Abstract
Acute gastroenteritis accounts for a significant burden of medically attended illness in children under the age of five. For this study, four multiplex reverse transcription PCR assays were used to determine the incidence of adenovirus, astrovirus, coronavirus, norovirus GI and GII, rotavirus, and sapovirus in stool samples submitted for viral electron microscopy (EM) to the Children's Hospital Colorado. Of 1105 stool samples available, viral RNA/DNA was detected in 247 (26.2%) of 941 pediatric samples (median age = 2.97 years, 54% male) with 28 (3.0%) positive for more than one virus. Adenovirus, astrovirus, norovirus GI, norovirus GII, rotavirus, and sapovirus were detected in 95 (10.0%), 33 (3.5%), 8 (0.9%), 90 (9.6%), 49 (5.2%), and 2 (0.2%) of the pediatric samples, respectively. No coronaviruses were identified. Sequencing of norovirus positive samples indicated an outbreak of norovirus strain GII.4 in 2006 with evidence of numerous circulating strains. Multiple samples from the same immunocompromised patients demonstrated symptomatic shedding of norovirus for up to 32 weeks and astrovirus for 12 weeks. RT‐PCR detected 99 of 111 (89%) adenovirus‐positive samples versus 12 (11%) by EM, and 186 of 192 (97%) sapovirus/astrovirus/norovirus‐positive samples versus 21 (11%) by EM. Noroviruses and adenoviruses are common causes of gastroenteritis in children. Immunocompromised patients can be infected with multiple viruses and shed viruses in their stools for prolonged periods. This data support the superiority of RT‐PCR compared to EM for diagnosis of viral gastroenteritis. J. Med. Virol. 87:931–939, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: norovirus, pediatric, RT‐PCR, epidemiology, immunocompromised, shedding
INTRODUCTION
Acute gastroenteritis in children, particularly under the age of five, remains a major cause of morbidity and mortality worldwide, with an estimated incidence of 2.5 billion cases annually [Unicef WHO, 2009; Gautam et al., 2013]. Acute gastroenteritis is the second leading cause of mortality in study patients outside the neonatal period and accounts for an estimated 1.5 million deaths each year. In the United States, acute gastroenteritis in children under five is responsible for approximately 300 deaths, 200,000 hospitalizations, and 1.5 million outpatient visits, an estimated $250 million in direct medical costs, and $1 billion in indirect medical costs [de Bruin et al., 2006; Churgay and Aftab, 2012].
In children, viruses are the major causes of acute gastroenteritis [Parashar et al., 2003; Terletskaia‐Ladwig et al., 2007]. Rotavirus, adenovirus, astrovirus, norovirus, and sapovirus, have all been established as etiological agents of acute gastroenteritis in children [Hammond et al., 1981; Wilhelmi et al., 2003; Claas et al., 2005; Colomba et al., 2006; Unicef WHO, 2009; Churgay and Aftab, 2012; Chhabra et al., 2013; Payne et al., 2013]. Additionally, some clinical studies have suggested that coronaviruses may be a cause of acute gastroenteritis [Payne et al., 1986; van Maarseveen et al., 2010; Jevšnik et al., 2013]. Clinical data indicates that severe acute respiratory syndrome coronavirus (SARS‐CoV) caused significant diarrhea [Booth et al., 2003; Choi et al., 2003; Hsu et al., 2003; Peiris et al., 2003; Hsueh et al., 2004; Grant et al., 2012]. However, the true significance of endemic coronaviruses as gastrointestinal pathogens has not been fully elucidated [Trujillo et al., 2006; Dominguez et al., 2009; Esper et al., 2010; Jevšnik et al., 2013].
Electron microscopy (EM) has been the gold standard diagnostic method for acute gastroenteritis, but this method requires a high concentration of viral particles per gram of stool, is labor‐intensive, [Kuypers et al., 2007; Wei et al., 2013] and is now infrequently used. Many laboratories use commercially available immunoassays for the diagnosis of rotavirus, norovirus, and adenovirus diseases; however, these tests are limited by their ability to detect a single viral pathogen at a time, poor sensitivity and specificity, and limited availability outside suspected outbreaks [de Bruin et al., 2006; Terletskaia‐Ladwig et al., 2007; Harris et al., 2009; Mans et al., 2010; Chhabra et al., 2013; Gautam et al., 2013]. Laboratories have been transitioning toward molecular‐based techniques like RT‐PCR for detection of etiologic viruses in patients with diarrheal illness.
The primary objective of this study was to determine the epidemiology of six common known enteric viruses (adenovirus, astrovirus, norovirus GI and GII, rotavirus, and sapovirus) as well as assess the role of the known endemic human coronaviruses in acute gastroenteritis in children. The secondary objective of this study was to compare the sensitivities of electron microscopy and RT‐PCR for the diagnosis of viral acute gastroenteritis. This is the first hospital‐based, multi‐year, longitudinal study evaluating multiple viral etiologies of acute gastroenteritis in pediatric patients in the United States.
MATERIALS AND METHODS
Study Patients
All stool samples from September 2006 to June 2009 submitted for viral diagnostic testing by electron microscopy (EM) at Children's Hospital Colorado (CHCO) in Aurora, CO were diluted in sterile deionized water, vortexed, centrifuged, further diluted to 2.5% v/v of original specimen, and archived at −70 °C. For EM, samples were concentrated by ultracentrifugation for 30 min at 22 psi, using an Airfuge and EM90 rotor (Beckman Coulter, Fullerton CA) [Hammond et al., 1981; Payne et al., 2013] negatively stained and scanned at 25,000 × magnification for virus particles. Use of the banked specimens and clinical data was approved by the Colorado Multiple Institutional Review Board.
DNA/RNA Extraction From Fecal Specimens
Nucleic acids from each sample were extracted on a Qiagen Biorobot EZ1 using the EZ1 viral RNA mini kit (Qiagen, Valencia, CA) according to the manufacturer's instructions, was eluted in RNase‐free water, and stored at −80 °C. Samples were divided into groups of 6 samples, and 10 microliters of each sample was combined. Each 60 microliter pool was stored at −80 °C.
