Abstract
ABSTRACT: The ability of spermatogenic cells to evade the host immune system and the ability of systemic inflammation to inhibit male reproductive function represent two of the most intriguing conundrums of male reproduction. Clearly, an understanding of the underlying immunology of the male reproductive tract is crucial to resolving these superficially incompatible observations. One important consideration must be the very different immunological environments of the testis, where sperm develop, and the epididymis, where sperm mature and are stored. Compared with the elaborate blood‐testis barrier, the tight junctions of the epididymis are much less effective. Unlike the seminiferous epithelium, immune cells are commonly observed within the epithelium, and can even be found within the lumen, of the epididymis. Crucially, there is little evidence for extended allograft survival (immune privilege) in the epididymis, as it exists in the testis, and the epididymis is much more susceptible to loss of immune tolerance. Moreover, the incidence of epididymitis is considerably greater than that of orchitis in humans, and susceptibility to sperm antibody formation after damage to the epididymis or vas deferens increases with increasing distance of the damage from the testis. Although we still know relatively little about testicular immunity, we know less about the interactions between the epididymis and the immune system. Given that the epididymis appears to be more susceptible to inflammation and immune reactions than the testis, and thereby represents the weaker link in protecting developing sperm from the immune system, it is probably time this imbalance in knowledge was addressed.
Keywords: Sperm, autoimmunity, orchitis, epididymitis, leukocytes, antibodies
The ability of the developing male germ cells and mature sperm to avoid the immune system of the host and the ability of inflammation arising outside the male reproductive tract to inhibit male fertility are among the most intriguing conundrums of male reproductive function. Clearly, resolving these two observations, which on first glance would appear to be incompatible, depends upon a comprehensive understanding of the underlying immunology of the male reproductive tract. Unfortunately, the nature of the interactions that exist between the immune system and the male reproductive system remains poorly understood. One fact that is apparent, however, is that immune responses within the testis, where sperm develop, and immune responses in the epididymis, where mature sperm concentrate and mature, are very different. This implies that the mechanisms underlying immunity in these 2 tissues are different and that a more thorough examination of these differences may be very productive.
In the following review, I aim to summarize briefly what is known about the innate and adaptive immune systems as they relate to the testis and epididymis in order to draw some general principles, make some predictions, and suggest where attention might best be directed in the future. In the interests of full disclosure, these represent the personal opinions of a researcher with a long‐standing interest in the mechanisms of male reproductive tract immunology in general, but one who does not work primarily on the epididymis. It will not be possible to cover every aspect or previous study relating to the topic, and interested readers are directed to a more comprehensive review (Hedger and Hales, 2006).
The Effect of Infection and Inflammation on the Testis and Epididymis
In developed societies, it is estimated that 5%–10% of male factor infertility of known etiology has a suspected inflammatory or autoimmune involvement, which encompasses sperm antibody formation, orchitis, epididymitis and epididymo‐orchitis, and at least some “idiopathic” infertility (Dohle et al, 2005; Schuppe et al, 2008). More importantly, the incidence of immunological infertility tends to be much higher among populations in which access to health care or education is poor—in other words, the larger proportion of humanity (Ekwere, 1995). The World Health Organization has estimated that reproductive tract infections are responsible for about 3% of the global burden of ill health among men (AbouZahr and Vaughan, 2000). The epithelia of the testis and epididymis are not regenerative and infections within these organs and their inflammatory sequelae frequently result in permanent damage leading to a loss of fertility (Dohle et al, 2005). Moreover, approximately 1% of men suffer from debilitating and frequently intractable persistent, antibiotic‐resistant scrotal or perineal pain (“sterile” orchitis, epididymitis, vasitis, or prostatitis), a condition that remains largely mysterious but almost certainly involves an underlying inflammatory cause (Alexander et al, 1998; Nickel et al, 2005; Nariculam et al, 2007). Finally, although most testicular cancers have a developmental, rather than an inflammatory, origin and epididymal cancer is extremely rare, the role of inflammatory and immune mechanisms in determining the incidence, severity, and/or course of cancers in these tissues should not be neglected, simply for lack of information.
Epididymitis is the most common intrascrotal inflammation and is a significant cause of urological consults. In the United States, a country with an estimated male population of around 150 million, the National Institutes of Health records 600 000 cases annually (Trojian et al, 2009). The symptoms and consequences of epididymitis include pain, nodules, edema, urinary difficulties, fever, urethral discharge, and infertility. However, epididymitis can also be asymptomatic, meaning that it is also underreported. Acute epididymitis is usually due to retrograde ascent of urethral pathogens and sexually transmitted bacterial infections: notably, Chlamydia trachomatis and Neisseria gonorrhoeae, but also Ureaplasma urealyticum, Escherichia coli, Enterococcus faecalis, and Pseudomonas aeruginosa. Less commonly, systemic bacterial infections (eg, Mycobacterium tuberculosis, Haemophilus influenzae type B, Salmonella, and Brucella) or nonbacterial infections (eg, Cytomegalovirus and Filariasis) may be responsible, particularly in children or immunocompromised individuals. Noninfectious causes of epididymitis include various medical procedures, the arrhythmia drug amiodarone, trauma, physical stress, vasectomy, urinary reflux, neoplasia, postinfectious and systemic inflammation, vasculitis, and autoimmunity (Hagley, 2003; Haidl et al, 2008; Trojian et al, 2009).
Crucially, the incidence of epididymitis is considerably higher than the incidence of orchitis, which most frequently presents as epididymo‐orchitis (Krieger, 1984). In contrast to epididymitis, isolated orchitis is generally due to bloodborne dissemination of pathogens, usually viruses (eg, mumps, Coxsackie B, Varicella, Epstein‐Barr, Dengue, the severe acute respiratory syndrome coronavirus), but also mycobacteria (eg, Mycobacterium leprae and M tuberculosis) and parasites, such as Malaria and Filariasis. Non‐infectious causes of orchitis include physical trauma, vasectomy, neoplasia and autoimmunity (Salomon et al, 1982; Flickinger, 1985; Pannek and Haupt, 1997; Vitolo et al, 2008). In general, data on the incidence of autoimmune orchitis among human populations is extremely poor, but its relative prevalence may be surmised from various animal models exhibiting this condition, as well as from biopsies of infertile men showing evidence of past immunological events (Suominen and Soderstrom, 1982; Schuppe et al, 2008).
In summary, differences between the incidence and the causes of infection and inflammation in the testis and epididymis are significant, in spite of the fact that they are connected by the efferent ducts and linked to the external environment via the genitourinary tract and that there would appear to be no significant physical impediment to infections progressing from one tissue into the other. One obvious explanation for this difference is the underlying differences in the immune environment within the 2 tissues.
Effects of Systemic Inflammation and Illness on the Testis and Epididymis
It is well‐established that systemic infection and even illnesses that do not have an infectious basis can have inhibitory effects on male reproductive function (Dong et al, 1992; Baker, 1998). The mechanisms responsible for this observation are poorly defined, but the fact that disease external to the reproductive tract can inhibit fertility suggests that the inhibition is due to something more than simple cell damage caused by the activity of antimicrobial cells and secretions. The effects of illness on reproduction have been most extensively studied using bacterial lipopolysaccharide (LPS) to induce systemic or localized inflammation in experimental animals. The most common observation is a loss of androgen production, which can be attributed to the direct effects of pathogen‐specific molecules like LPS, as well as molecules stimulated by LPS, such as the proinflammatory cytokines interleukin 1 beta (IL1B) and tumor necrosis factor (TNF), reactive oxygen species, nitric oxide (NO) and corticosteroids, at various levels of the hypothalamic–pituitary–Leydig cell axis (Hedger and Hales, 2006). Although reduction in androgen production caused by systemic inflammation alone might not be sufficient to cause spermatogenic disruption (O'Bryan et al, 2000; Liew et al, 2007), there is no doubt that reduced androgen production by the testis will have negative effects on epididymal function, as well as on general health.
In addition to inhibition of steroidogenesis, a growing body of evidence indicates that infection and inflammation are able to affect the seminiferous epithelium directly (O'Bryan and Hedger, 2008; Hedger, 2011b). During infection, activation of inflammation and immunity is triggered by the recognition of specific motifs, or pathogen‐associated molecular patterns, found on bacterial, viral, fungal, and protozoan pathogens, mediated by specific pattern recognition receptors. The best characterized of these receptors are the Toll‐like receptors (TLR), which recognize bacterial and viral nucleic acids and other molecules unique to pathogens, including LPS, bacterial lipopeptides and peptidoglycans (Kawai and Akira, 2010). The TLRs are mainly found on myeloid cells (monocytes, macrophages, and dendritic cells) but are also expressed by epithelial and connective tissue cells.
The Sertoli cells express most of the TLRs (the Table), including TLR4, the receptor for LPS, and stimulation of Sertoli cells by TLR ligands activates inflammatory signaling pathways leading to the mitogen‐activated protein (MAP) kinases and the inflammatory transcription factors, nuclear factor kappa B (NFκB), and interferon regulatory factor 3 (Riccioli et al, 2006; Bhushan et al, 2008; Starace et al, 2008). This leads to activation of a subset of genes that are normally induced by inflammation, including IL1 alpha (the cell‐associated variant of IL1B), IL6 and nitric oxide synthase 2 (NOS2, also known as inducible NOS), and the immunoregulatory cytokine, activin A (Gérard et al, 1991; Stéphan et al, 1995; Syed et al, 1995; Okuma et al, 2005a; Riccioli et al, 2006; Wu et al, 2008; Winnall et al, 2009, 2011). Moreover, Sertoli cells are also able to respond to many of these endogenous inflammatory mediators, most notably IL1A and IL1B, TNF, NO, transforming growth factor beta 3 (TGFB3), and type 1 and type 2 interferons (Mauduit et al, 1993; Okuda et al, 1994; Stéphan et al, 1995; Dejucq et al, 1997; Bauché et al, 1998; Lui et al, 2003; Okuma et al, 2005b). Evidence is considerable that these molecules, which are usually associated with inflammation, and the pathways through which they exert their effects play fundamental roles in mediating intercellular communication within the seminiferous epithelium (O'Bryan and Hedger, 2008; Hedger, 2011b). These inflammatory molecules regulate mitosis and meiosis of the spermatogenic cells, organization of the Sertoli cell cytoskeleton and intercellular junctions, and various Sertoli cell activities throughout the cycle of the seminiferous epithelium. Consequently, the presence of pathogenic molecules, cytokines, and other mediators of inflammation produced within the testis, or entering from the circulation during systemic inflammation, impinge upon the normal functions of the Sertoli cells and spermatogenic cells, leading to disturbances in spermatogenesis.
Table Table.
. Toll‐like receptor (TLR) expression in the epithelium of male reproductive tract of the rat and mouse a
| Receptor | Principal Ligands | Principal Pathogens | Cellular Location | Sertoli Cells | Initial Segment | Epididymis | Vas Deferens |
|---|---|---|---|---|---|---|---|
| TLR1 | Triacyl lipopeptides | Bacteria, mycobacteria | Cell surface | +++ | ++ | ++ | +++ |
| TLR2 | Lipoproteins, peptidoglycans | Bacteria, mycobacteria, viruses | Cell surface | ++++ | +++ | ++ | + |
| TLR3 | dsRNA | Viruses | Endosomes | ++++ | ++++ | +++ | ++ |
| TLR4 | Lipopolysaccharides | Bacteria, viruses | Cell surface | ++++ | ++++ | ++ | + |
| TLR5 | Flagellin | Bacteria | Cell surface | +++ | +++ | +++ | +++ |
| TLR6 | Diacyl lipopeptides, zymosan | Bacteria, fungi (yeast) | Cell surface | +++ | +++ | +++ | + |
| TLR7 | ssRNA | Viruses | Endosomes | +/– | +/– | + | + |
| TLR8b | ssRNA | Viruses | Endosomes | – | +/– | +/– | – |
| TLR9 | CpG DNA | Bacteria, viruses, protists (Plasmodium) | Endosomes | – | ++ | ++ | ++ |
| TLR10c | Unknown | Bacteria | Cell surface | + | +/– | + | – |
| TLR11d | Profilin | Bacteria | Cell surface | + | ++ | +++ | +++ |
| TLR12d | Unknown | ND | ND | – | ND | ND | ND |
| TLR13d | Unknown | ND | ND | + | ND | ND | ND |
Abbreviation: ND, insufficient data available.
aDisclaimer: Table values are entirely subjective and based on occasionally contradictory data (Riccioli et al, 2006; Palladino et al, 2007, 2008; Bhushan et al, 2008; Rodrigues et al, 2008; Starace et al, 2008; Wu et al, 2008; Zhao et al, 2008; Sun et al, 2010; Winnall et al, 2011).
bTLR not functional in rodents.
cTLR not expressed in mouse.
dTLR not expressed in human.
