Short abstract
Defensins and LL‐37 play key roles in maintaining mucosal barriers against invasive infection and initiating immune responses after infection or injury to mucosal surfaces.
Keywords: retrocyclin, innate immunity
Abstract
Defensins are widespread in nature and have activity against a broad range of pathogens. Defensins have direct antimicrobial effects and also modulate innate and adaptive immune responses. We consider the role of human defensins and the cathelicidin LL‐37 in defense of respiratory, gastrointestinal, and genitourinary tracts and the oral cavity, skin, and eye. Human β‐defensins (hBDs) and human defensins 5 and 6 (HD5 and −6) are involved most obviously in mucosal responses, as they are produced principally by epithelial cells. Human α‐defensins 1–4 (or HNPs 1–4) are produced principally by neutrophils recruited to the mucosa. Understanding the biology of defensins and LL‐37 is the beginning to clarify the pathophysiology of mucosal inflammatory and infectious diseases (e.g., Crohn's disease, atopic dermatitis, lung or urinary infections). Challenges for these studies are the redundancy of innate defense mechanisms and the presence and interactions of many innate defense proteins in mucosal secretions.
Abbreviations
- –/–
knockout
- ARDS
acute respiratory distress syndrome
- BALF
bronchoalveolar lavage fluid
- BD
β‐defensin
- BV
bacterial vaginosis
- CD
Crohn's disease
- CF
cystic fibrosis
- CRAMP
cathelin‐related antimicrobial peptide
- DC
dendritic cell
- EGFR
epidermal growth factor receptor
- FPRL‐1
formyl peptide receptor‐like 1
- hBD
human β‐defensins
- hCAP18
human cathelicidin antimicrobial peptide 18
- HD5/6
human α‐defensins 5 and 6
- HNP
human neutrophil peptide
- IAV
influenza A virus
- LTB4
leukotriene B4
- mBD
mouse β‐defensin
- MMP7
matrix metalloproteinase 7
- NOD2
nucleotide‐binding oligomerization domain 2
- PLA2
phospholipase A2
- RSV
respiratory syncytial virus
- SARS
severe acute respiratory syndrome
- SP‐D
surfactant protein D
- sPLA2
secretory PLA2
- TAP
tracheal antimicrobial peptide
- Tcf‐4
transcription factor 4
Introduction
This review will focus specifically on the role of defensins and the antimicrobial peptide LL‐37 in human mucosal immunity. There have been excellent recent reviews that cover the structure, genomics, evolution, and mechanisms of action of these antimicrobial peptides [1, 2, 3, 4]. We refer the reader to these for more extensive discussion of these aspects. We will consider these topics as they relate to action of the antimicrobial peptides at specific mucosal sites. In general, defensins and LL‐37 have two major functions in host defense: direct inhibition of pathogens and modulation of other innate and adaptive immune responses. The human defensins include hBDs, which are expressed widely in skin and mucosal epithelia, and six α‐defensins, which include HNPs 1–4 and epithelial HD5 and HD6. hBDs and HD5 and −6 are expressed predominantly by mucosal epithelial cells, where they contribute to initial host defense against infection and also to maintenance of epithelial integrity. HNPs are expressed predominantly by neutrophils and to a lesser extent, in other bone marrow‐derived cells that are resident in, or recruited to, inflamed or infected sites in the body. The α‐defensins are expressed constitutively and generally packaged in granules (azurophil granules of neutrophils for HNPs and small intestinal Paneth cell granules for HDs) and are released by degranulation. HDs are also released by epithelial cells in other mucosal sites (e.g., in the genitourinary tract). HNPs are generally processed to the mature peptide prior to release, whereas HDs must be cleaved by extracellular proteases to achieve the mature, active form. In general, hBDs are expressed by secretion from epithelial cells rather than by degranulation and are secreted in mature form on to the surface of, or lining fluid surrounding, these cells. We will consider hBDs 1–4 in this review, although at least two others have been described. hBD1 is widely expressed in a constitutive manner by epithelial cells, but expression is induced, to a small degree, by inflammatory stimuli in some epithelia. hBDs 2–4 are also widely expressed by epithelial cells, but their expression is inducible in most of these tissues in response to infectious or inflammatory stimuli.
We include the hCAP18/LL‐37, as it has similar biology and plays a central role in mucosal defense as well. Like the defensins, hCAP18/LL‐37 is a member of a large family of cationic antimicrobial peptides expressed in many species and has broad‐spectrum antimicrobial activity and many immunomodulatory effects. The cathelicidins contain a signal peptide, a cathelin‐like domain, and antimicrobial domain (LL‐37). hCAP18/LL‐37 is produced by neutrophils, macrophages, and various epithelial cells as well. The antimicrobial domain is released by cleavage by proteases, and this domain is termed LL‐37. We will generally use the term LL‐37 subsequently in this review.
We also consider another class of defensins, called θ‐defensins or retrocyclins. These have a cyclic structure [5] and are expressed in nonhuman primates, such as the rhesus macaque and olive baboon (Papio anubis) [6, 7]. Humans express θ‐defensin mRNA but lack the corresponding peptides, as the human θ‐defensin gene contains a stop codon in the signal sequence that aborts translation. Retrocyclins are synthetic, humanized θ‐defensin peptides, whose sequences are based on those found in human θ‐defensin genes. The DEFTs also have broad‐spectrum antibacterial and antiviral activity and are relevant to this review because of their therapeutic potential and also, as it was shown recently that their expression can be rescued in human cervico‐vaginal epithelial cells by treatment with an aminoglycoside [8].
We will focus on the major mucosal systems, including the respiratory tract, the gastrointestinal tract, the oral cavity, the genitourinary tract, the skin, and the eye. The discussion of the respiratory tract will be most extensive, as some general themes and concepts will be laid out there. The mucosa within each system and even within different locales within a mucosal system (e.g., the nasal cavity, upper and lower respiratory tract) have different functions for the body and different needs in terms of host defense. Some sites are normally sterile, and others contain commensal bacteria. By necessity, we will not do full justice to the different domains within each mucosal system and the diversity of roles that defensins and LL‐37 play within specific sites; however, we hope to convey the extraordinary detail and intricacy of the systems that have evolved to allow our mucosal surfaces to carry out their job of allowing exchange with the external environment, while protecting us from myriad pathogens and other potential injuries. Table 1 summarizes the major known associations of human diseases with alterations in defensins or LL‐37.
Table 1.
Association of Defensins and LL‐37 with Various Diseases
| Respiratory Tract |
| Cystic fibrosis |
| ‐ reduced hBDs; elevated HNPs and LL‐37 associated with inflammation; inhibition of defensin activity by high ionic concentrations in respiratory lining fluid; degradation of defensins by proteases |
| Bronchiolitis obliterans and diffuse panbronchiolitis |
| ‐ elevated hBDs |
| ARDS and α1‐antitrypsin deficiency |
| ‐ elevated HNPs |
| Reactive airways disease |
| ‐ reduced hBD2 and LL‐37 in mouse model |
| Tobacco smoking |
| ‐ reduced hBD2 |
| Lung infections |
| ‐ Low LL‐37 associated with vitamin D deficiency increases risk for tuberculosis. |
| ‐ Defensins and LL‐37 contribute to host defense against other bacteria and respiratory viruses based on in vitro and mouse models. |
| ‐ Hyper‐IgE syndrome is associated with risk for lung and skin infections, in part, as a result of lack of production of antimicrobial peptides by bronchial epithelial cells or keratinocytes. |
| Gastrointestinal Tract |
| CD of ileum |
| ‐ reduced HD5 and HD6 as a result of impaired NOD2 and/or Wnt/Tcf‐4 signaling |
| CD of colon |
| ‐ reduced hBD2 and LL‐37 response; reduced hBD copy number |
| Ulcerative colitis |
| ‐ elevated hBD2 and LL‐37 |
| Helicobacter pylori infection of stomach |
| ‐ hBD2, hBD3, and LL‐37 participate in host response in vitro. |
| Bacterial dysentery |
| ‐ HD5 overexpression protects mice from Salmonella infection in the gut. |
| ‐ Mouse cryptdin expression correlates with inhibition of Shigella infection. |
| ‐ Shigella and enteropathogenic Escherichia coli interfere with hBD or LL‐37 expression; Salmonella alters LPS structure to avoid binding to antimicrobial peptides. |
| Oral Cavity |
| ‐ Morbus Kostmann syndrome is characterized by severe periodontitis associated with low levels of LL‐37 in neutrophils and saliva. |
| Genitourinary Tract |
| Bacterial vaginosis |
| ‐ reduced HNP and hBD levels in vaginal fluid |
| Neisseria gonorrhoeae urethritis and cervicitis |
| ‐ elevated HD and HNP levels in urethral and vaginal secretions; HD enhances HIV infectivity |
| HIV |
| ‐ reduced vaginal hBD levels |
| Urinary tract infection |
| ‐ mBD and CRAMP gene deletion in mice increase risk. |
| Skin |
| Atopic dermatitis |
| ‐ reduced hBD2 and LL‐37 levels in lesions |
| Hyper‐IgE syndrome |
| ‐ reduced levels of antimicrobial peptide production by keratinocytes associated with atopy and recurrent staphyloccal infections |
| Chronic skin ulcers |
| ‐ reduced antimicrobial peptide expression in ulcers |
| Psoriasis |
| ‐ increased hBD2 and LL‐37 in lesions; elevated hBD gene copy number |
| Acne rosacea |
| ‐ increased LL‐37 in lesions |
RESPIRATORY TRACT
There are distinctive features of the respiratory tract that make innate defense particularly important. The respiratory epithelium has a huge surface (estimated to be the size of a tennis court), and the alveolar epithelium in particular must be very thin and delicate to allow exchange of oxygen from air to the blood. This delicate mucosa must be protected from toxins and infectious agents with a minimum of inflammation. The upper respiratory tract functions to conduct air but also to filter out and prevent penetration of particulates and infectious agents to the alveoli. It is clear that defensins and cathelicidins play key roles in innate defense in the respiratory tract; however, it has been challenging to isolate their specific contributions as a result of the complexity and redundancy of the innate defense mechanisms that have evolved. Defensins and cathelicidins are produced by respiratory epithelial cells and immune cells that are resident in, or recruited to, the respiratory tract. We will first consider the role of the respiratory epithelium per se in defense and then how bone marrow‐derived inflammatory cells contribute to defense and interact with the epithelium. As this is a complex topic, we will focus on key points and seminal findings that have led us to our current state of understanding.
The respiratory epithelium itself is felt to be an important component of the innate response system to lung infection. Shornick et al. [9] recently confirmed the importance of the epithelium per se (as opposed to bone marrow‐derived cells) in initiation of the immune response. In this study, mice that lacked Stat1 in the epithelium but had bone marrow containing Stat1 had a strongly impaired response to respiratory viral infection, and the inverse was not true. In 1991, Diamond et al. [10] first reported production of a defensin (TAP) by respiratory epithelial cells (bovine tracheal epithelium), and there has been tremendous progress in the field since then. TAP was subsequently shown to be a member of the BD family (homologue of hBD2) and to be induced through a NF‐κB‐dependent mechanism triggered by bacteria or LPS [11, 12, 13]. It has now been demonstrated that human respiratory epithelial cells produce hBDs 1–4 and LL‐37 [14, 15, 16, 17]. hBD1 is constitutively expressed and not generally up‐regulated, whereas the others are up‐regulated during infections or inflammation [18]. All of the peptides are expressed in a variety of other epithelia and have broad‐spectrum antimicrobial activity. hBD2 is most highly expressed in lung, whereas hBD4 is expressed most highly in testes and stomach, LL‐37 in bone marrow, and hBD3 in skin and tonsil [15, 17, 19, 20]. An important question that has not been evaluated in depth is relative levels of secretion of the defensins at different levels of the respiratory tract during infection.
Regulation of hBD2 expression by respiratory epithelial cells has been evaluated fairly extensively. It can be induced by LPS in a manner that involves CD14, TLR4, and NF‐κB [12, 21]. In A549 lung epithelial cells, hBD2 expression requires not only LPS but also IL‐1β and TNF‐α produced by mononuclear phagocytes [22], possibly because A549 cells lack TLR4. RSV also stimulates production of hBD2 in A549 cells through a NF‐κB‐dependent process that involves RSV‐stimulated generation of TNF‐α by these cells [23]. hBD3 is induced by bacteria and TNF‐α in primary tracheal epithelial cells [20]. Recent studies have demonstrated that vitamin D is important in regulation of LL‐37 expression. Respiratory epithelial cells convert vitamin D to its active metabolites, contain vitamin D receptors, and elaborate cathelicidins and CD14 in response to active metabolites of vitamin D [24]. This response is potentiated by dsRNA. Figure 1 provides an overview of production of hBDs and LL‐37 by respiratory epithelia.
Figure 1.
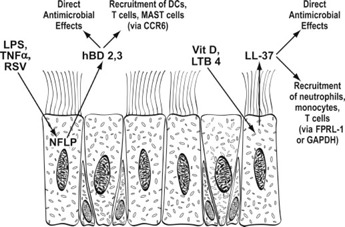
Schematic overview of BD and LL‐37 production by respiratory epithelia.