PCR and Sequencing
Four separate multiplex RT‐PCR assays, based on modifications of previously published assays, were used to screen for the following viruses: adenovirus (family: Adenoviridae, genus: Mastadenovirus, species: Human adenovirus A‐F) [Claas et al., 2005; Payne et al., 2013] and rotavirus (family: Reoviridae, subfamily: Sedoreovirinae, genus: Rotavirus, species: rotavirus A) [van Maarseveen et al., 2010; Sánchez‐Fauquier et al., 2011], astrovirus (family: Astroviridae, genus: Mamastrovirus) and sapovirus (family: Caliciviridae, genus: Sapovirus, species: Sapporo virus) [Hsueh et al., 2004; De Benedictis et al., 2011; Grant et al., 2012], norovirus groups I and II (family: Caliciviridae, genus: Norovirus, species: Norwalk virus, genogroups GI and GII) [Trujillo et al., 2006; Finkbeiner et al., 2008], and the four known endemic humans coronavirus (order: Nidovirales, family: Coronaviridae, genus: Alphacoronavirus species: human coronavirus 229E and NL63; genus: Betacoronavirus, species: human coronavirus HKU1 and OC43) [Kuypers et al., 2007; Dominguez et al., 2009; Finkbeiner et al., 2009a, 2009b]. Each pool containing RNA from six samples was run on each protocol. If a pool was positive for a given assay, RNA from each of the six samples was run individually to determine which of the samples were positive. Negative and positive controls were included in each run for all protocols. Due to concern for PCR inhibitors in stool, samples were spiked with 10 µL of extracted RNA from each pool with a known quantity of pandemic 2009 H1N1 influenza A RNA, and then assessed for influenza RNA by RT‐PCR (Supplemental Table).
Norovirus‐positive samples were randomly selected for sequencing during the peak of activity, and conventional PCR targeting a 343‐nucleotide region of the capsid gene was performed using a previously published assay [Mans et al., 2010; De Benedictis et al., 2011]. Amplicons were analyzed by agarose gel electrophoresis and sequenced on an ABI 3730 DNA sequencer (Applied Biosystems Technologies, Carlsbad, CA) at the University of Colorado School of Medicine Cancer Center DNA Sequencing and Analysis Core.
Data Analysis
Specimens were decoded, and medical chart review was performed on virus‐positive subjects using a standardized form. In order to assess typical clinical features of the viruses, clinical data comparisons were limited to patients who were not immunocompromised and who were positive for only one virus. Study data were collected and managed using REDCap electronic data capture tools hosted at the UCSOM [Harris et al., 2009; Chhabra et al., 2013]. Statistical computations were conducted with SAS software, version 9.1.3. Significance was determined using the Wilcoxon Signed Rank test or Chi Square.
RESULTS
Patients
From September 2006 to June 2009, 1777 stool samples were submitted for viral diagnostic testing. Of these, 1105 (62%) had enough residual sample to permit inclusion in the study. There were no differences in the age, gender, or order location of patients with samples included in or excluded from the study. The majority (941, 85%) of samples were from pediatric patients (median age = 2.97 years, 54% male) (Table I). The remaining 164 samples were referral specimens submitted from adults seen at the University of Colorado Hospital (UCH) from September 2006 to December 2008, of which 141 (86%) were submitted between September 2006 and January 2007.
Table I.
Characteristics and Patient Location on Submission of Stool Samples
| Total (n = 247) | Adenovirus (n = 95) | Astrovirus (n = 33) | Norovirus GI (n = 8) | Norovirus GII (n = 90) | Rotavirus (n = 49) | Co‐infections (n = 28) | |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Male | 122 (49.3) | 48 (50.5) | 18 (54.5) | 5 (62.5) | 46 (51.1) | 25 (51.0) | 18 |
| Median age (years) | 2.97 | 2.61 | 1.99 | 1.97 | 2.56 | 1.13 | 1.14 |
| Outpatient (n = 366) | 100 (40.4) | 42 (44.2) | 14 (42.4) | 3 (37.5) | 36 (40.0) | 22 (44.9) | 10 (35.7) |
| Clinic (n = 238) | 69 (27.9) | 26 (27.3) | 11 (33.3) | 0 (0.0) | 22 (24.4) | 17 (34.7) | 8 (28.6) |
| ED/Urgent Care (n = 138) | 31 (12.6) | 16 (16.8) | 3 (9.0) | 3 (37.5) | 14 (15.6) | 5 (10.2) | 2 (7.1) |
| Inpatient (n = 364) | 80 (32.4) | 34 (35.8) | 9 (27.3) | 2 (25.0) | 26 (28.9) | 18 (36.7) | 10 (35.7) |
| Medical Floor (n = 303) | 68 (27.5) | 29 (30.5) | 8 (24.2) | 2 (25.0) | 22 (24.4) | 15 (30.6) | 9 (32.1) |
| ICU (n = 61) | 12 (4.8) | 5 (5.3) | 1 (3.0) | 0 (0.0) | 4 (4.4) | 3 (6.1) | 1 (3.5) |
| Immunocompromised (n = 211) | 67 (27.1) | 25 (26.3) | 10 (30.3) | 3 (37.5) | 28 (31.1) | 9 (18.4) | 8 (28.6) |
Virus Detection
All pools were positive for influenza, indicating the absence of PCR inhibitors. Viral RNA/DNA was detected in 247 (26.2%) of the 941 pediatric samples. Of these, 95 (10.0%) were positive for adenovirus, 33 (3.5%) for astrovirus, 8 (0.9%) for norovirus GI, 90 (9.6%) for norovirus GII, 49 (5.2%) for rotavirus, and 2 (0.2%) for sapovirus. Coronavirus RNA was not detected in any sample (Table II). Overall, 100 (27.3%) outpatient samples, 80 (32.4%) inpatient samples, and 67 (27.1%) samples from immunocompromised patients were positive for at least one virus (Table II). Evidence of co‐infection was present in 28 (3.0%) of pediatric samples (Table III).
