Abstract
The human colorectal adenocarcinoma‐derived Caco‐2 cell line was evaluated as a means isolating common respiratory viruses from nasopharyngeal aspirates for the diagnosis of respiratory diseases. One hundred eighty‐nine direct immunofluorescence positive nasopharyngeal aspirates obtained from patients with various viral respiratory diseases were cultured in the presence of Caco‐2 cells or the following conventional cell lines: LLC‐MK2, MDCK, HEp‐2, and A549. Caco‐2 cell cultures effectively propagated the majority (84%) of the viruses present in nasopharyngeal aspirate samples compared with any positive cultures obtained using the panel cells (78%) or individual cell line MDCK (38%), HEp‐2 (21%), LLC‐MK2 (27%), or A549 (37%) cell lines. The differences against individual cell line were statistically significant (P = < 0.000001). Culture in Caco‐2 cells resulted in the isolation of 85% (36/42) of viruses which were not cultivated in conventional cell lines. By contrast, 80% (24/30) of viruses not cultivated in Caco‐2 cells were isolated using the conventional panel. The findings indicated that Caco‐2 cells were sensitive to a wide range of viruses and can be used to culture a broad range of respiratory viruses. J. Med. Virol. 85:874–879, 2013. © 2013 Wiley Periodicals, Inc.
Keywords: cell culture, Caco‐2, viral isolation, respiratory viruses
INTRODUCTION
Influenza A, influenza B, respiratory syncytial virus (RSV), parainfluenza virus (PIV), and adenovirus (AdV) represent the most common viruses causing acute respiratory diseases resulting in significant morbidity and mortality. Conventional culture methods used to isolate respiratory viruses are based on tissue culture methods utilizing a panel of cell lines that include MDCK (Madin–Darby canine kidney), LLC‐MK2 (Rhesus monkey kidney), HEp‐2 (laryngeal cancer), A549 (human lung carcinoma), and RD (Rhabdomyosarcoma). Maintenance of different cell lines is complicated, cumbersome, requires long turnaround times, and is expensive. The RhMK (primary rhesus monkey kidney) cell line has been used for isolation of various respiratory viruses. However, the availability of primary cells, varying susceptibility to infection with different respiratory viruses, the potential of harboring endogenous foamy virus, and high costs limit the use of this cell line in diagnostic virology laboratories. Commercial R‐Mix (Mix cells with A549 and Mint Lung) and super E‐Mix (genetically engineered BGMK and Caco‐2 cells) have replaced the conventional cell line panel for the isolation of respiratory viruses [Lee et al., 1992; Buck et al., 2002; Weinberg et al., 2004]. Caco‐2 cells have also been used to isolate enteroviruses, enteric viruses, and influenza viruses [Reigel, 1985; Pintó et al., 1994; Yoshino et al., 1998; Chiapponi et al., 2010; Jahangir et al., 2010]. Several studies have also showed that Caco‐2 cells have the ability to propagate coronaviruses NL63 and SARS from culture isolates [Spiegel and Weber, 2006; Müller et al., 2010]. However, the efficacy of Caco‐2 cells in isolating common respiratory viruses directly from clinical samples remains unknown. In this study, the efficacy of isolating respiratory viruses from Caco‐2 cells was compared to isolation from the conventional cell line panel.
MATERIALS AND METHODS
Nasopharyngeal Aspirate Samples
Nasopharyngeal aspirates were sent to the virology laboratory for routine direct immunofluorescence antigen testing to diagnose infections caused by respiratory viral diseases and residual samples were used for this study. One hundred eighty‐nine nasopharyngeal aspirate specimens positive by direct immunofluorescence antigen confirmation were evaluated. The specimens were collected from 94 males and 95 females with a mean age of 30.4 years (range 1 month to 102 years of age). The positive direct immunofluorescence antigen testing identified 27 RSV, 38 influenza A, 32 influenza B, 20 PIV‐1, 11 PIV‐2, 30 PIV‐3, 18 PIV‐4, and 13 AdV isolates. This study was approved by the Institutional Review Board of the University of Hong Kong Hospital Authority, Hong Kong, West Cluster.
