Abstract
Human bocavirus (HBoV) infection is reported worldwide and may cause severe respiratory tract infections. The aim of the present study was to assess the prevalence of HBoV, and other respiratory viral pathogens, in a 2‐year retrospective study of children admitted to hospital, and to investigate whether viral loads of HBoV DNA were associated with severity of infection. Between April 2007 and March 2009, 891 respiratory samples from 760 children admitted to hospital with acute respiratory tract infection were tested for the presence of respiratory viruses by real‐time PCR or direct immunofluorescence testing. HBoV DNA was detected by using internally controlled real‐time quantitative PCR assay and 25 samples selected at random were sequenced. The virus detected most frequently was rhinovirus, followed by respiratory syncytial virus, HBoV, and human metapneumovirus. HBoV DNA was detected in 18.4% of children admitted to hospital. HBoV was the only viral pathogen detected in 66/164 (40.2%) of HBoV DNA‐positive children and in 7.4% of all 891 samples. Ninety‐seven percent (64/66) of children with an HBoV single infection were diagnosed as having lower respiratory tract infection. Median HBoV DNA viral load was significantly higher in children when HBoV was detected as a single pathogen. Higher HBoV DNA viral loads were associated with prematurity and age. HBoV seems to be an important and frequent pathogen in respiratory tract infections in children, and it is likely that the severity of illness is comparable to the severity of RSV illness. J. Med. Virol. 84:99–108, 2011. © 2011 Wiley Periodicals, Inc.
Keywords: human bocavirus, respiratory viral infection, infection severity, viral load
INTRODUCTION
Human bocavirus (HBoV) was discovered in 2005 in nasopharyngeal aspirates of Swedish children with lower respiratory tract infection [Allander et al., 2005]. Respiratory infection with HBoV occurs worldwide with frequencies ranging from 1.5% to 19.3% in respiratory samples of children [Bastien et al., 2006; Weissbrich et al., 2006; Neske et al., 2007; Bonzel et al., 2008; Brieu et al., 2008; von Linstow et al., 2008; Albuquerque et al., 2009; Fabbiani et al., 2009; Calvo et al., 2010; Kaida et al., 2010; Moriyama et al., 2010; Midulla et al., 2010; Miron et al., 2010; Song et al., 2010]. HBoV was also detected in stool specimens of children admitted to hospital for acute gastroenteritis [Albuquerque et al., 2007; Lee et al., 2007; Chieochansin et al., 2008; Kapoor et al., 2009, 2010; Szomor et al., 2009; Chow et al., 2010]. Infections are known in children and adults, but children younger than 2 years appear to be most at risk of HBoV infection. HBoV was found recently in children with lower respiratory tract infection, and several studies show an association between HBoV and acute respiratory symptoms, which is consistent with a causal role [Allander et al., 2005; Kesebir et al., 2006; Manning et al., 2006; Fry et al., 2007; Maggi et al., 2007]. Most studies confirmed a high rate of coinfections, where HBoV occurs simultaneously with other respiratory viruses, thereby making the association of HBoV with disease more complex [Allander, 2008; Schildgen et al., 2008]. There are also a few reports of the persistence of HBoV DNA in respiratory and blood samples, or prolonged shedding of HBoV in infected patients [Allander et al., 2007; von Linstow et al., 2008; Martin et al., 2010].
The aim of this study was to assess the frequency and clinical features of HBoV infections in Slovenian children admitted to hospital with respiratory tract infections. Viral loads of HBoV DNA were determined in respiratory samples, and sequencing of amplified HBoV DNA was used to characterize virus strains present in the population. Clinical features of HBoV infections were compared with infections with other frequently detected respiratory viruses, such as respiratory syncytial virus (RSV), human rhinoviruses (hRV), human metapneumoviruses (hMPV), and human coronaviruses (hCoV).
PATIENTS AND METHODS
Study Population and Clinical Specimens
Between April 2007 and March 2009, 891 respiratory samples from 760 pediatric patients admitted to hospital with respiratory tract infection were submitted for routine testing of respiratory viruses from University Children's Hospital in Ljubljana and University Medical Centre in Ljubljana. Virtually all of the patients from Pulmonology department of Pediatric Children's hospital that were admitted to hospital between April 2007 and March 2009 were included in the study. A majority of children with lower respiratory tract infection which require hospital treatment and are located in central part of Slovenia are treated in this department every year. Each of the 891 samples represented a different hospitalization event. During the study, 664 patients were hospitalized once, 76 patients twice, and 20 patients three times or more.
Of 891 respiratory samples, 94% were nasopharyngeal swabs, 4% were throat swabs, and 2% were tracheal aspirates and bronchoalveolar lavages. Flocked swabs were transported in 2 ml of liquid viral transport medium (Copan Italia S.p.A., Brescia, Italy). Clinical and laboratory data were collected retrospectively from medical records and anonymized.
The medical records were reviewed for all studied patients and episodes, regardless of diagnostic results, including patients negative for all viruses tested. Since the study was retrospective, informed consent from the patients was not obtained, but the research was approved by the National Medical Ethics Committee of the Republic of Slovenia. Also, the principles of the Helsinki Declaration, the Oviedo Convention on Human Rights and Biomedicine and the Slovene Code of Medical Deontology were followed in the conduct of this research. No additional sample was taken from the patients for the purpose of the study.
Nucleic Acid Extraction and HBoV Quantitative Real‐Time PCR
Automatic nucleic acid extraction was carried out using a total nucleic acid isolation kit on a MagNa Pure Compact instrument (Roche Applied Science, Mannheim, Germany), according to the manufacturer's instructions. One hundred ninety‐five microliters of respiratory sample was used for extraction, and 5 µl of equine herpesvirus 1 (EHV1) was added as an internal control, to give a final volume of 200 µl. Nucleic acids were eluted in 100 µl of elution buffer. EHV1 was added and titrated to yield a threshold cycle (Ct) value of approximately 30 in real‐time PCR.
Multiplex real‐time PCR for HBoV and EHV1 was performed using Platinum Quantitative PCR SuperMix‐UDG kit (Invitrogen, Carlsbad, CA). Amplification of part of the NS1 gene of HBoV and part of the EHV1 glycoprotein B gene was performed using previously described assays [Diallo et al., 2006; Lu et al., 2006]. Each 25 µl of PCR mixture contained 2.5 µl of nucleic acid extract. Amplification was conducted using a StepOne instrument (Applied Biosystems, Foster City, CA). For standard curve generation, parts of the NS1 and NP1 genes of HBoV DNA were cloned previously and used as standards in serial dilutions [Lu et al., 2006].