In addition to the testis, TLRs are found throughout the epithelium of the reproductive tract, including that of the epididymis (the Table). However, combined data from several studies in rats and mice would seem to suggest regional differences in expression of the various pattern‐recognition receptors, with the result that TLRs 1–6 are expressed more strongly in the testis, whereas TLR 7, 9, and 11 expression tends to be higher in the epididymis, vas deferens, or both (Riccioli et al, 2006; Palladino et al, 2007, 2008; Bhushan et al, 2008; Rodrigues et al, 2008; Starace et al, 2008; Wu et al, 2008; Sun et al, 2010; Winnall et al, 2011). These observations need to be confirmed by a consistent, quantitative analysis. Moreover, it is not clear what this might mean in terms of pathogenicity because there is no obvious difference in viral vs bacterial sensors that might correspond to the differential susceptibility or exposure of the 2 organs to viral and bacterial infections, as mentioned in the previous section.
In contrast to the testis, there have been relatively few studies on inflammatory signaling in the epididymal epithelium or the effects of systemic inflammation on epididymal function. A study by Rodrigues et al (2008) reported that systemic administration (IP) of LPS induced the activation of NFκB and up‐regulation of several inflammation‐responsive genes, including IL1B, in the rat epididymis. However, TLR4 expression was also localized to macrophages in the epididymal stroma, so it remains to be seen whether this was a response of the epithelium itself. In our own studies using a similar model, LPS administration caused an increase in round cells in the epididymal lumen because of premature shedding of spermatogenic cells from the seminiferous epithelium, but the epididymis itself had no gross morphological changes, at least at the light microscope level (O'Bryan et al, 2000; Liew et al, 2007). However, many potential functional changes would not be readily detectable at this level, and this issue deserves further examination. In another more recent study, injection of LPS directly into the caput epididymis caused an increase in IL1B expression, hyperemia, edema, and interstitial leukocyte accumulation, along with unspecified inflammatory damage localized to the epithelium of the corpus epididymis, reduced sperm motility, and decreased expression of several beta defensins, which are endogenously produced antimicrobial proteins (Cao et al, 2010). In vitro, the murine caput epididymal epithelial cell line PC1 expresses TLR4 and several of its coreceptor proteins, and treatment of these cells with LPS induced NFκB activation and expression of another pattern recognition receptor, nucleotide‐binding oligomerization domain 2 (NOD2), which detects bacterial peptidoglycan (Mühlbauer et al, 2008). Stimulation of these cells with LPS and the peptidoglycan muramyl dipeptide enhanced TNF message expression. Infection of cultured rat cauda epididymal epithelial cells with Staphylococcus aureus stimulated signaling via NFκB and MAP kinase 14 (also called p38); production of IL1B, TNF, and NO; and expression of mRNA for NOS2 and TLR2 (Zhao et al, 2008). Receptors for IL1 have been detected in the mouse epididymal epithelium, indicating a capacity to respond directly to these fundamental inflammatory cytokines as well (Takao et al, 1990; Gomez et al, 1997).
As can be deduced from the preceding data, infection and inflammation can affect both testicular and epididymal function via suppression of steroidogenesis and by direct actions on the activity of the epithelial cells to inhibit fertility. However, because sperm are antigenic a particular consideration needs to be made when assessing the effects of the activated immune system on fertility, and it is this aspect that will be considered next.
Blood‐Epithelial Barriers and Compartmentalization in the Testis and Epididymis
Both the testis and epididymis have the same basic structural organization, comprising tubules lined by a highly heterogeneous epithelium, surrounded by a peritubular cellular layer, and an interstitial tissue containing the vasculature and lymphatics. However, the similarities are largely superficial. With the exception of the tubuli recti and rete testis, the epithelium of the testis comprises a single somatic cell type (the Sertoli cell) supporting a population of rapidly differentiating and proliferating spermatogenic cells, whereas the epididymal epithelium consists of a number of relatively stable epithelial cell types, including principal cells, clear cells, basal cells, and halo cells, as well as more regionally restricted cell types (apical cells and narrow cells). In the epididymis, the sperm are confined to the lumen of the tubule and are less dependent upon the epithelium for their survival, compared with spermatogenic cells in the testis. The tubules in the testis are surrounded by specialized peritubular myoid cells, which display a close dynamic interaction with the Sertoli cells (Skinner et al, 1985). In the epididymis, the surrounding cells are typical smooth muscle cells. The interstitial tissue of both organs comprises a loose connective tissue, but the testis interstitium also contains highly specialized, androgen‐producing Leydig cells and large numbers of resident macrophages (Hedger, 2002), whereas the interstitium of the epididymis appears less remarkable, dominated by mesenchymal fibroblastic cells and relatively fewer macrophages (Wang and Holstein, 1983; Nashan et al, 1989; Flickinger et al, 1995).
There is a very efficient blood‐testis barrier, consisting of basally located tight junctions and elaborate membrane and cytoskeletal specializations between adjacent Sertoli cells. The function of this barrier is to completely exclude all cellular and molecular traffic via the extracellular space between the Sertoli cells (Setchell et al, 1969; Dym and Fawcett, 1970). It is absolutely essential for creating a highly specialized biochemical environment for the meiotic and postmeiotic cells, but this barrier also means that immune cells are completely excluded from the epithelium and lumen of the seminiferous tubules. By comparison, the barrier functions of the epididymis are more comparable to those found in other epithelia, with apically located tight junctions that are less exclusive than the highly specialized tight junctions of the seminiferous epithelium (Friend and Gilula, 1972; Cyr et al, 1995; Hinton and Palladino, 1995). Consequently, immune cells are common among the cells of the epididymal epithelium (Wang and Holstein, 1983; Ritchie et al, 1984). In this, the epididymis much more resembles the other tissues of the mucosal immune system and, accordingly, shares a number of properties with those tissues (Beagley et al, 1998).
Distribution of Immune Cells in the Testis and Epididymis
In the normal testis, leukocytes are found almost exclusively within the interstitial tissue, peritubular zone, and testicular capsule. These cells chiefly comprise resident macrophages, dendritic cells, and circulating lymphocytes, although variable numbers of mast cells and eosinophils are also present, depending upon the species (el‐Demiry et al, 1987; Wang et al, 1994; Anton et al, 1998). The intratesticular lymphocyte population is skewed toward major histocompatibility complex (MHC) class I restricted (CD8+) cells and includes significant numbers of cytotoxic T cells and natural killer (NK) cells (Ritchie et al, 1984; Wang et al, 1994; Tompkins et al, 1998; Hedger and Meinhardt, 2000), but examination of rat and mouse testes indicate the presence of significant numbers of immunoregulatory T‐cell subsets, in the form of NK T cells and CD4+CD25+ Treg cells, as well (Hedger, unpublished data). Additionally, it is well established that the majority of resident testicular macrophages have reduced pro‐inflammatory activity, display a preference for immunosuppressive cytokine (ie, TGFB and IL10) production when stimulated and express surface markers consistent with an alternatively activated (immunoregulatory) macrophage or M2 phenotype (Kern and Maddocks, 1995; Hayes et al, 1996; Hedger, 2002; Bryniarski et al, 2004; Maresz et al, 2008). These macrophages can be presumed to play an important role in regulating antigen‐specific responses within the testicular environment, in concert with the immunoregulatory T‐cell subsets. The dendritic cells of the normal testis have yet to be fully characterized, but evidence from the rat suggests that the phenotype and functions of these crucial antigen‐presenting cells is consistent with the immunoregulatory functions of the resident macrophages and T cells of the testis (Rival et al, 2006, 2007).
In contrast to the testis, macrophages and lymphocytes are frequently observed within both the epithelium and the interstitial tissue of the epididymis and are commonly identified as halo cells in epithelial sections (Dym and Romrell, 1975; Wang and Holstein, 1983; Ritchie et al, 1984; Nashan et al, 1989; Hooper et al, 1995; Flickinger et al, 1997; Serre and Robaire, 1999). Macrophages are the main leukocyte present and are most prominent within the interstitial tissue and peritubular zone. These interstitial tissue macrophages express MHC class II antigens, whereas macrophages within the epididymal epithelium mostly do not (Nashan et al, 1989, 1990). Studies on the distribution of T‐cell subsets in the epididymis are complicated by the fact that the CD4 and CD8 antigens are also expressed by rat and human monocytes and macrophages (Crocker et al, 1987; Gibbings et al, 2007). Studies in the mouse, in which these antigens are more restricted to lymphocyte subsets, indicate an excess of MHC class II restricted CD4+ T cells (helper and regulatory T cells) over the CD8+ T‐cell (cytotoxic T cell) subset in the interstitial tissue, typical of that observed in blood and most other tissues (Nashan et al, 1989). By contrast, the intraepithelial lymphocytes in the epididymis of rats, mice, and humans are predominantly CD8+ T cells, which is a common feature of mucosal epithelia (Ritchie et al, 1984; Nashan et al, 1989; Flickinger et al, 1997; Yakirevich et al, 2002). Leukocytes are found in all regions of the epididymis, although the tendency is toward greater numbers of all leukocyte subsets in the peritubular zone and epithelium of the caput compared with the cauda (Nashan et al, 1989; Flickinger et al, 1997; Seiler et al, 1999), and intraepithelial macrophage and CD8+ T‐cell numbers increase preferentially in the more proximal regions of the epithelium during aging and increased spermatogenic disturbance in rats (Serre and Robaire, 1999). The strikingly different distribution of leukocytes in the epididymis compared with the testis clearly indicates the existence of a different immunological environment, one in which separation between the antigenic sperm and the local immune cells is less robust. In fact, it has been speculated that macrophages within the epididymal duct might even be responsible for phagocytosis of senescent and excess sperm, although the evidence for this is equivocal (Tomlinson et al, 1992; Cooper et al, 2002).
Studies have suggested that some basal cells of the epididymal epithelium exhibit structural and antigenic properties typical of macrophages (Yeung et al, 1994; Seiler et al, 1999). The numbers of the basal cells expressing macrophage‐specific markers in the mouse are increased by the presence of damaged sperm, and it has been speculated that these cells are actually resident macrophages that could be playing a role in regulating immunity in the epididymis (Seiler et al, 1999, 2000). Moreover, exciting new data from the mouse indicate that dendritic cells are a major feature of the epididymal epithelium (Da Silva et al, 2011). These cells form a dense network in the basal region of the epithelium and extend their processes up toward the apical tight junctions between epithelial cells. The intraepithelial dendritic cells express normal antigen‐presenting surface molecules and have antigen‐presenting activity in vitro. The extent of overlap between these dendritic cells, the macrophage‐like basal cells, and conventional intraepithelial macrophages remains to be established, but all display an increased distribution toward the more proximal regions of the normal epididymis. It is self‐evident that some or all of these cells will play important roles in controlling immunity within the epididymis.