Viral Cultures
MDCK, LLC‐MK2, HEp‐2, A549, and Caco‐2 (ATCC HTB 37) cell monolayers grown in culture tubes were inoculated with 200 µl of each Nasopharyngeal aspirate sample and incubated at 35°C for 1 hr [Chan et al., 2008; Li et al., 2009]. MDCK and LLC‐MK2 cells were fed with 1 ml of serum‐free minimum essential medium (MEM) (GibcoBRL, Grand Island, NY) containing TPCK (tosylsulfonyl phenylalanyl chloromethyl ketone)‐treated trypsin (2 µg/ml) (Sigma, St. Louis, MO), and antibiotics (Garamycin, 0.02 mg/ml, Schering‐Plough Corporation, Heist‐op‐den‐Berg, Belgium; penicillin–streptomycin, 100 units/ml, GibcoBRL; nystatin, 20 units/ml, Sigma). Caco‐2; HEp‐2, and A549 cells were fed with 1 ml of MEM supplemented with 1% fetal calf serum (GibcoBRL) and antibiotics. Culture tubes were incubated using a roller apparatus at a speed of 12–15 revolutions per hour at 35°C. The cultures were then examined for virus‐induced cytopathic effect (CPE) daily for up to 10 days. At the end of the incubation period, or when CPE was detected, a cell scraper was used to collect cells that were mounted subsequently on Teflon‐coated slides, fixed, and immunostained with IMAGEN™ respiratory screen and typing reagents (Oxoid, Hampshire, UK) specific for viral antigens.
Direct Immunofluorescence Antigen Testing
Direct immunofluorescence antigen testing was carried out on nasopharyngeal aspirate specimens or culture‐infected cells as described previously [Chan et al., 2002]. Briefly, nasopharyngeal aspirate or infected cells were centrifuged, and the cell pellet washed in phosphate‐buffered saline (PBS). The cell pellet was then spotted on 6‐mm teflon‐coated slide wells, air dried, and fixed in ice‐cold acetone for 10 min. Smears were stained with IMAGEN™ respiratory screen and typing reagents for influenza virus type A and B, RSV, PIV screen and typing, AdV (Oxoid), and PIV‐4 (Millipore, Temecula, CA) and viewed at a magnification of 400× under epi‐fluorescence illumination using the fluorescein isothiocyanate (FITC) filter of a fluorescence microscope (Euroimmun, Luebeck, Germany).
Nucleic Acid Extraction and PCR for Respiratory Viruses
Nucleic acids were extracted using the NucliSens EasyMAG automatic robotic platform (bioMerieux, Marcy‐l'Etoile, France) according to the manufacturer's instructions. Briefly, 250 µl of a nasopharyngeal aspirate sample was added to 2 ml of lysis buffer and the mixture incubated for 10 min at room temperature. Total nucleic acid was recovered in 55 µl of elution buffer after magnetic separation [Chan et al., 2008]. PIV were identified by a set of multiplex primers used to amplify the hemagglutinin‐neuraminidase gene of PIV‐1, ‐2, and ‐3 or the phosphoprotein gene of PIV‐4 [Aguilar et al., 2000].
Data Analysis
Sensitivity of detection for a particular cell line was defined as the number of positive cultures divided by the total number of direct immunofluorescence antigen test positive samples. Chi‐square analysis was used to compare the sensitivity between different cell lines. A P‐value of <0.05 was considered as statistically significant.
RESULTS
One hundred eighty‐five nasopharyngeal aspirate samples were direct immunofluorescence antigen positive for eight common respiratory viruses subsequently cultured in five cell lines: Caco‐2, A549, HEp‐2, LLC‐MK2, and MDCK (Table I). Overall the sensitivity of Caco‐2 cells [84% (159/189)] for recovery of these respiratory viruses was higher than the sensitivity of the cell panel [78% (147/189)], however, this difference was not statistically significant (P = 0.150). When the sensitivity was compared to individual cell lines in the panel (MDCK [38%], HEp‐2 [21%], LLC‐MK2 [27%], and A549 [37%]) detection differences were statistically significant (P = < 0.000001).
Table I.
