Abstract
As one of the most important mosquito‐borne viral diseases, dengue infection is now becoming a global concern due to its rapid spread and rise in incidence. Currently, there is no approved vaccine or effective antiviral drug for dengue virus (DENV) infection. Glycyrrhetinic acid (GNa) and its related derivatives have been reported to inhibit a broad spectrum of viruses. However, it is unknown whether Carbenoxolone disodium (CBX), one of the GNa derivatives, affects DENV infection. Here, we found that the production of infectious DENV particles was significantly decreased by CBX treatment in DENV‐permissive cells, while the viral RNA and viral protein synthesis were not affected. Moreover, results from time‐of‐addition study showed that the inhibitory effect of CBX on DENV was exhibited by targeting the virus itself, not the host cells. Directly incubating DENV with CBX resulted in a remarkable reduction of virus titer and virus infectivity. Furthermore, DENV RNA from progeny virions in the supernatants was significantly decreased by CBX treatment in a dose‐dependent manner. Taken together, these data indicate that the antiviral activity of CBX against DENV may be mainly due to a virucidal effect exerted by the compound itself. Our work, for the first time, demonstrates that CBX has antiviral activity against DENV infection, providing useful information for development of potential therapeutic interventions against dengue. J. Med. Virol. 89:571–581, 2017 . © 2016 Wiley Periodicals, Inc.
Keywords: dengue virus, carbenoxolone, antiviral compound, virucidal effect
Abbreviations
- DENV
dengue virus
- CBX
Carbenoxolone disodium
- DF
dengue fever
- DHF
dengue hemorrhagic fever
- DSS
dengue shock syndrome
- GNa
glycyrrhetinic acid
- GRa
glycyrrhizic acid
- HIV
human immunodeficiency virus
- VSV
vesicular stomatitis virus
- HSV
herpes simplex virus
- MOI
multiplicity of infection
- CPE
cytopathic effects
- PBMC
peripheral blood mononuclear cells
INTRODUCTION
Dengue virus (DENV), a member of the genus Flavivirus in the family Flaviviridae, causes one of the most widespread mosquito‐borne diseases in human. There are four serotypes of dengue virus, types 1 (DENV‐1) to 4 (DENV‐4), which have similar clinical manifestations and epidemiology in tropical and subtropical regions of the world [Wang et al., 2000]. DENV attacks a set of target cells, including dendritic cells, monocytes/macrophages, endothelial cells, and disseminates in other non‐immune cells [Pagni and Fernandez‐Sesma, 2012]. Once DENV enters cytoplasm, its single‐stranded, positive‐sense RNA genome is directly translated into a single polyprotein which is eventually cleaved into the 10 individual viral proteins, including three structural proteins (C, prM, and E) and seven non‐structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) [Chambers et al., 1990; Filomatori et al., 2006; Zybert et al., 2008; Idrees and Ashfaq, 2012].
Dengue infection either results in a mild illness known as dengue fever (DF) or the more severe and even life‐threatening diseases, dengue hemorrhagic fever (DHF), and dengue shock syndrome (DSS) [Guzman and Kouri, 2002]. Currently, it is estimated that over 50 million cases of dengue infection occur annually, which have swept through more than 100 countries, with potential for further spread [Simmons et al., 2012]. From September to December 2014, an unexpected large outbreak of dengue infection with a significant proportion of severe cases was reported in Pearl River Delta of Guangdong Province, China. It has been the most severe dengue epidemic over the years, with more than 40,000 cases was diagnosed, including five deaths (http://www.cdcp.org.cn). The emergence of the unprecedented magnitude of dengue outbreak has aroused widespread social concern. In this situation, the WHO considers the development of an effective dengue vaccine to be a high priority. Due to great endeavor of scientific researchers from around the world, Dengvaxia (Sanofi Pasteur, Lyon, Rh) has become the first licensed vaccine available to prevent DF. However, for now there is no available antiviral agent approving for combating DENV [Idrees and Ashfaq, 2012]. Therefore, under current circumstance, the pressing task is to develop safe and effective antiviral drugs against dengue.
Currently, a series of natural compounds and their derivatives are reported as important source of antiviral drugs [Pompei et al., 1979, 2009; Chen et al., 2004; Fiore et al., 2008]. For example, triterpenic compounds, such as glycyrrhizic acid (GRa), glycyrrhetinic acid (GNa), and some of their derivatives have been demonstrated to block the replication of a broad spectrum of viruses, including human immunodeficiency virus (HIV), SARS related coronavirus, Epstein–Barr virus, flaviviruses, vesicular stomatitis virus (VSV), hepatitis B and C virus, influenza virus, rotavirus, adenovirus, etc. [Dargan et al., 1992a,b; Takahara et al., 1994; Matsuo et al., 2001; Cinatl et al., 2003; Crance et al., 2003; Lin, 2003; Orlent et al., 2006; Cos et al., 2008; Ashfaq et al., 2011; Hardy et al., 2012; Smirnov et al., 2012]. Carbenoxolone disodium (CBX), one of the GNa derivatives, is similar to substances found in the root of licorice plant. With a steroid‐like structure, CBX has been clinically applied in the treatment of gastric ulcers and other types of inflammation [Connors, 2012]. Besides, CBX is best known in cellular physiology as an effective, water‐soluble blocker of gap junctions [Rozental et al., 2001]. In earlier studies, CBX was reported to exhibit complex activities against herpes simplex virus (HSV), including synthesis, processing, and transportation of viral gene products [Dargan and Subak‐Sharpe, 1985, 1986; Dargan et al., 1988]. Nonetheless, whether CBX has an inhibitory effect on DENV remains unknown.