Immune Privilege in the Testis and Epididymis
The testicular interstitial tissue in several rodent species is able to support the survival of allografts and xenografts for extended periods—even indefinitely in some cases—in spite of the fact that this tissue has an extensive and effective lymphatic drainage to local lymph nodes and contains significant populations of antigen‐presenting cells (macrophages and dendritic cells) and T cells (Ferguson and Scothorne, 1977; Whitmore and Gittes, 1978; Head et al, 1983; Head and Billingham, 1985). The parameters that determine the survival of grafts in the testis include structural, experimental, and species differences that remain poorly explained, and the precise mechanisms responsible for this “immune privilege” of the testis are yet to be fully determined, but it is evident that tissue‐specific immunoregulatory mechanisms are involved (Hedger, 2007; Meinhardt and Hedger, 2011). Teleologically, it is generally assumed that testicular immune privilege exists to protect the developing spermatogenic cells from the host immune system.
By contrast, immune privilege in the epididymis has received little attention, and the limited available evidence indicates that the epididymis does not provide the same level of protection afforded by the testis, if at all. Some data indicate that allografts can survive slightly extended periods within the rat epididymis, but this could simply be related to regional differences in lymphatic drainage between animals, rather than local immunoregulation (Kazeem, 1988). Given the large variability in the historical success rate of studies on testicular immune privilege, it might be wise to reserve final judgment until more comprehensive data are available. However, experimental evidence is considerable that the epididymis might be much more susceptible to inflammation and subsequent leukocytic infiltration than is the testis. Interstitial tissue infiltrates of lymphocytes and neutrophils are frequently observed in the epididymis and vas deferens, but rarely in the testis, of aging mice (Itoh et al, 1999a). Similarly, in mice injected (IV) with Bordetella pertussis and adjuvant, neutrophils invade the stroma of the epididymis and vas deferens, as well as the accessory organs, but the testis is not affected (Itoh et al, 1995). In the alymphoplastic (aly) mouse, which is deficient in lymphoid tissue, eosinophils and macrophages spontaneously accumulate in the stroma of the epididymis and vas deferens, but not the testis (Itoh et al, 1999b).
Immunoregulation in the Testis and Epididymis
To understand how immune privilege can be sustained, it is essential to know how antigen‐specific (adaptive) immunity is regulated. In the immune system, the ability to discriminate self antigens from foreign antigens relies on the induction of tolerance. This involves central tolerance, whereby T and B cells directed against self antigens are deleted from the immune repertoire in the thymus and bone marrow (Kappler et al, 1987; Nossal, 1994; Liston et al, 2003). Central tolerance is established before birth or during the perinatal period in different species but clearly occurs long before the appearance of antigens associated with all but the earliest stages of spermatogenic development. Peripheral tolerance, on the other hand, is maintained throughout life by interactions between antigen‐presenting cells and T cells under “tolerizing” conditions, in which some of the necessary costimulatory signals between the cells are absent or in the presence of immunoregulatory cytokines, ligands, leukocyte subsets, or a combination of these elements (Mueller, 2010). This tolerizing interaction results in responses ranging from destruction of the antigen‐specific T cell entirely to the generation of actively immunosuppressive antigen‐specific T‐cell subsets, such as CD4+CD25+ Treg cells or CD8+ suppressor T cells (Gilliet and Liu, 2002; Toda and Piccirillo, 2006). Furthermore, the immune response itself can be directed toward either an aggressive cellular immunity mediated by type 1 helper cells (Th1 cell), which is primarily responsible for graft rejection, or an alternatively activated or type 2 (Th2 cell) response, which favors antibody responses but reduced cell‐mediated immunity. In general, Th2 cells and alternatively activated (M2) macrophages have been associated with immune privilege and tolerance in a number of systems (Cobbold and Waldmann, 1998; Stein‐Streilein and Streilein, 2002; Martinez et al, 2008).
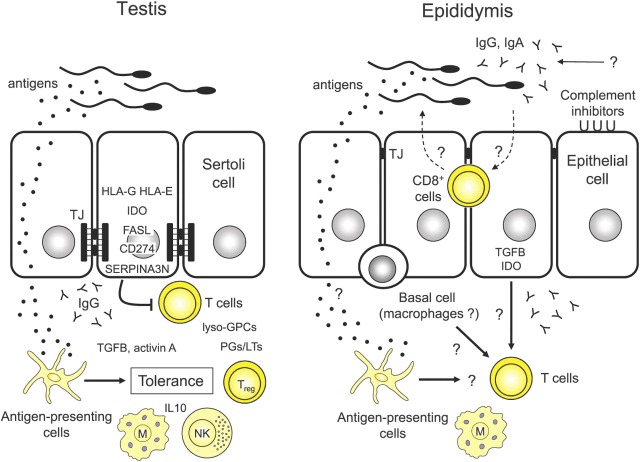
Evidence is accumulating that a number of complementary mechanisms are involved in testicular immune privilege (the Figure). Significantly, Head et al (1983) discovered that intratesticular allograft survival depended upon antigen exposure being confined to the testis environment because active immunization of the host against the graft alloantigens caused the intratesticular grafts to be rapidly rejected. It already has been noted that the testicular interstitial tissue contains dendritic cells and macrophages with an alternatively activated phenotype. Direct evidence that interactions between these antigen‐presenting cells and T cells within the testicular environment, which would also include the local draining lymph nodes, can produce peripheral tolerance comes from studies showing that introduction of spinal cord, retinal, or arthritogenic antigens via the testis route can suppresses immune responses to these antigens in the periphery (Li et al, 1997; Ditzian‐Kadanoff, 1999; Veräjänkorva et al, 2002). This concept has recently received compelling support from the discovery that transgenic mice, which specifically express high levels of a surrogate autoantigen, ovalbumin, in late spermatids and subsequently release this antigen into the interstitial tissue, were resistant to T‐cell– or antibody‐mediated immune responses after active immunization with exogenous ovalbumin (K. Tung in Krawetz et al, 2009). Altogether, these data suggest that continuous release of spermatogenic cell antigens is responsible for maintaining peripheral tolerance to these autoantigens. Accordingly, immunoregulatory NK T cells and Treg cells are prominently represented in the rat and mouse testis (Hedger, unpublished data), and there is evidence that activated, memory T cells, or both, which would normally effect an immunological response, are instead targeted for destruction when they enter the testicular environment (Dai et al, 2005; Nasr et al, 2005). A crucial role for Treg‐cell–mediated tolerance in the human testis is also indicated by the autoimmune polyendocrinopathy syndrome type 2 (APS2), a condition of spontaneous autoimmunity in a number of endocrine glands, including the testes in a subset of patients, which is characterized by defective Treg‐cell function (Kriegel et al, 2004).
Figure Figure.
. Comparison of proposed immunoregulatory mechanisms in the testis and epididymis. Adjacent epithelial Sertoli cells of the testis produce basally located extensive tight junctional (TJ) specializations that completely restrict the movement of immune cells, immunoglobulin, and other molecules into the epithelium and lumen of the seminiferous tubules. Consequently, the majority of developing spermatogenic cells and intraluminal sperm are completely isolated from the immune system within the seminiferous tubules. Regions where this restriction is less effective, notably the tubuli recti and rete testis, are zones of increased susceptibility to inflammatory and autoimmune responses. Antigens within the testis, including spermatogenic cell antigens, are picked up by antigen‐presenting cells in the interstitium (dendritic cells and macrophages) and are presented to circulating T cells in a tolerizing environment that involves the resident macrophages (M), natural killer (NK), and NK T cell subsets and immunoregulatory cytokines, such as TGFB, activin A, and IL10. This leads to production of immunoregulatory T cells, such as CD4+CD25+ Treg cells, thereby maintaining ongoing tolerance to antigens within the testicular environment. In addition, Sertoli cells, and possibly other testicular cells, express a number of immunoregulatory molecules that inhibit T‐cell activity, survival, or both in the interstitial space: nonclassical major histocompatibility complex class molecules (HLA‐G and HLA‐E), the negative costimulatory ligand CD274 antigen, indoleamine 2,3 dioxygenase (IDO), FASL, the granzyme B inhibitor serine peptidase inhibitor A3N (SERPINA3N), anti‐inflammatory prostaglandins (PG) and leukotrienes (LT), and lyso‐glycerophosphatidylcholine species (lyso‐GPCs). In the epididymis, the interepithelial junctions are less specialized and apically located, so that immune cells (dendritic cells, macrophages, and CD8+ lymphocytes) and other immune products are able to enter the epithelium, where they may have contact with the luminal contents under certain conditions. Entry of antibody (IgA and IgG) into the epididymis can occur either by transepithelial transport or via the ascending tract from the accessory glands. Antigen‐presenting cells and basal cell macrophages in the epididymal epithelium could play a role in regulating T‐cell responses. Epithelial cells of the epididymis express IDO and TGFB and might possess immunoregulatory functions similar to those of the Sertoli cells, although this remains speculative.
The testicular cell that appears to be central to maintaining testicular immune privilege is the Sertoli cell. Studies have established that isolated Sertoli cells possess inherent immunosuppressive properties and can be transplanted into a variety of tissues across both allogeneic and xenogeneic barriers (Selawry and Cameron, 1993; Sanberg et al, 1996; Korbutt et al, 1997). Moreover, these cells are able to provide an immunoprotective environment for various allografts and xenografts in cotransplantation studies. The Sertoli cell expresses several molecules that have the capacity to induce apoptosis in antigen‐specific T cells, including Fas ligand (FASL, also called CD95L; Bellgrau et al, 1995) and the negative costimulatory ligand CD274 antigen (also called B7‐H1; Dal Secco et al, 2008). A recent study has shown that blocking the activity of the tryptophan‐metabolizing enzyme, indoleamine 2,3 dioxygenase (IDO), which inhibits T‐cell–mediated autoimmunity and stimulates Treg‐cell functions, abrogated the protective activity of porcine Sertoli cells in a cotransplantation model in mice (Fallarino et al, 2009). Additionally, Sertoli cells produce inhibitors of complement, an important inducer of the inflammatory response (Lee et al, 2007), and secrete inhibitors of granzyme B, which is part of the destructive arsenal of cytotoxic lymphocytes (Bladergroen et al, 2001; Sipione et al, 2006). Sertoli cells also produce several immunoregulatory cytokines of the TGFB family, specifically activin A, which directs the development of macrophages and T cells toward type 2 responses (Hedger, 2011a), and TGFB1, which has been implicated in graft survival promoted by the Sertoli cells (Suarez‐Pinzon et al, 2000).
Other testicular immunoregulatory mechanisms include expression of the nonclassical MHC molecules human leukocyte antigen (HLA)‐G and HLA‐E, which inhibit T‐cell and NK‐cell activity, in the seminiferous epithelium of the rhesus monkey and human testis (Fiszer et al, 1997; Slukvin et al, 1999; Ryan et al, 2002). Endogenous elevation of IL10, which is induced in the testis during inflammation (O'Bryan et al, 2005; Rival et al, 2007), has been shown to reduce inflammation, autoimmunity, and spermatogenic damage in a mouse model of autoimmune orchitis (Watanabe et al, 2005). Finally, a role for bioactive lipids in testicular immunoregulation is gradually emerging. Prostanoid biosynthesis occurs in most testicular cells (Winnall et al, 2007); prostaglandins of the D, E, and I series and thromboxane A, in particular, display anti‐inflammatory, immunosuppressive functions, or both (Hata and Breyer, 2004). Moreover, several medium–chain length lysoglycerophosphatidylcholine species, which are produced during prostanoid biosynthesis and are present in testicular interstitial fluid, possess potent immunoregulatory activities (Foulds et al, 2008).
