Abstract
Syndromic diagnosis by multiplex nucleic acid amplification tests is the most practical approach to respiratory tract infections since the symptoms are rarely agent‐specific. The aim of this study was to investigate the respiratory viruses in children admitted to a university hospital with acute respiratory tract infection during the last 8 years by a multiplex polymerase chain reaction (PCR) assay. A total of 3162 respiratory samples collected from children between April 2011 and April 2018 tested by a multiplex real‐time PCR assay. Two different commercial assays were used during the study period, "AusDiagnostics/Respiratory Pathogens 12 (AusDiagnostics)" used between April 2011 and December 2015, which changed to "Fast Track Diagnostics/Respiratory Pathogens 21 (Fast Track Diagnostics)" after January 2016 to cover more viruses. Nucleic acid extraction was done by EZ1 Advanced XL platform (QIAGEN). Respiratory pathogens detected in 1857 of the 3162 (58.7%) samples. The most prevalent viruses during the 8‐year period were rhinovirus/enterovirus (RV/EV; 36.2%), respiratory syncytial virus (RSV; 19%), and influenza virus A/B (14.7%). Rhinovirus was the main contributor to the RV/EV group as shown by the assay used during the 2016‐2018 period. RV/EV and adenoviruses detected throughout the year. Influenza virus was most frequently detected during January to March when both RSV and metapneumovirus were also in circulation. The coinfection percentage was 10.2%. Rhinovirus was the most common virus in coinfections while RSV plus rhinovirus/enterovirus were the most frequent combination. RSV and metapneumovirus showed a similar seasonal distribution to the influenza virus, which made it necessary to use a virological diagnostic assay during the influenza season.
Keywords: adult, child, multiplex polymerase chain reaction, respiratory tract infections, viruses
Abbreviations
- ARTI
Acute respiratory tract infections
- IAV
Influenza A virus
- IBV
influenza B virus
- RSV
respiratory syncytial virus
- PIVs
parainfluenza viruses
- AdV
adenovirus
- HMPV
human metapneumovirus
- RV
rhinovirus
- mPCRs
Multiplex PCR tests
- DEUH
Dokuz Eylul University Hospital
- RV/EV
rhinovirus/enterovirus
- HCoV‐NL63
human coronaviruses NL63
- HCoV 229E
human coronaviruses 229E
- HCoV OC43
human coronaviruses OC43
- HCoV HKU1
human coronaviruses HKU1
- M. pneu
Mycoplasma pneumoniae
- HboV
human bocavirus
- HpeV
human parechovirus
- ILI
influenza‐like illness
1. INTRODUCTION
Acute respiratory tract infections (ARTIs) are the leading cause of global mortality and morbidity.1 Incidence in children is similar in developed and developing countries, but mortality rates are higher in developing countries.2 Nearly 70% of children between the ages of one and four with respiratory disease and 90% of infants under the age of one require hospitalization.3
Viruses are the etiologic agent pathogen in approximately 80% of ARTIs.4 Influenza A virus (IAV), influenza B virus (IBV), respiratory syncytial virus (RSV), parainfluenza viruses (PIVs), adenovirus (AdV), human metapneumovirus (HMPV), and rhinovirus (RV) are frequently reported agents.5, 6, 7 Clinical differentiation is not possible since there are no pathogen‐specific symptoms. Therefore, a specific diagnosis can only be made by microbiological tests.8
Viral pathogens can be detected by viral culture, direct immunofluorescence tests, rapid antigen tests, and/or nucleic acid tests. Although viral culture is traditionally the gold standard method, it is often slow and technically demanding, which causes problems in patient management. Direct immunofluorescence tests and rapid antigen assays can produce results in a relatively short‐time frame compared with viral cultures; however, their sensitivity and specificity are lower and can only be used for specific viruses.9 Nucleic acid—based methods are often preferred for the diagnosis of viral respiratory infections, especially for hospitalized patients. These tests quickly identify the agent with high sensitivity and help to facilitate the appropriate management for both the patient and the infection control measures.10 Specific polymerase chain reactions (PCRs; monoplex) can be used to identify single viral agents such as influenza A/B and RSV, however, their role is limited. Multiplex PCR tests (mPCRs) allow a fast detection of a wide range of respiratory viruses, therefore their importance and use are increasing with several commercial options.11
This study aimed to assess the role, distribution, and characteristics of viral respiratory agents in children, who were evaluated by mPCR in a single university hospital for the last 8 years.
