Abstract
This report describes the occurrence of non‐weightbearing lameness caused by Mycoplasma felis monoarthritis in two, immunocompetent, European, shorthair adult cats with a suspected history of trauma. Clinical signs recurred after conservative treatment. The joints were treated surgically and M felis was identified as the causative agent for the monoarthritis. Medication with 10 mg/kg doxycycline twice daily was initiated according to susceptibility testing. One cat underwent further joint flushing after two weeks; both the cats recovered completely after eight and nine weeks, respectively. The findings suggest that M felis, in addition to being an agent associated with conjunctivitis in cats, is able to act as a pathogen in other tissues and cause arthritis even in immunocompetent cats.
Introduction
Mycoplasma species are prokaryotic bacteria without a cell wall and are considered to be normal flora of mucous membranes in cats and dogs. They appear to be part of the physiological pharyngeal flora in one‐third of the feline population (Randolph and others 1993) and have been isolated from the conjunctiva, upper respiratory tract and urogenital tract in this species (Heyward and others 1969, 1979). They cannot be classified as completely benign as they have been isolated in conjunction with lower respiratory tract infections and conjunctivis (Campbell and others 1973, 1998, 2004a, 2004b). Mycoplasma felis and Mycoplasma gateae are the species most often found in clinically affected animals. An extensive literature search revealed two reports describing Mycoplasma polyarthritis in cats (Moise and others 1985, 1983). In the current case M felis was found in the elbow joint of one cat and in the tarsal joint of another with painful septic monoarthritis. Surgical treatment and medication of the patients with doxycycline (Vibramycin; Pfizer) resulted in resolution of the disorder.
Case Histories
Case 1
A 17‐year‐old, male, neutered, European shorthair cat weighing 3·8 kg was referred with a non‐weightbearing lameness of the left hind leg. The cat had been outside, unattended, the day before and the owner reported a sudden onset of lameness. The cat had no history of illness and had always been active. Clinical evaluation revealed a swollen, painful left tarsal joint and a slight crepitus could be provoked during careful manipulation. Rectal temperature was elevated (39·6°C); the cat was depressed, mildly dehydrated and panting; and a holosystolic heart murmur grade II/VI was heard during auscultation over the left heart base.
Radiographs revealed a lateral slab fracture of the calcaneus, with a free joint body and pronounced joint effusion (Fig 1). Thoracic radiographs were normal. Electrocardiographic findings showed a normal sinus rhythm (180 bpm). Two‐dimensional echocardiography, from the right parasternal long axis view, revealed a left ventricular concentric hypertrophy and a moderate left atrial dilation. Doppler examination showed a mild transmitral systolic regurgitation. No signs of a vegetative valvular lesion could be detected.
Figure 1.
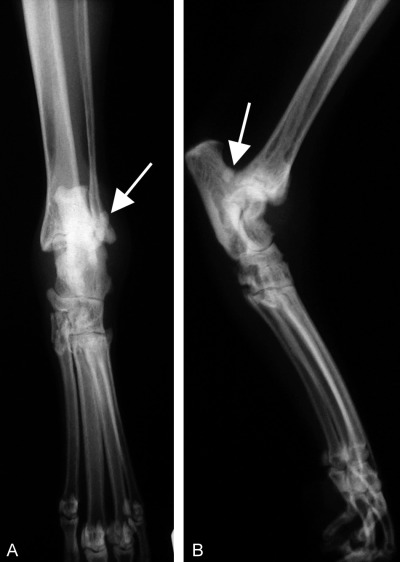
Craniocaudal (A) and mediolateral (B) views of the tarsal joint of cat 1. The free joint body is indicated with an arrow
Results of a complete blood count (CBC) showed leucocytosis (22·6×109 cells/l [reference range 6·0 to 18·0×109 cells/l]), with elevated numbers of segmented neutrophils (20·2 × 109 cells/l [reference range 3·6 to 12·8×109 cells/l]). A serum biochemistry profile revealed hyperproteinaemia (92·5 g/l [reference range 60 to 75 g/l]), hyperglycaemia (13·8 mmol/l [reference range 3·1 to 5·6 mmol/l]), elevated alanine aminotransferase activity (82 units/l [reference range <50 units/l]) and elevated glutamate dehydrogenase activity (12·32 units/l [reference range <6 units/l]). Measurement of thyroxine was performed to rule out hyperthyroidism, and it was found to be 12·9 mmol/l (reference range 19 to 46 mmol/l). Serological testing for antibodies to feline coronavirus and feline immunodeficiency virus (FIV) and an ELISA test for feline leukaemia group antigen p27 were negative.
