Abstract.
Background. The pathophysiology of severe acute respiratory syndrome (SARS) is at present poorly understood, but advanced age and serum total lactate dehydrogenase (LD) activity >300 U L−1 have been associated with adverse clinical outcomes. Blood leucocytes and lymphocyte subsets were reported to decrease, respectively, in 47% and up to 100% of 38 patients in Beijing. However, their prognostic implications have not been thoroughly investigated.
Objective. To investigate serum total LD, LD isoenzymes, and other parameters including blood lymphocyte subsets as prognostic indicators in SARS patients for adverse clinical outcomes in terms of admission to intensive care unit (ICU) and death.
Design. Retrospective analysis.
Subjects and methods. A total of 109 patients with a clinical diagnosis of SARS according to the modified World Health Organization case definition of SARS were recruited from two major acute hospitals in Hong Kong. They were either involved in the initial outbreak of SARS, or cases from the community outbreak of Amoy Gardens between 10 March and 5 May 2003. The clinical diagnosis was subsequently confirmed by serological test and/or molecular analysis. Serum total LD and LD isoenzyme activities, complete blood picture with total leucocyte count and differential counts, absolute counts of CD3+, CD4+, CD8+, natural killer cells and B lymphocytes were measured daily upon admission. Receiver operating characteristic curve analysis was used to determine and compare different cut‐offs for various biochemical and immunological parameters at peak serum total LD concentration in predicting adverse clinical outcomes.
Results. Of a total of 109 patients, 41 were admitted to ICU and 42 died. Of 42 fatal patients, 24 died in ICU and 18 died in general medical wards. Age was found to be an independent prognostic indictor for death with an area under curve (AUC) of 0.96 [95% confidence interval (CI) = 0.90–0.99] but not for admission to ICU [AUC = 0.61 (CI = 0.51–0.70)]. Whilst serum total LD could only achieve AUC of 0.68 (CI = 0.59–0.77) for predicting death, LD1 isoenzyme was found to be the best biochemical prognostic indicator with AUC of 0.84 (CI = 0.75–0.90), sensitivity of 62% (CI = 46–76%), specificity of 93% (CI = 83–98%) at cut‐off activity of ≥80 U L−1. CD3+, CD4+, CD8+ and natural killer cell counts were promising immunological prognostic indicators for predicting admission to ICU with AUC of 0.94 (CI = 0.86–0.98), 0.91 (CI = 0.81–0.96), 0.93 (CI = 0.85–0.98), and 0.87 (CI = 0.76–0.94), respectively.
Conclusions. Apart from age, serum LD1 activity was the best prognostic indicator for predicting death in patients with SARS compared with serum total LD activity, haemoglobin concentration, leucocyte and lymphocyte counts. Its release could possibly be from blood erythrocytes and body tissues other than the myocardium. Blood CD3+, CD4+, CD8+ and natural killer cell counts were found to be good prognostic indicators for predicting admission to ICU in patients with SARS compared with age, leucocyte count and LD isoenzymes. The suppressed CD3+, CD4+, CD8+, and natural killer cell counts were also implicated in the pathophysiology of SARS. Patients with increased serum LD1 should be closely monitored to ensure prompt management, and preparation for admission to ICU could be planned ahead for patients with suppressed lymphocyte subsets.
Keywords: lactate dehydrogenase isoenzyme, lymphocyte subsets, multiple ROC analysis, pathophysiology, prognosis, severe acute respiratory syndrome (SARS)
Introduction
Severe acute respiratory syndrome (SARS) is a potentially lethal illness caused by the infection of a newly discovered coronavirus [1]. It has caused outbreak infections in Hong Kong and worldwide [2, 3, 4]. The World Health Organization (WHO) has revised the case fatality ratio from the previous experience in Vietnam of 8% to that of 15% in Hong Kong on 7 May 2003 [5]. Influencing these mean fatality rates, there is a disproportional increase for patients aged 65 or older, many with co‐morbidity [2]. Therefore, age itself can no doubt function as an independent risk factor for fatality. However, there is no information about other biochemical and immunological markers for helping clinicians to predict adverse clinical outcomes other than serum total lactate dehydrogenase (LD) activity alone [2]. Blood leucocytes and lymphocyte subsets were reported to decrease, respectively, in 47% and up to 100% of a 38‐patient cohort in Beijing [6]. However, their prognostic implications have not been thoroughly investigated. This study was conducted to assess the prognostic values of these parameters in SARS.