Testing for Other Respiratory Viruses
The samples were tested routinely for the presence of the antigens of respiratory viruses, by direct immunofluorescence assay (DFA). The antigens of adenovirus (AdV), RSV, influenza viruses A and B (Flu A/B), and parainfluenza viruses 1–3 (PIV 1–3) were detected using monoclonal antibodies (Oxoid, Cambridge, UK). Aliquots of samples were used for detection of viruses by DFA, and all samples were stored frozen prior to nucleic acid extraction and further analysis.
All samples were tested by RT‐PCR for hRV, hMPV, and hCoV, as described previously [Maertzdorf et al., 2004; Scheltinga et al., 2005; Kuypers et al., 2007].
Sequencing of HBoV DNA
Twenty‐five HBoV DNA‐positive samples (viral load >105 copies/ml of sample) were selected randomly for direct sequencing. The 1,136 bp VP1/VP2 gene region of the HBoV genome was amplified separately using Promega PCR Master Mix (Promega, Madison, WI). PCR products were purified and sequenced subsequently using BigDye terminator chemistry on an ABI PRISM 310 genetic analyzer (Applied Biosystems).
Statistical Analysis
Statistical analysis was carried out using the SPSS (15.0) software package (SPSS, Inc., Chicago, IL). Categorical variables between groups were compared using the chi square test or the two‐tailed Fisher's exact test. For continuous variables, comparisons were made using the nonparametric Mann–Whitney U‐test. P < 0.05 was considered to be significant.
RESULTS
Patient Characteristics
During the study period, there were 891 different admissions to hospital of 760 children aged 0–17 years. The majority of children were below the age of 3 years, with 36.2% of children aged between 0 and 12 months, 32.7% of children aged between 13 and 24 months, and 19.9% of children aged between 25 and 36 months. Only 11.2% of the study population was older than 3 years of age. The median age of the study population was 17 months, and 56.1% were males. Lower respiratory tract infection was diagnosed in 96% of the study population, and bronchiolitis was diagnosed in 59.6% of cases, followed by radiologically confirmed pneumonia in 19% of cases. An underlying condition (cardiac and pulmonary conditions, prematurity, and congenital immunodeficiency) was observed in 27.3% of cases. Recurrent wheezing and asthma were identified in 9% and 6.7% of cases, respectively. In this study, 2.7% of children admitted to hospital were transferred to the intensive care unit.
Virus Identification
At least one potential viral pathogen was detected in 75% (668/891) of respiratory samples. Single respiratory viral infections were detected in 75.3% (503/668) of the positive samples and coinfections in 24.7% (165/668). Infection with hRV was detected in 33.1% of all patients admitted to hospital, RSV in 23.5%, and HBoV in 18.4%. Infections with hMPV and hCoV were less frequent and were detected only in 9% and 8.4% of patients, respectively. Adenoviruses, influenza virus A and B, and parainfluenza viruses 1 and 3 were detected in 0.8%, 0.3%, 0.6%, and 0.9% of cases, respectively. Seasonal variation was observed in the respiratory viruses detected most frequently, with a high HBoV infection rate in late winter and early spring, and very low or no HBoV infection rate in summer. A significant difference in the annual frequency of HBoV infections was observed between the two winter seasons of 2007–2008 (111/503, 22.1%) and 2008–2009 (53/388, 13.7%) (P = 0.001). Seasonal variations in HBoV, and the other respiratory viruses detected most frequently, are shown in Figure 1.
Figure 1.
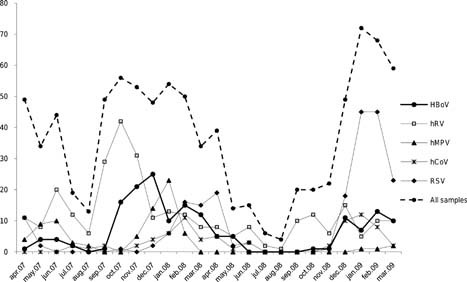
Seasonal distribution of HBoV, hRV, RSV, hMPV, and hCoV, five respiratory viruses detected most frequently among children admitted to hospital are shown.
HBoV was detected as a single pathogen in 40.2% (66/164) of HBoV DNA‐positive respiratory samples. hRV, RSV, hMPV, and hCoV were found as single pathogens in 71.5% (211/295), 68.4% (143/209), 61.2% (49/80), and 28% (21/75) of individual virus‐positive samples, respectively (Fig. 2).
Figure 2.
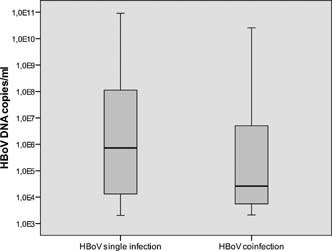
Comparison of HBoV median viral load between children where HBoV was found as a sole pathogen and children with HBoV coinfection.
The viruses found most frequently in HBoV coinfections were hRV in 53.1% of respiratory samples (52), RSV in 28.6% (28), human metapneumovirus in 17.3% (17), hCoV in 10.2% (10), influenza virus A in 1% (1), and parainfluenza virus 3 in 1% (1).
Clinical Characteristics of Children With an HBoV Single Infection
HBoV DNA was detected in 18.4% (164/891) of respiratory samples, which represented 20.8% (158/760) of the patients studied. HBoV was detected as a single pathogen in 7.4% (66/891) of respiratory samples.
The median age of children with single HBoV infection was 17 months, and males were infected more frequently (P = 0.027). The median duration of hospitalization and oxygen therapy was 4.5 and 2 days, respectively. The majority of HBoV‐positive children (53%) were aged in the range of 13–24 months (P = 0.003). Bronchiolitis was the leading diagnosis (63.6%), followed by pneumonia (24.2%) and recurrent wheezing (9.1%). Only two (3%) children with HBoV single infection had upper respiratory tract infection.
The symptoms reported most frequently were dyspnea, cough, hypoxia, rhinorrhea, and wheezing. Fever, gastrointestinal symptoms, and conjunctivitis were present, but less frequently. Fourteen patients (21.2%) had a single HBoV infection with underlying diseases. Five patients had cardiac/pulmonary or other congenital underlying diseases. There were six cases of prematurity (<37 weeks), two cases of prematurity and cardiac/pulmonary or other congenital underlying diseases, and one patient with congenital immunodeficiency (Table I).
Table I.