A joint involvement from a bite wound was suspected. Ringer’s solution was administered intravenously (iv), and after correction of the fluid imbalance, the cat was premedicated with 0·4 mg/kg butorphanol (Butomidor; Richter Pharma), 0·2 mg/kg midazolam (Midazolam; Mayerhofer) and 1 mg/kg ketamine (Ketasol; Graeub), induced with propofol (Propofol; Fresenius Kabi); and anaesthesia was maintained with isoflurane (Forane; Abbott) administered to effect. Before surgery, the cat received an epidural injection of lidocaine (Xylanaest purum; Gebro Pharma) and bupivacain (Carbostesin; AstraZeneca). No traces of a bite wound and scars were detected after clipping the leg. Left tarsal joint arthrotomy yielded large quantities of yellow‐grey, turbid joint fluid. The oedematous joint capsule and the periarticular tissue contained large amounts of fibrin. The free intra‐articular chip was removed, synovectomy was performed, the joint was lavaged with isotonic Ringer’s solution, and swabs for bacterial culture and sensitivity testing were taken. For three days, a Penrose drain was placed intra‐articularly and an immobilising splint was applied.
The swabs were examined for bacteria including mycoplasmas and fungi. Mycoplasmas were recovered abundantly from swab samples. Other bacteria and fungi were not detected. For identification of Mycoplasma isolates, the colony immunoblot technique using specific rabbit hyperimmune sera against feline Mycoplasma species was performed. Using this method, isolates were identified as M felis.
Perioperative medication consisted of 20 mg/kg cefazolin (Cefazolin; Sandoz) iv every eight hours and an opioid‐agonist, 5 mg tramadol (Tramal; Gruenenthal) given orally every 24 hours.
The cat received 2·2 mg/kg carprofen (Rimadyl; Pfizer) every second day for six days beginning on the day of surgery. After isolation and identification of M felis, antibiotic therapy was changed appropriately to 10 mg/kg doxycyline orally twice daily and this was continued for four weeks. Prescott (2000) recommended that the doxycycline be given in two doses of 10 mg/kg orally, followed by 5 mg/kg orally. Antibiotics are not generally recommended to be injected intra‐articularly since this may provoke chemical synovitis. In order to keep the antibiotic level as high as possible in the affected joint, the high dose of 10 mg/kg was also continued for the maintenance therapy. The cat did not show any side effects during the administration period. For treatment of the hypertrophic cardiomyopathy diltiazem (Dilzem; Goedecke), 8 mg orally twice daily was chosen because of its negative inotropic effect and positive lusitropic effect. The angiotensin‐converting enzyme inhibitor, 1·25 mg benazepril hydrochloride (Fortekor; Novartis) orally once daily, was added to alleviate the effects of the secondary mitral valve insufficiency by reducing cardiac pre‐ and afterload.
The cat recovered well from anaesthesia and surgery and showed some improvement, although it was still lame two weeks after surgery. As there was an amount of joint swelling present, the joint was again flushed. The immobilising splint was placed for an additional four weeks. The lameness resolved completely nine weeks after initial surgery.
Case 2
A seven‐year‐old, male, neutered, European shorthair cat, weighing sustained a bite wound in the left elbow region. It was treated by the referring veterinarian, including wound debridement and administration of antibiotics and analgesics. Although the skin wound healed well and the cat was initially able to walk normally, after six days, a non‐weightbearing lameness developed on the injured foreleg.
The cat was presented to the clinic seven days after the initial trauma, and at this time, swelling and pain were found in the left elbow region. Similar to cat 1, this cat was also depressed, febrile (39·9°C) and hyperproteinaemic (83 g/l). A CBC showed monocytosis (1·1×109 cells/l [reference of <0·5×109 cells/l]); however, leucocytosis was not present. Serological testing for antibodies to feline coronavirus and FIV and an ELISA test for feline leukaemia group antigen p27 were negative. Radiographs and ultrasound showed a periarticular swelling but did not reveal involvement of the joint. The cat was treated with 0·1 mg/kg meloxicam (Metacam; Boehringer Ingelheim) orally once daily. The cat showed no improvement after a period of 10 days, and radiographic evaluation was repeated: the periarticular swelling was more pronounced, and two radiodensities, 2 mm in diameter, were visible proximolateral to the tuber olecrani.