Comparison of the Caco‐2 Cell Line With Conventional Cell Lines Used in the Recovery of Respiratory Viruses From Nasopharyngeal Aspirate
| Direct immunofluorescence antigen test positive | Number of nasopharyngeal aspirates | Caco‐2 number positive (%) | A549 number positive (%) | HEp‐2 number positive (%) | LLC‐MK2 number positive (%) | MDCK number positive (%) |
|---|---|---|---|---|---|---|
| RSV | 27 | 20 (74%) | 14 (52%) | 22 (81%) | 5 (19%) | 0% |
| Flu A | 38 | 35 (92%) | 4 (11%) | 0% | 3 (8%) | 36 (95%) |
| Flu B | 32 | 25 (78%) | 7 (22%) | 0% | 3 (9%) | 31 (97%) |
| PIV‐1 | 20 | 16 (80%) | 20 (100%) | 0% | 20 (100%) | 3 (15%) |
| PIV‐2 | 11 | 11 (100%) | 5 (45%) | 1 (9%) | 7 (64%) | 1(9%) |
| PIV‐3 | 30 | 22 (73%) | 6 (20%) | 4 (13%) | 11 (37%) | 0% |
| PIV‐4 | 18 | 18 (100%) | 1 (6%) | 1 (6%) | 2 (11%) | 1 (6%) |
| AdV | 13 | 12 (92%) | 13 (100%) | 12 (92%) | 0% | 0% |
| Total | 189 | 159 (84%) | 70 (37%) | 40 (21%) | 51 (27%) | 72 (38%) |
RSV, respiratory syncytial virus; Flu A, influenza A; Flu B, influenza B; PIV, parainfluenza virus; AdV, adenovirus.
CPE was observed for all virus‐infected Caco‐2 cells except PIV between days 3 and 8 (Fig. 1). CPE was observed for RSV, PIV‐1, ‐2, and AdV but not influenza virus‐infected A549 cells between days 3 and 6. CPE was only observed for RSV, PIV‐4, and AdV infected HEp‐2 cells between days 2 and 5. CPE was not discernable in virus‐infected LLC‐MK2 cells except for RSV and PIV‐1 that induced CPE between days 3 and 7. Only influenza virus was able to induce CPE in MDCK cells between days 2 and 5. All virus‐infected cells developing or not developing CPE were also stained with a panel of fluorescein labeled monoclonal antibodies to confirm infection as described in Materials and Methods Section (Fig. 2).
Figure 1.
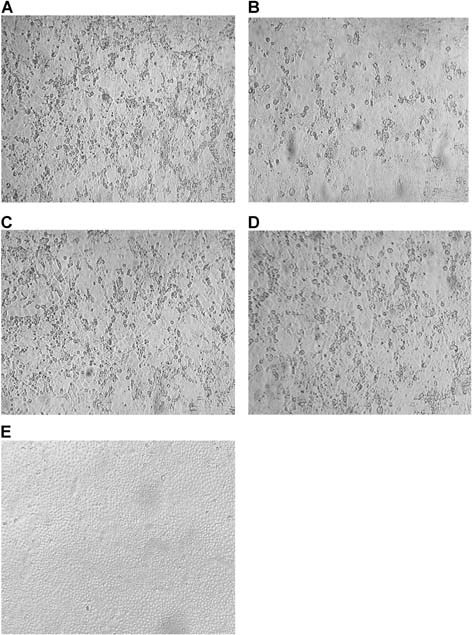
CPE in Caco‐2 cells following infections with (A) RSV, (B) AdV, (C) Flu A, (D) Flu B, and (E) a virus negative nasopharyngeal aspirate.
Figure 2.