In addition to their direct antimicrobial and antiviral activities, hBDs and LL‐37 function as “alarmins” to stimulate recruitment of inflammatory cells to the mucosa. This is again a large topic that has been the subject of excellent recent reviews [1, 2]. In brief, hBDs are chemotactic for DCs and T cells via interaction with CCR6 [25]. hBD2 can activate DCs via binding to TLR4 [26], and hBD3 can similarly activate APCs by interacting with TLR1 and −2 [27]. hBDs can also stimulate Mast cell migration and activation [28]. LL‐37 stimulates chemotaxis of neutrophils, monocytes, and T cells via FPRL‐1 [29] and down‐regulates monocyte inflammatory cytokine production in response to LPS and other TLR ligands [30]. Recently, it was also shown that LL‐37 binds to GAPDH at an intracellular location in monocytes, leading to generation of chemokines and IL‐10 [31]. Hence, it is clear that these peptides not only contribute to solidifying the initial mucosal barrier against infection but also modulate further innate and adaptive immune responses.
The recruitment of neutrophils and mononuclear phagocytes brings into play HNPs and further increases in LL‐37. These peptides are again important, not only for their direct antimicrobial effects but also for their signaling properties. In addition, there are complex interactions between phagocytes and epithelial cells that trigger further response from the epithelial cells. HNPs are produced most abundantly by neutrophils, although more limited production by eosinophils has been reported [32]. Although macrophages store only minor amounts of HNPs, a fascinating recent paper demonstrated that they can acquire HNPs via ingestion of neutrophils and that these HNPs colocalize with Mycobacterium tuberculosis in early endosomes and contribute to inhibition of bacterial growth [33]. It is possible that this finding is representative of a more widespread phenomenon in which defensins or LL‐37 can be taken up by various cell types and exert inhibition on growth of intracellular pathogens. For example, HD5 has been shown to be taken up by cervical epithelial cells [34].
HNPs also have chemotactic effects for immature DCs, T cells, and Mast cells [35, 36, 37]. In addition, HNPs stimulate IL‐8 and IL‐1β mRNA and IL‐8 protein production by human bronchial epithelial cells [38]. High concentrations (>20 μg/ml) of HNPs also can be cytotoxic for respiratory epithelial cells [38]. HNP1 induces mucin production in NCI‐H292 cells (with additive increases in the presence of LPS) [39]. HNPs also induced oxidant production in lung explants, and the combination of lung explant tissue and defensins had markedly greater antibacterial activity than the HNPs alone [40]. HNPs also can induce aggregation of bacteria and viruses and promote uptake of these pathogens by neutrophils or macrophages [41, 42]. Hence, there are various ways that HNPs could promote immune responses and inflammation in the lung. LL‐37 is also produced by neutrophils and mononuclear phagocytes [43] and can activate epithelial cells by interacting with EGFRs [44]. LL‐37 can promote inflammatory responses of macrophages in concert with IL‐1β [45]; however, LL‐37 also binds LPS and can inhibit responses to it through this mechanism and through direct interactions with monocytic cells that reduce TLR‐mediated activation [30]. Figures 2 and 3 summarize sources and actions of defensins and LL‐37 derived from myeloid cells.
Figure 2.
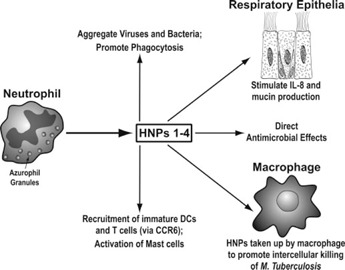
Overview of production and actions of HNPs. Note that macrophages (and possibly other cells) can take up HNPs from the extracellular milieu and use them to inhibit intracellular pathogens.
Figure 3.
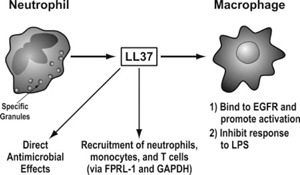
Overview of production of LL‐37 by neutrophils and actions of LL‐37.
Mouse models have been helpful for analysis of the role of hBDs and LL‐37. Mouse homologues for hBDs and LL‐37 have been identified as follows: hBD1 = mBD1, hBD2 = mBD3, hBD3 = mBD14, and hCAP/LL‐37 = CRAMP [19, 46, 47, 48]. Knockout of the mBD1 and CRAMP genes have been accomplished. The results from the mBD1−/− mouse models (developed in two laboratories) have shown no obvious phenotypic changes in the absence of infection. Absence of mBD1 did result in delayed clearance of Hemophilus influenzae from the lung, although inflammatory responses and survival were unchanged [46]. No difference in response to Streptococcus pneumoniae or Staphylococcus aureus lung infection was noted in mBD−/− mice [46, 49]. The subtlety of the defects in these mice raised the question of whether deletion of several mBDs would be needed to show greater effects. The host defense defects documented in CRAMP−/− mice thus far have been for bacterial infections of skin and urinary tract [50, 51]. One limitation of mouse models is that mouse neutrophils do not express peptides homologous to HNPs.
Animal models have been useful to study up‐regulation of mBDs and CRAMP during respiratory bacterial infections [11, 52]. Viral infections also increase expression of epithelial defensins. For instance, IAV infection up‐regulates expression of mBD3 and −4 in conducting airway epithelial cells in mice [53], and parainfluenza virus increases expression of sheep BD1 in neonatal lambs [54]. In each case, up‐regulation of BDs coincided with increased expression of SP‐D (another innate immune protein with broad‐spectrum antibacterial and antiviral activity).
Association of defensins with human respiratory diseases
CF.
Studies of CF reveal the complexity of trying to determine the role of defensins in lung disease. Children with CF are born with normal lung function but undergo steady deterioration marked by recurrent respiratory and bacterial viral infections and chronic lung infections with organisms such as Pseudomonas aeruginosa and Burkholderia cenocepacia. BALF from CF patients has been shown to have reduced antibacterial activity, and this appears in part to be a result of high salt concentrations in the fluid and inhibition of defensin activity (which is salt‐sensitive) [14, 55]. Up‐regulation of hBD2 during infection may be impaired in CF [56]. Levels of hBDs and HNPs can be affected by gene copy numbers, which can be highly variable for genes encoding these proteins [57, 58]; however, no association was found between hBD copy numbers and progression of CF [59]. hBDs have been shown to be proteolytically degraded by cathepsins present in BALF of CF patients [60]. In addition, the LPS of B. cenocepacia renders it resistant to antimicrobial peptides [61]. Hence, there appear to be impaired activity, impaired production, and accelerated breakdown of hBDs in CF, which could in part account for reduced antibacterial activity.
Salt‐insensitive defects in the antibacterial activity of the fluid have also been demonstrated [62]. Furthermore, there are increased levels of HNPs and LL‐37 in CF BALF [56, 63], associated with persistent inflammation and infection. These peptides could actually promote deleterious inflammation in several ways, including stimulating mucin [39] and LTB4 production [64] and interfering with activity of SP‐D [65]. Neutrophil proteases levels are elevated in concert with HNPs in CF and other inflammatory lung conditions (e.g., α1‐antitrypsin deficiency) and act in a concerted manner to reduce SP‐D levels [65, 66] or stimulate LTB4 production [64]. Note that instillation of HNPs into mouse lung caused inflammation and deterioration of lung function [67]. These findings suggest that in some conditions, high levels of HNPs may be deleterious. In contrast, a recent study showed that HNPs released from dying and necrotic neutrophils dampen inflammation in vitro and in vivo, probably by inhibiting proinflammatory cytokine production by macrophages while promoting macrophage phagocytosis [68].
Other inflammatory lung diseases.
Elevated levels of hBD2 have been found in diffuse panbronchiolitis, a progressive inflammatory disease also associated with recurrent episodes of lung infection [69]. Similarly elevated hBD2 levels were noted in bronchiolitis obliterans complicating lung transplantation [70]. Elevated levels HNPs have been found in idiopathic pulmonary fibrosis [71] and ARDS [72], in these cases, correlating with the degree of lung inflammation or injury. Further studies obviously are needed to determine if the defensins are causative of inflammation or a response to other insults. The contributions of defensins or LL‐37 to asthma have not been studied extensively, although subjects with asthma do have increased susceptibility to respiratory viral infections. Of interest, hBD2 and LL‐37 expression can be suppressed by Th2 cytokines, leading to reduced antimicrobial activity [73]. Corticosteroid treatment also reduces antimicrobial peptide production in calves [74].
Lung infections.
Bacterial pneumonia is associated with elevations in hBD2 and −3 levels in the lung fluids and blood [75, 76, 77]. Consequent to neutrophil recruitment, massive amounts of HNPs and LL‐37 are also present during the acute phase of pneumonia. The extent to which these peptides act within phagocyte vacuoles or are released into the respiratory fluid has not been determined; however, high levels (e.g., up to 100 ug/ml) have been measured in blood during sepsis and in BALF of patients with bacterial pneumonia or tuberculosis [78]. It is unclear how these defensins are processed or removed, although one mechanism of inhibition of HNPs in the lung involved ADP‐ribosylation on arginine residues [79, 80]. HNP activities are also inhibited by serum proteins. Pathogenic bacteria have evolved ways to evade killing by respiratory antimicrobial peptides, including modification of their LPS structure (e.g., B. cenocepacia [61, 81] and H.influenzae [82]) or secretion of other bacterial surface proteins (e.g., penicillin‐binding protein by Group B streptococcus [83]).
LL‐37 appears to play an important role in host defense against tuberculosis [84]. African Americans have been shown to have increased susceptibility to tuberculosis, which may relate to low levels of vitamin D and consequent, reduced ability of macrophages to kill M. tuberculosis [43]. Treatment of macrophages with TLR agonists increases vitamin D receptors and results in increased production of LL‐37 and other vitamin D‐responsive genes. HNPs also have activity against mycobacteria and may contribute to host defense mediated by macrophages [33] or eosinophils [32].
Antimicrobial peptides likely play important roles in respiratory viral infections as well. HNPs, HDs, hBD2, and retrocyclins all have antiviral activity against IAV [42, 85]. Of interest, LTB4 treatment increases cathelicidin and mBD3 production in mouse lung, and this treatment results in improved outcome of IAV infection [86]. SP‐D also plays important roles in defense against IAV [87, 88]. A potential adverse effect of severe respiratory IAV or SARS infection associated with extensive neutrophil influx is degradation of SP‐D through action of proteases and HNPs, and HNPs 1–3 bind strongly to SP‐D, generally reduce its antiviral activity, and can cause it to precipitate out of BALF [85]. We have shown recently that retrocyclins and hBDs do not interfere with antiviral activities of SP‐D but rather, have additive effects [42]. Defensins also have activity against a variety of other respiratory viruses, including RSV [23], adenovirus, and parainfluenza virus, and contribute to the respiratory epithelial cell response to rhinovirus [89].
Tobacco smoking.
Tobacco smoking predisposes to infection, emphysema, and lung cancer. A recent study demonstrated that current or former smoking is associated with reduced levels of hBD2 in pharyngeal washes and sputum of patients with acute pneumonia [90]. Of note, smoking and chronic obstructive lung disease are also associated with reduced levels of SP‐A and SP‐D [91, 92], which may impair innate defense further.
GASTROINTESTINAL TRACT
Some of the most compelling in vivo and clinical evidence for the importance of defensins in maintaining mucosal defense derive from studies of the role of Paneth cell α‐defensins, including HD5 and −6 in humans and the related cryptdins in mice. Paneth cells are secretory cells present at the base of luminal crypts in the small intestine. These cells degranulate in response to microbial stimuli such as muramyl peptide, LPS, or CpG DNA. In humans, HD5 and HD6 constitute the most abundant released granule components, although other antimicrobial products released include sPLA2, lysozyme, trypsin, and α1 antitrypsin. Murine studies point to an important role of the Paneth cell α‐defensins in intestinal host defense. Deletion of MMP7 results in impaired production of Paneth cell defensins in mice and decreased resistance to oral bacterial challenge [93]. Overexpression of HD5 in intestine of mice results in graded changes in microbial flora [94] and increased protection against challenge with Salmonella typhimurium [95]. In mice, appearance of Paneth cells early in life correlates with ability to inhibit growth of Shigella species in the gut [96].
Inflammatory bowel diseases
Studies of human subjects with ileal Crohn's disease (CD) have revealed consistent evidence for a deficiency of HD5 and HD6 in disease pathogenesis [94, 97]. CD is a chronic inflammatory condition that can affect the small bowel (especially the ileum) or colon leading to strictures, fistula formation, intestinal obstruction, and cancer. The main approaches to treatment are immune suppression or surgery; however, there is a longstanding observation that antibiotics can cause improvement. Changes in gut bacterial flora and adherence of bacteria to the small intestinal lining have been found in CD, leading to the hypothesis that some primary defect in antibacterial defense is present, leading to chronic inflammation. A major breakthrough in the field was the discovery of a link between frameshift mutations of NOD2/caspase recruitment domain 15 and a subset of patients with CD [98, 99]. NOD2 is highly expressed in Paneth cells and is a cytoplasmic receptor for a bacterial muramyl peptide (a cell‐wall component of many bacteria) that leads to NF‐κB activation and production of α‐defensins. The NOD2 mutation is associated with marked deficiency of HD5 and −6 and is linked specifically to ileal CD and not colonic CD or ulcerative colitis [94, 100, 101, 102]. NOD2−/− mice show decreases in a subset of cryptdins and increased susceptibility to Listeria infection [103].