Compared with the testis, immunoregulatory mechanisms and induction of tolerance in the epididymis have much less data, and many more question marks remain (the Figure). However, the possibility that macrophages and dendritic cells, either in the epithelia or those in the peritubular or interstitial tissue, play a crucial role in maintaining tolerance to sperm antigens, similar to that proposed for these cells in the testis, is tantalizing. Moreover, evidence is limited that the principal cells or other epididymal epithelial cells might have similar immunoregulatory functions as those displayed by the Sertoli cell. The immunoregulatory cytokine TGFB1 has been found in principal cells and apical cells of the caput and corpus epididymis of the marmoset (Bomgardner et al, 1999). Significantly, IDO is highly expressed in the principal cells and apical cells of the mouse caput epididymis, although it remains to be confirmed that this enzyme plays a role in epididymal immunoregulation (Britan et al, 2006). Alternatively, such immunoregulatory mechanisms might be unnecessary in the epididymis because peripheral tolerance created in the testis environment could be sufficient to provide protection to sperm antigens throughout the male reproductive tract, under normal conditions. However, a more prominent role could exist for complement inhibitors in the epididymal epithelium and sperm than in the seminiferous epithelium (Harris et al, 2006; Mizuno et al, 2006; Ito et al, 2007), which could reflect the more ready access of immunoglobulins to the lumen of the epididymal duct (Weininger et al, 1982; Yule et al, 1988; Beagley et al, 1998; Knee et al, 2005).
Consequences of Immunological Events
The testis and epididymis would appear to differ significantly in terms of several immunological parameters. Clinical evidence that immunoregulation in the epididymis might actually be influenced by proximity to the testis comes from studies of sperm autoantibody formation in men with obstructive azoospermia, with congenital absence of the vas, or following vasectomy (de Kretser et al, 1998). In these patients, the incidence of sperm antibodies increased with increasing distance of the lesion from the testis: the presence of sperm antibody ranged from 66% to 100% in patients with a vasectomy or obstructions in the cauda epididymis, whereas lesions in the rete testis or caput are associated with low or negligible sperm antibody levels. Together with the observation that the number or activity of intraepithelial macrophages and dendritic cells, or both, are highest in the more proximal regions of the epididymis (Seiler et al, 1999; Da Silva et al, 2011), suggesting that the testis exerts an influence on the immune environment of the adjacent epididymis that attenuates along the epididymal duct.
Autoimmune regulator (AIRE) is a promiscuous thymic transcription factor involved in controlling expression of antigens for presentation during the establishment of central tolerance in the thymus (Pitkanen and Peterson, 2003). The loss of this protein is responsible for the autoimmune polyendocrinopathy syndrome type 1, which predisposes patients to testicular autoimmunity. In mice with a targeted deletion of the Aire gene, testicular function appeared normal, but fertility was reduced because of sperm antibody formation and because the interstitial tissue of the epididymis contained frequent leukocytic infiltrates (Hubert et al, 2009; Scott and O'Bryan, personal communication). Furthermore, neonatal (day 3) thymectomy in mice, which leads to a loss of peripheral tolerance mediated by Treg cells (Thornton and Shevach, 1998), initially causes epididymovasitis in the postpubertal period, followed by an increasing incidence of orchitis many weeks later (Taguchi and Nishizuka, 1981; Tung et al, 1987a). Subsequently, the incidence of orchitis remains considerably lower than the incidence of the epididymovasitis, even in mature adult mice. Both these observations indicate a much greater susceptibility of the epididymis to inflammation and autoimmunity when compared with the testis in models of tolerance failure.
In experimental models of autoimmune orchitis (EAO) induced by active immunization with testicular extracts in the presence of suitable adjuvants in rats, mice, and guinea pigs, epididymitis is also commonly observed, most frequently involving the cauda epididymis (Tung et al, 1970, 1987b; Kohno et al, 1983; Zhou et al, 1989). Curiously, subcutaneous injection of syngeneic testicular spermatogenic cells into mice in the absence of adjuvant causes orchitis without epididymitis (Itoh et al, 1991a), but passive transfer of lymphocytes from mice immunized against these cells and subsequently stimulated in vitro actually favors the induction of epididymitis (Itoh et al, 1991b, 1992). The reasons for these differences remain unresolved (Naito and Itoh, 2008), but significantly, the transfer of T cells activated specifically against spermatid antigens induce epididymitis in this model. A similar result was observed after the passive transfer of T cells from EAO mice, with testicular involvement initially confined to the tubuli recti, rete testis, and efferent ducts (Mahi‐Brown et al, 1987; Tung et al, 1987b; Yule and Tung, 1993). In an analogous manner, syngeneic testicular spermatogenic cells or epididymal spermatozoa caused spermatic granuloma formation when injected into the epididymides, but not when injected into the testes, of adult mice (Itoh et al, 1999c). In fact, spermatic granulomata are rarely seen in the human testis but are common in the epididymis and vas deferens (Nistal et al, 1997). Furthermore, detailed studies in mice have established that the immunogenetics underlying susceptibility to autoimmune orchitis and epididymitis are quite distinct (Roper et al, 1998). Altogether, these studies further highlight the particular susceptibility of the epididymis to inflammation when responses to spermatogenic antigens occur.
Vasectomy generally causes the formation of sperm antibodies, a response that is believed to be due to sperm retention and increased intraluminal pressure in the reproductive tract, leading to increased sperm degradation in situ, epithelial rupture, sperm granuloma formation, and exposure of sperm antigens to the immune system, particularly at the vasectomy site (Alexander and Tung, 1977; Flickinger et al, 1995). Species and strain differences in susceptibility to orchitis after vasectomy are striking: lesions are common in the testes of monkeys, guinea pigs, rabbits, and some strains of rats and mice but are rare in humans (Bigazzi et al, 1976; Kojima and Spencer, 1983; Flickinger, 1985; Herr et al, 1987). In humans, chronic pain of inflammatory origin can occur (McMahon et al, 1992). Postvasectomy, epididymal inflammation is characterized by infiltration of macrophages, lymphocytes, and neutrophils into the interstitial and peritubular zones in particular, but also in the epithelium, along with epididymal granulomata (Nashan et al, 1990; Flickinger et al, 1995; Hooper et al, 1995). However, these epididymal changes might also occur secondary to the development of autoimmune orchitis (Flickinger et al, 1990), and clearly separating cause and effect is difficult. Nonetheless, vasectomized A/J mice immunized with syngeneic testicular spermatogenic cells develop epididymitis without orchitis (Qu et al, 2008, 2010), suggesting that the epididymis can be the primary site of immune reactions after vasectomy.
In summary, although the mechanisms underlying experimental autoimmune orchitis and epididymitis remain to be fully characterized, studies suggest direct exposure to spermatogenic cell antigens, loss of tolerance, or passive transfer of activated T cells against spermatogenic antigens preferentially leads to epididymitis. By contrast, full‐blown orchitis develops only against favorable background genetics or requires more aggressive immunization protocols. This would tend to suggest that central tolerance is the most important immunoprotective mechanism operating in the epididymis and that, compared with the testis, this tissue could have fewer effective back‐up mechanisms. These differences would also tend to fit with the observation that the epididymis probably lacks the ability to support foreign tissue grafts, raising questions about the actual role of the intraepithelial antigen‐presenting cells of the epididymis (Da Silva et al, 2011), which only more work can resolve.
Innate Immunity in the Testis and Epididymis
If normal adaptive immune mechanisms are modulated within the testis and epididymis to minimize responses against spermatogenic cell antigens, then innate immunity becomes potentially more important for protecting the testis and epididymis against infection and tumors. As outlined earlier, innate immunity in the testis is mediated via pattern recognition receptors expressed primarily by macrophages and Sertoli cells acting as sentinels against infections from the circulation and ascending tract, respectively. However, the resident effector cells of innate immunity, the testicular macrophages, NK cells, mast cells, and eosinophils, are located entirely within the interstitial tissue, pointing to potential problems during infection of the seminiferous tubules. Studies of retrograde infections in rats and mice using live E coli introduced directly into the seminiferous tubules, or via the epididymis or proximal vas deferens, leads to inflammation and invasion of the seminiferous epithelium by leukocytes, particularly neutrophils, resulting in long‐term damage to the epithelium even after the infection has been cleared (Lucchetta et al, 1983; Demir et al, 2007). Notably, aggressively uropathogenic bacteria are able to subvert the innate immune response in rat testicular macrophages and Sertoli cells by suppressing pro‐inflammatory NFκB signaling, directing them instead to mount an antiviral response (Bhushan et al, 2008). How the leukocytes gain access to the seminiferous tubules in intraluminal infections remains to be resolved, but evidence suggests that the inter‐Sertoli tight junctions might be disrupted, and a possibility that leukocytes traverse the less restrictive epithelium of the tubuli recti and rete testis. Significantly, this leukocytic infiltration was not observed if dead bacteria or LPS alone were introduced into the tubules, suggesting that the invasion of the seminiferous tubules is an option of last resort in response to actively proliferating infections (Nagaosa et al, 2009). Ongoing balancing mechanisms could involve the resident immune cells and somatic cells of the testis, which calibrate the ability to resist infection or neoplasms, while attempting to minimize intratesticular inflammation and immunity to reduce collateral damage to the developing spermatogenic cells.
In the epididymis, the situation is probably a little different. Adaptive immune responses might not be compromised, as they appear to be in the testis, and clearly leukocytes have access to the epididymal epithelium, where they could have close contact with intraluminal infections. Likely, the intraepithelial macrophages and CD8+ cells, which are presumably cytotoxic lymphocytes (but could also include immunoregulatory T cells), have an important role in protecting against infection in the epididymis. Appropriately, injection of E coli into the epididymis of adult mice via the vas deferens route induces an increase in macrophage and CD8+ lymphocyte numbers and activity in the epididymal interstitium, peritubular zone, and epithelium (Nashan et al, 1993). However, these cells have yet to be studied in any detail, and their phenotype and functions remain poorly understood.
One mechanism that does appear to be particularly important for innate immune protection in the epididymis is the production of beta defensins. Defensins are small, positively charged peptides that disrupt bacteria, fungi, parasites, and some enveloped viruses by forming multimeric pores in the pathogen membrane (Selsted and Ouellette, 2005). Most epithelial cells, including those of the testis and epididymis, produce beta defensins, but a large number of epididymal‐specific beta defensins with distinct regional distributions have been identified in the mouse and the rat (Li et al, 2001; Yamaguchi et al, 2002; Com et al, 2003; Yenugu et al, 2004; Zhou et al, 2004; Jalkanen et al, 2005; Patil et al, 2005). Many of these epididymal defensins are developmentally and hormonally regulated, with evidence that some might also play a role in sperm maturation (Li et al, 2001; Zhou et al, 2004). Normally, epithelial defensin production is stimulated by TLR activation and cytokines during inflammation, but a recent study in rats discovered that LPS actually causes the expression of some epididymal beta defensins to fall (Cao et al, 2010). The precise functions of these peptides in the epididymis are obviously a topic of considerable ongoing interest.
Looking to the Future
The underlying immunology of the testis and epididymis appears to be different in several fundamental aspects on the basis of 1) the ability to support grafts, 2) organization of the blood‐epithelial barrier, 3) distribution of immune cells and pattern recognition receptors, 4) susceptibility to different types of infection, and 5) autoimmune responses to damage, immunization, or loss of central tolerance. Immunoregulatory mechanisms are apparently effective in generating peripheral tolerance to sperm antigens in the testis, whereas data for similar mechanisms in the epididymis are lacking. It is even possible that the epididymis does not need to provide this type of specific immunoregulatory protection and simply acts to minimize the effect of any responses that might occur. However, the barrier functions and active immunosuppressive mechanisms of the epididymis also appear to be less effective compared with the testis, which might explain the greater susceptibility of the epididymis to inflammation after trauma, infection, or immune activation.
