Abstract
Background
Human metapneumovirus (hMPV) is a recently detected virus, which can cause mild to severe respiratory tract infections. Through this study, we aimed to detail the outcomes of hMPV infections.
Materials/methods
Between January 2012 and November 2017, patients who had hMPV detected in nasopharyngeal or bronchoalveolar lavage by molecular respiratory pathogen tests were evaluated. The Food and Drug Administration cleared multiplexed‐polymerase chain reaction system (Idaho Technology, Salt Lake City, UT) was used for diagnosis. Chest radiography (CR) and computed tomography (CT) were evaluated by an expert radiologist.
Results
In total 100 patients were included, the mean age was 22.9 (0‐87) years, and 50% were male. The hospitalization rate was 52%. Lower respiratory system infection (LRTI) was diagnosed in 44 patients with clinical findings, and in 31 patients out of 44 the radiological findings supported the diagnosis. The LRTI rate was significantly higher in adults than children (66.7%‐32.8%; P = 0.001). In CR, peribronchovascular infiltration (PI) was the most common feature seen in 14 out of 18 patients and was generally bilateral (13 out of 18 patients). In CT imaging, ground‐glass opacity was the most common finding seen in 11 out of 16 patients and nodular consolidation in five patients. Ribavirin was given to four patients, three of whom were severe and required respiratory support. None of the patients died of hMPV infection.
Conclusions
The ground‐glass opacity in CT was similar to other respiratory virus infections, and PI in CR was very common and typical; however, nodular consolidation that may mimic bacterial infection was seen in one‐fourth of CT.
Keywords: human metapneumovirus, influenza virus, pathogenesis, respiratory tract, virus classification
Highlights
Human metapneumovirus (hMPV) is a recently detected virus which can cause mild to severe respiratory tract infections. Nodular consolidation that may mimics bacterial infection was detected in one fourth of CT administered patients. Clinicians should be alert that hMPV infection may present like a bacterial LRTI in radiologic imaging.
1. INTRODUCTION
Human metapneumovirus (hMPV) was defined in 2001. It causes mild to severe respiratory system infections.1, 2, 3 Due to the increasing availability of molecular based tests, reporting of hMPV infection is increasing all over the world.4, 5, 6 The hMPV has been reported to be one of the most commonly detected viruses that cause influenza‐like illness7 and community‐acquired pneumonia6 in both adults and children.
Radiologic findings of hMPV infection have not been reported to be different from other viral pathogens in studies with high sample sizes;8 however, it has been reported that studies including radiologic findings of hMPV infections were commonly done among immunocompromised hosts.9
hMPV infection may cause fatality in patients with hematologic malignancy.3, 10 The infection presents with a wide variation of clinical conditions from asymptomatic to fatal. We aimed to describe the clinical course of the disease by detailing the clinical and radiological findings and the outcome.
2. METHODS
2.1. Study population and design
We included patients from inpatient and outpatient departments who had hMPV in nasopharyngeal or bronchoalveolar lavage, diagnosed using the molecular respiratory pathogen test. The study was conducted in a 265‐bed private hospital in Istanbul between January 2012 and November 2017.
This was a retrospective study performed by reviewing the patient charts. The clinical features of the patients, demographic characteristics, chronic diseases including malignant disorders, diabetes mellitus, chronic kidney and liver diseases, and detailed information about antibiotic and antiviral drugs were collected by chart review. Complete blood count, C‐reactive protein, and liver and kidney function tests were studied among all patients. Bacterial cultures (throat, sputum, blood, and urine) were obtained, and procalcitonin (PCT) was studied in patients with suspicion of bacterial infection and/or in critical patients. Chlamydia pneumoniae and Mycoplasma pneumoniae were included in multiplex polymerase chain reaction (PCR) tests, and urine Legionella antigen test was performed in case of suspicion.