In this study, we investigated whether CBX treatment inhibits dengue infection by measuring the production of progeny virions and viral RNA as well as viral protein expression. Our data showed a dose‐dependent inhibition of DENV‐1 and DENV‐2 progeny virions following treatment with indicated concentration of CBX. Moreover, pre‐incubating DENV‐2 with CBX remarkably abolished virus infectivity and decreased virus titer. Furthermore, treatment with CBX significantly decreased DENV RNA from progeny virions in the supernatants. Our results for the first time identify the old drug CBX as a novel anti‐DENV compound, and it might be developed as a potential treatment for dengue infection.
MATERIALS AND METHODS
Cells and Virus
A549 cells (ATCC; CCL‐185) were cultured in DMEM (GIBCO, Carlsbad, CA) complemented with 5% FBS at 37°C. THP‐1 cells (ATCC; TIB‐202) were cultured in RPMI‐1640 (GIBCO) with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT) and 1% 2‐mercaptoethanol (Invitrogen, Carlsbad, CA) at 37°C. HUVEC cells (Clonetics Corp., San Diego, CA) were cultured in M199 medium (JRH Biosciences, Lenexa, KS) containing 20% FBS, 50 mg/ml endothelial cell growth supplement (Collaborative Biomedical Products, Bedford, MA), and 50 mg/ml heparin (Sigma, St. Louis, MO) at 37°C. Mosquito cell line C6/36 cells (ATCC; CRL‐1660) were cultured in RPMI‐1640 with 5% FBS at 28°C. All media were supplemented with 1% sodium pyruvate, 100 μg/ml of penicillin and 100 U/ml streptomycin (Invitrogen).
The DENV‐1 Hawaii strain and DENV‐2 New Guinea C strain were provided by Guangzhou Centers for Disease Control, and propagated in C6/36 cells. C6/36 cells were inoculated with DENV at a multiplicity of infection (MOI) of 0.01, and incubated at 35°C for 5–6 days (DENV‐1) and 2–3 days (DENV‐2). The supernatants were collected and clarified by centrifugation. Virus titer was measured by TCID50 assay in C6/36 cells and viral stocks were stored at −80°C. Vesicular stomatitis virus (VSV) was kindly provided by Dongyan Jin [Kok et al., 2011], and propagated in Vero cells.
Cell Viability Assay
MTT (3‐(4,5‐dimethyl‐2‐thiazolyl)‐2,5‐diphenyl‐2H‐tetrazolium bromide) was purchased from Sigma–Aldrich (St Louis, MO). A549 cells were cultured in 96‐well plates with different concentrations of CBX for 24 hr. Then 10 µl of PBS containing MTT (0.5 mg/ml) was added to each well. After 4 hr of incubation at 37°C, the supernatant was removed and 150 µl of DMSO was added to each well to solubilize the formazan crystals. Absorbance was measured in a microplate reader at 490 nm after vigorous shaking.
Infection of Cells
Adherent A549 and HUVEC cells were seeded on cell culture plates 1 day prior to infection. For the suspension cell line, THP‐1 cells were seeded on the day of infection before being infected by DENV. Virions were diluted in DMEM (A549 and HUVEC) or RPMI‐1640 (THP‐1) without FBS, and inoculated to cell monolayers or suspensions at MOI of 3 (DENV‐1) or MOI of 1 (DENV‐2). After 1 hr incubation at 37°C to allow virus attachment, supernatants were removed and cells were washed three times with PBS to remove residual virus. Infected cells were then incubated at 37°C in culture medium containing 2% FBS for indicated time.
Time‐of‐Addition Study
CBX (3β‐hydroxy‐11‐oxoolean‐12‐en‐30‐oic acid 3‐hemisuccinate) was purchased from Sigma–Aldrich and dissolved according to product instruction. Appropriate volumes of medium containing specific concentration of CBX were added to cells at different time frames: starting at 1 hr prior to DENV infection (pre‐treatment); 0 hr concurrently with viral infection till 24 hr post‐infection (post‐treatment); from 1 hr prior to viral infection till 24 hr post‐infection (continuous‐treatment); from 1 hr prior to viral infection till 16 hr post‐infection (short‐treatment). The cells were then incubated at 37°C for indicated time of infection before they were harvested.
Quantitative RT‐PCR
Total RNAs were extracted and purified from cells using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Reverse transcription was carried out by using 1 μg of RNA. For quantitative PCR, the reactions were performed in triplicate with each cDNA template by using IQ SYBR green Supermix (Bio‐Rad, Hercules, CA) and performed on Bio‐Rad CFX96 real‐time Detection System (Bio‐Rad). The mRNA expression levels were normalized to GAPDH expression. Primer pairs used were as follows: GAPDH (glyceraldehyde‐3‐phosphate dehydrogenase), forward primer GCCTTCCGTGTCCCCACTG, reverse primer CGCCTGCTTCACCACCTTC; DENV‐C gene, forward primer TCCTAACAATCCCACCAACAGCA, reverse primer AGTTCTGCGTCTCCTGTTCAAGA.
Immunofluorescence Assay
Cells were seeded onto coverslips (30–50% confluency) and infected with DENV‐2 (MOI of 1) or DENV‐1 (MOI of 3) for 24 hr, or left uninfected. Then the cells were fixed with 4% paraformaldehyde at 4°C for 30 min, and permeabilized with PBS containing 0.1% Triton X‐100. The virus protein was detected by using a monoclonal antibody against DENV prM glycoprotein (Abcam, Cambridge, MA) and a secondary anti‐mouse antibody (Invitrogen). Cell nuclei were stained with DAPI. Stained samples were visualized using Zeiss fluorescence microscope.
