Abstract
Background: Influenza outbreaks in the childcare setting are a significant cause of excess winter morbidity. This study explored methods of follow up and sample collection for a proposed randomised controlled trial of influenza vaccination in children attending childcare.
Methods: The study was conducted in four Sydney childcare centres during 2007. Healthy children aged 6–59 months eligible for vaccination were recruited in two centres, with another two acting as controls. Data on influenza‐like illness (ILI: ≥37.8°C plus at least one respiratory symptom) occurrence were collected weekly. In those children with an ILI, parents were asked to collect nasal swabs and send via surface mail for viral polymerase chain reaction. Vaccine efficacy (VE) for ILI was estimated overall and for subgroups aged 6–23 and 24–59 months using the formula VE = 1 − relative risk (RR).
Results: Sixty‐three per cent (151/238) of eligible children had parents give consent. Sixty‐three children received influenza vaccine and 88 participated as controls. Of 26 specimens returned, a virus was detected in 18 (69%); none with influenza. Two symptomatic children had positive near‐patient influenza tests in general practice (one a vaccine failure). The RR with 95% confidence interval in all children and those aged 6–23 months were less than one, 0.56 (0.32–1.02) and 0.46 (0.15–1.45), respectively.
Conclusions: This study demonstrated the feasibility and utility of parent‐collected and mailed respiratory specimens for VE research in the childcare setting. Two‐thirds of parent‐collected swabs proved positive for at least one virus. Finding ways to reduce reluctance of parents to submit samples could improve the representativeness of samples collected and the power of the study. No evidence was found for influenza VE, but point estimates were in the direction of protection.
Keywords: children, daycare centres, influenza, pilot, vaccine effectiveness
Introduction
Influenza is a seasonal, vaccine‐preventable disease which causes excess morbidity and mortality during winter in temperate climates. The health and economic costs associated with childhood influenza are substantial. 1 For example, in Australia during 2002–2005, there were reports of 25 433 hospitalisations and four deaths for influenza and pneumonia among children aged under 5 years. 2 The annual cost due to influenza‐related diseases in Australia is estimated to exceed $115 million. 3
The World Health Organization recommends annual influenza vaccination as the cornerstone for prevention and control. Efficacious influenza vaccines have been available for over 50 years, and yet, routine use in childhood remains the exception in most countries. The effectiveness of influenza vaccine for children in childcare has been demonstrated for children aged ≥24 months. 4 , 5 , 6 , 7 , 8 Although the US Advisory Committee on Immunisation Practices has recommended children aged 6–23 months to be vaccinated with influenza vaccine since 2004, 9 there is ongoing debate about vaccine effectiveness in this age group. Three recent systematic reviews 10 , 11 , 12 concluded that either influenza vaccine was not effective in children ≤24 months of age or that there were insufficient data to form a conclusion.
Influenza is transmitted from person to person through contact and respiratory droplets; however, the droplets do not remain suspended in the air for long nor do they travel far. 13 Transmission of influenza generally requires close contact with an infected person or contact with a contaminated surface or object. 14 , 15 The childcare setting provides enhanced opportunities for transmission of infections including influenza as there is prolonged close interaction between young children and the sharing of toys and other objects. Further to this, young children are particularly susceptible to infection as they are immunologically naïve to many viruses.
Commercial childcare in Australia is available in two broad categories: daycare centres (DCC) for children aged 6 weeks until 6 years and pre‐school centres (PSC) for children aged 3 to 6 years. Commercial childcare usage in Australia is increasing. The median attendance time for Australian children who use childcare is 10 h per week, but 13% attend 35 h a week or more. 16 Children in childcare are known to be more likely to contract respiratory illnesses, including influenza, 17 , 18 , 19 , 20 , 21 and are considered to be major transmitters of influenza to their siblings, parents, extended families and care workers. 6 , 8 , 22 , 23
The 2007 influenza season in Australia ran from late May until October and notifications peaked during August. 24 Australia witnessed antigenically drifted influenza virus (A/Brisbane/59/2007 (H1N1)‐like and A/Brisbane/10/2007 (H3N2)‐like), and it was the most severe influenza season since a national influenza reporting system was established in 2001. 25
With this study, the primary process issues of conducting influenza vaccine research in the childcare environment were evaluated, including recruitment, retention, vaccination and specimen handling. While this pilot study was not powered to assess an efficacy end point, preliminary vaccine efficacy (VE) data were also examined.