2.2. Diagnosis
The Food and Drug Administration cleared multiplexed‐respiratory PCR system BioFireFilmArray (Idaho Technology), which detects 17 viral pathogens including hMPV, and three bacterial species were used for the molecular detection of hMPV.
Chest radiography (CR) and computed tomography (CT) were done if lower respiratory system infection (LRSI) was suspected. The radiological imaging of patients was evaluated by a single dedicated radiologist.
2.3. Definitions
The upper respiratory system infection (URSI) was defined based on the modified CENTOR score that was developed for acute tonsillitis/pharyngitis11 and deduced from the recommendation of the Centers for Disease Control and Prevention and Infectious Diseases Society of America.12, 13
The LRSI is defined as infectious inflammation of the lower respiratory tract and refers to acute pneumonia, acute bronchitis, and acute bronchiolitis. Clinical LRSI is a syndrome characterized by symptoms consistent with respiratory tract infection (such as fever, cough, sputum, and dyspnea) and lung auscultation findings (crackles, rhonchus, and decreased lung sounds).14 Radiological LRSI is defined as radiological findings of the lung, including consolidation, cavitation, peribronchial ground‐glass opacity (GGO), airspace consolidation, and small nodules.
2.4. Data analysis
In the statistical analysis, the t test for continuous variables and the χ 2 test for the comparison of categorical variables were used. In the analysis, STATA 11 (StataCorp, College Station, TX) was used, and P < 0.05 was set as significant. The Institutional Review Board of Koç University approved the study.
3. RESULTS
Our study included 100 patients (Figure 1). The mean age of the patients was 22.9 (0‐87) years, and 50% of them were male (Table 1). Two‐third (67%) of the patients were under 18 years old. In 14 patients, more than one virus was detected. Concomitant agents were as follows: seven rhino/enterovirus, five influenza, two coronavirus, and one respiratory syncytial virus. The cases were most commonly seen between November and June, and 21% of the cases were seen in December (Figure 2).
Figure 1.
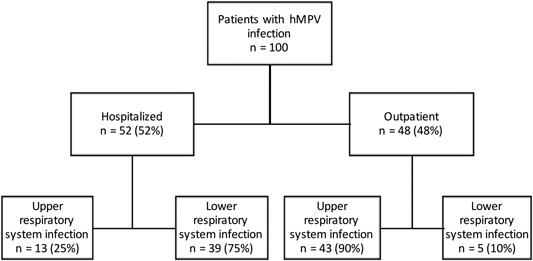
Study population. hMPV, human metapneumovirus
Table 1.
Demographic features and outcomes of the patients
| Features | n = 100 (%) |
|---|---|
| Age | 22.9 (0‐87) y |
| Sex, male | 50% |
| Comorbid diseases | 13 (13) |
| Hypertension | 11 (11) |
| Solid organ malignancy | 4 (4) |
| Rheumatologic disease | 3 (3) |
| Hematologic malignancy | 3 (3) |
| Diabetes mellitus | 2 (2) |
| Chronic obstructive lung disease | 1 (1) |
| Oxygen requirement | 21 (21) |
| Nasal/mask oxygen | 20 (20) |
| Noninvasive ventilation | 1 (1) |
| Mechanic ventilation | 0 |
| ICU transfer | 0 |
| Median length of stay among hospitalized patients | 4 (1‐211) d |
| hMPV related rehospitalization | 0 |
Abbreviation: hMPV, human metapneumovirus.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 2.