2. MATERIALS AND METHODS
Respiratory tract samples were collected from pediatric patients with ARTI symptoms and tested for viral pathogens at the Core Laboratory of Dokuz Eylul University Hospital (DEUH) between April 2011 and April 2018. The children were either seen at the outpatient departments or admitted to the pediatric wards of the same hospital. Results of 3162 patients between 0 and 18 years of age were evaluated retrospectively. Two different commercial kits based on multiplex real‐time PCR were used during the study period. An AusDiagnostics/Respiratory Pathogens 12 kit (AusDiagnostics, Beaconsfield, Australia) was used between April 2011 and December 2015, while a Fast Track Diagnostics/Respiratory Pathogens 21 kit (FTD 21; Fast Track Diagnostics, Junglinster, Luxemburg) was used between January 2016 and April 2018.
The AusDiagnostics test is based on multiplex tandem PCR method and identifies IAV, IBV, HMPV, PIV‐1, ‐2, ‐3, RSV, AdV, rhinovirus/enterovirus (RV/EV), and Bordetella spp. The FTD 21 test, using different primers and probes, detects 20 agents in five reaction tubes by mPCR. These agents are IAV, IAV subtype H1N1 (pandemic H1N1), IBV, human RV, human coronaviruses NL63 (HCoV‐NL63), 229E (HCoV 229E), OC43 (HCoV OC43) and HKU1 (HCoV HKU1), PIV‐1, ‐2, ‐3 and ‐4, Mycoplasma pneumoniae, human bocavirus (HBoV), HMPVA/B, RSVA/B, AdV, EV, and human parechovirus (HpeV). Both assays include internal control as well as negative and positive run controls. EZ1 Virus Mini Kit V 2.0 (QIAGEN, Hamburg, Germany) was used for nucleic acid extraction which was performed on the EZ1 Advanced XL (QIAGEN) device according to the manufacturer's instructions.
This study was approved by the Ethical Committee for Research of the Dokuz Eylul University (ref: 2018/20‐33).
3. RESULTS
Among the 3162 tested samples of patients with ARTI symptoms, 85.4% (n = 2699) were from the patients admitted to the wards or intensive care units, while 14.6% (n = 463) were from outpatients. Study samples consisted of nasopharyngeal swabs (90.6%, 2865 of 3162), nasopharyngeal aspirates (8.6%, 272 of 3162), and bronchoalveolar lavage samples (0.8%, 25 of 3162). Among 3162 patients, 44.9% (1421 of 3162) were female and 55% (1741 of 3162) were male. The median age was 12 months (0‐211 months). Samples were grouped according to the age of the patients, Patients under the 2 years of age group accounted for 50% (1584 of 3162) of the study group while 24% (759 of 3162) were from 2 to 4 years, 15.1% (479 of 3162) were from 5 to 9 years and 10.7% (340 of 3162) were of 10 to 17 years age group.
A pathogen was detected in 1857 (58.7%) of the study samples. Distribution of PCR positive samples according to age groups was found as follows; 53% (978 of 1857) in less than 2 years age group, 24% (454 of 1857) in 2 to 4 years group, 15% (270 of 1857) in the 5 to 9 years group, and 8% (155 of 1857) in 10 to 17 years group. The positivity rate decreased significantly above 5 years of age (P < 0.05).
The mean positivity rate was 44.4% during study period when the AusDignostics assay was used compared with 69.8% of the second part of the study using the Fast Track Diagnostics assay. The difference is statistically significant (P < 0.001; Figure 1A). RV/EV (17.6%) and RSV (9.2%) were the most commonly detected agents followed by IAV (4.8%; Table 1). When the distribution of the agents according to years were examined, RV/EV was the most common agent for each year. RSV and coinfections took the second and third place in the frequency order except 2011 in which they are replaced by PIV‐3 and AdV. The study covered only the first 4 months of 2018 where coinfections were the most frequent cause of the respiratory infections in the studied group and followed by RV/EV and influenza A/B, respectively (Figure 1B). When the distribution according to age groups was determined, The first three pathogens were RV/EV, RSV, and coinfections for less than 2 years; RV/EV, coinfections, and influenza A/B for 2 to 4 years; RV/EV, influenza A/B, and coinfections for 5 to 9 and 10 to 17 years (Table 2).
Figure 1.