What is already known on this topic
-
1
Children in childcare are more likely to contract influenza and transmit infection to their siblings, parents, extended families and child‐care workers.
-
2
USA, Canada and Western Australia currently have a routine influenza vaccine policy in place that includes children 6 months of age and older.
-
3
Evidence for the effectiveness of influenza vaccine in children aged less than 24 months is limited and high quality, appropriately powered, randomised controlled trials are needed.
What this study adds
-
1
It is feasible to follow children weekly for 3 months to obtain swabs for influenza‐like illness.
-
2
Two‐thirds of parent‐collected swabs were positive for at least one virus demonstrating the utility of this approach for future studies. Reluctance of parents to submit swabs for analysis may be a limitation of this approach.
Methods
Subjects
From July to August 2007, children aged 6–59 months attending four childcare centres in New South Wales were recruited for this study: two DCC caring for children aged 0–59 months and two PSC caring for children aged 36–59 months. The four DCC were chosen by convenience (proximity to The Children's Hospital at Westmead) with equal number of children between DCC and PSC. One DCC and one PSC were allocated to influenza vaccination, and one DCC and one PSC were allocated to be controls.
This study was approved by The Royal Alexandra Hospital for Children Ethics Committee, and informed parental consent for participation was obtained prior to study procedures. The participating children were evaluated in two age groups based on age at enrolment: 6–23 months and 24–59 months.
Vaccine and schedule
The influenza vaccine administered was a 2007 Southern Hemisphere preparation, purified, inactivated, split vaccine (VAXIGRIP JUNIOR, provided by Sanofi Pasteur, Lyon, France), incorporating:
-
•
A/New Caledonia/20/99 (H1N1)‐like strain (A/New Caledonia /20/99 IVR‐116);
-
•
A/Wisconsin/67/2005 (H3N2)‐like strain (A/Wisconsin/67/2005 NYMCX‐161B);
-
•
B/Malaysia/2506/2004‐like strain.
Children were administrated the vaccine according to the standard recommended dose and schedule for age. 26 As all children at the centres randomised to receive vaccine were influenza vaccine naïve, each received two doses of vaccine 1 month apart – 0.25 mL intramuscular dose for those less than 36 months of age and 0.5 mL intramuscular dose for those aged 36–59 months at the time of their first dose. Vaccines were administered between 11 July 2007 and 19 September 2007.
Influenza‐like illness surveillance and specimen collection
We defined influenza‐like illness (ILI) as an illness with fever >37.8°C and with one or more respiratory symptoms (cough, blocked nose or runny nose) to maximise sensitivity. As a protective level of antibody is usually detectable within 2 weeks of the second dose of vaccine, 13 , 27 ILI surveillance was commenced in vaccinated children at this time point. In control children, ILI surveillance was arbitrarily commenced from the week ending 26 August 2007: at this time, just over half (32/62) of the children eventually fully vaccinated had received vaccine, and from that week, the ratio of child‐weeks of follow up in vaccinated and unvaccinated children was similar (Fig. 2). Parent education for ILI surveillance was provided at study entry. Households received a weekly email or telephone call from 30 July until 21 October 2007 (12 weeks) to monitor the study children for ILI symptoms.
Figure 2.