Clinical, Laboratory, and Demographic Characteristics of Studied Population and HBoV DNA‐Positive Children, Classified According to HBoV Single or HBoV Coinfection
| Characteristics | HBoV single infection | HBoV mixed infection | All HBoV infected | Study population |
|---|---|---|---|---|
| Demographic data | ||||
| Number of subjects | n = 66 | n = 98 | n = 164 | n = 891 |
| Age, median (months) | 17 | 16 | 16 | 17 |
| Gender—F:M ratio | 1:1.75 | 1:1.51 | 1:1.56 | 1:1.28 |
| Hospitalization in days, median (range) | 4.5 (1–13) | 4 (1–15) | 4 (1–15) | 4 (1–25) |
| Oxygen therapy in days, median (range) | 2 (0–12) | 2 (0–14) | 2 (0–14) | 2 (0–22) |
| Underlying disease | ||||
| Prematurity (<37 weeks) | 8 (12.1%) | 8 (8.2%) | 16 (9.8%) | 97 (10.9%) |
| Immunodeficiency | 1 (1.5%) | 0 | 1 (0.6%) | 11 (1.2%) |
| Other important underlying diseases (cardiac, pulmonary, congenital disorders) | 7 (10.6%) | 14 (14.3%) | 21 (12.8%) | 172 (19.3%) |
| Clinical findings | ||||
| Fever >38°C | 23 (34.8%) | 26 (26.5%) | 49 (29.9%) | 241 (27%) |
| Cough | 59 (89.4%) | 86 (87.8%) | 145 (88.4%) | 718 (80.6%) |
| Rhinorrhea | 44 (66.7%) | 70 (71.4%) | 114 (69.5%) | 538 (60.4%) |
| Dyspnea | 59 (89.4%) | 93 (94.9%) | 152 (92.7%) | 792 (88.9%) |
| Wheezing | 37 (56.1%) | 63 (64.3%) | 100 (61%) | 507 (56.9%) |
| Conjunctivitis | 3 (4.5%) | 8 (8.2%) | 11 (6.7%) | 38 (4.3%) |
| Vomiting/diarrhea | 7 (10.6%) | 11 (11.2%) | 18 (11%) | 74 (8.3%) |
| Otitis | 4 (6.1%) | 7 (7.1%) | 11 (6.7%) | 34 (3.8%) |
| Laboratory findings | ||||
| CRP >8 mg/L | 38/63 (60.3%) | 52/98 (53%) | 90/161 (55.9%) | 455/856 (53.2%) |
| WBC × 109 cells/L, median | n = 65, 13.5 | n = 98, 11.65 | n = 163, 12.8 | n = 868, 12.4 |
| Hypoxia (sat. O2 <94%) | 53 (80.3%) | 80 (81.6%) | 133 (81.1%) | 650 (73%) |
| Chest radiographic findings | ||||
| X‐ray performed | 17 (25.8%) | 19 (19.3%) | 36 (21.9%) | 257 (28.8%) |
| X‐ray pathology (infiltrates, hyperinflation) | 16 (24.2%) | 18 (18.4%) | 34 (20.7%) | 251 (28.2%) |
| Final diagnosis | ||||
| Upper respiratory tract infection | 2 (3%) | 2 (2%) | 4 (2.4%) | 36 (4%) |
| Lower respiratory tract infection | 64 (97%) | 96 (98%) | 160 (97.6%) | 855 (96%) |
| Pneumonia | 16 (24.2%) | 14 (14.3%) | 30 (18.3%) | 169 (19%) |
| Bronchiolitis | 42 (63.6%) | 71 (72.4%) | 113 (68.9%) | 531 (59,6%) |
| Bronchitis | 0 | 1 (1.1%) | 1 (0.6%) | 10 (11.2%) |
| Recurrent wheezing | 6 (9.1%) | 7 (7.1%) | 13 (7.9%) | 82 (9%) |
| Asthma | 0 | 3 (3.1%) | 3 (1.8%) | 70 (6.7%) |
HBoV, human bocavirus; F:M, female:male; CRP, C reactive protein; WBC, white blood cells.
HBoV Viral Load
HBoV viral loads in the respiratory samples of children with acute respiratory tract infection ranged from 2.0 × 103 to 9.1 × 1010 copies/ml of sample material.
In this study, the HBoV viral loads of children with HBoV as a single pathogen were compared with the viral loads of children with coinfections. A significant difference was found between the median value of HBoV viral load for the two groups (7.4 × 105 copies/ml vs. 2.6 × 104 copies/ml; P = 0.005) (Fig. 3, Table II).
Figure 3.
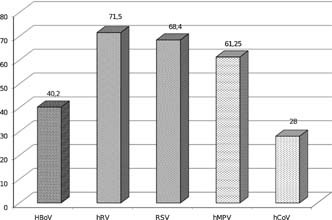
Frequencies of respiratory viral single infections among viruses detected most frequently in children are shown, 72% of all hCoV infections and only 28.5% of all hRV infections have been found as coinfection.
Table II.
Comparison of the Median Value of the HBoV DNA Viral Load in Children With a Single HBoV Infection and Children With HBoV Coinfection
| Characteristics | Median viral load in children with HBoV infection (copies/ml) | P‐value | |
|---|---|---|---|
| HBoV single infection (n = 66) | HBoV coinfection (n = 98) | ||
| Demographic data | |||
| Gender | |||
| Female | 1.8 × 107 (n = 24) | 1.0 × 104 (n = 39) | 0.005 |
| Male | 5.4 × 105 (n = 42) | 4.3 × 104 (n = 59) | 0.293 |
| Age groups | |||
| 0–12 months | 2.6 × 104 (n = 16) | 2.3 × 104 (n = 24) | |
| 24 months | 4.1 × 107 (n = 35) | 1.8 × 106 (n = 54) | 0.005 |
| ≥25 months | 1.9 × 104 (n = 15) | 1.1 × 104 (n = 20) | |
| Underlying disease | |||
| Prematurity, congenital immunodeficiency, or other congenital (pulmonary, cardiac) disorders | 4.1 × 107 (n = 14) | 1.2 × 104 (n = 19) | 0.001 |
| Laboratory findings | |||
| CRP <8 mg/L | 5.2 × 106 (n = 25) | 1.4 × 104 (n = 46) | 0.001 |
| WBC >10 × 109 cells/ml | 7.4 × 105 (n = 54) | 4.3 × 104 (n = 65) | 0.033 |
| Hypoxia (sat. O2 <94%) | 5.3 × 105 (n = 53) | 3.2 × 104 (n = 80) | 0.044 |
| Final diagnosis | |||
| Upper respiratory tract infection | 1.8 × 104 (n = 2) | 6.0 × 107 (n = 2) | 0.121 |
| Lower respiratory tract infection | 8.9 × 105 (n = 64) | 2.5 × 104 (n = 96) | 0.003 |
| Pneumonia | 2.2 × 107 (n = 16) | 1.3 × 104 (n = 14) | 0.096 |
| Bronchiolitis | 7.2 × 105 (n = 42) | 3.4 × 104 (n = 71) | 0.044 |
| Bronchitis | 0 | 2.3 × 105 (n = 1) | ND |
| Recurrent wheezing | 8.6 × 104 (n = 6) | 1.9 × 104 (n = 7) | 0.391 |
| Asthma | 0 | 3.5 × 103 (n = 3) | ND |
HBoV, human bocavirus; CRP, C‐reactive protein; WBC, white blood cells; ND, not done.
