Abstract
Dental disease due to osteoclast over‐activity reaches epidemic proportions in older domestic cats and has also been reported in wild cats. Feline osteoclastic resorptive lesions (FORL) involve extensive resorption of the tooth leaving it liable to root fracture and subsequent tooth loss. The aetio‐pathogenesis of FORL is not known. Recent work has shown that systemic acidosis causes increased osteoclast activation and that loci of infection or inflammation in cat mouth are likely to be acidotic. To investigate this, we generated osteoclasts from cat blood and found that they formed in large numbers (∼400) in cultures on bovine cortical bone slices. Acidosis caused an increase in the size of cells—in cultures maintained up to 14 days at basal pH 7.25, mean osteoclast area was 0.01 ± 0.003 mm2, whereas an 8.6‐fold increase was observed in cells cultured between 11 and 14 days at pH 7.15 (0.086 ± 0.004 mm2). Acidosis caused a modest increase in the number of osteoclasts. Exposure to pH 6.92 exhibited a 5‐fold increase in the area of bone slices covered by resorption lacunae (∼70% bone slice resorbed). In line with this finding, significant increases were observed in the expression of cathepsin K and proton pump enzymes (both approximately 3‐fold) that are key enzymes reflective of resorptive activity in osteoclasts. These results demonstrate that acidosis is a major regulator of osteoclast formation and functional activation in the cat, and suggest that local pH changes may play a significant role in the pathogenesis of FORL. J. Cell. Physiol. 213: 144–150, 2007. © 2007 Wiley‐Liss, Inc.
As veterinary science has developed, it has been realized that musculo‐skeletal diseases are a significant cause of morbidity and mortality (Leonard and Tillson, 2001), and several conditions involve increased bone resorption by osteoclasts. These include; metabolic bone diseases (e.g., hypervitaminosis A, nutritional, and secondary hyperparathyroidism) (Clark, 1970; Barber and Elliott, 1998), neoplasia (Heldmann et al., 2000), arthritis (Bennett, 1995), disuse osteopenia and non‐union fractures (Hill, 1977). “Feline osteoclastic resorption lesions” (FORL) (Okuda and Harvey, 1992; Reiter, 1998; Lommer and Verstraete, 2000)—external resorptive lesions or “neck” lesions of the tooth—are common in domestic cats. The characteristic lesions leave an “apple core” shape that is liable to root fracture and tooth loss with subsequent pain and morbidity (Lyon, 1992; Gengler et al., 1995; Heaton et al., 2004). Epidemiological studies have shown that up to 75% of cats are affected with at least one lesion during life, and there appears to be an increased incidence of disease after 4 years of age. FORL initiate on the external surface of the root, and extend to involve dentine and enamel and rarely involve the pulp, except in advanced stages of the disease (Okuda and Harvey, 1992). At early stages of FORL, lesions appear as small, localized pits at the gingival margin of the crown of the tooth (Okuda and Harvey, 1992; DeLaurier et al., 2005). Resorption is associated with gingival inflammation and proliferation, which may involve the presence of extensive dental plaque, and the formation of vascular granulation tissue filling the expanding lesion. At advanced stages of disease, the lesion extends into the underlying dentine, and the tooth structure is significantly resorbed. Ultimately, the crown fractures, and the remaining fragments of root are resorbed (Okuda and Harvey, 1992; Reiter, 1998; DeLaurier et al., 2005). Histological studies have demonstrated the presence of large numbers of osteoclasts on the external surface of the tooth root at early stages of the disease, whereas few are detected at advanced stages of disease and during the initiation of repair response (Okuda and Harvey, 1992).
The consensus is that FORL is not related to caries lesions or to periodontal diseases. There is no equivalent condition in other carnivores, although FORL have been reported in large wild cat species (Gengler et al., 1995; Okuda et al., 1995; Berger et al., 1996). In man, a similar, though rare, condition has been reported and is described as “idiopathic resorption” (Moody and Muir, 1991). The specific underlying factor or factors involved in initiating pathological tooth resorption in the cat remain unknown (Reiter, 1998). Many factors have been implicated, such as dietary texture, mechanical stress, dietary deficiencies, excessive vitamin A intake, periodontal disease, developmental tooth defects, breeding, and local and systematic viral disease (e.g. FCV, feline calicivirus; FcoV, feline coronavirus; FeLV, feline leukemiavirus; FHV, feline herpesvirus; FPV, feline parvovirus) (Reiter and Mendoza, 2002, for review). However, none of these have been definitively proven to be the direct cause of tooth resorption; moreover, compared with other species, little is known about the biology of osteoclasts in the cat, contributing to the frustration in managing this disease.
Acidosis has been implicated in the pathogenesis of various metabolic diseases (Arnett, 2003). Acid ingestion is known to stimulate osteoclastic resorption, but the mechanism(s) remain(s) unclear. Arnett and colleagues (Arnett and Dempster, 1986; Arnett and Spowage, 1996; Meghji et al., 2001) have investigated the effects of small shifts in extra‐cellular pH on the differentiation and resorptive activity of rodent osteoclasts in vitro. Hypoxia also stimulates resorption by increasing osteoclast formation (Arnett et al., 2003), a situation that would also occur at sites of “inflammatory” bone or tooth destruction; we have recently demonstrated similar responses with feline osteoclasts (Muzylak et al., 2006). Because of the striking effects of acidosis on osteoclasts from other species, and since sites of inflammation and infection in the mouth are likely to be acidic, we hypothesize that “acidic” conditions could exist in the oral cavity of the adult cat; and that, as a known stimulator of osteoclast differentiation and activation of bone resorption, these micro‐environmental conditions would contribute to the pathogenesis of FORL.