Virus Titration
DENV titer in harvested supernatants was determined by TCID50 assay. Samples were serially diluted and inoculated into C6/36 cells in 96‐well plates. After 5‐day (DENV‐2) or 7‐day (DENV‐1) incubation at 35°C with 5% CO2, cells were examined for cytopathic effects (CPE) under a light microscope. The virus titer (TCID50/ml) was calculated using the Reed–Muench method [Reed and Muench, 1938]. One TCID50/ml was equivalent to 0.69 PFU/ml. The titer of VSV was quantified using plaque assay. Ninety percent confluent Vero cells in 12‐well plates were infected with optimally diluted VSV and then covered with low melting temperature agarose (Sangon Biotech, Shanghai, China) after rinsing with phosphate buffered saline (PBS). At 48 hr post‐infection, 1% crystal violet was used to stain the Vero cells and plaques were quantified.
Virucidal Activity Assay
DENV‐2 suspension containing 1 × 108 PFU/ml or DENV‐1 suspension containing 1 × 107 PFU/ml was incubated with an equal volume of DMEM with or without indicated concentrations of CBX for indicated time at 37°C. The mixtures were then diluted in DMEM, and were used for infection in C6/36 cells to test viral titer or in A549 cells to test residual infectivity by TCID50 assay, immunofluorescence assay, or quantitative RT‐PCR.
Statistical Analysis
For each separate set of assays, at least three independent experiments were evaluated. Data are represented as mean ± SD. Statistical significance was determined by two‐tailed, unpaired Student's t‐test; P < 0.05 was considered statistically significant.
RESULTS
Inhibitory Effect of CBX on DENV Infection
Chemical structure of CBX is shown in Figure 1a. Cytotoxicity of CBX was first determined by the 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl tetrazolium bromide (MTT) method in A549 cells, one of the dengue‐permissive cells, to establish suitable dosages for further studies. As shown in Figure 1b, cell viability was close to control in treatments with 10, 25, 50, and 100 µM CBX, whereas only 80% cells survived in the presence of 200 µM CBX. Therefore, CBX concentrations ranging from 10 to 100 µM were considered for the following assay. To examine the effect of CBX on DENV infection, A549 cells were infected with DENV‐1 Hawaii strain and DENV‐2 New Guinea C strain, respectively, and incubated for 24 hr in the presence or absence of CBX. As shown in Figure 1c and d, the production of infectious DENV particles was significantly decreased by CBX treatment in a dose‐dependent manner. At concentrations of 50 and 100 µM, CBX treatment led to 500‐ and 1500‐fold reduction of DENV‐1 titer, respectively (Fig. 1c). Likewise, at 25, 50, and 100 µM CBX, around 20‐, 2000‐, and 5000‐fold lower levels of DENV‐2 titer were observed than control, respectively (Fig. 1d). Taken together, these results suggest that CBX has a dose‐dependent inhibition effect on progeny DENV production in A549 cells, and the anti‐DENV effect of CBX is not limited to a specific DENV serotype.
Figure 1.
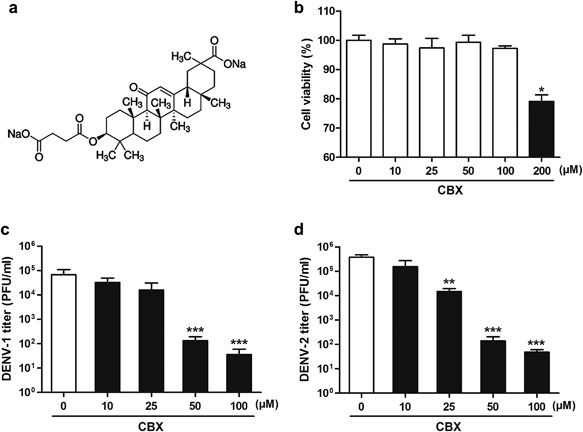
Antiviral activity of CBX against DENV in A549 cells. a: Chemical structure of CBX. b: Cell viability. A549 cells were treated with indicated concentrations of CBX for 24 hr and then tested by MTT assay for cell viability. c and d: A549 cells were treated with indicated concentrations of CBX from 1 hr prior to DENV‐1 (c) or DENV‐2 (d) infection till 24 hr post‐infection, and the titer of progeny virions was measured by TCID50 assay. Data are shown as mean ± SEM of at least three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
To determine whether CBX inhibits the production of infectious progeny DENV in other dengue‐permissive cells, TCID50 assay was performed to measure the virus titer in CBX‐treated THP‐1 and HUVEC cells, both of which are widely used in DENV studies [Halstead, 1988; Wu et al., 2000]. As shown in Figure 2a and b, both THP‐1 and HUVEC cells were well infected with DENV‐2, with about 30% of cells being infected. In THP‐1 cells, treated with 25, 50, and 100 µM CBX resulted in 100‐, 400‐, and 4000‐fold reduction of virus titer, respectively (Fig. 2c). In HUVEC cells, treated with 25, 50, and 100 µM CBX led to 15‐, 400‐, and 1000‐fold reduction of virus titer, respectively (Fig. 2d). These results indicate that the anti‐DENV activity of CBX is not cell‐specific.
Figure 2.
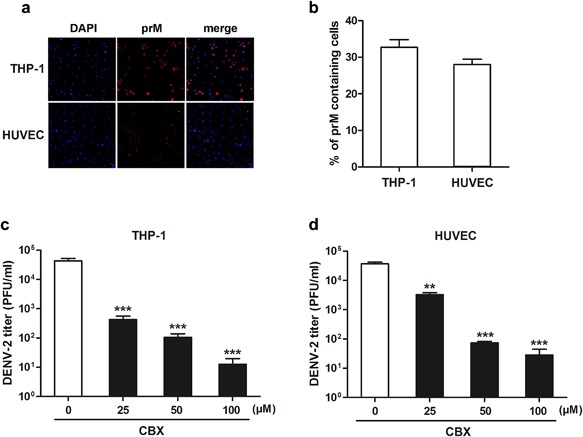
Inhibitory effect of CBX on DENV‐2 in THP‐1 and HUVEC cells. a and b: Immunofluorescence assay using DENV prM glycoprotein antibody was performed to determine the infection rates of THP‐1 and HUVEC cells. THP‐1 (c) and HUVEC (d) cells were treated with indicated concentrations of CBX from 1 hr prior to DENV‐2 infection till 24 hr post‐infection, and the titer of progeny virions was measured by TCID50 assay. Data are shown as mean ± SEM of at least three independent experiments. **P < 0.01; ***P < 0.001.