The NOD2 mutation provides a genetic mechanism for a subset of patients with ileal CD; however, only approximately one‐third of CD patients has this mutation in Western countries, and it is rare in Japanese CD patients [104]. Despite this, deficiency in antibacterial activity in intestinal mucosal extracts and specifically in Paneth cell α‐defensins has been demonstrated in the majority of patients with ileal CD [94]. The deficiency of HD5 and −6 is specific, as other Paneth cell products, including lysozyme and sPLA2, are not affected. These findings led to a search for other explanations for Paneth cell α‐defensins in CD patients with wild‐type NOD2. Recent studies have demonstrated a deficiency of Wnt/Tcf‐4 in ileal CD [101]. Wnt signaling is involved in differentiation and maturation of Paneth cells and regulates specifically expression of MMP7 and Paneth cell α‐defensins in mice, as demonstrated with Tcf‐4−/− mice [105]. Decreased Tcf‐4 mRNA was found in ileal mucosa of ileal CD patients, and this decrease correlated with decreased HD5 expression [101]. There was no association of Tcf‐4 expression with an inflammation score, IL‐8 levels, or NOD2 mutations in these subjects. The Tcf‐4 deficiency was again specific for ileal and not colonic CD. Heterozygous Tcf‐4+/− mice showed decreased cryptdin‐1 and –4, as well as decreased small intestinal killing of S. aureus and E. coli. More recently, the same group demonstrated a genetic association of one polymorphism in the Tcf‐4 promoter region with ileal CD [106]. This genetic association was independent of NOD2 status. Hence, Tcf‐4 alterations appear to provide another genetic basis for Paneth cell α‐defensin deficiency in ileal CD.
Of interest, associations between decreased hBD2 expression or copy number have been made with colonic CD, suggesting that this form of the disease also relates to a defect in defensin‐mediated host defense [107]. Expression of hBD2 is inducible in colonic mucosa through activation of NOD2 and NF‐κB. In contrast to ulcerative colitis, where hBD2 expression is elevated in areas of inflammation, expression is not elevated in colonic CD [108], and luminal secretions in colonic CD have reduced antimicrobial activity [102]. A similar pattern was found with colonic cathelicidin expression: increased in inflamed areas in ulcerative colitis but not increased in colonic CD [109]. Hence, colonic CD may also result from localized deficiencies in antimicrobial peptide expression and impaired epithelial antibacterial defense. CRAMP−/− mice show increased adherence of pathogenic intestinal bacteria in the gut [51], supporting the concept that cathelicidins contribute to intestinal host defense.
Overall, these results highlight the importance of defensins as part of the initial barrier against infection of mucosal surfaces. It appears likely that the chronic intestinal inflammation that characterizes the different forms of CD does not, therefore, reflect a simple hyperinflammatory phenotype but rather, a failure of the first line of host defense, leading to a second level of innate or adaptive immune response that is in fact less effective. This raises the question of whether other chronic inflammatory conditions (e.g., asthma) result from failure of initial defense barriers.
Gastric infection
The stomach is a gastrointestinal site that is normally sterile apart from certain highly adapted bacteria such as H. pylori infection, which is responsible for a variety of human diseases, including peptic ulcer disease and gastric cancers (including gastric lymphomas and adenocarcinomas). Over 50% of the human population is infected with H. pylori, and the majority is asymptomatic. It is therefore an important goal of research to determine which factors are responsible for colonization or disease induction by H. pylori. One important factor is variation between pathogenicity of different H. pylori strains; however, variations in innate defense against H. pylori may also contribute. H. pylori induces hBD mRNA and protein expression in vitro and in vivo in gastric epithelia, and hBD2 and hBD3 have strong bactericidal activity against H. pylori [110]; H. pylori LPS does not appear to be a strong stimulus for NF‐κB activation in gastric epithelial cells, implying that TLR4 may not be a key pathway involved in response of these cells to the bacteria. Boughan et al. [111] have found that H. pylori stimulates hBD2 and hBD3 by distinct mechanisms in these cells. hBD2 was stimulated through NOD1, whereas hBD3 was up‐regulated through a NOD1‐independent, EGFR‐mediated pathway.
Cathelicidin expression is also induced in gastric epithelia by H. pylori. LL‐37 has independent antimicrobial activity against this bacteria and synergizes with hBDs [112]. It is not yet known how these events relate to colonization or disease induction by H. pylori, but the results provide the basis for future clinical research. It is tempting to speculate that impairments in early defensin‐mediated responses may allow for colonization and for ongoing inflammation similar to the findings in CD. The results of Boughan et al. [111] are also revealing about the complexity of regulation of different defensins. The authors speculate that hBD2 may be more involved in the early phase of host response to infection, whereas hBD3 induction may relate more to the later wound‐healing phase of the response, as EGFR (which is up‐regulated by H. pylori infection) is involved in wound healing.
Bacterial diarrhea
As noted above, expression of defensins in the mouse intestinal epithelium is correlated with the ability to inhibit growth of Salmonella and Shigella species in the gut [96]. Bacterial pathogens also elicit defensin expression to variable extents in intestinal epithelium, perhaps accounting for differences in pathogenicity [113]. The importance of antimicrobial peptides to host defense in the intestine is highlighted by the ability of some important intestinal bacterial pathogens to counteract production or action of the peptides. As examples, cholera toxin and labile toxin of enteropathogenic E. coli reduce expression of hBD1 and LL‐37 in intestinal epithelial cells [114], virulent Shigella flexneri release plasmid DNA to turn off expression of antimicrobial peptides by epithelial cells and monocytes [115], and Salmonella species can modify the structure of their LPS to reduce the ability of antimicrobial peptides to bind and kill them (see ref. [2] for an excellent review of this topic).
ORAL CAVITY
The role of antimicrobial peptides in the oral cavity was the subject of a recent excellent review [116]. We showed that intrinsic antiviral activity of saliva against IAV is in part mediated by HNPs and by another antimicrobial peptide, histatin [117], although other inhibitors contribute as well. There are fairly high levels of HNPs in saliva of normal volunteers, presumably derived from neutrophils. Edentulous subjects have markedly reduced levels of HNPs in saliva as a result of absence of creviculae (sites of neutrophil ingress), and this may account for increased risk for oral infections in these subjects [118]. HIV is also inhibited by saliva, and HIV does not appear to be transmitted through oral contact. hBD2 and −3 can inhibit HIV at the concentrations found in normal saliva. Of interest, levels of hBD2 were found to be reduced in saliva of HIV‐positive subjects, possibly predisposing them to other types of infection [119]. Morbus Kostmann is a form of congenital neutropenia characterized by frequent infections, including severe periodontitis [120]. Although neutrophil counts can be increased with G‐CSF treatment, some infections persist associated with a defect in the ability of neutrophils to produce LL‐37 and HNPs and lack of LL‐37 in saliva [121].
GENITOURINARY TRACT
The female reproductive tract is a portal of entry for bacterial, fungal, and viral infections. Cervico‐vaginal secretions contain many innate inhibitors that could contribute to host defense against bacteria, viruses, and fungi, including HNPs, hBDs 1–3 [122], HD5 and −6, and other proteins such as LL‐37, serum leukocyte protease inhibitor, and lysozyme. Levels of HNPs in vaginal lavage from normal controls are ∼2 μg/ml, and levels of hBD1, hBD2, and HD5 are in the range of 10–40 ng/ml based on a study by Valore et al. [123]. The major source of the HNPs is probably neutrophils, perhaps accounting for the comparatively high concentrations of HNPs. The constituents of cervico‐vaginal secretions vary with menstrual cycling or estrogen treatment [124] and in the presence of infections (see below).
Sexually transmitted diseases
There has been intense study about host defense factors that may contribute to protection or vulnerability to sexually transmitted viral infections [124]. Cationic antimicrobial peptides are a key factor contributing to the ability of cervico‐vaginal secretions to inhibit HIV [125]. Of interest, hBD3 not only has direct HIV inhibitory activity but also binds to and down‐regulates the HIV‐1 coreceptor CXCR4 [126]. Cervico‐vaginal fluid of healthy subjects inhibits HSV (including protecting mice from lethal infection with HSV), whereas fluids from HIV‐positive subjects were less inhibitory [127]. The inhibition of HSV was correlated with levels of HNPs in the fluid.
There is an increased risk of HIV and HSV infection in the presence of concurrent sexually transmitted bacterial or viral infections or bacterial vaginosis (BV), which is characterized by changes in bacterial flora in the vagina with loss of the normal commensal, Lactobacillus, and acquisition of other bacteria such as Gardnerella vaginalis. Valore et al. [123] demonstrated that antimicrobial activity of vaginal fluids from patients with BV is reduced. BV fluids had lower concentrations of hBDs 1 and 2 and HNPs compared with normal controls or patients with vulvovaginal candidiasis. The low levels of HNPs correlated with reduced neutrophil influx and IL‐8. Hence, there appears to be a deficiency in defensins associated with BV; however, this was not an intrinsic defect, as in the case of CD, as the defensin levels rose after treatment of BV. The low levels of defensins may contribute to acquisition of HIV or HSV by the subjects. The reasons for low levels of defensins in BV may relate to failure of G. vaginalis to induce immune responses. For instance, G. vaginalis did not induce hBD2, IL‐1, or IL‐8 in vaginal epithelial cells, whereas these were all induced by Lactobacillus jensenii. This may relate to lack of LPS and low concentrations of peptidoglycan in the coat of G. vaginalis. BV, therefore, represents an example in which the commensal flora provoke a balanced innate immune response that limits introduction of other organisms, whereas a superinfecting organism does not induce this response. There are other examples of bacteria failing to induce defensin responses, allowing the bacteria to persist in mucosa. For instance, S. aureus strains from chronic nasal carriers were found not to induce hBD2 and −3 in nasal mucosa, and S. flexneri inhibits antimicrobial peptide expression in gut epithelia [115, 128]. Hence, acquired deficits in defensin‐mediated barriers can result from bacterial infection.
HD5 and −6 are induced in vaginal secretions and the male urethra in response to infection. As noted, cleavage of the precursor of HDs is necessary for them to acquire antimicrobial activity. A distinctive method of cleavage of HD5 was found in studies of urethral secretions of subjects with N. gonorrhoeae or Chlamydia trachomatis infection [129], in which neutrophil proteases were shown to affect cleavage. HD5 and −6 are also produced by vaginal epithelial cells in response to N. gonorrhoeae infection [130]. Although HNPs, hBDs, and retrocyclins inhibit HIV, a surprising finding was that HD5 and −6 actually increased HIV infection of CD4 cells [130]. This effect was mediated by a direct interaction with the virus and occurred with purified peptides or supernatants of N. gonorrhoeae‐infected vaginal epithelial cells. The HIV‐enhancing effect occurred at HD concentrations of ≥10 μg/ml. Although levels of HDs in vaginal lavage are less than this, the lavage is probably diluted considerably, and local concentrations may be much higher. It will be interesting in the future to determine if subjects with NOD or Tcf2 mutations also have reduced HD levels at mucosal sites other than the intestine and whether this is associated with any change in disease susceptibility. Note that HNP levels are considerably higher in urethral or cervico‐vaginal secretions, and hence, their effect may predominate over effects of HDs. For instance, urethral levels of HNPs and HD5 were particularly elevated in N. gonorrhoeae infection (∼20 and 0.5 μg/ml, respectively).
The finding that HD5 and −6 can promote HIV infection was unexpected [130]. We have found, in contrast, that HDs are inhibitory for IAV [42]. It will be important to determine the reasons for divergent effects of HDs on infection by these two enveloped viruses. Another paradoxical finding is that cervico‐vaginal secretions that do contain high levels of HNPs and LL‐37 (which contribute to anti‐HIV activity of the fluid) have been associated in one study with increased risk of HIV transmission [131]. This was related to the fact that these samples came from subjects with sexually transmitted bacterial infections. In such cases, other factors promoting HIV transmission presumably outweigh the impact of the increased antimicrobial peptides. Of interest, increased hBD gene copy numbers have been associated with reduced risk of HIV infection in preliminary studies [132]. Further studies of this kind will be of interest.
Other genitourinary infections and cancers
hBD1−/− mice have been developed, and these mice show increased carriage of bacteria in their bladder [49]. Similarly, CRAMP−/− mice have greater susceptibility to bacterial urinary tract infection [50]. BK virus is a nonenveloped polyomavirus that establishes lifelong infection in ∼90% of the human population. In general, infection is asymptomatic but becomes a problem in some immunocompromised hosts, causing hemorrhagic cystitis or kidney infection, leading to kidney transplant rejection. A recent study demonstrated that HNP1 and HD5 act directly on the virions, in part, through viral aggregation [133]. As noted above, we have similarly reported aggregation of IAV by defensins. Future studies may determine if differences in α‐defensin expression account for susceptibility of some subjects to complications of BK infection during immune suppression.