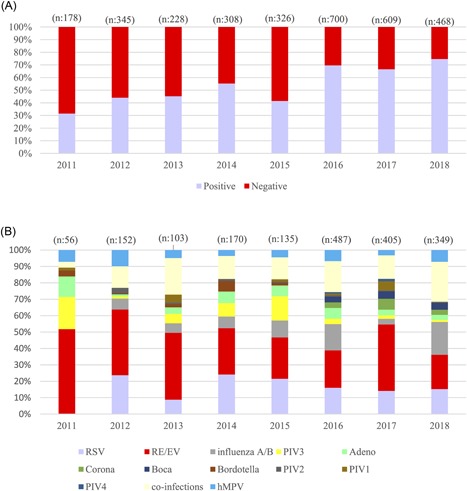
A, Positive and negative results according to the years. B, Distributions of the respiratory agents according to the years. EV, enterovirus; hMPV, human metapneumovirus; PIV, parainfluenza virus; RSV, respiratory syncytial virus; RV, rhinovirus
Table 1.
Results (n) of 3162 respiratory samples analyzed by multiplex real‐time polymerase chain reaction in relation to month of the year during the study period (April 2011‐April 2018)
| Month | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agents | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | Total, n (%) | % of positive |
| Rhinovirus/enterovirus | 43 | 41 | 53 | 75 | 45 | 27 | 27 | 18 | 34 | 76 | 70 | 47 | 556 (17.6) | 36.2 |
| Respiratory syncytial virus | 65 | 90 | 68 | 31 | 10 | 2 | 1 | 0 | 0 | 1 | 3 | 21 | 292 (9.2) | 19 |
| Influenza A virus | 85 | 43 | 16 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 3 | 151 (4.8) | 9.8 |
| Metapneumovirus | 25 | 32 | 18 | 19 | 4 | 2 | 2 | 0 | 0 | 0 | 1 | 1 | 104 (3.3) | 6.8 |
| Adenovirus | 7 | 6 | 9 | 19 | 17 | 13 | 4 | 1 | 1 | 6 | 3 | 4 | 90 (2.8) | 5.8 |
| Parainfluenza virus type 3 | 2 | 0 | 2 | 12 | 11 | 6 | 7 | 8 | 9 | 12 | 6 | 7 | 82 (2.6) | 5.3 |
| Influenza B virus | 22 | 26 | 18 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 75 (2.4) | 4.9 |
| Coronavirus b | 5 | 5 | 7 | 8 | 11 | 0 | 2 | 0 | 3 | 2 | 9 | 2 | 54 (1.7) | 3.5 |
| Coronavirus (Cor‐43) | 5 | 0 | 1 | 1 | 8 | 0 | 1 | 0 | 2 | 1 | 4 | 0 | 23 | … |
| Coronavirus (Cor‐229E) | 0 | 2 | 3 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | … |
| Coronavirus (HKU1) | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 9 | … |
| Coronavirus (NL63) | 0 | 3 | 1 | 3 | 2 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 14 | … |
| Bocavirus b | 11 | 11 | 7 | 3 | 4 | 2 | 2 | 1 | 0 | 3 | 3 | 3 | 50 (1.6) | 3.3 |
| Parainfluenza virus type 1 | 5 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 5 | 16 | 7 | 1 | 37 (1.2) | 2.4 |
| Bordotella spp a | 0 | 0 | 0 | 1 | 2 | 5 | 4 | 4 | 1 | 0 | 0 | 1 | 18 (0.6) | 1.2 |
| Parainfluenza virus type 2 | 1 | 1 | 0 | 0 | 1 | 1 | 2 | 1 | 0 | 2 | 4 | 2 | 15 (0.5) | 1.0 |
| Parainfluenza virus type 4 | 3 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 2 | 2 | 11 (0.3) | 0.7 |
| Total positive with one agent | 274 | 255 | 198 | 176 | 106 | 60 | 54 | 34 | 54 | 118 | 109 | 97 | 1535 (48.5) | 100 |
| Samples with two agents positive | 50 | 63 | 48 | 40 | 19 | 12 | 5 | 2 | 4 | 13 | 12 | 25 | 293 (9.3) | |
| Samples with three agents positive | 10 | 2 | 8 | 1 | 3 | 0 | 0 | 0 | 0 | 3 | 1 | 1 | 29 (0.9) | |
| Total positive with two and three agents | 60 | 65 | 56 | 41 | 22 | 12 | 5 | 2 | 4 | 16 | 13 | 26 | 322 (10.2) | |
| Total positive samples | 334 | 320 | 254 | 217 | 128 | 72 | 59 | 36 | 58 | 134 | 122 | 123 | 1857 (58.7) | |
| Total negative samples | 147 | 113 | 138 | 171 | 121 | 89 | 59 | 82 | 75 | 78 | 113 | 119 | 1305 (41.3) | |
| % of total positive | 69.5 | 73.9 | 64.8 | 55.9 | 51.4 | 44.7 | 50 | 30.5 | 43.6 | 63.2 | 51.9 | 50.8 | ||
| Total samples | 481 | 433 | 392 | 388 | 249 | 161 | 118 | 118 | 133 | 212 | 235 | 242 | 3162 (100) | |
Data between April 2011‐ December 2015.
