1. Summary, 759
2. Introduction, 759
3. Epidemiological features, 760
3.1 Viral gastroenteritis, 760
3.1.1 Norwalk‐like viruses, 761
3.1.2 Rotavirus, 762
3.1.3 Astrovirus, 762
3.2 Hepatitis, 762
3.2.1 Hepatitis A, 762
3.2.2 Hepatitis E, 763
4. Properties of foodborne viruses, 763
5. Detection, 763
6. Routes of contamination, 764
7. Survival, 764
7.1 Survival of viruses in water, 764
7.2 Survival of viruses in soil, 765
7.3 Survival of viruses on surfaces, 765
7.4 Survival of viruses on fruits and vegetables, 766
8. Fresh produce washing, 767
8.1 Susceptibility of foodborne viruses to disinfectants and processing aids used in the fresh produce industry, 767
8.2 Chlorine, 767
8.3 Chlorine dioxide, 767
8.4 Organic acids, 768
8.5 Surfactants, 768
8.6 Ozone, 768
9. Control, 768
10. Conclusions, 769
11. Acknowledgements, 770
12. References, 770
1. SUMMARY
Raw and minimally processed fruits and vegetables are typically sold to the consumer in a ready‐to‐use or ready‐ to‐eat form. These products do not generally contain preservatives or antimicrobial agents and rarely undergo any heat processing prior to consumption. For many years raw fruits and vegetables have been implicated as vehicles for transmission of infectious micro‐organisms. Although fresh produce can support the growth and/or survival of many pathogenic bacteria there is little published information on the stability of human pathogenic viruses on these food products. Viruses cannot grow in or on foods but may sometimes be present on fresh produce as a result of faecal contamination. This contamination can arise at source in the growth and harvesting area from contact with polluted water and inadequately or untreated sewage sludge used for irrigation and fertilization. Alternatively, fruits or vegetables handled by an infected person might become contaminated with virus and transmit infection. The most frequently reported foodborne viral infections are viral gastroenteritis and hepatitis A: both have been associated with the consumption of fresh fruit or vegetables.
2. INTRODUCTION
In recent years it has been recognized that viruses are an important cause of foodborne disease. Unlike bacteria, viruses do not grow or multiply in or on foods, but foods may become contaminated with human viruses and transmit infection. There are many groups of viruses which could contaminate food items, but the major foodborne viral pathogens are those that infect via the gastrointestinal tract, such as the gastroenteritis viruses and hepatitis A virus. It is these viruses that are the main subject of this review.
Viruses that infect via the gastrointestinal tract are excreted in faeces and may also be present in vomit. Foods become contaminated either directly by infected people or through sewage pollution. Those enteric viruses, which are commonly associated with foodborne outbreaks, either cannot be cultured in the laboratory or can only be cultured with difficulty. Hence information and experimental studies on survival and recovery of viruses from foods often relates to other virus types that are readily cultured. Some of these viruses may be good models for important foodborne viral pathogens, but others may have widely differing characteristics. Enteroviruses, such as Coxsackieviruses and vaccine strains of poliovirus, have been used particularly. They infect via the gastrointestinal tract, occur in the environment as a result of sewage contamination and are relatively stable. However, there are very few published reports of foodborne outbreaks of illness caused by enteroviruses.
There is the potential for contamination with other virus groups, such as respiratory viruses, although transmission to humans through foods has not been recognized. Infrequently endogenous contamination of meat and milk can occur with viruses such as tick‐borne encephalitis. Such zoonotic infections have been reviewed by Sattar and Tetro (2001) and need not be considered in this review.
3. EPIDEMIOLOGICAL FEATURES
Viruses are usually transmitted directly from person to person, but epidemiological and laboratory investigations indicate that viral diseases can on occasions be transmitted via foods, particularly those that are minimally processed. These include seafood, especially the bivalve molluscan shellfish, and fresh fruits, vegetables and salad items. It might be expected that any virus that infects via the gastrointestinal tract could be foodborne. In practice, however, the most commonly reported foodborne viral infections are viral gastroenteritis and less frequently hepatitis A.
It is believed that the incidence of both is greatly under reported, but for different reasons. Viral gastroenteritis is a relatively mild disease and most people do not consult a medical practitioner; hence, the majority of cases are not investigated and are not reported. A recent national study funded by the Department of Health of infectious intestinal disease (IID) in the community indicated that for every case of IID detected by national laboratory surveillance, there are 136 cases in the community. The study also concluded that a smaller proportion of cases due to common viral pathogens are reported than cases due to common bacterial pathogens (Wheeler et al. 1999; Food Standards Agency, 2000). Viral gastroenteritis has a short incubation period of 1–4 days depending on the type of virus. This means that when cases are investigated, the possibility of foodborne transmission is likely to be considered. In contrast, hepatitis A is often a more severe disease and is more likely to be reported. However, the incubation period is 3–6 weeks and hence an association with a food source is unlikely to be made, unless there is a very clearly defined outbreak.
3.1 Viral gastroenteritis
Several different viruses cause gastroenteritis: the most important include rotavirus, the small round‐structured viruses (SRSV) otherwise known as Norwalk‐like viruses (NLV), astrovirus and adenovirus types 40 and 41. However, in almost all foodborne outbreaks where a virus is identified, it is an NLV. Rotavirus and astrovirus are only rarely implicated. Adenovirus has not been associated with food or waterborne transmission.
Viral gastroenteritis is usually regarded as a mild self‐limiting disease lasting 24–48 h. However, people can feel debilitated for 2 or 3 weeks, which has considerable economic implications in terms of working days lost and impaired performance. Symptoms include malaise, abdominal pain, pyrexia, diarrhoea and/or vomiting. A range of symptoms occurring in an outbreak should alert investigators to the possibility of a viral cause. The viruses are usually transmitted by the faecal–oral route, but they are also present in vomitus. Onset of viral gastroenteritis may be sudden and can commence with projectile vomiting. Virus will be disseminated over a wide area in aerosol droplets, which is a particular hazard where food is being prepared and laid out. Although most transmission is directly from person to person, contaminated food and water can give rise to common source outbreaks. The infective doses are not known, but the evidence from volunteer studies and the typically high attack rates observed in outbreaks suggest that they are very low (Westwood and Sattar 1976; Caul et al. 1988). For instance, it has been estimated that NLVs have an infective dose of between 10 and 100 virus particles (Caul 1994; Stolle and Sperner 1997).
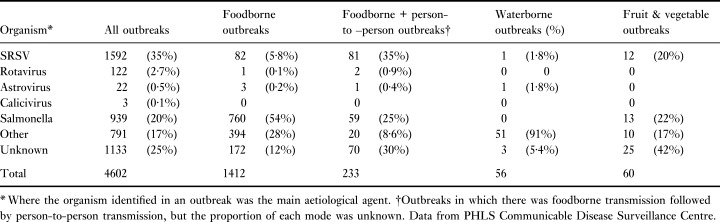
Viruses account for 6% of foodborne and 5% of waterborne outbreaks occurring in England and Wales reported to the PHLS Communicable Disease Surveillance Centre (CDSC) (Appleton, 2000; O’Brien et al. 2000) (Table 1). A recent paper prepared by the Public Health Laboratory Service (PHLS) reported on the microbiological status of ready‐to‐eat fruit and vegetables between 1992 and 1999 in England and Wales (O’Brien et al. 2000). Fruit and vegetables accounted for 4·3% (60/1408) of the total foodborne outbreaks reported during that period. The most commonly identified aetiological agent was the NLVs, which were linked to 20% of the outbreaks. The causative organism of a further 42% of outbreaks was reported as unknown, although the clinical and epidemiological features of these outbreaks suggested that the majority (64%) were also viral.
Table 1.
Outbreaks of infectious intestinal disease in England and Wales 1992–99
3.1.1 Norwalk‐like viruses.
This group of viruses infects all age groups. There is a variable incubation period of 12–60 h, which is thought to be dose‐dependent. It occurs all year round, although in temperate climates most infections occur over the winter months. These viruses are responsible for both sporadic cases of gastroenteritis in the community and for outbreaks in schools, hospitals, old people’s homes, hotels and cruise ships. The national IID study indicated that viruses are the most common cause of IID in the community, with NLV the most frequently reported organism (Wheeler et al. 1999; Food Standards Agency, 2000). From 1992 to 1997, NLVs accounted for one‐third of all gastroenteritis outbreaks reported to the PHLS Communicable Disease Surveillance Centre (CDSC) and the number of outbreaks of NLV gastroenteritis exceeded the number of outbreaks of salmonellosis. Unlike the salmonellosis outbreaks, however, only 6% of the NLV outbreaks were known to be food or waterborne (Appleton, 2000). The virus is extremely infectious and secondary cases are a characteristic feature of foodborne outbreaks. Therefore, it is not always possible to determine whether illness is acquired from a foodborne source or by person‐to‐person transmission (see Table 1). This accounts partly for under‐recognition of the extent of foodborne transmission of these viruses. In a review of NLV (SRSV) infection in England and Wales between 1990 and 1995 by the PHLS, direct foodborne transmission could be ascertained in only 14% of outbreaks (Dedman et al. 1998).
There have been reports of outbreaks where NLVs have been epidemiologically associated with various items of fresh produce, such as washed salads (Lieb 1985; Lo et al. 1994), imported frozen raspberries (Ponka et al. 1999), coleslaw (Currier 1996), green salads (Griffin et al. 1982), fresh cut fruits (Herwaldt et al. 1994) and potato salad (Patterson et al. 1997).