Subjects and methods
Study population
All patients who met a modified WHO case definition of SARS [7] admitted to two major acute hospitals in Hong Kong from 10 March to 5 May 2003 were included in the study. The two major acute hospitals were the Prince of Wales Hospital (PWH) and United Christian Hospital (UCH) in Hong Kong. PWH is located in the New Territories East region where the initial outbreak affected 69 health care professionals [2]. UCH is situated in the Kowloon East region within the catchment area of the Amoy Gardens community outbreak cases [8].
Definition of SARS
The clinical case definition for SARS was fever with body temperature of 38 °C or higher, cough or shortness of breath, and new pulmonary infiltrates on chest radiograph, with either a history of exposure to a confirmed patient with SARS or a lack of response to empirical antimicrobial therapy for typical and atypical pneumonia including beta‐lactams, macrolides, fluoroquinolones or tetracyclines [7]. All patient diagnoses were subsequently confirmed either serologically by detection of antibody against the SARS‐associated coronavirus (SARS‐CoV) or demonstration of SARS‐CoV using reverse‐transcriptase polymerase chain reaction technique [9].
Treatment of SARS
The SARS patients were given the following treatment according to our protocol in Hong Kong [10]. On the first day of admission, ribavirin was given either orally at 1.2 g three times daily after a loading dose of 2.4 g or intravenously 400 mg every 8 h for a complete 14‐day course. Levofloxacin or cefotaxime was supplemented to cover any bacterial chest infection. If there was persistent fever >38 °C at the third day of admission, a maintenance steroid therapy would be started using either oral prednisolone 0.5–1.0 mg kg−1 day−1 or intravenous hydrocortisone 100 mg every 8 h with step‐down titration starting at the third week of admission. For patients with clinical deterioration including desaturation (oxygen saturation <90% using pulse oximetry) or development of new infiltrates in chest radiograph, pulse steroid using methylprednisolone 0.5 g daily for 3 days will be given.
Blood sample collection
Serial ethylenediaminetetraacetic acid (EDTA) and clotted blood samples were collected daily from the first day of hospital admission, through acute disease at the general medical ward and ICU (if applicable), till just before death, or discharge to home, or to another convalescent hospital.
Laboratory tests
The EDTA blood samples were used for assessment of complete blood picture including total and differential leucocyte counts (GEN‐S blood cell counter; Beckman‐Coulter Inc., Miami, FL, USA). Lymphocyte subsets were enumerated by flow cytometry (MultiTEST IMK kit, four‐colour FACSCalibur Flow Cytometer; Becton‐Dickinson Corp., San Jose, CA, USA). The immune fluorescence reagent contained monoclonal antibodies against cluster of differentiation (CD) antigens including CD3 (clone SK7, total T lymphocytes), CD4 (clone SK3, T‐helper lymphocytes), CD8 (clone SK1, T‐suppressor lymphocytes and cytotoxic T lymphocytes), CD56 (clone NCAM 16.2, natural killer cells) and CD19 (clone SJ25 C1, B lymphocytes).
After centrifugation of clotted blood samples at 1600 g for 10 min, sera were aliquoted within a bio‐safety cabinet for the measurement of serum total LD activity using lactate‐to‐pyruvate enzymatic assay (DP Modular Analyser; Roche Diagnostics Corp., Indianapolis, IN, USA). Patient sera with peak LD activity were subjected to isoenzyme study by agarose gel electrophoresis followed by densitometry scanning (Paragon Electrophoresis System; Beckman‐Coulter Inc., LA, CA, USA). Isoenzyme (LD1–LD5) activities were calculated by multiplying total LD activity with the respective scanning percentages. Serum cardiac troponin T (cTnT) concentration was measured for all patients using the concurrent serum samples for LD isoenzymes to determine their possible origin (Elecsys 2010 Analyser; Roche Diagnostics Corp.). Derived parameters including blood CD4+ to CD8+ cell count ratio (CD4+/CD8+) and LD1 to LD2 isoenzyme ratio (LD1/LD2) were computed using their respective measured data.