Data between January 2016 – April 2018.
Bold entries are showing the total (cumulative) number of the specified group of subjects shown in above lines.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 2.
All of the agents according to the age groups
| Age group | |||||
|---|---|---|---|---|---|
| Agents | <2, n | 2‐4, n | 5‐9, n | 10‐17, n | Total |
| Rhinovirus/enterovirus | 268 | 155 | 77 | 56 | 556 |
| Respiratory syncytial virus | 215 | 45 | 20 | 12 | 292 |
| Influenza A/B virus | 79 | 58 | 56 | 33 | 226 |
| Metapneumovirus | 49 | 35 | 14 | 6 | 104 |
| Adenovirus | 35 | 21 | 24 | 10 | 90 |
| Parainfluenza virus 3 | 59 | 12 | 7 | 4 | 82 |
| Coronavirus | 25 | 10 | 11 | 8 | 54 |
| Coronavirus (Cor‐43) | 12 | 3 | 5 | 3 | 23 |
| Coronavirus (Cor‐229E) | 4 | 2 | 0 | 2 | 8 |
| Coronavirus (HKU1) | 1 | 4 | 4 | 0 | 9 |
| Coronavirus (NL63) | 8 | 1 | 2 | 3 | 14 |
| Parainfluenza virus 1 | 21 | 11 | 3 | 2 | 37 |
| Bocavirus | 23 | 11 | 13 | 3 | 50 |
| Bordotella spp | 17 | 1 | 0 | 0 | 18 |
| Parainfluenza virus 2 | 10 | 3 | 1 | 1 | 15 |
| Parainfluenza virus 4 | 7 | 2 | 2 | 0 | 11 |
| Coinfections | 182 | 84 | 40 | 16 | 322 |
| Total positive samples, n | 990 | 448 | 268 | 151 | 1857 |
| Positive samples, % | 62.9 | 57.2 | 58 | 43.6 | 58.7 |
| Coinfections, % | 11.6 | 10.7 | 8.6 | 4.6 | 10.2 |
| Total samples, n | 1572 | 782 | 462 | 346 | 3162 |
Bold entries are showing the total (cumulative) number of the specified group of subjects shown in above lines.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Among the parainfluenza viruses, PIV‐3 was the most frequently detected subtype, followed by PIV‐1, ‐2, and ‐4, respectively (Table 1).
Bocavirus was positive in 94 cases (monoinfection 50 cases and coinfection 44 cases). Coinfections of bocaviruses with rhinovirus (15 of 44), RSV (9 of 44), and influenza A/B (5 of 44) were found to be frequent. Coronaviruses were positive in 98 cases (monoinfection 54 cases and coinfection 44 cases). HCoV OC43 was seen in 43% (42 of 98), HCoV‐NL63 in 31% (30 of 98), HCoV HKU1 in 15% (15 of 98), and HCoV 229E in 11% (11 of 98) of the patients. Coinfections were often associated with rhinovirus (17 of 44) and RSV (14 of 44). There was no positive result for HpeV during the study period.
In this study, Bordetella spp, which was the only bacterial agent in the test panel used in 2011‐2015 period. It was detected in a total of 28 cases as 18 monoinfections and 10 coinfections. The majority (96.4%; 27 of 28) of them were children younger than 3 months.
