Abstract
Emerging viruses such as Ebola virus (EBOV), Lassa virus (LASV), and avian influenza virus H5N1 (AIV) are global health concerns. Since there is very limited options (either vaccine or specific therapy) approved for humans against these viruses, there is an urgent need to develop prophylactic and therapeutic treatments. Previously we reported a high‐throughput screening (HTS) protocol to identify entry inhibitors for three highly pathogenic viruses (EBOV, LASV, and AIV) using a human immunodeficiency virus–based pseudotyping platform which allows us to perform the screening in a BSL‐2 facility. In this report, we have adopted this screening protocol to evaluate traditional Chinese Medicines (TCMs) in an effort to discover entry inhibitors against these viruses. Here we show that extracts of the following Chinese medicinal herbs exhibit potent anti‐Ebola viral activities: Gardenia jasminoides Ellis, Citrus aurantium L., Viola yedoensis Makino, Prunella vulgaris L., Coix lacryma‐jobi L. var. mayuen (Roman.) Stapf, Pinellia ternata (Thunb.) Breit., and Morus alba L. This study represents a proof‐of‐principle investigation supporting the suitability of this assay for rapid screening TCMs and identifying putative entry inhibitors for these viruses. J. Med. Virol. 89:908–916, 2017 . © 2016 Wiley Periodicals, Inc.
Keywords: antiviral drugs, high‐throughput screening, Traditional Chinese Medicine, entry inhibitors, Ebola virus
INTRODUCTION
Emerging and reemerging viruses pose severe global health concerns. The four influenza pandemics in last 100 years are the examples of how these viruses impact humans. Avian influenza viruses pose significant threats to animal and human health. They are a source of genetic diversity that permits the emergence of pandemic influenza by means of genetic reassortment with prevailing human influenza viruses. A highly pathogenic avian influenza virus, H5N1, caused disease outbreaks in poultry in China and seven other east Asian countries after 2003 [Guan et al., 2004]. The recent Ebola virus (EBOV) outbreak in West Africa underscores the unpreparedness of humans dealing with such deadly virus. EBOV and Marburg virus (MARV) belong to the filovirus family Filoviradae and can cause a rapidly lethal hemorrhagic fever in humans. Since 1967, both outbreaks of EBOV and MARV occurred repeatedly and unpredictedly, with new strains showing high mortality rates [Towner et al., 2006]. Lassa virus, a member of the arenaviridae family, represent two important etiological agents of viral hemorrhagic fevers [Nichol et al., 2000]. Since clinically approved antiviral therapeutics or vaccines against these viruses are limited, there is an urgent need to discover and develop antivirals, either targeting one particular virus or broadly targeting multiple viruses. Traditional Chinese medicine has been playing an important role in human healthcare in China and Asia for thousands of years, and now it is also a valuable source for new drug discovery in modern medicine. As our knowledge on the therapeutic benefits of these Chinese medicines increases, research efforts on the previously serendipitous treatments to discover new medicines are greatly intensified. Natural products including Chinese medicines have been and continue to be a rich source for new drug discover [Newman and Cragg, 2007] since they have a large structural diversity and complexity that remains unmatched by other drug formats [Rosen et al., 2009]. Artemisinin, also known as Qing Hao Su in Chinese which was originally isolated from the plant Artemisia annua L, and its semi‐synthetic derivatives are a group of drugs that possess the most rapid action of all current drugs against Plasmodium falciparum malaria [White, 1997], are an illustrious example of how new antimicrobial drugs can be discovered from Chinese Medicine herbs. Tetrandrine, which was isolated and identified as an active ingredient in a Chinese medicinal herb, Stephaniae Tetrandrae Radix, was reported to inhibit EBOV infection both in vitro and in vivo [Sakurai et al., 2015]. Also anti‐influenza drug discovery efforts on natural products have seen a rapid increase in the recent years [Wang et al., 2006].