Influence of CBX on DENV Viral RNA Synthesis and Protein Expression
To examine the effect of CBX on the synthesis of DENV viral RNA, quantitative RT‐PCR was performed. As shown in Figure 3a and b, both DENV‐1 and DENV‐2 viral RNA (C protein) synthesis were not affected by CBX treatment even at the highest concentration (100 µM). Meanwhile, immunofluorescence assay was performed to analyze the effect of CBX on DENV viral protein expression upon infection. Antibody specific to the DENV structural prM protein was used in this part of the study. The percentages of prM containing cells were calculated to determine the expression of viral protein (Fig. 3d and f). As shown in Figure 3c and e, CBX treatment at the highest concentration (100 µM) did not reduce DENV‐1 or DENV‐2 viral protein expression. These results suggest that both DENV viral RNA synthesis and protein expression are not regulated by CBX treatment.
Figure 3.
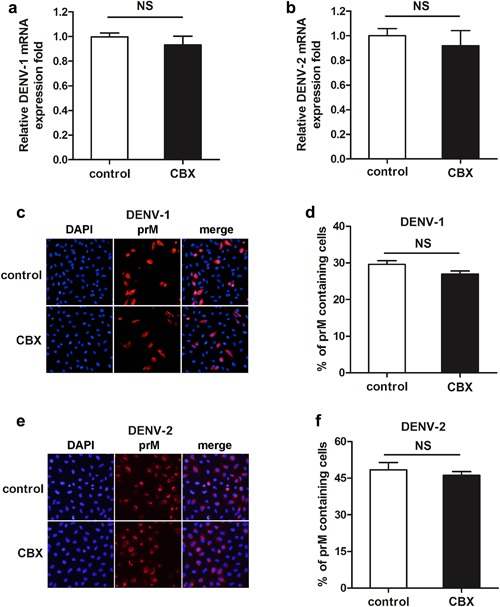
Roles of CBX on DENV viral RNA and viral protein synthesis. A549 cells were treated with 100 µM CBX from 1 hr prior to DENV‐1 or DENV‐2 infection till 24 hr post‐infection. a and b: The total RNA was isolated from DENV‐1(a)‐ or DENV‐2(b)‐infected cells and analyzed by quantitative RT‐PCR. c and d: The expression of DENV‐1(c) or DENV‐2(e) viral protein was determined by immunofluorescence assay using DENV prM glycoprotein antibody, and the percentages of DENV‐1(d) or DENV‐2(f) prM containing cells were determined by PicCnt 100×. Data are shown as mean ± SEM of at least three independent experiments. NS, non‐significant by t‐test (n = 3).
Time‐of‐Addition Study of CBX on DENV Infection
To further identify the step(s) of progeny DENV production inhibited by CBX, time‐course of the inhibitory effect was analyzed by a time‐of‐addition experiment in A549 cells. 100 µM CBX was added into the media at different time frames (Fig. 4a). Starting at 1 hr prior to DENV infection (pre‐treatment); 0 hr concurrently with viral infection till 24 hr post‐infection (post‐treatment); from 1 hr prior to viral infection till 24 hr post‐infection (continuous‐treatment), or till 16 hr post‐infection (short‐treatment). Cell supernatants were harvested for TCID50 assay. As shown in Figure 4b, both post‐treatment and continuous‐treatment of cells by CBX led to approximately 3000‐fold reduction of progeny virus titer. By contrast, pre‐treatment with CBX did not have a significant impact on DENV‐2 yields (Fig. 4b). As CBX can freely shuttle into cytoplasm by all three treatments mentioned above [Parke, 1983; Connors, 2012], this result indicates that the inhibitory effect of CBX on DENV is not exhibited by targeting the host cells. When eliminating CBX from the media at 16 hr post‐infection (short‐treatment), the titer of progeny DENV‐2 particles also was not affected (Fig. 4b). In our DENV‐2‐infected system, newly formed virions began to secret from A549 cells at 16 hr post‐infection (data not shown). So at this point, short‐treatment as mentioned can prevent progeny virions from contacting with CBX. Therefore, these results from time‐of‐addition study suggest that CBX inhibits DENV by directly targeting the infectious virions extracellularly.
Figure 4.
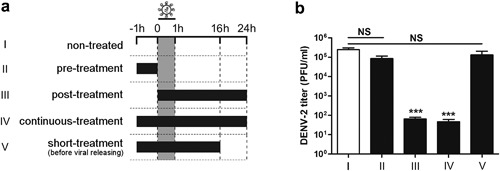
Time course of CBX‐addition experiment during DENV‐2 infection. a: Infected with DENV‐2, A549 cells were treated with 100 µM CBX at different time frames: starting at 1 hr before DENV‐2 infection (pre‐treatment); 0 hr concurrently with viral infection till 24 hr post‐infection (post‐treatment); from 1 hr prior to viral infection till 24 hr post‐infection (continuous‐treatment) or till 16 hr post‐infection (short‐treatment). b: The titer of progeny DENV‐2 particles was measured by TCID50 assay. Data are shown as mean ± SEM of at least three independent experiments. ***P < 0.001. NS, non‐significant by t‐test (n = 3).
