Abstract
A cat was presented with a history of vomiting, decreased appetite and weight loss. Abnormal findings were poor body condition, pale mucous membranes, dehydration and a palpable abdominal mass. Abdominal ultrasound showed lymph node enlargement, a mass of uncertain origin, thickening of the muscularis layer of the small bowel, focal thickening of the ileum with loss of layering and free peritoneal fluid. Cytology revealed a piogranulomatous infiltrate and numerous macrophages containing oval or round yeast‐like cells 2 to 5 µm diameter with a central, spherical, lightly basophilic body surrounded by a clear halo, compatible with Histoplasma capsulatum, within the cytoplasm. Post‐mortem examination revealed cavity effusions, granulomatous nodules in lungs, intestine and omentum, thickened intestinal walls and intestinal perforation. Staining with Grocott and immunohistochemistry (IHC) revealed numerous organisms within the granulomatous reaction. H. capsulatum has a worldwide distribution in temperate and subtropical climates. To the author’s knowledge, this is the first report of feline histoplasmosis in Europe.
Introduction
Histoplasmosis, caused by fungus Histoplasma capsulatum, can affect both human beings and animals. H. capsulatum occurs in soil, especially that contaminated with bird and bat droppings, and infection is usually by inhalation of infective mycelial forms. Histoplasmosis occurs in specific endemic areas, including North, Central and South America, Africa, India and Southeast Asia (Panackal and others 2002). Feline histoplasmosis is considered one of the more common deep mycotic infections in cats and several case reports/series have been published from endemic areas (Clinkenbeard and others 1987, Davis & Troy 1996, Brömel & Sykes 2005). Although histoplasmosis has been reported sporadically in human beings and animals (dogs, badger) from several European countries, including Italy, Confalonieri and others 1994, Confalonieri and others 1995 (Antinori and others 1997, Confalonieri and others 1997, Ashbee and others 2008), this is the first case of feline histoplasmosis both in Italy and in Europe to the authors’ knowledge.
Case report
A six‐year‐old, neutered male domestic shorthaired cat was referred to the Veterinary Teaching Hospital of Parma University with a five‐month history of episodic vomiting, decreased appetite and weight loss. The cat was allowed access to the outdoors and had been administered with annual booster vaccinations against feline panleukopenia virus, feline herpesvirus and feline calicivirus several months before presentation.
Abnormal findings were limited to poor body condition, pale mucous membranes, mild dehydration and a palpable abdominal mass. Complete blood count revealed mild normocytic, normocromic, non‐regenerative anaemia and mild leucocytosis with neutrophilia. Serum biochemistry profile was unremarkable and serologic testing for FeLV and FIV infections was negative (Table 1). Abdominal ultrasound showed diffuse thickening of the muscularis layer of the small bowel, focal thickening of a jejunal loop with loss of layering, abdominal lymph node enlargement, a rounded hypoechoic heterogeneous mass (4 cm × 4 cm), and small amount of free peritoneal fluid (Fig 1). Thoracic radiographs revealed only enlargement of the retrosternal lymph node (Fig 2). Polymerase chain reaction testing (PCR) testing for the detection of feline coronavirus RNA in the peritoneal fluid was negative.
Table 1.
Laboratory results at presentation
| Haematological parameters | Measured value | Reference range |
|---|---|---|
| WBC count(109/l) | 17·5 | 6·0 to 17·0 |
| RBC count (109/l) | 6·3 | 5·0 to 10·0 |
| Haemoglobin (g/dl) | 8·4 | 9·0 to 15·0 |
| Haematocrit (l/l) | 0·24 | 0·30 to 0·45 |
| MCV (fl) | 39·4 | 40 to 54 |
| MCH (pg) | 13·4 | 14 to 18 |
| MCHC (g/dl) | 34·1 | 31 to 36 |
| Platelet count(109/l) | 159 | 250 to 750 |
| Neutrophils (%) | 86 | 50 to 75 |
| Lymphocytes (%) | 11 | 20 to 50 |
| Monocytes (%) | 0 | 1 to 4 |
| Eosinophils (%) | 2 | 2 to 6 |
| Basophils (%) | 0 | 0 to 0·5 |
| Aggregate reticulocytes (%) | 0·4 | 0·5 to 2·0 |
| Punctate reticulocytes (%) | 8·5 | >10 |
| Biochemical parameters | ||
| Alanine aminotransferase (IU/l) | 50 | 0·0 to 45 |
| Alkaline phosphatase (IU/l) | 5 | 0·0 to 50 |
| Total bilirubin (μmol/l) | 2·39 | 0·0 to 3·4 |
| Glucose (mmol/l) | 8·04 | 3·05 to 6·66 |
| Urea (mmol/l) | 7 | 3·33 to 9·15 |
| Creatinine (μmol/l) | 142·3 | 0·0 to 141 |
| Total protein (g/l) | 70·1 | 65 to 85 |
| Albumin (g/l) | 28·5 | 23 to 33 |
| Globulin (g/l) | 41·6 | 42 to 52 |
| Calcium (mmol/l) | 2·29 | 1·99 to 2·98 |
| Ab FeLV (Immunochromatographic test—Agrolabo®) | Negative | |
| Ab FIV (Immunochromatographic test—Agrolabo®) | Negative | |
WBC White blood cell, RBC Red blood cell, MCV Mean cell volume, MCH Mean cell haemoglobin, MCHC Mean cell haemoglobin concentration.