In this study, we adopted a previously developed high‐throughput screening (HTS) assay to identify entry inhibitors of three highly pathogenic viruses from Chinese Medicine extracts. We demonstrate that this protocol allows one to identify specific and “shared” entry inhibitors for different viruses with a greatly reduced number of false positives, alleviating a major problem in HTS. This HTS format should be amenable for quickly screening for antiviral inhibitors from natural products including Chinese medicinal herbs.
MATERIALS AND METHODS
Cell Culture and Plasmids
Human 293T embryonic kidney cells and A549 human lung epithelial cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM; Cellgro, Manassas, VA) supplemented with 10% fetal bovine serum (SIGMA, Louis, MO), 100 µg/ml streptomycin, and 100 U penicillin (GIBCO, Grand Island, NY). The pseudovirions for HTS were created by the following plasmids: hemagglutinin (HA), isolated from a highly pathogenic avian influenza virus, A/Goose/Qinghai/59/05 (H5N1) strain [Guo et al., 2009a], Ebola virus Zaire envelope glycoprotein (GP) [Manicassamy and Rong, 2009], LASV envelope GP [Radoshitzky et al., 2007], and the HIV‐1 proviral vector pNL4‐3.Luc.R−E− [He et al., 1995] which was obtained through the National Institutes of Health (NIH) AIDS Research and Reference Reagent program. Pseudovirion of these three viruses were amplified in 293T cells.
Plant Material Extraction and Control
Traditional Chinese herbs were purchased from the Zhonglu Hospital of Shandong University of Traditional Chinese Medicine (Jinan, China). The extracts from these medicinal plants were obtained according to the Chinese Pharmacopoeia (Pharmacopoeia of the People's Republic of China 2010). Four hundreds and twenty‐seven extracts with different polarity were obtained from different herb plants. They were dried in vacuum at 50°C and redissolved with DMSO or PBS by different polar solvents. One milliliter of this solution was equal to 0.1–1 g dried herb material. Two hundred and ten unique extracts were arrayed in a 384‐well plate at a 10–50 mg/ml stock concentration in solvent, leaving columns 1, 2, 23, and 24 with DMSO or PBS. The positive control for this assay was azidothymidine (AZT; Sigma, St. Louis, MO), which was solubilized at 10 mM in DMSO.
Production of Pseudovirions
All three types of pseudovirions: Avian influenza virus (AIV)/HIV, EBOV/HIV, and LASV/HIV were produced by transient cotransfection of human 293T cells using a polyethylenimine–based transfection protocol. Plasmids encoding HA/neuraminidase (NA) and replication‐defective HIV vector (pNL4‐3.Luc.R−E−) were used for transient cotransfection into 293T‐producing cells. Five hours after transfection, cells were washed with phosphate‐buffered saline, and 20 ml of fresh medium was added to each plate (150 mm). Forty‐eight hours after transfection, the supernatants were collected and filtered through a 0.45‐µm pore size filter (Nalgene, Rochester, NY). The pseudovirion stocks were stored at 4°C prior to use.
High‐Throughput Screen
Target A549 cells were seeded into white, flat‐bottom, 384‐well plates (CulturPlate; PerkinElmer, Waltham, MA) at a density of ∼1,000 cells/well in a 30 µl assay medium using JANUS liquid handler MDT (Modular Dispense Technology; PerkinElmer) and incubated at 37°C, 5% CO2, with high humidity. Twenty‐four hours later, 40 µl pseudovirions per well and 0.2 µl of each extract were added to 384‐well plate through a pin tool (V&P Scientific, San Diego, CA). This resulted in a final concentration of 10–60 µg/ml for the extracts. Plates were incubated at 37°C, 5% CO2 for 48 hr. After incubation, 20 µl of neolite (PerkinElmer) was added to each well using the JANUS liquid handler MDT, and plates were incubated at room temperature for 5–10 min. Luciferase activity was measured by an EnVision plate reader (PerkinElmer).
