Abstract
Objectives
Bronchitis is a common respiratory tract infection of humans mainly caused by influenza virus, rhinovirus, adenovirus, coronavirus and respiratory syncytial virus. The aim of this review was to gather fragmented literature on ethnomedicinal plants used against bronchitis in the Himalayan region and their in-vitro validation against bronchitis causing viral pathogens.
Key findings
Present review contains ethnomedicines of total 55 plants from different countries of the Himalayas. Most of the literature reported was from India followed by Pakistan, China and Nepal. Familiarly used plant families for bronchitis treatment in the Himalayan region were Leguminosae (six plants) and Lamiaceae (five plants). Leaves and roots were the most common parts used in ethnomedicines against bronchitis. Of these 55 plants, only six plants have been studied in vitro against viral pathogens causing bronchitis. Different compounds like monoterpenoids, flavonoids, triterpenoids, iridoid glycosides, sesquiterpenes, benzoic and phenolic compounds were reportedly isolated from these plant extracts having strong antiviral potential.
Summary
The Himalayan regions possess variety of ethnomedicinal plants used against respiratory diseases, but still there are only few studies related with their in-vitro validation. We invite the attention of researchers for detailed ethnopharmacological and phytochemical studies on unexplored plants used to treat bronchitis for the development of novel antiviral drugs.
Keywords: antiviral activity, bronchitis, the Himalaya region, traditional knowledge
Introduction
Bronchitis is a respiratory disease of humans, in which mucous membrane of the bronchial tubes become inflamed that carry oxygen from trachea to lungs. The main aetiological agents for this disease are viruses including influenza, rhinoviruses, adenoviruses, coronavirus and respiratory syncytial virus[1,2] while some bacteria including Mycoplasma pneumoniae, Chlamydophila pneumoniae and Bordetella pertussis account for about 10% of cases.[3] Cigarette smoking, air pollution, occupational exposure to dust particles, irritants, fumes, polluted air particles and biomass fuels may also cause bronchitis.[4] Bronchitis is slowly progressive and largely irreversible disease with clinical signs and symptoms like sore throat, runny nose, airway obstruction, tiredness, sensation of tightness, burning or dull pain in the chest under the breast bone, shortness of breath, fever, sputum production and sputum tenacity.[5] According to World Health Organization report, respiratory tract infections are foremost cause of deaths among all contagious disease.[6] Viral diseases are life-threatening diseases with high degree of complications to the humans because of its rapid outbreak throughout the developing world.[7–9] Treatment of viral diseases is great challenge for the peoples even today because of different reasons like easily adaptations of virus, emergence of resistant viral pathogens, development of new viral strains, high cost and side effects of medicine, and host resistance to antiviral drugs.[10,11] Antibiotics are ineffective against viral infections because viral envelope and set of replicating machinery are completely different than that of bacteria.[3] The common treatment for viral diseases includes antiviral drugs that do not destroy their target pathogen; instead, they inhibit their development, shorten infection and help prevent complications.[12] Bacteria involved in causing bronchitis have also shown resistance against commonly used antibiotics (azithromycin, clarithromycin, tosufloxacin, minocycline, rifamycins and telithromycin) against respiratory tract infections.[13,14] In recent years, emergence of resistant potential of viral and bacterial pathogens against commonly used drugs particularly for respiratory tract infections has increased, which is a major public health problem and great hurdle in the treatment of these diseases.[12,15] Generally, resistance of viral pathogens to chemotherapy has been shown in immune-compromised individuals.[16,17] The pathogenic resistance patterns and high cost of antiviral drugs encourage the use of medicinal plants all over the world.[18]
In Asia, the medicinal plant species are abundantly found in the Himalayan forests that play a key role in the rural livelihood, by supplying diversity of valuable products for food and medicine.[19] The Himalayan region is the largest mountain system blessed with large number of valuable medicinal plants because of its unique topographical, ecological, geographical, climatic and physiographical conditions.[20] The Himalayan medicinal plants have very immense historical background, earliest evidences and records of using plants as medicines originated from the Himalayas and can found in old texts of ‘Rigveda’ written 6500 year ago.[21] Local healers of the region have tremendous skills and knowledge of preparing traditional medicines for treating different kind of ailments. There is no exact figure about the number of the Himalayan medicinal plants being use for primary health care and livelihood, but most of the researchers documented that more than 10 000 Himalayan medicinal plants are supporting about 600million people in the region.[22]
Approximately 70–80% people worldwide depend on medicinal plants to cure various human ailments including viral diseases.[12] Herbal drugs have gained much importance due to their easily adaptability, low cost and fewer side reactions on patients.[23] Various traditional medicine systems like Ayurveda and Chinese medicine have gained popularity and used in various parts of the world.[24] Most of the people in the Himalayan region used medicinal plants traditionally to treat various diseases including bronchitis. Medicinal plants are considered as an important source of various bioactive compounds having antiviral properties, yet fewer studies have been done on antiviral potential of medicinal plants.[15]
Literature is limited regarding the Himalayan medicinal plants used against viral diseases especially bronchitis. Main objectives of the present review were to gather utmost fragmented literature on the ethnomedicinal plants used to treat bronchitis in the Himalayan region and their in-vitro activity against viral pathogens and phytochemistry. This review will provide baseline information to the researchers about potential antiviral medicinal plants and will also disclose the scientific gaps in current knowledge that could lead towards the development of novel drugs for the welfare of human beings.
Methodology
Published data on traditionally used medicinal plants for bronchitis and their antiviral activity were collected from the online bibliographical databases: PubMed, Scopus, Google Scholar, Web of Science and floras of the Himalayan countries. Inside the databases, we used the keywords like medicinal plants, Himalaya, antiviral plant extracts and isolated compounds for bronchitis. Plant databases such as ‘Tropicos’ and ‘The Plant List’ (http://www.theplantlist.org) were searched for plant accepted names, synonyms and families.[25] Total 87 articles were reviewed for this study, which were mostly published in English. We selected only those articles, in which complete information was given regarding the traditional use of medicinal plants in the Himalayan region and their antiviral activity. Two tables were developed using Microsoft Excel 2007 and Microsoft Word 2007. Table 1 contains information on the ethnomedicinal uses of selected plants used to treat bronchitis in the Himalayan region including information such as plant name, family, local name, habit, part used, study area and recipe formulation. Table 2 consisted of antiviral activity of plant extracts, concentration (μg/ml), inhibition (%) and active compounds tested against viral pathogens. Chemical structures of compounds were drawn using ChemDraw software (CambridgeSoft\ChemOffice2004\ChemDraw).
Table 1.