Although we still know relatively little about the innate and acquired immune systems as they relate to the testis, we know even less about the interaction between these systems and the epididymis. Given that the epididymis could be more susceptible to inflammation and immune events, and hence a weak link in sperm protection from the immune system, it is probably time this imbalance in our knowledge was addressed. So, what is the knowledge that we lack that is needed to make progress?
-
1
Epididymal immune cell phenotypes and immune functions need to be properly characterized. Although the presence of these cells has long been established and their general characteristics have been identified, the functional properties of these cells remain very poorly understood. One obvious deficiency is an apparent lack of consensus over the identification of cells, specifically the degree of overlap between basal cells, halo cells, macrophages and dendritic cells, and T‐cell subsets in different studies.
-
2
The parameters of tolerance to antigens within the epididymis need to be established. In the testis, we know that allografts survive for extended periods and that testis tissue can survive transplantation under appropriate conditions, and this immune privilege is related to a number of specific mechanisms. Apparently, no analogue of immune privilege exists in the epididymis, but definitive studies are lacking.
-
3
The effects of activating innate immunity and signaling pathways in the normal and infected/inflamed epididymis need to be determined. A small number of studies to date indicate that activation of innate immunity in the epididymis affects its physiological functions and, consequently, the ability to support or protect the sperm or both. Such studies need to be extended to investigate the responses of the various epithelial cells of the epididymis to inflammation and infection.
-
4
Local immunoregulatory mechanisms in the epididymis need to be identified and verified. It would be important to know if any of the several epithelial cell types of the epididymis have immunoregulatory properties, similar to those of the Sertoli cell. Although some attention has been paid to the basal cells, the evidence that these cells have immunoregulatory functions is so far circumstantial. The recent observation that the epithelium of the epididymis is invested with dendritic cells, especially in the caput region, will be followed with interest.
Acknowledgment
This review is a summary of a presentation given at the Fifth International Conference on the Epididymis, Ãguas de São Pedro, Brazil, October 2010.
Footnotes
This work has been supported by grants from the National Health and Medical Research Council and the Australian Research Council, and by the Victorian Government's Operational Infrastructure Support Program.
References
- AbouZahr C., Vaughan JP. Assessing the burden of sexual and reproductive ill‐health: questions regarding the use of disability‐adjusted life years. Bull W H O 2000; 78: 655–666. [PMC free article] [PubMed] [Google Scholar]
- Alexander NJ, Tung KS. Immunological and morphological effects of vasectomy in the rabbit. Anat Rec. 1977; 188: 339–350. [DOI] [PubMed] [Google Scholar]
- Alexander RB, Ponniah S., Hasday J., Hebel J.R.. Elevated levels of proinflammatory cytokines in the semen of patients with chronic prostatitis/chronic pelvic pain syndrome. Urology. 1998; 52: 744–749. [DOI] [PubMed] [Google Scholar]
- Anton F., Morales C., Aguilar R., Bellido C., Aguilar E., Gaytan F.. A comparative study of mast cells and eosinophil leukocytes in the mammalian testis. Zentbl Veterinarmed [A]. 1998; 45: 209–218. [DOI] [PubMed] [Google Scholar]
- Baker HW. Reproductive effects of nontesticular illness. Endocrinol Metab Clin North Am. 1998; 27: 831–850. [DOI] [PubMed] [Google Scholar]
- Bauché F., Stéphan JP, Touzalin AM, Jégou B.. In vitro regulation of an inducible‐type NO synthase in the rat seminiferous tubule cells. Biol Reprod. 1998; 58: 431–438. [DOI] [PubMed] [Google Scholar]
- Beagley KW, Wu ZL, Pomering M., Jones RC. Immune responses in the epididymis: implications for immunocontraception. J Reprod Fertil Suppl. 1998; 53: 235–245. [PubMed] [Google Scholar]
- Bellgrau D., Gold D., Selawry H., Moore J., Franzusoff A., Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995; 377: 630–632. [DOI] [PubMed] [Google Scholar]
- Bhushan S., Tchatalbachev S., Klug J., Fijak M., Pineau C., Chakraborty T., Meinhardt A.. Uropathogenic Escherichia coli block MyD88‐dependent and activate MyD88‐independent signaling pathways in rat testicular cells. J Immunol. 2008; 180: 5537–5547. [DOI] [PubMed] [Google Scholar]
- Bigazzi PE, Kosuda LL, Hsu KC, Andres GA. Immune complex orchitis in vasectomized rabbits. J Exp Med. 1976; 143: 382–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladergroen BA, Strik MC, Bovenschen N., van Berkum O., Scheffer GL, Meijer CJ, Hack CE, Kummer JA. The granzyme B inhibitor, protease inhibitor 9, is mainly expressed by dendritic cells and at immune‐privileged sites. J Immunol. 2001; 166: 3218–3225. [DOI] [PubMed] [Google Scholar]
- Bomgardner D., Wehrenberg U., Rune GM. TGF‐β could be involved in paracrine actions in the epididymis of the marmoset monkey (Callithrix jacchus). J Androl. 1999; 20: 375–383. [PubMed] [Google Scholar]
- Britan A., Maffre V., Tone S., Drevet J.R.. Quantitative and spatial differences in the expression of tryptophan‐metabolizing enzymes in mouse epididymis. Cell Tissue Res. 2006; 324: 301–310. [DOI] [PubMed] [Google Scholar]
- Bryniarski K., Szczepanik M., Maresz K., Ptak M., Ptak W.. Subpopulations of mouse testicular macrophages and their immunoregulatory function. Am J Reprod Immunol. 2004; 52: 27–35. [DOI] [PubMed] [Google Scholar]
- Cao D., Li Y., Yang R., Wang Y., Zhou Y., Diao H., Zhao Y., Zhang Y., Lu J.. Lipopolysaccharide‐induced epididymitis disrupts epididymal β‐defensin expression and inhibits sperm motility in rats. Biol Reprod. 2010; 83: 1064–1070. [DOI] [PubMed] [Google Scholar]
- Cobbold S., Waldmann H.. Infectious tolerance. Curr Opin Immunol. 1998; 10: 518–524. [DOI] [PubMed] [Google Scholar]
- Com E., Bourgeon F., Evrard B., Ganz T., Colleu D., Jégou B., Pineau C.. Expression of antimicrobial defensins in the male reproductive tract of rats, mice, and humans. Biol Reprod. 2003; 68: 95–104. [DOI] [PubMed] [Google Scholar]
- Cooper TG, Yeung CH, Jones R., Orgebin‐Crist MC, Robaire B.. Rebuttal of a role for the epididymis in sperm quality control by phagocytosis of defective sperm. J Cell Sci. 2002; 115: 5–7. [DOI] [PubMed] [Google Scholar]
- Crocker PR, Jefferies WA, Clark SJ, Chung LP, Gordon S.. Species heterogeneity in macrophage expression of the CD4 antigen. J Exp Med. 1987; 166: 613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr DG, Robaire B., Hermo L.. Structure and turnover of junctional complexes between principal cells of the rat epididymis. Microsc Res Tech. 1995; 30: 54–66. [DOI] [PubMed] [Google Scholar]
- Da Silva N., Cortez‐Retamozo V., Reinecker HC, Wildgruber M., Hill E., Brown D., Swirski F., Pittet M., Breton S.. A dense network of dendritic cells populates the murine epididymis. Reproduction. 2011; 141: 653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z., Nasr IW, Reel M., Deng S., Diggs L., Larsen CP, Rothstein DM, Lakkis FG. Impaired recall of CD8 memory T cells in immunologically privileged tissue. J Immunol. 2005; 174: 1165–1170. [DOI] [PubMed] [Google Scholar]
- Dal Secco V., Riccioli A., Padula F., Ziparo E., Filippini A.. Mouse Sertoli cells display phenotypical and functional traits of antigen‐presenting cells in response to interferon gamma. Biol Reprod. 2008; 78: 234–242. [DOI] [PubMed] [Google Scholar]
- de Kretser DM, Huidobro C., Southwick GJ, Temple‐Smith PD. The role of the epididymis in human infertility. J Reprod Fertil Suppl. 1998; 53: 271–275. [PubMed] [Google Scholar]
- Dejucq N., Chousterman S., Jégou B.. The testicular antiviral defense system: localization, expression, and regulation of 2′5′ oligoadenylate synthetase, double‐stranded RNA‐activated protein kinase, and Mx proteins in the rat seminiferous tubule. J Cell Biol. 1997; 139: 865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir A., Türker P., Önol FF, Sirvanci S., Findik A., Tarcan T.. Effect of experimentally induced Escherichia coliepididymo‐orchitis and ciprofloxacin treatment on rat spermatogenesis. Int J Urol. 2007; 14: 268–272. [DOI] [PubMed] [Google Scholar]
- Ditzian‐Kadanoff R.. Testicular‐associated immune deviation and prevention of adjuvant‐induced arthritis by three tolerization methods. Scand J Immunol. 1999; 50: 150–158. [DOI] [PubMed] [Google Scholar]
- Dohle GR, Colpi GM, Hargreave TB, Papp GK, Jungwirth A., Weidner W.. EAU guidelines on male infertility. Eur Urol. 2005; 48: 703–711. [DOI] [PubMed] [Google Scholar]
- Dong Q., Hawker F., McWilliam D., Bangah M., Burger H., Handelsman DJ. Circulating immunoreactive inhibin and testosterone levels in men with critical illness. Clin Endocrinol. 1992; 36: 399–404. [DOI] [PubMed] [Google Scholar]
- Dym M., Fawcett DW. The blood‐testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970; 3: 308–326. [DOI] [PubMed] [Google Scholar]
- Dym M., Romrell LJ. Intraepithelial lymphocytes in the male reproductive tract of rats and rhesus monkeys. J Reprod Fertil. 1975; 42: 1–7. [DOI] [PubMed] [Google Scholar]
- Ekwere PD. Immunological infertility among Nigerian men: incidence of circulating antisperm auto‐antibodies and some clinical observations: a preliminary report. Br J Urol. 1995; 76: 366–370. [DOI] [PubMed] [Google Scholar]
- el‐Demiry MI, Hargreave TB, Busuttil A., Elton R., James K., Chisholm GD. Immunocompetent cells in human testis in health and disease. Fertil Steril. 1987; 48: 470–479. [DOI] [PubMed] [Google Scholar]
- Fallarino F., Luca G., Calvitti M., Mancuso F., Nastruzzi C., Fioretti MC, Grohmann U., Becchetti E., Burgevin A., Kratzer R., van Endert P., Boon L., Puccetti P., Calafiore R.. Therapy of experimental type 1 diabetes by isolated Sertoli cell xenografts alone. J Exp Med. 2009; 206: 2511–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J., Scothorne RJ. Extended survival of pancreatic islet allografts in the testis of guinea‐pigs. J Anat. 1977; 124: 1–8. [PMC free article] [PubMed] [Google Scholar]