IC50 and CC50 Determination
Tissue culture plates (96‐well) were seeded with 5 × 103cells/well (A549 cells) and incubated for 24 hr at 37°C under 5% CO2 with high humidity. DMSO or PBS were used as a negative control. For the IC50, cells were infected with influenza pseudovirus along with serial dilutions of each extract. Forty‐eight hours post infection, luciferase was read using 50 μl of NeoLite substrate. For the CC50, cells were treated with fresh media plus the same serial dilutions of each extract. Forty‐eight hours later, cell viability was measured using 50 μl CellTiter‐Glo Substrate (Promega, Madison, WI). IC50 and CC50 were determined by fitting the dose‐response curves against pseudovirus or control wells with four‐parameter logistic regression in GraphPad prism. Selectivity index (SI) was calculated from the following equation (CC50/IC50) and extracts with SI >10 was considered a hit.
Time of Addition Assay
A549 cells were seeded in a 96 well plate (5,000 cells/well) 24 hr prior to the experiment. After that, cells were incubated with pseudovirions at 4°C for 1 hr to allow virus attachment to the cells. Then, virus was removed and cells were washed with cold PBS two times before fresh media was added. Temperature was shifted to 37°C to trigger virus entry. At different time points of virus entry, Extract of TCMs or AZT (5 µM) was introduced to assess their impact on virus entry. Triplicate wells were used for each time point. Control infected cell cultures were treated with drug vehicle (DMSO or PBS) only. Virus infection was measured 48 hr post‐infection as described above, luciferase levels were determined.
Data Analysis
The luminescence signal in each well was measured using the EnVision plate reader. The data were exported as excel style files, and the median of luminescence signals of all samples in each plate was calculated, and the signal in each well was normalized by the plate sample median. Percentage of inhibition (% inhibition) was calculated as 100 × (1 − normalized signal). Hit extract lists were generated separately from screens of three viruses by applying various % inhibition cutoffs, and shared hits and virus‐specific hits were picked by comparing these lists.
RESULTS
Summary of Cell‐Based High‐Throughput Screen for Herbal Extracts
Avian influenza virus H5N1, Ebola virus, and Lassa virus are classified as highly pathogenic viruses, thus studies on these viruses require biosafety level 3 or 4 containment (BSL‐3 or BSL‐4). To overcome this obstacle in our effort to identify antiviral entry inhibitors of these viruses, we adopted a surrogate system which allows us to perform the initial screening in a BSL‐2 facility [Wang et al., 2014]. This human immunodeficiency virus‐1 (HIV‐1)‐based surrogate assay has been widely used by us and others to investigate the entry mechanisms of highly pathogenic enveloped viruses such as filoviruses [Manicassamy et al., 2005; Manicassamy and Rong, 2009], avian influenza virus H5N1 [Guo et al., 2009a], and severe acute respiratory syndrome coronavirus (SARS‐CoV) [Guo et al., [Link]]. This system has also been used to identify and develop entry inhibitors as antivirals [Guo et al., [Link]; Basu et al., 2014].
In this study, as described in the Materials and Methods, the following three types of pseudovirions, EBOV (HIV with Ebola glycoprotein GP incorporated on the surface), AIV (HIV with avian influenza virus H5N1 HA), and LASV (HIV with Lassa arenavirus GP), were produced and used to evaluate Chinese medicinal herbal extracts. The effect of each extract on three types of pseudovirions could be directly compared, thus putatively virus‐specific and “shared” hit extracts could be easily identified with one round screen.
Technically, as depicted in Figure 1, approximately 1,000 A549 cells were seeded for each well. Twenty‐four hours later, each set of the 384‐well plates with A549 cells was replaced with one type of the HIV pseudovirions (EBOV, AIV, or LASV) with the predetermined amounts individually, each of the 427 herbal extracts was robotically dispensed into the 384‐well plates. After 48 hr incubation, the luciferase substrate was directly added to the wells and the luciferase activity of each well measured.
Figure 1.
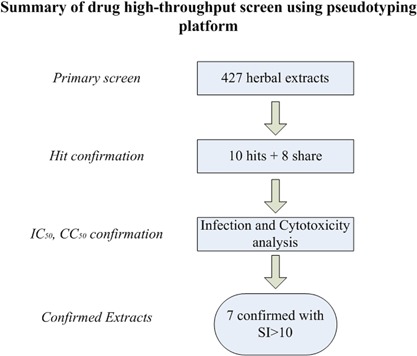
Flow chart of the Chinese medicinal herb extract screening in a 384‐well plate format.
