Abstract
Krebs von den Lungen 6 antigen (KL‐6) has been shown to be a useful biomarker of the severity of Respiratory syncytial virus bronchiolitis. To assess the correlation between the clinical severity of acute bronchiolitis, serum KL‐6, and the causative viruses, 222 infants with acute bronchiolitis presenting at the Pediatric Emergency Department of Estaing University Hospital, Clermont‐Ferrand, France, were prospectively enrolled from October 2011 to May 2012. Disease severity was assessed with a score calculated from oxygen saturation, respiratory rate, and respiratory effort. A nasopharyngeal aspirate was collected to screen for a panel of 20 respiratory viruses. Serum was assessed and compared with a control group of 38 bronchiolitis‐free infants. No significant difference in KL‐6 levels was found between the children with bronchiolitis (mean 231 IU/mL ± 106) and those without (230 IU/mL ± 102), or between children who were hospitalized or not, or between the types of virus. No correlation was found between serum KL‐6 levels and the disease severity score. The absence of Human Rhinovirus was a predictive factor for hospitalization (OR 3.4 [1.4–7.9]; P = 0.006). Older age and a higher oxygen saturation were protective factors (OR 0.65[0.55–0.77]; P < 0.0001 and OR 0.67 [0.54–0.85] P < 0.001, respectively). These results suggest that in infants presenting with bronchiolitis for the first time, clinical outcome depends more on the adaptive capacities of the host than on epithelial dysfunction intensity. Many of the features of bronchiolitis are affected by underlying disease and by treatment. J. Med. Virol. 86:1944–1952, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: KL‐6, acute bronchiolitis, clinical score, children, severity prediction
INTRODUCTION
Acute viral bronchiolitis is one of the most common respiratory diseases in early childhood and is a major health problem worldwide [Zorc and Hall, 2010]. The seasonal burden of the disease, the number of hospitalizations each year and the risk of subsequent asthma [Koponen et al., 2012] bring about substantial costs in developed countries [Pelletier et al., 2006].
Respiratory syncytial virus (RSV) and Human Rhinovirus (HRV) seem to be the most frequent etiologic agents, but other viruses such as human Metapneumovirus (hMPV), Influenza virus (IV), and Parainfluenza virus (PIV) can also be involved [Marguet et al., 2009; Feuillet et al., 2012].
The spectrum of clinical outcomes is wide, but bronchiolitis is more severe when caused by RSV [Marguet et al., 2009]. In contrast, while HRV is involved in milder forms, it is more likely to be associated with recurrent wheezing in infancy [Marguet et al., 2009; Valkonen et al., 2009; Gern, 2010; Midulla et al., 2012].
Over the past few years, many significant studies have focused on understanding the pathology but the respective influence of each virus on the larger spectrum of outcomes remains unclear. In a murine model of human RSV disease, viral infection was shown to cause major peri‐bronchiolar inflammation that leads to epithelial barrier alteration [Bern et al., 2011]. However, the results of experimental studies on the pathogenesis of the specific features of bronchiolitis cannot always be extended to humans because of host specificity.
Most major recent clinical studies have limitations because they were conducted in a selected population of hospitalized children and there are few data on children seen as outpatients [Kieninger and Regamey, 2012; Koponen et al., 2012]. It is likely that the potential severity of the symptoms and the risk of wheezing can be explained by the initial epithelial injury. Differences in the inflammatory cascade, depending on which virus is involved, may also play a role [Ioannidis et al., 2012].
Krebs von den Lungen 6 antigen (KL‐6) is a high‐molecular‐weight mucinous glycoprotein which was first described as a pulmonary adenocarcinoma‐related antigen. This protein is normally present on type II pneumocytes and respiratory bronchiolar epithelial cells [Hirasawa et al., 1997]. Elevation of serum KL‐6 levels is associated with increased permeability of the alveolar–capillary barrier. Serum KL‐6 has recently been shown to be a useful marker of the severity of RSV bronchiolitis in Japan [Kawasaki et al., 2009].