The virus was discovered in 1972 by electron microscopy (Kapikian et al. 1972) (Fig. 1). The first virus originated from the town of Norwalk in the United States, and became the prototype of the group. The name small round‐structured virus (SRSV), which describes the morphology of the virus particle (Caul and Appleton 1982, Appleton, 2000), was used in the United Kingdom and elsewhere until recently. It has now been agreed informally by virologists working with this group to adopt the name Norwalk‐like virus, for the present, so as to bring some conformity to the nomenclature of these viruses. However, formal names have not yet been agreed by the International Committee on Virus Nomenclature. The NLVs form a complex group of viruses. They have formally been classified with the Caliciviridae and are often referred to as human caliciviruses. They are split into two broad genogroups. Most viruses in these two groups have the typical morphology of a 30–35 nm diameter particle with an amorphous surface and ragged outline, as originally described for the SRSV group (Caul and Appleton 1982). Within the Caliciviridae there is a second genus of human gastroenteritis viruses, provisionally named Sapporo‐like viruses (SLVs). The SLVs have the morphology of classical caliciviruses and are genomically distinct from the NLVs (Green et al. 2000). On sequencing, some strains with classical calicivirus morphology fall into the NLV genus. There are suggestions that the epidemiology of SLVs differs from NLVs, in that SLVs mainly cause infections of young children. It is not clear whether SLVs are of significance in foodborne infections and more studies are needed to clarify their role. Genotypic analysis is being used increasingly to investigate the epidemiology of these two groups of viruses (Fankhauser et al. 1998; Maguire et al. 1999; Hale et al. 2000; Hedlund et al. 2000; Koopmans et al. 2000). There are several serotypes of human caliciviruses, which correspond broadly with the genotypic groups. Most studies have been carried out with NLVs, and indicate that immunity is complex and short‐lived (Matsui and Greenberg, 2000). Volunteer studies have shown that people can be infected repeatedly with the same virus strain (Parrino et al. 1977; Johnson et al. 1990).
Figure 1.
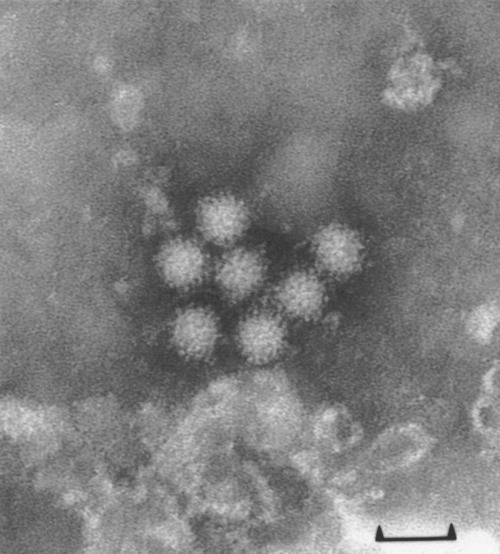
Electron micrograph of Norwalk‐like viruses negatively stained with phosphotungstic acid. Bar represents 50 nm
3.1.2 Rotavirus.
Rotaviruses mainly infect young children (Sattar et al. 2001). It is estimated that they cause one million deaths a year in children under 5 years of age, mostly in developing countries. In developed countries deaths are relatively rare, but rotavirus gastroenteritis is the most frequent reason for admission of young children to hospital. Rotaviruses consistently account for around 80% of all gastroenteritis viruses reported to CDSC, although this figure is probably biased by reports of hospitalized children, rather than the occurrence in the community, where NLVs are of greater significance. Foodborne and particularly waterborne spread are probably a significant route of transmission in developing countries, but in developed countries reports are rare (Table 1).
3.1.3 Astrovirus.
The astroviruses form a morphologically distinct group of viruses, and are named from the five‐ or six‐point star seen by electron microscopy on the surface of some particles. Astroviruses have mainly been associated with illness in young children, often under 1 year of age. Reports of astrovirus infection in older children and adults are infrequent, although outbreaks have been reported in the elderly. This may reflect testing policy: detection normally relies on electron microscopy, which is insensitive and often not performed on sporadic samples from adults. The use of more sensitive molecular detection methods is required to assess the incidence and epidemiology of these viruses. Astroviruses have been seen in some adults following the consumption of shellfish or contaminated water, but these incidents appear to be comparatively rare (Kurtz and Lee 1987; Kurtz 1994) (Table 1).
3.2 Hepatitis
There are two forms of enterically transmitted hepatitis – hepatitis A and hepatitis E (Cromeans et al. 2001).
3.2.1 Hepatitis A.
The most characteristic symptom of hepatitis A is jaundice, but milder symptoms of nausea and general malaise without jaundice are common. Patients may feel unwell for several weeks, but recovery is complete. Deaths are rare. Some infections, particularly in children, may be asymptomatic. Like viral gastroenteritis, transmission is by the faecal–oral route, but the primary site of viral replication is the liver. Virus excretion may commence up to a week before symptoms are apparent, making control difficult.
The epidemiology of foodborne hepatitis A is essentially similar to that of viral gastroenteritis. Food‐ and waterborne outbreaks have been recognized for over 40 years, but are infrequently reported. Epidemiological evidence to link hepatitis infection to food and water sources is sparse, because of the long incubation period. Between 1992 and 1997, 228 outbreaks occurring in England and Wales were reported to CDSC, but only one of these was known to be foodborne. That outbreak was associated with shellfish. From 1992 to 1999, CDSC received 19 747 laboratory‐confirmed reports of cases of hepatitis A. The source of most of these infections was unknown and just 155 were recorded as foodborne. In the United States, the Centers for Disease Control and Prevention (CDC) placed hepatitis A as the sixth leading cause of foodborne disease from 1988 to 1992 (Centers for Disease Control and Prevention 1996).
Outbreaks associated with fresh produce, particularly soft fruits and salads, have been reported from several countries. Iceberg lettuce (Rosenblum et al. 1990), strawberries (Niu et al. 1992), diced tomatoes (Williams et al. 1995) and salad items (Pebody et al. 1998) have all been implicated. A recent outbreak of hepatitis A in the United States was associated with consumption of food items containing frozen strawberries imported from Mexico (Anon 1997; Cliver 1997). In the United Kingdom, outbreaks have been traced to frozen raspberries. There was evidence that the raspberries in these outbreaks were contaminated by infected fruit‐pickers (Noah 1981; Reid and Robinson 1987; Ramsay and Upton 1989).
There is only one serotype of hepatitis A. Following infection immunity is lifelong. An effective vaccine is available. Currently it is used for persons at high risk, such as travellers (Department of Health et al. 1996). Food packagers and food handlers in the United Kingdom have not been associated with HAV transmission sufficiently often to justify routine immunization, except in outbreaks. The incidence of hepatitis A in developed countries has fallen in recent years and hence a susceptible population has built up. As endemic infection declines, it is possible that an increase in foodborne outbreaks will be seen. The year‐round global distribution of fruit and vegetable products poses a risk of infection, particularly when these products are imported from countries with a high incidence of hepatitis A.
3.2.2 Hepatitis E.
Hepatitis E has been associated with large waterborne outbreaks in some developing countries, notably in Asia, Africa and Central America. Foodborne transmission has been suggested, but not proved conclusively. Illness appears more severe than hepatitis A, particularly in pregnant women where a death rate of 17–33% has been observed (Cromeans et al. 2001). The primary source of infection appears to be contaminated water rather than person‐to‐person spread. Secondary person‐to‐person transmission is estimated at only 0·7–8% (Cromeans et al. 2001). Cases in the United Kingdom are reported infrequently and are mainly imported from endemic areas. With the worldwide distribution of foods, vigilance should be maintained.
4. PROPERTIES OF FOODBORNE VIRUSES
Viruses are very small micro‐organisms, and basically comprise a nucleic acid core of either DNA or RNA, surrounded by a protein coat. They require living cells in order to replicate and generally have a very restricted host range. Viruses do not multiply in foods or water, or in or on any other environmental sample. However, viruses can survive outside living cells and remain infectious.
Enteric viruses are hardy and survive well in the environment. These viruses survive on inanimate surfaces, on hands and in dried faecal suspensions (Green et al. 1998a; Barry‐Murphy et al. 2000; Bidawid et al. 2000; Sattar et al. 2000). Lingering outbreaks have occurred in hospitals, in residential homes and on cruise ships, probably as a result of environmental contamination. NLVs have been detected by PCR in environmental swabs from hospital lockers and hotel carpets supposedly cleaned after incidents of vomiting (Green et al. 1998a). The viruses survive just as well on kitchen surfaces and food preparation areas. In one reported outbreak, a kitchen worker vomited into a sink. The following day the sink, which had been cleaned with a chlorine‐based disinfectant, was used for washing salad and an outbreak of gastroenteritis associated with NLVs ensued (Patterson et al. 1997).
Enteric viruses are acid stable and so are able to survive in the gastrointestinal tract. It is likely that they will survive food processes designed to produce the low pH that inhibits bacterial spoilage organisms (e.g. pickling in vinegar and fermentation processes that produce foods such as yoghurt). Both NLVs and hepatitis A virus retain activity after exposure to acidity levels below pH 3 (Dolin et al. 1972; Scholtz et al. 1989).
Most viruses remain infectious after refrigeration and freezing. Frozen foods, that have not received further cooking, have been implicated in a number of incidents of viral gastroenteritis and hepatitis A (Noah 1981; Reid and Robinson 1987; Ramsay and Upton 1989; Niu et al. 1992). Gastroenteritis viruses and hepatitis A virus are inactivated by conventional cooking processes, but retain their infectivity after heating to 60°C for 30 min (Dolin et al. 1972; Parry and Mortimer 1984; Fleming et al. 1985; Millard et al. 1987; Slomka and Appleton 1998). It is uncertain whether they would be inactivated completely in some pasteurization processes.
5. DETECTION
NLVs cannot be cultured in the laboratory and until recently detection relied on the use of electron microscopy. This technique is fairly insensitive and requires a minimum of 106 virus particles per ml of sample. It has been used widely for detection of virus in faecal samples from patients, but cannot be used for looking for the lower concentration of virus particles present in contaminated food, water and environmental samples. Sequencing of the genome of the Norwalk virus has led to the development of PCR assays, with greatly enhanced sensitivity for virus detection (Ando et al. 2000). However, there is great genomic diversity among the NLVs and one set PCR primers will not detect all strains (Norcott et al. 1994). PCR assays are being used for the examination of food samples, particularly shellfish, but far more complex nucleic acid extraction techniques are required than when working with clinical specimens from patients (Atmar et al. 1993; Green et al. 1998b). There are also greater problems with naturally occurring inhibitors to the PCR reaction in these types of samples. NLVs have been detected in samples of raspberries associated with an outbreak of gastroenteritis in Quebec. Sequence analysis demonstrated that the strain of NLV identified in the raspberries was identical to that found in the patients (Gaulin et al. 1999). Expression of recombinant virus capsids in yeast and insect cells is allowing the development of ELISA‐based diagnostic assays, but reagents are not widely available and so far the tests only detect a very limited number of NLV strains (Jiang et al. 2000). Further development and more widespread use of ELISA tests will greatly facilitate the detection of NLVs in clinical samples, although such tests may not be sufficiently sensitive to detect virus in food samples. At the present time, PCR and ELISA assays for NLVs are only available in specialist laboratories and are not used for the routine testing of food samples. Commercial test kits are not yet available.