Table II.
Incidence of Viral Gastroenteritis in Pediatric and Adult Patients
| Total (n = 1105) | Pediatric (n = 941) | Adult (n = 164) | |
|---|---|---|---|
| Overall | 311 (28.1) | 247 (26.2) | 65 (39.6) |
| Adenovirus | 99 (9.0) | 95 (10.0) | 4 (2.4) |
| Astrovirus | 35 (3.2) | 33 (3.5) | 2 (1.2) |
| Coronavirus | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Norovirus GI | 14 (1.3) | 8 (0.9) | 6 (3.7) |
| Norovirus GII | 141 (12.8) | 90 (9.6) | 51 (31.1) |
| Rotavirus | 50 (4.5) | 49 (5.2) | 1 (0.6) |
| Sapovirus | 2 (0.2) | 2 (0.2) | 0 (0.0) |
Table III.
Mixed Infections Identified in 28 Patients With Gastrointestinal Symptoms
| Viruses | No. of samples |
|---|---|
| Adenovirus, astrovirus | 1 |
| Adenovirus, norovirus GI | 1 |
| Adenovirus, norovirus GII | 6 |
| Adenovirus, rotavirus | 14 |
| Astrovirus, norovirus GII | 1 |
| Norovirus GII, rotavirus | 2 |
| Adenovirus, rotavirus, norovirus GII | 2 |
| Norovirs GI, norovirus GII, rotavirus | 2 |
Each virus demonstrated seasonal and variable changes from year to year (Fig. 1). Adenovirus was detected throughout the year in children with acute gastroenteritis, with highest prevalence (44.5%) in April 2009. Astrovirus was detected from January to August 2007, with highest prevalence (15%) in April 2007; however, in 2008 and 2009 was only detected January to March. Norovirus GII was most prevalent from October 2006 to April 2007, and then was not detected until April 2008 with an increase in prevalence in December 2008. Rotavirus was highly prevalent from October 2006 to April 2007 with decreasing prevalence thereafter.
Figure 1.
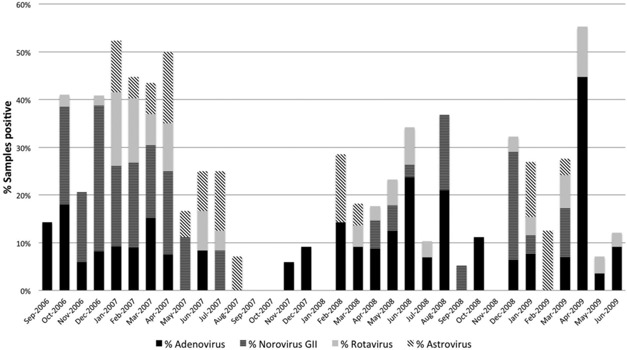
Distribution of samples from pediatric patients positive for any virus submitted for viral diagnostic testing at Children's Hospital Colorado from September 2006 through June 2009.
Of the 164 adult samples tested, 64 (39%) were positive, of which 51 (80%) contained norovirus GII nucleic acid, 4 (2.4%) adenovirus, 2 (1.2%) astrovirus, 6 (3.6%) norovirus GI, and 1 (0.6%) rotavirus as shown in Table II. All 51 samples positive for norovirus GII were collected between October 2006 and January 2007, and 8 of 14 (57%) samples were positive in October, 11 of 27 (41%) in November, 22 of 58 (38%) in December, and 10 of 41 (24%) in January. During this timeframe, 12 adult and 7 pediatric, norovirus positive samples were sequenced: 16 (84%) were norovirus GII.4. and 3 (16%), all from pediatric patients, were norovirus GII.14.
Persistent Infection
329 samples were collected from 112 patients. Of these, 18 patients had multiple samples positive for at least one virus. Five immunocompromised patients in this study had multiple, serial stool samples positive for the same virus. Details of these clinical cases are outlined in Table V.
Table V.
Duration of Viral Shedding in Consecutive Stool Samples in Five Immunocompromised Hosts
| Patient | Age/Sex | Underlying medical condition | Related treatment & complications | Virus | No. Samples | No. Weeks | Clinical Course |
|---|---|---|---|---|---|---|---|
| 1 | 11 years, female | Beta thalassemia | URCB‐HSCT, GVHD | Norovirus GII | 6 | 25 | Diarrhea, cramping abdominal pain, emesis January – April 2007, new onset diarrhea, abdominal pain in July 2007 |
| 2 | 2 years, male | Severe infantile osteoporosis | URB‐HCST, GVHD | Norovirus GII | 4 | 32 | Symptomatic gastroenteritis October 2006 – May 2007 with failure to thrive |
| 3 | 5 months, female | Severe combined immunodeficiency | Bone marrow transplants (2) | Norovirus GII | 2 | 5 | Hospitalized May 2008 – August 2009 with persistent diarrhea, intermittent adenovirus, rotavirus, and norovirus GII positive samples; 2 consecutive norovirus GII samples |
| 4 | 6 years, male | Severe aplastic anemia | URCB‐HCST | Norovirus GII | 2 | 18 | Diarrhea, emesis March 2007; recurrent diarrhea in July 2007 |
| 5 | 20 months, male | Atypical rhabdoid tumor | Chemotherapy, multiple ASCTs | Astrovirus | 4 | 12 | Chemotherapy associated diarrhea and emesis, requiring multiple admissions, one episode associated with hypovolemic shock |
URCB‐HSCT, unrelated cord blood hematopoietic stem cell transplant; GVHD, graft‐versus‐host disease; AGE: acute gastroenteritis; ASCT, autologous stem cell transplant.
Clinical Data
The demographic and clinical characteristics of the 135 non‐immunocompromised patients with singly positive samples and clinical data available are shown in Table IV. The median duration of diarrhea was 6 days (IQR 3‐14), with a median of 6 stools per day (IQR 4‐10) per patient. In total, 56% of patients had emesis and 41.6% had fever. Approximately one‐quarter of the patients had fecal leukocytes and erythrocytes present. Patients with adenovirus were more likely to have decreased urine output (28.6%). There were no other statistically significant differences in symptoms or laboratory values among the different enteric viruses.
