Abstract
Infection with pantropic canine coronavirus was detected during outbreaks in France and Belgium. This was concurrent in most cases with canine parvovirus 2c. One outbreak was a deadly acute systemic disease with a single pantropic canine coronavirus infection. This is the first report of a fatality associated with pantropic canine coronavirus alone outside Italy.
INTRODUCTION
Viruses are often implicated in haemorrhagic enteritis in puppies (Yesilbag and others 2007). Canine parvovirus type 2 (CPV‐2) emerged as a new virus in dogs in 1978 and was soon replaced by variant viruses (CPV‐2a and CPV‐2b), which continue to coexist in the dog population (Truyen 1999). Recently, a CPV‐2 mutant was detected in Italy (Buonavoglia and others 2001). The mutation occurred in residue 426 (Asp was replaced by Glu), so it was called mutant Glu‐426 or CPV‐2c. This mutation has also been detected in other countries such as Spain, Great Britain, Germany and Portugal (Decaro and others 2007a).
Canine coronavirus (CCoV) is another virus implicated in puppy enteritis (Pratelli and others 2003). In some cases, enteritis can be more severe and associated with mortality (Evermann and others 2005). There are two genotypes of CCoV, namely, CCoV type I (CCoV‐I) and type II (CCoV‐II). These two genotypes are now regarded as members of the species Geselavirus within the genus Alphacoronavirus, together with swine transmissible gastroenteritis virus and feline coronavirus type I (FCoV‐I) and type II (FCoV‐II) (de Groot and others 2008). CCoV infection is usually restricted to the enteric tract, although, in Italy, CCoV was recently associated with a systemic fatal disease in pups (Buonavoglia and others 2006). Severe clinical signs were observed in the affected pups, and necropsy examination revealed remarkable gross lesions in lungs, liver, spleen and kidneys. CCoV type II RNA was detected at very high titres in the internal organs of these dead pups. The association of CCoV with a severe, sometimes fatal disease in dogs, together with the isolation of the virus from internal organs with severe lesions, strongly suggests that CCoV tropism has evolved to acquire the ability to spread from the intestinal tract to other organs (Decaro and others 2007b).
Co‐infection with CCoV and CPV‐2 has been reported previously (Yesilbag and others 2007). CCoV infection with puppy mortality but without co‐infection with parvovirus had also been reported previously (Evermann and others 2005), but in this report infection was restricted to the intestine. Fatal cases of pantropic CCoV infection alone have only ever been observed in Italy (Buonavoglia and others 2006). We report on outbreaks of pantropic CCoV in France and Belgium, including the first case of a lethal infection with pantropic CCoV in a European country other than Italy, thus extending the area of distribution of this lethal viral infection.
CASE REPORT
Between March 2008 and August 2009, five outbreaks of a fatal systemic disease occurred in the north of France and Belgium. In March and June 2008, the first and the second outbreaks occurred in dogs belonging to a breeder of miniature pinchers in Bourg Fidèle, France (three pups affected and died and nine pups affected of which eight died, respectively). These were the first French cases. Clinical signs were observed at two months of age and consisted of lethargy, vomiting, anorexia, diarrhoea and convulsions. The attending veterinarian of the cases suspected parvovirus infection. Despite supportive treatment (fluid therapy and ω interferon 2 million IU/kg), the puppies died within 3 days. Eight (three from the first outbreak and five from the second one) of the dead puppies were frozen and submitted to the authors institution for further investigation. In addition, faecal samples were taken for analysis from two diarrhoeic animals (dogs ULg 100 and ULg 103) from another litter at the breeder after the second outbreak. Outbreaks 3 and 4 occurred in a pet shop in Péruwelz, Belgium, in September and November 2008 (in total seven puppies died); these were the first cases in Belgium. Outbreak 5 occurred in Oudenburg, Belgium, in August 2009 (two puppies died). In each of these last three outbreaks, one puppy was submitted for necropsy and virological investigation. Clinical signs were the same in all animals but one showed non‐haemorrhagic enteritis and survived. Necropsies were performed and organs (intestine, spleen, liver, lung and brain) were removed separately, crushed and analysed by polymerase chain reaction (PCR). In addition, histopathologic evaluation was performed.
Viral nucleic acid was extracted using the QIAamp viral RNA mini spin protocol (QIAGEN) allowing simultaneous extraction of both RNA and DNA.