Quality Control of the Screen
Figure 2 shows that the luciferase reporter gave a high level of signals compared to the basal/background signal with signal‐to‐noise ratio (S/B) of three pseudoviruses from 3,900‐fold to 25,000‐fold, respectively. Coefficient of variation (CV) was calculated from duplicate wells and used as an indicator of assay reproducibility. The median CVs in three screens ranged from 12% to 14%, indicating that screen reproducibility was excellent. Since Z′ factor is the most commonly used statistical parameter to assess screen quality [Zhang et al., 1999], it was used here to assess the quality of overall screen. The Z′ factor was calculated from the normalized signals from DMSO and AZT control wells on each plate with the following equation: 1–3 (StdDMSO + StdAZT)/(MeanDMSO − MeanAZT). As shown in Figure 2, the Z′ factor values of three parallel screens were all above 0.5, indicating that the overall quality of screen was excellent.
Figure 2.
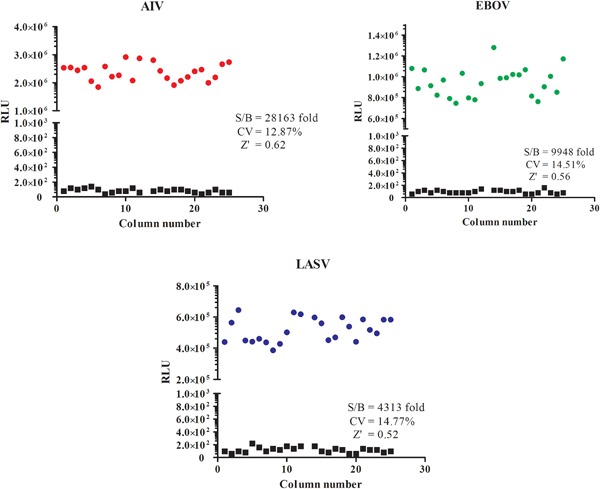
Scatter plot of the results from signal to basal level. The vertical axis indicates the relative luciferase units (RLU) of samples with basal level or signal in scale. Red circles are AIV/HIV signals, green circles are EBOV/HIV signals, blue circles are LASV/HIV signals, black squares are basal signals. The signal‐to‐basal ratio (S/B), CV and Z′ factor are all showed in scatter plot.
Normalized Luciferase Signal
The signals in DMSO, PBS, or AZT control wells were normalized by plate median, AZT was used as a positive control because it is an HIV reverse transcriptase inhibitor, so it can inhibit the infection of HIV‐based pseudotype viruses. DMSO and PBS were used as negative controls. As shown in Figure 3, DMSO or PBS has no significant difference as negative controls, and the positive control AZT has a more than 150‐fold lower signals compared with DMSO or PBS controls.
Figure 3.
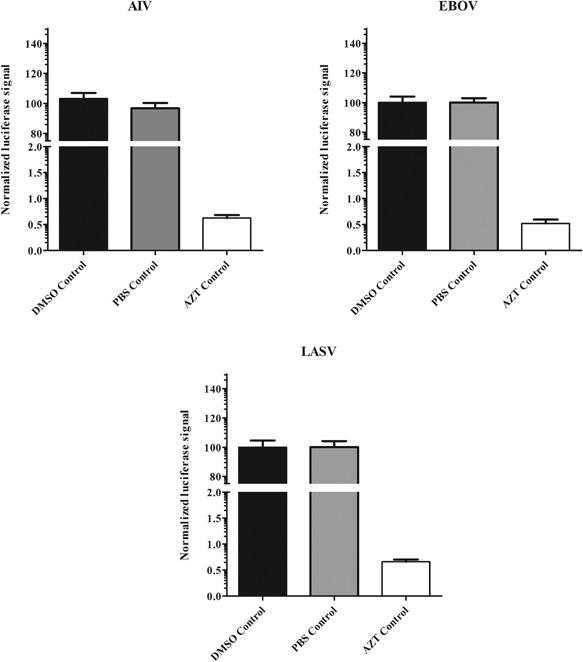
Quality control of solvent (DMSO or PBS) and AZT. The signals in DMSO, PBS or azidothymidine (AZT) control wells were normalized by plate median and are shown in black columns (DMSO), gray columns (PBS) and white columns (AZT). Results are the means ± standard deviation.