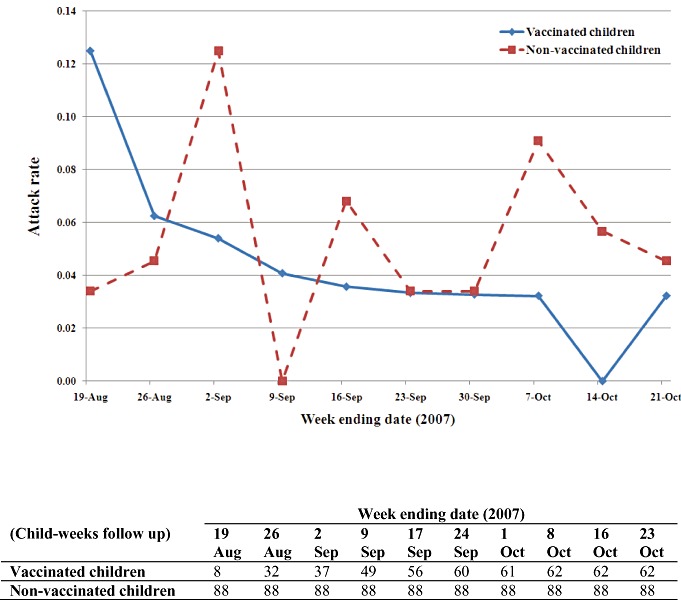
Weekly attack rate and child‐weeks follow up in vaccinated and non‐vaccinated children (ILI episodes/child‐weeks).
Parent training for the collection of nasal swabs was conducted by study nurses after the second immunisation. Nasal swabs were collected using the Virocult collection system (MW950) consisting of a rayon swab on a plastic shaft, with viral transport medium‐soaked foam pad in the base of the transport tube (COPAN Italia, Brescia, Italy), and were returned to the Queensland Paediatric Infectious Diseases Laboratory using a pre‐addressed, postage‐paid envelope.
Laboratory methods
Returned specimens were tested using previously reported, real‐time polymerase chain reaction assays with reverse transcription for RNA viruses. A total of 16 viruses were investigated: human rhinoviruses (HRV), 28 influenza A, influenza B, RSV, adenoviruses, HMPV, parainfluenza viruses I, II and III, 29 bocavirus 30 hPyV‐WU, hPyV‐KI, 31 and human coronaviruses: OC43, 229E, NL63 32 and HKU1. 33 While it was not part of the study protocol, some children had near‐patient influenza tests performed by their general practitioners and these were reported by parents to study staff.
Statistical analyses and VE
ILI incidence rates were calculated using child‐weeks in the denominator. Rate ratios (RR) and 95% confidence intervals were calculated comparing vaccinated and unvaccinated groups. These values were used to estimate VE using the formula VE = (1 − RR) × 100%. Comparisons were performed in three age groups, 6–59 months (all children), 6–23 months and 24–59 months.
Results
Subjects and influenza vaccination
The average ages of the children in the vaccine and control centre were 43.4 (7.7–65.4) and 44.1 (7.9–66.0) months, respectively, while the proportions who were males were 50.8 and 58.0%. Data on non‐enrolled children were not recorded.
There were 239 children in total attending the four childcare centres, and 151 children were enrolled giving a recruitment rate of 63%. Complete information was available for analysis in 150 children, with one vaccinated child lost to follow up during the study period (Fig. 1).
Figure 1.
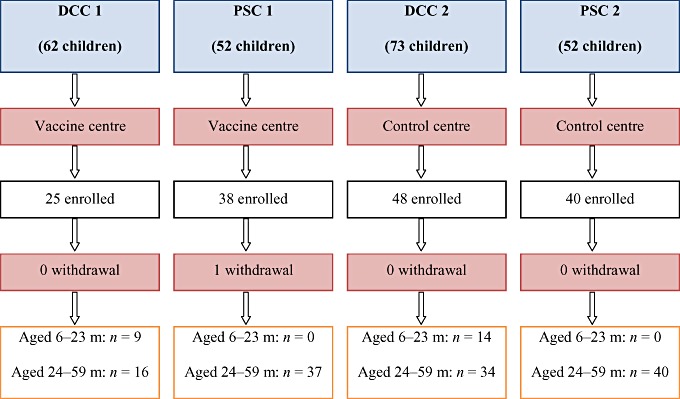
Flow chart of study participants. DCC, daycare centre; m, months; PSC, pre‐school centre.
