Abstract
Acute respiratory infections (ARIs), with viral pathogens as the major contributors, are the most common illnesses worldwide, and increase the morbidity and mortality among the elderly population. The clinical and pathological features of elderly people with ARIs need to be identified for disease intervention. From January 1, 2012 through December 31, 2015, respiratory specimens from patients above 60 years old with ARIs were collected from the outpatient and inpatient settings of six sentinel hospitals in Pudong New Area. Each specimen was tested via multiplex polymerase chain reaction (PCR) for eight target viral etiologies including influenza, human rhinovirus (HRV), human para‐influenza virus (PIV), adenovirus (ADV), respiratory syncytial virus (RSV), human metapneumovirus (hMPV), human coronavirus (hCoVs), and human bocavirus (hBoV). A total of 967 elderly patients with ARIs were enrolled, including 589 (60.91%) males, and the median age was 73 years old. 306 (31.64%) patients were tested positive for any one of the eight viruses, including 276 single infections and 30 co‐infections. Influenza was the predominant virus (14.17%, 137/967), detected from 21.35% (76/356) of the outpatients and 9.98% (61/611) of the inpatients. Influenza infections presented two annual seasonal peaks during winter and summer. Compared with non‐influenza patients, those with influenza were more likely to have fever, cough, sore throat, and fatigue. This study identified influenza as the leading viral pathogen among elderly with ARIs, and two seasonal epidemic peaks were observed in Shanghai. An influenza vaccination strategy needs to be advocated for the elderly population.
Keywords: influenza virus, public policy, respiratory track
1. INTRODUCTION
Acute respiratory infections (ARIs) are the most common illnesses worldwide. They cause nearly four million deaths per year, at a rate of more than 60 deaths/100 000 people.1, 2 Among all the individuals with ARIs, the elderly suffer disproportional morbidity and mortality rates, mostly due to viral respiratory infections.3, 4, 5
The rates of ARIs increase with age in adults and are highest among individuals aged >65 years.6 During the past decades, some nations, including China, have faced a rapid increase in the proportion of older people.7 Because acute lower respiratory infections, including pneumonia, are a primary source of morbidity and mortality among the elderly, a related disease control strategy is of paramount importance for this population.8
A majority of the previous research has focused on the evaluation of the disease burden associated with ARIs such as pneumonia and influenza among the elderly9, 10, 11; however, the clinical and etiological features among elderly who present with ARIs have not been well described in China.
A laboratory‐based surveillance system was established in 2012 in Pudong New Area, the largest district in Shanghai City, Eastern China. The current study seeks to describe the demographic and clinical characteristics of the elderly patients enrolled in this system, understand the clinical features of elderly patients with ARIs and identify the dominant viral pathogens among these patients in Shanghai.
2. MATERIALS AND METHODS
2.1. Study site and patient enrollment
Six general hospitals were selected as sentinel sites for this study based on their location, catchment area, and patient volume.12 Patients were considered eligible for enrollment when they had at least one of the following symptoms: cough, sore throat, runny nose, or shortness of breath, with or without fever. The inclusion criteria was formulated in accordance with the surveillance guidelines of acute respiratory infectious disease in the whole Shanghai city. For each month, approximately five patients with ARIs over 60 years old in each of the sentinel hospitals were enrolled in this study via convenience sampling. At two sentinel hospitals, surveillance was conducted in outpatient settings: the respiratory clinic and the emergency room. The other four sentinels conducted surveillance at inpatient settings, including the respiratory medicine ward and the intensive care unit.
2.2. Specimen collection and testing
The attending physician collected and stored the throat swab or sputum from the enrolled patient in 2‐mL viral transport media (VTM, Yocon, Beijing, China), which was transported at 2°C − 8°C within 24 h to Shanghai Pudong New Area Center for Disease Control and Prevention for lab testing. Specimens that could not be transported immediately were preserved at −20°C temporarily.