Table IV.
Clinical Characteristics, Laboratory Findings, and Patient Management of Non‐Immunocompromised Patients Where Data was Available
| Total (n = 135) | Adenovirus (n = 42) | Astrovirus (n = 10) | Norovirus GII (n = 57) | Rotavirus (n = 26) | P‐value * | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Sex (male) | 64 (51.2) | 23 (54.8) | 4 (40.0) | 3 (53.1) | 10 (38.5) | 0.34 |
| Age (years)a | 1.46 (0.58–5.2) | 1.24 (0.51–4.9) | 0.97 (0.08–5.0) | 1.74 (0.58–5.2) | 1.04 (0.61–6.8) | 0.91 |
| Underlying medical condition | 56 (44.8) | 17 (40.5) | 3 (30.0) | 29 (50.9) | 10 (38.5) | 0.45 |
| Known sick contacts | 22 (17.6) | 8 (19.0) | 2 (20.0) | 11 (19.3) | 3 (11.5) | 0.66 |
| History and Physical Exam | ||||||
| Duration of symptoms (days)a | 6 (3–13) | 7 (4–20) | 6 (4–17) | 5 (3–16) | 6 (3–11) | 0.77 |
| Diarrhea | 121 (89.6) | 40 (95.2) | 8 (80.0) | 49 (86.0) | 24 (92.3) | 0.28 |
| Blood visible in stool | 36 (26.7) | 13 (31.0) | 3 (30.0) | 15 (26.3) | 5 (19.2) | 0.56 |
| Mucous visible in stool | 19 (15.2) | 6 (14.3) | 1 (10.0) | 10 (17.5) | 3 (11.5) | 0.76 |
| Duration of diarrhea (days)a | 6 (3–14) | 6 (0.5–12) | 9 (1.5–49) | 5 (3–16) | 7 (4–13) | 0.30 |
| Max no. of stools per daya | 6 (4–10) | 6 (4–12) | 5 (3–7) | 6 (4–10) | 7 (4–10) | 0.91 |
| Emesis | 70 (56.0) | 24 (57.1) | 7 (70.0) | 30 (52.6) | 16 (61.5) | 0.74 |
| Duration of emesis (days)a | 4 (2–6) | 4 (2.5–7) | 4 (3–6) | 2 (2–5) | 6 (4–8) | 0.10 |
| Max no. of emesis per daya | 3 (2–5) | 3 (2–4) | 5 (1–6) | 3 (2–5) | 4 (3–6) | 0.52 |
| Fever (> 38.3 °C) | 52 (41.6) | 18 (42.9) | 5 (50.0) | 24 (42.1) | 10 (38.5) | 0.93 |
| Abdominal cramping | 15 (12.0) | 4 (9.52) | 2 (20.0) | 8 (14.0) | 3 (11.5) | 0.79 |
| Abdominal distention | 23 (18.4) | 7 (16.7) | 0 (0.0) | 13 (22.8) | 3 (11.5) | 0.44 |
| Abdominal tenderness | 20 (16.0) | 7 (16.7) | 1 (10.0) | 12 (21.0) | 1 (3.9) | 0.14 |
| Lethargic | 41 (32.8) | 12 (28.6) | 2 (20.0) | 21 (36.8) | 8 (30.8) | 0.67 |
| Decreased oral intake prior to testing | 65 (52.0) | 22 (52.4) | 6 (60.0) | 29 (50.9) | 14 (53.9) | 0.97 |
| Decreased urine output | 21 (16.8) | 12 (28.6) | 1 (10.0) | 7 (12.3) | 2 (7.7) | 0.038 |
| Hypotension (out of normal range for age) | 8 (6.4) | 3 (7.1) | 1 (10.0) | 3 (5.3) | 2 (7.7) | 0.89 |
| Tachycardia | 37 (29.6) | 14 (33.3) | 1 (10.0) | 18 (28.1) | 11 (24.4) | 0.80 |
| Stool studies | ||||||
| Red blood cells present | 29 (23.2) | 11 (26.2) | 2 (20.0) | 14 (24.6) | 4 (15.4) | 0.56 |
| White blood cells present | 32 (25.6) | 12 (28.6) | 1 (10.0) | 17 (29.8) | 3 (11.5) | 0.18 |
| Bacterial culture positive | 7 (5.6) | 3 (7.1) | 0 (0.0) | 4 (7.0) | 0 (0.0) | 0.38 |
| Positive for C. difficile | 6 (4.8) | 0 (0.0) | 0 (0.0) | 4 (7.0) | 2 (7.7) | 0.20 |
| Management | ||||||
| Received IV fluids | 32 (25.6) | 11 (26.2) | 3 (30.0) | 13 (22.8) | 8 (31.0) | 0.74 |
| Admitted to hospital | 79 (63.2) | 27 (64.3) | 6 (60.0) | 34 (59.7) | 18 (69.2) | 0.85 |
| Admitted for GI reasons | 60 (48.0) | 21 (50.0) | 6 (60.0) | 26 (45.6) | 13 (50.0) | 0.89 |
| Admitted to pediatric ICU | 17 (13.6) | 4 (9.5) | 1 (10.0) | 9 (15.8) | 4 (15.4) | 0.64 |
| Length of stay (days)a | 8 (4–15) | 7 (4–20) | 4 (3–6) | 9 (5–15) | 11 (4–23) | 0.48 |
indicates median number and interquartile range.
statistical analysis performed on adenovirus, rotatvirus, and norovirus GII samples as clinical data was available on sufficient number of patients.