The prevalence of coinfections, where two or more agents were associated, was 10.2% (322 of 3162) in this study, detected especially in children under the age of 5 years. Prevalence was 11.6% (182 of 1572), 10.7% (84 of 782), 8.6% (40 of 462), and 4.6% (16 of 346), respectively, according to the increasing age groups (Table 2). RV/EV (32%, 103 of 322) and RSV (25.2%, 81 of 322) were responsible for the majority of the coinfections (Figure 2). Frequent coinfections were RV/EV with RSV (12.4%, 40 of 322), RV/EV with AdV (6.8%, 22 of 322), RSV with AdV (5.6%, 18 of 322), rhinovirus with coronavirus (5.3%, 17 of 322), rhinovirus with bocavirus (4.7%, 15 of 322) and RSV with coronavirus (4.3%, 14 of 322).
Figure 2.
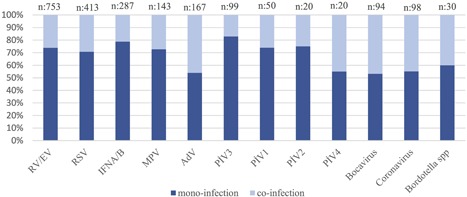
Evaluation of the monoinfection and coinfection distribution. AdV, adenovirus; EV, enterovirus; hMPV, human metapneumovirus; MPV, metapneumovirus; PIV, parainfluenza virus; RSV, respiratory syncytial virus; RV, rhinovirus
When Table 1 carefully examined two different distributions of pathogens determined, the first one was between RV/EV and AdV showing detection throughout the year (Figure 3A). The second one was among influenza A/B, RSV, and HMPV, showing a similar seasonal distribution although there were some shifts between months of different years (Figure 3B). These similar distributions make it difficult to predict the virus by the season and point out the importance of microbiological evaluation for an accurate diagnosis. Although RV/EV was detected in all months during the study period, the frequency increased in October and November as seen in Table 3.
Figure 3.
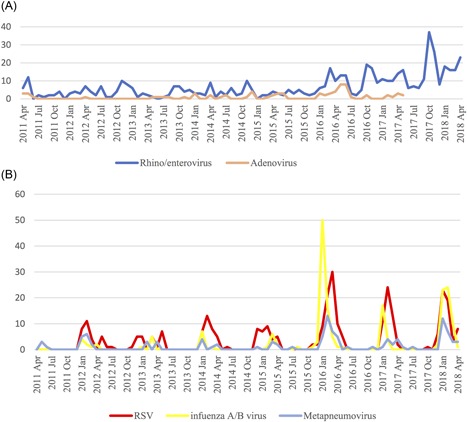
A, Distribution of rhinovirus/enterovirus and adenovirus according to the month. B, Distribution of RSV, metapneumovirus and influenza A/B according to the months. RSV, respiratory syncytial virus
Table 3.
Distribution of the rhinovirus and enterovirus according to the months
| Month | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agents | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | Total, n (%) |
| Rhinovirus/enterovirus a | 12 | 11 | 15 | 32 | 20 | 9 | 20 | 10 | 17 | 19 | 28 | 30 | 223 (40.1) |
| Rhinovirus b | 29 | 30 | 37 | 42 | 25 | 18 | 8 | 7 | 17 | 57 | 41 | 17 | 328 (58.9) |
| Enterovirus b | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 5 (0.9) |
| Total | 43 | 41 | 53 | 75 | 45 | 27 | 28 | 17 | 34 | 76 | 70 | 47 | 556 (100) |
AusDiagnostics assay results, RV and EV are grouped as one.
Fast Track Diagnostics assay results, RV and EV are separated.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
4. DISCUSSION
The role and importance of respiratory viruses have become more easily detectable by the use of nucleic acid—based tests in clinical laboratories. Thus it became possible to obtain detailed epidemiological data and to manage the patients better.