In this study we used a previously established method to generate feline osteoclasts from blood mononuclear cells stimulated by Macrophage Colony Stimulating Factor (M‐CSF) and Receptor Activator of NFκB Ligand (RANKL) (Muzylak et al., 2002). These cultured osteoclasts have a phenotype typical of osteoclasts from other species. We examined the effect of altering pH on the in vitro development and resorptive activity of feline osteoclasts and found that large numbers of osteoclasts of great size developed from peripheral blood monocytes under acidic culture conditions and that these were highly active in in vitro bone resorption. We conclude that these innate characteristics could account for the pathology of FORL in the absence of other causative factors, and for the apparent rarity of this disease in other species such as humans.
Materials and Methods
Cat peripheral blood mononuclear cells
Peripheral blood was obtained from healthy adult cats, aged 2 years (Waltham Centre for Pet Nutrition, Leicestershire, UK). Ten milliliters of blood, obtained by jugular puncture, was heparinized (200 µl per 10 ml of blood PUMP‐HEP, Leo Labs Ltd, Bucks, UK) and transported on wet ice. Peripheral blood mononuclear cells (PBMCs) were isolated by density centrifugation over Ficoll Hypaque (density 1.077, Amersham Pharmacia Biotech, Little Chalfont, Bucks, UK) for 30 min at 1,460 g at room temperature. Mononuclear cells were collected and washed twice in α Minimum Essential Medium (MEM; Sigma‐Aldrich Ltd, Gillingham, Dorset, UK) by centrifugation at 1,000g for 10 min.
Generation of cat osteoclasts from peripheral blood
Osteoclasts were generated from feline PBMCs using methods developed for human osteoclasts (Lader and Flanagan, 1998; Massey and Flanagan, 1999; Lader et al., 2001) and described previously (Muzylak et al., 2002). Isolated PBMCs were re‐suspended in αMEM supplemented with 10% heat‐inactivated fetal bovine serum (Sera Laboratories Int. Ltd, Crawley, W. Sussex, UK), 2 mM l‐glutamine, 100 IU benzyl penicillin per ml and 100 mg streptomycin per ml (Gibco BRL, Paisley, Scotland, UK) and plated (2 × 105) on bovine bone slices (day 1) in 96‐well plates in a final volume of 200 µl. Cultures were maintained for the first 4 days in culture medium and M‐CSF (25 ng/ml) (kindly provided by Genetics Institute, Boston, MA) at 37°C in 5% CO2/95% air and fed twice weekly. On day 4, 90% of the medium was removed and replaced with fresh medium containing additional soluble RANKL (30 ng/ml) (kindly provided by Amgen, Thousand Oaks, CA); subsequently, cultures were fed twice weekly with both growth factors after demi‐depletion of medium and the cultures were terminated after up to 14 days.
Growth factor manipulation
The growth factor concentrations used under “standard” culture conditions were found by prior experimentation (Muzylak et al., 2002). Cultures were performed at ambient oxygen concentration in the absence of all growth factors or with the addition of M‐CSF (concentration range 25–100 ng/ml) and RANKL (concentration range 30–120 ng/ml) to arrive at minimum factor doses for use in the study.
Modifying culture pH
Prior to altering the culture pH, medium and growth factors were added when the cultures were established and then changed by demi‐depletion at control pH, as described (Muzylak et al., 2002), until pH manipulation on day 7 or 11. The pH of the culture medium was adjusted to a nominal starting value in the range pH 5.5–8.5 for the specified culture periods, days 7–14 or 11–14. Prior to pH modification, cultures were exposed to ambient CO2 conditions for the shortest time possible during medium changes and growth factor addition to keep pH fluctuations to a minimum. Culture medium acidification or alkalinization was achieved by adding small amounts of concentrated HCl or NaOH, respectively, to the αMEM medium, as described by Murrills et al. (1998). The pH and pCO2 of the culture medium was measured at each medium exchange and at the termination of an experiment using a blood gas analyzer (ABL 330; ABL 705, Radiometer, Copenhagen, Denmark) as described (Meghji et al., 2001). The pH referred to in the data was that at termination of the culture.
Immunocytochemistry
For confocal microscopy (Leica TCS NT, Heidelberg, Germany), osteoclasts on the bone slices were fixed for 5 min in a 50:50 mixture of αMEM with fixation buffer (3.5% paraformaldehyde and 2% sucrose in phosphate buffered saline (PBS; Sigma‐Aldrich Ltd), washed in PBS and placed in ice‐cold permeabilization buffer (20 mM HEPES, 300 mM sucrose, 50 mM NaCl, 3 mM MgCl2, 0.5% Triton X‐100, and 0.5% sodium azide in PBS) for a further 5 min (Nesbitt and Horton, 1997). To stain for the osteoclast proton pump ATPase, cells were fixed in ice cold methanol for 5 min. Osteoclasts were then incubated in TRITC‐phalloidin conjugate (Molecular Probes, Eugene, OR) (5 U/ml) in PBS to identify resorbing osteoclasts by their characteristic F‐actin “ring” structure (Nesbitt and Horton, 1997). Cell markers were identified using monoclonal antibodies: 23C6 for the human integrin αvβ3 vitronectin receptor (VNR) (Horton et al., 1985; Nesbitt and Horton, 1997; Horton et al., 2002), cathepsin K (gift of SmithKline Beecham, Pennsylvania, PA) and MMP9 (Chemicon, Temecula, CA), tartrate‐resistant acid phosphatase (TRAP; gift of SA Nesbitt), proton pump (gift of SA Nesbitt). The secondary antibody was FITC‐conjugated goat anti‐mouse IgG (Dako Ltd, Ely, UK). Osteoclasts were defined as cells expressing the VNR and F‐actin rings (Nesbitt and Horton, 1997). Confocal micrographs shown are merged through‐focus images for a stack of xy images (as in Fig. 1C,D). Twenty osteoclasts were examined on each bone slice and the intensity of staining evaluated in arbitrary units (pixel intensity per unit area under standardized conditions as in Horton et al., 2003).