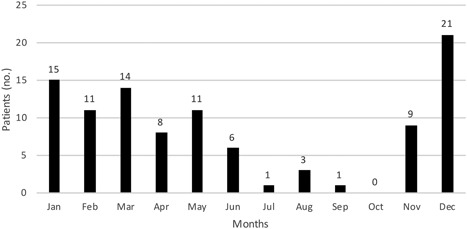
Seasonal distribution of cases
All the inpatients with hMPV infection were hospitalized because of hMPV. There was no nosocomial hMPV infection. The rate of hospitalization among the children was 46% (31 out of 67 patients), while it was 64% (21 out of 33 patients) among adult patients. Among the hospitalized patients, the rate of LRSI was 68% (21 out of 31 patients) in children and 86% (18 out of 21 patients) in adults. Among patients with LRSI, comorbid disease was seen in 50%; however, among patients with URSI, comorbid disease was detected in 22% (P = 0.16). The mean leukocyte, C reactive protein (CRP), and PCT levels on admission were 8.496/μL (3.4 to 17.29), 38.9 mg/L (5.3 to 193), and 0.1 ng/mL (0.04 to 0.28) in adults and 11.251/μL (3.72 to 25), 36.45 mg/L (0.5 to 218), and 0.38 ng/mL (0.06 to 1.2) in children.
In total, LRSI was diagnosed in 44 patients with clinical findings, and in 31 patients out of 44, radiological findings supported the diagnosis. Thirty‐nine out of 44 LRSI were hospitalized, and five were followed up in the outpatient department. The LRSI rate was significantly higher in adults than children (66.7%‐32.8%; P = 0.001), but when we compared monoviral or polyviral infections in terms of the presence of LRSI, there was no significant difference (46% in monoviral to 31% in polyviral; P = 0.26).
Radiological imaging, including CR and/or CT, was done among 34 patients, and radiologic LRSI findings were detected in 31 out of 34 patients. The CR was used among 28 patients: 11 of them were adults and 17 were children. Among the children, radiologic findings were detected in 76% of patients; among the adults, it was seen in 45% of the patients (P = 0.094). Among patients with LRSI, CR was normal in 10 patients and radiologic findings were detected in 18 patients. Among patients with normal CR, the time from onset of symptoms to hospital admission was 2.5 days; however among patients with abnormal CR, it was 3.6 days (P = 0.05).
In CR, peribronchovascular infiltration (PI) was the most common feature seen in 14 out of 18 patients and radiological infiltrations were generally bilateral (13 out of 18 patients; Figure 3). The CT was performed among 17 adult patients. In CT imaging, GGO was the most common finding, seen in 11 out of 17 patients (65%) and nodular consolidation in five out of 17 patients (29%). Centrilobular nodules were detected in seven out of 17 patients (41%; Table 2; Figure 4).
Figure 3.
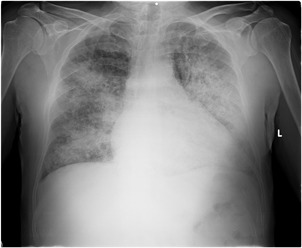
Chest radiograph shows bilateral patchy consolidation with fine nodular appearance
Table 2.
Radiologic features of hMPV infection
| Chest X‐ray | n = 28 (%) |
|---|---|
| Normal | 10 (36) |
| Peribronchovascular infiltration | 14 (50) |
| Unilateral | 5 (18) |
| Bilateral | 13 (46) |
| Computer tomography | n = 17 (%) |
| Normal | 1 (6) |
| Ground‐glass opacity | 11 (65) |
| Nodular consolidation | 5 (29) |
| Centrilobular nodules | 7 (41) |
| Unilateral | 7 (41) |
| Bilateral | 9 (53) |
Abbreviation: hMPV, human metapneumovirus.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 4.
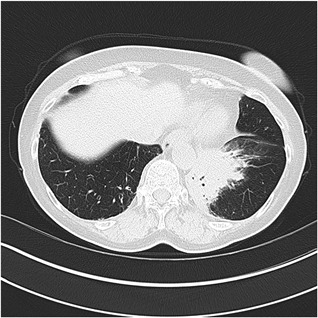
The computer tomography image demonstrates left basal consolidation with air bronchograms. In addition, mild bronchial wall thickening is seen in the right lower lobe
The antibiotics were given in 28% of the patients, despite detection of virus. The rate of antibiotic prescription was higher among LRTI when compared with URSI (54%‐21%; P = 0.003). The third generation cephalosporins, β‐lactam/β‐lactamase inhibitor combination, and quinolones were the most commonly used antibiotics (17%, 11%, and 10%, respectively).