P‐values <0.05 are significant. Statistical values are in bold.
In 66 children with single HBoV infection, a relationship was observed with prematurity, child age, and HBoV DNA load. Prematurely born children had a higher median HBoV DNA load compared with children born at term (P = 0.015). Children aged between 13 and 24 months had a higher median HBoV DNA load compared with children in the other two age groups (age group 0–12 months: P = 0.031; age group >25 months: P = 0.007). The median HBoV DNA viral load was higher in females (1.8 × 107 copies/ml), children with pneumonia (2.2 × 107 copies/ml), and children with underlying diseases (4.1 × 107 copies/ml), but the differences were not significant (P = 0.54; P = 0.6; P = 0.1), data not shown.
In children with HBoV single infection, stratification of clinical data on the basis of HBoV DNA load did demonstrate significant differences between children with high (>105 HBoV DNA copies/ml, n = 39) and low (<105 HBoV DNA copies/ml, n = 27) HBoV DNA load in their respiratory samples. In the group of children with high HBoV DNA load wheezing was detected more frequently compared to children with low HBoV DNA load (P = 0.037) and the majority of children were within age group between 12 and 24 months (P = 0.002).
HBoV Single Infection Versus hRV, RSV, hMPV, and hCoV Single Infections
Duration of hospitalization and oxygen therapy in patients with HBoV infection were longer compared with children with hRV infection (P = 0.001, P = 0.009). Children with HBoV infection had less frequently underlying diseases (P = 0.05). Among children infected with HBoV, fever (>38°C), cough, hypoxia, and conjunctivitis were present more frequently compared with children infected with hRV (P = 0.006, P = 0.034, P = 0.01, P = 0.043). Asthma was diagnosed more frequently in children with hRV infection compared with HBoV, where no subjects were diagnosed with asthma (P = 0.001).
Compared with RSV infection, HBoV‐infected children were older (P < 0.001), and there was a tendency to more male infections (P = 0.052). There was no significant difference in the duration of hospitalization and/or oxygen therapy. The median value of the WBC count was significantly lower in RSV‐positive patients compared with HBoV‐positive patients (P = 0.001).
Compared with hMPV‐infected children, the children with HBoV infection had a cough and rhinorrhea more frequently (P = 0.045, P = 0.038), and the median value of leukocytes was significantly higher (P < 0.001).
The duration of oxygen therapy was shorter in hCoV‐infected patients compared with children with HBoV infection (P = 0.012). Children with HBoV infection required more frequently oxygen therapy (P = 0.021), but had less frequently underlying diseases (P = 0.005), when compared with hCoV‐infected children. Cough and rhinorrhea were more frequent in children with HBoV infection (P = 0.007, P = 0.005), and asthma was diagnosed more frequently in hCoV‐infected children (P = 0.013). The clinical, laboratory, and demographic characteristics of children with HBoV single infections were compared with those with RSV, hRV, hMPV, and hCoV single infections (Table III).
Table III.
Comparison of Clinical Features of Children With Different Single‐Virus Respiratory Infections
| Characteristics | HBoV | hRVa | RSVb | hMPVc | hCoVd | P‐value |
|---|---|---|---|---|---|---|
| Demographic data | ||||||
| Number of subjects | n = 66 | n = 211 | n = 143 | n = 49 | n = 21 | |
| Age (median) | 17 | 20 | 7 | 21 | 12 | 0.07a, <0.001 b , 0.289c, 0.161d |
| Age groups | ||||||
| 0–12 months | 16 (24.2%) | 59 (28%) | 95 (66.4%) | 14 (28.6%) | 11 (52.4%) | 0.01 a , <0.001 b , 0.039 c , 0.015 d |
| 13–24 months | 35 (53%) | 70 (33.2%) | 21 (14.7%) | 15 (30.6%) | 4 (19%) | |
| ≥25 months | 15 (22.7%) | 82 (38.9%) | 27 (18.9%) | 20 (40.8%) | 6 (28.6%) | |
| Gender—F:M ratio | 1:1.75 | 1:1.37 | 1.1:1 | 1.1:1 | 1:1 | 0.393a, 0.052b, 0.131c, 1d |
| Hospitalization in days, median (range) | 4.5 (1–13) | 4 (1–25) | 5 (1–14) | 6 (1–14) | 4 (2–12) | 0.001 a , 0.1b, 0.201c, 0.54d |
| Oxygen therapy in days, median (range) | 2 (0–12) | 1 (0–22) | 3 (0–10) | 3 (0–10) | 1 (0–6) | 0.009 a , 0.52b, 0.721c, 0.012 d |
| Underlying disease | ||||||
| Prematurity (<37 weeks), congenital immunodeficiency and/or other cardiac, pulmonary, congenital disorders | 14 (21.20%) | 74 (35.1%) | 29 (20.3%) | 13 (26.5%) | 12 (57.1%) | 0.05 a , 0.856b, 0.514c, 0.005 d |
| Clinical findings | ||||||
| Fever >38°C | 23 (34.8%) | 38 (18%) | 49 (34.3%) | 18 (36.7%) | 5 (23.8%) | 0.006 a , 1b, 0.846c, 0.428d |
| Cough | 59 (89.4%) | 162 (76.8%) | 124 (86.7%) | 36 (73.5%) | 13 (61.9%) | 0.034 a , 0.658b, 0.045 c , 0.007 d |
| Rhinorrhea | 44 (66.7%) | 131 (62.1%) | 104 (72.7%) | 23 (46.9%) | 6 (28.6%) | 0.56a, 0.414b, 0.038 c , 0.005 d |
| Dyspnea | 59 (89.4%) | 196 (92.9%) | 135 (94.4%) | 44 (89.8%) | 16 (76.2%) | 0.433a, 0.248b, 1c, 0.152d |
| Wheezing | 37 (56.1%) | 133 (63%) | 73 (51%) | 27 (55.1%) | 12 (57.1%) | 0.315a, 0.552b, 1c, 1d |
| Conjunctivitis | 3 (4.5%) | 1 (0.5%) | 10 (7%) | 4 (8.2%) | 2 (9.5%) | 0.043 a , 0.759b, 0.457c, 0.59d |
| Vomiting/diarrhea | 7 (10.6%) | 12 (5.7%) | 14 (9.8%) | 4 (8.2%) | 1 (4.8%) | 0.171a, 0.81b, 0.756c, 0.673d |
| Otitis | 4 (6.1%) | 8 (3.8%) | 4 (2.8%) | 2 (4.1%) | 2 (9.5%) | 0.488a, 0.265b, 1c, 0.628d |
| Laboratory findings | ||||||
| CRP >8 mg/L | 38/63 (60.3%) | 119/202 (58.9%) | 68/136 (50%) | 25/47 (53.2%) | 11/21 (52.4%) | 0.561a, 0.285b, 0.559c, 0.323d |