Papillomavirus infections are linked to genital warts and to cervical, penile, anal, and head and neck cancers. HNPs and HD5 have been shown to have strong antiviral activity against human papillomaviruses in vitro, whereas hBDs lacked activity [134]. HNP2 and hBD2 were shown in another study to mediate recruitment of DCs into preneoplastic epithelial lesions induced by papillomavirus [135]. Hence, defensins may modulate the immune response to papillomavirus‐related cancers or preneoplastic lesions. It is possible that antimicrobial peptides are protective against prostatic carcinomas. For instance, prostatic cancer is associated with loss of hBD1 expression, and re‐expression of hBD1 induced cell death in prostate cancer cell lines [136]. In contrast, there is evidence that LL‐37 facilitates the development of ovarian cancer [137].
SKIN
The skin provides an essential barrier against electrolyte loss and infection. It is not surprising, therefore, that skin is heavily invested with host defense peptides. Studies of the skin have been extremely valuable in characterizing the regulation and role of antimicrobial peptides in host defense, wound healing, and barrier function. hBDs and LL‐37 are expressed by epithelial cells in the skin. Regulation of expression is surprisingly complex. hBD2 and −3 are strongly inducible in keratinocytes but by distinct mechanisms. hBD2 expression in skin is induced by LPS or other bacterial products in combination with IL‐1β derived from resident monocyte‐derived cells, whereas induction of hBD3 results from transactivation of EGFR [138, 139]. Transactivation refers to local release of surface‐bound EGFR ligands via activation of metalloproteases. Hence, this is a distinct role for metalloproteases in defensin biology from the cleavage of defensin precursors discussed previously. Increased expression of hBDs in response to TGF‐α or other EGFR ligands suggests that the defensins play a role in wound healing [140, 141]. hBD1 can also be induced in skin to a lesser extent by similar stimuli [138]. hBDs also were found to have distinct anatomic loci of expression within the skin. These findings suggest that the different hBDs may have distinct roles in response to skin infection and injury.
LL‐37 or the murine homologue CRAMP is also induced in keratinocytes by skin wounding [142]. mBD3 is increased by skin wounding in mice in a manner that is coordinated with increases in CRAMP and lipid metabolic responses. These findings are consistent with the fact that these antimicrobial peptides are copackaged with lipids in lamellar bodies in the epidermis. Furthermore, the up‐regulation of antimicrobial peptides in response to interruption of the skin barrier demonstrates the inter‐relationship of skin‐barrier function, wound healing, and defense from infection. CRAMP−/− mice have been developed and have impaired restoration of skin barrier function after wounding but also increased susceptibility to streptococcal skin infection [143]. hBD2 and LL‐37 are increased in human skin wounds and both stimulate angiogenesis to promote wound healing by different signaling mechanisms. LL‐37 stimulates keratinocyte migration via transactivation of EGFR and activates endothelial cells via binding to FPRL‐1 [144].
Vitamin D is critical for regulation of cathelicidin and LL‐37 in skin. Levels of LL‐37 are markedly elevated in skin lesions of psoriasis. One driving factor for this induction is IL‐17 produced by lesional T cells [145]. Glucocorticoids down‐regulate expression of CRAMP and mBD‐3 in mouse skin [146]. IL‐4 and IL‐13 inhibit LL‐37 and hBD2 activation in human keratinocytes, and TNF increases them [147].
Associations of antimicrobial peptides with skin diseases
The complex regulation of antimicrobial peptide expression in skin is relevant to a variety of important skin diseases. Atopic dermatitis is characterized by increased frequency of skin infections with viral, bacterial, and fungal organisms. Expression of hBD2 and LL‐37 was reduced greatly in atopic dermatitis lesions as compared with normal controls or psoriasis (where these peptides are increased) [147]. This decrease in antimicrobial peptide expression was independent of recent steroid use. Hyper‐IgE syndrome is a primary immunodeficiency in which subjects have atopy and recurrent staphyloccal skin and lung infections [148]. The condition has been found to relate to defects in Th17 cell differentiation, raising the question of why infections are confined principally to the lung and skin. A recent study demonstrated that this may relate to the fact that keratinocytes and bronchial epithelial cells (in contrast to other epithelia) require IL‐17 and classic cytokines to generate chemokines and antimicrobial peptides [149]. Chronic skin ulcers have been found to have low levels of LL‐37 expression as well [150], perhaps reflecting the importance of LL‐37 in wound healing.
As noted, psoriasis is characterized by markedly increased expression of hBD2 and LL‐37, and in this case, LL‐37 may contribute to disease pathogenesis by binding to self‐DNA, resulting in autoimmune responses directed by DCs [151]. Of interest, hBD gene copy numbers are increased in psoriasis, and serum levels of hBDs have been proposed as a marker for the disease [152, 153]. Increased LL‐37 concentrations have also been found to promote inflammation in roseacea [154]. Glucocorticoid elevation resulting from psychological stress in mice causes down‐regulation of mBD3 and CRAMP in mouse skin, resulting in greater susceptibility to streptococcal skin infections [146]. Psychological stress can predispose to respiratory infection as well [155], and it would be of interest to determine if this, in part, is mediated by stress‐induced decreases in antimicrobial peptide expression in the lung.
EGFR inhibitors are being used increasingly in treatment of cancer, and their principle toxicity is dermatologic, including acne‐like rashes and fissuring of skin [156]. It will be of interest to determine if these toxicities relate to inhibition of defensin or LL‐37 production in the skin.
EYE
The eye is another site where having an effective first‐line host defense and minimizing inflammation are important. P. aeruginosa is an important organism capable of causing destructive infection of the cornea. Using a mouse model of corneal infection with pseudomonas infection, mBD2 was found to be important for reducing bacterial counts and neutrophil infiltration [157]. mBD2 also modulated expression of a variety of inflammatory cytokines and reduced NF‐κB activation. Although mBD1 was also expressed in the cornea, silencing of mBD1 did not alter infection or inflammation. Tear fluid also contains SP‐D, which inhibits P. aeruginosa infection in mice [158, 159]. Hence, this is another site (like the respiratory tract) where SP‐D and defensins may interact. The eye is also a potential portal of entry for IAV and a site for other viral infections. Application of a θ‐defensin has been shown to be protective against HSV‐induced keratitis [160].
THERAPEUTIC CONSIDERATIONS
Although we will not discuss in depth the potential therapeutic uses of defensins or LL‐37, some possibilities are relevant to this review. Defensins have potential for therapy, given their broad‐spectrum antimicrobial and antiviral activity and the fact that they can be synthesized with minor modifications to enhance activity further. Direct application to sites such as the skin or cervico‐vaginal mucosa for treatment of wounds or infection is of interest, particularly in conditions where antimicrobial peptide generation is reduced. One concern for direct applications of defensins would be the possibility of inducing excessive inflammation, as occurred with instillation of HNPs into the lung [67]. Retrocyclins are of interest for HIV therapy, as they strongly inhibit viral replication [161]. We have found that retrocyclins have strong antiviral activity against IAV and unlike HNPs, do not interfere with the antiviral activity of SP‐D, despite binding to it [42]. Hence, retrocyclins may be a better candidate than HNPs for instillation in the respiratory tract. A recent study demonstrated reduced weight loss and mortality in mice treated with a retrocyclin by intranasal instillation 15 min before infection with a mouse‐adapted version of the SARS coronavirus [162]. Surprisingly, the protection was not the result of inhibition of viral replication. In this model, the retrocyclin did induce some level of inflammation in the lung on its own, although it reduced generation of some proinflammatory cytokines triggered by the virus. Hence, retrocyclins may exert beneficial effects through modulation of inflammatory responses in the lung. Another fascinating, recent finding is that retrocyclins can be produced in human cervico‐vaginal secretions in response to aminoglycoside treatment [8].
Treatment of some diseases through direct application of defensins may be impractical (e.g., how to deliver defensins for treatment of inflammatory bowel diseases). Adenoviral vectors for delivery of antimicrobial peptides have been tested in animal models with mixed results [163, 164, 165]. Another approach would be to increase endogenous defensin generation through use of known regulatory stimuli. In a mouse model, administration of LTB4 increased defensin and cathelicidin generation and improved outcome of IAV infection [86]. Vascular endothelial growth factor promotes BD and SP‐D production in the lung, resulting in improved outcome of RSV infection [166]. The importance of vitamin D in LL‐37 generation and other aspects of innate defense indicate that measurement of vitamin D levels and supplementation could be important for improving the outcome of some infections.
CONCLUSIONS
One general theme that emerges from the study of defensins and other first‐line host defense proteins is that success of the initial barrier to invasive pathogens can prevent deleterious inflammation and that breakdown in functions of the initial barriers can not only lead to infection but also to chronic inflammatory conditions. This is most obvious for inflammatory bowel diseases but could account for a variety of other conditions such as gastric diseases induced by H. pylori and inflammatory diseases of the lung. It is of interest in this regard that BDs and LL‐37 are imbedded in other physiological processes such as wound healing and angiogenesis, in addition to their roles in defense against infection. This is reminiscent of the roles of surfactant proteins A and D in the lung phospholipid metabolism as well as host defense. Another recurring theme is that pathogenic bacteria have evolved a variety of mechanisms to circumvent defensin‐mediated immunity to create invasive infections. Important subjects for future study will include how defensins interact with other components of innate defense, since clearly they do not act in isolation. Some preliminary studies have explored this question [65, 85, 117, 167, 168, 169, 170], but further studies are needed. Furthermore, it is currently hard to determine the relative importance of direct antimicrobial or antiviral effects of defensins or LL‐37 as compared with their immunomodulatory effects in many instances; hence, this should be an important focus of future investigations.
REFERENCES
- 1. Yang, D. , Biragyn, A. , Hoover, D. M. , Lubkowski, J. , Oppenheim, J. J. (2004) Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil‐derived neurotoxin in host defense. Annu. Rev. Immunol. 22, 181–215. [DOI] [PubMed] [Google Scholar]
- 2. Lai, Y. , Gallo, R. L. (2009) AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 30, 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lehrer, R. I. (2007) Multispecific myeloid defensins. Curr. Opin. Hematol. 14, 16–21. [DOI] [PubMed] [Google Scholar]