Statistical analysis
Multiple receiver operating characteristic (ROC) curve comparison and nonparametric Kruskal–Wallis test were applied using the MedCalc 7.1 programme (http://www.medcalc.be). For ROC curve analysis, the programme chooses the respective cut‐off values for all input variables automatically, so that false negative and false positive rates were minimized [11]. Positive and negative likelihood ratios were also determined at the chosen cut‐off values by the programme. Confidence intervals of the central 95% distribution for AUC used in ROC analysis, sensitivity and specificity at cut‐off values, and medians of various biochemical and immunological markers were also computed by the programme. A P‐value of <0.05 was chosen to be of statistical significance. All probabilities were two‐tailed.
Results
A total of 109 patients were included in the present study. There were 19 patients from UCH, and 90 patients from PWH; with 43 males and 66 females aged 21–100 years with a median of 43 [inter‐quartile range (IQR) = 29–69]. Five patients from PWH were not given ribavirin and pulse steroid due to regression of fever before the establishment of the treatment protocol. Oral and intravenous ribavirin were given to 39 and 65 patients, respectively. Oral prednisolone and intravenous hydrocortisone were given to 46 and 58 patients, respectively. There were a total of 80 patients who received pulse steroid. The median cumulative dose of pulse methylprednisolone was 2.0 g (IQR = 2.0–4.0). Of a total of 109 patients, 41 were admitted to ICU and 42 died. Of a total of 42 fatal patients, 24 died in ICU and 18 died in general medical wards. Details of patient demographics, therapeutic intervention and adverse clinical outcomes are summarized in Table 1.
Table 1.
Demographic information, therapeutic intervention and adverse clinical outcomes of 109 clinically confirmed SARS patients in the study
| Prince of Wales Hospital (n = 90) | United Christian Hospital (n = 19) | |
|---|---|---|
| Sex (M : F) | 34 : 56 | 9 : 10 |
| Age [median (IQR)] | 44 (27–73) | 42 (33–54) |
| Co‐morbiditya (M : F) | 23 : 9 | 2 : 4 |
| Admission to ICU (M : F) | 13 : 9 | 9 : 10 |
| Death (M : F) | 24 : 8 | 5 : 5 |
| Ribavirin therapy (Nil : p.o. : i.v.) | 5 : 39 : 46 | 0 : 0 : 19 |
| Steroid maintenance therapy (Nil : P : HC) | 5 : 46 : 39 | 0 : 0 : 19 |
| Pulse steroid therapy [median (IQR)]b | 2.0 (1.5–3.0) | 5.0 (5.0–5.0) |
SARS, severe acute respiratory syndrome; IQR, inter‐quartile range; Nil, no drug given; p.o., 1.2 g oral ribavirin three times daily after an oral loading of 2.4 g; i.v., 400 mg intravenous ribavirin every 8 h daily; P, oral prednisolone at 0.5–1.0 mg kg−1 day−1; HC, 100 mg intravenous hydrocortisone three times daily. aCo‐morbidity included diabetes mellitus, significant cardiovascular, respiratory, hepato‐biliary, renal, haematological and malignant diseases. bTotal accumulated dose in gram(s) of methylprednisolone from admission to discharge or death.