ILI surveillance
There were 481 (62 children) and 792 (88 children) child‐weeks of follow up in vaccine and control centres, respectively. A total of 59 ILIs were identified in all study children, and weekly ILI incidence rates are provided (Fig. 2).
Laboratory results
Of the 26 swabs received during the study period, 18 (69%) had at least one virus identified, with bocavirus being the most common virus found in six swabs (Table 1), followed by HRV in five swabs. One swab contained three viruses (HRV, adenovirus and bocavirus). Thirteen of these swabs were from 12 vaccinated children, with the other half from 13 controls.
Table 1.
Viruses detected in 26 parent‐collected, mailed nasal swabs from study children with ILI
| DCC 1 | PSC 1 | DCC 2 | PSC 2 | ||
|---|---|---|---|---|---|
| Vaccine centre | Vaccine centre | Control centre | Control centre | ||
| Near‐patient test | Influenza A | 1 | 0 | 0 | 1 |
| PCR | Influenza A | 0 | 0 | 0 | 0 |
| HRV | 1 | 3 | 1 | 0 | |
| Adenovirus | 2 | 0 | 1 | 0 | |
| Bocavirus | 4 | 0 | 2 | 0 | |
| Parainfluenza 1 | 0 | 1 | 0 | 0 | |
| Parainfluenza 2 | 0 | 0 | 1 | 0 | |
| Parainfluenza 3 | 1 | 0 | 2 | 0 | |
| HMPV | 0 | 1 | 1 | 0 | |
| KI | 0 | 0 | 1 | 0 | |
| RSV | 0 | 0 | 0 | 1 | |
| HKU1 | 0 | 0 | 0 | 1 | |
Viruses not listed were not detected in study children. DCC, daycare centre; ILI, influenza‐like illness; PCR, polymerase chain reaction; PSC, pre‐school centre.
Two positive near‐patient influenza tests (one a vaccine failure) were reported by parents to the study staff: one was from an unvaccinated child in a control centre; the other child was vaccinated with the test done 14 days post‐second vaccination (Table 1).
Estimate of VE against ILI
There were a total of 59 ILIs identified with efficacy point estimates in the direction of protection for all age‐groups but not significant (Table 2).
Table 2.
Number of ILI episodes and person‐time follow up, relative risk, and vaccine efficacy for ILI prevention in each age group
| Age group | Vaccination status | Rate (ILI episodes/child‐weeks) | Relative rate (95% CI) |
|---|---|---|---|
| Vaccine efficacy estimate | |||
| 6–23 months | Vaccinated | 0.05 (4/78) | 0.46 (0.15–1.45) |
| Controls | 0.11 (14/126) | 54% | |
| 24–59 months | Vaccinated | 0.03 (11/403) | 0.61 (0.30–1.22) |
| Controls | 0.05 (30/666) | 39% | |
| Total | Vaccinated | 0.03 (15/481) | 0.56 (0.32–1.02) |
| Controls | 0.06 (44/792) | 44% |
CI, confidence interval; ILI, influenza‐like illness.
Discussion
The key findings of this pilot study were that a high recruitment rate could be achieved, that recruited families were tolerant of regular weekly follow up over an extended period (3 months) and that there was no evidence of protective efficacy, but point estimates of VE for the less‐specific end point of ILI were in the direction of protection.