PCR was performed with primer pair 555for/555rev (Buonavoglia and others 2001) to detect CPV‐2 infection. After PCR confirmation, stools or intestinal tissue in pups from outbreak 1 were further characterised by minor groove binding probe technology (Decaro and others 2006). Similarly, CPV‐2 genomes were quantified and typed in pooled samples from spleen and liver of pups from outbreaks 2 to 4.
CCoV detection was by means of the AccessQuick RT‐PCR System from PROMEGA according to the manufacturer’s instructions. Primer pairs CCV1 and CCV2 (Pratelli and others 1999) amplified a 409 base pair (bp) sequence in the gene encoding the transmembrane protein M. Confirmation of the presence of CCoV and differentiation between CCoV‐I and CCoV‐II was made by genotype‐specific fluorogenic real‐time PCR (RT‐PCR) assays (Decaro and others 2005).
Post‐mortem examination revealed a range of findings in the intestines and major organs. Gastro‐intestinal contents were typically scant, mucoid and yellow to brown. Macroscopic lesions consistent with acute mild to moderate enteritis were generally confined to the small intestine. The affected areas showed pink to red intestinal walls and sometimes slightly dry and rough surface with rare petechiae. Regional mesenteric lymph nodes were enlarged (1·5 to 2‐fold); some were congested and showed either petechiae or more extensive haemorrhage. One puppy had gastric ulcers associated with melaena. Another presented a venous infarction of a colic segment secondary to intussusception. Many puppies (11/15) presented with light to severe hepatosis. The spleen was slightly enlarged and congested. Pulmonary congestion was detected in some animals. Brains could not be investigated because of liquefaction after freeze‐thawing.
Histopathologic evaluation showed that intestinal villi were reduced in length and often fused. Villous epithelial lesions were not interpretable because of autolysis. Numerous crypts were dilated and contained degenerated and necrosed cells particularly at their bases (Fig 1). Subacute enteritis was characterised by moderate infiltrate of mononuclear inflammatory cells in the lamina propria. Lymphoid tissues were oedematous and lymphoid depletion was evident in some nodes, while lymphoid hyperplasia was not observed. Mild to severe hepatocyte degenerative changes were observed; cytoplasm was clear, showed microvacuoles and was negative to Periodic Acid Schiff staining (confirming hepatocellular microvesicular steatosis) (Fig 2). There was marked pulmonary congestion. Multifocal to diffuse subacute interstitial pneumonia and alveolar oedema were observed in five dogs (Fig 3).
Figure 1.
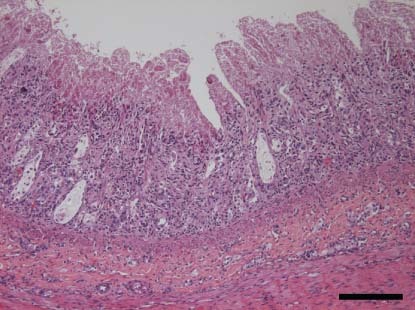
Ileum. Villi were shortened and fused. Some crypt lumina were dilated, distorted and filled with necrotic debris (pup co‐infected with canine coronavirus type I and canine parvovirus 2c from outbreak 2. H&E. ×100; bar: 200 μm)
Figure 2.
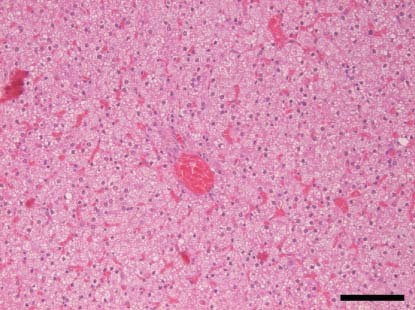
Liver. Diffuse hepatocellular microvesicular steatosis observed in a pup co‐infected with canine coronavirus type II and canine parvovirus 2c from outbreak 1 (H&E. ×200; bar: 100 μm)
Figure 3.