Finally, ribavirin was given to four patients, three of whom were severe and required respiratory support. None of the patients died of hMPV infection.
4. DISCUSSION
Our study demonstrates the radiologic findings of hMPV infections in patients with LRSI (Figures 3 and 4). One of the most interesting radiologic findings was the presence of nodular consolidation in CT, which is generally expected to be seen in bacterial infection.
The PI in CR and GGO in CT are the most common radiologic findings, which are frequently seen in viral pneumonia. In a recent review for radiologic imaging of viral agents that may cause pneumonia, the general radiologic findings of hMPV infections were followed as bilateral centrilobular nodules, GGO, and multilobar infiltrations; however, there was no information about nodular consolidation. In addition, they noted that the radiologic findings of hMPV infections were most commonly reported in patients with hematologic malignancy, but there were limited data on immunocompetent patients.9 Similar radiologic findings in hMPV infection among immunocompromised patients have been reported in different studies.15, 16, 17 Karimata et al18 recently reported 105 nonimmunocompromised cases of hMPV infection in their study and bronchial wall thickenings were the most commonly seen radiologic findings in both CR and CT. Multilobar distribution was seen in all cases and lobular opacity was one of the leading findings in CT in the same study.Our populations were mostly composed of immunocompetent patients and nodular consolidation, which is generally seen in bacterial infection and was seen in 29% of CT. Multilobar infiltrations were detected in half of the patients, which is lower when compared with the study of Karimata et al.18
The time from onset of symptoms to hospital admission may affect radiologic findings. In our study, among patients with normal CR, which were LRSI‐based on their clinical findings, time from onset of symptoms to hospital admission was lower (2.5 to 3.6 days; P = 0.05). By this finding, we concluded that normal CR does not rule out LRSI, particularly in early stages of the disease. LRSI was diagnosed based on symptoms and lung auscultation findings in the setting of negative imaging.
Concomitant bacterial infection was also evaluated based on relevant cultures, molecular tests for atypical bacterial infection, and PCT. We could not detect any evidence of bacterial pneumonia in any cases when we checked their laboratory and microbiological tests. Despite the fact that there was no bacterial coinfection, the antibiotic prescription rate was still high, which was 54% among patients with LRSI.
There is no confirmed antiviral treatment in hMPV infection. Ribavirin use in hMPV infections is limited to case reports in immunocompromised patients.17 In a recent study, ribavirin use was not associated with a better clinical outcome. They reported that the duration of viral shedding was shorter in patients using ribavirin.10 A recent review also concluded that there is no evidence to recommend ribavirin in immunocompromised patients.19 The fourth European Conference on Infections in Leukemia (ECIL‐4) guideline also commented that general recommendations for treatment of hMPV infection could not be made with current evidence.20 In our study, ribavirin was used in four cases, three of whom were severe and required respiratory support and none of them died.
In conclusion, GGO in CT was similar to other respiratory virus infections and the PI in CR was very common and typical; however, nodular consolidation, which may mimic bacterial infection, was detected in one‐fourth of CT‐administered patients. Clinicians should be alert that hMPV infection may present like a bacterial LRTI in radiologic imaging. Despite detection of virus without evidence of bacterial infection, antibiotic use is still high in hMPV infection, particularly in patients with LRSI. Further studies could be performed to show the efficacy of ribavirin in hMPV infections.