Frequency Distribution of Three Pseudovirions
Figure 4 represents Gaussian distribution of the normalized signals for each screen. The normalized signal was obtained by dividing the measured signal in each well by the median luciferase signal of all sample wells in a plate. The AIV screen shows a broad bell‐shaped normal signal distribution. In contrast the LASV screen shows a slightly positively skewed distribution, while the EBOV screen shows a greatly positively skewed distribution. The great positively skewed distribution suggests that some samples of the EBOV screen have greatly reduced the luciferase activity, implicating inhibition by these samples on EBOV.
Figure 4.
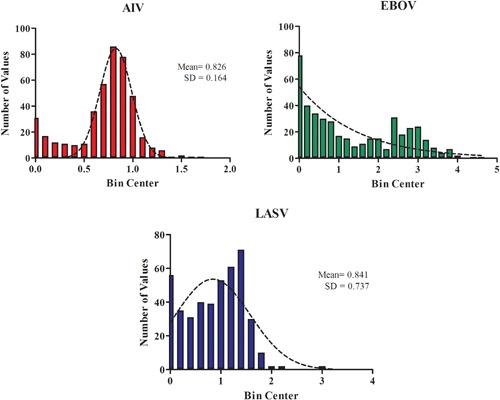
Gaussian Distribution of signals for each screen. The x‐axis shows bin center of the number of values, y‐axis shows the number of values of each bin in a frequency distribution. Red color bin is AIV, blue color bin is LASV, green color bin is EBOV.
Hit Selection to Identify Active Herbal Extracts
Analysis of the three parallel screens allowed us to rapidly identify and classify putative entry inhibitors for EBOV, AIV, and LASV. As shown in Table I, we used 80% inhibition as the cutoff, 19, 9, and 9 hit herbal extracts were identified for EBOV, AIV, and LASV based on the three individual screens respectively. However, three‐way comparisons revealed that only 10 herbal extracts could be classified as putative EBOV‐specific entry inhibitors. Table I also shows that 8 herbal extracts belonged to “shared” hits (with >80% inhibition in three pseudovirus screens). It suggests that these herbal extractseither block entry of these three viruses, or block HIV replication, or they are toxic at the tested concentration (10–60 µg/ml). This class of extracts will be evaluated further in the future.
Table I.
Summary of Putative Entry Inhibitors for Three Viruses From the Herbal Extracts
“Shared” means: the number of herbal extracts showing >80% inhibition of EBOV, AIV, and LASV.
“Hit” means: the number of herbal extracts showing >80% inhibition of EBOV, but both AIV, and LASV inhibitions <50%.
Time of Addition Experiments Give Insight Into the Mechanism of Action
To determine the step(s) during viral entry, a time‐of‐addition experiment was performed. For this experiment, we selected Prunella vulgaris L. with the highest SI (more than 124), which was tested at various time points along with azidothymidine (AZT), and PBS as controls. Briefly, cells were incubated with Ebola pseudovirus for one hour at 4°C to allow viral attachment to cells. Virus was washed from cells and the temperature was shifted 37°C to trigger virus entry. Extracts and controls were added to the wells at various time points (−1, 0, 1, 2, 3, 4, 5, 6, 7, and 8 hr). As shown in Figure 5, AZT works post‐fusion on the reverse transcriptase of HIV, it still has inhibitory effects at the later time points (>4 hr). Extract of Prunella vulgaris L., showed inhibition during early time points (−1, 0, and 1 hr). This suggests that this extract is working at the early attachment step of filoviral entry. The remainder of the extract exhibited their inhibitory effects at a earlier than AZT. This suggests that this extract is working at a post‐attachment step, but prior to the membrane fusion step.