It was thus decided to perform a prospective study in a non‐selected population of children with bronchiolitis during the winter epidemic season to assess the correlation between the clinical severity of acute bronchiolitis, serum KL‐6 levels, and the causative viruses.
MATERIALS AND METHODS
Cases
Consecutive infants with acute bronchiolitis, aged under 1 year and examined in the Pediatric Emergency Department of the Estaing University Hospital, Clermont‐Ferrand, France during one epidemic season from October 2011 to May 2012 were considered for inclusion.
Bronchiolitis was diagnosed by the presence of a recent history of an upper respiratory tract infection followed by onset of respiratory distress with cough, tachypnea, retraction and diffuse crackles, or wheezing on auscultation, in accordance with the international recommendations on the diagnosis and management of acute bronchiolitis [Subcommittee on diagnosis and management of bronchiolitis, 2006].
Children were not included if they had suspected or confirmed underlying chronic diseases (i.e., cystic fibrosis, chronic pulmonary disease, congenital heart disease, bronchopulmonary disease, prematurity) or had already had more than one wheezing episode.
Detailed demographic data were obtained from the parents by a structured questionnaire. Studied variables included age, gender, exposure to tobacco smoke, familial (parents or sister or brother) or personal history of atopy, and nursery attendance.
Weight and vital signs (heart rate, respiratory rate, tympanic temperature, oxygen saturation breathing ambient air measured by pulse oximetry—SpO2) were recorded in the emergency room.
Each infant's condition was classified as mild, moderate or severe according to a severity score calculated from SpO2, respiratory rate, and respiratory effort on admission [Wainwright et al., 2003].
Patients were admitted to hospital (inpatient group) on the basis of French national guidelines [ANAES, [Link]] that is, aged under 6 weeks, major deterioration in general condition, apnoea or cyanosis, respiratory rate over 60/min, persistence of an SpO2 under 94%, gastrointestinal disorders compromising hydration, dehydration with weight loss over 5%, atelectasis detected by a chest radiograph carried out because of clinical signs, or psychosocial problems.
If the infant was hospitalized, the following data were recorded: C‐reactive protein (CRP), days of oxygen therapy, need for and duration of respiratory support (mechanical or non‐invasive ventilation), need for and duration of stay in the Pediatric Intensive Care Unit (PICU) or Pediatric Department, days of gastroenteral feeding or parenteral nutrition, occurrence of a respiratory complication defined as a secondary pulmonary infection documented by a blood sample and chest radiograph, and/or atelectasis. All the radiological findings were reviewed by a pediatric pulmonologist. Occurrence of acute respiratory distress syndrome or death was also recorded.
Infants who did not require hospitalization (outpatient group) were cared for at home after their parents had been taught chest physiotherapy and how to relieve nasal obstruction, according to French recommendations [ANAES, [Link]].
Controls
Infants aged under 12 months were enrolled after the anesthesia consultation carried out before a planned surgical intervention (e.g., surgery for inguinal hernia or clubfoot), as the control group to measure KL‐6 levels. A blood sample to determine KL‐6 was processed at the same time as the pre‐operative blood test. At this point, health status was evaluated by a complete medical check‐up, which included a clinical questionnaire. Children with a previous history of pulmonary disease (i.e., history of a wheezing episode prior to inclusion or cardiopulmonary disease or chronic pulmonary disease or prematurity) or with any infectious process, particularly a recent history of an upper or lower respiratory tract infection, were excluded.
Detection of Respiratory Viruses for Cases
A nasopharyngeal aspirate was collected either in the emergency room or within the first 24 h following admission to detect respiratory viruses.