Multiple ROC curve comparison for the prediction of adverse clinical outcomes is summarized in Table 2. Age was found to be an independent prognostic indictor for predicting death with AUC of 0.96 [95% confidence interval (CI) = 0.90–0.99] but not for predicting admission to ICU with AUC of 0.61 (CI = 0.51–0.70). Whilst serum total LD activity could only achieve AUC of 0.68 (CI = 0.59–0.77) for predicting death, LD1 was found to be the best biochemical prognostic indicator for predicting death with AUC of 0.84 (CI = 0.75–0.90) at cut‐off activity of ≥80 U L−1. There were statistically significant differences in AUC for death prediction for serum LD1 activity compared with the rest of the LD isoenzymes (LD2–LD5), LD1/LD2 ratio, cTnT concentration, blood haemoglobin concentration, total leucocyte and lymphocyte counts. The multiple ROC curve comparison for predicting death is presented in Fig. 1.
Table 2.
Multiple ROC curve comparison of various prognostic indictors for the prediction of adverse clinical outcomes
| Death | Admission to ICU | |||
|---|---|---|---|---|
| AUC (95% CI) | Cut‐off (LR+; LR−) | AUC (95% CI) | Cut‐off (LR+; LR−) | |
| Age | 0.96 (0.90–0.99)a | ≥61 (54.2; 0.19) | 0.61 (0.51–0.70)b | ≥41 (1.6; 0.57) |
| LD (87–213 U L−1) | 0.68 (0.59–0.77)a | ≥352 (2.8; 0.56) | 0.62 (0.52–0.71)b | ≥272 (1.7; 0.51) |
| LD1 (15–66 U L−1) | 0.84 (0.75–0.90) | ≥80 (8.3; 0.41) | 0.58 (0.48–0.67)b | ≥64 (1.4; 0.70) |
| LD2 (30–102 U L−1) | 0.80 (0.72–0.87) | ≥109 (3.8; 0.32) | 0.66 (0.57–0.75)b | ≥99 (2.0; 0.45) |
| LD3 (13–62 U L−1) | 0.65 (0.55–0.74)a | ≥86 (4.8; 0.69) | 0.63 (0.53–0.72)b | ≥54 (1.7; 0.57) |
| LD4 (<20 U L−1) | 0.65 (0.55–0.73)a | ≤7 (6.1; 0.59) | 0.53 (0.43–0.62)b | ≤5 (2.8; 0.79) |
| LD5 (<21 U L−1) | 0.66 (0.56–0.75)a | ≤8 (22.3; 0.68) | 0.52 (0.42–0.61)b | ≥34 (1.6; 0.70) |
| LD1/LD2 ratio (0.4–0.9) | 0.51 (0.41–0.61)a | ≥1.2 (1.2; 0.61) | 0.65 (0.55–0.74)b | ≥1.5 (2.0; 0.59) |
| cTnT (AMI cut‐off≥ 0.1 μg L−1) | 0.67 (0.58–0.76)a | >0.03 (23.9; 0.65) | 0.58 (0.46–0.70)b | ≤0.08 (1.1; 0.41) |
| Haemoglobin concentrationc | 0.65 (0.55–0.74)a | ≤10.6 (3.7; 0.55) | 0.65 (0.55–0.74)b | ≤10.6 (2.6; 0.64) |
| Leucocyte count (4.0–10.8 × 103 μL−1) | 0.58 (0.48–0.67)a | ≥9.9 (2.2; 0.80) | 0.54 (0.44–0.64)b | ≥4.8 (1.3; 0.55) |
| Lymphocyte count (0.8–3.2 × 103 μL−1) [27] | 0.64 (0.54–0.73)a | ≤0.5 (2.0; 0.63) | 0.57 (0.47–0.67)b | ≤1.0 (1.2; 0.38) |
| CD3+ cell count (690–2540 μL−1) | ND | ND | 0.94 (0.86–0.98) | ≤253 (6.0; 0.10) |
| CD4+ cell count (410–1590 μL−1) | ND | ND | 0.91 (0.81–0.96) | ≤131 (3.5; 0.11) |
| CD8+ cell count (190–1140 μL−1) | ND | ND | 0.93 (0.85–0.98) | ≤79 (10.1; 0.14) |
| CD4+/CD8+ ratio (0.8–3.5) [27] | ND | ND | 0.59 (0.47–0.71)b | ≥1.7 (2.6; 0.66) |
| CD19+ (B lym) cell count (90–660 μL−1) | ND | ND | 0.74 (0.62–0.84) | ≤61 (4.1; 0.44) |
| CD56+ (NK) cell count (90–590 μL−1) | ND | ND | 0.87 (0.76–0.94) | ≤45 (6.1; 0.24) |
Normal range of biochemical and immunological parameters are shown in brackets. AUC, area under ROC curve; LR+, positive likelihood ratio; LR−, negative likelihood ratio; B lym, B lymphocyte; NK, natural killer; ND, not done due to inadequate number of fatal cases in statistical analysis. aStatistical difference in AUC for predicting death compared with LD1. bStatistical difference in AUC for predicting admission to ICU compared with either CD3+, CD4+, CD8+ or CD56+ cell count. cMale (13.2–16.7 g dL−1), female (11.5–14.3 g dL−1).