- 4. Klotman, M. E. , Chang, T. L. (2006) Defensins in innate antiviral immunity. Nat. Rev. Immunol. 6, 447–456. [DOI] [PubMed] [Google Scholar]
- 5. Ganz, T. (2003) Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3, 710–720. [DOI] [PubMed] [Google Scholar]
- 6. Garcia, A. E. , Osapay, G. , Yuan, J. , Selsted, M. E. (2008) Isolation, synthesis, and antimicrobial activities of natural occurring θ‐defensin isoforms from baboon leukocytes. Infect. Immun. 76, 5883–5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang, Y. Q. , Yuan, J. , Osapay, G. , Osapay, K. , Tran, D. , Miller, C. J. , Ouellette, A. J. , Selsted, M. E. (1999) A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated α‐defensins. Science 286, 498–502. [DOI] [PubMed] [Google Scholar]
- 8. Venkataraman, N. , Cole, A. L. , Ruchala, P. , Waring, A. J. , Lehrer, R. I. , Stuchlik, O. , Pohl, J. , Cole, A. M. (2009) Reawakening retrocyclins: ancestral human defensins active against HIV‐1. PLoS Biol. 7, e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shornick, L. P. , Wells, A. G. , Zhang, Y. , Patel, A. C. , Huang, G. , Takami, K. , Sosa, M. , Shukla, N. A. , Agapov, E. , Holtzman, M. J. (2008) Airway epithelial versus immune cell Stat1 function for innate defense against respiratory viral infection. J. Immunol. 180, 3319–3328. [DOI] [PubMed] [Google Scholar]
- 10. Diamond, G. , Zasloff, M. , Eck, H. , Brasseur, M. , Maloy, W. L. , Bevins, C. L. (1991) Tracheal antimicrobial peptide, a cysteine‐rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc. Natl. Acad. Sci. USA 88, 3952–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caverly, J. M. , Diamond, G. , Gallup, J. M. , Brogden, K. A. , Dixon, R. A. , Ackermann, M. R. (2003) Coordinated expression of tracheal antimicrobial peptide and inflammatory‐response elements in the lungs of neonatal calves with acute bacterial pneumonia. Infect. Immun. 71, 2950–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diamond, G. , Kaiser, V. , Rhodes, J. , Russell, J. P. , Bevins, C. L. (2000) Transcriptional regulation of β‐defensin gene expression in tracheal epithelial cells. Infect. Immun. 68, 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diamond, G. , Russell, J. P. , Bevins, C. L. (1996) Inducible expression of an antibiotic peptide gene in lipopolysaccharide‐challenged tracheal epithelial cells. Proc. Natl. Acad. Sci. USA 93, 5156–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goldman, M. J. , Anderson, G. M. , Stolzenberg, E. D. , Kari, U. P. , Zasloff, M. , Wilson, J. M. (1997) Human β‐defensin‐1 is a salt‐sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 88, 553–560. [DOI] [PubMed] [Google Scholar]
- 15. Bals, R. , Wang, X. , Wu, Z. , Freeman, T. , Bafna, V. , Zasloff, M. , Wilson, J. M. (1998) Human β‐defensin 2 is a salt‐sensitive peptide antibiotic expressed in human lung. J. Clin. Invest. 102, 874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bals, R. , Wang, X. , Zasloff, M. , Wilson, J. M. (1998) The peptide antibiotic LL‐37/hCAP‐18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. USA 95, 9541–9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garcia, J. R. , Krause, A. , Schulz, S. , Rodriguez‐Jimenez, F. J. , Kluver, E. , Adermann, K. , Forssmann, U. , Frimpong‐Boateng, A. , Bals, R. , Forssmann, W. G. (2001) Human β‐defensin 4: a novel inducible peptide with a specific salt‐sensitive spectrum of antimicrobial activity. FASEB J. 15, 1819–1821. [PubMed] [Google Scholar]
- 18. Singh, P. K. , Jia, H. P. , Wiles, K. , Hesselberth, J. , Liu, L. , Conway, B. D. , Greenberg, E. P. , Valore, E. V. , Welsh, M. J. , Ganz, T. , Tack, B. F. , McCray Jr., P. B. (1998) Production of β‐defensins by human airway epithelia. Proc. Natl. Acad. Sci. USA 95, 14961–14966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hinrichsen, K. , Podschun, R. , Schubert, S. , Schroder, J. M. , Harder, J. , Proksch, E. (2008) Mouse β‐defensin‐14, an antimicrobial ortholog of human β‐defensin‐3. Antimicrob. Agents Chemother. 52, 1876–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harder, J. , Bartels, J. , Christophers, E. , Schroder, J. M. (2001) Isolation and characterization of human β‐defensin‐3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276, 5707–5713. [DOI] [PubMed] [Google Scholar]
- 21. Becker, M. N. , Diamond, G. , Verghese, M. W. , Randell, S. H. (2000) CD14‐dependent lipopolysaccharide‐induced β‐defensin‐2 expression in human tracheobronchial epithelium. J. Biol. Chem. 275, 29731–29736. [DOI] [PubMed] [Google Scholar]
- 22. Tsutsumi‐Ishii, Y. , Nagaoka, I. (2003) Modulation of human β‐defensin‐2 transcription in pulmonary epithelial cells by lipopolysaccharide‐stimulated mononuclear phagocytes via proinflammatory cytokine production. J. Immunol. 170, 4226–4236. [DOI] [PubMed] [Google Scholar]
- 23. Kota, S. , Sabbah, A. , Chang, T. H. , Harnack, R. , Xiang, Y. , Meng, X. , Bose, S. (2008) Role of human β‐defensin‐2 during tumor necrosis factor‐α/NF‐κB‐mediated innate antiviral response against human respiratory syncytial virus. J. Biol. Chem. 283, 22417–22429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hansdottir, S. , Monick, M. M. , Hinde, S. L. , Lovan, N. , Look, D. C. , Hunninghake, G. W. (2008) Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J. Immunol. 181, 7090–7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang, D. , Chertov, O. , Bykovshaia, S. , Chen, Q. , Buffo, M. , Shogan, J. , Anderson, M. , Schroder, J. , Wang, J. , Howard, O. , Oppenheim, J. (1999) β Defensins: linking innate and adaptive immunity through dendritic cells and T cell CCR6. Science 286, 525–528. [DOI] [PubMed] [Google Scholar]
- 26. Biragyn, A. , Ruffini, P. A. , Leifer, C. A. , Klyushnenkova, E. , Shakhov, A. , Chertov, O. , Shirakawa, A. K , Farber, J. M. , Segal, D. M. , Oppenheim, J. J. , Kwak, L. W. (2002) Toll‐like receptor 4‐dependent activation of dendritic cells by β‐defensin 2. Science 298, 1025–1029. [DOI] [PubMed] [Google Scholar]
- 27. Funderburg, N. , Lederman, M. M. , Feng, Z. , Drage, M. G. , Jadlowsky, J. , Harding, C. V. , Weinberg, A. , Sieg, S. F. (2007) Human‐defensin‐3 activates professional antigen‐presenting cells via Toll‐like receptors 1 and 2. Proc. Natl. Acad. Sci. USA 104, 18631–18635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen, X. , Niyonsaba, F. , Ushio, H. , Hara, M. , Yokoi, H. , Matsumoto, K. , Saito, H. , Nagaoka, I. , Ikeda, S. , Okumura, K. , Ogawa, H. (2007) Antimicrobial peptides human β‐defensin (hBD)‐3 and hBD‐4 activate mast cells and increase skin vascular permeability. Eur. J. Immunol. 37, 434–444. [DOI] [PubMed] [Google Scholar]
- 29. De Yang, Chen, Q. , Schmidt, A. P. , Anderson, G. M. , Wang, J. M. , Wooters, J. , Oppenheim, J. J. , Chertov, O. (2000) LL‐37, the neutrophil granule‐ and epithelial cell‐derived cathelicidin, utilizes formyl peptide receptor‐like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 192, 1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mookherjee, N. , Brown, K. L. , Bowdish, D. M. , Doria, S. , Falsafi, R. , Hokamp, K. , Roche, F. M. , Mu, R. , Doho, G. H. , Pistolic, J. , Powers, J. P. , Bryan, J. , Brinkman, F. S. , Hancock, R. E. (2006) Modulation of the TLR‐mediated inflammatory response by the endogenous human host defense peptide LL‐37. J. Immunol. 176, 2455–2464. [DOI] [PubMed] [Google Scholar]
- 31. Mookherjee, N. , Lippert, D. N. , Hamill, P. , Falsafi, R. , Nijnik, A. , Kindrachuk, J. , Pistolic, J. , Gardy, J. , Miri, P. , Naseer, M. , Foster, L. J. , Hancock, R. E. (2009) Intracellular receptor for human host defense peptide LL‐37 in monocytes. J. Immunol. 183, 2688–2696. [DOI] [PubMed] [Google Scholar]
- 32. Driss, V. , Legrand, F. , Hermann, E. , Loiseau, S. , Guerardel, Y. , Kremer, L. , Adam, E. , Woerly, G. , Dombrowicz, D. , Capron, M. (2009) TLR2‐dependent eosinophil interactions with mycobacteria: role of α‐defensins. Blood 113, 3235–3244. [DOI] [PubMed] [Google Scholar]
- 33. Tan, B. H. , Meinken, C. , Bastian, M. , Bruns, H. , Legaspi, A. , Ochoa, M. T. , Krutzik, S. R. , Bloom, B. R. , Ganz, T. , Modlin, R. L. , Stenger, S. (2006) Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J. Immunol. 177, 1864–1871. [DOI] [PubMed] [Google Scholar]
- 34. Hazrati, E. , Galen, B. , Lu, W. , Wang, W. , Ouyang, Y. , Keller, M. J. , Lehrer, R. I. , Herold, B. C. (2006) Human α‐ and β‐defensins block multiple steps in herpes simplex virus infection. J. Immunol. 177, 8658–8666. [DOI] [PubMed] [Google Scholar]
- 35. Yang, D. , Chen, Q. , Chertov, O. , Oppenheim, J. J. (2000) Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J. Leukoc. Biol. 68, 9–14. [PubMed] [Google Scholar]
- 36. Chertov, O. , Michiel, D. F. , Xu, L. , Wang, J. M. , Tani, K. , Murphy, W. J. , Longo, D. L. , Taub, D. D. , Oppenheim, J. J. (1996) Identification of defensin‐1, defensin‐2, and CAP37/azurocidin as T‐cell chemoattractant proteins released from interleukin‐8‐stimulated neutrophils. J. Biol. Chem. 271, 2935–2940. [DOI] [PubMed] [Google Scholar]
- 37. Grigat, J. , Soruri, A. , Forssmann, U. , Riggert, J. , Zwirner, J. (2007) Chemoattraction of macrophages, T lymphocytes, and mast cells is evolutionarily conserved within the human a‐defensin family. J. Immunol. 179, 3958–3965. [DOI] [PubMed] [Google Scholar]
- 38. Sakamoto, N. , Mukae, H. , Fujii, T. , Ishii, H. , Yoshioka, S. , Kakugawa, T. , Sugiyama, K. , Mizuta, Y. , Kadota, J. , Nakazato, M. , Kohno, S. (2005) Differential effects of α‐ and β‐defensin on cytokine production by cultured human bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 288, L508–L513. [DOI] [PubMed] [Google Scholar]
- 39. Ishimoto, H. , Mukae, H. , Sakamoto, N. , Amenomori, M. , Kitazaki, T. , Imamura, Y. , Fujita, H. , Ishii, H. , Nakayama, S. , Yanagihara, K. , Kohno, S. (2009) Different effects of telithromycin on MUC5AC production induced by human neutrophil peptide‐1 or lipopolysaccharide in NCI‐H292 cells compared with azithromycin and clarithromycin. J. Antimicrob. Chemother. 63, 109–114. [DOI] [PubMed] [Google Scholar]
- 40. Porro, G. A. , Lee, J. , Azavedo, J. D. , Crandall, I. , Whitehead, T. , Ganz, E. T. T. , Liu, M. , Slutsky, A. S. , Zhang, H. (2001) Direct and indirect bacterial killing functions of neutrophil defensins in lung explants. Am. J. Physiol. Lung Cell. Mol. Physiol. 281, L1240–L1247. [DOI] [PubMed] [Google Scholar]
- 41. Tecle, T. , White, M. R. , Gantz, D. , Crouch, E. C. , Hartshorn, K. L. (2007) Human neutrophil defensins increase neutrophil uptake of influenza A virus and bacteria and modify virus‐induced respiratory burst responses. J. Immunol. 178, 8046–8052. [DOI] [PubMed] [Google Scholar]
- 42. Doss, M. , White, M. , Tecle, T. , Gantz, D. , Crouch, E. , Jung, G. , Ruchala, P. , Waring, A. , Lehrer, R. , Hartshorn, K. (2009) Interactions of α‐, β‐and θ‐defensins with influenza A virus and surfactant protein D. J. Immunol. 182, 7878–7887. [DOI] [PubMed] [Google Scholar]
- 43. Liu, P. T. , Stenger, S. , Li, H. , Wenzel, L. , Tan, B. H. , Krutzik, S. R. , Ochoa, M. T. , Schauber, J. , Wu, K. , Meinken, C. , Kamen, D. L. , Wagner, M. , Bals, R. , Steinmeyer, A. , Zugel, U. , Gallo, R. L. , Eisenberg, D. , Hewison, M. , Hollis, B. W. , Adams, J. S. , Bloom, B. R. , Modlin, R. L. (2006) Toll‐like receptor triggering of a vitamin D‐mediated human antimicrobial response. Science 311, 1770–1773. [DOI] [PubMed] [Google Scholar]
- 44. Tjabringa, G. S. , Aarbiou, J. , Ninaber, D. K. , Drijfhout, J. W. , Sorensen, O. E. , Borregaard, N. , Rabe, K. F. , Hiemstra, P. S. (2003) The antimicrobial peptide LL‐37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J. Immunol. 171, 6690–6696. [DOI] [PubMed] [Google Scholar]