Rotavirus and astrovirus can both be grown in cell cultures in the laboratory. However, it is unreliable and time‐consuming for isolation from primary specimens and is not normally used. Rotavirus is frequently detected using commercial ELISA or latex agglutination tests and PCR assays are available. Rotavirus has been detected in lettuce (Hernandez et al. 1997) and shellfish samples, but it is not clear if these were of human origin. Electron microscopy is still the most usual method for the detection of astroviruses, although PCR assays are used in a few laboratories.
Diagnosis of hepatitis A infection in patients is by detection of specific IgM antibody, since virus excretion has largely ceased by the time illness becomes apparent. The virus can be cultured in the laboratory, but this is a long and unreliable procedure. In one outbreak at a summer camp in the United States, virus was isolated from the drinking water supply, but this took 21 weeks. PCR assays have been developed and have been used for detecting virus in water, shellfish and other food and environmental samples. Due to the long incubation period of hepatitis A, food items are not usually available for testing, even if suspected as the source of illness. In particular, minimally processed fruits and vegetables have a short shelf life.
There have been a few experimental studies to investigate seeding and recovery of viruses from fresh produce. Transfer of hepatitis A virus to lettuce leaves has been investigated (Bidawid et al. 2000; Sattar et al. 2000). Rotavirus and poliovirus were recovered from the surface of vegetables in a method described by Badawy et al. (1985b). Average recovery rates of 80 and 65%, respectively, were obtained from lettuce; however, recovery of rotavirus from non‐leafy vegetables was lower, averaging 44%. Ward et al. (1982) also recovered poliovirus and adenovirus from vegetable surfaces, obtaining mean efficiencies of approximately 55–58%. There is a need to develop more effective quantitative methods in order to assess the survival of viruses on fresh produce and to determine the decontamination efficiencies of current commercial washing systems for fruit and vegetables.
6. ROUTES OF CONTAMINATION
Fruits and vegetables may become contaminated with viruses in two ways. First, they may be contaminated in their growing area before harvest by coming into contact with inadequately treated sewage or sewage polluted water. Secondly, contamination can arise during processing, storage, distribution or final preparation either directly from infected people or by contact with a contaminated environment. In most outbreaks of foodborne viral disease involving fresh produce, it is not known whether contamination took place before, during or after harvest.
Guidelines issued by the World Health Organization state that fruits and vegetables to be eaten raw should not be fertilized with sewage or irrigated with contaminated water (Beuchat 1998). Sewage sludge is sometimes applied to agricultural land, with the benefit that useful plant nutrients and organic matter are recycled to the soil. However, the UK government is proposing more stringent controls for harvesting vegetables from land where conventionally processed sewage sludge is applied (ADAS, 2000). The transmission of viruses is thought to be mainly by surface contamination. There are relatively few reported studies on the possible uptake of viruses within damaged plant tissues during primary growth. Studies with poliovirus report that virus can infiltrate into the roots and body of plants from the soil (Oron et al. 1995), but there is no evidence of illness from this source. Viruses from sewage do not bind readily with soil particles and can enter groundwaters leading to contamination of water sources.
The viruses causing gastroenteritis and hepatitis A appear to be extremely infectious in very low doses. Large numbers of virus particles can be excreted in the faeces from an infected person. Levels of the order of 106–1011 infective units per gram have been estimated (Feachem et al. 1983). Poor personal hygiene is therefore a major route through which viruses can directly reach foods. Virus can be transferred from faecally contaminated fingers to foods or to work surfaces and door handles. There is a significant risk of contamination from field workers who do not have adequate on‐site toilet and hand‐washing facilities. Even when these facilities are put in place, the workers need to be supervised in such a way as to ensure that the facilities are used (Cliver 1994). In an outbreak of hepatitis A, associated with frozen raspberries, infection was confirmed in a fruit picker on the farm where the raspberries were cultivated (Ramsay and Upton 1989). A number of outbreaks have also been linked to contamination of fresh produce from the vomitus of infected food handlers (Caul et al. 1988). It has been suggested that between 20 and 30 million virus particles are liberated during vomiting (Reid et al. 1988; Caul 1994). As well as direct transmission, aerosols produced by vomiting can contaminate exposed food, or surfaces with subsequent transfer to foods.
7. SURVIVAL
7.1 Survival of viruses in water
Human enteric viruses will potentially be present in any type of water contaminated by human faecal material and by sewage. The Department of Environment, Transport and Regions (DETR) jointly with UK Water Industry Research Limited and the Environment Agency, recently commissioned four reviews on enteroviruses. These covered the source (Sellwood et al. 1999) and survival (Irving and Morris 1999) in the environment and their fate through sewage treatment processes (Merrett and Weatherley 1999). Mounting evidence suggests that viruses can survive long enough and in high enough numbers to cause human diseases through direct contact with polluted water or contaminated foods (Nasser 1994; Bosch 1995). Irving and Morris (1999) concluded that a thorough and valid assessment of the occurrence and significance of viruses in natural waters is hampered by lack of reliable information. They suggested that this is due to inefficient analytical techniques, and because different analytical methods have been used so that comparisons on survival data between different studies cannot be made. However, it does appear that different types of viruses are inactivated at different rates under identical conditions, and therefore no single microorganism can be expected to be an indicator for all viruses.
Temperature is an important factor, with low temperatures favouring viral survival in natural waters. Raphael et al. (1985) found no significant drop in rotavirus titre after 64 days at 4°C in raw water, treated tap water or filtered water. However, a 99% drop in titre was observed after 10 days at 20°C. Astrovirus survival has been demonstrated in drinking water after 90 days at 4°C (Abad et al. 1997b). Nasser et al. (1993) concluded that hepatitis A virus and poliovirus could survive in wastewater and groundwater for 90 days or more at 10°C . Hepatitis A virus and poliovirus were shown to survive in excess of one year in mineral water stored at 4°C (Biziagos et al. 1988). Hepatitis A virus can survive in fresh or salt water for up to a year (Sobsey et al. 1988).
Evidence suggests that adsorption of viruses to particulate matter and sediments confers substantial protection against inactivating influences. Salinity and pH do not appear to have a significant direct effect on virus survival under conditions normally found in natural waters, but may have indirect effects by modifying interaction of viruses with particulates. There is some evidence that solar radiation promotes inactivation of viruses, but the effects have not been extensively studied.
Development of PCR‐based assays has allowed the detection of NLVs in both river water and seawater (Sellwood et al. 2000; Wyn‐Jones et al. 2000). In 1997, an outbreak of NLV gastroenteritis occurred among canoeists and was associated with river water at a water sports centre (Gray et al. 1997). When hepatitis A virus was detected in lettuce from Costa Rica, it was suggested that the possible source of contamination was the discharge of untreated sewage into river water used to irrigate crops, which is common practice in some less well‐developed countries (Hernandez et al. 1997). In another outbreak of hepatitis A, traced to commercially distributed lettuce or tomatoes, it was hypothesized that contamination may have occurred in the fields from dirty water used for growing or irrigation, or possibly from the use of night soil, although this was difficult to prove (Rosenblum et al. 1990).
7.2 Survival of viruses in soil
Enteric viruses may contaminate soil through the land disposal of sewage sludge and dirty irrigation water. A number of research studies have investigated the survival of human pathogenic viruses in soils with conflicting results. Survival appears to depend on a number of different variables, particularly the growing season, soil temperature, rainfall, soil type and composition. For example, viable poliovirus was recovered from spray‐irrigated soil after 96 days during the winter season (Sullivan and Tierney 1976; Tierney et al. 1977). This compared to a maximum survival period of only 11 days during the summer, which suggested the higher temperature and solar radiation levels in the warmer seasons accelerated viral inactivation. The possibility also exists that viruses may be mechanically transmitted to fruits and vegetables during harvest. Sadovski et al. (1978) noted the persistence of inoculated poliovirus in drip irrigation pipes and soil. Moderate environmental conditions and alluvial‐type soil, which restricts water infiltration, enhanced viral recoveries in the upper soil layers. Oron et al. (1995) found that relatively high soil temperature (30°C) and a low moisture content hindered poliovirus survival. Wet soil conditions are frequently associated with low soil temperature. Gerba (1983) reported that a large proportion of outbreaks of waterborne disease in the Unite States resulted from contaminated groundwater. Climate, the nature of the soil and the nature of the resident microflora determine virus survival and retention within soil particles. Both electrostatic and hydrophobic interactions are thought to contribute towards virus adsorption and are controlled by the characteristics of the soil.