Figure 1.
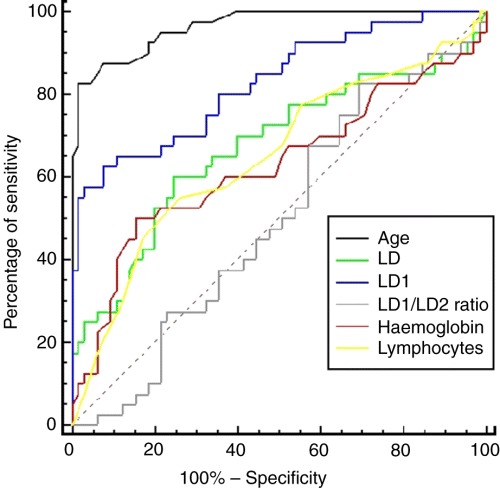
Multiple ROC curve comparison of age, serum total LDH activity, serum LD1 activity, serum LD1/LD2 ratio, blood total haemoglobin concentration, and blood absolute lymphocyte count for the prediction of death.
For the prediction of admission to ICU, all CD3+, CD4+, CD8+ and natural killer cell counts but not B lymphocytes were promising immunological prognostic indicators with AUC of 0.94 (CI = 0.86–0.98), 0.91 (CI = 0.81–0.96), 0.93 (CI = 0.85–0.98) and 0.87 (CI = 0.76–0.94), respectively. They are superior to age, serum total LD and LD isoenzymes activities, blood haemoglobin concentration, total leucocyte and lymphocyte counts, as well as CD4+/CD8+ ratio, as predictors for admission to ICU with statistical significant differences in AUC. The multiple ROC curve comparison for predicting death is presented in Fig. 2.
Figure 2.
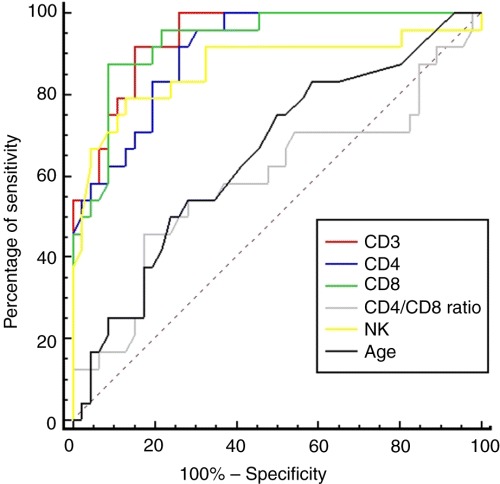
Multiple ROC curve comparison of age, serum total LDH activity, blood absolute CD3+, CD4+ and CD8+ cell counts, and the CD4+/CD8+ ratio for the prediction of admission to intensive care unit.