Medicinal plants used to treat bronchitis in the Himalayan region
| Plant/Family/Local name | Study area | Habit/Part used | Recipe |
|---|---|---|---|
| Abies pindrow (Royle ex D. Don) Royle/Pinaceae/Raisul[30] | Nepal, Garhwal Himalayas | Tree/Bark | Extract of bark is given orally |
| Acacia nilotica (L.) Delile/Leguminosae/Babool[59] | Bageshwar Valley (Kumaun Himalayas) of Uttarakhand, India | Tree/Bark | Bark's extract is used |
| Achyranthes aspera L./Amaranthaceae/Lich Kura[30] | Garhwal Himalayas | Herb/Whole Plant | Plant decoction is given |
| Achyranthes bidentata Blume/Amaranthaceae/Kuru[59] | Bageshwar Valley (Kumaun Himalayas) of Uttarakhand, India | Herb/Root | Juice of root is used |
| Acorus calamus L./Acoraceae/Bojho, Katara, Bach[60] | Terai Forest in western Nepal | Herb/Root | Juice of root is given orally |
| Angelica glauca Edgew./Apiaceae/Chura[61] | Kashmir Himalaya | Herb/Root | Roots are used and combined with tonics for bronchitis |
| Artemisia indica Willd./Asteraceae/Tite Pati[60] | Terai Forest in western Nepal | Herb/Leaves | Leaf juice is given orally |
| Barleria cristata L./Acanthaceae/Porcupine Flower, Barleria[36] | Pauri Garhwal Himalayas | Shrub/Root | Root decoction is given |
| Betula utilis D. Don/Betulaceae/Bhojpatra, Bhooj, Himalayan Silver Birch[62] | Himachal Pradesh | Tree/Bark | Infusion of bark is given |
| Bidens pilosa L./Asteraceae/Blackjack, Spanish Needle[36] | Pauri Garhwal Himalayas | Herb/Whole Plant | Plant extract along with honey is given |
| Boerhavia diffusa L./Nyctaginaceae/Pundera[30] | Garhwal Himalayas | Herb/Whole Plant | Plant infusion is given |
| Bombax ceiba L./Malvaceae/Simal, Semar[60] | Terai Forest in western Nepal | Tree/Bark | Bark decoction is given orally |
| Cannabis sativa L./Cannabaceae/Bhang[39,59] | Vaidyas in Ukhimath Block, Uttarakhand | Herb/Leaves, Seeds | Leaves and seed extract with pepper, cumin seeds and cardamom is given |
| Cassia fistula L./Leguminosae/Amaltas, Raj Briksha[46] | Garhwal Himalayas | Tree/Fruit | Fruit pulp is given |
| Celastrus paniculatus Willd./Celastraceae/Sankhiran[62] | Himachal Pradesh, India | Shrub/Seeds | Powder of seeds is given |
| Desmodium gangeticum (L.) DC./Leguminosae/Shalparni[59] | Bageshwar Valley (Kumaun Himalayas) of Uttarakhand, India | Herb/Root | Extract of root is given |
| Desmodium elegans DC./Leguminosae/Bhatul[59] | Bageshwar Valley (Kumaun Himalayas) of Uttarakhand, India | Herb/Whole Plant | Extract of plant is used |
| Ephedra gerardiana Wall. ex Stapf/Ephedraceae/Ephedra, Somlata[63,64] | Pakistan, Himachal Pradesh | Shrub/Whole Plant | Powder of the crushed plant and some time its tea is used for relaxation of bronchial muscles |
| Euphorbia hirta L./Euphorbiaceae/Jatli-Dodal[65] | Sewa River Catchment Area in the north-west Himalaya | Herb/Whole Plant | Decoction of plant is used |
| Euphorbia neriifolia L./Euphorbiaceae/Sheund[66] | Tarai Region of Kumaun, Uttarakhand, India | Shrub/Whole Plant | Cooked vegetable is given |
| Hedychium spicatum Sm./Zingiberaceae/Ban-Haldi, Pankha Phool, Sara[30,40] | Garhwal Himalayas, Uttarakhand | Herb/Rhizome | Rhizome extract is taken orally |
| Hibiscus rosa-sinensis L./Malvaceae/Gurhal[59] | Bageshwar Valley (Kumaun Himalayas) of Uttarakhand, India | Shrub/Stem, Flower | Extract of stem and flower is used |
| Hippophae rhamnoides L./Elaeagnaceae/Buru[67] | Gilgit District of northern Pakistan | Shrub/Fruit | Syrup made from fruit mixed with Morus nigra L. fruit and sugar is used for bronchial congestion |
| Hyoscyamus niger L./Solanaceae/Henbane, Khorasani Ajwain[62] | Himachal Pradesh, India | Herb/Seeds | Seeds is principally employed as sedative in bronchitis |
| Indigofera tinctoria L./Leguminosae/Neel[65] | Sewa River Catchment Area in the north-west Himalaya | Shrub/Whole Plant | Extract of plant is used |
| Jurinea ceratocarpa (Decne.) Benth. & Hook.f./Compositae/Turjit, Chholmong[68] | Nubra Valley – a cold arid zone of Himalaya | Shrub/Leaves | Leaf extract is given |
| Justicia adhatoda L./Acanthaceae/Asuro, Ross, Benkar, Brehankar, Baiker[60,70] | Terai Forest in western Nepal, Allai Valley, western Himalaya, Pakistan | Shrub/Whole Plant | Dried powder of entire plant parts is given. Roots and leaves are either given in decoction or powder form |
| Lantana camera L./Verbenaceae/Phoolwari[70] | Kumaun Himalayas of Uttarakhand | Shrub/Whole Plant | Decoction is given |
| Musa balbisiana Colla./Musaceae/Ban Kera[71] | Sikkim Himalayas of India | Herb/Leaves | Syrup is made from leaves and given |
| Myrica esculenta Buch.-Ham. ex D. Don/Myricaceae/Kafal, Kaphal[30] | Nepal Himalaya | Tree/Bark | Bark decoction is used |
| Myrtus communis Linn/Myrtaceae/Manoo[69] | Allai Valley, western Himalaya, Pakistan | Shrub/Whole Plant | Plant is taken internally in the treatment of bronchial congestion |
| Oberonia falconeri Hook.f./Orchidaceae/Hirvi Chapti Amri[30] | Garhwal Himalayas | Herb/Whole Plant | Plant extract is given |
| Ocimum sanctum L. (Ocimum tenuiflorum L.)/Lamiaceae/Tulsi[70] | Kumaun Himalayas of Uttarakhand | Herb/Leaves | Leave juice is used |
| Ocimum basilicum L./Lamiaceae/Niazbo[51,72] | Morgah Biodiversity Park, Rawalpindi, China |
Herb/Leaves | Decoction of leaves is given |
| Origanum vulgare L./Lamiaceae/Ban Tulsi[61] | Western Himalaya, India | Herb/Leaves | Tea is made from leaves and used |
| Oroxylum indicum (L.) Kurz/Bignoniaceae/Sonpatha[66] | Tarai Region of Kumaun, Uttarakhand, India |
Tree/Bark | Decoction of bark is given |
| Papaver somniferum L./Papaveraceae/Posht[37] | Kashmir Himalaya, India | Herb/Flowers | Syrup made from sepals and ovary given to the kids for the cure of bronchitis |
| Perilla frutescens (L.) Britton/Lamiaceae/Bhang, Jeera[30] | Garhwal Himalayas | Herb/Whole Plant | Plant extract or powder of dried plant parts is used |
| Pinus roxburghii Sarg./Pinaceae/Chir Pine[36] | Pauri Garhwal Himalayas | Tree/Bark | Saw dust is given |
| Piper Longum L./Piperaceae/Pipla[71] | Sikkim Himalayas of India | Herb/Fruit | Mature and dried fruits are taken orally |
| Plantago major L./Plantaginaceae/Jabai[69] | Allai Valley, western Himalaya, Pakistan | Herb/Roots | Decoction of roots is used |
| Punica granatum L./Lythraceae/Anar[27,73] | Neelam Valley, Azad Jammu Kashmir, Kumaun Himalayas of Uttarakhand | Shrub/Whole Plant | Decoction of flower bud is given |
| Rheum nobile Hooker. f. & Thomson/Polygonaceae/Kenjo[74,75] | Himachal Pradesh India | Herb/Roots | The watery extract of roots is given orally |