Figure 1.
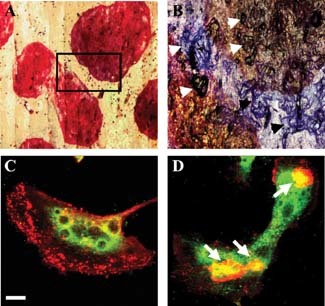
Gigantic osteoclasts formed in vitro under acidic culture conditions. A: Low power transmitted light micrograph of TRAP‐stained osteoclasts on a bone slice formed during culture from day 7 to 14 under acid conditions (pH 6.8) showing extreme size (approximately 2 mm diameter) (numerous nuclei, >200, are present but obscured by the intense TRAP staining) (mag. 5×). B: Higher power reflected light micrograph of combined TRAP‐ and toluidine blue‐stained osteoclasts; detail of the area marked in (A) showing numerous resorption lacunae under and associated with two osteoclasts (examples are identified by arrows) (mag. 10×). C,D: Immunostaining of osteoclasts for cathepsin K formed under control (pH 7.4) (C) and acidic (pH 6.8) conditions (D). F‐actin rings are indicated by arrows in (D). Scale bar (in C) = 20 µm. These cells are representative examples used for the analyses summarized in Table 1.
Tartrate resistant acid phosphatase staining
Experiments were terminated by fixing the bone slices in 2% glutaraldehyde, followed by staining for 35 min to demonstrate TRAP (Sigma‐Aldrich Kit 387‐A).
Assessment of osteoclast numbers and spread area
The number of osteoclasts attached to the bone slices was determined after staining for TRAP by taking pictures of the slices using a JVC color video camera and the images analyzed using LEICA QWin software to give cell number; mean, maximum, and total cell area and percent bone surface covered by osteoclasts.
Assessment of osteoclastic resorption
Devitalized cortical bovine bone slices (20 mm2; 4 × 5 × 0.1 mm3) (Muzylak et al., 2002) were used as substrate for osteoclastic bone resorption. After counting the number and assessing the area of osteoclasts attached to the substrate, as above, cells were removed by rubbing on filter paper or by treatment with TRISOL and rubbing on filter paper. Resorption lacunae were visualized by biotin‐conjugated WGA lectin staining (Vector Laboratories, Burlingame, CA) and reaction with TRITC‐streptavidin (Sigma‐Aldrich). Fluorescence images were captured using a Leica fluorescence microscope and analyzed using Leica QWin software, as for the TRAP‐stained cultures, producing values for resorption pit “area” assessed as “mean pixel intensity per unit area.”
Statistical analysis
Graphical and numerical data for osteoclast numbers, size, and bone resorption are shown from one of the three replicate experiments where values under each experimental condition tested represent data obtained from at least four bone slices. The results were analyzed using one‐way ANOVA, where significance was accepted as P < 0.05. Results are displayed as means ± standard deviation. Control conditions, 20% oxygen and medium pH of ∼7.2, were used as a baseline for statistical comparisons.
Results
General characterization of osteoclasts generated from feline PBMCs
PBMCs were cultured with M‐CSF (25 ng/ml) and RANKL (30 ng/ml) and examined over a 14 day culture period. As in our earlier work (Muzylak et al., 2002), cells with osteoclastic morphology appeared from day 7. This coincided with the appearance of TRAP‐positive polykaryons and the first resorption lacunae, identified in bone slices by reflection microscopy or WGA lectin staining of exposed bone matrix proteins.
By 14 days of culture (Muzylak et al., 2002), the cells were multinucleated (not shown), had multiple F‐actin enriched “rings” (arrows in Fig. 1D), and expressed the osteoclast enzymes TRAP (Fig. 1A), proton pump, and cathepsin K, and the membrane αvβ3 integrin VNR (Fig. 1C,D and Table 1). The myeloid antigen, CD18, and the megakaryocyte/platelet integrin, CD41, were absent (not shown).
Table 1.
Quantification of marker proteins in osteoclasts (expressed as mean pixel intensity per mm2 cell area in arbitrary units assessed by immunocytochemistry and confocal microscopy) in cultures grown under modified pH conditions from day 7 to 14
| Marker | Mean pixel intensity per mm2 | % changes in mean pixel intensity | Significance | |
|---|---|---|---|---|
| pH 7.4 | pH 6.8 | |||
| VNR | 19,818 ± 9,746 | 160,11 ± 10,324 | −19 | P = 0.032 |
| TRAP | 283,800 ± 115,287 | 108,863 ± 39,900 | −61 | P = 0.008 |
| Proton pump | 199,393 ± 124,358 | 595,057 ± 294,425 | +298 | P = 0.01 |
| Cathepsin K | 176,002 ± 91,707 | 5600,860 ± 107,428 | +319 | P = 0.001 |
Control cultures contained up to 400 osteoclasts per bone slice (Fig. 2A,B) and these resorbed bone, forming 4,794 ± 1,780 lacunae per 1 mm2 bone slice (mean ± SD) after 14 days and resorbed 3.5 ± 1.4% of the bone surface (Fig. 4A).