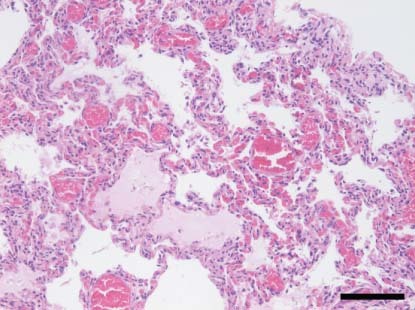
Lung. Diffuse thickening of alveolar walls by mononuclear infiltrate and oedema in few alveoli (pup co‐infected with canine coronavirus type I and canine parvovirus 2c from outbreak 2. H&E. ×200; bar: 100 μm)
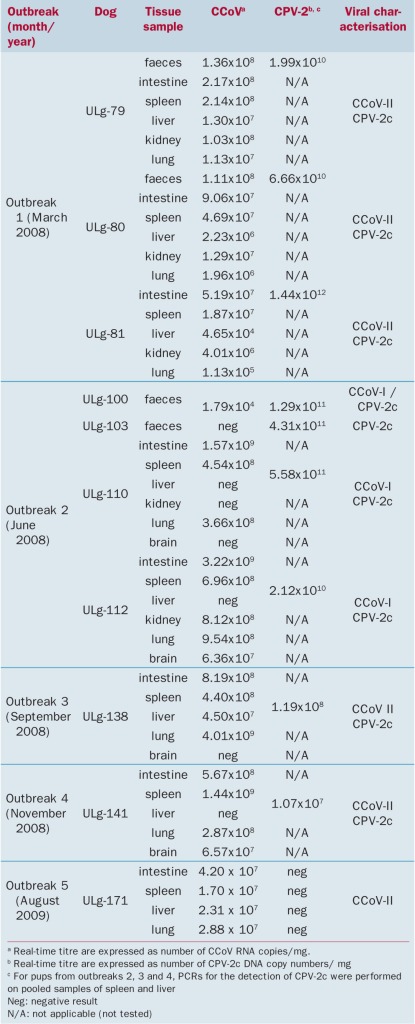
In outbreaks 1 to 4, a co‐infection of CPV‐2c and CCoV‐I or II was confirmed (Table 1). Among the puppies from those outbreaks, a single pup was infected with CPV‐2c only. This pup had non‐haemorrhagic diarrhoea and survived, unlike the other co‐infected puppies. High genomic levels of CPV‐2c were detected in faeces (1·99×1010 to 4·31×1011 DNA copies/mg) and organs from these puppies were also positive for CCoV‐II (4·65×104 to 4·01×109 RNA copies/mg) (Table 1). In outbreak 2, puppies showed infection with CCoV‐I (6·36×107 to 3·22×109 RNA copies/mg in intestinal tissue, spleen and lung) and CPV‐2c (Table 1). In one of the puppies, the brain was also positive for CCoV and in another, CCoV was found in the kidney sample. Outbreak 5 was of particular interest as it was caused by a mono‐infection of CCoV‐II, which was associated with the death of one of the affected puppies. Virus was found in all tissues examined (intestine, spleen, liver and lung) (Table 1).
Table 1.
Quantification by real‐time PCR of infection with canine coronavirus (CCoV) type I, type II and canine parvovirus 2c (CPV‐2c) during the five outbreaks
DISCUSSION
This is the first report of outbreaks of disease caused by a co‐infection with CCoV and CPV‐2c outside Italy. It is also the second report anywhere of a lethal pansystemic disease in dogs caused by CCoV‐II infection alone. The clinical signs and organ pathology were similar to those observed previously during the Italian outbreak with the CCoV‐II pantropic variant (Buonavoglia and others 2006). In most of the reported cases, the veterinarian suspected a CPV‐2 infection. Although seizures can be present with CPV‐2 infections alone, they were also systematically observed in association with the pantropic CCoV infections both during dual infection with CPV‐2 and mono CCoV infections.
A surprising finding is represented by the detection of a CCoV‐I strain in the internal organs of two pups in the second outbreak. Currently, there are no reports on the association of this CCoV genotype with systemic infections. Sequence analyses and experimental infections with CCoV‐I strains would be needed to further characterise this virus as a pantropic strain. In addition, it could be suggested that a concurrent CPV‐2 infection might facilitate the spread of enteric CCoV to non‐target tissues (Decaro and others 2009, Ntafis and others 2011).
This outbreak involving CCoV‐I followed a previous one that occurred in the same breeding facility 3 months earlier and was associated with CCoV‐II infection. These two outbreaks were most likely the consequence of two independent events of viral introduction to the breeding facility, probably attributable to the breeder’s participation in several dog shows.
Pantropic CCoV infection appears to be a new emerging disease in European countries other than Italy, thus extending its distribution.
Acknowledgements
The authors thank the Belgian veterinary practitioners who provided us with the cases reported in this study.