| Rhododendron anthopogon D. Don/Ericaceae/Dhoopi[75] | Himachal Pradesh India | Shrub/Flowers, Leaves | Given orally |
| Tagetes minuta L./Asteraceae/Genda[65] | Sewa River Catchment Area in the north-west Himalaya | Herb/Flowers | Volatile oil of flower is used |
| Taxus wallichiana Zucc./Taxaceae/Himalayan Yew[76] | India, Nepalese Himalaya | Tree/Leaves | Leaves extract is given |
| Terminalia chebula Retz./Combretaceae/Harad[59] | Bageshwar Valley (Kumaun Himalayas) of Uttarakhand, India | Herb/Fruit | Fruit powder is used |
| Thymus linearis Benth./Lamiaceae/Ban-Ajwain[77] | Himachal Pradesh, India | Herb/Whole Plant | Plant infusion or syrup is used |
| Toona ciliate M. Roem/Meliaceae/Toon[70] | Kumaun Himalayas of Uttarakhand | Tree/Bark | The extract of stem bark is given |
|
Trifolium pratense L./Leguminosae/Trepatra, Chita-Batta[67] |
Gilgit District of northern Areas | Herb/Flower | Powder of dried flowers is given |
| Urtica dioica L./Urticaceae/Jalbang[69] | Allai Valley, western Himalaya | Herb/Roots, Leaves | The juice of the roots or leaves mixed with honey or sugar is given |
| Verbascum thapsus L./Scrophulariaceae/Mullein, Akalbir, Cows Lungwort[30] | Garhwal Himalayas | Herb/Whole Plant | Plant extract is used |
| Viola pilosa Blume./Violaceae/Kaura[30] | Garhwal Himalayas | Herb/Whole Plant | Decoction of plant is given orally |
| Viola canescens Wall./Violaceae/Banafsha[75] | Neelum Valley, Azad Jammu Kashmir | Herb/Leaves, Flowers | The decoction of leaves and flowers is given |
| Zingiber officinale Roscoe./Zingiberaceae/Aduwa, Suntho[60] | Terai Forest of western Nepal | Herb/Rhizome | Rhizome is chewed in bronchial infections |
Table 2.
In-vitro screening of antiviral plants against viruses causing bronchitis
| Plant name | Part used | Extract/ Compound/ Control drug | Chemical class | %age of composition of major comp. in the extracts | Concentration (μg/ml) | Inhibition (%) | Viral type | Techniques |
|---|---|---|---|---|---|---|---|---|
| Hyoscyamus niger | Flower | Methanol | 18.7 | 40 | 50 | Influenza A | Dye uptake assay[78] | |
| Acyclovir | No effect | |||||||
| Amantadine HC | 16.8 | 50 | ||||||
| Justicia adhatoda | Leaves | Methanol | Vasicine Alkaloids | 0.026 | 10 000 | 100 | Influenza | Simultaneous assay[50] |
| Aqueous | 0.023 | 5000 | 16.67 | |||||
| 10 000 | 33 | |||||||
| Ocimum basilicum | Whole plant | Aqueous | 174.1 | 50 | Adenovirus (ADV-3) | Cytopathic effect reduction assay[28] | ||
| Ethanol | >1000 | |||||||
| Linalool | Monoterpenoids | >95 | 24.4 | |||||
| Apigenin | Flavonoids | >95 | 11.1 | |||||
| Ursolic acid | Triterpenoids | >95 | >200 | |||||
| 2′,3′-Dideoxycytidine | 26.7 | 50 | ||||||
| Aqueous | 129.6 | 50 | Adenovirus (ADV-8) | |||||
| Ethanol | >200 | |||||||
| Linalool | >95 | 26.4 | ||||||
| Apigenin | >95 | 8.0 | ||||||
| Ursolic acid | >95 | 4.2 | ||||||
| 2′,3′-Dideoxycytidine | 12 | 50 | ||||||
| Aqueous | 129.1 | 50 | Adenovirus (ADV-11) | |||||
| Ethanol | 91.9 | |||||||
| Linalool | >95 | 16.9 | ||||||
| Apigenin | >95 | 20.9 | ||||||
| Ursolic acid | >95 | 24.5 | ||||||
| Hot aqueous | 2500 | 32 | ||||||
| Methanol | 78 | 28 | ||||||
| Hydro-methanol | 1250 | 30 | ||||||
| 2′,3′-Dideoxycytidine | 28.2 | 50 | ||||||
| Plantago major | Whole plant | Aqueous | >1000 | 50 | Adenovirus (ADV-3) | XTT assay [10] | ||
| Aucubin | Iridoid glycoside | NA | >200 | |||||
| Baicalein | Flavonoids | >20 | ||||||
| Baicalin | >50 | |||||||
| Luteolin | >25 | |||||||
| Caffeic acid | Phenolic compounds | 14.2 | ||||||
| Chlorogenic acid | 76.0 | |||||||
| Ferulic acid | >100 | |||||||
| p-coumaric acid | >200 | |||||||
| Oleanolic acid | Triterpenoids | >40 | ||||||
| Ursolic acid | >20 | |||||||
| Vanillic acid | Benzoic compounds | >200 | ||||||
| ddC | 5.3 | |||||||
| Aqueous | >1000 | 50 | Adenovirus (ADV-8) | |||||
| Aucubin | >200 | |||||||
| Baicalein | >20 | |||||||
| Baicalin | >50 | |||||||
| Caffeic acid | >200 | |||||||
| Chlorogenic acid | 108 | |||||||
| Ferulic acid | 52.5 | |||||||
| Luteolin | >25 | |||||||
| Oleanolic acid | >40 | |||||||
| p-coumaric acid | >200 | |||||||
| Ursolic acid | >20 | |||||||
| Vanillic acid | >200 | |||||||
| ddC | 10 | 50 | ||||||
| Aqueous | >1000 | 50 | Adenovirus (ADV-11) | |||||
| Aucubin | >200 | |||||||
| Baicalein | >20 | |||||||
| Baicalin | >50 | |||||||
| Caffeic acid | >200 | |||||||
| Chlorogenic acid | 13.3 | |||||||
| Ferulic acid | 23.3 | |||||||
| Luteolin | >25 | |||||||
| Oleanolic acid | >40 | |||||||
| p-coumaric acid | 43.8 | |||||||
| Ursolic acid | >20 | |||||||
| Vanillic acid | >200 | |||||||
| ddC | 14 | |||||||
| Verbascum thapsus | Aerial parts | Methanol | Phenylethanoid and lignan glycosides | 6.25 | 50 | Influenza A | Dye uptake assay [50] | |
| Zingiber officinale | Rhizome | ar-curcumene | Sesquiterpenes | 20.4 | 50 | Rhinovirus IB | Plaque reduction test [30] | |
| β-Sesquiphellandrene | 0.90 | |||||||
| α-Zingiberene | 1.90 | |||||||
| β-Bisabolene | 14.3 | |||||||
| Flavan | Flavonoids | 0.27 | ||||||
| 4, 6-Dichloroflavan | 0.02 | |||||||
| Flavan 5 | 0.02 |
Discussion
Ethnomedicinal plants used to treat bronchitis in the Himalayas
The Himalayan region is considered to be the rich source of medicinal plants. Local peoples have strong traditional beliefs regarding high efficacy of ethnomedicinal plants used to treat bronchitis. Traditional healers believe that ethnomedicinal plants are more effective with fewer side effects as compared to modern allopathic drugs. Present review shows that 38 families are being used in the Himalayan belt across the different countries. Most of studies related to traditional uses have been carried out in India while rarely from Pakistan, China and Nepal due to less availability of published data (Table 1). Possible reasons for the high availability of ethnomedicinal data from India are; medicinal plants from India have been reported to be very useful in curing various human ailments including bronchitis, rich medicinal plants diversity as Himalaya region is mostly covered by India,[26] most popular traditional systems of this country like Ayurveda, Siddha and Unani, strong traditional believe and dependency of local community on medicinal plants for curing diseases.[26] The most widely used plant families for the treatment of bronchitis in the Himalayan region are Leguminosae (six plants), Lamiaceae (five plants) and Asteraceae (four plants). High number of medicinal plants in these families might be due to their higher abundance in the study area, presence of most active secondary metabolites like flavonoids in the Leguminosae[27]; monoterpenoids, flavonoids and triterpenoids of Lamiaceae[28]; and sesquiterpene lactones in the Asteraceae.