7.3 Survival of viruses on surfaces
Some investigations have focused on the survival of viruses on inanimate environmental surfaces such as stainless steel, glass and plastics. Abad et al. (1994) observed that a range of enteric viruses, including hepatitis A virus and rotavirus, persisted for extended periods (greater than 30 days) on several types of porous and nonporous surfaces. Greater virus survival was noted at 4°C than at 20°C. The effect of relative humidity on survival of hepatitis A virus on non‐porous surfaces has been investigated (Bidawid et al. 2000; Sattar et al. 2000). At 5°C, relative humidity had little effect on survival time, but at 20°C survival was longest when relative humidity was low. It is apparent from the lingering outbreaks that have occurred on cruise ships that NLVs survive well on environmental surfaces. NLVs have been detected in swabs of lockers in hospital outbreaks and from hotel carpets (Green et al. 1998a; Barry‐Murphy et al. 2000). A disinfectant formulation is considered to be effective if it is capable of inducing a 1000‐fold (99·9%) or greater reduction in the virus titre (Lloyd‐Evans et al. 1986; Springthorpe et al. 1986). Experimental studies have shown that a free chlorine level of 5000 ppm reduced the infectivity titre by more than 99·9% on stainless‐steel disks contaminated with the enteric viruses Coxsackievirus B3 and hepatitis A virus, and the respiratory viruses adenovirus type 5, parainfluenza virus type 3 and coronavirus 229E (Sattar et al. 1989; Mbithi et al. 1990). The virucidal action of sodium hypochlorite (1250 ppm at pH 9·56) was also tested against hepatitis A virus, human rotavirus and a Bacteroides fragilis bacteriophage dried on polystyrene (Abad et al. 1997a). Overall, a less than 1000‐fold titre reduction was achieved for all the viruses examined. Chlorine is a common disinfectant used by many fresh produce processors to wash fruits and vegetables (Beuchat 1998; Seymour 1999). However, chlorine levels greater than 200 ppm are thought to cause adverse discoloration (bleaching) and off flavours in the finished product (Hurst and Schuler 1992).
7.4 Survival of viruses on fruits and vegetables
Survival times for some enteric viruses have been determined on a range of different fruit and vegetable commodities. It is difficult to draw conclusions from the different studies, since experimental conditions and methods varied. However, most of these studies report viability in excess of the product shelf life. Konowalchuk and Speirs (1974) established no significant loss in Coxsackievirus B5 titre when contaminated lettuce was stored for 16 d under moist conditions at 4°C, but some inactivation took place during storage under dry conditions. They hypothesized that under conditions of low or no moisture the aqueous part of the virus inoculum evaporated leaving the virus exposed to air and/or salts. Celery, lettuce and carrots stored at 4°C supported the survival of a range of enteric viruses for up to 8 days (Konowalchuk and Speirs 1975a).
Badawy et al. (1985a) indicated that rotavirus SA‐11 (a simian rotavirus that can be cultured) survived on lettuce, radishes and carrots for up to 30 days at 4°C. Viral inactivation at room temperature (25°C) was significantly greater than at 4°C; however, viable rotavirus SA‐11 was still detected on lettuce after 25 days. They concluded that the rough or irregular surfaces present on lettuce might offer some additional protection for virus particles. Indeed, protected segments of plants such as the roots, closed leaves and internal fruit parts, may offer favourable conditions that increase survival time up to 60 days (Smith 1982). Viable poliovirus was recovered from effluent spray‐irrigated lettuce and radishes 23 days after inoculation (Tierney and Sullivan 1976; Tierney et al. 1977). Sadovski et al. (1978) isolated polioviruses from cucumbers grown in effluent‐irrigated soil for a full 8 days after inoculation. Oron et al. (1995) investigated the transmission of poliovirus from subsurface drip‐irrigated soil to tomato plant leaves and tomato fruits. Virus was not detected in the tomato fruits. However, a number of leaf samples were positive for poliovirus even though the virus was injected 10 cm below the surface of the soil. This result suggests that poliovirus can penetrate into plant tissue through the root system. The lack of viable poliovirus in the tomato fruits may be due to the presence of antiviral substances as witnessed by other authors.
Chilled storage temperatures (2–8°C) typically retard respiration, senescence, product browning, moisture loss, and microbial growth in minimally processed fruits and vegetables, but may contribute to the survival and transmission of viruses to the human host.
Konowalchuk and Speirs (1975b, 1976) described the potent antiviral properties of different fruit extracts at neutral pH, particularly strawberry. They found significant differences in viral recovery on strawberries, cherries and peaches held in a humid atmosphere at 4°C. Recoveries of Coxsackievirus and echovirus were also greater than those of poliovirus and reovirus, although the authors did not offer an explanation for this result. Most of the aqueous fruit extracts and infusions demonstrated notable antiviral properties. Although the active compounds were not isolated, these agents could be phenolic in nature. In grapes, these chemicals are thought to be located primarily in the skin (Konowalchuk and Speirs 1977). Fruits and vegetables are known to contain a vast array of antimicrobial substances, particularly organic acids, phenolic and sulphur compounds and small polypeptide proteins. Despite this, outbreaks of viral gastroenteritis and hepatitis A have been associated with fruits and fruit juices. In the absence of formal studies it could be inferred that the gastroenteritis and hepatitis A viruses are relatively resistant to these virucidal chemicals.
In view of the increasing use of ready‐to‐eat vegetables sold in modified atmosphere packaging (MAP), the survival of hepatitis A virus on lettuce in MAP, stored at room temperature and 4°C, has been investigated (Bidawid et al. 2000). Survival at 4°C was the same in MAP as under normal conditions of packaging. At room temperature viral survival was slightly better in MAP containing higher carbon dioxide levels. It was suggested that enhanced virus survival might be due to inhibition of ethylene by carbon dioxide, resulting in reduced physiological spoilage of vegetables such as lettuce and possibly less toxic effects on the virus. Studies have indicated that indigenous microflora in the water environment are deleterious to survival of enteric viruses (Raphael et al. 1985). These findings highlight the importance of avoiding contamination of food items before packaging in MAP (Sattar et al. 2000).
Bardell (1997) studied the survival of herpes simplex virus type 1, suspended in saliva, on the skin of tomatoes and the upper surface of lettuce. Although storage times of only 1 h were studied, temperature was shown to have a significant effect on virus titre. There was no loss of virus infectivity titre at 2°C compared to a 2‐log reduction at room temperature (22–24°C). Although not an enteric virus and not a virus that comes to mind as a possible foodborne infection, herpes viruses could invade through the mucous membranes of the mouth.
8. FRESH PRODUCE WASHING
Most fruit and vegetable washing systems are designed to remove gross contamination such as dirt, insects, and foreign matter. However, they are reported to be less successful at removing microbial contaminants (Beuchat 1998; Seymour 1999). Vigorous washing of fruits and vegetables with clean potable water typically reduces the number of micro‐organisms by 10–100‐fold (Beuchat 1998) and is often as effective as treatment with 100 mg l−1 chlorine, the current industry standard (Elphick 1998; Seymour 1999). Raw materials are typically immersed in cooled sanitized water and then dewatered to remove surface moisture and fruit and vegetable juices from the product (Simons and Sanguansri 1997). Product agitation is optimized by using water or air jets which enhance surface contact, carriage of product, and suspension of solids and vegetable debris (Simons and Sanguansri 1997). There has been recent concern over the possible migration of bacterial pathogens into the core tissue of fruits and vegetables during washing. Zhang and Farber (1996) found that uptake of bacterial cells was associated with a negative temperature differential between the water and the product. However, the uptake of human viruses by fruits and vegetables under similar conditions has not been studied. Lodging or attachment of microorganisms in tissue crevices may protect cells from direct contact with disinfectants and consequently aid in their survival (Beuchat 1992). Recent studies by Seo and Frank (1999) found that Escherichia coli O157:H7 could survive in the stomata and on cut edges of lettuce following chlorine treatment. Although there are no available data for viruses, cell surface structures are likely to offer some additional protection.
8.1 Susceptibility of foodborne viruses to disinfectants and processing aids used in the fresh produce industry
Numerous authors have reported on the efficacy of disinfectants for the inactivation of readily culturable human viruses using standard suspension tests, but data for gastroenteritis viruses and hepatitis A virus are lacking. The authors of this review were unable to find any data on the effectiveness of disinfectants and processing aids for the decontamination of enteric viruses on fruits and vegetables.
The most commonly used sanitizers and processing aids for cleaning fruits and vegetables are chlorine, chlorine dioxide, organic acids and surfactants, while ozone is receiving renewed interest. The mechanism of action of these disinfectants on viruses and their interaction with plant materials is poorly understood and there are conflicting reports on their efficacy. There are no positive lists of permitted processing aids in the United Kingdom or European Community, although an inventory has been produced by the Codex Alimentarius Commission (Codex Alimentarius General Requirements, volume 1 A, 2nd edition 1995. FAO/WHO ISBN 92–5‐103762–0). Directive 98/8/EC of the European Parliament contains a list of biocidal products, which have been approved and authorized for use. Council Regulation (EEC) no. 2092/91 highlights the food additives, processing aids and other substances permitted for the organic production of agricultural products. The use of chlorine, for the treatment of organically grown fruits and vegetables, has recently been banned in the United Kingdom and the fresh produce industry is currently trying to find viable and effective alternatives.
8.2 Chlorine
Chlorine is the most widely used disinfectant for washing fresh produce because it is relatively cheap, easy to use and exhibits rapid microbiological action in aqueous solution (Elphick 1998). The Food and Drug Administration (FDA) in the United States permits the use of chlorine as a disinfectant in wash, spray and flume waters in the raw fruit and vegetable industry (Garret 1992). However, in certain EC countries, chlorine is not permitted as a wash water additive. Disinfectants such as chlorine and ozone have a strong affinity for organic matter and are ‘used up’ rapidly in wash tanks containing dirty produce. Therefore, for any washing system, it is important to monitor and control the level of disinfectant at all times, to ensure that it is optimal. It is also imperative to maintain chlorine disinfectants within a suitable pH range (Boyette et al. 1993). Chlorine‐based disinfectants are usually considered to be the most effective against enteric viruses. However, NLVs and hepatitis A virus appear to be relatively resistant to chlorine (Grabow et al. 1983; Peterson et al. 1983; Keswick et al. 1985). NLVs are inactivated by 10 mg chlorine l−1, which is the concentration used to treat a water supply after a contamination incident. In the United States a level of 5 mg chlorine l−1 with a contact time of 1 min is recommended for inactivation of hepatitis A virus. In recent studies (Doultree et al. 1999) feline calicivirus was shown to be surprisingly resistant to chlorine, requiring 1000 mg l−1 freshly reconstituted granular hypochlorite for complete inactivation. Clearly there is a need for further studies on disinfection of these persistent organisms.