Performance Characteristics: RT‐PCR versus EM
Samples positive by RT‐PCR for adenovirus, rotavirus, and caliciviruses or astroviruses were compared with those positive by EM (Table VI). Of 111 samples positive for adenovirus by EM or PCR, 99 (89%) were positive by PCR and 12 (11%) were positive by EM. Of 192 samples positive for astrovirus, sapovirus, or norovirus by EM (designated collectively as “small round viruses”) or RT‐PCR, 186 (97%) were positive by RT‐PCR and 21 (11%) were positive by EM. For rotavirus, 50 (60%) were positive by RT‐PCR and 56 (67%) were positive by EM.
Table VI.
Performance Characteristics: RT‐PCR versus Electron Microscopy for Detection of Virus in Stool Samples
| Virus | EM + | EM − | Total |
|---|---|---|---|
| Adenovirus | |||
| PCR + | 12 | 87 | 99 |
| PCR − | 12 | 994 | 1006 |
| Total | 24 | 1081 | 1105 |
| Rotavirus | |||
| PCR + | 22 | 28 | 50 |
| PCR − | 34 | 1021 | 1055 |
| Total | 56 | 1049 | 1105 |
| SRV/Norovirus/Astrovirus | |||
| PCR + | 15 | 177 | 192 |
| PCR − | 6 | 907 | 913 |
| Total | 21 | 1084 | 1105 |
DISCUSSION
This is the first hospital‐based, 3‐year, longitudinal study evaluating multiple viral etiologies of acute gastroenteritis in pediatric patients in the United States. Overall, 26% of all samples from children with gastrointestinal symptoms showed evidence of viral infection by RT‐PCR, similar to what has been reported in most studies using molecular techniques [Esper et al., 2010; Chhabra et al., 2013; Jevšnik et al., 2013; Payne et al., 2013]. Of note, viral agents were not detected in 74% of samples, and few samples had bacterial agents identified, suggesting that diarrhea had a non‐infectious cause or was due to a pathogen not assessed in this study. Recently, Payne et al. prospectively evaluated the etiology of acute gastroenteritis in children less than 5 years of age for 12 months in three counties in the United States and found that 21% of cases were attributed to norovirus and 12% to rotavirus. When Chhabra et al. expanded this study to evaluate the roles of bocavirus, parechovirus, and aichivirus in acute gastroenteritis in addition to the viruses studied here, viruses were identified in two‐thirds of their patients. This increased identification of an etiological agent is likely due to their active surveillance and more comprehensive survey. Additionally, there are reports of diarrhea associated with rotavirus groups B in children in Asia [Sanekata et al., 2003] and group C worldwide [Jiang et al., 1995; Kumazaki and Usuku, 2013], as well as toroviruses [Koopmans et al., 1997; Jamieson et al., 1998] in children with gastroenteritis, which were not included in the RT‐PCR assay in this study. Nevertheless, these studies suggest that there are likely other, yet to be discovered, viruses that cause acute gastroenteritis in children.
As expected, the incidence of rotaviruses decreased over the course of this study, which corresponded with the implementation of the rotavirus vaccine, RotaTeq (Merck, USA) in 2006 and Rotarix (GlaxoSmithKline, Belgium) in 2008. Interestingly, norovirus GII was detected more frequently than rotavirus both prior to and following vaccine implementation, which indicates a significant, continuous burden of disease due to norovirus infections. This correlates with recently published data [Payne et al., 1986, 2013; Risku et al., 2010; Sánchez‐Fauquier et al., 2011]. A significant amount of adenovirus DNA was detected in children with gastrointestinal symptoms, and while other studies have shown variable detection rates of adenovirus 40 and 41 [Colomba et al., 2006; Chhabra et al., 2013] the assay in this study was able to detect multiple serotypes in addition to 40 and 41. Only serotypes 40 and 41, and occasionally 18 and 31 have been associated with gastroenteritis. Despite the fact that providers at this institution are discouraged from sending samples for EM unless the patient is symptomatic, the numbers in this study may overestimate the burden of gastroenteritis in children attributed to adenoviruses and could represent asymptomatic shedding of non‐enteric adenoviruses with other pathogens responsible for symptoms.
Astroviruses have long been known to cause gastroenteritis in humans [De Benedictis et al., 2011; Chhabra et al., 2013]. Recently, however, new astrovirus strains have been discovered and been associated with disease in humans [Zimmerman et al., 2001; Finkbeiner et al., 2008, 2009a, 2009b; Hall, 2011; Lindesmith et al., 2011]. The astrovirus protocol used in this study was not designed to detect these novel strains and likely underestimates the true burden of astrovirus disease in these study patients [Phan et al., 2007; De Benedictis et al., 2011]. Surprisingly, in contrast to other studies, very few cases of sapovirus were detected. In other studies, sapoviruses have been reported to cause more vomiting than diarrhea in children, and this study only evaluated stool samples [Chhabra et al., 2013]. Additionally, no cases were associated with the known endemic human coronaviruses. While similar results have been documented in the literature [Esper et al., 2010; Jevšnik et al., 2013], other studies have found endemic coronaviruses and coronavirus‐like particles in stool in patients with gastroenteritis [Payne et al., 1986; Risku et al., 2010]. The data from this study supports the hypothesis that known endemic coronaviruses do not cause clinically significant gastroenteritis in children.
All viruses demonstrated seasonal as well as annual variation throughout the study period. Adenovirus was present throughout the year, while astrovirus, rotavirus, and norovirus GII were present most frequently in the fall and winter months, similar to previously reported data [Zimmerman et al., 2001; Hall, 2011; Chhabra et al., 2013]. The data in this study indicate that there was an outbreak of norovirus GII.4 in this community during the winter of 2006–2007. This observation supports other data which suggests norovirus GII.4 strain is the leading cause of norovirus outbreaks due to its ability to rapidly evolve through antigenic variation, possibly driven by herd immunity [Lindesmith et al., 2011] as well as recombination [Bull et al., 2007; Phan et al., 2007; Unicef WHO, 2009]. These data also support the observation that multiple different strains of norovirus can circulate in the community at one time, which could facilitate evolution of new strains.
