376‐380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spibey, N. , Greenwood, N. M. , Sutton, D. , et al. (2008) Canine parvovirus type 2 vaccine protects against virulent challenge with type 2c virus. Veterinary Microbiology 128, 48‐55 [DOI] [PubMed] [Google Scholar]
- Srivastav, A. , Kass, P. H. , McGill, L. D. , et al. (2012) Comparative vaccine‐specific and other injectable‐specific risks of injection‐site sarcomas in cats. Journal of the American Veterinary Medical Association 241, 595‐602 [DOI] [PubMed] [Google Scholar]
- Stepita, M. E. , Bain, M. J. , Kass, P. H. (2013) Frequency of CPV infection in vaccinated puppies that attended puppy socialization classes. Journal of the American Animal Hospital Association 49, 95‐100 [DOI] [PubMed] [Google Scholar]
- Strasser, A. , May, B. , Teltscher, A. , et al. (2003) Immune modulation following immunization with polyvalent vaccines in dogs. Veterinary Immunology and Immunopathology 94, 113‐121 [DOI] [PubMed] [Google Scholar]
- Thiry, E. & Horzinek, M. C. (2007) Vaccination guidelines: a bridge between official requirements and the daily use of vaccines. Revue Scientifique et Technique de l'Office International des Épizooties 26, 511‐517 [DOI] [PubMed] [Google Scholar]
- Ueland, K. & Lutz, H. (1992) Prevalence of feline leukemia virus and antibodies to feline immunodeficiency virus in cats in Norway. Zentralblatt für Veterinärmedizin. Reihe B. Journal of Veterinary Medicine . Series B 39, 53‐58 [DOI] [PubMed] [Google Scholar]
- Wang, C. , Johnson, C. M. , Ahluwalia, S. K. , et al. (2010) Dual‐emission fluorescence resonance energy transfer (FRET) real‐time PCR differentiates feline immunodeficiency virus subtypes and discriminates infected from vaccinated cats. Journal of Clinical Microbiology 48, 1667‐1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijer, K. & Daams, J. H. (1976) The presence of leukaemia (lymphosarcoma) and feline leukaemia virus (FeLV) in cats in The Netherlands. Journal of Small Animal Practice 17, 649‐659 [DOI] [PubMed] [Google Scholar]
- Weijer, K. , UijtdeHaag, F. , Osterhaus, A. (1986) Control of feline leukaemia virus infection by a removal programme. Veterinary Record 119, 555‐556 [DOI] [PubMed] [Google Scholar]
- Weijer, K. , Uytdehaag, F. G. , Osterhaus, A. D. (1989) Control of feline leukaemia virus. Veterinary Immunology and Immunopathology 21, 69‐83 [DOI] [PubMed] [Google Scholar]
- Welborn, L. V. , DeVries, J. G. , Ford, R. , et al. (2011) 2011 AAHA canine vaccination guidelines. Journal of the American Animal Hospital Association 47, 1‐42 [DOI] [PubMed] [Google Scholar]
- Westman, M. E. , Malik, R. , Hall, E. et al. (2015) Determining the feline immunodeficiency virus (FIV) status of FIV‐vaccinated cats using point‐of‐care antibody kits. Comparative Immunology, Microbiology and Infectious Diseases; 10.1016/j.cimid.2015.07.004 [DOI] [PubMed] [Google Scholar]
- Wilson, S. , Stirling, C. , Borowski, S. , et al. (2013) Vaccination of dogs with Duramune DAPPi + LC protects against pathogenic canine parvovirus type 2c challenge. Veterinary Record 172, 662 [DOI] [PubMed] [Google Scholar]
- Wilson, S. , Stirling, C. , Thomas, A. , et al. (2013) Duration of immunity of a multivalent (DHPPi/L4R) canine vaccine against four Leptospira serovars. Vaccine 31, 3126‐3130 [DOI] [PubMed] [Google Scholar]
- Yamamoto, J. K. , Pu, R. , Sato, E. , et al. (2007) Feline immunodeficiency virus pathogenesis and development of a dual‐subtype feline‐immunodeficiency‐virus vaccine. AIDS 21, 547‐563 [DOI] [PubMed] [Google Scholar]
- Yilmaz, H. , Ilgaz, A. , Harbour, D. A. (2000) Prevalence of FIV and FeLV infections in cats in Istanbul. Journal of Feline Medicine and Surgery 2, 69‐70 [DOI] [PubMed] [Google Scholar]