8.3 Chlorine dioxide
Chlorine dioxide is not as susceptible to pH changes or the presence of organic matter as chlorine (Simons and Sanguansri 1997). Chlorine dioxide is unstable, must be generated on‐site and can be explosive when concentrated. The oxidizing power of chlorine dioxide is reported to be about 2·5 times that of chlorine (Beuchat 1998). However, Zhang and Farber (1996) found no significant difference in decontamination efficiency between chlorine and chlorine dioxide. Several authors have reported the sensitivity of a range of animal viruses to chlorine dioxide (Harakeh 1987; Chen and Vaughn 1990) although there is no information for NLVs and hepatitis A virus.
8.4 Organic acids
Organic acids can occur naturally in many fruits and vegetables and may retard the growth of some microorganisms and prevent the growth of others (Beuchat 1998). Most of these acids behave primarily as fungistatic agents, while others are more effective at inhibiting bacterial growth. These include acetic, citric, succinic, malic, tartaric, benzoic, propanoic and sorbic acids. Due to recent changes in legislation, washes and sprays containing organic acids are becoming more popular for the processing of organically grown fruits and vegetables. A number of products are now commercially available. However, Scholtz et al. (1989) found that hepatitis A virus remained infectious for 90 min at pH 1. NLVs and hepatitis A virus survive exposure to acidity levels below pH 3, while rotavirus is inactivated below pH 3 and above pH 10 (Dolin et al. 1972; Palmer et al. 1977; Weiss and Clark 1987; Scholtz et al. 1989). Viruses that infect via the gastrointestinal tract are acid stable since they have to survive the harsh low pH environment of the stomach. Organic acids are therefore unlikely to have a significant effect on the viability of hepatitis A virus and NLVs during the typically short contact times used for washing fruits and vegetables (Seymour 1999).
8.5 Surfactants
Several authors have reported the action of surfactants (wetting agents) in combination with disinfectants (Adams et al. 1989; Zhang and Farber 1996). However, these agents may reduce the antimicrobial effect of chlorine disinfectants and adversely affect product quality (Adams et al. 1989).
8.6 Ozone
Ozone is lethal to a wide variety of microorganisms, including enteric viruses (Finch and Fairbairn 1991). It is a potent oxidizing agent, is very reactive and unstable, leaves no residues in water and naturally decomposes into ordinary oxygen. This absence of toxicity is one of its major advantages over chlorine disinfectants. Ozone has recently been approved as a disinfectant for food applications in the United States (Graham 1997) and industry suppliers are working to develop appropriate systems for fresh produce washing. Ozone is unstable, cannot be stored and must therefore be generated on‐site. However, due to perceived safety problems and a lack of information on the efficacy of ozone, this technology has not yet been readily taken up in Europe. Although ozone can be used safely, it can cause irritation of the eyes, headache, dryness of the throat, and coughing at exposure levels in the range of 0·1–1·0 ppm (Boisrobert et al. 1998). The Occupational Safety and Health Administration (OSHA) has set the permissible exposure limit of workers to ozone at 0·1 ppm If ozone is to be used in aqueous or gaseous form it is essential that the air is monitored for ozone and that suitable control measures are in place to remove it.
9. CONTROL
The control of foodborne viral infection was considered in the report of the Advisory Committee on the Microbiological Safety of Food (1998). Sewage pollution is a major factor in the contamination of food and water. This is particularly pertinent to fruits and vegetables that will not be cooked before consumption. Untreated or inadequately treated sewage discharged into natural waters can cause contamination of crops. Sewage sludge is applied to agricultural land, with the benefit that useful plant nutrients are recycled to the soil. In April 2000, the Agricultural Development and Advisory Service (ADAS, UK) published the Safe Sludge Matrix (ADAS, 2000). This document gives clear guidance on the minimum acceptable level of treatment for any sewage sludge applied to agricultural land and provides a framework to ensure microbiological safety. As from 31 December 1999 application of all untreated sludge on agricultural land used to grow food crops has been prohibited in the United Kingdom. Use on all agricultural land will be prohibited from the end of 2001. Untreated sludge is produced by either the primary settlement or secondary biological stages of sewage treatment. Further processing may be undertaken to produce treated sludge, resulting in improved stability and a reduction in health hazards and odour problems. (MAFF 1998). Currently, treated sludge may be applied to land used for salad crops, but harvesting is not permitted for 30 months. Vegetables cannot be harvested for 12 months. A 10‐month harvest interval is required if enhanced treated sludge is used. All applications must comply with the Sludge (Use in Agriculture) Regulations 1989 and DETR Code of Practice 1986. These controls may help to reduce the risks of microbiological contamination of fruits and vegetables in the UK, but still do not address the potential problem of imported produce from countries with different standards for organic fertilisers or irrigation water.
The other major source of contamination is from infected people handling food. People with symptoms should be excluded from food handling. However, food‐handlers with only very minimal symptoms have been implicated in transmission of viral gastroenteritis. Current recommendations state that food‐handlers should be allowed to return to work 48 h after symptoms have ceased. (PHLS 1993). These recommendations appear to work satisfactorily, but were based on the rapid decrease in virus excretion observed by electron microscopy. Using more sensitive PCR assays, NLVs can be detected for longer periods than electron microscopy and, in some instances, for up to a week after onset of symptoms. It is not clear if people shedding virus detectable by PCR are infectious, but recommendations on how long to exclude people from food‐handling need to be kept under review. The main period of excretion for hepatitis A virus is before symptoms become apparent and therefore control is difficult. The wearing of disposable gloves is recommended if foods are to be manipulated by hand, but this does not prevent transfer of viruses to gloves by touching contaminated surfaces.
If vomiting occurs, virus may be spread over a wide area in aerosol droplets. Uncovered food that is not to receive cooking should be discarded. The environment should be thoroughly cleaned, including work surfaces, sinks and door handles. Recent studies by the PHLS demonstrated the presence of virus on surfaces and materials that had been cleaned by recommended decontamination methods. This suggests that the current recommendations for the removal of NLVs from contaminated surfaces are inadequate (Barry‐Murphy et al. 2000).
Cliver (1995) advised thorough cooking of virus‐contaminated foods. However, ready‐to‐eat fruits and vegetables are unlikely to withstand such harsh treatment and may show deleterious changes in sensory quality. Larkin (1981) suggested a wash at 80°C for 2 min to ensure the microbiological safety of fresh produce. He concluded that this heat treatment does not affect the appearance and taste of most fruits and vegetables provided the food is eaten within 24 h. However, the majority of minimally processed fruits and vegetables require a shelf life in excess of 1 day and therefore this heating step is not applicable.
Meticulous attention to good food handling practices and education is essential. There should be provision of adequate toilet and hand‐washing facilities, not only in the catering and retail industry, but also for farm workers.
There is an effective vaccine for hepatitis A and it has been suggested that food handlers should be vaccinated (Cliver 1997). Epidemiological data currently suggest that food‐handlers in the United Kingdom do not pose a significantly greater risk of transmitting hepatitis A infection than other people, and use of vaccine in this group may not be cost‐effective. No vaccines have been developed for NLVs but research studies with NLV recombinant capsid antigens offer the potential for future development of vaccines. There are challenges, however, in designing effective vaccines in that first, there are multiple types of NLVs and secondly, the mechanisms for inducing immunity to these agents is poorly understood (Estes et al. 2000).
10. CONCLUSIONS
Fresh produce contributes to the transmission of viral infections. There is a lack of information on the survival of viruses on fresh produce related to shelf life and types of packaging. Information is also lacking on the efficiency of current washing and decontamination processes for the removal of viruses. Studies are therefore required to provide this information for NLVs and hepatitis A virus in particular. There is a need for information on rotaviruses and astroviruses. Both these viruses have been implicated in foodborne and waterborne outbreaks in the United Kingdom, although only very infrequently. The role and extent of food or waterborne transmission needs to be more clearly defined for both these viruses.
The infectivity of NLVs and hepatitis A virus is difficult to study in the laboratory. NLVs cannot be cultured in vitro and culture techniques for hepatitis A virus are time‐consuming. Other viruses that can be cultured readily have been used as models for these pathogens in a number of studies. Although only the use of specific human pathogenic viruses will give clear and unequivocal data on survival, inactivation and removal, this is not always safe and practical. Model systems using similar non‐pathogenic human or mammalian viruses may be the most satisfactory alternative. For example, feline calicivirus has been used as a model system for NLVs in assessing the efficacy of commercially available disinfectants (Doultree et al. 1999). Feline calicivirus was also used as a model for NLVs in another study on the heat treatment of shellfish (Slomka and Appleton 1998). This organism would appear to be a good candidate for virus studies on fresh produce. In order to undertake studies on the survival and removal of viruses, there is a need to develop better methods for inoculation and recovery of virus from a range of fresh produce items.
There have been recent suggestions that faecal coliforms on fresh produce may be an indicator of the probable presence of enteric viruses. However, several authors have found no significant correlation (Keswick et al. 1982; Le Guyader et al. 1993). Despite the shortcomings of indicator organisms such as bacteriophages, there is a need for a safe and convenient model that can be used to help the food industry to assess and optimize new treatment processes. This is not intended as an indicator to monitor food samples directly. Other studies have shown that the behaviour and survival of bacteriophages mimic human viruses more closely than bacteria and would be suitable for such purposes. The optimization of washing and decontamination processes to remove viruses from fruits and vegetables will ultimately contribute to the overall safety of these food products.
11. Acknowledgements
The authors acknowledge the financial support of the Food Standards Agency for the production of this paper (Project: BO2014).
References
- 1. Abad, F.X. , Pinto, R.M. , Bosch, A. (1994) Survival of enteric viruses on environmental fomites. Applied and Environmental Microbiology 60 , 3704–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abad, F.X. , Pinto, R.M. , Bosch, A. (1997a) Disinfection of human enteric viruses on fomites. FEMS Microbiology Letters 156 , 107–111. [DOI] [PubMed] [Google Scholar]
- 3. Abad, F.X. , Pinto, R.M. , Villena, C. , Gajardo, R. , Bosch, A. (1997b) Astrovirus survival in drinking water. Applied and Environmental Microbiology 63 , 3119–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adams, M.R. , Hartley, A.D. , Cox, C.J. (1989) Factors affecting the efficacy of washing procedures used in the production of prepared salads. Food Microbiology 6 , 69–77. [Google Scholar]
- 5. ADAS (2000) The safe sludge matrix. Ref: AMPU 1234/B/1. Wolverhampton: ADAS.