It is unclear how long virus shedding can occur in the intestinal tract following natural infection. Several publications have shown variable duration of shedding in pediatric patients both with and without symptoms [Payne et al., 1986, 2013; Wilde et al., 1991; Mitchell et al., 1995; Richardson et al., 1998; Parashar et al., 2003; Wilhelmi et al., 2003; Colomba et al., 2006; Unicef WHO, 2009; Graham et al., 2010; Churgay and Aftab, 2012; Chhabra et al., 2013; Jevšnik et al., 2013]. Data from this study indicate that immunocompromised children, in particular those who have received HSCTs, may be infected and shed enteric viruses for up to 8 months and are at risk for persistent symptomatic infection. These findings have significant clinical and infection control implications and complicate the diagnosis of diarrhea in these study patients. Immunocompromised patients might also provide a reservoir for these viruses and a potential environment for recombination events and generation of novel viral strains [Booth et al., 2003; Choi et al., 2003; Hsu et al., 2003; Peiris et al., 2003; Hsueh et al., 2004; Schorn et al., 2010]. Furthermore, prolonged infection with enteric pathogens has been shown to put patients at risk for significant sequelae including severe weight loss, malnutrition, dehydration, and altered intestinal mucosa [Roddie et al., 2009; Schwartz et al., 2011; Jevšnik et al., 2013].
This study did not demonstrate statistically significant differences in history, physical examination, laboratory studies, or patient management among illnesses caused by different enteric viruses. However, while enteric viruses are generally thought not to elicit severe inflammatory gastroenteritis, nearly one quarter of patients with viral acute gastroenteritis in this study had erythrocytes and leukocytes present in their stool. Additionally, a significant number of pediatric patients with viral acute gastroenteritis were hospitalized due to gastrointestinal symptoms, and a small number were admitted to the intensive care unit, which indicates these infections can have significant financial impacts.
In the past decade, many clinical laboratories have been moving toward RT‐PCR multiplex assays to assess the etiology of multiple infectious diseases. At the time these samples were collected and submitted for diagnostic testing, this institution only employed EM for viral testing of stool samples. These results clearly demonstrate that RT‐PCR is superior in the detection of noroviruses, astroviruses, and adenoviruses in stool than EM. RT‐PCR did not perform significantly better than EM in the diagnosis of rotavirus, but this may have been due to the fact that the RT‐PCR assay in use detected only group A rotaviruses, while EM was able to detect groups A‐C. Additionally, the assays used in this study were designed for laboratory research and have not been fully vetted for clinical samples.
This study has several limitations. It was retrospective and conducted at a large tertiary care children's hospital, which likely biased sample collection toward more seriously ill patients and those with underlying medical conditions and therefore may not reflect the burden of disease in the wider pediatric population. Providers at the institution in this study are discouraged from routinely sending stool samples in patients with acute gastroenteritis unless it will impact clinical care, and this may have caused selection bias toward children with severe symptoms. Diluted stool samples were used, which might have reduced the ability to detect viral nucleic acid present in small amounts. Additionally, the presence of viral nucleic acid in stool may not reflect the cause of current symptoms but could instead reflect prolonged shedding from a previous infection or asymptomatic carriage [Kapusinszky et al., 2012].
In summary, a viral cause was identified in 26% of children with diarrheal disease, with the largest burdens of disease attributed to adenoviruses and noroviruses in children. This study indicates that immunocompromised patients can experience prolonged symptomatic infection and shedding of enteric virus. The data in this study also support the use of improved rapid, well‐standardized RT‐PCR diagnostic methods to establish the etiology of diarrhea. In addition to the advances made in the prevention of rotavirus disease, development of targeted therapies and vaccines towards other enteric viruses are needed to alleviate the significant burden of disease associated with diarrheal illness in children.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web‐site.
Supplementary Table S1.
ACKNOWLEDGMENTS
We thank the staff of the Children's Hospital Colorado Virology Laboratory for their assistance with this project.
Funding: NIH 1K08‐AI073525‐04A1 to SRD; University of Colorado School of Medicine‐Research Track to CMO; NIH/NCRR Colorado CTSI Grant Number UL1 TR001082 for RedCap use.
Competing interests: None declared.
Ethical approval: Colorado Multiple Institutional Review Board 05‐0101
REFERENCES
- Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, Walmsley SL, Mazzulli T, Avendano M, Derkach P, Ephtimios IE, Kitai I, Mederski BD, Shadowitz SB, Gold WL, Hawryluck LA, Rea E, Chenkin JS, Cescon DW, Poutanen SM, Detsky AS. 2003. Clinical features and short‐term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 289:2801–2809. [DOI] [PubMed] [Google Scholar]
- Bull RA, Tanaka MM, White PA. 2007. Norovirus recombination. J Gen Virol 88:3347–3359. [DOI] [PubMed] [Google Scholar]
- Chhabra P, Payne DC, Szilagyi PG, Edwards KM, Staat MA, Shirley SH, Wikswo M, Nix WA, Lu X, Parashar UD, Vinjé J. 2013. Etiology of viral gastroenteritis in children <5 years of age in the United States, 2008–2009. J Infect Dis 208:790–800. [DOI] [PubMed] [Google Scholar]