Nucleic acid was extracted from a 200 µL suspension using QIAxtractorTM workstation (Qiagen, Hilden, Germany) with QIAxtractor Virus Reagents (Cat NO. 85146, Qiagen, MD) according to the manufacturer's instruction. The elution volume of nucleic acid was 50 µL.
Each specimen was tested for the following eight viral etiologies: influenza virus (A, B, and C), human rhinovirus (HRVs), human para‐influenza virus (PIVs 1‐4), human adenovirus (ADV), respiratory syncytial virus (RSV‐A and B), human metapneumovirus (hMPV), human coronavirus (hCoVs‐229E, OC43, NL63, and HKU1), and human bocavirus (hBoV). Polymerase chain reaction (PCR) amplification was performed with an Applied Biosystems (Forster, CA) 9700 GeneampPCR system using a Seeplex® RV15 ACE Detection Kit. According to the reagent instructions, each set of reaction tubes consisted of 4 µL 5 × RV12 ACE PM (A, B, or C), 3 µL 8‐Mop Solution, 10 µL 2 × Multiplex Master Mix, and 3 µL sample cDNA. The PCR reaction program was 94°C for 15 min, 40 cycles of 94°C for 0.5 min, 60°C for 1.5 min, and 72°C for 1.5 min, with extension at 72°C for 10 min. For all the influenza‐A positive samples, the detection of A(H1N1)pdm09 (Cat NO.RR‐0145‐02), A(H3N2) (Cat NO.RR‐0052‐04), Influenza B (Cat NO.RR‐0053‐02), H7N9 (Cat NO.RR‐0049‐02) was performed using a real‐time (RT)‐PCR Detection Kit produced by Shanghai ZJ Bio‐Tech Co., LTD (Shanghai, China).
2.3. Data collection and statistical analyses
The hospital staff recorded the demographic and clinical information including gender, age, symptoms, and clinical diagnosis in case‐report forms. The data were entered into the standard informatics system established by the Chinese Centers for Disease Control and Prevention and analyzed using R 3.2.3 (R Core Team, R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria). We used chi‐square test (χ 2 test) or Fisher's exact tests for categorical variables and Wilcoxon rank‐sum or Kruskal‐Wallis tests for continuous variables as appropriate. The 95% binomial confidence intervals (95%CI) of proportions were calculated by Pearson‐Klopper method. The results were age‐adjusted by Mantel‐Haenszel method. P‐values <0.05 were considered statistically significant.
3. RESULTS
3.1. Demographics and clinical features
From January 1, 2012 through December 31, 2015, 967 patients above 60 years old were enrolled from six sentinel hospitals. Of these patients, 589 (60.91%) were male, and their median age was 73 years old (interquartile range [IQR] = 66‐80 years). A total of 356 (36.81%) were outpatients, and 611 (63.19%) were inpatients. The proportion of male inpatients (409, 66.94%) was much higher than that in outpatients (180, 50.56%) (P = 0.000). The median age of the inpatients was much older than that of the outpatients (75 vs. 70, Z = 6.55, P = 0.000, respectively). Specimens were continuously collected during the surveillance period, with an average of 20.15 specimens per month.
Fever, cough, and sputum production were the most common symptoms. However, the frequency of several symptoms varied between the outpatients and inpatients. Approximately 26.84% (164/611) of the inpatients presented with shortness of breath, and this percentage was significantly higher than that among the outpatients (52, 14.16%) (P = 0.001). In addition, more inpatients produced sputum (56.46 vs. 42.70%, P = 0.001). However, outpatients were more likely to have fever (307, 86.24%), sore throat (132, 37.8%), headache (97, 27.25%), fatigue (110, 30.90%), and runny nose (89, 25.00%) than inpatients (P = 0.000, Table 1).
Table 1.