Our study retrospectively assessed the respiratory tract viruses detected in children with ARTI symptoms of 0 to 18 years between 2011‐2018. Two different assays were used during the study period. Ausdiagnostics assay covered a panel of 12 pathogens, while the Fast Track Assay tested for 21 agents. The wider coverage of pathogens resulted in a significantly higher rate of positive test results. In the study, RV/EV (17.6%) was the most frequently identified pathogen for all age groups and for every year without any seasonal difference. Using an assay enabling to separate rhinovirus from enterovirus made it possible to detect the importance of rhinovirus as the dominant type. Rhinoviruses have been reported as the most frequently detected agents in different parts of the world. In a US study using a commercial multiplex test, 66% of samples taken from pediatric cases were positive and RV was the most frequent causative agent between January 2014 and 2015. They also observed that RV was detected throughout the year with a peak in spring and autumn. Another 8‐year study from Scotland reported RV as the most common etiologic agent detected in respiratory tract samples for a group of patients with a median age of 27. Similar to the previous study, RV detected throughout the year with a peak in September. A Korean study in which the majority of the patients were children also reported RV as the number one etiologic factor.6, 12, 13
RSV (9.2%) was the second most common viral agent after RV in this study. It was particularly detected during winter and spring months with a peak between January and March. Consistent with our study, RSV is observed more frequently in winter months in many studies from different regions.14 RSV and HMPV were usually seen in children under five years of age as the cause of bronchiolitis and pneumonia. In our study, the age relation was significant for RSV and HMPV which were detected in 89% (260 of 292) and 81% (84 of 104) of children under 5 years, respectively. Similar to RSV, HMPV infections were frequently seen in winter and spring months as it was the case in our study while 90% of the HMPV cases were detected between January and April.15
IAV and IBV were detected with a frequency of 4.8% and 2.4%, respectively, and peaked between January and March. The presence of RSV and HMPV in circulation during the same period was notable, which necessitates the use of virological methods for the differential diagnosis of influenza‐like illness (ILI). Circulation of other respiratory viruses during the influenza season was also reported by the reference laboratory of the Turkish Public Health Institution with a warning of the high possibility of noninfluenza viruses causing ILI at the beginning and end of the season.16
ADVs detected in 90 cases which were mostly (64.4%) seen during spring and early summer months. Although seasonal characteristics of adenoviral infections affected by the geographical region and genotype of the virus, such infections are reported to be more common between September and February in the literature.17, 18
There are four subtypes of PIVs (1‐4) which may cause croup, pharyngitis, laryngitis, tracheobronchitis, bronchiolitis, and/or pneumonia.19 PIV‐3 was the most commonly detected subtype in our study, followed by PIV‐1, ‐2, and ‐4. PIV‐3 is detected more frequently in April, May, and October. It was not possible to detect the seasonal relation of other PIV subtypes due to a small number of cases. In other studies, PIV‐3 was also reported as the most common subtype followed other PIVs similar to our findings.20, 21 They were frequently detected during spring and early summer months.
Bocavirus, after being identified in 2005 has been isolated from 1.5% to 19% of the respiratory tract samples of children who were admitted to the hospital due to respiratory viral disease.22 Bocavirus causes coinfections up to 90% of the cases with other viruses such as influenza, rhinovirus, parainfluenza, RSV, and metapneumovirus.23, 24 The prevalence of bocavirus was 2.8% in our study with a coinfection frequency of 48.8%, mainly associated with rhinovirus/enterovirus, RSV, and influenza A/B. Infections were seen throughout the year without a clear seasonal relation although 58% (29 of 50) of cases were between January and March which is parallel to the other reported studies.25, 26, 27
Coronaviruses were identified nearly 5% of the patients, with OC43 being the most frequent subtype followed by NL63, HKU1, and 229E. Coinfections with rhinovirus or RSV were seen in almost half of the samples. These infections were detected in almost all months except June and August. It was reported that coronavirus infections peaked mainly in winter without any significant difference between the four subtypes in terms of seasonality and origin (community or hospital‐acquired) of the infection.28, 29
Bordetella spp is an agent that can be mistaken as viral infection since can lead to prolonged cough and severe infections in infants, and young children. There were only 28 patients with Bordetella infections in our study which were seen in patients less than 3‐months old, except for one. Although can be seen at any age, pertussis is more frequent and cause more severe disease in children younger than 3‐months–old because of lack of primary vaccination.30 High vaccine coverage (at least 95%) is necessary to protect children against vaccine‐preventable diseases with the herd immunity. The importance of herd immunity is nicely described by an Italian study of three cases of B. pertussis, one 16‐month old and two less than a month old children showing that reduced immunity of the child's family over the years increases the risk of infection especially in infants lack maternal immunity with a more severe clinical presentation. In Italy, vaccination coverage dropped from 97% in 2000s to 92% in the areas where the cases were reported due to the parental opposition to vaccination.31
Nucleic acid—based multiplex tests detect viral coinfections more frequently, with a prevalence up to 40% depending on the method and the study. Coinfections were detected in 10.2% of the samples in this study group. The prevalence decreased significantly at the ≥ 5 years of age (P < 0.05). The most common viruses detected in coinfections were RV/EV and RSV. Clinical importance of the viral coinfections is not clear as several studies detected no effect on the severity of the disease.32, 33
The main limitation of this study is its retrospective design. It was not possible to evaluate the effect of multiplex PCR assay on patient management since the clinical data were limited. However, the increasing number of test orders over the years suggests that test results were supportive of the clinical decisions. Also, the 8‐year–long study period allowed a better assessment of the factors affecting respiratory virus prevalence such as age and season. Some of the agents such as bocavirus, HCoV, PIV‐4, and Bordetella spp. could only be detected by one of the two tests used during the study, therefore, evaluated only for the years related commercial kit was used.