Pre‐Incubation With CBX Decreases DENV Titer and Infectivity
To further study the direct effects of CBX on DENV particles, DENV‐1 and DENV‐2 suspensions were pre‐incubated with different concentrations of CBX, respectively for indicated time, and then the viral titer was determined by TCID50 assay. As shown in Figure 5a, DENV‐1 titer was significantly decreased by 10‐ and 1500‐fold when incubated with 50 or 100 µM CBX, respectively, but not with 25 µM CBX. When DENV‐1 suspensions were incubated with 50 or 100 µM CBX for 1, 6, and 12 hr, respectively, virus titer at each time point was significantly decreased (Fig. 5b). Likewise, CBX exhibited a similar behavior on DENV‐2. As shown in Figure 5c, treating DENV‐2 suspensions with 25, 50, and 100 µM CBX resulted in 10‐, 80‐, and 1000‐fold reduction of virus titer, respectively. Moreover, longer incubation of CBX correspondingly led to a lower titer of residue DENV‐2, peaking at 12 hr incubation (Fig. 5d). Surprisingly, the titer of DENV‐2 was almost undetectable when incubating with 100 µM CBX for 12 hr (Fig. 5d). Taken together, these data indicate that incubation with CBX effectively decreases DENV titer in a dose‐ and time‐dependent manner.
Figure 5.
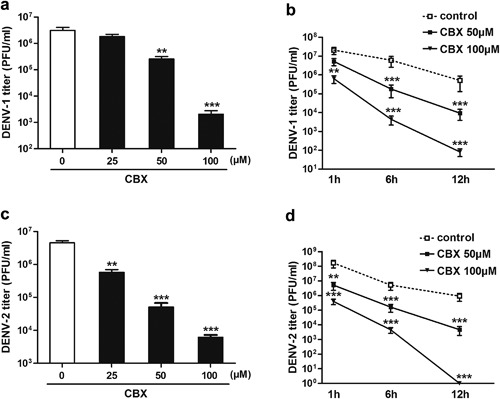
Pre‐incubation with CBX decreases the titer of DENV. DENV‐1 (a and b) or DENV‐2 (c and d) was directly incubated with indicated concentrations of CBX for 6 hr (a and c) or for indicated time (b and d) at 37°C. The titer of residual virus was determined by TCID50 assay. Data are shown as mean ± SEM of at least three independent experiments. **P < 0.01; ***P < 0.001.
Furthermore, we directly incubated DENV‐2 with 100 µM CBX at 37°C for 1 hr, and then followed by an infection in A549 cells for indicated time. The viral RNA synthesis, viral protein expression and progeny virus production were compared as indicated above. As shown in Figure 6a, the levels of viral RNA at 12 and 24 hr were barely detected in cells infected with CBX‐incubated DENV‐2. While in control cells, viral RNA synthesis was gradually increased post infection (Fig. 6a). Immunofluorescence microscopy images showed that no or just few cells were positively stained with DENV prM glycoprotein antibody in CBX‐incubating group, compared to approximate 52% of control cells were positively stained at 24 hr post DENV‐2 infection (Fig. 6b and c). Likewise, the production of progeny virions from CBX‐incubating group was dramatically decreased (Fig. 6d). Therefore, DENV infectivity is significantly inhibited by CBX pre‐incubation.
Figure 6.
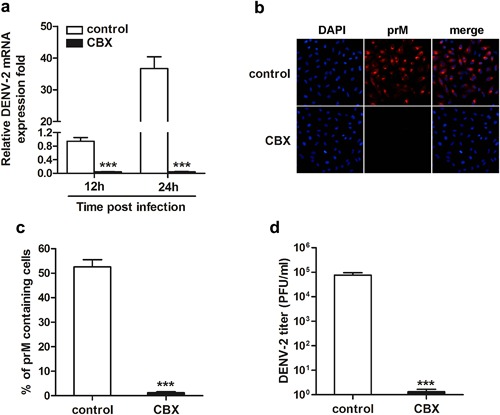
Infectivity of DENV‐2 in A549 cells is inhibited by CBX‐incubation. DENV‐2 particles were pre‐incubated with 100 µM CBX at 37°C for 1 hr, and then infected A549 cells for indicated time. a: The total RNA was isolated from the infected cells and analyzed by quantitative RT‐PCR using primers specific for the viral C protein (CP) mRNA. b: After 24 hr of infection, the expression of viral protein was determined by immunofluorescence assay using DENV prM glycoprotein antibody; c: the percentages of prM containing cells were determined by PicCnt 100×; d: the titer of progeny DENV‐2 particles was measured by TCID50 assay. Data are shown as mean ± SEM of at least three independent experiments. ***P < 0.001.
The Virucidal Activity of CBX Against DENV Infection
Data from Figures 5 and 6 suggested that the antiviral activity of CBX against DENV may be mainly due to a virucidal effect exerted by the compound itself. To test this assumption, A549 cells were infected with DENV‐2 and then incubated for 24 hr with different concentrations of CBX. The supernatants were collected for virus RNA detection. As shown in Figure 7a, DENV‐2 RNA from progeny virions in the supernatants was significantly decreased by CBX treatment in a dose‐dependent manner. At concentrations of 50 and 100 µM, CBX treatment led to 5‐ and 10‐fold reduction of DENV‐2 mRNA expression, respectively (Fig. 7a). It suggests that CBX has a virucidal activity to inactivate the progeny DENV particles. To further corroborate the virucidal effect of CBX, A549 cells were infected with a low MOI of DENV‐2 for 48 hr to complete at least two replication cycles, and incubated with 100 µM CBX till the end of infection. Quantitative RT‐PCR result showed that DENV‐2 viral RNA synthesis was significantly decreased by 50% in CBX‐treated group (Fig. 7b). Similarly, result from immunofluorescence assay showed that CBX treatment led to effective reduction of viral protein expression (Fig. 7c). The percentage of prM containing cells was half less than control group (Fig. 7d). Taken together, these results indicate that CBX inhibits the replication of progeny DENV particles through its virucidal activity.
Figure 7.