- 45. Yu, J. , Mookherjee, N. , Wee, K. , Bowdish, D. M. , Pistolic, J. , Li, Y. , Rehaume, L. , Hancock, R. E. (2007) Host defense peptide LL‐37, in synergy with inflammatory mediator IL‐1β, augments immune responses by multiple pathways. J. Immunol. 179, 7684–7691. [DOI] [PubMed] [Google Scholar]
- 46. Moser, C. , Weiner, D. J. , Lysenko, E. , Bals, R. , Weiser, J. N. , Wilson, J. M. (2002) β‐Defensin 1 contributes to pulmonary innate immunity in mice. Infect. Immun. 70, 3068–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bals, R. , Wang, X. , Meegalla, R. L. , Wattler, S. , Weiner, D. J. , Nehls, M. C. , Wilson, J. M. (1999) Mouse β‐defensin 3 is an inducible antimicrobial peptide expressed in the epithelia of multiple organs. Infect. Immun. 67, 3542–3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bals, R. , Goldman, M. J. , Wilson, J. M. (1998) Mouse β‐defensin 1 is a salt‐sensitive antimicrobial peptide present in epithelia of the lung and urogenital tract. Infect. Immun. 66, 1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morrison, G. , Kilanowski, F. , Davidson, D. , Dorin, J. (2002) Characterization of the mouse β defensin 1, Defb1, mutant mouse model. Infect. Immun. 70, 3053–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chromek, M. , Slamova, Z. , Bergman, P. , Kovacs, L. , Podracka, L. , Ehren, I. , Hokfelt, T. , Gudmundsson, G. H. , Gallo, R. L. , Agerberth, B. , Brauner, A. (2006) The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 12, 636–641. [DOI] [PubMed] [Google Scholar]
- 51. Iimura, M. , Gallo, R. L. , Hase, K. , Miyamoto, Y. , Eckmann, L. , Kagnoff M. F. (2005) Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J. Immunol. 174, 4901–4907. [DOI] [PubMed] [Google Scholar]
- 52. Braff, M. H. , Jones, A. L. , Skerrett, S. J. , Rubens, C. E. (2007) Staphylococcus aureus exploits cathelicidin antimicrobial peptides produced during early pneumonia to promote staphylokinase‐dependent fibrinolysis. J. Infect. Dis. 195, 1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chong, K. T. , Thangavel, R. R. , Tang, X. (2008) Enhanced expression of murine β‐defensins (MBD‐1, −2, −3, and −4) in upper and lower airway mucosa of influenza virus infected mice. Virology 380, 136–143. [DOI] [PubMed] [Google Scholar]
- 54. Grubor, B. , Gallup, J. M. , Meyerholz, D. K. , Crouch, E. C. , Evans, R. B. , Brogden, K. A. , Lehmkuhl, H. D. , Ackermann, M. R. (2004) Enhanced surfactant protein and defensin mRNA levels and reduced viral replication during parainfluenza virus type 3 pneumonia in neonatal lambs. Clin. Diagn. Lab. Immunol. 11, 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smith, J. J. , Travis, S. M. , Greenberg, E. P. , Welsh, M. J. (1996) Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell 85, 229–236. [DOI] [PubMed] [Google Scholar]
- 56. Chen, C. I. , Schaller‐Bals, S. , Paul, K. P. , Wahn, U. , Bals, R. (2004) β‐Defensins and LL‐37 in bronchoalveolar lavage fluid of patients with cystic fibrosis. J. Cyst. Fibros. 3, 45–50. [DOI] [PubMed] [Google Scholar]
- 57. Aldred, P. M. , Hollox, E. J. , Armour, J. A. (2005) Copy number polymorphism and expression level variation of the human α‐defensin genes DEFA1 and DEFA3. Hum. Mol. Genet. 14, 2045–2052. [DOI] [PubMed] [Google Scholar]
- 58. Hollox, E. J. , Armour, J. A. , Barber, J. C. (2003) Extensive normal copy number variation of a β‐defensin antimicrobial‐gene cluster. Am. J. Hum. Genet. 73, 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hollox, E. J. , Davies, J. , Griesenbach, U. , Burgess, J. , Alton, E. W. , Armour, J. A. (2005) β‐Defensin genomic copy number is not a modifier locus for cystic fibrosis. J. Negat. Results Biomed. 4, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Taggart, C. C. , Greene, C. M. , Smith, S. G. , Levine, R. L. , McCray Jr., P. B. , O'Neill, S. , McElvaney, N. G. (2003) Inactivation of human β‐defensins 2 and 3 by elastolytic cathepsins. J. Immunol. 171, 931–937. [DOI] [PubMed] [Google Scholar]
- 61. Loutet, S. A. , Flannagan, R. S. , Kooi, C. , Sokol, P. A. , Valvano, M. A. (2006) A complete lipopolysaccharide inner core oligosaccharide is required for resistance of Burkholderia cenocepacia to antimicrobial peptides and bacterial survival in vivo. J. Bacteriol. 188, 2073–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bals, R. , Weiner, D. J. , Meegalla, R. L. , Accurso, F. , Wilson, J. M. (2001) Salt‐independent abnormality of antimicrobial activity in cystic fibrosis airway surface fluid. Am. J. Respir. Cell Mol. Biol. 25, 21–25. [DOI] [PubMed] [Google Scholar]
- 63. Virella‐Lowell, I. , Poirier, A. , Chesnut, K. A. , Brantly, M. , Flotte, T. R. (2000) Inhibition of recombinant adeno‐associated virus (rAAV) transduction by bronchial secretions from cystic fibrosis patients. Gene Ther. 7, 1783–1789. [DOI] [PubMed] [Google Scholar]
- 64. Spencer, L. T. , Paone, G. , Krein, P. M. , Rouhani, F. N. , Rivera‐Nieves, J. , Brantly, M. L. (2004) Role of human neutrophil peptides in lung inflammation associated with α 1‐antitrypsin deficiency. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L514–L 1520. [DOI] [PubMed] [Google Scholar]
- 65. White, M. R. , Tecle, T. , Crouch, E. C. , Hartshorn, K. L. (2007) Impact of neutrophils on antiviral activity of human bronchoalveolar lavage fluid. Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L1293–L1299. [DOI] [PubMed] [Google Scholar]
- 66. Hirche, T. O. , Crouch, E. C. , Espinola, M. , Brokelman, T. J. , Mecham, R. P. , DeSilva, N. , Cooley, J. , Remold‐O'Donnell, E. , Belaaouaj, A. (2004) Neutrophil serine proteinases inactivate surfactant protein D by cleaving within a conserved sub‐region of the carbohydrate recognition domain. J. Biol. Chem. 279, 27688–27698. [DOI] [PubMed] [Google Scholar]
- 67. Zhang, H. , Porro, G. , Orzech, N. , Mullen, B. , Liu, M. , Slutsky, A. S. (2001) Neutrophil defensins mediate acute inflammatory response and lung dysfunction in dose‐related fashion. Am. J. Physiol. Lung Cell. Mol. Physiol. 280, L947–L954. [DOI] [PubMed] [Google Scholar]
- 68. Miles, K. , Clarke, D. J. , Lu, W. , Sibinska, Z. , Beaumont, P. E. , Davidson, D. J. , Barr, T. A. , Campopiano, D. J. , Gray, M. (2009) Dying and necrotic neutrophils are anti‐inflammatory secondary to the release of α‐defensins. J. Immunol. 183, 2122–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hiratsuka, T. , Mukae, H. , Iiboshi, H. , Ashitani, J. , Nabeshima, K , Minematsu, T. , Chino, N. , Ihi, T. , Kohno, S. , Nakazato, M. (2003) Increased concentrations of human β‐defensins in plasma and bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis. Thorax 58, 425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ross, D. J. , Cole, A. M. , Yoshioka, D. , Park, A. K. , Belperio, J. A. , Laks, H. , Strieter, R. M. , Lynch III, J. P. , Kubak, B. , Ardehali, A. , Ganz, T. (2004) Increased bronchoalveolar lavage human β‐defensin type 2 in bronchiolitis obliterans syndrome after lung transplantation. Transplantation 78, 1222–1224. [DOI] [PubMed] [Google Scholar]
- 71. Mukae, H. , Iiboshi, H. , Nakazato, M. , Hiratsuka, T. , Tokojima, M. , Abe, K , Ashitani, J. , Kadota, J. , Matsukura, S. , Kohno, S. (2002) Raised plasma concentrations of α‐defensins in patients with idiopathic pulmonary fibrosis. Thorax 57, 623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ashitani, J. , Mukae, H. , Arimura, Y. , Sano, A. , Tokojima, M. , Nakazato, M. (2004) High concentrations of α‐defensins in plasma and bronchoalveolar lavage fluid of patients with acute respiratory distress syndrome. Life Sci. 75, 1123–1134. [DOI] [PubMed] [Google Scholar]
- 73. Beisswenger, C. , Kandler, K , Hess, C. , Garn, H. , Felgentreff, K , Wegmann, M. , Renz, H. , Vogelmeier, C. , Bals, R. (2006) Allergic airway inflammation inhibits pulmonary antibacterial host defense. J. Immunol. 177, 1833–1837. [DOI] [PubMed] [Google Scholar]
- 74. Mitchell, G. B. , Al‐Haddawi, M. H. , Clark, M. E. , Beveridge, J. D. , Caswell, J. L. (2007) Effect of corticosteroids and neuropeptides on the expression of defensins in bovine tracheal epithelial cells. Infect. Immun. 75, 1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ishimoto, H. , Mukae, H. , Date, Y. , Shimbara, T. , Mondal, M. S. , Ashitani, J. , Hiratsuka, T. , Kubo, S. , Kohno, S. , Nakazato, M. (2006) Identification of hBD‐3 in respiratory tract and serum: the increase in pneumonia. Eur. Respir. J. 27, 253–260. [DOI] [PubMed] [Google Scholar]
- 76. Ashitani, J. , Mukae, H. , Nakazato, M. , Taniguchi, H. , Ogawa, K , Kohno, S. , Matsukura, S. (1998) Elevated pleural fluid levels of defensins in patients with empyema. Chest 113, 788–794. [DOI] [PubMed] [Google Scholar]
- 77. Hiratsuka, T. , Nakazato, M. , Date, Y. , Ashitani, J. , Minematsu, T. , Chino, N. , Matsukura, S. (1998) Identification of human β‐defensin‐2 in respiratory tract and plasma and its increase in bacterial pneumonia. Biochem. Biophys. Res. Commun. 249, 943–947. [DOI] [PubMed] [Google Scholar]
- 78. Ashitani, J. , Mukae, H. , Hiratsuka, T. , Nakazato, M. , Kumamoto, K , Matsukura, S. (2002) Elevated levels of α‐defensins in plasma and BAL fluid of patients with active pulmonary tuberculosis. Chest 121, 519–526. [DOI] [PubMed] [Google Scholar]
- 79. Paone, G. , Stevens, L. A. , Levine, R. L. , Bourgeois, C. , Steagall, W. K. , Gochuico, B. R. , Moss, J. (2006) ADP‐ribosyltransferase‐specific modification of human neutrophil peptide‐1. J. Biol. Chem. 281, 17054–17060. [DOI] [PubMed] [Google Scholar]
- 80. Paone, G. , Wada, A. , Stevens, L. A. , Matin, A. , Hirayama, T. , Levine, R. L. , Moss, J. (2002) ADP ribosylation of human neutrophil peptide‐1 regulates its biological properties. Proc. Natl. Acad. Sci. USA 99, 8231–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sahly, H. , Schubert, S. , Harder, J. , Rautenberg, P. , Ullmann, U. , Schroder, J. , Podschun, R. (2003) Burkholderia is highly resistant to human β‐defensin 3. Antimicrob. Agents Chemother. 47, 1739–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lysenko, E. S. , Gould, J. , Bals, R. , Wilson, J. M. , Weiser, J. N. (2000) Bacterial phosphorylcholine decreases susceptibility to the antimicrobial peptide LL‐37/hCAP18 expressed in the upper respiratory tract. Infect. Immun. 68, 1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jones, A. L. , Mertz, R. H. , Carl, D. J. , Rubens, C. E. (2007) A streptococcal penicillin‐binding protein is critical for resisting innate airway defenses in the neonatal lung. J. Immunol. 179, 3196–3202. [DOI] [PubMed] [Google Scholar]
- 84. Liu, P. T. , Stenger, S. , Tang, D. H. , Modlin, R. L. (2007) Cutting edge: vitamin D‐mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 179, 2060–2063. [DOI] [PubMed] [Google Scholar]
- 85. Hartshorn, K. L. , White, M. R. , Tecle, T. , Holmskov, U. , Crouch, E. C. (2006) Innate defense against influenza A virus: activity of human neutrophil defensins and interactions of defensins with surfactant protein D. J. Immunol. 176, 6962–6972. [DOI] [PubMed] [Google Scholar]
- 86. Gaudreault, E. , Gosselin, J. (2008) Leukotriene B4 induces release of antimicrobial peptides in lungs of virally infected mice. J. Immunol. 180, 6211–6221. [DOI] [PubMed] [Google Scholar]
- 87. LeVine, A. M. , Whitsett, J. A. , Hartshorn, K. L. , Crouch, E. C. , Korfhagen, T. R. (2001) Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J. Immunol. 167, 5868–5873. [DOI] [PubMed] [Google Scholar]
- 88. Hartshorn, K. L. , Crouch, E. C. , White, M. R. , Eggleton, P. , Tauber, A. I. , Chang, D. , Sastry, K. (1994) Evidence for a protective role of pulmonary surfactant protein D (SP‐D) against influenza A viruses. J. Clin. Invest. 94, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wiehler, S. , Proud, D. (2007) Interleukin‐17A modulates human airway epithelial responses to human rhinovirus infection. Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L505–L515. [DOI] [PubMed] [Google Scholar]