The anticoagulant used for haematology was ethylenediaminetetraacetic acid (EDTA). Analysis was performed using an inhouse automatic cell counter (Medonic CA 570; Delcon). Leucocyte differential count was made by microscopic examination of a stained blood film. The anticoagulant used for the biochemistry sample was lithium heparin. Analysis was performed using an inhouse analyser (Cobas Integra 400 plus; Roche).
Figure 1.

Ultrasound examination of the abdomen of the cat with disseminated histoplasmosis (a–c). Focal thickening of a jejunal loop with loss of layering (a). Mesenteric hypoechoic, heterogeneous rounded mass (b)
Figure 2.

Radiographic examination of the thorax of the cat with disseminated histoplasmosis. No pulmonary parenchyma abnormalities were obvious at the time of the examination. Enlargement of the retrosternal lymph node can be seen on the lateral view (arrow), as a soft tissue opacity dorsal to the cramial stomebrae.
Cytology was performed on fine‐needle aspirates from the abdominal mass and from several enlarged lymph nodes. A piogranulomatous infiltrate was observed and numerous macrophages containing oval or round yeast‐like cells 2 to 5 µm diameter within the cytoplasm. These had a central, spherical, lightly basophilic body surrounded by a clear halo (Fig 3a) and were considered compatible with H. capsulatum. The organisms stained positive with periodic acid Schiff (PAS) (Fig 3b). A diagnosis of intestinal histoplasmosis was made and systemic treatment with itraconazole (Itrafungol; Janssen Cilag) was initiated at 10 mg/kg PO every 12 hours.
Figure 3.
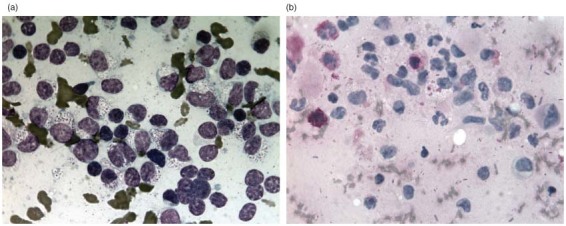
(a) Fine‐needle aspirate from the abdominal mass in a cat with disseminated histoplasmosis. There are numerous intracellular bodies within macrophages. Note the central, spherical, lightly basophilic body surrounded by a clear halo. Diff‐Quick, ×100 (b) Fine‐needle aspirate from the abdominal mass in a cat with disseminated histoplasmosis. Intracellular bodies stain positive with periodic acid Schiff stain (PAS) ×100
Six weeks later the owner reported that the cat was still lethargic and anorexic and that it was difficult to administer treatment. Ultrasound examination revealed that the lesions of the small bowel and the size of the abdominal mass were unchanged, while the amount of free fluid had increased. Debulking of the mass was proposed, but the owner refused. Cytology of the peritoneal fluid revealed the presence of numerous infected macrophages. Due to deteriorating conditions, the cat was euthanased.
Post‐mortem examination
Gross examination revealed yellowish, slightly heamorrhagic pleural and peritoneal effusion. There were several masses around the intestine and omentum and one small nodule in the lung. The intestinal walls were thickened and were presented in the areas of necrosis and intestinal perforation. Mesenteric lymph nodes were granulomatous and necrotic. Samples of several tissues, including the abdominal mass, lymph nodes, intestine and lung, were processed for routine histology and special staining for mycotic organisms. Histology of the enlarged retrosternal node revealed only hyperplasia and no signs of fungal infection. Examination of the abdominal mass and the small lung nodule revealed vast areas of necrosis and inflammatory infiltrate. Grocott staining revealed numerous organisms (Fig 4) and immunohistochemistry (IHC) (streptavidin–biotin horseradish peroxidase complex, diaminobenzidine as chromogen; DakoCytomation, Italy) with a specific polyclonal antibody (100601, goat anti‐Histoplasma, Meridian Bioscience Europe, Italy; dilution 1:50) was positive (Fig 5). Isolation in culture and molecular confirmation was not carried out due to the potential risks for human exposure to spores.
Figure 4.
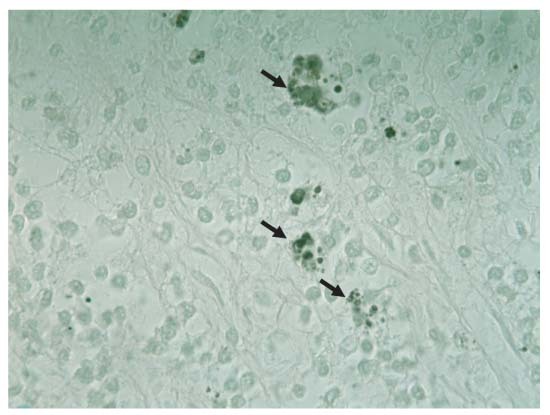
Grocott staining of same mass. Note the clusters of small, black organisms in unstained macrophages ×100
Figure 5.