The demographic and clinical features of the elderly with ARIs in Shanghai, China, 2012‐2015
| No. of cases (%) | ||||
|---|---|---|---|---|
| Characteristic | Total N = 967 (100) | Out patients N = 356 (36.81) | In patients N = 611 (63.19) | P‐value |
| Gender | ||||
| Male | 589 (60.91) | 180 (50.56) | 409 (66.94) | 0.000 |
| Female | 378 (39.09) | 176 (49.44) | 202 (33.06) | |
| Age, median (IQR, years) | 73 (66‐80) | 70 (64‐77) | 75 (68‐81) | |
| Age groups (years) | ||||
| 60‐69 | 357 (36.92) | 172 (48.31) | 185 (30.28) | |
| 70‐79 | 358 (37.02) | 122 (34.27) | 236 (38.63) | 0.000 |
| 80+ | 252 (26.06) | 62 (17.42) | 190 (31.10) | |
| Clinical features | ||||
| Cough | 824 (85.21) | 293 (82.30) | 531 (86.91) | 0.060 |
| Fever | 714 (73.84) | 307 (86.24) | 407 (66.61) | 0.000 |
| Sputum production | 497 (51.40) | 152 (42.70) | 345 (56.46) | 0.001 |
| Short of breath | 216 (22.34) | 52 (14.61) | 164 (26.84) | 0.001 |
| Sore throat | 157 (16.24) | 132 (37.08) | 25 (4.09) | 0.000 |
| Fatigue | 149 (15.41) | 110 (30.90) | 39 (6.38) | 0.000 |
| Headache | 116 (12.00) | 97 (27.25) | 19 (3.11) | 0.000 |
| Runny nose | 110 (11.38) | 89 (25.00) | 21 (3.44) | 0.000 |
| Chest pain | 33 (3.41) | 11 (3.09) | 22 (3.60) | 0.584 |
IQR, interquartile range.
3.2. Prevalence of viruses
Of the 967 enrolled patients, 306 (31.64%) tested positive for at least one of the eight target viruses. Of these patients, 276 presented with a single infection, whereas 30 (9.80%) were infected with more than one virus. The predominant etiologies of the respiratory viruses in single‐infection cases were influenza (137, 44.77%), HRV (44, 14.38%) and PIV (23, 7.52%). No hBoV positive samples were detected. Influenza was also the most frequently detected pathogen among multi‐infection cases; influenza plus HRV and influenza plus hMPV were the most frequent pathogen combinations among co‐infections, and these combinations were detected in five specimens (16.67%), followed by influenza plus RSV (4, 13.33%). Two patients were infected with three pathogens (Fig. 1).
Figure 1.
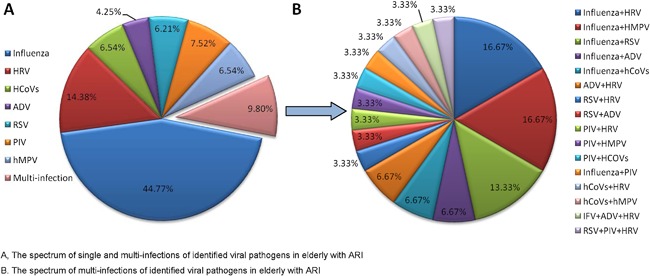
The percentages of identified viral pathogens in elderly with ARI in Shanghai, 2012‐2015
Of the 356 outpatients, 41.85% tested positive for at least one virus. This rate was much higher than that among inpatients (25.70% [157/611], P = 0.000). The most common viruses were influenza and HRV; 21.35% (76/356) and 6.18% (22/356) of outpatients tested positive for these viruses, respectively, whereas 9.98% (61/611) and 3.60% (22/611) of inpatients did so, respectively. Outpatients were more likely to test positive for influenza than inpatients (21.35 vs. 9.98%, P = 0.000). The proportion of patients with co‐infection was similar among outpatients (11, 3.09%) and inpatients (19, 3.11%, P = 0.469, Table 2).
Table 2.