| WBC × 109 cells/L, median | n = 65, 13.5 | n = 206, 14.45 | n = 138, 9.95 | n = 48, 9.15 | n = 20, 13.95 | 0.375a, <0.001 b , <0.001 c , 0.604d |
| Hypoxia (sat. O2 <94%) | 53 (80.3%) | 133 (63%) | 120 (83.9%) | 38 (77.5%) | 11 (52.4%) | 0.01 a , 0.557b, 0.818c, 0.021 d |
| Chest radiographic findings | ||||||
| X‐ray performed | 17 (25.8%) | 45 (21.3%) | 45 (31.5%) | 18 (36.7%) | 7 (33.3%) | 0.499a, 0.421b, 0.225c, 0.578d |
| X‐ray pathology (infiltrates, hyperinflation) | 16 (24.2%) | 42 (20.4%) | 45 (31.5%) | 18 (36.7%) | 7 (33.3%) | 0.488a, 0.327b, 0.215c, 0.571d |
| Final diagnosis | ||||||
| Upper respiratory tract infection | 2 (3%) | 6 (2.8%) | 2 (1.4%) | 4 (8.2%) | 2 (9.5%) | 1a, 0.592b, 0.399c, 0.244d |
| Lower respiratory tract infection | 64 (97%) | 205 (97.2%) | 141 (98.6%) | 45 (91.8%) | 19 (90.5%) | 1a, 0.592b, 0.399c, 0.244d |
| Pneumonia | 16 (24.2%) | 35 (16.6%) | 29 (20.3%) | 12 (24.5%) | 5 (23.8%) | 0.202a, 0.588b, 1c, 1d |
| Bronchiolitis | 42 (63.6%) | 116 (55%) | 104 (72.7%) | 26 (53.10%) | 11 (52.4%) | 0.255a, 0.197b, 0.338c, 0.443d |
| Bronchitis | 0 | 2 (0.9%) | 0 | 1 (2%) | 0 | 1a, NDb, 0.426c, NDd |
| Recurrent wheezing | 6 (9.10%) | 27 (12.8%) | 5 (3.5%) | 4 (8.2%) | 0 | 0.517a, 0.105b, 1c, 0.329d |
| Asthma | 0 | 25 (11.8%) | 3 (2.1%) | 2 (4.1%) | 3 (14.3%) | 0.001 a , 0.553b, 0.179c, 0.013 d |
HBoV, human bocavirus, hRV, human rhinoviruses; RSV, respiratory syncytial virus; hMPV, human metapneumovirus; hCoV, human corona viruses; F:M, female:male; CRP, C‐reactive protein; WBC, white blood cells.
P‐values <0.05 are significant. Statistical values are in bold.
Children with HBoV infection and children with hRV infection were compared.
Children with HBoV infection and children with RSV infection were compared.
Children with HBoV infection and children with hMPV infection were compared.
Children with HBoV infection and children with hCoV infection were compared.
The clinical features of children with an HBoV single infection versus HBoV + hRV, HBoV + RSV, and HBoV + hMPV coinfection pairs were analyzed to determine whether HBoV coinfections were more severe than HBoV single infections. This analysis revealed no significant differences in the duration of hospitalization and oxygen therapy between HBoV single and dual infections (Table IV).
Table IV.
Comparison of Clinical Features to Assess the Severity of Illness in Children With HBoV as a Sole Pathogen and Children With HBoV Coinfections
| Characteristics | HBoV single infection | hRV + HBoVa | RSV + HBoVb | hMPV + HBoVc | P‐value |
|---|---|---|---|---|---|
| Demographic data | |||||
| Number of subjects | n = 66 | n = 45 | n = 23 | n = 14 | |
| Age median (range) | 17 | 16 | 10 | 20 | 0.72a, 0.005 b , 0.358c |
| Gender—F:M ratio | 1:1.75 | 1:1.5 | 1.1:1 | 1:1.8 | 0.84a, 0.221b, 1c |
| Hospitalization in days, median (range) | 4.5 (1–13) | 4 (1–11) | 5 (2–7) | 5.5 (3–15) | 0.161a, 0.594b, 0.345c |
| Oxygen therapy in days, median (range) | 2 (0–12) | 2 (0–8) | 2 (0–8) | 2.5 (0–14) | 0.701a, 0.835b, 0.598c |
| Underlying disease | |||||
| Prematurity (<37 weeks), immunodeficiency and other cardiac, pulmonary, congenital disorders | 14 (21.2%) | 12 (26.7%) | 5 (21.7%) | 2 (14.3%) | 0.505a, 1b, 0.724c |
| Clinical findings | |||||
| Fever >38°C | 23 (34.8%) | 7 (15.6%) | 7 (30.4%) | 5 (35.7%) | 0.03 a , 0.801b, 1c |
| Cough | 59 (89.4%) | 39 (86.7%) | 23 (100%) | 8 (57.1%) | 0.766a, 0.183b, 0.009 c |
| Rhinorrhea | 44 (66.7%) | 23 (51.1%) | 19 (82.6%) | 11 (78.6%) | 0.319a, 0.188b, 0.531c |
| Dyspnea | 59 (89.4%) | 38 (84.4%) | 23 (100%) | 12 (85.7%) | 0.139a, 0.183b, 0.653c |
| Wheezing | 37 (56.1%) | 28 (62.2%) | 15 (65.2%) | 9 (64.3%) | 0.56a, 0.473b, 0.767c |
| Conjunctivitis | 3 (4.5%) | 2 (4.4%) | 2 (8.7%) | 2 (14.3%) | 0.685a, 0.601b, 0.209c |
| Vomiting/diarrhea | 7 (10.6%) | 3 (6.7%) | 4 (14.3%) | 1 (7.1%) | 1a, 0.465b, 1c |
| Otitis | 4 (6.1%) | 2 (4.4%) | 1 (4.3%) | 3 (21.4%) | 1a, 1b, 0.098c |
| Laboratory findings | |||||
| CRP >8 mg/L | 38/63 (60.3%) | 26 (57.8%) | 8 (39.1%) | 10 (71.4%) | 1a, 0.093b, 0.549c |
| WBC × 109 cells/L, median | n = 65, 13.5 | n = 45, 13 | n = 23, 10 | n = 14, 9.6 | 0.903a, 0.001 b , 0.003 c |
| Hypoxia (sat. O2 <94%) | 53 (80.3%) | 38 (84.4%) | 18 (78.3%) | 12 (85.7%) | 0.841a, 0.841b, 0.841c |
| Chest radiographic findings | |||||
| X‐ray performed | 17 (25.8%) | 2 (4.4%) | 3 (13%) | 4 (28.6%) | 0.363a, 0.258b, 1c |
| X‐ray pathology (infiltrates, hyperinflation) | 16 (24.2%) | 2 (4.4%) | 2 (8.7%) | 4 (28.6%) | 0.484a, 0.142b, 0.744c |
| Final diagnosis | |||||
| Upper respiratory tract infection | 2 (3%) | 1 (2.2%) | 0 | 0 | 1a, 1b, 1c |
| Lower respiratory tract infection | 64 (97%) | 44 (97.8%) | 23 (100%) | 14 (100%) | 1a, 1b, 1c |
| Pneumonia | 16 (24.2%) | 7 (15.6%) | 3 (13%) | 2 (14.3%) | 0.193a, 0.205b, 0.338c |
| Bronchiolitis | 42 (63.4%) | 29 (64.4%) | 20 (87%) | 11 (78.6%) | 0.547a, 0.029 b , 0.095c |
| Bronchitis | 0 | 1 (2.2%) | 0 | 0 | 0.405a, NDb, NDc |
| Recurrent wheezing | 6 (9.1%) | 4 (8.9%) | 0 | 1 (7.15%) | 0.624a, 0.156b, 0.646c |
| Asthma | 0 | 3 (6.7%) | 0 | 0 | 0.064a, NDb, NDc |
| HBoV median viral load (copies/ml) | 7.4 × 105 | 8.1 × 104 | 8.8 × 103 | 1.5 × 104 | 1a, 1b, 1c |
HBoV, human bocavirus; hRV, human rhinoviruse; RSV, respiratory syncytial virus; hMPV, human metapneumovirus; F:M, female:male; CRP, C‐reactive protein; WBC, white blood cells.
P‐values <0.05 are significant; hCoV + HBoV dual coinfections were excluded from analyses because of low sample sizes (n = 4). Statistical values are in bold.
Children with HBoV infection and children with HBoV and hRV double coinfection were compared.