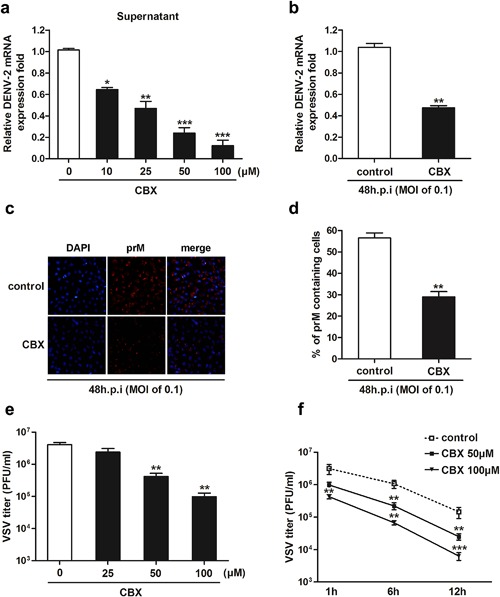
The virucidal effects of CBX against DENV, and other enveloped virus. a: A549 cells were treated with indicated concentrations of CBX from 1 hr prior to DENV‐2 infection till 24 hr post‐infection, and the expression of viral RNA from progeny virions in the supernatants was determined by quantitative RT‐PCR. b–d: A549 cells were treated with 100 µM CBX from 1 hr prior to DENV‐2 infection (MOI of 0.1) till 48 hr post‐infection. b: The total RNA was isolated from the infected cells and analyzed by quantitative RT‐PCR. c: The expression of viral protein was determined by immunofluorescence assay using DENV prM glycoprotein antibody. d: The percentages of prM containing cells were determined by PicCnt 100×. e and f: VSV was directly incubated with indicated concentrations of CBX for 6 hr (e) or for indicated time (f) at 37°C. The titer of residual virus was determined by plaque assay. Data are shown as mean ± SEM of at least three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Antiviral Effect of CBX Against Other Enveloped Virus
To confirm the specificity of the antiviral activity of CBX, VSV, another enveloped virus was evaluated. VSV suspensions were pre‐incubated with different concentrations of CBXm, respectively for indicated time, and then the viral titer was determined by plaque assay. As shown in Figure 7e, VSV titer was significantly decreased by 10‐ and 40‐fold when incubated with 50 or 100 µM CBX, respectively, but not with 25 µM CBX. When VSV suspensions were incubated with 50 or 100 µM CBX for 1, 6, and 12 hr, respectively, virus titer was significantly decreased in a time‐dependent manner (Fig. 7f). Potent inhibition against another enveloped virus (VSV) indicates that the antiviral effect of CBX is not specific to DENV.
DISCUSSION
The current epidemic of dengue infection poses a threat to public health worldwide, making it clear that safe and effective countermeasures are urgently needed against this infection. Unfortunately, there is almost no specific antiviral treatment currently available for dengue diseases. Given the complex pathology of the illness and the need to control four virus serotypes simultaneously, dengue vaccine development has been challenging too. In response to the urgent need for effective anti‐DENV drugs, our work for the first time identifies CBX, one of the glycyrrhetinic acid (GNa) derivatives, as a potential natural compound against different serotypes of DENV.
CBX treatment did not inhibit DENV‐1 or DENV‐2 RNA synthesis and protein expression in one replication cycle, but markedly reduced progeny virus production, suggesting that post‐translation stages of DENV lifecycle as possible targets. Time‐of‐addition experiment was also performed to narrow down the effective period of this compound. It is reported that CBX can freely shuttle into cytoplasm [Parke, 1983; Connors, 2012]. However, progeny DENV‐2 was inhibited only when CBX was added after virus attachment till 24 hr post‐infection (post‐treatment) or throughout the course of infection (continuous‐treatment), but not with pre‐treatment of cells (pre‐treatment) or till 16 hr post‐infection (short‐treatment), indicating that the inhibition of CBX is not exhibited by targeting the host cells. From these results, it was deduced that DENV itself is the target of CBX. To test this assumption, virucidal activity assay was performed. Both the titer and residual infectivity of DENV were affected as assumed by pre‐incubating with CBX, which manifested in the reduction of viral RNA and viral protein, and the drop in viral titer. Further study found that the level of viral RNA in the supernatants was significantly down‐regulated in CBX‐treated group, demonstrating CBX directly inactivates the progeny DENV particles via its virucidal activity. The virucidal effect of CBX on DENV might be exerted by irreversibly disrupting viral envelope intactness so that virus loses its protection and results in degradation.
In this study, we used three different cell lines (A549, THP‐1, and HUVEC) to investigate the effect of CBX on DENV. Given their high susceptibility to DENV, A549 cells were the main cell line used. THP‐1 cell line substituted peripheral blood mononuclear cells (PBMC) to mimic in vivo infection [Clyde et al., 2006]. HUVEC cells, a cell line of human umbilical vein endotheliocyte, were also tested as they are a representative of non‐cancerous, primary cell type. As a result, DENV‐2 was inhibited dose‐dependently with increasing CBX in all three cell lines, demonstrating that CBX effectively inhibits DENV‐2 in the various cell types targeted by the virus. Beside DENV‐2, DENV‐1 viruses were also used to verify the inhibitory effect of CBX. All the results have demonstrated that the antiviral activity of CBX has no specificity on cell type or DENV serotype, which might predict CBX as a new anti‐DENV compound to exhibit favorable application perspectives.