[29] Plants belonging to these families are not only used to treat bronchitis but also for many other human diseases in the Himalayan region.[30–32] These families are not only preferred in the Himalayan region but in other countries as well for medicinal purposes due to antimicrobial activity of their plants.[33,34] Medicinal plants used to treat bronchitis in the Himalayan region are mostly herbs (58%) as compared to shrubs (24%) and trees (18%) because they are easily collected, their higher abundance and efficacy against different human pathogens and popularity in traditional medicine system.[35,36] Mostly whole plants are used traditionally in recipe formation to treat bronchitis, which is major threat to the conservation status of these medicinal plants. Almost all plant parts are found to have antiviral activity but most preferred plant parts used are leaves, roots, bark and flowers (Table 1). Different additives like water, sugar and honey are used in the recipe formation in order to reduce the bitter taste of plants as well as to reduce their toxic effects.[37,38] Mixture of some plants is also used for recipes formulation in order to increase their efficacy due to synergistic effect of various compounds in these plants.[39,40] As an example, leaves and seed extract of Cannabis sativa with pepper, cumin seeds and cardamom are given orally to treat bronchitis (Table 1). Powder, infusion, decoction and extract are common techniques for recipe formation in the Himalayan region. The most preferred techniques are decoction and extract in the study area that might be due to their easy administration or efficacy of dissolved bioactive compounds in extract.
Antiviral activity of isolated compounds from plants
Data available on the Himalayan plants show that of 55 ethnomedicinally used plants, only six plants have been reportedly studied for their in-vitro activity against viral pathogens causing bronchitis. Whole plant and its different parts like leaves, flowers, aerial parts and rhizomes have been used for the preparation of extracts. Different solvents like aqueous, ethanol and methanol have been reportedly used for the extract preparation (Table 2). Methanol and ethanol are most preferable solvents for plant extraction due to their polar nature that ensures the release of wide variety of bioactive compounds from plants. Plants extracted with these solvents have been reported for their good antimicrobial activity.[41,42] The plant extracts have been taken in different concentrations ranging from 6.25 μg/ml to 10 000 μg/ml, which showed strong inhibition ranges from 16.67% to 100% against adenovirus and influenza virus. Cos et al.[43] studied the anti-infective potential of natural product and defined the criteria for good activity as 100 μg/ml for extracts and essential oils while 10 μg/ml for pure compounds and drugs. Among various techniques used for the detection of viral inhibition, dye uptake assay and cytopathic effect reduction assay were the most common techniques (Table 2). These techniques are also used in other countries for in-vitro antiviral study.[44,45] Zingiber officinale has been investigated using plaque reduction test. In plaque reduction assay, crude fractions (pg per plate) were derived from the dose–response curve and from this the specific activity in units lg, where one unit of activity (1 u) was defined as the activity that reduced the number of plaques by 50% as compared to the virus controls. Flavan 5 was used as the positive control in the plaque reduction test.[30]
Medicinal plants are the good source of various chemical compounds that provide basis for the development of new chemotherapeutic agents against various human pathogens.[10,46] The mechanism of antiviral potential of plants extract or compounds varies among different viruses. Some phytochemical compounds of plants target viral envelope or membrane protein; others inhibit the formation of viral genome or stop attachment of virus to the host cellular machinery for reproduction while some destroy enzymes necessary for viral encoding.[15,47]
Acute respiratory infections (ARI) remain serious problem and leading cause of death in young children throughout the world especially in developing countries. Most frequently reported viruses include rhinovirus, respiratory syncytial virus, influenza virus A and bocavirus. Anders et al.[48] demonstrated a high burden of ARI during the first year of life in southern Vietnam. Emerging drug-resistant potential of influenza virus led towards identification of novel antiviral agents. First-generation influenza antiviral agents such as amantadine and rimantadine act on viral M2 protein and stated as ion channel blockers. However, their side effects are associated with patient nervous system and gastrointestinal tract. Moreover, rapid emergence of viral resistance limited the benefits and usefulness of adamantanes in the prevention and treatment of influenza. Complementary and alternative medicines with ethnopharmacological background suggest novel platform for the development of antiviral drugs. Antiviral potential compounds present in medicinal plants work either alone or in synergistic manner. Effectiveness of several medicinal plants extracts is directly associated with synergistic properties of therapeutically active compounds and their derivatives. However, slight cytotoxicity observed in plant extracts investigated might be due to the presence of cytoprotective components. These phytocompounds and extracts could serve as potential source for the development of innovative antiviral drugs. Present review reported plant extracts likely to be promising candidates in the race of developing third-generation anti-influenza drugs, thus challenging the neuraminidase drug-resistant viruses in an effort to protect human health and the global economy.[49]
Present review showed that the Himalayan plants contain several bioactive substances like monoterpenoids, flavonoids, triterpenoids, iridoid glycosides, sesquiterpenes, benzoic and phenolic compounds having strong antiviral activity. Among traditionally used medicinal plants of the Himalayan region, phytochemicals of only four plants (Justicia adhatoda, Ocimum basilicum, Plantago major and Zingiber officinale) have been directly checked against viral pathogens (Table 2).