Children with HBoV infection and children with HBoV and RSV double coinfection were compared.
Children with HBoV infection and children with HBoV and hMPV double coinfection were compared.
Phylogenetic Analysis of HBoV DNA Sequences
Twenty‐five samples were selected at random from 66 HBoV single infections and the 1,136‐bp‐long region of the VP1/VP2 gene was amplified and sequenced. Of 25 sequences obtained, there were eight unique sequences, which were deposited in GenBank (acc. no. GQ907238–GQ907245). All sequences were variants of the HBoV ST1 or ST2 genotypes and shared sequence identity of between 98.6% and 99.9%. Two of the eight sequences showed 100% identity with the sequences deposited in GenBank.
DISCUSSION
This study aimed to evaluate respiratory viral infections in a 2‐year cohort of children hospitalized in University Children's hospital, with an emphasis on infections caused by the HBoV discovered recently.
Of the 891 children included in this study, a possible viral etiology was determined in 75% of patients. The viruses detected most frequently were hRV, RSV, and HBoV. A previous study of a population of children admitted to hospital found an identical frequency distribution of respiratory virus infections [Chung et al., 2007], although the median age of patients in that study was lower (8.5 months) than the cohort of patients in the current study. Comparison between different studies is always difficult, because many factors can influence the frequency of respiratory viruses detected, for example, climate, study duration, season, patient age and background, and laboratory methods used.
In this study, viral infections showed a clear seasonal distribution, as would be expected for the continental climate found in Slovenia. The current study confirmed that HBoV infections are distributed seasonally, with the highest infection rate in winter and early spring, as shown in other studies [Foulongne et al., 2006; Weissbrich et al., 2006; Margaret et al., 2008; von Linstow et al., 2008; Vallet et al., 2009; Calvo et al., 2010; Christensen et al., 2010]. The HBoV case distribution and frequency differed significantly between the two seasons studied. A previous study also reported seasonality for other respiratory viruses [Christensen et al., 2010].
Overall, the current study detected HBoV DNA in the respiratory samples of 18.4% of children admitted to hospital with respiratory tract infection. HBoV was detected as the third most commonly virus, after hRV and RSV. The prevalence of HBoV infection is among the highest compared with similar studies [Allander et al., 2007]. Interestingly, the highest HBoV infection rate was found in children aged between 13 and 24 months. This finding agrees with previous reports, which indicate that the median age of HBoV‐infected children is between 13 and 18 months [Foulongne et al., 2006; Volz et al., 2007; Bharaj et al., 2010; Calvo et al., 2010; Garcia‐Garcia et al., 2010; Miron et al., 2010; Moriyama et al., 2010]. Male children were more frequently infected with HBoV.
HBoV was found frequently with other respiratory viruses in the current study (60%), which agrees with the findings in previous reports [Christensen et al., 2008; Garcia‐Garcia et al., 2008; Jacques et al., 2008; Calvo et al., 2010; Miron et al., 2010]. However, the median level of viral loads appeared to be significantly higher in patients with a single HBoV infection than in patients coinfected with other respiratory viruses (P = 0.005). These findings corroborate the results of two other previous studies [Allander et al., 2007; Brieu et al., 2008]. Allander et al. [2007] suggested that high HBoV DNA loads are indicative of a causative role of HBoV in respiratory tract infections, whereas low viral loads indicate asymptomatic shedding. In the present study the age of the children (12–24 months) and wheezing were associated with high HBoV DNA load.
Children aged between 13 and 24 months, and prematurely born children, had a higher median viral load compared with children in the other two age groups and children born at term. The majority of HBoV‐positive samples were detected in children aged between 13 and 24 months. These data suggest that in this age group HBoV infections are a consequence of primary infection which may more likely result in a higher viral load and this in turn may manifest as a severe clinical presentation.
In the present study, the clinical features of children with single infections caused by HBoV, hRV, RSV, hMPV, and hCoV were also assessed. The most important observation was probably that HBoV infection can result in severe disease manifestation that is virtually indistinguishable from infections caused by RSV and hMPV, as was recently described by other researchers [Soderlund‐Venermo et al., 2009; Calvo et al., 2010; Garcia‐Garcia et al., 2010]. Patients with RSV and hMPV were hospitalized longer and needed longer oxygen therapy, but the differences were not statistically significant. Bronchiolitis, pneumonia, and recurrent wheezing were the most common clinical presentations of single bocavirus infections. These data agree with other published studies where these clinical presentations were detected commonly in HBoV‐infected patients. Direct comparison with other studies is difficult, because the inclusion criteria in different studies vary widely, ranging among patients suffering from acute bronchiolitis, recurrent wheezing, or unspecified respiratory tract infections.