- Fiszer D., Ulbrecht M., Fernandez N., Johnson JP, Weiss EH, Kurpisz M.. Analysis of HLA class Ib gene expression in male gametogenic cells. Eur J Immunol. 1997; 27: 1691–1695. [DOI] [PubMed] [Google Scholar]
- Flickinger CJ. The effects of vasectomy on the testis. N Engl J Med. 1985; 313: 1283–1285. [DOI] [PubMed] [Google Scholar]
- Flickinger CJ, Bush LA, Howards SS, Herr JC. Distribution of leukocytes in the epithelium and interstitium of four regions of the Lewis rat epididymis. Anat Rec. 1997; 248: 380–390. [DOI] [PubMed] [Google Scholar]
- Flickinger CJ, Herr JC, Caloras D., Sisak J.R., Howards SS. Inflammatory changes in the epididymis after vasectomy in the Lewis rat. Biol Reprod. 1990; 43: 34–45. [DOI] [PubMed] [Google Scholar]
- Flickinger CJ, Howards SS, Herr JC. Effects of vasectomy on the epididymis. Microsc Res Tech. 1995; 30: 82–100. [DOI] [PubMed] [Google Scholar]
- Foulds LM, Boysen RI, Crane M., Yang Y., Muir JA, Smith AI, de Kretser DM, Hearn MT, Hedger MP. Molecular identification of lyso‐glycerophosphocholines as endogenous immunosuppressives in bovine and rat gonadal fluids. Biol Reprod. 2008; 79: 525–536. [DOI] [PubMed] [Google Scholar]
- Friend DS, Gilula NB. Variations in tight and gap junctions in mammalian tissues. J Cell Biol. 1972; 53: 758–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérard N., Syed V., Bardin W., Genetet N., Jégou B.. Sertoli cells are the site of interleukin‐1α synthesis in rat testis. Mol Cell Endocrinol. 1991; 82: R13–16. [DOI] [PubMed] [Google Scholar]
- Gibbings DJ, Marcet‐Palacios M., Sekar Y., Ng MC, Befus AD. CD8α is expressed by human monocytes and enhances FcγR‐dependent responses. BMC Immunol. 2007; 8: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliet M., Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand‐activated plasmacytoid dendritic cells. J Exp Med. 2002; 195: 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez E., Morel G., Cavalier A., Lienard MO, Haour F., Courtens JL, Jégou B.. Type I and type II interleukin‐1 receptor expression in rat, mouse, and human testes. Biol Reprod. 1997; 56: 1513–1526. [DOI] [PubMed] [Google Scholar]
- Hagley M.. Epididymo‐orchitis and epididymitis: a review of causes and management of unusual forms. Int J STD AIDS. 2003; 14: 372–377. [DOI] [PubMed] [Google Scholar]
- Haidl G., Allam JP, Schuppe HC. Chronic epididymitis: impact on semen parameters and therapeutic options. Andrologia. 2008; 40: 92–96. [DOI] [PubMed] [Google Scholar]
- Harris CL, Mizuno M., Morgan BP. Complement and complement regulators in the male reproductive system. Mol Immunol. 2006; 43: 57–67. [DOI] [PubMed] [Google Scholar]
- Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Therapeut. 2004; 103: 147–166. [DOI] [PubMed] [Google Scholar]
- Hayes R., Chalmers SA, Nikolic‐Paterson DJ, Atkins RC, Hedger MP. Secretion of bioactive interleukin 1 by rat testicular macrophages in vitro. J Androl. 1996; 17: 41–49. [PubMed] [Google Scholar]
- Head J.R., Billingham RE. Immune privilege in the testis. II. Evaluation of potential local factors. Transplantation. 1985; 40: 269–275. [DOI] [PubMed] [Google Scholar]
- Head J.R., Neaves WB, Billingham RE. Immune privilege in the testis. I. Basic parameters of allograft survival. Transplantation. 1983; 36: 423–431. [DOI] [PubMed] [Google Scholar]
- Hedger MP. Macrophages and the immune responsiveness of the testis. J Reprod Immunol. 2002; 57: 19–34. [DOI] [PubMed] [Google Scholar]
- Hedger MP. Immunologically privileged environments In: Halberstadt CR, Emerich DF, eds. Cellular Transplantation from Laboratory to Clinic. Amsterdam, The Netherlands: Elsevier; 2007: 567–590. [Google Scholar]
- Hedger MP. The regulation and functions of activin and follistatin in inflammation and immunity. Vitam Horm. 2011a; 85: 255–297. [DOI] [PubMed] [Google Scholar]
- Hedger MP. Toll‐like receptors and signalling in spermatogenesis and testicular responses to inflammation—a perspective. J Reprod Immunol. 2011b; 88: 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedger MP, Hales DB. Immunophysiology of the male reproductive tract In: Neill JD, ed. Knobil and Neill's Physiology of Reproduction. Amsterdam, The Netherlands: Elsevier; 2006: 1195–1286. [Google Scholar]
- Hedger MP, Meinhardt A.. Local regulation of T cell numbers and lymphocyte‐inhibiting activity in the interstitial tissue of the adult rat testis. J Reprod Immunol. 2000; 48: 69–80. [DOI] [PubMed] [Google Scholar]
- Herr JC, Flickinger CJ, Howards SS, Yarbro S., Spell DR, Caloras D., Gallien TN. The relation between antisperm antibodies and testicular alterations after vasectomy and vasovasostomy in Lewis rats. Biol Reprod. 1987; 37: 1297–305. [DOI] [PubMed] [Google Scholar]
- Hinton BT, Palladino MA. Epididymal epithelium: its contribution to the formation of a luminal fluid microenvironment. Microsc Res Tech. 1995; 30: 67–81. [DOI] [PubMed] [Google Scholar]
- Hooper P., Smythe E., Richards RC, Howard CV, Lynch RV, Lewis‐Jones DI. Total number of immunocompetent cells in the normal rat epididymis and after vasectomy. J Reprod Fertil. 1995; 104: 193–198. [DOI] [PubMed] [Google Scholar]
- Hubert FX, Kinkel SA, Crewther PE, Cannon PZ, Webster KE, Link M., Uibo R., O'Bryan MK, Meager A., Forehan SP, Smyth GK, Mittaz L., Antonarakis SE, Peterson P., Heath WR, Scott HS. Aire‐deficient C57BL/6 mice mimicking the common human 13‐base pair deletion mutation present with only a mild autoimmune phenotype. J Immunol. 2009; 182: 3902–3918. [DOI] [PubMed] [Google Scholar]
- Ito K., Hasegawa A., Komori S., Koyama K.. Biochemical property and immunogenicity of mouse male reproductive tract CD52 (mrt‐CD52). J Reprod Immunol. 2007; 75: 32–39. [DOI] [PubMed] [Google Scholar]
- Itoh M., Chen XH, Takeuchi Y., Miki T.. Morphological demonstration of the immune privilege in the testis using adjuvants: tissue responses of male reproductive organs in mice injected with Bordetella pertussigens. Arch Histol Cytol. 1995; 58: 575–579. [DOI] [PubMed] [Google Scholar]
- Itoh M., Hiramine C., Hojo K.. A new murine model of autoimmune orchitis induced by immunization with viable syngeneic testicular germ cells alone. I. Immunological and histological studies. Clin Exp Immunol. 1991a; 83: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M., Hiramine C., Mukasa A., Tokunaga Y., Fukui Y., Takeuchi Y., Hojo K.. Establishment of an experimental model of autoimmune epididymo‐orchitis induced by the transfer of a T‐cell line in mice. Int J Androl. 1992; 15: 170–181. [PubMed] [Google Scholar]
- Itoh M., Miyamoto K., Ohga T., Takeuchi Y.. Spontaneous occurrence of vasculitis‐like lesions in male reproductive tissues in mice: a histological study. Arch Androl. 1999a; 42: 151–159. [DOI] [PubMed] [Google Scholar]
- Itoh M., Miyamoto K., Ooga T., Iwahashi K., Takeuchi Y.. Spontaneous accumulation of eosinophils and macrophages throughout the stroma of the epididymis and vas deferens in alymphoplasia (aly) mutant mice: I. A histological study. Am J Reprod Immunol. 1999b; 42: 246–253. [DOI] [PubMed] [Google Scholar]
- Itoh M., Mukasa A., Tokunaga Y., Hiramine C., Hojo K.. New experimental model for adoptive transfer of murine autoimmune orchitis. Andrologia. 1991b; 23: 415–420. [DOI] [PubMed] [Google Scholar]
- Itoh M., Xie Q., Miyamoto K., Takeuchi Y.. Major differences between the testis and epididymis in the induction of granulomas in response to extravasated germ cells. I. A light microscopical study in mice. Int J Androl. 1999c; 22: 316–323. [DOI] [PubMed] [Google Scholar]
- Jalkanen J., Huhtaniemi I., Poutanen M.. Discovery and characterization of new epididymis‐specific beta‐defensins in mice. Biochim Biophys Acta. 2005; 1730: 22–30. [DOI] [PubMed] [Google Scholar]
- Kappler JW, Roehm N., Marrack P.. T cell tolerance by clonal elimination in the thymus. Cell. 1987; 49: 273–280. [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S.. The role of pattern‐recognition receptors in innate immunity: update on Toll‐like receptors. Nat Immunol. 2010; 11: 373–384. [DOI] [PubMed] [Google Scholar]
- Kazeem AA. A critical consideration of the rat epididymis as an immunologically privileged site. Scand J Immunol. 1988; 27: 149–156. [DOI] [PubMed] [Google Scholar]
- Kern S., Maddocks S.. Indomethacin blocks the immunosuppressive activity of rat testicular macrophages cultured in vitro. J Reprod Immunol. 1995; 28: 189–201. [DOI] [PubMed] [Google Scholar]
- Knee RA, Hickey DK, Beagley KW, Jones RC. Transport of IgG across the blood‐luminal barrier of the male reproductive tract of the rat and the effect of estradiol administration on reabsorption of fluid and IgG by the epididymal ducts. Biol Reprod. 2005; 73: 688–694. [DOI] [PubMed] [Google Scholar]
- Kohno S., Munoz JA, Williams TM, Teuscher C., Bernard CC, Tung KS. Immunopathology of murine experimental allergic orchitis. J Immunol. 1983; 130: 2675–2682. [PubMed] [Google Scholar]
- Kojima A., Spencer CA. Genetic susceptibility to testicular autoimmunity: comparison between postthymectomy and postvasectomy models in mice. Biol Reprod. 1983; 29: 195–205. [DOI] [PubMed] [Google Scholar]
- Korbutt GS, Elliott JF, Rajotte RV. Cotransplantation of allogeneic islets with allogeneic testicular cell aggregates allows long‐term graft survival without systemic immunosuppression. Diabetes. 1997; 46: 317–322. [DOI] [PubMed] [Google Scholar]
- Krawetz SA, de Rooij DG, Hedger MP. Molecular aspects of male fertility. International Workshop on Molecular Andrology. EMBO Rep. 2009; 10: 1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel MA, Lohmann T., Gabler C., Blank N., Kalden J.R., Lorenz HM. Defective suppressor function of human CD4+ CD25+regulatory T cells in autoimmune polyglandular syndrome type II. J Exp Med. 2004; 199: 1285–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger JN. Epididymitis, orchitis, and related conditions. Sex Transm Dis. 1984; 11: 173–181. [DOI] [PubMed] [Google Scholar]
- Lee HM, Oh BC, Lim DP, Lee DS, Cho J., Lee G., Lee J.R.. Role of complement regulatory proteins in the survival of murine allotransplanted Sertoli cells. J Korean Med Sci. 2007; 22: 277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Ren J., Dhabuwala CB, Shichi H.. Immunotolerance induced by intratesticular antigen priming: expression of TGF‐β, Fas and Fas ligand. Ocul Immunol Inflamm. 1997; 5: 75–84. [DOI] [PubMed] [Google Scholar]