Discussion
The prognostic significance of serum total LD in SARS has been repeatedly demonstrated by several studies in Hong Kong [2, 12, 13]. This prompted us to further investigate the prognostic significance of LD isoenzymes in the present study. LD is a cytoplasmic zinc‐containing enzyme of ubiquitous tissue distribution in mammalian species. The human LD is a tetramer that has a molecular weight of about 140 kDa. For such a sizeable molecule to be released into the extracellular fluid through the cytoplasmic membrane, it must be due either to normal cellular turnover such as apoptosis, or tissue destruction such as acute myocardial infarction (AMI). Five LD isoenzymes can be found in human serum: LD1–LD5 of different tissue predominance [14]. In the myocardium, LD1 contributes to about 60% of the total LD activity; in blood erythrocytes, the contribution is about 40% [14]. These are the two major sources of LD1 found in human serum. In SARS‐associated elevation of serum total LD activity, we found LD1 to be the major contributor compared with other LD isoenzymes as evidenced by its high prognostic value in predicting death.
Whilst full evaluation of all the five LD isoenzymes requires electrophoresis, routine monitoring of serum LD1 is as simple as total LD assay, and can be easily performed by automated analysis in most laboratories [15].
The increase of serum LD1 in SARS is thought to originate from blood erythrocytes and body tissues other than the myocardium, as evidenced by only 9% of all samples had cardiac troponin T (cTnT) ≥0.1 μg L−1 (AMI cut‐off). Amongst these 10 patients with raised serum cTnT concentrations, eight of them had documented co‐morbidity related to the cardiovascular system including hypertension, chronic rheumatic heart disease, congestive heart failure, atrial fibrillation, complete heart block, a previous history of AMI and diabetes mellitus.
When serum cTnT concentration was subject to ROC curve analysis with serum LD1 activity, the AUC for predicting death was only 0.67 (CI = 0.58–0.76). There is statistical difference in AUC between serum cTnT concentration and LD1 activity for predicting death (Table 2). Moreover, after exclusion of all patients (n = 10) with raised serum cTnT concentrations, a repeat analysis of the data set still showed that serum LD1 activity could predict death at AUC of 0.81 (CI = 0.71–0.88). The cut‐off activity remained to be ≥80 U L−1 with sensitivity of 56% (CI = 38–74%), specificity of 93% (CI =83–98%), and positive and negative likelihood ratios of 7.9 and 0.45, respectively. In addition, there was no significant difference in LD1/LD2 ratio between the deceased and survivors (P = 0.89) and LD1/LD2 is a poor predictor for death with AUC of 0.51 (CI = 0.41–0.61). As the LD1/LD2 ratio is increased in myocardial damage, our findings make the origin of serum LD1 from the myocardium even more unlikely.
Increase in serum LD activity was postulated to be a result of massive tissue destruction during the acute phase of SARS‐CoV infection with immune hyperactivity [16]. As a further support to this hypothesis, our group has recently shown that there was a cytokine and chemokine storm with a significant elevation of T‐helper cell cytokine interferon‐γ, inflammatory cytokine interleukin (IL)‐1, IL‐6, IL‐12, neutrophil chemokine IL‐8, monocyte chemoattractant protein‐1, and Th1 interferon‐γ‐inducible protein‐10 during the early phase of SARS‐CoV infection [17]. In contrast to SARS, there exists no previous report on massive tissue destruction or increase in serum LD activity in H5N1 influenza A virus infection, although both infections cause lymphopenia [2, 18]. The latter absence of massive tissue destruction could be accounted by tissue tropism of the avian flu virus towards lung parenchyma predominantly [19] with diminished cytokine production [20].
As LD1 is the major contributor of elevated LD in our SARS patients, it is likely that the former has also originated from tissue destruction. However, as LD1 is abundantly distributed in erythrocytes, the haemolytic effect of ribavirin [21] cannot be excluded as another possible cause. The Centers for Disease Control and Prevention (CDC) indicated online on 13 May 2003 that preliminary in vitro experiments had suggested that ribavirin did not inhibit growth or cell‐to‐cell spread of the new coronavirus tested [22]. However, at the time of SARS outbreak in Hong Kong, there was no other known effective antiviral agent against the SARS‐CoV. It was definitely unethical to withhold the only antiviral treatment available with the requirement of using continuous and massive pulse corticosteroid therapy at the same time. Later, Knowles et al. reported that the use of high‐dose ribavirin was associated with significant haemolytic anaemia and prolonged hospital stay in a case–control study [23]. The efficacy and toxicity of ribavirin therapy against SARS requires further elucidation with randomized clinical trials.