Anti‐H. capsulatum immunohistochemistry. Notice the numerous positive‐staining fungal bodies scattered within the abdominal mass ×100
Discussion
Clinical and pathological features of feline histoplasmosis have been widely described and predominant clinical findings include anaemia, weight loss, lethargy, fever, anorexia and interstitial lung disease, usually present for several weeks and months before diagnosis (Clinkenbeard and others 1987). Interestingly, in one case series, eight cats with disseminated histoplasmosis had no obvious signs of respiratory tract disease, similar to our findings here (Wolf 1987). It has been suggested that even though the initial route of infection is via inhalation of infective particles in soil, it is possible that rapid diffusion takes place within the lymphatic system, leading to disseminated disease. In our case, lung lesions were visible only at necropsy. The most important clinical signs in the case reported here were gastrointestinal, including vomiting and anorexia. Intestinal involvement was severe in our case and subsequent necrosis and perforation of the intestine undoubtedly lead to the worsening of the cat’s condition and the poor response to treatment. Indeed, itraconazole is the therapy of choice for feline histoplasmosis and several authors have reported good/very good results following long‐term (4 to 8 months) treatment (Brömel & Sykes 2005).
Previous reports of autochthonous histoplasmosis in human beings and dogs in Italy have come from the Lombardy and Emilia‐Romagna (ER) regions. Our cat was from Parma, which is located in north‐east ER. According to the European Confederation of Medical Mycology (ECMM) Working Group, Italy, Germany and Turkey have geographical areas that could be considered endemic for H. capsulatum (Ashbee and others 2008). Even though there are no reports of animal–human transmission, infected pets may be a sentinel for human exposure. Veterinary practitioners working in these areas should be aware of the clinical features of feline histoplasmosis.
Acknowledgements
The authors thank Paola Gianelli for excellent technical assistance.
References
- Antinori, S. , Galimberti, L. , Bonaccorso, C. , Vago, L. , Nebulosi, M. & Esposito, R. (1997) A case of fatal disseminated histoplasmosis of autochthonous origin in an Italian AIDS patient. European Journal of Clinical Microbiology & Infectious Diseases 16, 545–546 [DOI] [PubMed] [Google Scholar]
- Ashbee, H. R. , Evans, E. G. , Viviani, M. A. , Dupont, B. , Chryssanthou, E. , Surmont, I. , Tomsikova, A. , Vachkov, P. , Enero, B. , Zala, J. & Tintelnot, K. (2008) ECMM Working Group on; histoplasmosis histoplasmosis in Europe: report on an epidemiological survey from the European Confederation of Medical Mycology Working Group. Medical Mycology 46, 57–65 [DOI] [PubMed] [Google Scholar]
- Brömel, C. & Sykes, J. E. (2005) Histoplasmosis in dogs and cats. Clinical Techniques in Small Animal Practice 20, 227–232 [DOI] [PubMed] [Google Scholar]
- Confalonieri, M. , Gandola, L. , Aiolfi, S. , Parigi, P. & Mazzoni, A. (1994) Histoplasmin sensitivity among a student population in Crema, Po Valley, Italy. New Microbiologica 17, 151–153 [PubMed] [Google Scholar]
- Confalonieri, M. , Aiolfi, S. , Gandola, L. , Scartabellati, A. , Colavecchio, A. , Cannatelli, G. & Mazzoni, A. (1995) Disseminated histoplasmosis and idiopathic CD4c T‐lymphocytopenia. An autochthonous Italian case. Presse Medicale 24, 459 [PubMed] [Google Scholar]
- Confalonieri, M. , Nanetti, A. , Gandola, L. , Colavecchio, A. , Aiolfi, S. , Cannatelli, G. , Parigi, P. , Scartabellati, A. , Della Porta, R. & Mazzoni, A. (1997) Histoplasmosis capsulati in Italy: Autochthonous or imported? European Journal of Epidemiology 10, 435–439 [DOI] [PubMed] [Google Scholar]
- Clinkenbeard, K. D. , Cowell, R. L. & Tyler, R. D. (1987) Disseminated histoplasmosis in cats: 12 cases (1981‐1986). Journal of the American Veterinary Medical Association 190, 1445–1448 [PubMed] [Google Scholar]
- Davies, C. & Troy, G. C. (1996) Deep mycotic infections in cats. Journal of the American Animal Hospital Association 32, 380–391 [DOI] [PubMed] [Google Scholar]
- Panackal, A. A. , Hajjeh, R. A. , Cetron, M. S. & Warnock, D. W. (2002) Fungal infections among returning travelers. Clinical Infectious Diseases 35, 1088–1095 [DOI] [PubMed] [Google Scholar]
- Wolf, A. M. (1987) Histoplasma capsulatum osteomyelitis in the cat. Journal of Veterinary Internal Medicine 1, 158–162 [DOI] [PubMed] [Google Scholar]