The percentages of patients positive for eight viruses among the elderly with ARIs categorized by outpatient and inpatient, Shanghai, China, 2012‐2015
| Virus detected | Total (%) N = 967 | Outpatients (%) N = 356 | Inpatients (%) N = 611 | P‐value |
|---|---|---|---|---|
| Positive for anyvirus | 306 (31.64) | 149 (41.85) | 157 (25.7) | 0.000 |
| Influenza | 137 (14.17) | 76 (21.35) | 61 (9.98) | 0.000 |
| HRV | 44 (4.55) | 22 (6.18) | 22 (3.6) | 0.285 |
| hCoVs | 20 (2.07) | 9 (2.53) | 11 (1.8) | 0.405 |
| RSV | 19 (1.96) | 9 (2.53) | 10 (1.64) | 0.269 |
| PIV | 23 (2.38) | 8 (2.25) | 15 (2.45) | 0.426 |
| ADV | 13 (1.34) | 5 (1.4) | 8 (1.31) | 0.882 |
| hMPV | 20 (2.07) | 9 (2.53) | 11 (1.8) | 0.392 |
| Co‐infections | 30 (3.10) | 11 (3.09) | 19 (3.11) | 0.469 |
HRV, human rhinovirus; hCoV, human coronavirus; RSV, respiratory syncytial virus; PIV, human para‐influenza virus; ADV, adenovirus; hMPV, human metapneumovirus.
The positive percentage of influenza virus presented two seasonal peaks, with a primary peak in the winter (January‐February) and a secondary peak in the summer (July‐August), with the positive percentage of 36.09% and 20.13% respectively. The winter and summer peaks among outpatients were much higher than those among inpatients (46.75 vs. 27.17%, P = 0.008; 32.14 vs. 13.09%, P = 0.000, Fig. 2). hCoVs (9.33% [7/75]) and hMPV (10.67% [8/75]) had the highest positive percentage in March. HRV was the most frequently detected virus in November (12.90% [12/93]) and June (12.50% [8/64]). No distinct seasonal patterns were observed for PIV or RSV.
Figure 2.
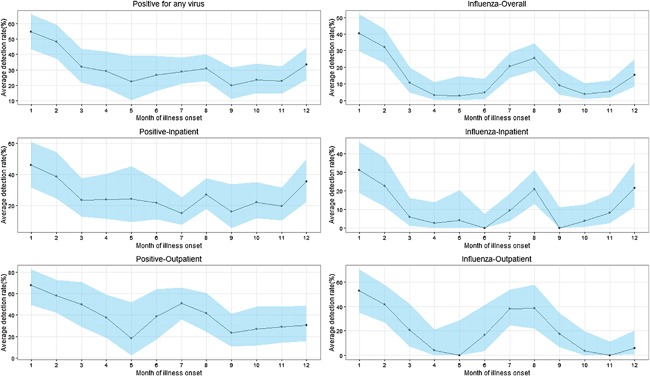
Seasonal trend of detection rates of respiratory viruses among elderly with ARI in Shanghai, 2012‐2015
3.3. Characteristics of patients with influenza
Males accounted for 53.50% of the 157 patients with influenza infection (including 137 single infections and 20 multi‐infections), whose median age was 70 years old.
Compared with the patients who tested negative for influenza, those with positive influenza were more likely to have fever (87.26 vs. 71.23%, P = 0.000), cough (94.27 vs. 83.46%, P = 0.000), sore throat (29.30 vs. 13.70%, P = 0.000) and headache (19.75 vs. 10.49%, P = 0.000). The percentage of influenza‐like illnesses (ILIs) among patients who tested positive for influenza was significantly higher than that among those who tested negative (82.17 vs. 56.42%, P = 0.000, Table 3).
Table 3.