In conclusion, the RV/EV group, namely rhinovirus, was the most common virus for all age groups during the study period. The second commonly detected virus was RSV for children younger than 2 years of age, influenza A/B for children older than 5 years of age. The seasonal cycle of influenza virus is well known. The same relation was also seen for RSV and metapneumovirus. Virological diagnosis of the pathogen was important since these three viruses caused infections in the same season. Surveillance and monitoring of the annual/seasonal characteristics of the respiratory viruses are critical in predicting and detecting epidemics and pandemics. In this way, it would be possible to diagnose the correct pathogen and prevent inappropriate antimicrobial treatment.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
Appak Ö, Duman M, Belet N, Sayiner AA. Viral respiratory infections diagnosed by multiplex polymerase chain reaction in pediatric patients. J Med Virol. 2019;91:731‐737. 10.1002/jmv.25379
References
REFERENCES
- 1. Panda S, Mohakud NK, Pena L, Kumar S. Human metapneumovirus: review of an important respiratory pathogen. Int J Infect Dis. 2014;25:45‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shapiro ED. Epidemiology of acute respiratory infections. Semin Pediatr Infect Dis. 1998;9:31‐36. [Google Scholar]
- 3. Pancer KW, Gut W, Abramczuk E, Lipka B, Litwińska B. Non‐influenza viruses in acute respiratory infections among young children. High prevalence of HMPV during the H1N1V.2009 pandemic in Poland. Przegl Epidemiol. 2014;68(4):627‐632. [PubMed] [Google Scholar]
- 4. Mahony JB, Petrich A, Smieja M. Molecular diagnosis of respiratory virus infections. Crit Rev Clin Lab Sci. 2011;48(5‐6):217‐249. [DOI] [PubMed] [Google Scholar]
- 5. Zhang D, He Z, Xu L, et al. Epidemiology characteristics of respiratory viruses found in children and adults with respiratory tract infections in southern China. Int J Infect Dis. 2014;25:159‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arbefeville S, Ferrieri P. Epidemiologic analysis of respiratory viral infections mainly in hospitalized children and adults in Midwest University Medical Center after the implementation of a 14‐virus multiplex nucleic acid amplification test. Am J Clin Pathol. 2017;147(1):43‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gulen F, Yildız B, Cicek C, Demir E, Tanac R. Ten year retrospective evaluation of the seasonal distribution of agent viruses in childhood respiratory tract infections. Turk Pediatri Ars. 2014;49(1):42‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lam WY, Yeung ACM, Tang JW, et al. Rapid multiplex nested PCR for detection of respiratory viruses. J Clin Microbiol. 2007;45(11):3631‐3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wishaupt JO, Russcher A, Smeets LC, Versteegh FGA, Hartwig NG. Clinical impact of RT‐PCR for pediatric acute respiratory infections: a controlled clinical trial. Pediatrics. 2011;128:e1113‐e1120. [DOI] [PubMed] [Google Scholar]
- 10. Yun SG, Kim MY, Choi JM, et al. Comparison of three multiplex PCR assays for detection of respiratory viruses: Anyplex II RV16, AdvanSure RV, and Real‐Q RV. J Clin Lab Anal. 2017;32:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krause JC, Panning M, Hengel H, Henneke P. The role of multiplex PCR in respiratory tract infections in children. Dtsch Arztebl Int. 2014;111:639‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nickbakhsh S, Thorburn F, Von Wissmann B, McMenamin J, Gunson RN, Murcia PR. Extensive multiplex PCR diagnostics reveal new insights into the epidemiology of viral respiratory infections. Epidemiol Infect. 2016;144(10):2064‐2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ko DH, Hyun J, Kim HS, Kim JS, Song W, Kim HS. Analysis of respiratory viral infections detected using multiplex real‐time PCR in Hwaseong, Korea from 2013 to 2015. Clin Lab. 2017;63(5):1003‐1007. [DOI] [PubMed] [Google Scholar]