Justicia adhatoda L
Justicia adhatoda is a well-known plant in Ayurvedic and Unani medicine, a shrub which is widespread throughout the tropical regions of south-east Asia. Leaves of this plant have been used extensively for the treatment of respiratory disorders. Simultaneous assay of leaves showed 33% reduction at a concentration of 10 000 μg/ml in aqueous extract and 16.67% reduction was observed from 1000 μg/ml to 5000 μg/ml, whereas 100% reduction was observed in methanolic extract at a concentration of 10 000 μg/ml. As the concentration decreased, inhibition also found to be decreased from 33.34% at 1 mg/ml to 16.67% at 5000 μg/ml. These data suggest that aqueous and methanolic extracts may directly interfere with protein envelope of viruses and not with sialic acid receptor at the cell surface. Only methanolic extract inhibited influenza virus infection by blocking viral attachment and inhibiting viral hemagglutinin (HA) protein. Vasicine alkaloids have been isolated from leaves, with percentage composition of 0.026% in methanol and 0.023% in aqueous extract which may be responsible for activity.[50]
Ocimum basilicum L
Sesquiterpenoid compounds like farnesol, caryophyllene, flavonoids such as apigenin (Figure 1), monoterpenes like linalool (Figure 2), cineole, fenchone, carvone, myrcene, geraniol and thujone, and triterpenoids such as ursolic acid (Figure 3) have been reportedly isolated from Ocimum basilicum. These compounds have been reported to inhibit various viral infections of DNA and RNA viruses.[28,51] Only three types of secondary metabolites apigenin, linalool and ursolic acid have been checked directly against different types of human adenovirus involved in bronchitis. Among these ursolic acid showed maximum inhibition (50%) against adenovirus (ADV-8) at minimum concentration (4.2 μg/ml). Composition of all the isolated compounds is >95%. 2′,3′-Dideoxycytidine is used as positive control and similar activity of ursolic acid (Table 2). Triterpenoid saponins of oleanane group inhibited the viral activity by inhibiting DNA synthesis, while urasane group inhibited viral protein capsid.[52] Other compounds need further investigation against viral pathogens involved in bronchitis. Toxicological studies showed that higher doses of plant cause stomach irritation.[53]
Figure 1.
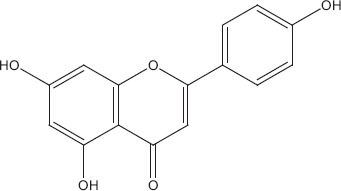
Apigenin.[79]
Figure 2.
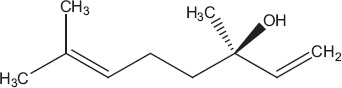
Linalool.[80]
Figure 3.
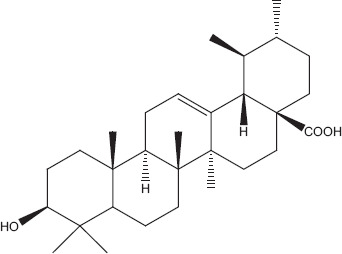
Ursolic acid.[81]
Plantago major L
Phytochemicals isolated from Plantago major contain five classes of biologically active compounds: aucubin (Figure 4) belongs to class iridoid glycoside, baicalein, baicalin and luteolin are derivatives of flavons (Figure 5), and vanillic acid (Figure 6) belongs to benzoic compounds and derivatives of cinnamic acid (Figure 7) like caffeic acid, chlorogenic acid, ferulic acid, p-coumaric acid, triterpenes ursolic acid (Figure 3) and oleanolic acid (Figure 8). In-vitro antiviral activity of these compounds has been checked against three types of human adenovirus at different concentrations. Although all compounds showed similar inhibition against adenovirus, but chlorogenic acid and caffeic acid had shown 50% inhibition against adenovirus at minimum concentration of about 13.3 and 14.2 μg/ml, respectively (Table 2). The strong antiviral activity of phenolic compounds might be correlated with the presence of two hydroxyl groups on chlorogenic acid and caffeic acid than ferulic acid and p-coumaric acid with only one hydroxyl group. Phenolic compounds found effective for their antiviral activity not only against adenoviruses but also against many other viral pathogens.[10,46] Many bioactive chemical classes like flavonoids, triterpenes, sesquiterpenes, phenolic and benzoic compounds isolated from many other plants have been investigated for their antiviral activity.[10,15,28,47,51] Toxicological studies showed no toxicity of methanolic extract with doses up 2 g/kg body weight in an acute toxicity assay. Contrary to this result, another study suggested that oral administration of an aqueous extract (1000 mg/kg body weight) of leaves had shown highest reduction in the total acidity of gastric fluid in rats.[54] Another study reported that oral administration of aqueous extract (2000 mg/kg) of Plantago major for total 40 days did result in mortality of model organism.[52]
Figure 4.
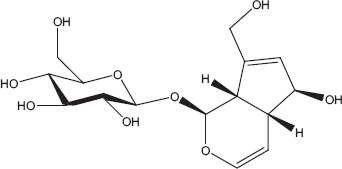
Aucubin.[82]
Figure 5.
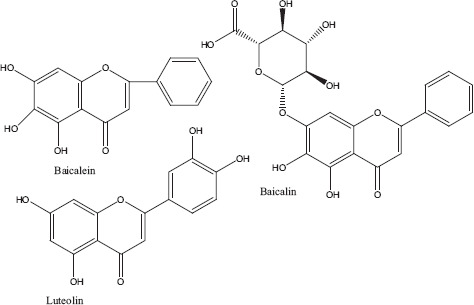
Derivatives of flavons.[83]
Figure 6.
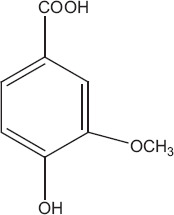
Vanillic acid.[84]
Figure 7.