Previous study has showed that, glycyrrhizin, another effective compound derived from glycyrrhiza, inhibited DENV infection in Vero cells [Crance et al., 2003]. This promoted us to suppose that CBX, one of the derivatives of glycyrrhetinic acid (GNa), may play an effective role in anti‐DENV infections. As observed in our study, CBX treatment significantly reduced the progeny virus yield of different serotype of DENV (1 and 2). Further study found that CBX inhibits DENV infection through its virucidal activity. Beside DENV, CBX has been previously found to inhibit another virus, HSV [Partridge and Poswillo, 1984; Dargan and Subak‐Sharpe, 1986]. However, the antiviral mechanisms of CBX on these two viruses are quite distinct. In regarding to HSV, CBX appeared to be active throughout the replication cycle: by reducing the synthesis of some polypeptides, affecting the transportation of certain proteins, and inhibiting the post‐translational processing. As a result, CBX treatment greatly diminished progeny HSV quality, and it is thought to inhibit HSV infection in a non‐specific manner, by altering cellular activities that can affect virus replication or activate host antiviral mechanisms. While in our model, CBX directly inhibited the infectivity of DENV particles without targeting the host cells. Likewise, further study found that pre‐incubation with CBX significantly decreased VSV titer. Taken together, it suggests that the antiviral activity of CBX was not limited to a specific type of virus, and the mechanisms might be something different.
To be pointed out, CBX is a widely used compound as an inhibitor of gap junctions [Connors, 2012]. Gap junctions are fast‐track intercellular communication routes. Genetic studies have revealed that connexin (Cx) protein is the molecular component of vertebrate gap junction channels [Paul, 1986]. Gap junctions comprise clusters of transcellular channels that allow small signaling molecules and inorganic ions to transfer rapidly and directly from one cell to another. Recent studies have reported that gap junctions play key roles in the physiological processes and pathological conditions of several viruses, such as HIV, cytomegalovirus (CMV), Borna disease virus, and Rous sarcoma virus [Eugenin, 2014]. Importantly, in order to increase virus production, infection with the latter two viruses above results in down‐regulation of Cx expression. As a non‐specific gap junction blocker, CBX inhibits various Cx proteins including Cx26, Cx32, Cx36, and Cx43 [Tonkin et al., 2015]. Therefore, the possibility that CBX inhibits DENV infection through blocking Cx proteins could not be ruled out.
To this end, much more work would be needed to elucidate exactly how CBX inactivates DENV through its virucidal activity. Further studies in suitable animal models would also be required to evaluate the reproducibility of the inhibitory effects of CBX in vivo. In conclusion, our work provides compelling data that CBX has inhibitory effect on DENV infection. Importantly, the antiviral activity of CBX is not limited to a specific cell type or a specific DENV serotype. Combined with its history as a clinical treatment for gastric ulcers or inflammation with a good safety profile [Turpie and Thomson, 1965; Ram et al., 2009], CBX might potentially be developed as a promising antiviral drug for dengue infection.
Conflicts of interest: none.
The work presented in this report is the subject of our patent (no. 201210588691.2) held by Sun Yat‐sen University in Guangzhou, China.
Contributor Information
Zhicong Yang, Email: yangzc@gzcdc.org.cn.
Xi Huang, Email: huangxi6@mail.sysu.edu.cn.
REFERENCES
- Ashfaq UA, Masoud MS, Nawaz ZJ, Riazuddin S. 2011. Glycyrrhizin as antiviral agent against hepatitis C virus. J Transl Med 9:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers TJ, Hahn CS, Galler R, Rice CM. 1990. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol 44:649–688. [DOI] [PubMed] [Google Scholar]
- Chen F, Chan KH, Jiang Y, Kao RY, Lu HT, Fan KW, Cheng VC, Tsui WH, Hung IF, Lee TS, Guan Y, Peiris JS, Yuen KY. 2004. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol 31:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. 2003. Glycyrrhizin, an active component of liquorice roots, and replication of SARS‐associated coronavirus. Lancet 361:2045–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyde K, Kyle JL, Harris E. 2006. Recent advances in deciphering viral and host determinants of dengue virus replication and pathogenesis. J Virol 80:11418–11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors BW. 2012. Tales of a dirty drug: Carbenoxolone, gap junctions, and seizures. Epilepsy Curr 12:66–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cos P, Maes L, Vlietinck A, Pieters L. 2008. Plant‐derived leading compounds for chemotherapy of human immunodeficiency virus (HIV) infection—An update (1998–2007). Planta Med 74:1323–1337. [DOI] [PubMed] [Google Scholar]
- Crance JM, Scaramozzino N, Jouan A, Garin D. 2003. Interferon, ribavirin, 6‐azauridine and glycyrrhizin: Antiviral compounds active against pathogenic flaviviruses. Antivir Res 58:73–79. [DOI] [PubMed] [Google Scholar]
- Dargan DJ, Aitken JD, Subak‐Sharpe JH. 1988. The effect of triterpenoid compounds on uninfected and herpes simplex virus‐infected cells in culture. III. Ultrastructural study of virion maturation. J Gen Virol 69:439–444. [DOI] [PubMed] [Google Scholar]
- Dargan DJ, Galt CB, Subak‐Sharpe JH. 1992a. The effect of cicloxolone sodium on the replication in cultured cells of adenovirus type 5, reovirus type 3, poliovirus type 1, two bunya viruses and Semliki Forest virus. J Gen Virol 73:407–411. [DOI] [PubMed] [Google Scholar]