Markers of lymphocyte subsets including CD3+, CD4+, CD8+ and CD56+ cell counts were promising indicators for predicting admission to ICU. They represent lymphocyte sub‐populations, namely, total (CD3+), T‐helper (CD4+) and cytotoxic T (CD8+) lymphocytes, as well as natural killer (CD56+) cells that are much involved in the development of humoral and cytotoxic immunity against viral infection. A marked reduction in their absolute counts could possibly be due to direct viral cytotoxicity. After steroid therapy, these immunological markers started to rise from their trough values [24]. The suppressed CD3+, CD4+, CD8+ and CD56+ cell counts may be implicated in the pathophysiology of SARS. In acquired immunodeficiency syndrome (AIDS), only a selected population of T‐lymphocytes (T‐helper cells) is attacked and destroyed by the human immunodeficiency virus (HIV). There exist published recommendations on the measurement of CD4+ cell counts by CDC to monitor the disease course and therapeutic efficacy for HIV infection and AIDS [25]. During the initial outbreak of SARS in PWH, we encountered difficulty in the allocation of limited number of ICU beds because SARS patients requiring ICU care could be as high as 20% [26]. Therefore, measurement of the above immunological markers should be useful in the planning and management of SARS patients, in particular, the estimation of ICU bed requirement.
In conclusion, serum LD1 activity was found to be a good prognostic indicator for predicting death in patients with SARS compared with serum total LD activity, haemoglobin concentration, leucocyte and lymphocyte counts. Its release could possibly be from blood erythrocytes and body tissues other than the myocardium. Routine monitoring of serum LD1 is as simple as total LD assay. Close monitoring of SARS patients using serum LD1 should be performed to ensure prompt management. Blood CD3+, CD4+, CD8+ and natural killer cell counts were found to be good prognostic indicators for predicting admission to ICU in patients with SARS compared with age, leucocyte count and LD isoenzymes. The suppressed CD3+, CD4+, CD8+ and natural killer cell counts were also implicated in the pathophysiology of SARS. Preparation for admission to ICU could be planned ahead for patients with suppressed lymphocyte subsets.
Conflict of interest statement
We are not aware of any conflicts of interest.
Acknowledgements
We are indebted to all medical, nursing and supportive health care workers in Hong Kong, particularly the late Dr Y M Tse, Dr H Y Cheng, Mr W K Lau, Ms H M Tang, Ms K Y Lau, and Ms K T Wong for their courageous, unselfish and altruistic contributions to fighting against this deadly disease. Technical support from Ms H Y P Iu, Mr D C W Leung and Dr R C K Cheung is gratefully acknowledged. We thank Ms Kelly Cheng of GreaterChina Technology Group Ltd for donation of a multi‐fluorescence flow cytometer for SARS research.
References
- 1. Peiris JSM, Lai ST, Poon LLM et al. Severe acute respiratory syndrome (SARS) is associated with a coronavirus. Lancet 2003; 361: 1319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee N, Hui D, Wu A et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med 2003; 348: 1986–94. [DOI] [PubMed] [Google Scholar]
- 3. Booth CM, Matukas LM, Tomlinson GA et al. Clinical features and short‐term outcomes of 144 patients with SARS in the Greater Toronto Area. JAMA 2003; 289: 2801–9. [DOI] [PubMed] [Google Scholar]
- 4. Anonymous . Outbreak of severe acute respiratory syndrome ‐ worldwide, 2003. MMWR 2003; 52: 226–8. [PubMed] [Google Scholar]
- 5. World Health Organization . Severe acute respiratory syndrome (SARS) – multi‐country outbreak – update 49. http://www.who.int/csr/don/2003_05_07a/en/ (accessed 19 May 2003).