Comparison of the characteristics between patients testing positive or negative for influenza in Shanghai, China, 2012‐2015
| No. of cases (%) | |||
|---|---|---|---|
| Characteristics | Influenza cases N = 157 | Non‐influenza cases N = 810 | P‐value |
| Gender | |||
| Male | 84 (53.50) | 505 (62.35) | 0.024 |
| Female | 73 (46.50) | 305 (37.65) | |
| Age, median (IQR, years) | 70 (65‐77) | 74 (66‐80) | 0.001 |
| Age groups (years) | |||
| 60‐69 | 69 (43.95) | 288 (35.56) | 0.016 |
| 70‐79 | 61 (38.85) | 297 (36.67) | |
| 80+ | 27 (17.20) | 225 (27.78) | |
| Clinical symptoms | |||
| Cough | 148 (94.27) | 676 (83.46) | 0.001 |
| Fever | 137 (87.26) | 577 (71.23) | 0.000 |
| ILI a | 129 (82.17) | 457(56.42) | 0.000 |
| Short of breath | 22 (14.01) | 194 (23.95) | 0.014 |
| Sore throat | 46 (29.3) | 111 (13.7) | 0.000 |
| Fatigue | 34 (21.66) | 115 (14.2) | 0.048 |
| Headache | 31 (19.75) | 85 (10.49) | 0.005 |
| Sputum production | 77 (49.04) | 420 (51.85) | 0.289 |
| Runny nose | 21 (13.38) | 89 (10.99) | 0.231 |
| Chest pain | 5 (3.18) | 30 (3.70) | 0.486 |
ILI: Influenza‐like illness: An acute respiratory infection with measured fever of ≥38°C and cough; with onset within the last 10 days.
Of all the patients with influenza, 93 (59.20%) were infected with A(H3N2), 36 (22.93%) were infected with influenza B, 27 (17.20%) were infected with A(H1N1)pdm09, and one was infected with (0.64%) A(H7N9). A(H3N2) predominated among the outpatient (60.71% [51/84]) and inpatient (57.53% [42/73]). Influenza B was more common than A(H1N1)pdm09 among outpatients (32.88 vs. 10.71%), whereas A(H1N1)pdm09 was more common among inpatient influenza patients (16.44 vs. 24.66%, respectively).
Compared with the subgroups younger than 80 years old, those older than 80 years were more likely to test positive for A(H1N1)pdm09, with a proportion of 29.63% [8/27], P = 0.007). However, A(H3N2) was more frequently detected (52.17% [36/69]) among patients between 60‐69 years than those over 70 years old (P = 0.030).
A(H3N2) was the most frequently identified strain during the study period, with the highest identified percentage (20.13% [61/303]) during the summer (July‐September) of each year. Influenza B positive cases were detected in every winter of the four surveillance years and accounted for 91.67% of the isolates collected between January and March. A(H1N1)pdm09 was primarily detected during the winters of 2014 (48.15% [13/27]) and 2015 (51.85% [14/27] Fig. 3).
Figure 3.
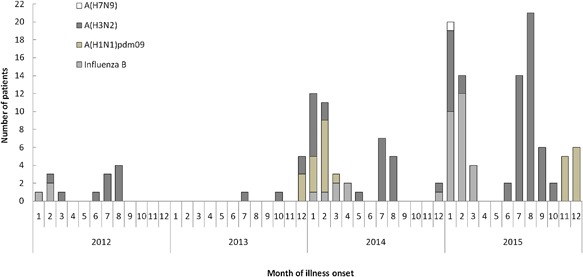
Number of influenza positive by type/subtype and by month of illness onset among elderly with ARI in Shanghai, 2012‐2015
In January 2015, the sample from a 66 years old female case was detected positive for A(H7N9). The patient presented the symptoms of fever (39.5°C), cough, phlegm, and dyspnea when admitted to hospital after 5 days of illness onset. She had a history of recent exposure to poultry and her chest radiography revealed diffuse opacities and consolidation. She died from the complications of acute respiratory distress syndrome and multiorgan failure after 7 days treatment.
4. DISCUSSION
This study identified common respiratory viruses among patients with ARI above 60 years old. Influenza was the predominant virus among both inpatients and outpatients. Despite the low number of collection at baseline, it was evidenced two seasonal peaks (in winter and summer) for influenza virus in Shanghai. Patients confirmed as having influenza infection were more likely to develop fever, cough, sore throat, and fatigue.