- 6. Advisory Committee on the Microbiological Safety of Food (1998) Report on Foodborne Viral Infections. London: HMSO.
- 7. Ando, T. , Noel, J.S. , Fankhauser, R.L. (2000) Genetic classification of ‘Norwalk‐like’ viruses. Journal of Infectious Diseases 181 (Suppl. 2), S336–S348. [DOI] [PubMed] [Google Scholar]
- 8. Anonymous (reported by: United States of America, Calhoun Department of Public Health) (1997) Hepatitis A associated with consumption of frozen strawberries – Michigan, March 1997. Journal of the American Medical Association 277 , 1271–1271. [Google Scholar]
- 9. Appleton, H. (2000) Control of food‐borne viruses. British Medical Bulletin 56 , 172–183. [DOI] [PubMed] [Google Scholar]
- 10. Appleton, H. (2001) Norwalk virus and the small round viruses causing foodborne gastro‐enteritis. In: Foodborne Disease Handbook, 2nd edn, Vol. 2. Viruses, Parasites, Pathogens and HACCP, eds Hui, Y.H., Sattar, S.A., Murrell, K.D., Nip, W.K. and Stanfield, P.S.), pp. 77–98. New York: Marcel Dekker.
- 11. Atmar, R.L. , Metcalf, T.G. , Neill, F.H. , Estes, M.K. (1993) Detection of enteric viruses in oysters by using the polymerase chain reaction. Applied and Environmental Microbiology 59 , 631–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Badawy, A.S. , Gerba, C.P. , Kelley, L.M. (1985a) Survival of rotavirus SA‐11 on vegetables. Food Microbiology 2 , 199–205. [Google Scholar]
- 13. Badawy, A.S. , Gerba, C.P. , Kelley, L.M. (1985b) Development of a method for recovery of rotavirus from the surface of vegetables. Journal of Food Protection 48 , 261–264. [DOI] [PubMed] [Google Scholar]
- 14. Bardell, D. (1997) Survival of herpes simplex virus type 1 on some common foods routinely touched before consumption. Journal of Food Protection 60 , 1259–1261. [DOI] [PubMed] [Google Scholar]
- 15. Barry‐Murphy, K. , Green, J. , Brown, D.W.G. , Cheesbrough, J.S. , Hoffman, P.N. (2000) Norwalk‐like viruses – investigation of patterns of environmental contamination on a hospital ward and evaluation of decontamination procedures. 25th PHLS Annual Scientific Conference Abstracts, pp. 22.
- 16. Beuchat, L.R. (1992) Surface disinfection of raw produce. Dairy, Food and Environmental Sanitation 12 , 6–9. [Google Scholar]
- 17. Beuchat, L.R. (1998) Surface decontamination of fruits and vegetables eaten raw: a review. WHO/FSF/FOS/98.2. Geneva: Food Safety Unit, World Health Organization.
- 18. Bidawid, S. , Farber, J.M. , Sattar, S.A. (2000) Contamination of foods by food handlers: experiments on hepatitis A transfer to food and its interruption. Applied and Environmental Microbiology 66 , 2759–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Biziagos, E. , Passagot, J. , Crance, J.M. , Deloince, R. (1988) Long‐term survival of hepatitis A virus and poliovirus type 1 in mineral water. Applied and Environmental Microbiology 54 , 2705–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boisrobert, C. , Yuan, J. , Novak, J. , Mrotek, M. (1998) Ozone: safety considerations. Fresh Cut December, 16–18, 23–23.
- 21. Bosch, A. (1995) The survival of enteric viruses in the water environment. Microbiologia 11 , 393–396. [PubMed] [Google Scholar]
- 22. Boyette, M.D. , Ritchie, D.F. , Carballo, S.J. , Blankenship, S.M. , Sanders, D.C. (1993) Chlorination and postharvest disease control. Horticultural Technology 3 , 395–400. [Google Scholar]
- 23. Caul, E.O. & Appleton, H. (1982) The electron microscopical and physical characteristics of small round human faecal viruses. Journal of Medical Virology 9 , 257–265. [DOI] [PubMed] [Google Scholar]
- 24. Caul, E.O. , Sellwood, N.J. , Brown, D.W. et al. (1988) Outbreaks of gastro‐enteritis associated with SRSVs. PHLS Microbiology Digest 10 , 2–8. [Google Scholar]
- 25. Caul, E.O. (1994) Small round structured viruses: airborne transmission and hospital control. Lancet 343 , 1240–1242. [DOI] [PubMed] [Google Scholar]
- 26. Centers for Disease Control and Prevention (1996) Ten leading nationally notifiable infectious diseases – United States, 1995. Morbidity and Mortality Weekly Report 45 , 883–884. [PubMed] [Google Scholar]
- 27. Chen, Y.S. & Vaughn, J.M. (1990) Inactivation of human and simian rotaviruses by chlorine dioxide. Applied and Environmental Microbiology 56 , 1363–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cliver, D.O. (1994) Epidemiology of viral foodborne disease. Journal of Food Protection 57 , 263–266. [DOI] [PubMed] [Google Scholar]
- 29. Cliver, D.O. (1995) Detection and control of foodborne viruses. Trends in Food Science and Technology 6 , 353–358.DOI: 10.1016/S0924-2244(00)89190-6 [DOI] [Google Scholar]
- 30. Cliver, D.O. (1997) Virus transmission via food. Food Technology 51 , 71–78. [Google Scholar]
- 31. Cromeans, T. , Favorou, M. , Nianan, O. , Margolis, H. (2001) Hepatitis A and hepatitis E viruses. In: Foodborne Disease Handbook, Vol. 2, Viruses, Parasites and HACCP, eds Hui, Y.H., Satter, S.A., Murrel, K.D. Nip, W.K. and Stanfield. P.S, pp. 23–76. New York: Marcel Dekker.
- 32. Currier, M. (1996) Foodborne outbreak. Dairy, Food and Environmental Sanitation 16 , 32–33. [Google Scholar]
- 33. Department of Health, Welsh Office, Scottish Office Department of Health, DHSS and Northern Ireland (1996) Immunisation Against Infectious Disease, eds Salisbury, D.M. and Begg, N.T., pp. 85–94. London: HMSO.
- 34. Dedman, D. , Laurichesse, H. , Caul, E.O. , Wall, P.G. (1998) Surveillance of small round structured virus (SRSV) infection in England and Wales 1990–5. Epidemiology and Infection 121 , 139–149.DOI: 10.1017/S0950268898001095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dolin, R. , Blacklow, N.R. , Dupont, H. , Buscho, R.F. , Wyalt, R.G. , Kasel, J.A. , Hornick, R. , Chanock, R.M. (1972) Biological properties of Norwalk agent of acute infectious nonbacterial gastroenteritis. Proceedings of the Society of Experimental Medicine 140 , 578–583. [DOI] [PubMed] [Google Scholar]
- 36. Doultree, J.C. , Druce, J.D. , Birch, C.J. , Bowden, D.S. , Marshall, J.A. (1999) Inactivation of feline calicivirus, a Norwalk virus surrogate. Journal of Hospital Infection 41 , 51–57. [DOI] [PubMed] [Google Scholar]
- 37. Elphick, A. (1998) Fruit and vegetable washing systems. Food Processing January, 22–23.
- 38. Estes, M.K. , Ball, J.M. , Guerrero, R.A. et al. (2000) Norwalk virus vaccines: challenges and progress. Journal of Infectious Diseases 181 (Suppl. 2), S367–73. [DOI] [PubMed] [Google Scholar]
- 39. Fankhauser, R.L. , Noel, J.S. , Monroe, S.S. , Ando, T. , Glass, R.I. (1998) Molecular epidemiology of Norwalk‐like viruses in outbreaks of gastroenteritis in the United States. Journal of Infectious Diseases 178 , 1571–1578. [DOI] [PubMed] [Google Scholar]
- 40. Feachem, R.G. , Bradley, D.J. , Garelick, H. , Mara, D.D. (1983). Sanitation and Disease: health aspects of excreta and wastewater management. Chichester: John Wiley and Sons.
- 41. Finch, G.R. & Fairbairn, N. (1991) Comparative inactivation of poliovirus type 3 and MS2 coliphage in demand‐free phosphate buffer by using ozone. Applied and Environmental Microbiology 57 , 3121–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fleming, B. , Billing, A. , Vallbracht, A. , Botzenhart, K. (1985) Inactivation of hepatitis A virus by heat and formaldehyde. Water Science and Technology 17 , 43–45. [Google Scholar]
- 43. Food Standards Agency (2000) A Report of the Study of Infectious Intestinal Disease in England. London: HMSO.
- 44. Garret, E. (1992) Chlorination of product wash water and effects of pH control. NAFPP Conference, May 2–5 , 1992–1992. [Google Scholar]
- 45. Gaulin, C. , Ramsay, D. , Cardinal, P. , D'Halewyn, M. , Carpenter, N. (1999) Outbreaks of gastroenteritis associated with imported raspberries in Quebec (July 1997). International Workshop on Human Caliciviruses. Centers for Disease Control and Prevention. Atlanta, Georgia. Abstracts, pp. S4–S5.