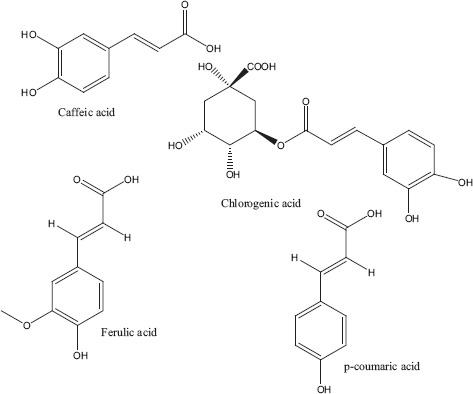
Derivatives of cinnamic acid.[10]
Figure 8.
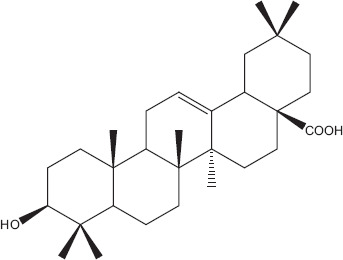
Oleanolic acid.[85]
Zingiber officinale Roscoe
Three types of sesquiterpene derivatives (Figure 9) ar-curcumene, β-sesquiphellandrene, α-zingiberene and β-bisabolene and two types of flavonoids (Figure 10) flavan and 4, 6-dichloroflavan have been isolated from Z. officinale and investigated for their antiviral activity. All these compounds showed 50% inhibition against viral pathogens at different concentrations. 4, 6-Dichloroflavan was considered to be the most effective compound as it showed 50% inhibition against rhinovirus at very lowest concentration about 0.02 μg/ml (Table 2). The lipophilic nature of 4, 6-dichloroflavan has been attributed to its antirhinoviral activity as Z. officinale is known to contain various lipophilic secondary metabolites.[30] Dichloroflavan isolated from other natural products has also been investigated against rhinovirus and showed its strongest ability to bind with most sensitive rhinovirus IB at their receptor sites. Many bioactive compounds isolated from higher plants have been checked for their antiviral activity particularly for their antirhinoviral activity. Available data showed that most of the antirhinoviral activity of plants attributed to the presence of flavonoids compounds.[55,56] Administration of aqueous extracts either individually or in combination altered the relative weights of the heart, liver and kidney of the animals which is an indication of a toxic effect.[57,58]
Figure 9.
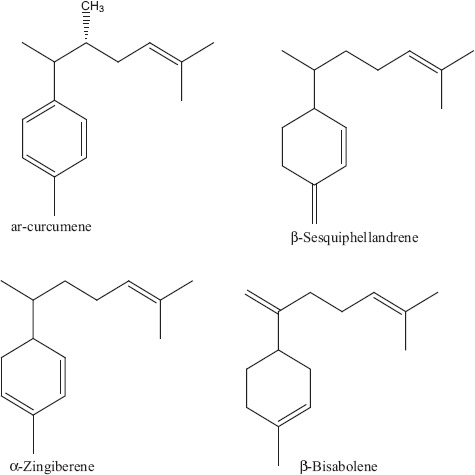
Derivatives of sesquiterpenes.[30]
Figure 10.
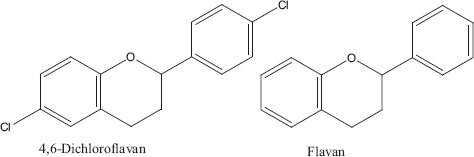
Flavonoids.[55]
Verbascum thapsus L
Methanolic extract of Verbascum thapsus showed strong efficacy about 50% at minimum concentration of 6.25 μg/ml against influenza A virus. Phenylethanoid and lignan glycosides have been isolated from methanolic extract of V. thapsus. Phytoconstituents responsible for anti-influenza viral activity depend on the amount of active compounds present in the plants which alternatively depends on the geographical distribution, collected season, climatic and ecological condition at the site of plant collection. Further fractionation and separation of extract(s) may reveal potent antiviral activity from this plant. No toxicity has been observed for higher doses.[53]
Conclusions and Future Considerations
The Himalayan region contains variety of medicinal plants traditionally being used by the local people to treat bronchitis. However, literature is scanty regarding recipe formulation techniques, effective dosage and side effects of the Himalayan medicinal plants used against bronchitis. Therefore, it is necessary to carry out detailed ethnomedicinal studies covering all aspects which could be helpful for the patients and researchers. In addition, more focus should be given to widely distributed plants that might lead towards extraction of novel compounds due to geographical variation. Most of the ethnomedicinal studies were reported from India, and it is highly recommended that research activity in regard to antiviral medicinal plants should also be carried out in other countries of the Himalayan region. Very few plants have been screened in vitro and in vivo against viral pathogens involved in bronchitis. It is imperative to screen unexplored plants in detail not only for their in-vitro and in-vivo activity but also for their phytochemistry, toxicology and mechanism of actions.
Declarations
Conflicts of interest
The Authors have no conflicts to report.
Disclosure statement
The study had no ethical approval requirements.
Acknowledgements
The authors are indebted to the departmental colleagues for support in the development of this manuscript.