There are also limitations in the current study. First, the retrospective nature of the study might introduce a bias into patient selection. Second, the prevalence of viruses detected by DFA testing (i.e., Flu A/B) might indicate an underestimation of the true prevalence, because of the low sensitivity of the DFA method when compared with PCR. However, the sensitivity of DFA in RSV detection was determined in other studies (unpublished data) and did not differ to any great extent [Kuypers et al., 2006; Aslanzadeh et al., 2008]. While HBoV shedding is very frequent, the true seasonality of symptomatic primary HBoV infection is difficult to study by this approach, although we found a clear seasonal distribution of infections caused by HBoV. Finally, a control group of children of the same age would be desirable. Thus, a new prospective study was commenced at the University Medical Centre in 2009, where an age‐matched control group of patients was included.
Acknowledgements
We are grateful to the Institute for Microbiology and Parasitology and the Virology Unit at the Veterinary Faculty, University of Ljubljana for providing an isolate of Equine herpes virus 1.
Conflicts of interest: none.
REFERENCES
- Albuquerque MC, Rocha LN, Benati FJ, Soares CC, Maranhao AG, Ramirez ML, Erdman D, Santos N. 2007. Human bocavirus infection in children with gastroenteritis, Brazil. Emerg Infect Dis 13:1756–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque MC, Pena GP, Varella RB, Gallucci G, Erdman D, Santos N. 2009. Novel respiratory virus infections in children, Brazil. Emerg Infect Dis 15:806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allander T. 2008. Human bocavirus. J Clin Virol 41:29–33. [DOI] [PubMed] [Google Scholar]
- Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung‐Lindell A, Andersson B. 2005. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA 102:12891–12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allander T, Jartti T, Gupta S, Niesters HG, Lehtinen P, Osterback R, Vuorinen T, Waris M, Bjerkner A, Tiveljung‐Lindell A, van den Hoogen BG, Hyypia T, Ruuskanen O. 2007. Human bocavirus and acute wheezing in children. Clin Infect Dis 44:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslanzadeh J, Zheng X, Li H, Tetreault J, Ratkiewicz I, Meng S, Hamilton P, Tang YW. 2008. Prospective evaluation of rapid antigen tests for diagnosis of respiratory syncytial virus and human metapneumovirus infections. J Clin Virol 46:1682–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien N, Brandt K, Dust K, Ward D, Li Y. 2006. Human bocavirus infection, Canada. Emerg Infect Dis 12:848–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharaj P, Sullender WM, Kabra SK, Broor S. 2010. Human bocavirus infection in children with acute respiratory tract infection in India. J Med Virol 82:812–816. [DOI] [PubMed] [Google Scholar]
- Bonzel L, Tenenbaum T, Schroten H, Schildgen O, Schweitzer‐Krantz S, Adams O. 2008. Frequent detection of viral coinfection in children hospitalized with acute respiratory tract infection using a real‐time polymerase chain reaction. Pediatr Infect Dis J 27:589–594. [DOI] [PubMed] [Google Scholar]
- Brieu N, Guyon G, Rodiere M, Segondy M, Foulongne V. 2008. Human bocavirus infection in children with respiratory tract disease. Pediatr Infect Dis J 27:969–973. [DOI] [PubMed] [Google Scholar]
- Calvo C, Pozo F, Garcia‐Garcia ML, Sanchez M, Lopez‐Valero M, Perez‐Brena P, Casas I. 2010. Detection of new respiratory viruses in hospitalized infants with bronchiolitis: A three‐year prospective study. Acta Paediatr 99:883–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieochansin T, Thongmee C, Vimolket L, Theamboonlers A, Poovorawan Y. 2008. Human bocavirus infection in children with acute gastroenteritis and healthy controls. Jpn J Infect Dis 61:479–481. [PubMed] [Google Scholar]
- Chow BD, Ou Z, Esper FP. 2010. Newly recognized bocaviruses (HBoV, HBoV2) in children and adults with gastrointestinal illness in the United States. J Clin Virol 47:143–147. [DOI] [PubMed] [Google Scholar]
- Christensen A, Nordbo SA, Krokstad S, Rognlien AG, Dollner H. 2008. Human bocavirus commonly involved in multiple viral airway infections. J Clin Virol 41:34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A, Nordbo SA, Krokstad S, Rognlien AG, Dollner H. 2010. Human bocavirus in children: Mono‐detection, high viral load and viraemia are associated with respiratory tract infection. J Clin Virol 49:158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JY, Han TH, Kim SW, Kim CK, Hwang ES. 2007. Detection of viruses identified recently in children with acute wheezing. J Med Virol 79:1238–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo IS, Hewitson G, Wright L, Rodwell BJ, Corney BG. 2006. Detection of equine herpesvirus type 1 using a real‐time polymerase chain reaction. J Virol Methods 131:92–98. [DOI] [PubMed] [Google Scholar]
- Fabbiani M, Terrosi C, Martorelli B, Valentini M, Bernini L, Cellesi C, Cusi MG. 2009. Epidemiological and clinical study of viral respiratory tract infections in children from Italy. J Med Virol 81:750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulongne V, Olejnik Y, Perez V, Elaerts S, Rodiere M, Segondy M. 2006. Human bocavirus in French children. Emerg Infect Dis 12:1251–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AM, Lu X, Chittaganpitch M, Peret T, Fischer J, Dowell SF, Anderson LJ, Erdman D, Olsen SJ. 2007. Human bocavirus: A novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis 195:1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Garcia ML, Calvo C, Pozo F, Perez‐Brena P, Quevedo S, Bracamonte T, Casas I. 2008. Human bocavirus detection in nasopharyngeal aspirates of children without clinical symptoms of respiratory infection. Pediatr Infect Dis J 27:358–360. [DOI] [PubMed] [Google Scholar]