- Choi KW, Chau TN, Tsang O, Tso E, Chiu MC, Tong WL, Lee PO, Ng TK, Ng WF, Lee KC, Lam W, Yu WC, Lai JY, Lai ST, Princess Margaret Hospital SARS Study Group . 2003. Outcomes and prognostic factors in 267 patients with severe acute respiratory syndrome in Hong Kong. Ann Intern Med 139:715–723. [DOI] [PubMed] [Google Scholar]
- Churgay CA, Aftab Z. 2012. Gastroenteritis in Children: Part I. diagnosis AFP 85:1059–1062. [PubMed] [Google Scholar]
- Claas ECJ, Schilham MW, de Brouwer CS, Hubacek P, Echavarria M, Lankester AC, van Tol MJD, Kroes ACM. 2005. Internally controlled real‐time PCR monitoring of adenovirus dna load in serum or plasma of transplant recipients. J Clin Microbiol 43:1738–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomba C, Grazia S, Giammanco GM, Saporito L, Scarlata F, Titone L, Arista S. 2006. Viral gastroenteritis in children hospitalised in Sicily, Italy. Eur J Clin Microbiol Infect Dis 25:570–575. [DOI] [PubMed] [Google Scholar]
- De Benedictis P, Schultz‐Cherry S, Burnham A, Cattoli G. 2011. Astrovirus infections in humans and animals ‐ molecular biology, genetic diversity, and interspecies transmissions. Infect Genet Evol 11:1529–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin E, Duizer E, Vennema H, Koopmans MPG. 2006. Diagnosis of Norovirus outbreaks by commercial ELISA or RT‐PCR. J Virol Methods 137:259–264. [DOI] [PubMed] [Google Scholar]
- Dominguez SR, Robinson CC, Holmes KV. 2009. Detection of four human coronaviruses in respiratory infections in children: A one‐year study in Colorado. J Med Virol 81:1597–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esper F, Ou Z, Huang YT. 2010. Human coronaviruses are uncommon in patients with gastrointestinal illness. J Clin Virol 48:131–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner SR, Allred AF, Tarr PI, Klein EJ, Kirkwood CD, Wang D. 2008. Metagenomic analysis of human diarrhea: Viral detection and discovery. PLoS Pathog 4:e1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner SR, Holtz LR, Jiang Y, Rajendran P, Franz CJ, Zhao G, Kang G, Wang D. 2009a. Human stool contains a previously unrecognized diversity of novel astroviruses. Virol J 6:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner SR, Li Y, Ruone S, Conrardy C, Gregoricus N, Toney D, Virgin HW, Anderson LJ, Vinjé J, Wang D, Tong S. 2009b. Identification of a novel astrovirus (astrovirus VA1) associated with an outbreak of acute gastroenteritis. J Virol 83:10836–10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam R, Lyde F, Esona MD, Quaye O, Bowen MD. 2013. Comparison of Premier™ Rotaclone®, ProSpecT™, and RIDASCREEN® rotavirus enzyme immunoassay kits for detection of rotavirus antigen in stool specimens. J Clin Virol 58:292–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JM, Fitzpatrick EA, Black KJL. 2010. “ My child can't keep anything down!” Interviewing parents who bring their preschoolers to the emergency department for diarrhea, vomiting, and dehydration. Pediatr Emerg Care 26:251–256. [DOI] [PubMed] [Google Scholar]
- Grant L, Vinjé J, Parashar U, Watt J, Reid R, Weatherholtz R, Santosham M, Gentsch J, O'Brien K. 2012. Epidemiologic and clinical features of other enteric viruses associated with acute gastroenteritis in American Indian infants. J Pediatr 161:110–115.e1. [DOI] [PubMed] [Google Scholar]
- Hall AJ, Rosenthal M, Gregoricus N, Greene SA, Ferguson J, Henao OL, Vinjé J, Lopman BA, Parashar UD, Widdowson MA. 2011. Incidence of acute gastroenteritis and role of norovirus, Georgia, USA, 2004–2005. Emerg Infect Dis 17:1381–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond GW, Hazelton PR, Chuang I, Klisko B. 1981. Improved detection of viruses by electron microscopy after direct ultracentrifuge preparation of specimens. J Clin Microbiol 14:210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research electronic data capture (REDCap)—A metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu L‐Y, Lee C‐C, Green JA, Ang B, Paton NI, Lee L, Villacian JS, Lim P‐L, Earnest A, Leo Y‐S. 2003. Severe acute respiratory syndrome (SARS) in Singapore: Clinical features of index patient and initial contacts. Emerg Infect Dis 9:713–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh P‐R, Chen P‐J, Hsiao C‐H, Yeh S‐H, Cheng W‐C, Wang J‐L, Chiang B‐L, Chang S‐C, Chang F‐Y, Wong W‐W, Kao CL, Yang PC, SARS Research Group of National Taiwan University College of Medicine and National Taiwan University Hospital . 2004. Patient data, early SARS epidemic, Taiwan. Emerg Infect Dis 10:489–493. [DOI] [PubMed] [Google Scholar]
- Jamieson FB, Wang EE, Bain C, Good J, Duckmanton L, Petric M. 1998. Human torovirus: A new nosocomial gastrointestinal pathogen. J Infect Dis 178:1263–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevšnik M, Steyer A, Zrim T, Pokorn M, Mrvič T, Grosek Š, Strle F, Lusa L, Petrovec M. 2013. Detection of human coronaviruses in simultaneously collected stool samples and nasopharyngeal swabs from hospitalized children with acute gastroenteritis. Virol J 10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Dennehy PH, Spangenberger S, Gentsch JR, Glass RI. 1995. First detection of group C rotavirus in fecal specimens of children with diarrhea in the United States. J Infect Dis 172:45–50. [DOI] [PubMed] [Google Scholar]
- Kapusinszky B, Minor P, Delwart E. 2012. Nearly constant shedding of diverse enteric viruses by two healthy infants. J Clin Microbiol 50:3427–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans MP, Goosen ES, Lima AA, McAuliffe IT, Nataro JP, Barrett LJ, Glass RI, Guerrant RL. 1997. Association of torovirus with acute and persistent diarrhea in children. Pediatr Infect Dis J 16:504–507. [DOI] [PubMed] [Google Scholar]
- Kumazaki M, Usuku S. 2013. Epidemiological and genetic analysis of human group C rotaviruses isolated from outbreaks of acute gastroenteritis in Yokohama, Japan, between 2006 and 2012. Arch Virol 159:761–771. [DOI] [PubMed] [Google Scholar]