A decision to perform exploratory surgery was made, and the cat received 20 μg/kg medetomidine (Domitor; Pfizer), 0·4 mg/kg butorphanol and 1 mg/kg ketamine for premedication and was induced with propofol iv and maintained with isoflurane and an iv continuous rate infusion of 2 μg/kg/hour fentanyl (Fentanyl; Janssen‐Cilag). Perioperative antibiotic treatment consisted of 20 mg/kg cefazolin iv every eight hours, and this was changed to oral medication of cefalexin (Ospexin; Sandoz) after recovery from anaesthesia.
Lateral arthrotomy of the left elbow of cat 2 revealed similar findings to that in cat 1. The joint was flushed and debrided as described in case 1. Postoperatively, the cat received 0·1 mg/kg methadone (Heptadon; Ebewe) intramuscularly and 0·2 mg/kg meloxicam subcutaneously. Meloxicam was continued orally for five days. After detection of M felis, oral antibiotic therapy with 10 mg/kg doxycycline orally twice daily was initiated and continued for five weeks. Similar to cat 1, no side effects of the high dose of doxycycline was seen in this cat. The immobilising splint was removed after three weeks, the cat was only slightly lame by the end of the first week following removal and was sound within two months.
Discussion
Commonly involved organisms in joint infections in cats are Pasteurella multocida and haemolytic strains of Escherichia coli (Pedersen and others 2000). Bacterial L‐form infections do occur and are manifested by fistulating subcutaneous wounds, which frequently spread to local and distant joints by local extension or haematogenously (Carro and others 1989). Two Mycoplasma species have been implicated as having pathogenic significance in the cat. While M gateae is usually recovered as a commensal of the respiratory and urogenital tract, Moise and others (1983) were able to infect healthy immunocompetent and immunosuppressed experimental cats with M gateae. Polyarthritis was induced in all the animals by iv inoculation; thus, a haematogenous spread is likely to be possible. This seems to occur in natural infections as well. M felis has been variously incriminated as a cause of feline conjunctivitis (Blackmore and Hill 1973, 1973, 1991), although this disease is often seen in conjunction with other more pathogenic agents (Blackmore and Hill 1973, 1973).
Hooper and others (1985) cultured M felis from the synovial fluid from septic joints in one cat. It had had a severe lymphocytic deficiency for its whole life and subsequently developed a multitude of illnesses culminating in severe polyarthritis. Bonilla and others (1997) reported arthritis of the left hip and right knee joint caused by M felis in a woman following exposure to cats. In both cases, there is no evidence of direct trauma, so again haematogenous spread seems to be the most likely explanation for the joint involvement. In cat 1, no joint‐penetrating injury could be confirmed, but injury due to acute blunt trauma cannot be excluded; thus, a systemic spread of M felis and subsequent manifestation in the possibly pre‐injured tarsus seem feasible. Although not reported previously, endocardial manifestation of the infection is possible; however, cardiac ultrasound did not reveal any signs of myocarditis and/or endocarditis.
According to radiographic and ultrasonographic findings in cat 2, no joint involvement was initially suspected, so the cat was treated conservatively. Since the disease did not respond adequately over a 10‐day period and the radiograph revealed increased swelling of the involved elbow region, the joint was also treated surgically. Only Mycoplasma organisms were present at the time of surgery. Taking into account that the cat had received antibiotic treatment for a period of time before surgery, the possibility that more pathogenic bacteria could have been the initial cause for the arthritis cannot be ruled out. It was concluded that M felis had at least perpetuated the arthritis, if not substantially caused it.
Conclusions
M felis was found to be the sole pathogenic agent causing monoarthritis in these immunocompetent cats. These findings suggest that M felis, in addition to being an agent causing conjunctivitis, can also become pathogenic for other tissues. Under yet unidentified predisposing factors, M felis could be expected to be a pathogen that can cross the mucosal barrier to cause systemic infections that could contribute to arthritis.
Acknowledgements
The authors would like to thank Dr A. Böhler and Dr K. Hittmayer, Department for Diagnostics, Clinic for Radiology, University of Veterinary Medicine, Vienna, for the provision of the radiographs; and Dr U. Kolm, Department for Small Animals and Horses, Clinic for Internal Medicine and Infectious Diseases, Cardiology Service, University of Veterinary Medicine, Vienna, for her help in revising this report.