- 6. Cui W, Fan Y, Wu W et al. Expression of lymphocytes and lymphocyte subsets in patients with severe acute respiratory syndrome. Clin Infect Dis 2003; 37: 857–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization . Severe acute respiratory syndrome (SARS). Wkly Epidemiol Rec 2003; 78: 81–3. [PubMed] [Google Scholar]
- 8. Tomlinson B, Cockram C. SARS: experience at Prince of Wales Hospital, Hong Kong. Lancet 2003; 361: 486–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization . PCR primers for SARS developed by WHO Network Laboratories. http://www.who.int/csr/sars/primers/en/print/html (accessed 20 September 2003).
- 10. Wong GWK, Hui DSC. Severe acute respiratory syndrome (SARS): epidemiology, diagnosis and management. Thorax 2003; 58: 558–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chan MHM, Chow KM, Chan ATC et al. Quantitative analysis of pleural fluid cell‐free DNA as a tool for the classification of pleural effusions. Clin Chem 2003; 49: 740–5. [DOI] [PubMed] [Google Scholar]
- 12. Choi KW, Chau TN, Tsang O et al. Outcomes and prognostic factors in 267 patients with severe acute respiratory syndrome in Hong Kong. Ann Intern Med 2003; 139: 715–23. [DOI] [PubMed] [Google Scholar]
- 13. Chan JWM, Ng CK, Chan YH et al. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS). Thorax 2003; 58: 686–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lott JA, Nemesánszky E. Lactate dehydrogenase In: Lott JA, Wolf PL, eds. Clinical Enzymology: A Case‐orientated Approach. New York: Field, Rich and Associates, Inc., 1986; 227–9. [Google Scholar]
- 15. Onigbinde TA, Wu AH, Johnson M et al. Clinical evaluation of an automated chemical inhibition assay for lactate dehydrogenase isoenzyme 1. Clin Chem 1990; 36: 1819–22. [PubMed] [Google Scholar]
- 16. Tsui PT, Kwok ML, Yuen H et al. Severe acute respiratory syndrome: clinical outcomes and prognostic correlates. Emerg Infect Dis 2003; 9: 1064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong CK, Lam CWK, Wu AKL et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 2004; 136: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yuen KY, Chan PKS, Peiris M et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 1998; 351: 467–71. [DOI] [PubMed] [Google Scholar]
- 19. Rimmelzwaan GF, Kuiken T, van Amerongen G et al. Pathogenesis of influenza A (H5N1) virus infection in a primate model. J Virol 2001; 75: 6681–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tumpey TM, Lu X, Morken T et al. Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. J Virol 2000; 74: 6105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Franceschi L, Fattovich G, Turrini F et al. Haemolytic anemia induced by ribavirin therapy in patients with chronic hepatitis C virus infection: role of membrane oxidative damage. Hepatology 2000; 31: 997–1004. [DOI] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention . Severe acute respiratory syndrome (SARS) – Is the use of ribavirin (or other antiviral drugs) effective in the treatment of patients with SARS? http://www.cdc.gov/ncidod/sars/qa/illness.htm (accessed24 May 2003).
- 23. Knowles SR, Phillips EJ, Dresser L et al. Common adverse events associated with the use of ribavirin for severe acute respiratory syndrome in Canada. Clin Infect Dis 2003; 37: 1139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wong RSM, Wu A, To KF et al. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ 2003; 326: 1358–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mandy FF, Nicholson JKA, McDougal JS. Guidelines for performing single‐platform absolute CD4+ T‐cell determinations with CD45 gating for persons infected with human immunodeficiency virus. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5202a1.htm (accessed 24 May 2003). [PubMed]
- 26. Lapinsky SE, Hawryluck L. ICU management of severe acute respiratory syndrome. Intensive Care Med 2003; 29: 870–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hannet I, Erkeller‐Yuksel F, Lydyard P et al. Developmental and maturational changes in human blood lymphocyte populations. Immunol Today 1992; 13: 215–8. [DOI] [PubMed] [Google Scholar]