Figure 2.
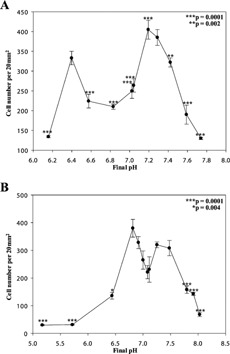
Numbers of osteoclasts formed in cultures grown under modified pH conditions. The number of TRAP‐positive osteoclasts per bone slice (per 20 mm2 area) in cultures exposed to altered pH from days 7 to 14 (A) and days 11–14 (B). *P = 0.004, **P = 0.002, ***P = 0.0001.
Figure 4.
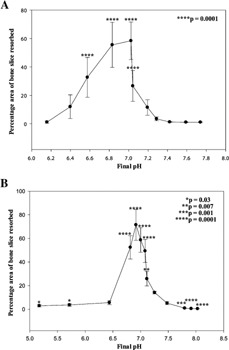
Percentage area of bone slice resorbed by osteoclasts formed in cultures grown under modified pH conditions. Percentage area of bone slice resorbed in cultures exposed to altered pH from day 7 to 14 (A) and 11–14 (B). *P = 0.03, **P = 0.007, ***P = 0.001, ****P = 0.0001.
The effect of pH change upon osteoclast differentiation
Over pH values of ∼6.5–7.5, 200–400 per 20 mm2 osteoclasts were observed in cultures when the pH was modified from either days 7–14 or 11–14 (Fig. 2A,B). At pHs more acidic or alkaline, osteoclast numbers were less, though there was still significant osteoclast differentiation at surprisingly extreme conditions (Fig. 2B, pHs 5.2 and 8 in cultures where the pH was changed from days 11–14 when the osteoclasts already present were “mature”; P = 0.0001).
Osteoclasts and their precursors fused over a wide range of pHs in both 7–14 and 11–14 day cultures (Fig. 3A,B), achieving a mean spread area of 0.05 ± 0.001 mm2 (at pH 6.8 in 7–14 day cultures, Fig. 3A) versus 0.015 ± 0.002 mm2 (under control conditions in 7–14 day cultures, pH 7.3), and 0.086 ± 0.0048 mm2 (at pH 7.15 in 11–14 day cultures, Fig. 3B) compared to 0.01 ± 0.003 (under control conditions in 11–14 day cultures, pH 7.25). A maximal 8.6‐fold stimulatory response to acidosis was seen if the pH was shifted at days 11–14 when the osteoclasts are of “equivalent maturity” to those isolated from neonatal bone (Fig. 3B, P = 0.0001). The pH range for maximal effect is more acidic (pH 6.83) and shows a less acute pH response profile if treatment occurs earlier (days 7–14), that is, when the cells are at the “precursor” stage (Fig. 3A).
Figure 3.
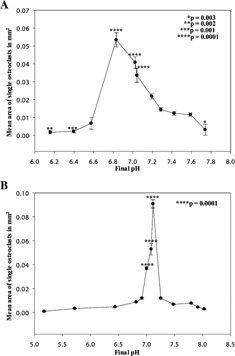
Area of osteoclasts formed in cultures grown under modified pH conditions. Mean area (mm2) of single osteoclasts in cultures exposed to altered pH from day 7 to 14 (A) and 11–14 (B). *P = 0.003, **P = 0.002, ***P = 0.001, ****P = 0.0001.
Osteoclasts achieved a maximum area of 5.9 and 13.2 mm2 after 7–14 days and 11–14 of culture, a 3.7‐ and 10.4‐fold increase over cultures at pH 6.8 and 7.15, respectively (data not shown, but examples of “gigantic osteoclasts” are illustrated in Fig. 1A). They covered 52.5% (10.5 mm2) and 66.4% (16 mm2) of the bone slice, respectively, increases of 3.4‐ and 4.7‐fold over basal conditions.
Stimulation of bone resorption by acidosis
Acidosis significantly stimulated bone resorption in cultures when the pH was modified from days 7 or 11–14 (Fig. 4A,B). Under control pH conditions, osteoclasts resorbed ∼3.5% (d7–14) and ∼14.1% (d11–14) of the surface of bone slices; this increased 16‐ and 5‐fold (∼58% and 72%, respectively, P = 0.0001) upon exposure to acid conditions during culture (pH 7.02 and pH 6.92, respectively). Similar results were observed for resorption pit number. Under basal pH conditions, osteoclast form 4,794 ± 1,780 (at pH 7.28 for d7–14) lacunae per 1 mm2 bone slice; this increased 10.9‐fold to 52,142 ± 11,607 upon exposure to acidic conditions during culture (pH 7.02; P = 0.0001). The mean pit area was 7.30 ± 3.93 µm2 (at pH 7.28 in 7–14 day cultures) compared to 16.74 ± 11.16 µm2 under acidic conditions (pH 7.04; P = 0.004). Significant differences were also observed at pHs 6.15–7.04 (data not shown). Whilst osteoclasts formed in significant numbers at very low and high pHs (Fig. 2A,B), these exhibited minimal resorption (Fig. 4A,B); at pH 6.44–5.17 this was still detectable, but not under extreme alkaline conditions (Fig. 4A,B).