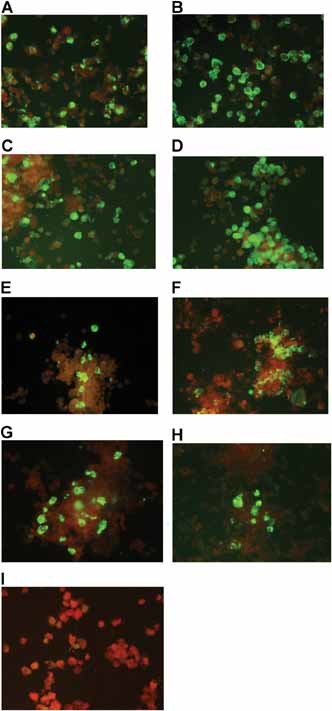
Antigen expression in Caco2 cells identified by direct immunofluorescence staining using a panel of monoclonal antibodies specific for respiratory viruses. A: RSV, (B) AdV, (C) Flu A, (D) Flu B, (E) PIV‐1, (F) PIV‐2, (G) PIV‐3, (H) PIV‐4, and (I) a virus negative nasopharyngeal aspirate.
The sensitivities of different combinations of conventional panel cell lines (LLC‐MK2, A549, MDCK, and HEp‐2) with Caco‐2 cells were 88%, 88%, 89%, and 87%, respectively (Table III). Of the 42 cultures that were negative using the conventional cell culture panel, 36 were positive when cultured with Caco‐2 cells. Conversely, 30 Caco‐2 cultures were negative and 24 were conventional panel positive (Table II). There were 6 PIV‐3 direct immunofluorescence antigen test positive specimens with negative viral cultures that were confirmed positive by RT‐PCR.
Table III.
Different Caco‐2 Combinations With Panel Cell Lines Used in the Recovery of Respiratory Viruses
| Cell lines | RSV (n = 27) | Flu A (n = 38) | Flu B (n = 32) | PIV‐1 (n = 20) | PIV‐2 (n = 11) | PIV‐3 (n = 30) | PIV‐4 (n = 15) | AdV (n = 13) | Total culture positive | Percentage positive (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Caco‐2 | 20 | 35 | 25 | 16 | 11 | 22 | 18 | 12 | 159 | 84 |
| Caco‐2 + A549 | 22 | 35 | 25 | 20 | 11 | 23 | 18 | 13 | 167 | 88 |
| Caco‐2 + MDCK | 20 | 38 | 32 | 16 | 11 | 22 | 18 | 12 | 169 | 89 |
| Caco‐2 + HEp‐2 | 25 | 35 | 25 | 16 | 11 | 22 | 18 | 13 | 165 | 87 |
| Caco‐2 + LLC‐MK2 | 23 | 35 | 25 | 20 | 11 | 23 | 18 | 12 | 167 | 88 |
RSV, respiratory syncytial virus; Flu A, influenza A; Flu B, influenza B; PIV, parainfluenza virus; AdV, adenovirus.
Table II.
Evaluation of Negative Cultures Following Culture With Panel Cells Compared to Culture With Caco‐2 Cells Resulting in Positive Viral Propagation and Vice Versa
| Virus type | Panel cells negative | Caco‐2 cells positive | Caco‐2 cells negative | Panel cells positive |
|---|---|---|---|---|
| RSV | 2 | 2 | 7 | 7 |
| Flu A | 2 | 2 | 3 | 3 |
| Flu B | 1 | 1 | 7 | 7 |
| PIV‐1 | 0 | 0 | 4 | 4 |
| PIV‐2 | 4 | 4 | 0 | 0 |
| PIV‐3 | 17 | 11 | 8 | 2 |
| PIV‐4 | 16 | 16 | 0 | 0 |
| AdV | 0 | 0 | 1 | 1 |
| Total | 42 | 36 | 30 | 24 |
RSV, respiratory syncytial virus; Flu A, influenza A; Flu B, influenza B; PIV, parainfluenza virus; AdV, adenovirus.