References
- Peiris JS et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 2003; 361: 1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandra P et al. Detection and typing by molecular techniques of respiratory viruses in children hospitalized for acute respiratory infection in Rome Italy. J Med Virol 2007; 79: 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabale AS. Vital ethnomedicinal plant species exploited in treating bronchitis. Indian J Appl Res 2014; 4: 30–32. [Google Scholar]
- Lanzetti M et al. Mate tea reduced acute lung inflammation in mice exposed to cigarette smoke. Nutrition 2008; 24: 375–381. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev 2007; 87: 1047–1082. [DOI] [PubMed] [Google Scholar]
- Beaglehole R et al. The World Health Report 2004 – changing history. J Adv Nurs 2004; 48: 542–542. [Google Scholar]
- Whitley RJ. HSV infections of women and their offspring: implications for a developed society. Proc Natl Acad Sci USA 1994; 91: 2441–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila MM et al. Viral etiology in acute low respiratory infections in children from a closed community. Am Rev Respir Dis 1989; 140: 634–637. [DOI] [PubMed] [Google Scholar]
- Sathya B. A primitive approach on review of Siddha herbs, herbo-mineral formulation exhibiting antiviral activity. Int J Pharma Bio Sci 2014; 5: 138–147. [Google Scholar]
- Chiang LC. Antiviral activity of Plantago major extracts and related compounds in vitro. Antiviral Res 2002; 55: 53–62. [DOI] [PubMed] [Google Scholar]
- Peera C, Efferth T. Antiviral medicinal herbs and phytochemicals. J Pharmacogn 2012; 3: 1106–1118. [Google Scholar]
- Wang X, Liu Z. Prevention and treatment of viral respiratory infections by traditional Chinese herbs. Chin Med J 2014; 127: 1344–1350. [PubMed] [Google Scholar]
- Cao Bin et al. High prevalence of macrolide resistance in Mycoplasma pneumoniae isolates from adult and adolescent patients with respiratory tract infection in China. Clin Infect Dis 2010; 51: 189–194. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H et al. Chlamydia pneumoniae resists antibiotics in lymphocytes. Antimicrob Agents Chemother 2003; 47: 1972–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruwali P et al. Antiviral potential of medicinal plants, an overview. Int Res J Pharm 2013; 4: 8–16. [Google Scholar]
- Zahradnik JM et al. Adenovirus infection in immunocompromised patients. Am J Med 1980; 68: 725–732. [DOI] [PubMed] [Google Scholar]
- Field HJ. Herpes simplex virus antiviral drug resistance-current trends and future prospects. J Clin Virol 2001; 21: 261–269. [DOI] [PubMed] [Google Scholar]
- Sumithra P et al. Antiviral and antioxidant activities of two medicinal plants. Int J Curr Sci 2012; 256–261. [Google Scholar]
- Kala CP. Ethnomedicinal botany of the Apatani in the eastern Himalayan region of India. J Ethnobiol Ethnomed 2005; 1: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kala CP, Mathur VB. Patterns of plant species distribution in the trans-Himalayan region of Ladakh, India. J Veg Sci 2002; 13: 751–754. [Google Scholar]
- Malla SB, Shakya PR. Medicinal plants. In: Majupuria TC, ed. Nepal Nature's Paradise. Bangkok, Thailand: White Lotus Co. Ltd, 1984: 261–297. [Google Scholar]
- Shengji P. Ethnobotanical approaches of traditional medicine studies: some experiences from Asia. Pharm Biol 2001; 39: 74–79. [DOI] [PubMed] [Google Scholar]
- Edziri H et al. Antiviral activity of leaves extract of Marrubium alysson L. J Med Plants Res 2011; 5: 360–363. [Google Scholar]
- Hudson JB. The use of herbal extract in use control of influenza. J Med Plants Res 2009; 3: 1189–1195. [Google Scholar]
- http://www.Theplantlist.org
- Gangwar RS, Joshi BD. Some medicinal flora in the riparian zone of river Ganga at Saptrishi Haridwar Uttaranchal. Himalayan J Environ Zool 2006; 20: 237–241. [Google Scholar]
- Chew YL et al. Assessment of phytochemical content, polyphenolic composition, antioxidant and antibacterial activities of Leguminosae medicinal plants in Peninsular Malaysia. BMC Complement Altern Med 2011; 11: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang LC et al. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin Exp Pharmacol Physiol 2005; 32: 811–816. [DOI] [PubMed] [Google Scholar]
- Fischer NH et al. The biogenesis and chemistry of sesquiterpene lactones. In: Herz W et al. eds Progress in the Chemistry of Organic Natural Products. Vienna: Springer-Verlag, 1979, 38: 47–320. [Google Scholar]
- Radha B et al. Diversity and availability status of ethno-medicinal plants in the lohba range of Kedarnath Forest Division (KFD), Garhwal Himalaya. Glob J Res Med Plants Indig Med 2013; 2: 198–212. [Google Scholar]
- Qureshi SJ et al. A survey of useful medicinal plants of Abbottabad in Northern Pakistan. J Sci 2008; 6: 39–51. [Google Scholar]
- Marilena M et al. Impedance measurements to study the antimicrobial activity of essential oils from Lamiaceae and Compositae. Int J Food Microbiol 2001; 67: 187–195. [DOI] [PubMed] [Google Scholar]
- Stephanie I et al. Pharmacological activities and biologically active compounds of Bulgarian medicinal plants, phytochemistry. Adv Res 2006; 2: 87–103. [Google Scholar]
- Rathore B et al. Indian herbal medicines: possible potent therapeutic agents for rheumatoid arthritis. J Clin Biochem Nutr 2007; 41: 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad H et al. Ethnobotanical study of upper Siran. J Herbs Spices Med Plants 2009; 15: 86–97. [Google Scholar]
- Nazir A et al. Traditional uses of medicinal plants of Pauri Garhwal Uttarkhand. Nat Sci 2010; 8: 57–61. [Google Scholar]
- Firdous A et al. Ethno-medicinal survey of Kajinaag range of Kashmir Himalaya, India. Int J Pharma Bio Sci 2012; 3: 442–449. [Google Scholar]
- Semwal DP et al. Medicinal plants used by local Vaidyas in Ukhimath block Uttarakhand. Indian J Tradit Knowl 2010; 9: 480–485. [Google Scholar]
- Bisht VK et al. Traditional use of medicinal plants in district Chamoli Uttarakhand, India. J Med Plants Res 2013; 7: 918–929. [Google Scholar]
- Lourens ACU et al. In vitro biological activity and essential oil composition of four indigenous South African Helichrysum species. J Ethnopharmacol 2004; 9: 253–258. [DOI] [PubMed] [Google Scholar]
- Parekh J et al. Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity. Turk J Biol 2005; 29: 203–210. [Google Scholar]
- Richa S. Antiviral activity of crude extracts of Eugenia Jambolana Lam against highly pathogenic avian influenza (H5N1) virus. Indian J Exp Biol 2012; 50: 179–186. [PubMed] [Google Scholar]
- Cos P et al. Anti-infective potential of natural products: how to develop a stronger in vitro ‘proof-of-concept’. J Ethnopharmacol 2006; 106: 290–302. [DOI] [PubMed] [Google Scholar]
- Waye MMY, Sing CW. Antiviral drugs for human Adenoviruses. Pharmaceuticals 2010; 3: 3343–3354. [Google Scholar]
- Harbourne JB. Phytochemical Methods, A Guide to Modern Techniques of Plant Analysis. London: Chapman and Hall, 1984. [Google Scholar]