- 14. Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta‐analysis. The Lancet. 2010;375:1545‐1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haynes AK, Fowlkes AL, Schneider E, Mutuc JD, Armstrong GL, Gerber SI. Human metapneumovirus circulation in the United States, 2008 to 2014. Pediatrics. 2016;137(5):e20152927. [DOI] [PubMed] [Google Scholar]
- 16. Altaş AB, Bayrakdar F, Korukluoğlu G. Influenza surveillance in five consecutive seasons during post pandemic period: results from National Influenza Center, Turkey. Mikrobiyol Bul. 2016;50(3):401‐417. [DOI] [PubMed] [Google Scholar]
- 17. Erdman D, Xu W, Gerber S, et al. Molecular epidemiology of adenovirus type 7 in the United States, 1966‐2000. Emerg Infect Dis. 2002;8(3):269‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheng CC, Huang LM, Kao CL, et al. Molecular and clinical characteristics of adenoviral infections in Taiwanese children in 2004‐2005. Eur J Pediatr. 2008;167(6):633‐640. [DOI] [PubMed] [Google Scholar]
- 19. Branche A, Falsey A. Parainfluenza virus infection. Semin Respir Crit Care Med. 2016;37(4):538‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fry AM, Curns AT, Harbour K, Hutwagner L, Holman RC, Anderson LJ. Seasonal trends of human parainfluenza viral infections: United States, 1990‐2004. Clin Infect Dis. 2006;43(8):1016‐1022. [DOI] [PubMed] [Google Scholar]
- 21. Zhao H, Harris RJ, Ellis J, Donati M, Pebody RG. Epidemiology of parainfluenza infection in England and Wales, 1998‐2013: any evidence of change? Epidemiol Infect. 2017;145(6):1210‐1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Allander T. Human bocavirus. J Clin Virol. 2008;41:29‐33. [DOI] [PubMed] [Google Scholar]
- 23. Martin ET, Fairchok MP, Kuypers J, et al. Frequent and prolonged shedding of bocavirus in young children attending daycare. J Infect Dis. 2010;201:1625‐1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moreno B, Abrego L, Carrera JP, et al. Detection of human bocavirus type 1 infection in Panamanian children with respiratory illness. J Med Virol. 2016;88:389‐394. [DOI] [PubMed] [Google Scholar]
- 25. Choi EH, Lee HJ, Kim SJ, et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000‐2005. Clin Infect Dis. 2006;43(5):585‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bastien N, Chui N, Robinson JL, et al. Detection of human bocavirus in Canadian children in a 1‐year study. J Clin Microbiol. 2007;45(2):610‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brieu N, Guyon G, Rodière M, Segondy M, Foulongne V. Human bocavirus infection in children with respiratory tract disease. Pediatr Infect Dis J. 2008;27:969‐973. [DOI] [PubMed] [Google Scholar]
- 28. Varghese L, Zachariah P, Vargas C, et al. Epidemiology and clinical features of human coronaviruses in the pediatric population. J Pediatric Infect Dis Soc. 2017;7:151‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Litwin CM, Bosley JG. Seasonality and prevalence of respiratory pathogens detected by multiplex PCR at a tertiary care medical center. Arch Virol. 2014;159(1):65‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Long SS. Pertussis (Bordetella pertussis and B. parapertussis) In: Behrman RE, Kliegman RM, Jenson HB, eds. Nelson Textbook of Pediatrics. 17th ed Philadelphia, PA: Saunders Company; 2004: pp. 908‐912. [Google Scholar]
- 31. Brindicci G, Carboni D, Genga R, et al. Report on three cases of pertussis in the Urbino area (Italy). Infez Med. 2018;26(1):85‐88. [PubMed] [Google Scholar]
- 32. Lim FJ, de Klerk N, Blyth CC, Fathima P, Moore HC. Systematic review and meta‐analysis of respiratory viral coinfections in children. Respirology. 2016;21(4):648‐655. [DOI] [PubMed] [Google Scholar]
- 33. Scotta MC, Chakr VCBG, De Moura A, et al. Respiratory viral coinfection and disease severity in children: a systematic review and meta‐analysis. J Clin Virol. 2016;80:45‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]