- 46. Gerba, C.P. (1983) Virus survival and transport in groundwater. Developments in Industrial Microbiology 24 , 247–254. [Google Scholar]
- 47. Grabow, W.O.K. , Gauss‐Müller, V. , Prozesky, O.W. , Deinhardt, F. (1983) Inactivation of hepatitis A virus and indicator organisms in water by free chlorine residuals. Applied and Environmental Microbiology 46 , 619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Graham, D.M. (1997) Use of ozone for food processing. Food Technology 51 , 72–75. [Google Scholar]
- 49. Gray, J.J. , Green, J. , Cunliffe, C. , Gallimore, C. , Lee, J.V. , Neal, K. , Brown, D.W.G. (1997) Mixed genogroup SRSV infections among a party of canoeists exposed to contaminated recreational water. Journal of Medical Virology 52 , 425–429. [PubMed] [Google Scholar]
- 50. Green, K.Y. , Ando, T. , Balayan, M.S. et al. (2000) Taxonomy of the caliciviruses. Journal of Infectious Diseases 181 (Suppl.), S322–S330. [DOI] [PubMed] [Google Scholar]
- 51. Green, J. , Henshilwood, K. , Gallimore, C.I. , Brown, D.W.G. , Lees, D.H. (1998b) A nested reverse transcriptase PCR assay for detection of small round structured viruses in environmentally contaminated shellfish. Applied and Environmental Microbiology 64 , 858–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Green, J. , Wright, P.A. , Gallimore, C.I. , Mitchell, O. , Morgan‐Capner, P. , Brown, D.W.G. (1998a) The role of environmental contamination with small round structured viruses in a hospital outbreak investigation by reverse‐transcription polymerase chain reaction assay. Journal of Hospital Infection 39 , 39–45. [DOI] [PubMed] [Google Scholar]
- 53. Griffin, M.R. , Surowiec, J.J. , McCloskey, D.I. et al. (1982) Foodborne Norwalk virus. American Journal of Epidemiology 115 , 178–184. [DOI] [PubMed] [Google Scholar]
- 54. Hale, A. , Mattick, K. , Lewis, D. et al. (2000) Distinct epidemiological patterns of Norwalk‐like viral gastroenteritis. Journal of Medical Virology 62 , 99–103.DOI: [DOI] [PubMed] [Google Scholar]
- 55. Harakeh, S. (1987) The behaviour of viruses on disinfection by chlorine dioxide and other disinfectants in effluent. FEMS Microbiology Letters 44 , 335–341. [Google Scholar]
- 56. Hedlund, K.O. , Rubilar‐Abreu, E. , Svensson, L. (2000) Epidemiology of calicivirus infections in Sweden 1994–98. Journal of Infectious Diseases 181 (Suppl. 2), S275–S280. [DOI] [PubMed] [Google Scholar]
- 57. Hernandez, F. , Monge, R. , Jimenez, C. , Taylor, L. (1997) Rotavirus and hepatitis A virus in market lettuce (Lactuca sativa) in Costa Rica. International Journal of Food Microbiology 37 , 221–223.DOI: 10.1016/S0168-1605(97)00058-5 [DOI] [PubMed] [Google Scholar]
- 58. Herwaldt, B.L. , Lew, J.F. , Moe, C.L. , Lewis, D.C. , Humphrey, C.D. , Monroe, S.S. , Pon, E.W. , Glass, R.I. (1994) Characterisation of a variant strain of Norwalk virus from a food‐borne outbreak of gastro‐enteritis on a cruise ship in Hawaii. Journal of Clinical Microbiology 32 , 861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hurst, W.A. & Schuler, G.A. (1992) Fresh produce processing – an industry perspective. Journal of Food Protection 55 , 842–847. [DOI] [PubMed] [Google Scholar]
- 60. Irving, T.E. & Morris, R. (1999) Review of the fate of enteroviruses in the environment. EC Bathing Waters Directive Enterovirus Research. Report Ref. No 99/WW/11/1. London: UK Water Industry Research Ltd.
- 61. Jiang, X. , Wilton, N. , Zhong, W.M. et al. (2000) Diagnosis of human caliciviruses by use of enzyme immunoassays. Journal of Infectious Diseases 181 (Suppl. 2), 349–359. [DOI] [PubMed] [Google Scholar]
- 62. Johnson, P.C. , Matthewson, J.J. , Dupont, H.L. , Greenberg, H.B. (1990) Multiple‐challenge study of host susceptibility to Norwalk gastroenteritis in US adults. Journal of Infectious Diseases 161 , 18–21. [DOI] [PubMed] [Google Scholar]
- 63. Kapikian, A.Z. , Wyatt, R.G. , Dolin, R. , Thornhill, T.S. , Kalica, A.R. , Channock, R.M. (1972) Visualisation by immune electron microscopy of a 27 nm particle associated with acute infectious nonbacterial gastroenteritis. Journal of Virology 10 , 1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Keswick, B.H. , Gerba, C.P. , Secor, S.L. , Cech, I. (1982) Survival of enteric viruses and indicator bacteria in groundwater. Journal of Environmental Science and Health A17 , 903–912. [Google Scholar]
- 65. Keswick, B.H. , Satterwhite, T.K. , Johnson, P.C. et al. (1985) Inactivation of Norwalk virus in drinking water by chlorine. Applied and Environmental Microbiology 50 , 261–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Konowalchuk, J. & Speirs, J.I. (1974) Recovery of coxsackievirus B5 from stored lettuce. Journal of Milk and Food Technology 37 , 132–134. [Google Scholar]
- 67. Konowalchuk, J. & Speirs, J.I. (1975a) Survival of enteric viruses on fresh vegetables. Journal of Milk and Food Technology 38 , 469–472. [Google Scholar]
- 68. Konowalchuk, J. & Speirs, J.I. (1975b) Survival of enteric viruses on fresh fruit. Journal of Milk and Food Technology 38 , 598–600. [Google Scholar]
- 69. Konowalchuk, J. & Speirs, J.I. (1976) Antiviral activity of fruit extracts. Journal of Food Science 41 , 1013–1017. [Google Scholar]
- 70. Konowalchuk, J. & Speirs, J.I. (1977) Virus detection on grapes. Canadian Journal of Microbiology 23 , 1301–1303. [DOI] [PubMed] [Google Scholar]
- 71. Koopmans, M. , Vinje, J. , De Wit, M. , Leenen, I. , Van Der Poel, W. , Van Duynhoven, Y. (2000) Molecular epidemiology of human enteric caliciviruses in the Netherlands. Journal of Infectious Diseases 181 (Suppl. 2), S262–S269. [DOI] [PubMed] [Google Scholar]
- 72. Kurtz, J.B. (1994) Astrovirus. In: Viral Infections of the Gastrointestinal Tract, 2nd edn, ed. Kapikian, A.Z., pp. 569–580. New York: Marcel Dekker.
- 73. Kurtz, J.B. & Lee, T.W. (1987) Astroviruses: human and animal. In: Novel Diarrhoea Viruses, Ciba Foundation Symposium 128, eds Bock, G and Whelan, J. pp. 92–107. Chichester: Wiley. [DOI] [PubMed]
- 74. Larkin, E.P. (1981) Food contaminants – viruses. Journal of Food Protection 44 , 320–325. [DOI] [PubMed] [Google Scholar]
- 75. Le Guyader, F. , Apaire‐Marchais, V. , Brillent, J. , Billaudel, S. (1993) Use of genomic probes to detect hepatitis A virus and enterovirus RNAs in wild shellfish and relationship of viral contamination to bacterial contamination. Applied and Environmental Microbiology 59 , 3963–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lieb, S. (1985) Norwalk virus gastro‐enteritis outbreak associated with a cafeteria at a college. American Journal of Epidemiology 121 , 259–268. [DOI] [PubMed] [Google Scholar]
- 77. Lloyd‐Evans, N. , Springthorpe, V.S. , Sattar, S.A. (1986) Chemical disinfection of human‐rotavirus‐contaminated inanimate surfaces. Journal of Hygiene 97 , 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lo, S.V. , Connolly, A.M. , Palmer, S.R. , Wright, D. , Thomas, P.D. , Joynson, D. (1994) The role of the pre‐symptomatic food handler in a common source outbreak of food‐borne SRSV gastro‐enteritis in a group of hospitals. Epidemiology and Infection 113 , 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Maguire, A. , Green, J. , Brown, D.W.G. , Desselberger, U. , Gray, J.J. (1999) Molecular epidemiology of outbreaks of gastroenteritis associated with small round structured viruses in East Anglia, United Kingdom, during the 1996–97 season. Journal of Clinical Microbiology 37 , 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Matsui, S.M. & Greenberg, H.B. (2000) Immunity to calicivirus infection. Journal of Infectious Diseases 181 (Suppl. 2), S331–S335. [DOI] [PubMed] [Google Scholar]
- 81. Mbithi, J.N. , Springthorpe, V.S. , Sattar, S.A. (1990) Chemical disinfection of hepatitis A virus on environmental surfaces. Applied and Environmental Microbiology 56 , 3601–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Merrett, H. & Weatherley, C. (1999) Review of the fate of enteroviruses through the sewage treatment process. EC Bathing Waters Directive Enterovirus Research. Report Ref. No 99/WW/11/1. London: UK Water Industry Research Ltd.
- 83. Millard, J. , Appleton, H. , Parry, J.V. (1987) Studies on heat inactivation of hepatitis A virus with special reference to shellfish. Epidemiology and Infection 98 , 397–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ministry of Agiculture, Fisheries and Food (MAFF) (1998) The Soil Code. Code of Good Agricultural Practice for the Protection of Soil. Ministry of Agriculture, Fisheries and Food, Welsh Agricultural Department.
- 85. Nasser, A.M. , Tchorch, Y. , Fattal, B. (1993) Comparative survival of E. coli, F super (+) bacteriophages, HAV and poliovirus 1 in wastewater and groundwater. In: Health Related Water Microbiology, Vol. 27, eds Morris, R.W., Grabow, W.O.K. and Dufour, A.P., pp. 401–407. Oxford: Pergamon Press.
- 86. Nasser, A.M. (1994) Prevalence and fate of hepatitis A virus in water. Critical Reviews in Environmental Science and Technology 24 , 281–323. [Google Scholar]
- 87. Niu, M.T. , Polish, L.B. , Robertson, B.H. et al. (1992) Multistate outbreak of hepatitis A associated with frozen strawberries. Journal of Infectious Diseases 166 , 518–524. [DOI] [PubMed] [Google Scholar]
- 88. Noah, N.D. (1981) Foodborne outbreaks of hepatitis A. Medical Laboratory Science 38 , 428–428. [Google Scholar]
- 89. Norcott, J.P. , Green, J. , Lewis, D. , Estes, M.K. , Barlow, K.L. , Brown, D.W.G. (1994) Genomic diversity of small round structured viruses in the United Kingdom. Journal of Medical Virology 44 , 280–286. [DOI] [PubMed] [Google Scholar]
- 90. O'Brien, S.J. , Mitchell, R.T. , Gillespie, I.A. , Adak, G.K. (2000) The microbiological status of ready to eat fruit and vegetables. Discussion paper: advisory Committee on the Microbiological Safety of Food. ACM/476, 1–34.