- Jassim SAA, Naji MA. Novel antiviral agents: a medicinal plant perspective. J Appl Microbiol 2003; 95: 412–427. [DOI] [PubMed] [Google Scholar]
- Duke JA. Handbook of Phytochemical Constituents of GRAS Herbs and Other Economic Plants. Boca Raton, FL: CRC Press, 1992. [Google Scholar]
- Anders KL et al. Epidemiology and virology of acute respiratory infections during the first year of life: a birth cohort study in Vietnam. Pediatr Infect Dis J 2015; 34: 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran D et al. Identification of traditional medicinal plant extracts with novel anti-influenza activity. PLoS One 2013; 8: 79293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavan R, Chowdhary A. In vitro inhibitory activity of Justicia adhatoda extracts against influenza virus infection and hemagglutination. Int J Pharm Sci Rev Res 2014; 25: 231–236. [Google Scholar]
- Lin YM. Antiviral activities of biflavonoids. Planta Med 1999; 65: 120–125. [DOI] [PubMed] [Google Scholar]
- Garcia GM et al. Sub-chronic toxicity and test of eye irritability of leaf aqueous extract from Plantago major (Plantaginaceae). Rev Biol Trop 2003; 51: 635–638. [PubMed] [Google Scholar]
- Escobar FM et al. Genotoxic evaluation of a methanolic extract of Verbascum thapsus using micronucleus test in mouse bone marrow. Nat Prod Commun 2011; 6: 989–991. [PubMed] [Google Scholar]
- Kobeasy MI et al. Gastroprotective effect of Plantago major L. against gastric injury induced by aspirin in rats. J Chem Acta 2013; 2: 86–91. [Google Scholar]
- Denyer CV et al. Isolation of antirhinoviral sesquiterpenes from ginger (Zingiber Offcinale). J Nat Prod 1994; 57: 658–662. [DOI] [PubMed] [Google Scholar]
- Bauer DJ et al. 4′, 6-Dichloroflavan (BW683C): a new anti-rhinovirus compound. Nature (London) 1981; 292: 369–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plengsuriyakarn T et al. Cytotoxicity, toxicity, and anticancer activity of Zingiber officinale roscoe against cholangiocarcinoma. Asian Pac J Cancer Prev 2012; 13: 4597–4606. [DOI] [PubMed] [Google Scholar]
- Sulaiman FA et al. Antimicrobial and toxic potential of aqueous extracts of Allium sativum, Hibiscus sabdariffa and Zingiber officinale in wistar rats. J Taibah Univ Sci 2014; 8: 315–322. [Google Scholar]
- Singh P, Attri BL. Survey on traditional uses of medicinal plants of Bageshwar valley (Kumaun Himalaya) of Uttarakhand, India. Int J Conserv Sci 2014; 5: 223–234. [Google Scholar]
- Singh AG et al. An ethnobotanical survey of medicinal plants used in Terai forest of western Nepal. J Ethnobiol Ethnomed 2012; 8: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht C, Badoni A. Medicinal strength of some alpine and sub-alpine zones of Western Himalaya India. New York Sci J 2009; 2: 41–46. [Google Scholar]
- Devi U, Thakur M. Exploration of ethno botanical uses of some wild plants from cold desert of Himachal Pradesh. Asian J Exp Biol Sci 2011; 2: 362–366. [Google Scholar]
- Arti S et al. Studies on traditional knowledge of ethnomedicinal plants in Jawalamukhi Himachal Pradesh, India. Int Res J Biological Sci 2014; 3: 6–12. [Google Scholar]
- Khan SM et al. Medicinal flora and ethnoecological knowledge in the Naran Valley, Western Himalaya, Pakistan. J Ethnobiol Ethnomed 2013; 9: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M et al. Medicinal plants of Sewa river catchment area in the Northwest Himalaya and its implication for conservation. Ethnobot Lealf 2009; 13: 13–39. [Google Scholar]
- Mathur A, Joshi H. Ethnobotanical studies of the Tarai region of Kumaun Uttarakhand, India. Ethnobot Res Appl 2013; 11: 175–203. [Google Scholar]
- Qureshi RA et al. Ethnobotanical studies of medicinal plants of Gilgit district and surrounding areas. Ethnobot Res Appl 2006; 5: 115–122. [Google Scholar]
- Kumar PG et al. Ethnobotanical studies of Nubra Valley – a cold arid zone of Himalaya. Ethnobot Leaflets 2009; 13: 752–765. [Google Scholar]
- Haq F. The ethno botanical uses of medicinal plants of Allai Valley, Western Himalaya Pakistan. Int J Plant Res 2012; 2: 21–34. [Google Scholar]
- Singh HB et al. Folk medicinal plants in the Sikkim Himalayas of India. Asian Folklore Stud 2002; 61: 295–310. [Google Scholar]
- Joshi M et al. Ethnomedicinal uses of plant resources of the Haigad watershed in Kumaun Himalaya India. Med Aromatic Plant Sci Biotechnol 2010; 4: 43–46. [Google Scholar]
- Husain SZ et al. Ethnobotanical properties and uses of medicinal plants of Morgah biodiversity park, Rawalpindi. Pak J Bot 2008; 40: 1897–1911. [Google Scholar]
- Ahmad KS, Habib S. Indigenous knowledge of some medicinal plants of Himalaya Region, Dawarian Village Neelum Valley Azad Jammu and Kashmir, Pakistan. Univers J Plant Sci 2014; 2: 40–47. [Google Scholar]
- Lepcha SR, Das AP. Ethno-medico-botanical exploration along the international borders to Tibet Autonomous Region of China and the kingdom of Bhutan with special reference to the Pangolakha Wildlife Sanctuary, East Sikkim. Recent Studies in Biodiversity and Traditional Knowledge in India Gour Mahavidyalaya, Malda 2011; 7: 257–270. [Google Scholar]
- Kharwal AD, Rawat DS. Ethnobotanical studies and distribution of different Rhododendron species in Himachal Pradesh, India. Plant Sci Feed 2013; 3: 46–49. [Google Scholar]
- Rahman S et al. Taxus wallichiana Zucc (Himalayan Yew): insights on its anti-microbial and pharmacological activities. Altern Med 2013; 1: 1–3. [Google Scholar]
- Sharma PK, Lal B. Ethnobotanical on some medicinal and aromatic plants of Himachal Pradesh. Indian J Tradit Knowl 2005; 4: 424–428. [Google Scholar]
- Rajbhandari M et al. Antiviral activity of some plants used in Nepalese traditional medicine. Adv Access Publ 2009; 6: 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beate I. Structural determinants for activation and block of CFTR-mediated chloride currents by apigenin. Am J Physiol Cell Physiol 2000; 279: 838–846. [DOI] [PubMed] [Google Scholar]
- Chnag MY, Shen YL. Linalool exhibits cytotoxic effects by activating antitumor immunity. Molecules 2014; 19: 6694–6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taralkar SV, Chattopadhyay S. A HPLC method for determination of ursolic acid and betulinic acids from their methanolic extracts of Vitex negundo Linn. J Anal Bioanal Tech 2012; 3: 1–6. [Google Scholar]
- Johanna S. Extraction of iridoid glycosides and their determination by micellar electrokinetic capillary chromatography. J Chromatogr 2000; 868: 73–83. [DOI] [PubMed] [Google Scholar]
- Yasunori N. Effects of Scutellariae radix extract and its components (Baicalein, Baicalin and Wogonin) on the experimental elevation of aqueous flare in pigmented rabbits. Jpn J Ophthalmol 2001; 45: 216–220. [DOI] [PubMed] [Google Scholar]
- Maria LAR et al. Degradation of vanillic acid and production of guaiacol by microorganisms isolated from cork samples. FEMS Microbiol Lett 2003; 220: 49–55. [DOI] [PubMed] [Google Scholar]
- Jie L. Review article, Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol 1995; 49: 57–68. [DOI] [PubMed] [Google Scholar]