It is inevitable that ILI would include non‐influenza infections which cause respiratory signs and symptoms, especially as we used a sensitive definition (at the expense of specificity), so it is not surprising that a range of other viral pathogens were identified in our study. A population‐based surveillance study showed that less than 10% of hospitalised children aged ≤59 months with ILI had confirmed influenza infection. 34
Our study has some limitations. The childcare centres were not randomised. The commencement midway through an influenza season limited the number of influenza cases identified. Less than half the episodes in children of ILI (26 out of 59, 44%) had a respiratory sample sent. This reduced sampling is probably due to the added burden on parents of sample collection (and posting) while a child is ill. In addition, as the childcare centres were in the suburbs with relatively lower Socio‐Economic Indexes for Areas 35 (and also involved larger families), this, too, may have limited parental cooperation. The participants were only followed from the 2nd half of August when the 2007 season was peaking, so some may have thought that sample collection in September or October was too late. Given the limited data that were collected on symptomatology and the relatively small number of specimens, it was not possible to do an extensive analysis comparing symptoms by virus type to address if there are differences in symptoms among various viruses. Furthermore, the demographic data (e.g. sex, age range) of those who did not participate in the study were not collected; therefore, it was not possible to identify if there was any recruitment bias. Greater efforts are required for future studies in (i) improving the proportion of swabs collected and sent by initiating ILI follow up before the influenza season starts and findings better ways to overcome parents’ reluctance in submitting swabs; (ii) obtaining more detailed data on symptomatology of respiratory infection; and (iii) collecting de‐identified demographic data on those who are not enrolled in the study.
This study showed no evidence for influenza VE. There was only a suggestion of protection in that all the point estimates were in that direction. Trivalent, live, cold‐adapted influenza vaccine (CAIV‐T) may be a better option for young children and has been demonstrated to have significantly higher efficacy than inactivated vaccine among young children during moderate 36 , 37 and high attack‐rate influenza seasons. 38 CAIV‐T was also able to provide protection even when the circulating influenza virus was an antigenically distinct strain. 37
There were two children with positive results for influenza A from the near‐patient test. This type of test is known to have only moderate sensitivity but high specificity, so a positive test is unlikely to be false. 39
Older children (24–59 months) are more likely to have developed natural immunity through previous infection and are more likely to have developed better personal hygiene. For these reasons, older children are less susceptible to influenza as well as other non‐influenza infections that may cause ILI.
Conclusions
This pilot study has shown the feasibility and value of parent‐collected (and mailed) nasal sample for influenza VE research in childcare. Two‐third of parent‐collected swabs proved positive for at least one virus. Means of lessening reluctance of parents to submit respiratory samples from their children need to be found to improve the representativeness of samples collected and the power of the study. No evidence was found for influenza VE, but point estimates were all in the direction of protection.
Reference
- 1. Lambert SB, Allen KM, Carter RC, Nolan TM. The cost of community‐managed viral respiratory illnesses in a cohort of healthy preschool‐aged children. Respir. Res. 2008; 9: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brotherton J, Wang H, Schaffer A et al Vaccine preventable diseases and vaccination coverage in Australia, 2003 to 2005. Commun. Dis. Intell. 2007; 31 (Suppl.): 152. [DOI] [PubMed] [Google Scholar]
- 3. Newall AT, Scuffham PA. Influenza‐related disease: the cost to the Australian healthcare system. Vaccine 2008; 26: 6818–23. [DOI] [PubMed] [Google Scholar]
- 4. Clements DA, Langdon L, Bland C, Walter E. Influenza A vaccine decreases the incidence of otitis media in 6‐ to 30‐month‐old children in day care. Arch. Pediatr. Adolesc. Med. 1995; 149: 1113–7. [DOI] [PubMed] [Google Scholar]