Detection was performed prospectively by one‐step real‐time Polymerase Chain Reactions (PCRs) using the Respiratory Multi‐Well System (MWS) r‐gene™ range of assays (Argène‐bioMérieux, Craponne, France). A panel of viruses from the same extracted sample was screened for by five duplex PCR kits (Influenza A/B r‐gene™, RSV/hMPV r‐gene™, HCoV/hPIV r‐gene™, AdV/hBoV r‐gene™, Rhino&EV/Cc r‐gene™). The viruses screened for included IV A and B, RSV A and B, hMPV, Coronaviruses 229E, NL63, OC43 and HKU1 (referred collectively as CoVs), PIV 1–4, Adenovirus (ADV), Bocavirus (BoV), HRVs, and Enteroviruses (EVs). HRVs and EVs are related closely to Picornaviruses and cannot be differentiated by this assay. Further identification of genus, species, and type was performed by sequencing of the VP4/VP2 genomic region, as described previously [Henquell et al., 2012]. After proteinase K (20 mg/mL) pre‐treatment, total nucleic acids were extracted from 400 µL of clinical sample using the NucliSENS® easyMAG™ extraction automated system (Argène‐bioMérieux, Craponne, France) and eluted in 100 µL. In accordance with the manufacturer's recommendations, 10 µL of the extracted sample were added to 15 µL of each ready‐to‐use amplification premix for the duplex detections. The protocol and amplification program, with a first step of reverse transcription for Ribonucleic Acid (RNA) targets, was common to all the respiratory MWS r‐gene™ kits. The real‐time one‐step reverse transcriptase‐PCRs were performed on the Rotor‐Gene system (Qiagen, Venlo, The Netherlands) following the manufacturer's specifications. All results were validated with positive and negative controls, and a cellular cell control (detected simultaneously with HRV/EV with the Rhino&EV/Cc r‐gene™ kit) validated the sample quality and the whole PCR process from extraction to amplification.
Viral co‐infection was defined as a positive result for the detection of more than one virus.
KL‐6 Determination for Cases and Controls
Venous blood samples were processed to serum and deep frozen at −20°C, checked for stability during the freezing period and then assayed. An Enzyme Linked Immunosorbent Assay (reference number: ABIN366553) from Immulab GmbH (Kassel, Germany) was used to determine serum KL‐6 concentrations. Each measurement was performed in duplicate with 100 µL of serum per measurement, according to the manufacturer's recommendations. The 96‐well microplate was read by measuring absorbance at 450 nm with a correction wavelength of 570 nm. The KL‐6 calibration curve shows 1000 IU/mL as the highest point. The minimum detectable level of the assay was 3.9 IU/mL according to the manufacturers. The determined coefficient of variation for duplicates across the entire concentration range for calibrators, controls and samples was <8%. No significant cross‐reactivity or interference was observed.
Ethics
All the infants' parents gave written informed consent for their infant to participate. The study was approved by the local Ethics Committee (Comité de Protection des Personnes Sud‐Est I, Saint‐Etienne, France) and was posted on Clinical Trials (http://www.clinicaltrials.gov/) under the ID number NCT01437956.
Statistical Analysis
Owing to the originality of the study and to the lack of information in the literature concerning the relationship between serum KL‐6 and disease severity, we believed that it would be difficult to give an a priori sample size estimation. According to the inclusion capacity and a previous study carried out in the Pediatric Emergency Department [Henquell et al., 2012], it was estimated that 200 subjects would be required to show a significant correlation coefficient of 0.3.
Statistics were computed with STATA V10 (Stata Corp., College Station, TX). Data were expressed as frequencies and associated percentages for categorical data and as mean ± SD and minimum–maximum, or as median and interquartile range for quantitative parameters.
Tests were two‐sided, with a type I error set at α of 5%.
The principal analysis (link between KL‐6 values and the severity score) was carried out using the Spearman correlation coefficient.
Secondary analysis, comparing groups (case vs. control), hospitalization (yes vs. no), and virus detection (yes vs. no) with categorical data were performed using the chi‐square test or the Fisher‐exact when appropriate. Differences concerning continuous data between independent groups were studied using the Student's t‐test or the Kruskal Wallis test when appropriate (normality verified by the Shapiro–Wilk test and homoscedasticity evaluated by the Fisher–Snedecor test).