Similar studies have demonstrated that influenza causes the great majority of ARIs, and the impact of influenza on the elderly is substantial. More than 2/3 of influenza‐related hospitalizations and more than 80% of the influenza‐related deaths in the United States are estimated to involve the elderly.13, 14 In addition to their increased morbidity and mortality rates, the clinical features of influenza among the elderly might differ from other populations. In a study targeting older adults hospitalized with influenza, cough was the most common symptom, followed by fever.15 These results are consistent with those from our study. The seasonal pattern of influenza observed in our study was similar to the results previously reported in South China16 and the recent ILI surveillance results in Shanghai.17 In addition to ILI,18 elderly patients with influenza were more likely to develop sore throat, shortness of breath, fatigue, and headache. A(H3N2) was the most frequently detected strain in the patients with influenza enrolled in our study. A(H3N2) was the predominant local influenza subtype between 2012 and 2013,17 and this result might be affected by the effectiveness of the influenza vaccine in China. A recent study showed that the vaccine effectiveness (VE) against A(H3N2) was lower than that against the A(H1N1)pdm09 strain over those years.19
Vaccination is the most effective strategy to prevent influenza. Although elderly might experience a declining immunogenicity after vaccination due to the immunosenescence,20 they are still an important target population for influenza vaccination because of its highest influenza‐related death burden. Seasonal influenza vaccination is recommended for this population worldwide.21, 22 A quantitative review of 31 vaccine antibody response studies showed that the protection rates of influenza vaccine against A(H1N1), A(H3N2), and B‐type influenza in the elderly were 69%, 74%, and 67% respectively.23 According to a study conducted in Brazil, no influenza virus was identified during the influenza season among a group of elderly patients with ARIs, with an influenza immunization coverage of 62.30%.24 Influenza vaccines specifically targeting this population have been developed and might facilitate additional efforts to prevent influenza among the elderly.25, 26 The elderly of several Chinese cities (ie, Beijing and Ningbo) are offered free annual influenza vaccinations.19, 27, 28 However, similar public health policies have not been offered in Shanghai yet.
Previous researches had showed that most of the H7N9 cases were older people with the median age over 60 years.29, 30 One case infected with A(H7N9) was identified by our surveillance system, which was helpful to increase the timeliness of both clinical treatment and public health disposal.
Several limitations exist in this study. First, because the surveillance system has been operating for only 4 years, and the quantity of the specimens was inadequate at the beginning period, seasonal trends cannot be well described yet. However, the predominant viral pathogen in elderly with ARIs and the characteristics of these individuals was clearly revealed by our preliminary results, which provide primary background information. Second, sampling bias might be caused due on convenience sampling method. Beside, although some inpatients were excluded because they previously visited outpatient clinics, the bias caused by repeated visits remains in our study, especially among inpatients. To improve future surveillance, more information (eg, detailed clinical symptoms, complications, vaccines, and the treatment histories of the patients) should be collected to evaluate the disease burden associated with target respiratory viruses and determine the risk factors.
The findings of the current study showed that influenza is the leading viral pathogen among both elderly inpatients and outpatients with ARIs. Furthermore, two seasonal epidemic peaks were identified in Shanghai, China. An influenza vaccination strategy should be advocated for the elderly population to reduce the disease burden of influenza among this population at high risk for complicated diseases.
ACKNOWLEDGMENTS
The authors would like to thank the staff of the six sentinel hospitals in Pudong New Area for their assistance with throat swab acquisition and data collection. We also thank Dr. Luzhao Feng and Dr. Zhibin Peng from Chinese Center for Disease Control and Prevention for their assistance. This study was supported by the fund of Key Discipline Construction of Health System in Pudong New Area (PWZx2014‐14).
Ye C, Zhu W, Yu J, et al. Viral pathogens among elderly people with acute respiratory infections in Shanghai, China: Preliminary results from a laboratory‐based surveillance, 2012‐2015. J Med Virol. 2017;89: 1700–1706. 10.1002/jmv.24751
Chuchu Ye, Weiping Zhu, and Jianxing Yu contributed equally to this work.