- Garcia‐Garcia ML, Calvo C, Falcon A, Pozo F, Perez‐Brena P, De Cea JM, Casas I. 2010. Role of emerging respiratory viruses in children with severe acute wheezing. Pediatr Pulmonol 45:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques J, Moret H, Renois F, Leveque N, Motte J, Andreoletti L. 2008. Human bocavirus quantitative DNA detection in French children hospitalized for acute bronchiolitis. J Clin Virol 43:142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida A, Kubo H, Takakura K, Iritani N. 2010. Detection and quantitative analysis of human bocavirus associated with respiratory tract infection in Osaka City. Jpn Microbiol Immunol 54:276–281. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Slikas E, Simmonds P, Chieochansin T, Naeem A, Shaukat S, Alam MM, Sharif S, Angez M, Zaidi S, Delwart E. 2009. A newly identified bocavirus species in human stool. J Infect Dis 199:196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Simmonds P, Slikas E, Li L, Bodhidatta L, Sethabutr O, Triki H, Bahri O, Oderinde BS, Baba MM, Bukbuk DN, Besser J, Bartkus J, Delwart E. 2010. Human bocaviruses are highly diverse, dispersed, recombination prone, and prevalent in enteric infections. J Infect Dis 201:1633–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesebir D, Vazquez M, Weibel C, Shapiro ED, Ferguson D, Landry ML, Kahn JS. 2006. Human bocavirus infection in young children in the United States: Molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. J Infect Dis 194:1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers J, Wright N, Ferrenberg J, Huang ML, Cent A, Corey L, Morrow R. 2006. Comparison of real‐time PCR assays with fluorescent‐antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol 44:2382–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers J, Martin ET, Heugel J, Wright N, Morrow R, Englund JA. 2007. Clinical disease in children associated with newly described coronavirus subtypes. Pediatria 119:70–76. [DOI] [PubMed] [Google Scholar]
- Lee JI, Chung JY, Han TH, Song MO, Hwang ES. 2007. Detection of human bocavirus in children hospitalized because of acute gastroenteritis. J Infect Dis 196:994–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Chittaganpitch M, Olsen SJ, Mackay IM, Sloots TP, Fry AM, Erdman DD. 2006. Real‐time PCR assays for detection of bocavirus in human specimens. J Clin Microbiol 44:3231–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertzdorf J, Wang CK, Brown JB, Quinto JD, Chu M, de Graaf M, van den Hoogen BG, Spaete R, Osterhaus AD, Fouchier RA. 2004. Real‐time reverse transcriptase PCR assay for detection of human metapneumoviruses from all known genetic lineages. J Clin Microbiol 42:981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi F, Andreoli E, Pifferi M, Meschi S, Rocchi J, Bendinelli M. 2007. Human bocavirus in Italian patients with respiratory diseases. J Clin Virol 38:321–325. [DOI] [PubMed] [Google Scholar]
- Manning A, Russell V, Eastick K, Leadbetter GH, Hallam N, Templeton K, Simmonds P. 2006. Epidemiological profile and clinical associations of human bocavirus and other human parvoviruses. J Infect Dis 194:1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaret IP, Nelson EA, Cheuk ES, Leung E, Sung R, Chan PK. 2008. Pediatric hospitalization of acute respiratory tract infections with human bocavirus in Hong Kong. J Clin Virol 42:72–74. [DOI] [PubMed] [Google Scholar]
- Martin ET, Fairchok MP, Kuypers J, Magaret A, Zerr DM, Wald A, Englund JA. 2010. Frequent and prolonged shedding of bocavirus in young children attending daycare. J Infect Dis 201:1625–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midulla F, Scagnolari C, Bonci E, Pierangeli A, Antonelli G, De Angelis D, Berardi R, Moretti C. 2010. Respiratory syncytial virus, human bocavirus and rhinovirus bronchiolitis in infants. Arch Dis Child 95:35–41. [DOI] [PubMed] [Google Scholar]
- Miron D, Srugo I, Kra‐Oz Z, Keness Y, Wolf D, Amirav I, Kassis I. 2010. Sole pathogen in acute bronchiolitis: Is there a role for other organisms apart from respiratory syncytial virus? Pediatr Infect Dis J 29:7–10. [DOI] [PubMed] [Google Scholar]
- Moriyama Y, Hamada H, Okada M, Tsuchiya N, Maru H, Shirato Y, Maeda Y, Hirose Y, Yoshida M, Omura Y, Honda T, Muto A, Hayashi K, Terai M. 2010. Distinctive clinical features of human bocavirus in children younger than 2 years. Eur J Pediatr 169:1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neske F, Blessing K, Tollmann F, Schubert J, Rethwilm A, Kreth HW, Weissbrich B. 2007. Real‐time PCR for diagnosis of human bocavirus infections and phylogenetic analysis. J Clin Microbiol 45:2116–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltinga SA, Templeton KE, Beersma MF, Claas EC. 2005. Diagnosis of human metapneumovirus and rhinovirus in patients with respiratory tract infections by an internally controlled multiplex real‐time RNA PCR. J Clin Virol 33:306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildgen O, Muller A, Allander T, Mackay IM, Volz S, Kupfer B, Simon A. 2008. Human bocavirus: Passenger or pathogen in acute respiratory tract infections? Clin Microbiol Rev 21:291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund‐Venermo M, Lahtinen A, Jartti T, Hedman L, Kemppainen K, Lehtinen P, Allander T, Ruuskanen O, Hedman K. 2009. Clinical assessment and improved diagnosis of bocavirus‐induced wheezing in children, Finland. Emerg Infect Dis 15:1423–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JR, Jin Y, Xie ZP, Gao HC, Xiao NG, Chen WX, Xu ZQ, Yan KL, Zhao Y, Hou YD, Duan ZJ. 2010. Novel human bocavirus in children with acute respiratory tract infection. Emerg Infect Dis 16:324–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szomor KN, Kapusinszky B, Riqo Z, Kis Z, Rozsa M, Farkas A, Szilaqyi A, Berencsi G, Takacs M. 2009. Detection of human bocavirus from fecal samples of Hungarian children with acute gastroenteritis. Intervirology 52:17–21. [DOI] [PubMed] [Google Scholar]
- Vallet C, Pons‐Catalano C, Mandelcwajg A, Wang A, Raymond J, Lebon P, Gendrel D. 2009. Human bocavirus: A cause of severe asthma exacerbation in children. J Pediatr 155:286–288. [DOI] [PubMed] [Google Scholar]
- Volz S, Schildgen O, Klinkenberg D, Ditt V, Muller A, Tillmann RL, Kupfer B, Bode U, Lentze MJ, Simon A. 2007. Prospective study of human bocavirus (HBoV) infection in a pediatric university hospital in Germany 2005/2006. J Clin Virol 40:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Linstow ML, Hogh M, Hogh B. 2008. Clinical and epidemiologic characteristics of human bocavirus in Danish infants: Results from a prospective birth cohort study. Pediatr Infect Dis J 27:897–902. [DOI] [PubMed] [Google Scholar]
- Weissbrich B, Neske F, Schubert J, Tollmann F, Blath K, Blessing K, Kreth HW. 2006. Frequent detection of bocavirus DNA in German children with respiratory tract infections. BMC Infect Dis 6:109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]