Conflict of interest
None of the authors of this article has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
References
- Buonavoglia, C. , Martella, V. , Pratelli, A. , Tempesta, M. , Cavalli, A. , Buonavoglia, D. , Bozzo, G. , Elia, G. , Decaro, N. & Carmichael, L. (2001) Evidence for evolution of canine parvovirus type 2 in Italy. Journal of General Virology 82, 3021–3025 [DOI] [PubMed] [Google Scholar]
- Buonavoglia, C. , Decaro, N. , Martella, V. , Elia, G. , Campolo, M. , Desario, C. , Castagnaro, M. & Tempesta, M. (2006) Canine coronavirus highly pathogenic for dogs. Emerging Infectious Diseases 12, 492–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot, R. J. , Ziebuhr, J. , Poon, L. L. , Woo, P. C. , Talbot, P. , Rottier, P. J. M. , Holmes, K. V. , Baric, R. , Perlman, S. , Enjuanes, L. & Gorbalenya, A. E . (2008) http://talk.ictvonline.org/media/p/1230.aspx. Accessed October 20, 2009
- Decaro, N. , Martella, V. , Ricci, D. , Elia, G. , Desario, C. , Campolo, M. , Cavaliere, N. , Di Trani, L. , Tempesta, M. & Buonavoglia, C. (2005) Genotype‐specific fluorogenic RT‐PCR assays for the detection and quantitation of canine coronavirus type I and type II RNA in faecal samples of dogs. Journal of Virological Methods 130, 72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro, N. , Elia, G. , Martella, V. , Campolo, M. , Desario, C. , Camero, M. , Cirone, F. , Lorusso, E. , Lucente, M. S. , Narcisi, D. , Scalia, P. & Buonavoglia, C. (2006) Characterisation of the canine parvovirus type 2 variants using minor groove binder probe technology. Journal of Virological Methods 133, 92–99 [DOI] [PubMed] [Google Scholar]
- Decaro, N. , Desario, C. , Addie, D. D. , Martella, V. , Vieira, M. J. , Elia, G. , Zicola, A. , Davis, C. , Thompson, G. , Thiry, E. , Truyen, U. & Buonavoglia, C. (2007a) The study molecular epidemiology of canine parvovirus, Europe. Emerging Infectious Diseases 13, 1222–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro, N. , Martella, V. , Elia, G. , Campolo, M. , Desario, C. , Cirone, F. , Tempesta, M. & Buonavoglia, C. (2007b) Molecular characterisation of the virulent canine coronavirus CB/05 strain. Virus Research 125, 54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro, N. , Mari, V. , Campolo, M. , Lorusso, A. , Camero, M. , Elia, G. , Martella, V. , Cordioli, P. , Enjuanes, L. & Buonavoglia, C. (2009) Recombinant canine coronaviruses related to transmissible gastroenteritis virus of swine are circulating in dogs. Journal of Virology 83, 1532–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evermann, J. F. , Abbott, J. R. & Han, S. (2005) Canine coronavirus‐associated puppy mortality without evidence of concurrent canine parvovirus infection. Journal of Veterinary Diagnostic Investigation 17, 610–614 [DOI] [PubMed] [Google Scholar]
- Ntafis, V. , Mari, V. , Decaro, N. , Papanastassopoulou, M. , Papaioannou, N. , Mpatziou, R. , Buonavoglia, C. & Xylouri, E. (2011) Isolation, tissue distribution and molecular characterization of two recombinant canine coronavirus strains. Veterinary Microbiology 151, 238–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli, A. , Tempesta, M. , Greco, G. , Martella, V. & Buonavoglia, C. (1999) Development of a nested PCR assay for the detection of canine coronavirus. Journal of Virological Methods 80, 11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli, A. , Martella, V. , Decaro, N. , Tinelli, A. , Camero, M. , Cirone, F. , Elia, G. , Cavalli, A. , Corrente, M. , Greco, G. , Buonavoglia, D. , Gentile, M. , Tempesta, M. & Buonavoglia, C. (2003) Genetic diversity of a canine coronavirus detected in pups with diarrhoea in Italy. Journal of Virological Methods 110, 9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truyen, U. (1999) Emergence and recent evolution of canine parvovirus. Veterinary Microbiology 69, 47–50 [DOI] [PubMed] [Google Scholar]
- Yesilbag, K. , Yilmaz, Z. , Ozkul, A. & Pratelli, A. (2007) Aetiological role of viruses in puppies with diarrhoea. Veterinary Record 161, 169–170 [DOI] [PubMed] [Google Scholar]