- Dargan DJ, Galt CB, Subak‐Sharpe JH. 1992b. The effect of cicloxolone sodium on the replication of vesicular stomatitis virus in BSC‐1 cells. J Gen Virol 73:397–406. [DOI] [PubMed] [Google Scholar]
- Dargan DJ, Subak‐Sharpe JH. 1985. The effect of triterpenoid compounds on uninfected and herpes simplex virus‐infected cells in culture. I. Effect on cell growth, virus particles and virus replication. J Gen Virol 66:1771–1784. [DOI] [PubMed] [Google Scholar]
- Dargan DJ, Subak‐Sharpe JH. 1986. The antiviral activity against herpes simplex virus of the triterpenoid compounds carbenoxolone sodium and cicloxolone sodium. J Antimicrob Chemother 18:185–200. [DOI] [PubMed] [Google Scholar]
- Eugenin EA. 2014. Role of connexin/pannexin containing channels in infectious diseases. FEBS Lett 588:1389–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filomatori CV, Lodeiro MF, Alvarez DE, Samsa MM, Pietrasanta L. 2006. A 5′ RNA element promotes dengue virus RNA synthesis on a circular genome. Genes Dev 20:2238–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore C, Eisenhut M, Krausse R, Ragazzi E, Pellati D. 2008. Antiviral effects of Glycyrrhiza species. Phytother Res 22:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman MG, Kouri G. 2002. Dengue: An update. Lancet Infect Dis 2:33–42. [DOI] [PubMed] [Google Scholar]
- Halstead SB. 1988. Pathogenesis of dengue: Challenges to molecular biology. Science 239:476–481. [DOI] [PubMed] [Google Scholar]
- Hardy ME, Hendricks JM, Paulson JM, Faunce NR. 2012. 18 beta‐glycyrrhetinic acid inhibits rotavirus replication in culture. Virol J 9:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrees S, Ashfaq UA. 2012. A brief review on dengue molecular virology, diagnosis, treatment and prevalence in Pakistan. Genet Vaccin Ther 10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok KH, Lui PY, Ng MH, Siu KL, Au SW, Jin DY. 2011. The double‐stranded RNA‐binding protein PACT functions as a cellular activator of RIG‐I to facilitate innate antiviral response. Cell Host Microbe 9:299–309. [DOI] [PubMed] [Google Scholar]
- Lin JC. 2003. Mechanism of action of glycyrrhizic acid in inhibition of Epstein‐Barr virus replication in vitro. Antivir Res 59:41–47. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Takenaka K, Shimomura H, Fujii N, Shinagawa K, Kiura K, Harada M. 2001. Lamivudine and glycyrrhizin for treatment of chemotherapy‐induced hepatitis B virus HBV) hepatitis in a chronic HBV carrier with non‐Hodgkin lymphoma. Leuk Lymphoma 41:191–195. [DOI] [PubMed] [Google Scholar]
- Orlent H, Hansen BE, Willems M, Brouwer JT, Huber R. 2006. Biochemical and histological effects of 26 weeks of glycyrrhizin treatment in chronic hepatitis C: A randomized phase II trial. J Hepatol 45:539–546. [DOI] [PubMed] [Google Scholar]
- Pagni S, Fernandez‐Sesma A. 2012. Evasion of the human innate immune system by dengue virus. Immunol Res 54:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parke DV. 1983. The biochemical pharmacology of carbenoxolone. Its possible mechanisms of action. Acta Gastroenterol Belg 46:437–447. [PubMed] [Google Scholar]
- Partridge M, Poswillo DE. 1984. Topical carbenoxolone sodium in the management of herpes simplex infection. Br J Oral Maxillofac Surg 22:138–145. [DOI] [PubMed] [Google Scholar]
- Paul DL. 1986. Molecular cloning of cDNA for rat liver gap junction protein. J Cell Biol 103:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompei R, Flore O, Marccialis MA, Pani A, Loddo B. 1979. Glycyrrhizic acid inhibits virus growth and inactivates virus particles. Nature 281:689–690. [DOI] [PubMed] [Google Scholar]
- Pompei R, Laconi S, Ingianni A. 2009. Antiviral properties of glycyrrhizic acid and its semisynthetic derivative. Mini Rev Med Chem 9:996–1001. [DOI] [PubMed] [Google Scholar]
- Ram A, Singh SK, Singh VP, Kumar S, Ghosh B. 2009. Inhaled carbenoxolone prevents allergic airway inflammation and airway hyperreactivity in a mouse model of asthma. Int Arch Allergy Immunol 149:38–46. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Muench H. 1938. A simple method of estimating fifty percent endpoints. Am J Hyg 27:493–497. [Google Scholar]
- Rozental R, Srinivas M, Spray DC. 2001. How to close a gap junction channel: Efficies and potencies of uncoupling agents. Methods Mol Biol 154:447–476. [DOI] [PubMed] [Google Scholar]
- Simmons CP, Farrar JJ, Nguyen vV, Wills B. 2012. Dengue. N Engl J Med 366:1423–1432. [DOI] [PubMed] [Google Scholar]
- Smirnov VS, Zarubaev VV, Anfimov PM, Shtro AA. 2012. Effect of a combination of glutamyl‐tryptophan and glycyrrhizic acid on the course of acute infection caused by influenza (H3H2) virus in mice. Vopr Virusol 57:23–27. [PubMed] [Google Scholar]
- Takahara T, Watanabe A, Shiraki K. 1994. Effects of glycyrrhizin on hepatitis B surface antigen: A biochemical and morphological study. J Hepatol 21:601–609. [DOI] [PubMed] [Google Scholar]
- Tonkin RS, Mao Y, O'Carroll SJ, Nicholson LF, Green CR, Gorrie CA, Moalem‐Taylor G. 2015. Gap junction proteins and their role in spinal cord injury. Front Mol Neurosci 7:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpie AG, Thomson TJ. 1965. Carbenoxolone sodium in the treatment of gastric ulcer with special reference to side‐effects. Gut 6:591–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Ni H, Xu R, Barrett AD, Watowich SJ. 2000. Evolutionary relationships of endemic/epidemic and sylvatic dengue viruses. J Virol 74:3227–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SJ, Grouard‐Vogel G, Sun W, Mascola JR, Brachtel E. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nat Med 6:816–820. [DOI] [PubMed] [Google Scholar]
- Zybert IA, van der Ende‐Metselaar H, Wilschut J, Smit JM. 2008. Functional importance of dengue virus maturation: Infectious properties of immature virions. J Gen Virol 89:3047–3051. [DOI] [PubMed] [Google Scholar]