Figure 5.
Time of addition experiments give insight into extract mechanism. Ebola pseudovirus was incubated with A549 cells at 4°C for 1 hr. After 1 hr of incubation, virus was removed and the temperature was shifted to 37°C to trigger internalization. Prunella vulgaris L. (15.6 μg/ml), AZT (5 μM), or extract vehicle (PBS) was introduced at different time points during infection and the extract effects were assessed as described in Materials and Methods. Data were normalized to vehicle (PBS). Error bars represent standard deviations.
We identified 10 putative EBOV hits using the criteria of maximal inhibition of >80% in the EBOV and inhibition of <50% in AIV, and LASV. These primary hits were then picked with confirmation, to eliminate the effect of cytotoxicity using an ATP content assay. The SI for each active herbal extract is reported as a ratio of the CC50/IC50. The seven confirmed hits with SI >10 are listed in Table II, and they can be divided into seven actions and indications based on Chinese Medicine: herbs that drain fire, to regulate the Qi, to clear heat and eliminate toxins, to drain fire, to transform phlegm‐cold, and to relieve coughing and wheezing (Table II). Further evaluation of these herbal extracts will be discussed below.
Table II.
Summary of CC50 and IC50 With Ebola Pseudovirus
| Extract | CC50(µg/ml) | IC50(µg/ml) | SI | Actions and indications |
|---|---|---|---|---|
| Gardenia jasminoides Ellis | >300 | 11.04 ± 1.66 | >27.2 | Herbs that drain fire |
| Citrus aurantium L. | >810 | 38.35 ± 3.25 | >21.1 | Herbs that regulate the Qi |
| Viola yedoensis Makino | >670 | 17.54 ± 5.93 | >38.2 | Herbs that clear heat and eliminate toxins |
| Prunella vulgaris L. | >62.5 | 0.50 ± 0.01 | >124 | Herbs that drain fire |
| Coix lacryma‐jobi L. var. mayuen (Roman.) Stapf | >116 | 5.46 ± 1.37 | >21.2 | Herbs that regulate water and drain dampnes |
| Pinellia ternata (Thunb.) Breit. | >117 | 5.47 ± 0.19 | >21.4 | Warm herbs that transform phlegm‐cold |
| Morus alba L. | >121 | 4.38 ± 1.10 | >27.6 | Herbs that relieve coughing and wheezing |
DISCUSSION
In this report, we adopted a previous HTS assay protocol to screen herbal extracts for entry inhibitors against several highly pathogenic viruses. We have identified eight herbal extracts that can block EBOV entry. Although the active components in these extracts need to be isolated, identified, and validated in the future work, our results represent a proof‐of‐principle investigation to demonstrate that this HTS assay can be used for rapid screening of EBOV, AIV, and LASV (and other viruses) for antiviral inhibitors from Chinese medicinal herbs and other natural products. This strategy is particularly useful for highly pathogenic viruses such as Ebola virus, avian influenza virus H5N1, and Lassa virus, since the HIV vector‐based surrogate system allows us to perform high throughput screens in the biosafety level 2 (BSL‐2) containment instead of the BSL‐3 or BSL‐4 for the infectious viruses with alleviated safety concerns. Using several viruses screen simultaneously or sequentially, it can easily identify putative entry inhibitors for different viruses and reduce the number of the false‐positive hits (see Table I).