Contributor Information
Qiao Sun, Email: qsun@pdcdc.sh.cn.
Genming Zhao, Email: gmzhao@shmu.edu.cn.
REFERENCES
- 1. Nicholson KG, Kent J, Hammersley V, Cancio E. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. BMJ. 1997; 315:1060–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO (World Health Organization). World Health Report 2004 Statistical Annex. Geneva: WHO; 2004. [Google Scholar]
- 3. Williams BG, Gouws E, Boschi‐Pinto C, Bryce J, Dye C. Estimates of world‐wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002; 2:25–32. [DOI] [PubMed] [Google Scholar]
- 4. Lieberman D, Lieberman D, Ben‐Yaakov M, Lazarovich Z, Ohana B, Friedman MG, Dvoskin B, Leinonen M, Boldur I. Infectious aetiologies in elderly patients hospitalised with non‐pneumonic lower respiratory tract infection. Age Ageing. 2003; 32:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003; 289:179–186. [DOI] [PubMed] [Google Scholar]
- 6. Nicholson KG, Abrams KR, Batham S, Medina MJ, Warren FC, Barer M, Bermingham A, Clark TW, Latimer N, Fraser M, Perera N, Rajakumar K, Zambon M. Randomised controlled trial and health economic evaluation of the impact of diagnostic testing for influenza, respiratory syncytial virus and Streptococcus pneumoniae infection on the management of acute admissions in the elderly and high‐risk 18‐ to 64‐year‐olds. Health Technol Assess. 2014; 18:1–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu T, Flöthmann EJ. The new aging society: Demographic transition and its effects on old‐age insurance and care of the elderly in China. Z Gerontol Geriatr. 2013; 46:465–475. [DOI] [PubMed] [Google Scholar]
- 8.WHO (World Health Organization). World Health Statistics (WHOSIS). 2015. Available: http://www.who.int/gho/publications/world_health_statistics/2015/en/
- 9. Cascini S, Agabiti N, Incalzi RA, Pinnarelli L, Mayer F, Arcà M, Fusco D, Davoli M. Pneumonia burden in elderly patients: A classification algorithm using administrative data. BMC Infect Dis. 2013; 13:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kobashi Y, Okimoto N, Matsushima T, Soejima R. Clinical analysis of community‐acquired pneumonia in the elderly. Intern Med. 2001; 40:703–707. [DOI] [PubMed] [Google Scholar]
- 11. Kovács G, Kaló Z, Jahnz‐Rozyk K, Kyncl J, Csohan A, Pistol A, Leleka M, Kipshakbaev R, Durand L, Macabeo B. Medical and economic burden of influenza in the elderly population in central and eastern European countries. Hum Vaccin Immunother. 2014; 10:428–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang JF, Reis BY, Hu MG, Christakos G, Yang WZ, Sun Q, Li ZJ, Li XZ, Lai SJ, Chen HY, Wang DC. Area disease estimation based on sentinel hospital records. PLoS ONE. 2011; 6:e23428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. File TM, Jr . Community‐acquired pneumonia. Lancet. 2003; 362:1991–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza‐associated hospitalizations in the United States. JAMA. 2004; 292:1333–1340. [DOI] [PubMed] [Google Scholar]
- 15. Walsh EE, Cox C, Falsey AR. Clinical features of influenza A virus infection in older hospitalized persons. J Am Geriatrsoc. 2002; 50:1498–1503. [DOI] [PubMed] [Google Scholar]
- 16. Huo X, Qin Y, Qi X, Zu R, Tang F, Li L, Hu Z, Zhu F. Surveillance of 16 respiratory viruses in patients with influenza‐like illness in Nanjing, China. J Med Virol. 2012; 84:1980–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fu Y, Pan L, Sun Q, Zhu W, Zhu L, Ye C, Xue C, Wang Y, Liu Q, Ma P, Qiu H. The clinical and etiological characteristics of influenza‐like illness (ILI) in outpatients in Shanghai, China, 2011 to 2013. PLoS ONE. 2015; 10:e0119513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO (World Health Organization). Global Epidemiological Surveillance Standards for Influenza. 2014. Available at: http://www.who.int/influenza/resources/documents/influenza_surveillance_manual/en/
- 19. Yang P, Thompson MG, Ma C, Shi W, Wu S, Zhang D, Wang Q. 2014. Influenza vaccine effectiveness against medically‐attended influenza illness during the 2012‐2013 season in Beijing, China. Vaccine. 2013; 32:5285–5289. [DOI] [PubMed] [Google Scholar]
- 20. Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2015‐16 influenza season. MMWR Morb Mortal Wkly Rep. 2015; 64:818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO (World Health Organization). Influenza‐vaccine use. 2012. Available at: http://www.who.int/influenza/vaccines/use/en/.