- 90. Herr, C. , Beisswenger, C. , Hess, C. , Kandler, K , Suttorp, N. , Welte, T. , Schroeder, J. M. , Vogelmeier, C. (2009) Suppression of pulmonary innate host defence in smokers. Thorax 64, 144–149. [DOI] [PubMed] [Google Scholar]
- 91. Honda, Y. , Takahashi, H. , Kuroki, Y. , Akino, T. , Abe, S. (1996) Decreased contents of surfactant proteins A and D in BAL fluids of healthy smokers. Chest 109, 1006–1009. [DOI] [PubMed] [Google Scholar]
- 92. Sims, M. W. , Tal‐Singer, R. M. , Kierstein, S. , Musani, A. I. , Beers, M. F. , Panettieri, R. A. , Haczku, A. (2008) Chronic obstructive pulmonary disease and inhaled steroids alter surfactant protein D (SP‐D) levels: a cross‐sectional study. Respir. Res. 9, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wilson, C. L. , Ouellette, A. J. , Satchell, D. P. , Ayabe, T. , Lopez‐Boado, Y. S. , Stratman, J. L. , Hultgren, S. J. , Matrisian, L. M. , Parks, W. C. (1999) Regulation of intestinal α‐defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286, 113–117. [DOI] [PubMed] [Google Scholar]
- 94. Wehkamp, J. , Salzman, N. H. , Porter, E. , Nuding, S. , Weichenthal, M. , Petras, R. E. , Shen, B. , Schaeffeler, E. , Schwab, M. , Linzmeier, R. , Feathers, R. W. , Chu, H. , Lima Jr., H. , Fellermann, K , Ganz, T. , Stange, E. F. , Bevins, C. L. (2005) Reduced Paneth cell α‐defensins in ileal Crohn's disease. Proc. Natl. Acad. Sci. USA 102, 18129–18134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Salzman, N. H. , Ghosh, D. , Huttner, K. M. , Paterson, Y. , Bevins, C. L. (2003) Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422, 522–526. [DOI] [PubMed] [Google Scholar]
- 96. Fernandez, M. I. , Regnault, B. , Mulet, C. , Tanguy, M. , Jay, P. , Sansonetti, P. J. , Pedron, T. (2008) Maturation of Paneth cells induces the refractory state of newborn mice to Shigella infection. J. Immunol. 180, 4924–4930. [DOI] [PubMed] [Google Scholar]
- 97. Bevins, C. L. , Stange, E. F. , Wehkamp, J. (2009) Decreased Paneth cell defensin expression in ileal Crohn's disease is independent of inflammation, but linked to the NOD2 1007fs genotype. Gut 58, 882–883. [PubMed] [Google Scholar]
- 98. Hugot, J. P. , Chamaillard, M. , Zouali, H. , Lesage, S. , Cezard, J. P. , Belaiche, J. , Almer, S. , Tysk, C. , O'Morain, C. A. , Gassull, M. , Binder, V. , Finkel, Y. , Cortot, A. , Modigliani, R. , Laurent‐Puig, P. , Gower‐Rousseau, C. , Macry, J. , Colombel, J. F. , Sahbatou, M. , Thomas, G. (2001) Association of NOD2 leucine‐rich repeat variants with susceptibility to Crohn's disease. Nature 411, 599–603. [DOI] [PubMed] [Google Scholar]
- 99. Ogura, Y. , Bonen, D. K , Inohara, N. , Nicolae, D. L. , Chen, F. F. , Ramos, R. , Britton, H. , Moran, T. , Karaliuskas, R. , Duerr, R. H. , Achkar, J. P. , Brant, S. R. , Bayless, T. M. , Kirschner, B. S. , Hanauer, S. B. , Nunez, G. , Cho, J. H. (2001) A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411, 603–606. [DOI] [PubMed] [Google Scholar]
- 100. Wehkamp, J. , Koslowski, M. , Wang, G. , Stange, E. F. (2008) Barrier dysfunction due to distinct defensin deficiencies in small intestinal and colonic Crohn's disease. Mucosal Immunol. 1 (Suppl. 1), S67—S74. [DOI] [PubMed] [Google Scholar]
- 101. Wehkamp, J. , Wang, G. , Kubler, I. , Nuding, S. , Gregorieff, A. , Schnabel, A. , Kays, R. J. , Fellermann, K , Burk, O. , Schwab, M. , Clevers, H. , Bevins, C. L. , Stange, E. F. (2007) The Paneth cell α‐defensin deficiency of ileal Crohn's disease is linked to Wnt/Tcf‐4. J. Immunol. 179, 3109–3118. [DOI] [PubMed] [Google Scholar]
- 102. Nuding, S. , Fellermann, K. , Wehkamp, J. , Stange, E. F. (2007) Reduced mucosal antimicrobial activity in Crohn's disease of the colon. Gut 56, 1240–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kobayashi, K. S. , Chamaillard, M. , Ogura, Y. , Henegariu, O. , Inohara, N. , Nunez, G. , Flavell, R. A. (2005) Nod2‐dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307, 731–734. [DOI] [PubMed] [Google Scholar]
- 104. Inoue, N. , Tamura, K , Kinouchi, Y. , Fukuda, Y. , Takahashi, S. , Ogura, Y. , Inohara, N. , Nunez, G. , Kishi, Y. , Koike, Y. , Shimosegawa, T. , Shimoyama, T. , Hibi, T. (2002) Lack of common NOD2 variants in Japanese patients with Crohn's disease. Gastroenterology 123, 86–91. [DOI] [PubMed] [Google Scholar]
- 105. van Es, J. H. , Jay, P. , Gregorieff, A. , van Gijn, M. E. , Jonkheer, S. , Hatzis, P. , Thiele, A. , van den Born, M. , Begthel, H. , Brabletz, T. , Taketo, M. M. , Clevers, H. (2005) Wnt signaling induces maturation of Paneth cells in intestinal crypts. Nat. Cell Biol. 7, 381–386. [DOI] [PubMed] [Google Scholar]
- 106. Koslowski, M. J. , Kubler, I. , Chamaillard, M. , Schaeffeler, E. , Reinisch, W. , Wang, G. , Beisner, J. , Teml, A. , Peyrin‐Biroulet, L. , Winter, S. , Herrlinger, K. R. , Rutgeerts, P. , Vermeire, S. , Cooney, R. , Fellermann, K , Jewell, D. , Bevins, C. L. , Schwab, M. , Stange, E. F. , Wehkamp, J. (2009) Genetic variants of Wnt transcription factor TCF‐4 (TCF7L2) putative promoter region are associated with small intestinal Crohn's disease. PLoS ONE 4, e4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Fellermann, K , Stange, D. E. , Schaeffeler, E. , Schmalzl, H. , Wehkamp, J. , Bevins, C. L. , Reinisch, W. , Teml, A. , Schwab, M. , Lichter, P. , Radlwimmer, B. , Stange, E. F. (2006) A chromosome 8 gene‐cluster polymorphism with low human β‐defensin 2 gene copy number predisposes to Crohn disease of the colon. Am. J. Hum. Genet. 79, 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wehkamp, J. , Harder, J. , Weichenthal, M. , Mueller, O. , Herrlinger, K. R. , Fellermann, K , Schroeder, J. M. , Stange, E. F. (2003) Inducible and constitutive β‐defensins are differentially expressed in Crohn's disease and ulcerative colitis. Inflamm. Bowel Dis. 9, 215–223. [DOI] [PubMed] [Google Scholar]
- 109. Schauber, J. , Rieger, D. , Weiler, F. , Wehkamp, J. , Eck, M. , Fellermann, K , Scheppach, W. , Gallo, R. L. , Stange, E. F. (2006) Heterogeneous expression of human cathelicidin hCAP18/LL‐37 in inflammatory bowel diseases. Eur. J. Gastroenterol. Hepatol. 18, 615–621. [DOI] [PubMed] [Google Scholar]
- 110. George, J. T. , Boughan, P. K. , Karageorgiou, H. , Bajaj‐Elliott, M. (2003) Host anti‐microbial response to Helicobacter pylori infection. Mol. Immunol. 40, 451–456. [DOI] [PubMed] [Google Scholar]
- 111. Boughan, P. K , Argent, R. H. , Body‐Malapel, M. , Park, J. H. , Ewings, K. E. , Bowie, A. G. , Ong, S. J. , Cook, S. J. , Sorensen, O. E. , Manzo, B. A. , Inohara, N. , Klein, N. J. , Nunez, G. , Atherton, J. C. , Bajaj‐Elliott, M. (2006) Nucleotide‐binding oligomerization domain‐1 and epidermal growth factor receptor: critical regulators of β‐defensins during Helicobacter pylori infection. J. Biol. Chem. 281, 11637–11648. [DOI] [PubMed] [Google Scholar]
- 112. Hase, K. , Murakami, M. , Iimura, M. , Cole, S. P. , Horibe, Y. , Ohtake, T. , Obonyo, M. , Gallo, R. L. , Eckmann, L. , Kagnoff, M. F. (2003) Expression of LL‐37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori . Gastroenterology 125, 1613–1625. [DOI] [PubMed] [Google Scholar]
- 113. Veldhuizen, E. J. , Koomen, I. , Ultee, T. , van Dijk, A. , Haagsman, H. P. (2009) Salmonella serovar specific upregulation of porcine defensins 1 and 2 in a jejunal epithelial cell line. Vet. Microbiol. 136, 69–75. [DOI] [PubMed] [Google Scholar]
- 114. Chakraborty, K. , Ghosh, S. , Koley, H. , Mukhopadhyay, A. K. , Ramamurthy, T. , Saha, D. R. , Mukhopadhyay, D. , Roychowdhury, S. , Hamabata, T. , Takeda, Y. , Das, S. (2008) Bacterial exotoxins downregulate cathelicidin (hCAP‐18/LL‐37) and human β‐defensin 1 (HBD‐1) expression in the intestinal epithelial cells. Cell. Microbiol. 10, 2520–2537. [DOI] [PubMed] [Google Scholar]
- 115. Sperandio, B. , Regnault, B. , Guo, J. , Zhang, Z. , Stanley Jr., S. L. , Sansonetti, P. J. , Pedron, T. (2008) Virulent Shigella flexneri subverts the host innate immune response through manipulation of antimicrobial peptide gene expression. J. Exp. Med. 205, 1121–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Diamond, G. , Beckloff, N. , Ryan, L. K. (2008) Host defense peptides in the oral cavity and the lung: similarities and differences. J. Dent. Res. 87, 915–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. White, M. R. , Helmerhorst, E. J. , Ligtenberg, A. , Karpel, M. , Tecle, T. , Siqueira, W. L. , Oppenheim, F. G. , Hartshorn, K. L. (2009) Multiple components contribute to ability of saliva to inhibit influenza viruses. Oral Microbiol. Immunol. 24, 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Fanali, C. , Inzitari, R. , Cabras, T. , Pisano, E. , Castagnola, M. , Celletti, R. , Manni, A. , Messana, I. (2008) α‐Defensin levels in whole saliva of totally edentulous subjects. Int. J. Immunopathol. Pharmacol. 21, 845–849. [DOI] [PubMed] [Google Scholar]
- 119. Sun, L. , Finnegan, C. M. , Kish‐Catalone, T. , Blumenthal, R. , Garzino‐Demo, P. , La Terra Maggiore, G. M. , Berrone, S. , Kleinman, C. , Wu, Z. , Abdelwahab, S. , Lu, W. , Garzino‐Demo, A. (2005) Human β‐defensins suppress human immunodeficiency virus infection: potential role in mucosal protection. J. Virol. 79, 14318–14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Carlsson, G. , Andersson, M. , Putsep, K. , Garwicz, D. , Nordenskjold, M. , Henter, J. I. , Palmblad, J. , Fadeel, B. (2006) Kostmann syndrome or infantile genetic agranulocytosis, part one: celebrating 50 years of clinical and basic research on severe congenital neutropenia. Acta Paediatr. 95, 1526–1532. [DOI] [PubMed] [Google Scholar]
- 121. Putsep, K. , Carlsson, G. , Boman, H. G. , Andersson, M. (2002) Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet 360, 1144–1149. [DOI] [PubMed] [Google Scholar]
- 122. Quinones‐Mateu, M. E. , Lederman, M. M. , Feng, Z. , Chakraborty, B. , Weber, J. , Rangel, H. R. , Marotta, M. L. , Mirza, M. , Jiang, B. , Kiser, P. , Medvik, K. , Sieg, S. F. , Weinberg, A. (2003) Human epithelial β‐defensins 2 and 3 inhibit HIV‐1 replication. AIDS 17, F39–F48. [DOI] [PubMed] [Google Scholar]
- 123. Valore, E. V. , Wiley, D. J. , Ganz, T. (2006) Reversible deficiency of antimicrobial polypeptides in bacterial vaginosis. Infect. Immun. 74, 5693–5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Cole, A. M. , Cole, A. L. (2008) Antimicrobial polypeptides are key anti‐HIV‐1 effector molecules of cervicovaginal host defense. Am. J. Reprod. Immunol. 59, 27–34. [DOI] [PubMed] [Google Scholar]
- 125. Venkataraman, N. , Cole, A. L. , Svoboda, P. , Pohl, J. , Cole, A. M. (2005) Cationic polypeptides are required for anti‐HIV‐1 activity of human vaginal fluid. J. Immunol. 175, 7560–7567. [DOI] [PubMed] [Google Scholar]
- 126. Feng, Z. , Dubyak, G. R. , Lederman, M. M. , Weinberg, A. (2006) Cutting edge: human β defensin 3—a novel antagonist of the HIV‐1 coreceptor CXCR4. J. Immunol. 177, 782–786. [DOI] [PubMed] [Google Scholar]
- 127. John, M. , Keller, M. J. , Fam, E. H. , Cheshenko, N. , Hogarty, K. , Kasowitz, A. , Wallenstein, S. , Carlucci, M. J. , Tuyama, A. C. , Lu, W. , Klotman, M. E. , Lehrer, R. I. , Herold, B. C. (2005) Cervicovaginal secretions contribute to innate resistance to herpes simplex virus infection. J. Infect. Dis. 192, 1731–1740. [DOI] [PubMed] [Google Scholar]