Traditional Chinese medicine holds a unique position among all traditional medicines because of its long history in China. Many extracts of traditional Chinese medicinal herbs have been proven to have antiviral activities [Jassim and Naji, 2003]. Most of these are generally of low toxicity, cheap, and readily accessible. By using this pseudovirions entry system, we have tested more than four hundreds Chinese herb extracts and show that extracts of Gardenia jasminoides Ellis, Citrus aurantium L., Viola yedoensis Makino, Prunella vulgaris L., Coix lacryma‐jobi L. var. mayuen (Roman.) Stapf, Pinellia ternata (Thunb.) Breit., and Morus alba L. all exhibited potent inhibitory activity against EBOV glycoprotein entry out of all the extracts tested (Table II). Some previous reports have showed that Citrus aurantium L. displays anti‐HCV activity [Ho et al., 2003], the extract of Viola yedoensis Makino has high inhibitory activity toward HIV‐1 [Ngan et al., 1988] and RSV [Ma et al., 2002] in vitro, the extract of Prunella vulgaris L. has specific activity against Herpes simplex virus (HSV) [Xu et al., 1999] and HIV‐1 [Yao et al., 1992], Morus alba L. showed some activity against Herpes simplex virus types 1 (HSV‐1) [Du et al., 2003], HIV, influenza virus and HBV [Geng et al., 2012]. Here we demonstrate that extracts of Pinellia ternata (Thunb.) Breit. display potential inhibitory activity against EBOV‐GP entry. These Chinese medicinal herbs merit further investigation as a promising antiviral due to its rich source, low costs, low cytotoxicity, wide acceptability, and strong antiviral properties. The possible mechanism of action of these drugs in inhibiting EBOV entry remains unknown, and further detailed chemical research to identify and purify the active ingredient(s) will facilitate its mechanistic analyses and applications.
Overall, although more experiments will be needed to fully understand the possible use of any of these herbal extracts against these viruses infection, here we describe a surrogate pseudovirus entry assay that can be used for HTS in a BSL‐2 facility for the rapid screening of extensive herbal collections. The identification of one extract of Prunella vulgaris L. [Zhang et al., 2016] previously known to inhibit Ebola virus entry in our repurposing screen further supports the usefulness of our approach. We also detected that Prunella vulgaris L. could block the early attachment step of Ebola entry with time of addition experiment. We hope that our screening efforts will result in the identification of novel herbal extracts for the development of drugs to treat Ebola virus infections or other highly pathogenic viruses. These Chinese medicinal herbs may be used for further drug discovery and development of anti‐EBOV.
Yong Yang and Han Cheng contributed equally to this work.
Contributor Information
Yong Yang, Email: yy7204@163.com.
Li‐Jun Rong, Email: lijun@uic.edu.
REFERENCES
- Basu A, Antanasijevic A, Wang M, Li B, Mills DM, Ames JA, Nash PJ, Williams JD, Peet NP, Moir DT, Prichard MN, Keith KA, Barnard DL, Caffrey M, Rong L, Bowlin TL. 2014. New small molecule entry inhibitors targeting hemagglutinin‐mediated influenza a virus fusion. J Virol 88:1447–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, He ZD, Jiang RW, Ye WC, Xu HX, But PP. 2003. Antiviral flavonoids from the root bark of Morus alba L. Phytochemistry 62:1235–1238. [DOI] [PubMed] [Google Scholar]
- Geng CA, Ma YB, Zhang XM, Yao SY, Xue DQ, Zhang RP, Chen JJ. 2012. Mulberrofuran G and isomulberrofuran G from Morus alba L.: Anti‐hepatitis B virus activity and mass spectrometric fragmentation. J Agric Food Chem 60:8197–8202. [DOI] [PubMed] [Google Scholar]