Keske Ş, Gümüş T, Köymen T, Sandıkçı S, Tabak L, Ergönül Ö. Human metapneumovirus infection: Diagnostic impact of radiologic imaging. J Med Virol. 2019;91:958–962. 10.1002/jmv.25402
References
REFERENCES
- 1. van den Hoogen BG, de Jong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7(6):719‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hasvold J, Sjoding M, Pohl K, Cooke CR, Hyzy RC. The role of human metapneumovirus in the critically ill adult patient. J Crit Care. 2016;31(1):233‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. El Chaer F, Shah DP, Kmeid J, et al. Burden of human metapneumovirus infections in patients with cancer: risk factors and outcomes. Cancer. 2017;123(12):2329‐2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolf DG, Zakay‐rones Z, Fadeela A, Greenberg D, Dagan R. High seroprevalence of human metapneumovirus among young children in Israel. J Infect Dis. 2003;188(12):1865‐1867. [DOI] [PubMed] [Google Scholar]
- 5. Ebihara T, Endo R, Kikuta H, et al. Seroprevalence of human metapneumovirus in Japan. J Med Virol. 2003;70(2):281‐283. [DOI] [PubMed] [Google Scholar]
- 6. Jain S, Self WH, Wunderink RG, et al. Community‐acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keske Ş, Ergönül Ö, Tutucu F, Karaaslan D, Palaoğlu E, Can F. The rapid diagnosis of viral respiratory tract infections and its impact on antimicrobial stewardship programs. Eur J Clin Microbiol Infect Dis. 2018;37(4):779‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hilmes MA, Daniel Dunnavant F, Singh SP, et al. Chest radiographic features of human metapneumovirus infection in pediatric patients. Pediatr Radiol. 2017;47(13):1745‐1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koo HJ, Lim S, Choe J, Choi SH, Sung H, Do KH. Radiographic and CT features of viral pneumonia. Radiographics. 2018;38(3):719‐739. [DOI] [PubMed] [Google Scholar]
- 10. Spahr Y, Tschudin‐Sutter S, Baettig V, et al. Community‐acquired respiratory paramyxovirus infection after allogeneic hematopoietic cell transplantation: a single‐center experience. Open Forum Infect Dis. 2018;5(5):ofy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choby BA. Diagnosis and treatment of streptococcal pharyngitis. Am Fam Physician. 2009;79(5):383‐390. [PubMed] [Google Scholar]
- 12. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54(8):e72‐e112. [DOI] [PubMed] [Google Scholar]
- 13. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55(10):1279‐1282. [DOI] [PubMed] [Google Scholar]
- 14. Greene G, Hood K, Little P, et al. Towards clinical definitions of lower respiratory tract infection (LRTI) for research and primary care practice in Europe: an international consensus study. Prim Care Respir J. 2011;20(3):299‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kamboj M, Gerbin M, Huang CK, et al. Clinical characterization of human metapneumovirus infection among patients with cancer. J Infect. 2008;57(6):464‐471. [DOI] [PubMed] [Google Scholar]
- 16. Syha R, Beck R, Hetzel J, et al. Human metapneumovirus (HMPV) associated pulmonary infections in immunocompromised adults–initial CT findings, disease course and comparison to respiratory‐syncytial‐virus (RSV) induced pulmonary infections. Eur J Radiol. 2012;81(12):4173‐4178. [DOI] [PubMed] [Google Scholar]
- 17. Godet C, Le Goff J, Beby‐Defaux A, et al. Human metapneumovirus pneumonia in patients with hematological malignancies. J Clin Virol. 2014;61(4):593‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karimata Y, Kinjo T, Parrott G, et al. Clinical features of human metapneumovirus pneumonia in non‐immunocompromised patients: an investigation of three long‐term care facility outbreaks. J Infect Dis. 2018;218:868‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shah DP, Shah PK, Azzi JM, El Chaer F, Chemaly RF. Human metapneumovirus infections in hematopoietic cell transplant recipients and hematologic malignancy patients: a systematic review. Cancer Lett. 2016;379(1):100‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL‐4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis. 2013;56(2):258‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]