DISCUSSION
In this study, Caco‐2 cells [84% (159/189)] were shown to be more efficient for propagating the most common respiratory viruses associated with clinical NPA samples compared to the conventional cell panel comprised of the MDCK [(38% (72/189)], HEp‐2 [21% (40/189)], A549 [38% (70/189)], and LLC‐MK2 [(27% (51/189)] cell lines used for virus propagation or positive by any cell line in the panel [78% (147/189)]. Caco‐2 cells were the most efficient cell line for isolating PIV‐2–4, and HEp2, MDCK, LLC‐MK2, and A549 were the most efficient cell lines for recovering RSV, influenza A and B, PIV‐1, and adenovirus, respectively (Table I). One of the major advantages of using Caco‐2 cells was that viruses were recovered from this cell line that could not be cultured using cell lines comprising the conventional cell panel (Table II). However, Caco‐2 cells required a 2–5 day‐longer incubation time for virus recovery compared to the other cell lines. The sensitivity was increased from 84% to 87–89% if Caco‐2 were used with any one of the cell lines comprising the conventional panel (Table III). In order to maximize the sensitivity and decrease costs, combining Caco‐2 and MDCK cells can be used during influenza seasons to isolate the maximum number of influenza viruses. Combinations of Caco‐2 with LLC‐MK2 or A549 cells can be used to culture other viruses at other times of the year.
Furthermore, the Caco‐2 cell line does not require the addition of trypsin to isolate influenza viruses because they already cleave viral HA0 into the HA1 and HA2 subunits [Yoshino et al., 1998; Chiapponi et al., 2010] and are susceptible to CPE [Zhirnov and Klenk, 2003]. PIV‐4 was reported to be quite difficult to isolate in cell culture [Laurichesse et al., 1999; Lau et al., 2005]. In this study, Caco‐2 cell cultures efficiently supported the replication of PIV, particularly PIV‐4 (Tables I and II). Human PIVs have often been associated with upper respiratory tract infections and other more severe disease, especially in immunocompromised patients [Woo et al., 2000; Cortez et al., 2001]. PIV‐4 was associated with an outbreak involving 38 institutionalized children and three staff members [Lau et al., 2005] and also played an important role in causing acute lower respiratory tract infections in children [Ren et al., 2011].
Although antigen detection using immunofluorescence and nucleic acid detection by RT‐PCR are widely used in clinical diagnostic laboratories, it is important to maintain viral cultures since viral isolates are important for carrying out detailed molecular studies that require sufficient amounts of viral nucleic acid. This is especially important during the first isolation and characterization of new viruses, such as the SARS coronavirus [Peiris et al., 2003]. A simple culture work flow will facilitate diagnostic virology services since clinical laboratories are the first to process these specimens and would therefore have the highest chance of isolating respective virus since sample storage may reduce viral culture yields if processed at a later time.
Conflicts of interest: None.
REFERENCES
- Aguilar JC, Perez‐Brena MP, Garcia ML, Cruz N, Erdman DD, Echevarria JE. 2000. Detection and identification of human parainfluenzaviruses 1, 2, 3, and 4 in clinical samples of pediatric patients by multiplexreverse transcription‐PCR. J Clin Microbiol 38:1191–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck GE, Wiesemann M, Stewart L. 2002. Comparison of mixed cell culture containing genetically engineered BGMK and CaCo‐2 cells (Super E‐Mix) with RT‐PCR and conventional cell culture for the diagnosis of enterovirus meningitis. J Clin Virol 25:S13–S18. [DOI] [PubMed] [Google Scholar]
- Chan KH, Maldeis N, Pope W, Yup A, Ozinskas A, Gill J, Seto WH, Shortridge KF, Peiris JS. 2002. Evaluation of the DirectigenFluA + B test for rapid diagnosis of influenza virus type A and B infections. J Clin Microbiol 40:1675–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KH, Peiris JS, Lim W, Nicholls JM, Chiu SS. 2008. Comparison of nasopharyngeal flocked swabs and aspirates for rapid diagnosis of respiratory viruses in children. J Clin Virol 42:65–69. [DOI] [PubMed] [Google Scholar]