- 22. Feng L, Yang P, Zhang T, Yang J, Fu C, Qin Y, Zhang Y, Ma C, Liu Z, Wang Q, Zhao G, Yu H. Technical guidelines for the application of seasonal influenza vaccine in China (2014–2015). Hum Vaccin Immunother. 2015; 11:2077–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine. 2006; 24:1159–1169. [DOI] [PubMed] [Google Scholar]
- 24. Watanabe AS, Carraro E, Candeias JM, Donalísio MR, Leal E, Granato CF, Bellei N. Viral etiology among the elderly presenting acute respiratory infection during the influenza season. Rev Soc Bras Med Trop. 2011; 44:18–21. [DOI] [PubMed] [Google Scholar]
- 25. Haq K, McElhaney JE. Immunosenescence: Influenza vaccination and the elderly. Curr Opin Immunol. 2014; 29:38–42. [DOI] [PubMed] [Google Scholar]
- 26. Dunning AJ, DiazGranados CA, Voloshen T, Hu B, Landolfi VA, Talbot HK. Correlates of protection against influenza in the elderly: Results from an influenza vaccine efficacy trial. Clin Vaccine Immunol. 2016; 23:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu Y, Wu JY, Wang X, Chen JT, Xia M, Hu W, Zou Y, Yin WD. Review of 10 years of clinical experience with Chinese domestic trivalent influenza vaccine Anflu® . Hum Vaccin Immunother. 2014; 10:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qin Y, Zhang Y, Wu P, Feng S, Zheng J, Yang P, Pan Y, Wang Q, Feng L, Pang X, Puig‐Barberà J, Yu H, Cowling BJ. 2016. Influenza vaccine effectiveness in preventing hospitalization among Beijing residents in China, 2016‐15. Vaccine. 2016; 34:2329–2333. [DOI] [PubMed] [Google Scholar]
- 29. Cowling BJ, Jin L, Lau EH, Liao Q, Wu P, Jiang H, Tsang TK, Zheng J, Fang VJ, Chang Z, Ni MY, Zhang Q, Ip DK, Yu J, Li Y, Wang L, Tu W, Meng L, Wu JT, Luo H, Li Q, Shu Y, Li Z, Feng Z, Yang W, Wang Y, Leung GM, Yu H. Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: A population‐based study of laboratory‐confirmed cases. Lancet. 2013; 382:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li Q, Zhou L, Zhou M, Chen Z, Li F, Wu H, Xiang N, Chen E, Tang F, Wang D, Meng L, Hong Z, Tu W, Cao Y, Li L, Ding F, Liu B, Wang M, Xie R, Gao R, Li X, Bai T, Zou S, He J, Hu J, Xu Y, Chai C, Wang S, Gao Y, Jin L, Zhang Y, Luo H, Yu H, He J, Li Q, Wang X, Gao L, Pang X, Liu G, Yan Y, Yuan H, Shu Y, Yang W, Wang Y, Wu F, Uyeki TM, Feng Z. Epidemiology of human infections with avian influenza A(H7N9) virus in China. N Engl J Med. 2014; 370:520–532. [DOI] [PMC free article] [PubMed] [Google Scholar]