- 5. Ghendon YZ, Kaira AN, Elshina GA. The effect of mass influenza immunization in children on the morbidity of the unvaccinated elderly. Epidemiol. Infect. 2006; 134: 71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hurwitz ES, Haber M, Chang A et al Effectiveness of influenza vaccination of day care children in reducing influenza‐related morbidity among household contacts. JAMA 2000; 284: 1677–82. [DOI] [PubMed] [Google Scholar]
- 7. Hurwitz ES, Haber M, Chang A et al Studies of the 1996–1997 inactivated influenza vaccine among children attending day care: immunologic response, protection against infection, and clinical effectiveness. J. Infect. Dis. 2000; 182: 1218–21. [DOI] [PubMed] [Google Scholar]
- 8. Teo SS, Nguyen‐Van‐Tam JS, Booy R. Influenza burden of illness, diagnosis, treatment, and prevention: what is the evidence in children and where are the gaps? [Review][70 refs]. Arch. Dis. Child. 2005; 90: 532–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harper SA, Fukuda K, Uyeki TM et al Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 2004; 53 (RR‐6): 1–40. [PubMed] [Google Scholar]
- 10. Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst. Rev. 2008; (16): CD004879. [DOI] [PubMed] [Google Scholar]
- 11. Manzoli L, Schioppa F, Boccia A, Villari P. The efficacy of influenza vaccine for healthy children: a meta‐analysis evaluating potential sources of variation in efficacy estimates including study quality. Pediatr Infect. Dis. J. 2007; 26: 97–106. [DOI] [PubMed] [Google Scholar]
- 12. Negri E, Colombo C, Giordano L, Groth N, Apolone G, La Vecchia C. Influenza vaccine in healthy children: a meta‐analysis. Vaccine 2005; 23: 2851–61. [DOI] [PubMed] [Google Scholar]
- 13. Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G et al Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009; 58 (RR‐8): 1–52. [PubMed] [Google Scholar]
- 14. Brankston G, Gitterman L, Hirji Z, Lemieux C, Gardam M. Transmission of influenza A in human beings. [Review][63 refs]. Lancet Infect. Dis. 2007; 7: 257–65. [DOI] [PubMed] [Google Scholar]
- 15. Glass LM, Glass RJ. Social contact networks for the spread of pandemic influenza in children and teenagers. BMC Public Health 2008; 8: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Australian Bureau of Statistics . Child Care, Australia, Jun 2005 (Reissue), Cat. no. 4402.0. 2006. Available from: http://www.abs.gov.au/ausstats/abs@.nsf/ProductsbyReleaseDate/A323B76DC590C5F7CA2576010016F8A8?OpenDocument[accessed March 2010].
- 17. Hurwitz ES, Gunn WJ, Pinsky PF, Schonberger LB. Risk of respiratory illness associated with day‐care attendance: a nationwide study. Pediatrics 1991; 87: 62–9. [PubMed] [Google Scholar]
- 18. Louhiala PJ, Jaakkola N, Ruotsalainen R, Jaakkola JJ. Form of day care and respiratory infections among Finnish children. Am. J. Public Health 1995; 85 (Pt 1): 1109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marbury MC, Maldonado G, Waller L. Lower respiratory illness, recurrent wheezing, and day care attendance. Am. J. Respir. Crit. Care Med. 1997; 155: 156–61. [DOI] [PubMed] [Google Scholar]
- 20. Nafstad P, Hagen JA, Oie L, Magnus P, Jaakkola JJ. Day care centers and respiratory health. Pediatrics 1999; 103 (Pt 1): 753–8. [DOI] [PubMed] [Google Scholar]
- 21. Wald ER, Guerra N, Byers C. Frequency and severity of infections in day care: three‐year follow‐up. J. Pediatr. 1991; 118 (Pt 1): 509–14. [DOI] [PubMed] [Google Scholar]
- 22. Fox JP, Cooney MK, Hall CE, Foy HM. Influenza virus infections in Seattle families, 1975–1979. II. Pattern of infection in invaded households and relation of age and prior antibody to occurrence of infection and related illness. Am. J. Epidemiol. 1982; 116: 228–42. [DOI] [PubMed] [Google Scholar]
- 23. Hall CE, Cooney MK, Fox JP. The Seattle virus watch. IV. Comparative epidemiologic observations of infections with influenza A and B viruses, 1965–1969, in families with young children. Am. J. Epidemiol. 1973; 98: 365–80. [DOI] [PubMed] [Google Scholar]
- 24. Zambon MC, Stockton JD, Clewley JP, Fleming DM. Contribution of influenza and respiratory syncytial virus to community cases ofinfluenza‐like illness: an observational study. Lancet 2001; 358: 1410–6. [DOI] [PubMed] [Google Scholar]
- 25. Owen R, Barr IG, Pengilley A et al Annual report of the national influenza surveillance scheme, 2007. Commun. Dis. Intell. 2008; 32: 208–26. [DOI] [PubMed] [Google Scholar]
- 26. Sanofi Pasteur Pty LTD . VAXIGRIP © JUNIOR. 2007; Available from: http://www.tdgp.com.au/pdfs/VAXIGRIPJuniorPI2007SeasonForAU.pdf[accessed 16 May 2010].