- Li P., Chan HC, He B., So SC, Chung YW, Shang Q., Zhang YD, Zhang YL. An antimicrobial peptide gene found in the male reproductive system of rats. Science. 2001; 291: 1783–1785. [DOI] [PubMed] [Google Scholar]
- Liew SH, Meachem SJ, Hedger MP. A stereological analysis of the response of spermatogenesis to an acute inflammatory episode in adult rats. J Androl. 2007; 28: 176–185. [DOI] [PubMed] [Google Scholar]
- Liston A., Lesage S., Wilson J., Peltonen L., Goodnow CC. Aire regulates negative selection of organ‐specific T cells. Nat Immunol. 2003; 4: 350–354. [DOI] [PubMed] [Google Scholar]
- Lucchetta R., Clavert A., Meyer JM, Bollack C.. Acute experimentalE. coli epididymitis in the rat and its consequences on spermatogenesis. Urol Res. 1983; 11: 117–120. [DOI] [PubMed] [Google Scholar]
- Lui WY, Wong CH, Mruk DD, Cheng CY. TGF‐β3 regulates the blood‐testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology. 2003; 144: 1139–1142. [DOI] [PubMed] [Google Scholar]
- Mahi‐Brown CA, Yule TD, Tung KS. Adoptive transfer of murine autoimmune orchitis to naive recipients with immune lymphocytes. Cell Immunol. 1987; 106: 408–419. [DOI] [PubMed] [Google Scholar]
- Maresz K., Ponomarev ED, Barteneva N., Tan Y., Mann MK, Dittel BN. IL‐13 induces the expression of the alternative activation marker Ym1 in a subset of testicular macrophages. J Reprod Immunol. 2008; 78: 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Sica A., Mantovani A., Locati M.. Macrophage activation and polarization. Front Biosci. 2008; 13: 453–461. [DOI] [PubMed] [Google Scholar]
- Mauduit C., Jaspar JM, Poncelet E., Charlet C., Revol A., Franchimont P., Benahmed M.. Tumor necrosis factor‐α antagonizes follicle‐stimulating hormone action in cultured Sertoli cells. Endocrinology. 1993; 133: 69–76. [DOI] [PubMed] [Google Scholar]
- McMahon AJ, Buckley J., Taylor A., Lloyd SN, Deane RF, Kirk D.. Chronic testicular pain following vasectomy. Br J Urol. 1992; 69: 188–191. [DOI] [PubMed] [Google Scholar]
- Meinhardt A., Hedger MP. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol Cell Endocrinol. 2011; 335: 60–68. [DOI] [PubMed] [Google Scholar]
- Mizuno M., Harris CL, Morgan BP. Spermatogenic cells distal to the blood‐testis barrier in rats lack C3 convertase regulators and may be at risk of complement‐mediated injury. J Reprod Immunol. 2006; 69: 23–34. [DOI] [PubMed] [Google Scholar]
- Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2010; 11: 21–27. [DOI] [PubMed] [Google Scholar]
- Mühlbauer M., Cheely AW, Yenugu S., Jobin C.. Regulation and functional impact of lipopolysaccharide induced Nod2 gene expression in the murine epididymal epithelial cell line PC1. Immunology. 2008; 124: 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaosa K., Nakashima C., Kishimoto A., Nakanishi Y.. Immune response to bacteria in seminiferous epithelium. Reproduction. 2009; 137: 879–888. [DOI] [PubMed] [Google Scholar]
- Naito M., Itoh M.. Patterns of infiltration of lymphocytes into the testis under normal and pathological conditions in mice. Am J Reprod Immunol. 2008; 59: 55–61. [DOI] [PubMed] [Google Scholar]
- Nariculam J., Minhas S., Adeniyi A., Ralph DJ, Freeman A.. A review of the efficacy of surgical treatment for and pathological changes in patients with chronic scrotal pain. BJU Int. 2007; 99: 1091–1093. [DOI] [PubMed] [Google Scholar]
- Nashan D., Cooper TG, Knuth UA, Schubeus P., Sorg C., Nieschlag E.. Presence and distribution of leucocyte subsets in the murine epididymis after vasectomy. Int J Androl. 1990; 13: 39–49. [DOI] [PubMed] [Google Scholar]
- Nashan D., Jantos C., Ahlers D., Bergmann M., Schiefer HG, Sorg C., Nieschlag E.. Immuno‐competent cells in the murine epididymis following infection with Escherichia coli. Int J Androl. 1993; 16: 47–52. [DOI] [PubMed] [Google Scholar]
- Nashan D., Malorny U., Sorg C., Cooper T., Nieschlag E.. Immunocompetent cells in the murine epididymis. Int J Androl. 1989; 12: 85–94. [DOI] [PubMed] [Google Scholar]
- Nasr IW, Wang Y., Gao G., Deng S., Diggs L., Rothstein DM, Tellides G., Lakkis FG, Dai Z.. Testicular immune privilege promotes transplantation tolerance by altering the balance between memory and regulatory T cells. J Immunol. 2005; 174: 6161–6168. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Teichman JM, Gregoire M., Clark J., Downey J.. Prevalence, diagnosis, characterization, and treatment of prostatitis, interstitial cystitis, and epididymitis in outpatient urological practice: the Canadian PIE Study. Urology. 2005; 66: 935–940. [DOI] [PubMed] [Google Scholar]
- Nistal M., Mate A., Paniagua R.. Granulomatous epididymal lesion of possible ischemic origin. Am J Surg Pathol. 1997; 21: 951–956. [DOI] [PubMed] [Google Scholar]
- Nossal GJ. Negative selection of lymphocytes. Cell. 1994; 76: 229–239. [DOI] [PubMed] [Google Scholar]
- O'Bryan MK, Gerdprasert O., Nikolic‐Paterson DJ, Meinhardt A., Muir JA, Foulds LM, Phillips DJ, de Kretser DM, Hedger MP. Cytokine profiles in the testes of rats treated with lipopolysaccharide reveal localized suppression of inflammatory responses. Am J Physiol Regul Integr Comp Physiol. 2005; 288: R1744–R1755. [DOI] [PubMed] [Google Scholar]
- O'Bryan MK, Hedger MP. Inflammatory networks in the control of spermatogenesis. Chronic inflammation in an immunologically privileged tissue? Adv Exp Med Biol. 2008; 636: 92–114. [DOI] [PubMed] [Google Scholar]
- O'Bryan MK, Schlatt S., Phillips DJ, de Kretser DM, Hedger MP. Bacterial lipopolysaccharide‐induced inflammation compromises testicular function at multiple levels in vivo. Endocrinology. 2000; 141: 238–246. [DOI] [PubMed] [Google Scholar]
- Okuda Y., Sun XR, Morris PL. Interleukin‐6 (IL‐6) mRNAs expressed in Leydig and Sertoli cells are regulated by cytokines, gonadotropins and neuropeptides. Endocr J. 1994; 2: 617–624. [Google Scholar]
- Okuma Y., O'Connor AE, Muir JA, Stanton PG, de Kretser DM, Hedger MP. Regulation of activin A and inhibin B secretion by inflammatory mediators in adult rat Sertoli cell cultures. J Endocrinol. 2005a; 187: 125–134. [DOI] [PubMed] [Google Scholar]
- Okuma Y., Saito K., O'Connor AE, Phillips DJ, de Kretser DM, Hedger MP. Reciprocal regulation of activin A and inhibin B by interleukin‐1 (IL‐1) and follicle‐stimulating hormone (FSH) in rat Sertoli cells in vitro. J Endocrinol. 2005b; 185: 99–110. [DOI] [PubMed] [Google Scholar]
- Palladino MA, Johnson TA, Gupta R., Chapman JL, Ojha P.. Members of the Toll‐like receptor family of innate immunity pattern‐recognition receptors are abundant in the male rat reproductive tract. Biol Reprod. 2007; 76: 958–964. [DOI] [PubMed] [Google Scholar]
- Palladino MA, Savarese MA, Chapman JL, Dughi MK, Plaska D.. Localization of Toll‐like receptors on epididymal epithelial cells and spermatozoa. Am J Reprod Immunol. 2008; 60: 541–555. [DOI] [PubMed] [Google Scholar]
- Pannek J., Haupt G.. Orchitis due to vasculitis in autoimmune diseases. Scand J Rheumatol. 1997; 26: 151–154. [DOI] [PubMed] [Google Scholar]
- Patil AA, Cai Y., Sang Y., Blecha F., Zhang G.. Cross‐species analysis of the mammalian beta‐defensin gene family: presence of syntenic gene clusters and preferential expression in the male reproductive tract. Physiol Genomics. 2005; 23: 5–17. [DOI] [PubMed] [Google Scholar]
- Pitkanen J., Peterson P.. Autoimmune regulator: from loss of function to autoimmunity. Genes Immun. 2003; 4: 12–21. [DOI] [PubMed] [Google Scholar]
- Qu N., Naito M., Terayama H., Hirai S., Li J., Ogawa Y., Kitaoka M., Itoh M.. Developmental ontogeny of autoantigens associated with localized autoimmunity in murine testis and epididymis. J Reprod Immunol. 2010; 87: 45–51. [DOI] [PubMed] [Google Scholar]
- Qu N., Terayama H., Naito M., Ogawa Y., Hirai S., Kitaoka M., Yi SQ, Itoh M.. Caput epididymitis but not orchitis was induced by vasectomy in a murine model of experimental autoimmune orchitis. Reproduction. 2008; 135: 859–866. [DOI] [PubMed] [Google Scholar]
- Riccioli A., Starace D., Galli R., Fuso A., Scarpa S., Palombi F., de Cesaris P., Ziparo E., Filippini A.. Sertoli cells initiate testicular innate immune responses through TLR activation. J Immunol. 2006; 177: 7122–7130. [DOI] [PubMed] [Google Scholar]
- Ritchie AW, Hargreave TB, James K., Chisholm GD. Intra‐epithelial lymphocytes in the normal epididymis. A mechanism for tolerance to sperm auto‐antigens? Br J Urol. 1984; 56: 79–83. [DOI] [PubMed] [Google Scholar]
- Rival C., Guazzone VA, von Wulffen W., Hackstein H., Schneider E., Lustig L., Meinhardt A., Fijak M.. Expression of co‐stimulatory molecules, chemokine receptors and proinflammatory cytokines in dendritic cells from normal and chronically inflamed rat testis. Mol Hum Reprod. 2007; 13: 853–861. [DOI] [PubMed] [Google Scholar]
- Rival C., Lustig L., Iosub R., Guazzone VA, Schneider E., Meinhardt A., Fijak M.. Identification of a dendritic cell population in normal testis and in chronically inflamed testis of rats with autoimmune orchitis. Cell Tissue Res. 2006; 324: 311–318. [DOI] [PubMed] [Google Scholar]
- Rodrigues A., Queiróz DB, Honda L., Silva EJ, Hall SH, Avellar MC. Activation of toll‐like receptor 4 (TLR4) by in vivo and in vitro exposure of rat epididymis to lipopolysaccharide from Escherichia coli. Biol Reprod. 2008; 79: 1135–1147. [DOI] [PubMed] [Google Scholar]
- Roper RJ, Doerge RW, Call SB, Tung KS, Hickey WF, Teuscher C.. Autoimmune orchitis, epididymitis, and vasitis are immunogenetically distinct lesions. Am J Pathol. 1998; 152: 1337–1345. [PMC free article] [PubMed] [Google Scholar]
- Ryan AF, Grendell RL, Geraghty DE, Golos TG. A soluble isoform of the rhesus monkey nonclassical MHC class I molecule Mamu‐AG is expressed in the placenta and the testis. J Immunol. 2002; 169: 673–683. [DOI] [PubMed] [Google Scholar]
- Salomon F., Saremaslani P., Jakob M., Hedinger CE. Immune complex orchitis in infertile men. Immunoelectron microscopy of abnormal basement membrane structures. Lab Invest. 1982; 47: 555–567. [PubMed] [Google Scholar]
- Sanberg PR, Borlongan CV, Saporta S., Cameron DF. Testis‐derived Sertoli cells survive and provide localized immunoprotection for xenografts in rat brain. Nat Biotech. 1996; 14: 1692–1695. [DOI] [PubMed] [Google Scholar]
- Schuppe HC, Meinhardt A., Allam JP, Bergmann M., Weidner W., Haidl G.. Chronic orchitis: a neglected cause of male infertility? Andrologia. 2008; 40: 84–91. [DOI] [PubMed] [Google Scholar]