Induction of cathepsin K and proton pump in osteoclasts by acidosis
The expression of two enzymes, essential for the functionally activated osteoclasts, is up‐regulated in cultures maintained under acidic conditions, at pH 6.8, from days 7 to 14. Cathepsin K, the key acid protease for collagenolysis (Fig. 1C,D), and proton pump ATPase that is involved in proton extrusion into the sub‐osteoclast resorption lacuna, were analyzed by quantitative confocal microscopy after immunostaining. Expression of both enzymes increased approximately threefold in line with the observed increase in bone resorption (Table 1; P = 0.01 and 0.001, respectively). TRAP and αvβ3 expression, other marker proteins of osteoclasts, were somewhat decreased under the same culture conditions (Table 1).
Discussion
Commitment and differentiation of osteoclasts from their precursors is highly complex, and is usually based on studies using rodent or human cells. Osteoclasts are known to be formed from cells of the “monocytoid lineage,” and precursors have been shown to reside in both the immature colony forming unit population and more mature CD14+ monocytes; a distinct osteoclast lineage precursor, CFU‐O, has been proposed to be capable of responding rapidly to RANKL (Atkins et al., 2006). Cell‐to‐cell contact is also necessary for osteoclast formation since they form by cell fusion rather than endomitosis (Suda et al., 1992). Currently, several cell surface molecules have been reported to be involved in this process and it had been established that RANK signaling together with additional signaling through c‐Fms, the receptor for M‐CSF, promotes survival, proliferation, and fusion of mononuclear osteoclast precursors (Boyle et al., 2003; Ross and Teitelbaum, 2005; Vignery, 2005). In both co‐cultures and M‐CSF/RANKL stimulated systems, osteoclast formation proceeds in stages (Quinn and Gillespie, 2005), and at least 20 genes have been shown to regulate osteoclastogenesis and osteoclast activation. Some act during the formation and/or survival of the osteoclast precursor cell (e.g., PU.1, op/CSF‐1), whereas others mediate either the ability of the precursor cell to undergo differentiation (e.g., RANK, fos) or the adherence and lytic function of mature osteoclasts (e.g., src, CATK) (reviewed in Boyle et al., 2003). Furthermore, the interaction between RANKL, produced by osteoblasts, and its receptor, RANK, on the surface of osteoclast precursors, leads to activation of at least five signaling cascades (inhibitor of NF‐κB kinase (IKK), c‐Jun N‐terminal kinase (JNK), p38, extra‐cellular signal‐regulated kinase (ERK), and Src) that culminate in activation of transcription factors NF‐κB and nuclear factor of activated T‐cells (NFAT‐2) (reviewed in Boyle et al., 2003). Additionally, stromal cell‐derived factor‐1 (SDF‐1 or CXCL12) is a chemokine highly expressed by bone endothelium, marrow stromal cells, and immature osteoblasts and directly promotes early osteoclast development by stimulating precursor proliferation, fusion, TRAP activity, and cell survival by induction of anti‐apoptotic transcription factors (Wright et al., 2005).
Relatively little research has been carried out on osteoclast development and regulation of bone resorption in the domestic cat. Early studies (Addison, 1978; Allen et al., 1981; Ibbotson et al., 1984; Pharoah and Heersche, 1985; Horton et al., 1988; Shigeyama et al., 1996) investigated cat osteoclasts in situ or disaggregated directly from bone and they were shown to express TRAP and the αvβ3 integrin, and to resorb bone. Recently (Muzylak et al., 2002) demonstrated that the multinucleated cells produced by culture of feline PBMCs in the presence of M‐CSF and RANKL, conditions inductive of osteoclast differentiation in other species, are genuine osteoclasts; they express TRAP, resorb bone, and have high levels of αvβ3 and of the proteolytic enzymes, cathepsin K and MMP9. Cells with an identical phenotype, were also observed to form in vitro in this study, confirming our earlier results.
Under basal pH conditions, cultured feline osteoclasts were larger than human PMBC‐derived osteoclasts unless stimulated by TGFβ (Massey et al., 2001). Previous histological studies (Addison, 1979, 1980) have shown that feline osteoclasts are larger than those from other species, of the order of 300 µm in diameter, with the number of nuclei often exceeding 100 (Allen et al., 1981; Ibbotson et al., 1984; Pharoah and Heersche, 1985). In contrast, osteoclasts isolated from neonatal mouse bone or grown in vitro are small and frequently have less than five nuclei. These observations are in line with our culture experiments where the largest osteoclasts that we observed had a maximum cell area of up to ∼13 mm2 after 14 days of culture and often contained several hundred nuclei. These gigantic osteoclasts were functionally highly active, each associated with numerous F‐actin rings and resorption lacunae, and frequently resorbed the entire surface of bone slices (the mean area resorbed at pH <7.1 after 14 days culture being greater than 60%). Osteoclasts even formed at the extremes of culture pH studied, especially when “mature” osteoclast cultures had their pH modified; these still resorbed bone though to a diminished extent compared with basal conditions. Further studies are needed to determine the mechanism by which the exaggerated effects of acidosis upon feline osteoclast formation and function are mediated.