All the other analyses were performed with the same statistical methods. Only the comparison analysis between the various viral co‐infections with continuous variables was performed with ANOVA. Post‐hoc tests using Marascuillo (for categorical data) or Tukey–Kramer (for quantitative data) were performed when significance was found for more than two groups.
Multivariate analysis was performed using logistic regression (hospitalization as dependent variable) with a forward selection method and a logic link function. Variables were tested in the multivariate analysis model if P < 0.15 in the univariate analysis [Shtatland et al., 2001] and according to clinically significant parameters [Steyerberg et al., 2000]. Results were expressed as odds ratios (ORs) and 95% confidence intervals (95% CIs). Multivariate analysis was carried out with an adjustment for age, SpO2, and respiratory rate, considered as continuous variables.
RESULTS
Characteristics of the Study Patients
Two hundred twenty‐two infants were enrolled in the study group, of whom 131 (59%) were boys. Mean age at onset was 4.05 months ± 2.79 [0.26–11.82] (Table I).
Table I.
Characteristics of the Study Patients (n = 222)
| Male, n (%) | 131 (59) |
| Age (months) mean, SD [min–max] | 4.05 ± 2.79 [0.26–11.82] |
| Weight (kg) mean, SD [min–max] | 6.073 ± 1.96 [2.61–12.06] |
| Tobacco exposure, n (%) | 92 (41) |
| Family history of atopy, n (%) | 102 (46) |
| Nursery care attendance, n (%) | 103 (46) |
| CRPa (mg/L) mean, SD [min–max] | 14.5 ± 23.8 [0–110] |
| Hospitalization in Pediatric Intensive Care Unit, n (%) | 11 (6) |
| Severity score, n (%) | |
| Mild | 83 (37) |
| Moderate | 84 (38) |
| Severe | 55 (25) |
Available from 104 patients.
One hundred three children (46%) were attending a nursery, 72 (32%) had a history of eczema, and 92 (41%) were exposed to tobacco smoke. There was a familial history of atopy in 102 cases (46%). Bronchiolitis was classified as mild in 83 cases (37%), moderate in 84 (38%) and severe in 55 (25%) on the basis of the Wainwright severity score. There was no statistical difference in severity according to age, nursery attendance, history of eczema, exposure to tobacco smoke or familial history of atopy. The mean serum KL‐6 value was 231 IU/mL ± 106 [41–599].
The control group comprised 38 infants. No statistical difference was observed between the case and control groups in terms of age, gender, nursery attendance, history of eczema, exposure to tobacco smoke, or familial history of atopy.
One hundred seventy‐two children (79%) were hospitalized after the medical examination in the emergency room and constituted the inpatient group. Eighty‐six of these patients (50%) required oxygen therapy, with a mean duration of 3.1 days ± 2.1 [0–9]. Mean duration of stay was 5 days ± 3.5 [1–17]. Eighty‐nine of them (51%) required gastroenteral feeding, with a mean duration of 4.1 days ± 3.1 [1–14]. Twenty‐three (13%) developed a respiratory complication during hospitalization: 14 (8%) had an atelectasis on the chest radiograph and 9 (5%) had a documented secondary pulmonary infection.
The children in the inpatient group were significantly younger than the outpatients (3.5 months ± 2.6 vs. 6 months ± 2.7; P = 0.0001) and presented a more severe form of bronchiolitis according to assessment using the Wainwright severity score (P < 0.0001). SpO2 was significantly lower (96.9% ± 3.2 vs. 98.4% ± 1.6; P = 0.002). Factors associated with disease severity are summarized in Table II.
Table II.