- Seiler P., Cooper TG, Nieschlag E.. Sperm number and condition affect the number of basal cells and their expression of macrophage antigen in the murine epididymis. Int J Androl. 2000; 23: 65–76. [DOI] [PubMed] [Google Scholar]
- Seiler P., Cooper TG, Yeung CH, Nieschlag E.. Regional variation in macrophage antigen expression by murine epididymal basal cells and their regulation by testicular factors. J Androl. 1999; 20: 738–746. [PubMed] [Google Scholar]
- Selawry HP, Cameron DF. Sertoli cell–enriched fractions in successful islet cell transplantation. Cell Transplant. 1993; 2: 123–129. [DOI] [PubMed] [Google Scholar]
- Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005; 6: 551–557. [DOI] [PubMed] [Google Scholar]
- Serre V., Robaire B.. Distribution of immune cells in the epididymis of the aging Brown Norway rat is segment‐specific and related to the luminal content. Biol Reprod. 1999; 61: 705–714. [DOI] [PubMed] [Google Scholar]
- Setchell BP, Voglmayr JK, Waites GM. A blood‐testis barrier restricting passage from blood into rete testis fluid but not into lymph. J Physiol. 1969; 200: 73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipione S., Simmen KC, Lord SJ, Motyka B., Ewen C., Shostak I., Rayat GR, Dufour JM, Korbutt GS, Rajotte RV, Bleackley RC. Identification of a novel human granzyme B inhibitor secreted by cultured Sertoli cells. J Immunol. 2006; 177: 5051–5058. [DOI] [PubMed] [Google Scholar]
- Skinner MK, Tung PS, Fritz IB. Cooperativity between Sertoli cells and testicular peritubular cells in the production and deposition of extracellular matrix components. J Cell Biol. 1985; 100: 1941–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slukvin II, Watkins DI, Golos TG. Tissue distribution of the mRNA for a rhesus monkey major histocompatibility class Ib molecule, Mamu‐AG. Tissue Antigens. 1999; 53: 282–291. [DOI] [PubMed] [Google Scholar]
- Starace D., Galli R., Paone A., Cesaris PD, Filippini A., Ziparo E., Riccioli A.. Toll‐like receptor 3 activation induces antiviral immune responses in mouse Sertoli cells. Biol Reprod. 2008; 79: 766–775. [DOI] [PubMed] [Google Scholar]
- Stein‐Streilein J., Streilein JW. Anterior chamber associated immune deviation (ACAID): regulation, biological relevance, and implications for therapy. Int Rev Immunol. 2002; 21: 123–152. [DOI] [PubMed] [Google Scholar]
- Stéphan JP, Guillemois C., Jégou B., Bauché F.. Nitric oxide production by Sertoli cells in response to cytokines and lipopolysaccharide. Biochem Biophys Res Comm. 1995; 213: 218–224. [DOI] [PubMed] [Google Scholar]
- Suarez‐Pinzon W., Korbutt GS, Power R., Hooton J., Rajotte RV, Rabinovitch A.. Testicular Sertoli cells protect islet β‐cells from autoimmune destruction in NOD mice by a transforming growth factor‐β1–dependent mechanism. Diabetes. 2000; 49: 1810–1818. [DOI] [PubMed] [Google Scholar]
- Sun B., Qi N., Shang T., Wu H., Deng T., Han D.. Sertoli cell–initiated testicular innate immune response through toll‐like receptor‐3 activation is negatively regulated by Tyro3, Axl, and mer receptors. Endocrinology. 2010; 151: 2886–2897. [DOI] [PubMed] [Google Scholar]
- Suominen J., Soderstrom KO. Lymphocyte infiltration in human testicular biopsies. Int J Androl. 1982; 5: 461–466. [DOI] [PubMed] [Google Scholar]
- Syed V., Stéphan JP, Gérard N., Legrand A., Parvinen M., Bardin CW, Jégou B.. Residual bodies activate Sertoli cell interleukin‐1α (IL‐1α) release, which triggers IL‐6 production by an autocrine mechanism, through the lipoxygenase pathway. Endocrinology. 1995; 136: 3070–3078. [DOI] [PubMed] [Google Scholar]
- Taguchi O., Nishizuka Y.. Experimental autoimmune orchitis after neonatal thymectomy in the mouse. Clin Exp Immunol. 1981; 46: 425–434. [PMC free article] [PubMed] [Google Scholar]
- Takao T., Mitchell WM, Tracey DE, de Souza EB. Identification of interleukin‐1 receptors in mouse testis. Endocrinology. 1990; 127: 251–258. [DOI] [PubMed] [Google Scholar]
- Thornton AM, Shevach EM. CD4+CD25+immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998; 188: 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda A., Piccirillo CA. Development and function of naturally occurring CD4+CD25+ regulatory T cells. J Leuk Biol. 2006; 80: 458–470. [DOI] [PubMed] [Google Scholar]
- Tomlinson MJ, White A., Barratt CL, Bolton AE, Cooke ID. The removal of morphologically abnormal sperm forms by phagocytes: a positive role for seminal leukocytes? Hum Reprod. 1992; 7: 517–522. [DOI] [PubMed] [Google Scholar]
- Tompkins AB, Hutchinson P., de Kretser DM, Hedger MP. Characterization of lymphocytes in the adult rat testis by flow cytometry: effects of activin and transforming growth factor β on lymphocyte subsets in vitro. Biol Reprod. 1998; 58: 943–951. [DOI] [PubMed] [Google Scholar]
- Trojian TH, Lishnak TS, Heiman D.. Epididymitis and orchitis: an overview. Am Fam Physician. 2009; 79: 583–587. [PubMed] [Google Scholar]
- Tung KS, Smith S., Teuscher C., Cook C., Anderson RE. Murine autoimmune oophoritis, epididymoorchitis, and gastritis induced by day 3thymectomy. Immunopathology. Am J Pathol. 1987a; 126: 293–302. [PMC free article] [PubMed] [Google Scholar]
- Tung KS, Unanue ER, Dixon FJ. The immunopathology of experimental allergic orchitis. Am J Pathol. 1970; 60: 313–328. [PMC free article] [PubMed] [Google Scholar]
- Tung KS, Yule TD, Mahi‐Brown CA, Listrom MB. Distribution of histopathology and Ia positive cells in actively induced and passively transferred experimental autoimmune orchitis. J Immunol. 1987b; 138: 752–759. [PubMed] [Google Scholar]
- Veräjänkorva E., Setälä N., Teros T., Salmi AA, Pgöllänen P.. Testicular‐associated immune deviation: flushing of the testicular lymph sinusoids induces immunosuppression and inhibits formation of EAE in SJL mice. Scand J Immunol. 2002; 55: 478–483. [DOI] [PubMed] [Google Scholar]
- Vitolo U., Ferreri AJ, Zucca E.. Primary testicular lymphoma. Crit Rev Oncol-Hematol. 2008; 65: 183–189. [DOI] [PubMed] [Google Scholar]
- Wang J., Wreford NG, Lan HY, Atkins R., Hedger MP. Leukocyte populations of the adult rat testis following removal of the Leydig cells by treatment with ethane dimethane sulfonate and subcutaneous testosterone implants. Biol Reprod. 1994; 51: 551–561. [DOI] [PubMed] [Google Scholar]
- Wang YF, Holstein AF. Intraepithelial lymphocytes and macrophages in the human epididymis. Cell Tissue Res. 1983; 233: 517–521. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Kashiwakura Y., Kusumi N., Tamayose K., Nasu Y., Nagai A., Shimada T., Daida H., Kumon H.. Adeno‐associated virus‐mediated human IL‐10 gene transfer suppresses the development of experimental autoimmune orchitis. Gene Ther. 2005; 12: 1126–1132. [DOI] [PubMed] [Google Scholar]
- Weininger RB, Fisher S., Rifkin J., Bedford JM. Experimental studies on the passage of specific IgG to the lumen of the rabbit epididymis. J Reprod Fertil. 1982; 66: 251–258. [DOI] [PubMed] [Google Scholar]
- Whitmore WF III, Gittes RF. Intratesticular grafts: the testis as an exceptional immunologically privileged site. Trans Am Assoc Genitourin Surg. 1978; 70: 76–80. [PubMed] [Google Scholar]
- Winnall WR, Ali U., O'Bryan MK, Hirst JJ, Whiley PA, Muir JA, Hedger MP. Constitutive expression of prostaglandin–endoperoxide synthase 2 by somatic and spermatogenic cells is responsible for prostaglandin E2 production in the adult rat testis. Biol Reprod. 2007; 76: 759–768. [DOI] [PubMed] [Google Scholar]
- Winnall WR, Muir JA, Hedger MP. Differential responses of epithelial Sertoli cells of the rat testis to Toll‐Like receptor 2 and 4ligands: implications for studies of testicular inflammation using bacterial lipopolysaccharides. Innate Immun. 2011; 17: 123–136. [DOI] [PubMed] [Google Scholar]
- Winnall WR, Okuma Y., Saito K., Muir JA, Hedger MP. Regulation of interleukin 1α, activin and inhibin by lipopolysaccharide in Sertoli cells from prepubertal rats. Mol Cell Endocrinol. 2009; 307: 169–175. [DOI] [PubMed] [Google Scholar]
- Wu H., Wang H., Xiong W., Chen S., Tang H., Han D.. Expression patterns and functions of toll‐like receptors in mouse Sertoli cells. Endocrinology. 2008; 149: 4402–4412. [DOI] [PubMed] [Google Scholar]
- Yakirevich E., Yanai O., Sova Y., Sabo E., Stein A., Hiss J., Resnick MB. Cytotoxic phenotype of intra‐epithelial lymphocytes in normal and cryptorchid human testicular excurrent ducts. Hum Reprod. 2002; 17: 275–283. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Nagase T., Makita R., Fukuhara S., Tomita T., Tominaga T., Kurihara H., Ouchi Y.. Identification of multiple novel epididymis‐specific β‐defensin isoforms in humans and mice. J Immunol. 2002; 169: 2516–2523. [DOI] [PubMed] [Google Scholar]
- Yenugu S., Hamil KG, Radhakrishnan Y., French FS, Hall SH. The androgen‐regulated epididymal sperm‐binding protein, human beta‐defensin 118(DEFB118) (formerly ESC42), is an antimicrobial β‐defensin. Endocrinology. 2004; 145: 3165–3173. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Nashan D., Sorg C., Oberpenning F., Schulze H., Nieschlag E., Cooper TG. Basal cells of the human epididymis—antigenic and ultrastructural similarities to tissue‐fixed macrophages. Biol Reprod. 1994; 50: 917–926. [DOI] [PubMed] [Google Scholar]
- Yule TD, Montoya GD, Russell LD, Williams TM, Tung KS. Autoantigenic germ cells exist outside the blood testis barrier. J Immunol. 1988; 141: 1161–1167. [PubMed] [Google Scholar]
- Yule TD, Tung KS. Experimental autoimmune orchitis induced by testis and sperm antigen‐specific T cell clones: an important pathogenic cytokine is tumor necrosis factor. Endocrinology. 1993; 133: 1098–1107. [DOI] [PubMed] [Google Scholar]
- Zhao YT, Guo JH, Wu ZL, Xiong Y., Zhou WL. Innate immune responses of epididymal epithelial cells to Staphylococcus aureus infection. Immunol Lett. 2008; 119: 84–90. [DOI] [PubMed] [Google Scholar]
- Zhou CX, Zhang YL, Xiao L., Zheng M., Leung KM, Chan MY, Lo PS, Tsang LL, Wong HY, Ho LS, Chung YW, Chan HC. An epididymis‐specific β‐defensin is important for the initiation of sperm maturation. Nat Cell Biol. 2004; 6: 458–464. [DOI] [PubMed] [Google Scholar]
- Zhou ZZ, Zheng Y., Steenstra R., Hickey WF, Teuscher C.. Actively‐induced experimental allergic orchitis (EAO) in Lewis/NCR rats: sequential histo‐ and immunopathologic analysis. Autoimmunity. 1989; 3: 125–134. [DOI] [PubMed] [Google Scholar]