Normal extra‐cellular pH in bone has not been measured but it is likely to be somewhat less than blood pH; in normal skin, for example, interstitial pH has been measured at ∼7.1 (Martin and Jain, 1994), which approximates to the half‐maximal activation pH of dissociated rodent osteoclasts (Arnett and Spowage, 1996). In the diseased oral cavity, pH is likely to be even lower and this raises the question of how “acidosis” impacts the levels of control of osteoclast development and function, the available data only rarely coming from felines. Frick and Bushinsky (2003) found that metabolic acidosis significantly increased the expression of RANKL RNA, correlating with calcium efflux from bone; indomethacin blocked this effect, indicating a role for prostaglandins. Acidosis also increases osteoblastic secretion of PGE2, which is known to stimulate osteoclastic bone resorption (Krieger et al., 2000). Several classes of protein could function as extra‐cellular pH sensor(s) within the pH range at which extra‐cellular acidification activates and stimulates osteoclasts to resorb bone, but they are yet to be studied in cat osteoclasts. They include the H+‐sensing G‐protein‐coupled receptor, OGR1, which is expressed by osteoclasts and osteoblasts (Ludwig et al., 2003); the acid‐sensing ion channels (ASICS), several of which are expressed in bone (Jahr et al., 2005); the P2X2 receptor for extra‐cellular ATP (North, 2002), also present in osteoclasts; and the TRP cation channel (van der Eerden et al., 2005). Although the mechanism by which osteoclasts detect pH changes is still elusive, progress has been made in understanding the response of the key resorptive enzymes to extra‐cellular acidification, reflected in the data from this study. Acidosis rapidly stimulates the activity of the vacuolar‐type H+‐ATPase in osteoclasts (Nordstrom et al., 1997) and induces mRNA for carbonic anhydrase II (Biskobing and Fan, 2000). Furthermore, a recent study by Brandao‐Burch et al. (2003) shows that acidosis up‐regulates mRNA for TRAP and cathepsin K in organ cultures.
In conclusion, we consider that acidosis could be the locally acting exogenous factor that leads to the development of FORL, and contributes to the propensity of cat osteoclast precursors to develop into large mature cells with high resorptive activity. There also may be inherent, possibly genetic, differences in the cat that result in a substantially different osteoclast response compared to that observed in other species. That is, the cat reacts in a qualitatively or quantitatively different manner—an exaggerated osteoclast number and/or size, degree of activation (and hence resorption), growth factor sensitivity, or a response at a different range of pH may be observed.
Acknowledgements
The work was carried out with the financial help of a grant from the Waltham Centre for Pet Nutrition, Waltham‐on‐the‐Wolds, Leicestershire, UK. JSP and MAH are supported by a programme grant from The Wellcome Trust and TRA is supported by the Arthritis Research Campaign (UK), Novartis Pharma and the Wellcome Trust. The authors are grateful for the expert advice and help of Jenny Utting and Andrea Brandao‐Burch, UCL.
Literature Cited
- Addison WC. 1978. Enzyme histochemical properties of kitten osteoclasts in bone imprint preparations. Histochem J 10: 645–656. [DOI] [PubMed] [Google Scholar]
- Addison WC. 1979. The distribution of nuclei in imprints of feline osteoclasts. J Anat 129: 63–68. [PMC free article] [PubMed] [Google Scholar]
- Addison WC. 1980. The effect of parathyroid hormone on the numbers of nuclei in feline odontoclasts in vivo. J Periodontal Res 15: 536–543. [DOI] [PubMed] [Google Scholar]
- Allen TD, Testa NG, Suda T, Schor SL, Onions D, Jarrett O, Boyde A. 1981. The production of putative osteoclasts in tissue culture—ultrastructure, formation and behaviour. Scan Electron Microsc 33: 347–354. [PubMed] [Google Scholar]
- Arnett T. 2003. Regulation of bone cell function by acid‐base balance. Proc Nutr Soc 62: 511–520. [DOI] [PubMed] [Google Scholar]
- Arnett TR, Dempster DW. 1986. Effect of pH on bone resorption by rat osteoclasts in vitro. Endocrinol 119: 119–124. [DOI] [PubMed] [Google Scholar]
- Arnett TR, Spowage M. 1996. Modulation of the resorptive activity of rat osteoclasts by small changes in extracellular pH near the physiological range. Bone 18: 277–279. [DOI] [PubMed] [Google Scholar]
- Arnett TR, Gibbons DC, Utting JC, Orriss IR, Hoebertz A, Rosendaal M, Meghji S. 2003. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J Cell Physiol 196: 2–8. [DOI] [PubMed] [Google Scholar]
- Atkins GJ, Kostakis P, Vincent C, Farrugia AN, Houchins JP, Findlay DM, Evdokiou A, Zannettino ACW. 2006. RANK expression as a cell surface marker of human osteoclast precursore in peripheral blood, bone marrow, and giant cell tumors of bone. J Bone Miner Res 21: 1339–1349. [DOI] [PubMed] [Google Scholar]