- 91. Oron, G. , Goemans, M. , Manor, Y. , Feyen, J. (1995) Poliovirus distribution in the soil–plant system under reuse of secondary wastewater. Water Research 29 , 1069–1078. [Google Scholar]
- 92. Palmer, E.L. , Martin, M.L. , Murphy, F.A. (1977) Morphology and stability of infantile gastroenteritis virus: comparison with reovirus and bluetongue virus. Journal of General Virology 35 , 403–414. [Google Scholar]
- 93. Parrino, T.A. , Schreiber, D.S. , Trier, J.S. , Kapikian, A.Z. , Blacklow, N.R. (1977) Clinical Immunity in acute gastroenteritis caused by Norwalk agent. New England Journal of Medicine 297 , 86–89. [DOI] [PubMed] [Google Scholar]
- 94. Parry, J.V. & Mortimer, P.P. (1984) The heat sensitivity of hepatitis A virus determined by a simple tissue culture method. Journal of Medical Virology 14 , 277–283. [DOI] [PubMed] [Google Scholar]
- 95. Patterson, W. , Haswell, P. , Fryers, P.T. , Green, J. (1997) Outbreak of small round structured virus gastro‐enteritis arose after kitchen assistant vomited. Communicable Disease Report 7 , R101–3. [PubMed] [Google Scholar]
- 96. Pebody, R.G. , Leino, T. , Ruutu, P. et al. (1998) Foodborne outbreaks of hepatitis A in a low endemic country: an emerging problem? Epidemiology and Infection 120 , 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Peterson, D.A. , Hurley, T.R. , Hoff, J.C. , Wolff, L.G. (1983) Effect of chlorine treatment on infectivity of hepatitis A virus. Applied and Environmental Microbiology 45 , 223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. PHLS Viral Gastroenteritis Sub‐Committee (1993) Outbreaks of gastroenteritis associated with SRSVs. PHLS Microbiology Digest 10 , 2–8. [Google Scholar]
- 99. Ponka, A. , Maunula, L. , Von Bonsdorff, C.‐H. , Lyytikainen, O. (1999) An outbreak of calicivirus associated with consumption of frozen raspberries. Epidemiology and Infection 123 , 469–474.DOI: 10.1017/S0950268899003064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ramsay, C.N. & Upton, P.A. (1989) Hepatitis A and frozen raspberries. Lancet i , 43–44. [DOI] [PubMed] [Google Scholar]
- 101. Raphael, R.A. , Sattar, S.A. , Springthorpe, V.S. (1985) Long‐term survival of human rotavirus in raw and treated river water. Canadian Journal of Microbiology 31 , 124–128. [DOI] [PubMed] [Google Scholar]
- 102. Reid, J.A. , Caul, E.O. , White, D.G. , Palmer, S.R. (1988) Role of infected food handler in hotel outbreak of Norwalk‐like viral gastro‐enteritis: implications for control. Lancet ii , 321–323. [DOI] [PubMed] [Google Scholar]
- 103. Reid, T.M.S. & Robinson, H.G. (1987) Frozen raspberries and hepatitis A. Epidemiology and Infection 98 , 109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Rosenblum, L.S. , Mirkin, I.R. , Allen, D.T. , Safford, S. , Hadler, S.C. (1990) A multifocal outbreak of hepatitis A traced to commercially distributed lettuce. American Journal of Public Health 80 , 1075–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sadovski, A.Y. , Fattal, B. , Goldberg, D. , Katzenelson, E. , Shuval, H.I. (1978) High levels of microbial contamination of vegetables irrigated with wastewater by the drip method. Applied and Environmental Microbiology 36 , 824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sattar, S.A. , Springthorpe, V.S. , Karim, Y. , Loro, P. (1989) Chemical disinfection of non‐porous inanimate surfaces experimentally contaminated with four human pathogenic viruses. Epidemiology and Infection 102 , 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sattar, S.A. , Springthorpe, V.S. , Tetro, J.A. (2001) Rotavirus. In Foodborne Disease Handbook, 2nd edn, Vol. 2. Viruses, Parasites, Pathogens and HACCP, eds Hui, Y.H., Sattar, S.A., Murrell, K.D., Nip, W.K. and Stanfield, P.S., pp. 99–125. New York: Marcel Dekker.
- 108. Sattar, S.A. & Tetro, J.A. (2001) Other foodborne viruses. In Foodborne Disease Handbook, 2nd edn, Vol. 2. Viruses, Parasites, Pathogens and HACCP, eds Hui, Y.H., Sattar, S.A., Murrell, K.D., Nip, W.K. and Stanfield, P.S., pp. 127–136. New York: Marcel Dekker.
- 109. Sattar, S.A. , Tetro, J. , Bidawid, S. , Farber, J. (2000) Foodborne spread of hepatitis A: recent studies on virus survival, transfer and inactivation. Canadian Journal of Infectious Disease 11 , 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Scholtz, E. , Heinricy, U. , Flemhig, B. (1989) Acid stability of hepatitis A virus. Journal of General Virology 70 , 2481–2485. [DOI] [PubMed] [Google Scholar]
- 111. Sellwood, J. , Appleton, H. , Wyn‐Jones, P. (1999) Review of sources of enteroviruses in the environment. EC Bathing Waters Directive Enterovirus Research Report. Ref. No 99/WW/11/1. London: UK Water Industry Research Ltd.
- 112. Sellwood, J. , Shore, J. , Green, J. (2000) Small round structured viruses in environmental water samples. PHLS 25th Annual Scientific Conference Abstracts, pp. 112.
- 113. Seo, K.H. & Frank, J.F. (1999) Attachment of Escherichia coli O157: H7 to lettuce leaf surface and bacterial viability in response to chlorine treatment as demonstrated by using confocal scanning laser microscopy. Journal of Food Protection 62 , 3–9. [DOI] [PubMed] [Google Scholar]
- 114. Seymour, I.J. (1999) Review of current industry practice on fruit and vegetable decontamination. CCFRA Review 14. Chipping Campden, Gloucestershire: Campden and Chorleywood Food Research Association (CCFRA). [Google Scholar]
- 115. Simons, L.K. & Sanguansri, P. (1997) Advances in the washing of minimally processed vegetables. Food Australia 49 , 75–80. [Google Scholar]
- 116. Slomka, M. & Appleton, H. (1998) Feline calicivirus as a model system for heat inactivation studies on small round structured viruses in shellfish. Epidemiology and Infection 121 , 401–407.DOI: 10.1017/S0950268898001290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Smith, M.A. (1982) Retention of Bacteria, Viruses and Heavy Metals on Crops Irrigated with Reclaimed Water. Canberra: Australian Water Resource Council, pp. 308.
- 118. Sobsey, M.D. , Shields, P.A. , Hauchman, F.S. , Davis, A.L. , Rullman, V.A. , Bosch, A. (1988) Survival and persistence of hepatitis A in environmental samples. In: Viral Hepatitis and Liver Disease, ed. Zuckerman, A.J., pp. 121–124. New York: Alan Liss Inc.
- 119. Springthorpe, V.S. , Grenier, J.L. , Lloyd‐Evans, N. , Sattar, S.A. (1986) Chemical disinfection of human rotaviruses: efficacy of commercially available products in suspension tests. Journal of Hygiene 97 , 139–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Stolle, A. & Sperner, B. (1997) Viral infections transmitted by food of animal origin: the present situation in the European Union. Archives of Virology 13 , 219–228. [DOI] [PubMed] [Google Scholar]
- 121. Sullivan, R. & Tierney, J.T. (1976) Persistence of poliovirus 1 in soil irrigated with inoculated sewage sludge and effluent. Abstracts of the Annual Meeting of the American Society for Microbiology, pp. 192.
- 122. Tierney, J.T. & Sullivan, R. (1976) Persistence of poliovirus 1 on vegetables grown in soil previously flooded with sewage sludge or effluent. Abstracts of the Annual Meeting of the American Society for Microbiology, pp. 192. [DOI] [PMC free article] [PubMed]
- 123. Tierney, J.T. , Sullivan, R. , Larkin, E.P. (1977) Persistence of poliovirus 1 in soil and on vegetables grown in soil previously flooded with inoculated sewage sludge or effluent. Applied and Environmental Microbiology 33 , 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ward, B.K. , Chenoweth, C.M. , Irving, L.G. (1982) Recovery of viruses from vegetable surfaces. Applied and Environmental Microbiology 44 , 1389–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Weiss, C. & Clark, H.F. (1987) Rapid inactivation of rotaviruses by exposure to acid buffer or acid gastric juice. Journal of General Virology 66 , 2725–2730. [DOI] [PubMed] [Google Scholar]
- 126. Westwood, J.C.N. & Sattar, S.A. (1976) The minimal infective dose. In: Viruses in Water, eds Berg, G., Bodily, H.L., Lennette, E.H., Melnick, J.L. and Metcalf, T.G., pp. 61–69. Washington, DC: American Public Health Association.
- 127. Wheeler, J.G. , Sethi, D. , Cowden, J.M. et al. on behalf of the Infectious Intestinal Disease Study Executive (1999) Study of infectious disease in England: rates in the community, presenting to general practice, and reported to national surveillance. British Medical Journal 318 , 1046–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Williams, I.T. , Bell, B. , Berry, D. , Shapiro, C. (1995) Foodborne outbreak of hepatitis A, Arkansas. Epidemic Intelligence Service 44th Annual Conference, 27–31 March 1994, p. 19. Atlanta, Georgia: Centers for Disease Control and Prevention.
- 129. Wyn‐Jones, A.P. , Pallin, R. , Dedoussis, C. , Shore, J. , Sellwood, J. (2000) The detection of small round structured viruses in water and environmental materials. Journal of Virology Methods 87 , 99–107. [DOI] [PubMed] [Google Scholar]
- 130. Zhang, S. & Farber, J.M. (1996) The effects of various disinfectants against Listeria monocytogenes on fresh‐cut vegetables. Food Microbiology 13 , 311–321. [Google Scholar]