References
- Blackmore, D. K. & Hill, A . (1973) The experimental transmission of various mycoplasmas of feline origin to domestic cats (Felis catus). Journal of Small Animal Practice 14, 7‐13 [DOI] [PubMed] [Google Scholar]
- Bonilla, H. F. , Chenoweth, C. E. , Tully, J. G. , Blythe, L. K. , Robertson, J. A. , Ogenovski, V. M. & Kauffman, C. A . (1997) Mycoplasma felis septic arthritis in a patient with hypogammaglobulinemia. Clinical Infectious Diseases 24, 222‐225 [DOI] [PubMed] [Google Scholar]
- Campbell, L. H. , Snyder, M. S. , Reed, V. M. D. & Fox, J. G . (1973) Mycoplasma felis‐associated conjunctivitis in cats. Journal of the American Veterinary Medical Association 163, 991‐995 [PubMed] [Google Scholar]
- Carro, T. , Pedersen, N. C. , Beaman, B. L. & Munn, R . (1989) Subcutaneous abscesses and arthritis caused by a probable bacterial L‐form in cats. Journal of the American Veterinary Medical Association 194, 1583‐1588 [PubMed] [Google Scholar]
- Foster, S. F. , Barrs, V. R. , Martin, P. & Malik, R . (1998) Pneumonia associated with Mycoplasma spp in three cats. Australian Veterinary Journal 76, 460‐464 [DOI] [PubMed] [Google Scholar]
- Foster, S. F. , Martin, P. , Allan, G. S. , Barrs, V. R. & Malik, R . (2004a) Lower respiratory tract infections in cats: 21 cases (1995‐2000). Journal of Feline Medicine and Surgery 6, 167‐180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, S. F. , Martin, P. , Braddock, J. A. & Malik, R . (2004b) A retrospective analysis of feline bronchoalveolar lavage cytology and microbiology (1995‐2000). Journal of Feline Medicine and Surgery 6, 189‐198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haesebrouck, F. , Devriese, L. A. , Van Rijssen, B. & Cox, E . (1991) Incidence and significance of Mycoplasma felis from conjunctival swabs of cats. Veterinary Microbiology 26, 95‐101 [DOI] [PubMed] [Google Scholar]
- Heyward, J. T. , Sabry, M. Z. & Dodle, W. R . (1969) Characterization of mycoplasma species of feline origin. American Journal of Veterinary Research 30, 615‐622 [PubMed] [Google Scholar]
- Hooper, P. T. , Ireland, L. A. & Carter, A . (1985) Mycoplasma polyarthritis in a cat with probable severe immune deficiency. Australian Veterinary Journal 62, 352 [DOI] [PubMed] [Google Scholar]
- Moise, N. S. , Crissman, W. , Fairbrother, J. F. & Baldwin, C . (1983) Mycoplasma gateae arthritis and tenosynovitis in cats: case report and experimental reproduction of the disease. American Journal of Veterinary Research 44, 16‐22 [PubMed] [Google Scholar]
- Pedersen, N. C. , Morgan, J. P. & Vasseur, P. B . (2000) Joint diseases of dogs and cats In: Textbook of Veterinary Internal Medicine. 5th edn Eds Ettinger S. J. and Feldmann E. C. W. B. Saunders, Philadelphia, PA, USA. pp 1862‐1886 [Google Scholar]
- Prescott, J. F. (2000) Tetracyclines In: Antimicrobial Therapy – Veterinary Medicine 3rd edn. Eds Prescott J. F., Baggot J. D. and Walker R. D. Iowa state University Press, Ames, IA, USA. pp 275‐289 [Google Scholar]
- Randolph, J. F. , Moise, N. S. , Scarlett, J. M. , Shin, S. J. , Blue, J. T. & Corbett, J. R . (1993) Prevalence of mycoplasmal and ureaplasmal recovery from tracheobronchial lavages and prevalence of mycoplasmal recovery from pharyngeal swab specimens in cats with or without pulmonary disease. American Journal of Veterinary Research 54, 897‐900 [PubMed] [Google Scholar]
- Rosendal, S . (1979) Canine and feline mycoplasmas In: The Mycoplasmas II. Eds Tully J. G. and Whitcomb R. F. Academic Press, New York, NY, USA. pp 217‐233 [Google Scholar]