- Barber PJ, Elliott J. 1998. Feline chronic renal failure: Calcium homeostasis in 80 cases between 1992 and 1995. J Small Anim Pract 39: 108–116. [DOI] [PubMed] [Google Scholar]
- Bennett D. 1995. Diseases of the musculoskeletal system. In: Chandler EA, Gaskell CJ, Gaskell RM, editors. Feline medicine and therapeutics. Oxford: Blackwell Science. pp 150–191. [Google Scholar]
- Berger M, Schawalder P, Stich H, Lussi A. 1996. Feline dental resorptive lesions in captive and wild leopards and lions. Schweiz Arch Tierheikd 138: 546–551. [PubMed] [Google Scholar]
- Biskobing DM, Fan D. 2000. Acid pH increases carbonic anhydrase II and calcitonin receptor mRNA expression in mature osteoclasts. Calcif Tissue Int 67: 178–183. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WC, Lacey DL. 2003. Osteoclast differentiation and activation. Nature 423: 337–342. [DOI] [PubMed] [Google Scholar]
- Brandao‐Burch A, Meghji S, Arnett TR. 2003. Acidosis strongly upregulates mRNA for cathepsin K, TRAP and TRAF‐6 in bone. Calcif Tissue Int 72: 364. [Google Scholar]
- Clark L. 1970. The effect of excess vitamin A on long bone growth in kittens. J Comp Pathol 80: 625–634. [DOI] [PubMed] [Google Scholar]
- DeLaurier A, Boyde A, Horton MA, Price JS. 2005. A scanning electron microscopy study of idiopathic external tooth resorption in the cat. J Periodontol 76: 1106–1112. [DOI] [PubMed] [Google Scholar]
- Frick KK, Bushinsky DA. 2003. Metabolic acidosis stimulates RANKL RNA expression in bone through a Cyclo‐oxygenase‐Dependent mechanism. J Bone Miner Res 18: 1317–1325. [DOI] [PubMed] [Google Scholar]
- Gengler W, Dubielzig R, Ramer J. 1995. Physical examination and radiographic analysis to detect dental and mandibular bone resorption in cats: A study of 81 cases from necropsy. J Vet Dent 12: 97–100. [PubMed] [Google Scholar]
- Heaton M, Wilkinson J, Gorrel C, Butterwick R. 2004. A rapid screening technique for feline odontoclastic resorptive lesions. J Small Anim Pract 45: 598–601. [DOI] [PubMed] [Google Scholar]
- Heldmann E, Anderson MA, Wagner‐Mann C. 2000. Feline osteosarcoma: 145 cases (1990–1995). J Am Anim Hosp Assoc 36: 518–521. [DOI] [PubMed] [Google Scholar]
- Hill FW. 1977. A survey of bone fractures in the cat. J Small Anim Pract 18: 457–463. [DOI] [PubMed] [Google Scholar]
- Horton MA, Lewis D, McNulty K, Pringle JA, Chambers TJ. 1985. Monoclonal antibodies to osteoclastomas (giant cell bone tumours): definition of osteoclast‐specific cellular antigens. Cancer Res 45: 5663–5669. [PubMed] [Google Scholar]
- Horton MA, Fowler P, Simpson A, Onions D. 1988. Monoclonal antibodies to human antigens recognise feline myeloid cells. Vet Immunol and Immunopathol 18: 213–217. [DOI] [PubMed] [Google Scholar]
- Horton MA, Nesbitt SA, Bennett JH, Stenbeck G. 2002. Integrins and other cell surface attachment molecules of bone cells. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of bone biology, 2nd edition. San Diego, USA: Academic Press. pp 265–286. [Google Scholar]
- Horton MA, Massey HM, Rosenberg N, Nicholls B, Seligsohn U, Flanagan AM. 2003. Upregulation of osteoclast alpha2beta1 integrin compensates for lack of alphavbeta3 vitronectin receptor in Iraqi‐Jewish‐type Glanzmann thrombasthenia. Br J Haematol 122: 950–957. [DOI] [PubMed] [Google Scholar]
- Ibbotson KJ, Roodman GD, Mcmanus LM, Mundy GR. 1984. Identification and characterization of osteoclast‐like cells and their progenitors in cultures of feline marrow mononuclear cells. J Cell Biol 99: 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr H, van Driel M, van Osch GJ, Weinans H, van Leeuwen JP. 2005. Identification of acid‐sensing ion channels in bone. Biochem Biophys Res Commun 337: 349–354. [DOI] [PubMed] [Google Scholar]
- Krieger NS, Parker WR, Alexander KM, Bushinsky DA. 2000. Prostaglandins regulate acid‐induced cell‐mediated bone resorption. Am J Physiol 279: F1077–F1082. [DOI] [PubMed] [Google Scholar]
- Lader CS, Flanagan AM. 1998. Prostaglandin E2, interleukin 1α, and tumor necrosis factor‐α increase human osteoclast formation and bone resorption in vitro. Endocrinol 139: 3157–3164. [DOI] [PubMed] [Google Scholar]
- Lader CS, Scopes J, Horton MA, Flanagan AM. 2001. Generation of human osteoclasts in stromal cell‐free and stromal cell‐rich cultures: Differences in osteoclast CD11c/CD18 integrin expression. Br J Haematol 112: 430–437. [DOI] [PubMed] [Google Scholar]
- Leonard CA, Tillson M. 2001. Feline lameness. Vet Clin North Am Small Anim Pract 31: 143–163. [DOI] [PubMed] [Google Scholar]
- Lommer MJ, Verstraete FJ. 2000. Prevalence of odontoclastic resorption lesions and peripheral radiographic lucencies in cats: 265 cases (1995–1998). J Am Vet Med Assoc 217: 1866–1869. [DOI] [PubMed] [Google Scholar]
- Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K. 2003. Proton‐sensing G‐protein‐coupled receptors. Nature 425: 93–98. [DOI] [PubMed] [Google Scholar]