- Kuypers J, Martin ET, Heugel J, Wright N, Morrow R, Englund JA. 2007. Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics 119:e70–76. [DOI] [PubMed] [Google Scholar]
- Lindesmith LC, Donaldson EF, Baric RS. 2011. Norovirus GII.4 strain antigenic variation. J Virol 85:231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans J, de Villiers JC, Plessis du NM, Avenant T, Taylor MB. 2010. Emerging norovirus GII.4 2008 variant detected in hospitalised paediatric patients in South Africa. J Clin Virol 49:258–264. [DOI] [PubMed] [Google Scholar]
- Mitchell DK, Monroe S, Jiang XJ, Matson DO, Pickering LK. 1995. Virologic features of an astrovirus diarrhea outbreak in a day care center revealed by reverse transcriptase‐polymerase chain reaction. J Infect Dis 172:1437–1444. [DOI] [PubMed] [Google Scholar]
- Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. 2003. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 9:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne CM, Ray CG, Borduin V, Minnich LL, Lebowitz MD. 1986. An eight‐year study of the viral agents of acute gastroenteritis in humans: Ultrastructural observations and seasonal distribution with a major emphasis on coronavirus‐like particles. Diagn Microbiol Infect Dis 5:39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne DC, Vinjé J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, Hall CB, Chappell J, Bernstein DI, Curns AT, Wikswo M, Shirley SH, Hall AJ, Lopman B, Parashar UD. 2013. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med 368:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, Chan KH, Ng JS, Zheng BJ, Ng WL, Lai RW, Guan Y, Yuen KY, HKU/UCH SARS Study Group . 2003. Clinical progression and viral load in a community outbreak of coronavirus‐associated SARS pneumonia: A prospective study. Lancet 361:1767–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan TG, Kaneshi K, Ueda Y, Nakaya S, Nishimura S, Yamamoto A, Sugita K, Takanashi S, Okitsu S, Ushijima H. 2007. Genetic heterogeneity, evolution, and recombination in noroviruses. J Med Virol 79:1388–1400. [DOI] [PubMed] [Google Scholar]
- Richardson S, Grimwood K, Gorrell R, Palombo E, Barnes G, Bishop R. 1998. Extended excretion of rotavirus after severe diarrhoea in young children. Lancet 351:1844–1848. [DOI] [PubMed] [Google Scholar]
- Risku M, Lappalainen S, Räsänen S, Vesikari T. 2010. Detection of human coronaviruses in children with acute gastroenteritis. J Clin Virol 48:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddie C, Paul JPV, Benjamin R, Gallimore CI, Xerry J, Gray JJ, Peggs KS, Morris EC, Thomson KJ, Ward KN. 2009. Allogeneic hematopoietic stem cell transplantation and norovirus gastroenteritis: A previously unrecognized cause of morbidity. Clin Infect Dis 49:1061–1068. [DOI] [PubMed] [Google Scholar]
- Sanekata T, Ahmed MU, Kader A, Taniguchi K, Kobayashi N. 2003. Human group B rotavirus infections cause severe diarrhea in children and adults in Bangladesh. J Clin Microbiol 41:2187–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez‐Fauquier A, Montero V, Colomina J, Gonzalez‐Galan V, Aznar J, Aisa ML, Gutierrez C, Sainz de Baranda C, Wilhelmi I. 2011. Global study of viral diarrhea in hospitalized children in Spain: Results of Structural Surveillance of Viral Gastroenteritis Net Work (VIGESS‐net) 2006–2008. J Clin Virol 52:353–358. [DOI] [PubMed] [Google Scholar]
- Schorn R, Höhne M, Meerbach A, Bossart W, Wüthrich RP, Schreier E, Müller NJ, Fehr T. 2010. Chronic norovirus infection after kidney transplantation: molecular evidence for immune‐driven viral evolution. Clin Infect Dis 51:307–314. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Vergoulidou M, Schreier E, Loddenkemper C, Reinwald M, Schmidt‐Hieber M, Flegel WA, Thiel E, Schneider T. 2011. Norovirus gastroenteritis causes severe and lethal complications after chemotherapy and hematopoietic stem cell transplantation. Blood 117:5850–5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terletskaia‐Ladwig E, Leinmüller M, Schneider F, Meier S, Enders M. 2007. Laboratory approaches to the diagnosis of adenovirus infection depending on clinical manifestations. Infection 35:438–443. [DOI] [PubMed] [Google Scholar]
- Trujillo AA, McCaustland KA, Zheng DP, Hadley LA, Vaughn G, Adams SM, Ando T, Glass RI, Monroe SS. 2006. Use of TaqMan real‐time reverse transcription‐PCR for rapid detection, quantification, and typing of norovirus. J Clin Microbiol 44:1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unicef WHO . 2009. Diarrhoea: Why children are still dying and what can be done. http://www.unicef.org/media/files/Final_Diarrhoea_Report_October_2009_final.pdf
- van Maarseveen NM, Wessels E, de Brouwer CS, Vossen ACTM, Claas ECJ. 2010. Diagnosis of viral gastroenteritis by simultaneous detection of Adenovirus group F, Astrovirus, Rotavirus group A, Norovirus genogroups I and II, and Sapovirus in two internally controlled multiplex real‐time PCR assays. J Clin Virol 49:205–210. [DOI] [PubMed] [Google Scholar]
- Wei H, Zeng J, Deng C, Zheng C, Zhang X, Ma D, Yi Y. 2013. A novel method of real‐time reverse‐transcription loop‐mediated isothermal amplification developed for rapid and quantitative detection of human astrovirus. J Virol Methods 188:126–131. [DOI] [PubMed] [Google Scholar]
- Wilde J, Yolken R, Willoughby R, Eiden J. 1991. Improved detection of rotavirus shedding by polymerase chain reaction. Lancet 337:323–326. [DOI] [PubMed] [Google Scholar]
- Wilhelmi I, Roman E, Sánchez‐Fauquier A. 2003. Viruses causing gastroenteritis. Clin Microbiol Infect 9:247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman CM, Bresee JS, Parashar UD, Riggs TL, Holman RC, Glass RI. 2001. Cost of diarrhea‐associated hospitalizations and outpatient visits in an insured population of young children in the United States. Pediatr Infect Dis J 20:14–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's web‐site.
Supplementary Table S1.