- 27. Australian Government Department of H, Ageing C. National Health and Medical Research Council . The Australian immunisation handbook, 9th edition. Available from: http://www.health.gov.au/internet/immunise/publishing.nsf/Content/Handbook-home2008[accessed 16 May 2010].
- 28. Gama RE, Horsnell PR, Hughes PJ et al Amplification of rhinovirus specific nucleic acids from clinical samples using the polymerase chain reaction. J. Med. Virol. 1989; 28: 73–7. [DOI] [PubMed] [Google Scholar]
- 29. Lambert SB, Whiley DM, O’Neill NT et al Comparing nose‐throat swabs and nasopharyngeal aspirates collected from children with symptoms for respiratory virus identification using real‐time polymerase chain reaction. Pediatrics 2008; 122: e615–e20. [DOI] [PubMed] [Google Scholar]
- 30. Tozer SJ, Lambert SB, Whiley DM et al Detection of human bocavirus in respiratory, fecal, and blood samples by real‐time PCR. J. Med. Virol. 2009; 81: 488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bialasiewicz S, Whiley DM, Lambert SB, Gould A, Nissen MD, Sloots TP. Development and evaluation of real‐time PCR assays for the detection of the newly identified KI and WU polyomaviruses. J. Clin. Virol. 2007; 40: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gunson RN, Collins TC, Carman WF. Real‐time RT‐PCR detection of 12 respiratory viral infections in four triplex reactions. J. Clin. Virol. 2005; 33: 341–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dare RK, Fry AM, Chittaganpitch M, Sawanpanyalert P, Olsen SJ, Erdman DD. Human coronavirus infections in rural Thailand: a comprehensive study using real‐time reverse‐transcription polymerase chain reaction assays. J. Infect. Dis. 2007; 196: 1321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lambert SB, Allen KM, Druce JD et al Community epidemiology of human metapneumovirus, human coronavirus NL63, and other respiratory viruses in healthy preschool‐aged children using parent‐collected specimens. Pediatrics 2007; 120: e929–e37. [DOI] [PubMed] [Google Scholar]
- 35. Australian Bureau of Statistics . Census of population and housing: socio‐economic indexes for areas (SEIFA), Australia. 2008. Available from: http://www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/2033.0.55.001Main+Features12006?OpenDocument[accessed December 2010].
- 36. Belshe RB, Edwards KM, Vesikari T et al Live attenuated versus inactivated influenza vaccine in infants and young children.[Erratum appears in N. Engl. J. Med. 2007 Mar 22; 356 (12): 1283]. N. Engl. J. Med. 2007; 356: 685–96. [DOI] [PubMed] [Google Scholar]
- 37. Hirota Y, Takeshita S, Ide S et al Various factors associated with the manifestation of influenza‐like illness. Int. J. Epidemiol. 1992; 21: 574–82. [DOI] [PubMed] [Google Scholar]
- 38. Piedra PA, Gaglani MJ, Kozinetz CA et al Trivalent live attenuated intranasal influenza vaccine administered during the 2003–2004 influenza type A (H3N2) outbreak provided immediate, direct, and indirect protection in children. Pediatrics 2007; 120: e553–e64. [DOI] [PubMed] [Google Scholar]
- 39. Dwyer DE, Smith DW, Catton MG, Barr IG. Laboratory diagnosis of human seasonal and pandemic influenza virus infection. Med. J. Aust. 2006; 185 (Suppl.): S48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]