- Lyon KF. 1992. Subgingival odontoclastic resorptive lesions: Classification, treatment, and results in 58 cats. Vet Clin North Am Small Anim Pract 22: 1417–1432. [DOI] [PubMed] [Google Scholar]
- Martin GR, Jain RK. 1994. Nonivasive measurement of interstitial pH profiles in normal and neoplastic tissue using fluorescence ratio imaging microscopy. Cancer Res 54: 5670–5674. [PubMed] [Google Scholar]
- Massey HM, Flanagan AM. 1999. Human osteoclasts derive from CD14‐positive monocytes. Br J Haematol 106: 167–170. [DOI] [PubMed] [Google Scholar]
- Massey HM, Scopes J, Horton MA, Flanagan AM. 2001. Transforming growth factor‐beta1 (TGF‐beta) stimulates the osteoclast‐forming potential of peripheral blood hematopoietic precursors in a lymphocyte‐rich microenvironment. Bone 28: 577–582. [DOI] [PubMed] [Google Scholar]
- Meghji S, Henderson B, Morrison MS, Arnett TR. 2001. pH dependence of bone resorption: Mouse calvarial osteoclasts are activated by acidosis. Am J Physiol Endocrinol Metab 280: E112–119. [DOI] [PubMed] [Google Scholar]
- Moody GH, Muir KF. 1991. Multiple idiopathic root resorption. A case report and discussion of pathogenesis. J Clin Periodontol 18: 577–580. [DOI] [PubMed] [Google Scholar]
- Murrills DJ, Dempster DW, Arnett TR. 1998. Isolation and culture of osteoclasts and osteoclast resorption assays. In: Arnett TR, Henderson B, editors. Methods in bone biology. London, UK: Chapman and Hall. pp 64–105. [Google Scholar]
- Muzylak M, Flanagan AM, Ingham K, Gunn N, Price J, Horton MA. 2002. A feline assay using osteoclasts generated in vitro from peripheral blood for screening anti‐resorptive agents. Res Vet Sci 73: 283–290. [DOI] [PubMed] [Google Scholar]
- Muzylak M, Price JS, Horton MA. 2006. Hypoxia induces giant osteoclast formation and extensive bone resorption in the cat. Calcif Tissue Int 79: 301–309. [DOI] [PubMed] [Google Scholar]
- Nesbitt SA, Horton MA. 1997. Trafficking of matrix collagens through bone‐resorbing osteoclasts. Science 276: 266–269. [DOI] [PubMed] [Google Scholar]
- Nordstrom T, Shrode LD, Rotstein OD, Romanek R, Goto T, Heersche JN, Manolson MF, Brisseau GF, Grinstein S. 1997. Chronic extracellular acidosis induces plasmalemmal vacuolar type H+ ATPase activity in osteoclasts. J Biol Chem 272: 6354–6360. [DOI] [PubMed] [Google Scholar]
- North RA. 2002. Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067. [DOI] [PubMed] [Google Scholar]
- Okuda A, Harvey CE. 1992. Etiopathogenesis of feline dental resorptive lesions. Vet Clin North Am Small Anim Pract 22: 1385–1404. [DOI] [PubMed] [Google Scholar]
- Okuda A, Asari M, Harvey CE. 1995. Challenges in treatment of external odontoclastic resorptive lesions in cats. Comp Cont Educ Pract Vet 17: 1461–1469. [Google Scholar]
- Pharoah MJ, Heersche JN. 1985. 1,25‐Dihydroxyvitamin D3 causes an increase in the number of osteoclast like cells in cat bone marrow cultures. Calcif Tissue Int 37: 276–281. [DOI] [PubMed] [Google Scholar]
- Quinn JMW, Gillespie MT. 2005. Modulation of osteoclast formation. Biochem Biophys Res Commun 328: 739–745. [DOI] [PubMed] [Google Scholar]
- Reiter AM. 1998. Feline “odontolysis” in the 1920s: The forgotten histopathological study of feline odontoclastic resorptive lesions (FORL). J Vet Dent 15: 35–41. [DOI] [PubMed] [Google Scholar]
- Reiter AM, Mendoza KA. 2002. Feline odontoclastic resorptive lesions an unsolved enigma in veterinary dentistry. Vet Clin North Am Small Anim Pract 32: 791–837. [DOI] [PubMed] [Google Scholar]
- Ross FP, Teitelbaum SL. 2005. alphavbeta3 and macrophage colony‐stimulating factor: Partners in osteoclast biology. Immunol Rev 208: 88–105. [DOI] [PubMed] [Google Scholar]
- Shigeyama Y, Grove TK, Strayhorn C, Somerman MJ. 1996. Expression of adhesion molecules during tooth resorption in feline teeth: A model system for aggressive osteoclastic activity. J Dent Res 75: 1650–1657. [DOI] [PubMed] [Google Scholar]
- Suda T, Takahashi N, Martin TJ. 1992. Modulation of osteoclast differentiation. Endocr Rev 13: 66–80. [DOI] [PubMed] [Google Scholar]
- van der Eerden BC, Hoenderop JG, de Vries TJ, Schoenmaker T, Buurman CJ, Uitterlinden AG, Pols HA, Bindels RJ, van Leeuwen JP. 2005. The epithelial Ca2+ channel TRPV5 is essential for proper osteoclastic bone resorption. Proc Natl Acad Sci USA 102: 17507–17512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignery A. 2005. Macrophage fusion: Are somatic and cancer cells possible partners? Trends Cell Biol 15: 188–193. [DOI] [PubMed] [Google Scholar]
- Wright LM, Maloney W, Yu X, Kindle L, Collin‐Osdoby P, Osdoby P. 2005. Stromal cell derived factor‐1 binding to its chemokine receptor CXCR4 on precursor cells promotes the chemotactic recruitment, development and survival of human osteoclasts. Bone 36: 840–853. [DOI] [PubMed] [Google Scholar]