- Guan Y, Poon L, Cheung C, Ellis T, Lim W, Lipatov A, Chan K, Sturm‐Ramirez K, Cheung C, Leung Y. 2004. H5N1 influenza: A protean pandemic threat. Proc Natl Acad Sci USA 101:8156–8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Rumschlag‐Booms E, Wang J, Xiao H, Yu J, Guo L, Gao GF, Cao Y, Caffrey M, Rong L. 2009a. Analysis of hemagglutinin‐mediated entry tropism of H5N1 avian influenza. Virol J 6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Tisoncik J, McReynolds S, Farzan M, Prabhakar BS, Gallagher T, Rong L, Caffrey M. Identification of a new region of SARS‐CoV S protein critical for viral entry. J Mol Biol 394:600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Choe S, Walker R, Di Marzio P, Morgan DO, Landau NR. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol 69:6705–6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TY, Wu SL, Lai IL, Cheng KS, Kao ST, Hsiang CY. 2003. An in vitro system combined with an in‐house quantitation assay for screening hepatitis C virus inhibitors. Antiviral Res 58:199–208. [DOI] [PubMed] [Google Scholar]
- Jassim S, Naji MA. 2003. Novel antiviral agents: A medicinal plant perspective. J Appl Microbiol 95:412–427. [DOI] [PubMed] [Google Scholar]
- Ma SC, Du J, But PP, Deng XL, Zhang YW, Ooi VE, Xu HX, Lee SH, Lee SF. 2002. Antiviral Chinese medicinal herbs against respiratory syncytial virus. J Ethnopharmacol 79:205–211. [DOI] [PubMed] [Google Scholar]
- Manicassamy B, Rong L. 2009. Expression of Ebolavirus glycoprotein on the target cells enhances viral entry. Virol J 6:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicassamy B, Wang J, Jiang H, Rong L. 2005. Comprehensive analysis of ebola virus GP1 in viral entry. J Virol 79:4793–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. 2007. Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:461–477. [DOI] [PubMed] [Google Scholar]
- Ngan F, Chang RS, Tabba HD, Smith KM. 1988. Isolation, purification and partial characterization of an active anti‐HIV compound from the Chinese medicinal herb viola yedoensis. Antiviral Res 10:107–116. [DOI] [PubMed] [Google Scholar]
- Nichol ST, Arikawa J, Kawaoka Y. 2000. Emerging viral diseases. Proc Natl Acad Sci USA 97:12411–12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radoshitzky SR, Abraham J, Spiropoulou CF, Kuhn JH, Nguyen D, Li W, Nagel J, Schmidt PJ, Nunberg JH, Andrews NC, Farzan M, Choe H. 2007. Transferrin receptor 1 is a cellular receptor for New World haemorrhagic fever arenaviruses. Nature 446:92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J, Gottfries J, Muresan S, Backlund A, Oprea TI. 2009. Novel chemical space exploration via natural products. J Med Chem 52:1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y, Kolokoltsov AA, Chen C‐C, Tidwell MW, Bauta WE, Klugbauer N, Grimm C, Wahl‐Schott C, Biel M, Davey RA. 2015. Two‐pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science 347:995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towner JS, Khristova ML, Sealy TK, Vincent MJ, Erickson BR, Bawiec DA, Hartman AL, Comer JA, Zaki SR, Stroher U, Gomes da Silva F, del Castillo F, Rollin PE, Ksiazek TG, Nichol ST. 2006. Marburgvirus genomics and association with a large hemorrhagic fever outbreak in Angola. J Virol 80:6497–6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Cheng H, Ratia K, Varhegyi E, Hendrickson WG, Li J, Rong L. 2014. A comparative high‐throughput screening protocol to identify entry inhibitors of enveloped viruses. J Biomol Screen 19:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Jia W, Zhao A. 2006. Anti‐influenza agents from plants and traditional Chinese medicine. Phytother Res 20:335–341. [DOI] [PubMed] [Google Scholar]
- White NJ. 1997. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob Agents Chemother 41:1413–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HX, Lee SH, Lee SF, White RL, Blay J. 1999. Isolation and characterization of an anti‐HSV polysaccharide from Prunella vulgaris. Antiviral Res 44:43–54. [DOI] [PubMed] [Google Scholar]
- Yao XJ, Wainberg MA, Parniak MA. 1992. Mechanism of inhibition of HIV‐1 infection in vitro by purified extract of Prunella vulgaris. Virology 187:56–62. [DOI] [PubMed] [Google Scholar]
- Zhang J‐H, Chung TD, Oldenburg KR. 1999. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 4:67–73. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ao Z, Bello A, Ran X, Liu S, Wigle J, Kobinger G, Yao X. 2016. Characterization of the inhibitory effect of an extract of Prunella vulgaris on Ebola virus glycoprotein (GP)‐mediated virus entry and infection. Antiviral Res 127:20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