- 128. Quinn, G. A. , Cole, A. M. (2007) Suppression of innate immunity by a nasal carriage strain of Staphylococcus aureus increases its colonization on nasal epithelium. Immunology 122, 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Porter, E. , Yang, H. , Yavagal, S. , Preza, G. C. , Murillo, O. , Lima, H. , Greene, S. , Mahoozi, L. , Klein‐Patel, M. , Diamond, G. , Gulati, S. , Ganz, T. , Rice, P. A. , Quayle, A. J. (2005) Distinct defensin profiles in Neisseria gonorrhoeae and Chlamydia trachomatis urethritis reveal novel epithelial cell‐neutrophil interactions. Infect. Immun. 73, 4823–4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Klotman, M. E. , Rapista, A. , Teleshova, N. , Micsenyi, A. , Jarvis, G. A. , Lu, W. , Porter, E. , Chang, T. L. (2008) Neisseria gonorrhoeae‐induced human defensins 5 and 6 increase HIV infectivity: role in enhanced transmission. J. Immunol. 180, 6176–6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Levinson, P. , Kaul, R. , Kimani, J. , Ngugi, E. , Moses, S. , MacDonald, K. S. , Broliden, K. , Hirbod, T. (2009) Levels of innate immune factors in genital fluids: association of α defensins and LL‐37 with genital infections and increased HIV acquisition. AIDS 23, 309–317. [DOI] [PubMed] [Google Scholar]
- 132. Milanese, M. , Segat, L. , Arraes, L. C. , Garzino‐Demo, A. , Crovella, S. (2009) Copy number variation of defensin genes and HIV infection in Brazilian children. J. Acquir. Immune Defic. Syndr. 50, 331–333. [DOI] [PubMed] [Google Scholar]
- 133. Dugan, A. S. , Maginnis, M. S. , Jordan, J. A. , Gasparovic, M. L. , Manley, K. , Page, R. , Williams, G. , Porter, E. , O'Hara, B. A. , Atwood, W. J. (2008) Human α‐defensins inhibit BK virus infection by aggregating virions and blocking binding to host cells. J. Biol. Chem. 283, 31125–31132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Buck, C. B. , Day, P. M. , Thompson, C. D. , Lubkowski, J. , Lu, W. , Lowy, D. R. , Schiller, J. T. (2006) Human α‐defensins block papillomavirus infection. Proc. Natl. Acad. Sci. USA 103, 1516–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hubert, P. , Herman, L. , Maillard, C. , Caberg, J. H. , Nikkels, A. , Pierard, G. , Foidart, J. M. , Noel, A. , Boniver, J. , Delvenne, P. (2007) Defensins induce the recruitment of dendritic cells in cervical human papillomavirus‐associated (pre)neoplastic lesions formed in vitro and transplanted in vivo. FASEB J. 21, 2765–2775. [DOI] [PubMed] [Google Scholar]
- 136. Bullard, R. S. , Gibson, W. , Bose, S. K. , Belgrave, J. K. , Eaddy, A. C. , Wright, C. J. , Hazen‐Martin, D. J. , Lage, J. M. , Keane, T. E. , Ganz, T. A. , Donald, C. D. (2008) Functional analysis of the host defense peptide human β defensin‐1: new insight into its potential role in cancer. Mol. Immunol. 45, 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Coffelt, S. B. , Marini, F. C. , Watson, K. , Zwezdaryk, K. J. , Dembinski, J. L. , LaMarca, H. L. , Tomchuck, S. L. , Honer zu Bentrup, K. , Danka, E. S. , Henkle, S. L. , Scandurro, A. B. (2009) The pro‐inflammatory peptide LL‐37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc. Natl. Acad. Sci. USA 106, 3806–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sorensen, O. E. , Thapa, D. R. , Rosenthal, A. , Liu, L. , Roberts, A. A. , Ganz, T. (2005) Differential regulation of β‐defensin expression in human skin by microbial stimuli. J. Immunol. 174, 4870–4879. [DOI] [PubMed] [Google Scholar]
- 139. Liu, L. , Roberts, A. A. , Ganz, T. (2003) By IL‐1 signaling, monocyte‐derived cells dramatically enhance the epidermal antimicrobial response to lipopolysaccharide. J. Immunol. 170, 575–580. [DOI] [PubMed] [Google Scholar]
- 140. Miller, L. S. , Sorensen, O. E. , Liu, P. T. , Jalian, H. R. , Eshtiaghpour, D. , Behmanesh, B. E. , Chung, W. , Starner, T. D. , Kim, J. , Sieling, P. A. , Ganz, T. , Modlin, R. L. (2005) TGF‐α regulates TLR expression and function on epidermal keratinocytes. J. Immunol. 174, 6137–6143. [DOI] [PubMed] [Google Scholar]
- 141. Sorensen, O. E. , Cowland, J. B. , Theilgaard‐Monch, K. , Liu, L. , Ganz, T. , Borregaard, N. (2003) Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J. Immunol. 170, 5583–5589. [DOI] [PubMed] [Google Scholar]
- 142. Aberg, K. M. , Man, M. Q. , Gallo, R. L. , Ganz, T. , Crumrine, D. , Brown, B. E. , Choi, E. H. , Kim, D. K. , Schroder, J. M. , Feingold, K. R. , Elias, P. M. (2008) Co‐regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J. Invest. Dermatol. 128, 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nizet, V. , Ohtake, T. , Lauth, X. , Trowbridge, J. , Rudisill, J. , Dorschner, R. A. , Pestonjamasp, V. , Piraino, J. , Huttner, K. , Gallo, R. L. (2001) Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414, 454–457. [DOI] [PubMed] [Google Scholar]
- 144. Carretero, M. , Escamez, M. J. , Garcia, M. , Duarte, B. , Holguin, A. , Retamosa, L. , Jorcano, J. L. , Rio, M. D. , Larcher, F. (2008) In vitro and in vivo wound healing‐promoting activities of human cathelicidin LL‐37. J. Invest. Dermatol. 128, 223–236. [DOI] [PubMed] [Google Scholar]
- 145. Peric, M. , Koglin, S. , Kim, S. M. , Morizane, S. , Besch, R. , Prinz, J. C. , Ruzicka, T. , Gallo, R. L. , Schauber, J. (2008) IL‐17A enhances vitamin D3‐induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J. Immunol. 181, 8504–8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Aberg, K. M. , Radek, K. A. , Choi, E. H. , Kim, D. K. , Demerjian, M. , Hupe, M. , Kerbleski, J. , Gallo, R. L. , Ganz, T. , Mauro, T. , Feingold, K. R. , Elias, P. M. (2007) Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J. Clin. Invest. 117, 3339–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ong, P. Y. , Ohtake, T. , Brandt, C. , Strickland, I. , Boguniewicz, M. , Ganz, T. , Gallo, R. L. , Leung, D. Y. (2002) Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 347, 1151–1160. [DOI] [PubMed] [Google Scholar]
- 148. Minegishi, Y. , Karasuyama, H. (2008) Genetic origins of hyper‐IgE syndrome. Curr. Allergy Asthma Rep. 8, 386–391. [DOI] [PubMed] [Google Scholar]
- 149. Minegishi, Y. , Saito, M. , Nagasawa, M. , Takada, H. , Hara, T. , Tsuchiya, S. , Agematsu, K. , Yamada, M. , Kawamura, N. , Ariga, T. , Tsuge, I. , Karasuyama, H. (2009) Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper‐IgE syndrome. J. Exp. Med. 206, 1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Heilborn, J. D. , Nilsson, M. F. , Kratz, G. , Weber, G. , Sorensen, O. , Borregaard, N. , Stahle‐Backdahl, M. (2003) The cathelicidin anti‐microbial peptide LL‐37 is involved in re‐epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J. Invest. Dermatol. 120, 379–389. [DOI] [PubMed] [Google Scholar]
- 151. Lande, R. , Gregorio, J. , Facchinetti, V. , Chatterjee, B. , Wang, Y. H. , Homey, B. , Cao, W. , Su, B. , Nestle, F. O. , Zal, T. , Mellman, I. , Schroder, J. M. , Liu, Y. J. , Gilliet, M. (2007) Plasmacytoid dendritic cells sense self‐DNA coupled with antimicrobial peptide. Nature 449, 564–569. [DOI] [PubMed] [Google Scholar]
- 152. Jansen, P. A. , Rodijk‐Olthuis, D. , Hollox, E. J. , Kamsteeg, M. , Tjabringa, G. S. , de Jongh, G. J. , van Vlijmen‐Willems, I. M. , Bergboer, J. G. , van Rossum, M. M. , de Jong, E. M. , den Heijer, M. , Evers, A. W. , Bergers, M. , Armour, J. A. , Zeeuwen, P. L. , Schalkwijk, J. (2009) β‐Defensin‐2 protein is a serum biomarker for disease activity in psoriasis and reaches biologically relevant concentrations in lesional skin. PLoS ONE 4, e4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hollox, E. J. , Huffmeier, U. , Zeeuwen, P. L. , Palla, R. , Lascorz, J. , Rodijk‐Olthuis, D. , van de Kerkhof, P. C. , Traupe, H. , de Jongh, G. , den Heijer, M. , Reis, A. , Armour, J. A. , Schalkwijk, J. (2008) Psoriasis is associated with increased β‐defensin genomic copy number. Nat. Genet. 40, 23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Yamasaki, K. , Di Nardo, A. , Bardan, A. , Murakami, M. , Ohtake, T. , Coda, A. , Dorschner, R. A. , Bonnart, C. , Descargues, P. , Hovnanian, A. , Morhenn, V. B. , Gallo, R. L. (2007) Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat. Med. 13, 975–980. [DOI] [PubMed] [Google Scholar]
- 155. Cohen, S. , Tyrrell, D. A. , Smith, A. P. (1991) Psychological stress and susceptibility to the common cold. N. Engl. J. Med. 325, 606–612. [DOI] [PubMed] [Google Scholar]
- 156. Wilkes, G. , Hartshorn, K. (2009) Colon, rectal, and anal cancers. Semin. Oncol. Nurs. 25, 32–47. [DOI] [PubMed] [Google Scholar]
- 157. Wu, M. , McClellan, S. A. , Barrett, R. P. , Hazlett, L. D. (2009) β‐Defensin‐2 promotes resistance against infection with P. aeruginosa . J. Immunol. 182, 1609–1616. [DOI] [PubMed] [Google Scholar]
- 158. Ni, M. , Tam, C. , Verma, A. , Ramphal, R. , Hawgood, S. , Evans, D. J. , Fleiszig, S. M. (2008) Expression of surfactant protein D in human corneal epithelial cells is upregulated by Pseudomonas aeruginosa . FEMS Immunol. Med. Microbiol. 54, 177–184. [DOI] [PubMed] [Google Scholar]
- 159. Ni, M. , Evans, D. J. , Hawgood, S. , Anders, E. M. , Sack, R. A. , Fleiszig, S. M. (2005) Surfactant protein D is present in human tear fluid and the cornea and inhibits epithelial cell invasion by Pseudomonas aeruginosa . Infect. Immun. 73, 2147–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Brandt, C. R. , Akkarawongsa, R. , Altmann, S. , Jose, G. , Kolb, A. W. , Waring, A. J. , Lehrer, R. I. (2007) Evaluation of a θ‐defensin in a murine model of herpes simplex virus type 1 keratitis. Invest. Ophthalmol. Vis. Sci. 48, 5118–5124. [DOI] [PubMed] [Google Scholar]
- 161. Cole, A. L. , Herasimtschuk, A. , Gupta, P. , Waring, A. J. , Lehrer, R. I. , Cole, A. M. (2007) The retrocyclin analogue RC‐101 prevents human immunodeficiency virus type 1 infection of a model human cervicovaginal tissue construct. Immunology 121, 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Wohlford‐Lenane, C. L. , Meyerholz, D. K , Perlman, S. , Zhou, H. , Tran, D. , Selsted, M. E. , McCray Jr., P. B. (2009) Rhesus θ‐defensin prevents death in a mouse model of SARS coronavirus pulmonary disease. J. Virol., Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 163. Harvey, S. A. , Romanowski, E. G. , Yates, K. A. , Gordon, Y. J. (2005) Adenovirus‐directed ocular innate immunity: the role of conjunctival defensin‐like chemokines (IP‐10, I‐TAC) and phagocytic human defensin‐α . Invest. Ophthalmol. Vis. Sci. 46, 3657–3665. [DOI] [PubMed] [Google Scholar]
- 164. Meyerholz, D. K , Grubor, B. , Gallup, J. M. , Lehmkuhl, H. D. , Anderson, R. D. , Lazic, T. , Ackermann, M. R. (2004) Adenovirus‐mediated gene therapy enhances parainfluenza virus 3 infection in neonatal lambs. J. Clin. Microbiol. 42, 4780–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Bals, R. , Weiner, D. J. , Moscioni, A. D. , Meegalla, R. L. , Wilson, J. M. (1999) Augmentation of innate host defense by expression of a cathelicidin antimicrobial peptide. Infect. Immun. 67, 6084–6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Sow, F. B. , Gallup, J. M. , Meyerholz, D. K , Ackermann, M. R. (2009) Gene profiling studies in the neonatal ovine lung show enhancing effects of VEGF on the immune response. Dev. Comp. Immunol. 33, 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Cole, A. M. , Liao, H. I. , Stuchlik, O. , Tilan, J. , Pohl, J. , Ganz, T. (2002) Cationic polypeptides are required for antibacterial activity of human airway fluid. J. Immunol. 169, 6985–6991. [DOI] [PubMed] [Google Scholar]
- 168. Cole, A. M. , Wu, M. , Kim, Y. , Ganz, T. (2000) Microanalysis of antimicrobial properties of human fluids. J. Microbiol. Methods 41, 135–143. [DOI] [PubMed] [Google Scholar]
- 169. Cole, A. M. , Dewan, P. , Ganz, T. (1999) Innate antimicrobial activity of nasal secretions. Infect. Immun. 67, 3267–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. White, M. R. , Crouch, E. , van Eijk, M. , Hartshorn, M. , Pemberton, L. , Tornoe, I. , Holmskov, U. , Hartshorn, K. L. (2005) Cooperative anti‐influenza activities of respiratory innate immune proteins and neuraminidase inhibitor. Am. J. Physiol. Lung Cell. Mol. Physiol. 288, L831–L840. [DOI] [PubMed] [Google Scholar]