- Chiapponi C, Zanni I, Garbarino C, Barigazzi G, Foni E. 2010. Comparison of the usefulness of the CACO‐2 cell line with standard substrates for isolation of swine influenza A viruses. J Virol Methods 163:162–165. [DOI] [PubMed] [Google Scholar]
- Cortez KJ, Erdman DD, Peret TC, Gill VJ, Childs R, Barrett AJ, Bennett JE. 2001. Outbreak of human parainfluenza virus 3 infections in a hematopoietic stem cell transplant population. J Infect Dis 184:1093–1097. [DOI] [PubMed] [Google Scholar]
- Jahangir A, Ruenphet S, Hara K, Shoham D, Sultana N, Okamura M, Nakamura M, Takehara K. 2010. Evaluation of human intestinal epithelial differentiated cells (Caco‐2) for replication, plaque formation and isolation of avian influenza viruses. J Virol Methods 169:232–238. [DOI] [PubMed] [Google Scholar]
- Lau SKP, To WK, Tse PWT, Chan AKH, Woo PCY, Tsoi HW, Leung AFY, Li KSM, Chan PKS, Lim WWL, Yung RWH, Chan KH, Yuen KY. 2005. Human parainfluenza virus 4 outbreak and the role of diagnostic tests. J Clin Microbiol 43:4515–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurichesse H, Dedman D, Watson JM, Zambon MC. 1999. Epidemiological features of parainfluenza virus infections: Laboratory surveillance in England and Wales, 1975–1997. Eur J Epidemiol 15:475–484. [DOI] [PubMed] [Google Scholar]
- Lee SH, Boutilier JE, MacDonald MA, Forward KR. 1992. Enhanced detection of respiratory viruses using the shell vial technique and monoclonal antibodies. J Virol Methods 39:39–46. [DOI] [PubMed] [Google Scholar]
- Li IW, Chan KH, To KW, Wong SS, Ho PL, Lau SK, Woo PC, Tsoi HW, Chan JF, Cheng VC, Zheng BJ, Chen H, Yuen KY. 2009. Differential susceptibility of different cell lines to swine‐origin influenza A H1N1, seasonal human influenza A H1N1, and avian influenza A H5N1 viruses. J Clin Virol 46:325–330. [DOI] [PubMed] [Google Scholar]
- Müller MA, van der Hoek L, Voss D, Bader O, Lehmann D, Schulz AR, Kallies S, Suliman T, Fielding BC, Drosten C, Niedrig M. 2010. Human coronavirus NL63 open reading frame 3 encodes a virion‐incorporated N‐glycosylated membrane protein. Virol J 7: article no. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, Nicholls J, Yee WK, Yan WW, Cheung MT, Cheng VC, Chan KH, Tsang DN, Yung RW, Ng TK, Yuen KY, SARS Study Group . 2003. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361:1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintó RM, Diez JM, Bosch A. 1994. Use of the colonic carcinoma cell line CaCo‐2 for in vivo amplification and detection of enteric viruses. J Med Virol 44:310–315. [DOI] [PubMed] [Google Scholar]
- Reigel F. 1985. Isolation of human pathogenic viruses from clinical material on CaCo2 cells. J Virol Methods 12:323–327. [DOI] [PubMed] [Google Scholar]
- Ren L, Gonzalez R, Xie Z, Xiong Z, Liu C, Xiang Z, Xiao Y, Li Y, Zhou H, Li J, Yang Q, Zhang J, Chen L, Wang W, Vernet G, Paranhos‐Baccalà G, Shen K, Wang J. 2011. Human parainfluenza virus type 4 infection in Chinese children with lower respiratory tract infections: A comparison study. J Clin Virol 51:209–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel M, Weber F. 2006. Inhibition of cytokine gene expression and induction of chemokine genes in non‐lymphatic cells infected with SARS coronavirus. Virol J 3:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Brewster L, Clark J, Simoes E, ARIVAC consortium . 2004. Evaluation of R‐Mix shell vials for the diagnosis of viral respiratory tract infections. J Clin Virol 30:100–105. [DOI] [PubMed] [Google Scholar]
- Woo PC, Young K, Tsang KW, Ooi CG, Peiris M, Yuen KY. 2000. Adult croup: A rare but more severe condition. Respiration 67:684–688. [DOI] [PubMed] [Google Scholar]
- Yoshino S, Yamamoto S, Kawabata N. 1998. Use of Caco‐2 cells for isolation of influenza virus. Kansenshogaku Zasshi 72:347–351. [DOI] [PubMed] [Google Scholar]
- Zhirnov O, Klenk HD. 2003. Human influenza A viruses are proteolytically activated and do not induce apoptosis in CACO‐2 cells. Virology 313:198–212. [DOI] [PubMed] [Google Scholar]