Factors Associated With the Disease Severity
| Mild bronchiolitis (n = 83) | Moderate bronchiolitis (n = 84) | Severe bronchiolitis (n = 55) | P‐value | |
|---|---|---|---|---|
| Male, n (%) | 49 (59) | 53 (63) | 29 (53) | 0.48 |
| Age (months) | ||||
| Mean, SD | 4.3 ± 2.8 | 3.9 ± 2.6 | 3.9 ± 3.1 | 0.33 |
| Familial history of atopy, n (%) | 31 (46) | 43 (57) | 26 (54) | 0.41 |
| Exposure to tobacco smoke, n (%) | 29 (49) | 42 (61) | 20 (47) | 0.25 |
| Personal history of atopy, n (%) | 27 (40) | 27 (36) | 14 (29) | 0.46 |
| No nursery care attendance, n (%) | 29 (49) | 37 (56) | 24 (59) | 0.60 |
| Serum KL‐6 level (IU/ml) | ||||
| Mean, SD | 223 ± 109 | 238 ± 102 | 236 ± 109 | 0.48 |
| Hospitalization, n (%) | 49 (59) | 68 (81) | 55 (100) | <0.001 |
| Length of stay (days) mean, SD | 4.2 ± 3.2 | 4.8 ± 3.6 | 5.9 ± 3.5 | 0.016 |
| Hospitalization in Pediatric Intensive Care Unit, n (%) | 0 (0) | 3 (4) | 8 (15) | <0.001 |
| Oxygen saturation (%) mean, SD | 98.3 ± 1.8 | 97.6 ± 2.1 | 95.1 ± 4.2 | <0.001 |
| Need for oxygen therapy, n (%) | 16 (19) | 28 (33) | 41 (75) | <0.001 |
| Duration of oxygen therapy (days) mean, SD | 0.7 ± 1.8 | 1.1 ± 1.9 | 2.2 ± 2 | <0.001 |
| Respiratory rate (min−1) mean, SD | 43.4 ± 9.6 | 46.9 ± 11.8 | 57.9 ± 16.6 | <0.001 |
| CRP (mg/ml)a mean, SD | 13.5 ± 18.4 | 12.4 ± 18.9 | 17.1 ± 30 | 0.77 |
| RSV only, n (%) | 36 (61) | 45 (70) | 30 (70) | 0.49 |
| RSV + other virus, n (%) | 23 (39) | 19 (30) | 13 (30) | |
| HRV only, n (%) | 9 (31) | 7 (26) | 6 (38) | 0.73 |
| HRV + other virus, n (%) | 20 (69) | 20 (74) | 10 (62) | |
| Need for gastroenteral feeding, n (%) | 19 (40) | 35 (52) | 35 (64) | 0.06 |
RSV, Respiratory syncytial virus; HRV, Human Rhinovirus.
Available from 104 patients.
Eleven children (6%) were admitted to the Pediatric Intensive Care Unit (PICU). Of these, 10 (6%) required respiratory support: six by non‐invasive ventilation, one by mechanical ventilation, and three with both. They were significantly younger than the children hospitalized in the Pediatric Department (1.1 months ± 0.8 vs. 3.6 ± 2.6; P = 0.0001), had a higher Wainwright severity score (P = 0.008), a lower SpO2 (92.1% ± 6.2 vs. 97.2% ± 2.6; P = 0.003), and needed oxygen therapy for a longer period of time (2.8 ± 2 days vs. 1.5 ± 2.1; P = 0.003). The mean duration of their hospital stay was longer (10.6 days ± 3.8 vs. 4.6 ± 3.1; P = 0.0001). All the children admitted to the PICU required enteral nutrition, statistically more than those hospitalized in the Pediatric Department (P = 0.001). Documented secondary pulmonary infection was more frequent in patients in the PICU (36%, n = 4, vs. 3%, n = 5; P = 0.001), as was atelectasis (27%, n = 3 vs. 7%, n = 11; P = 0.048). No acute respiratory distress syndrome or death was recorded during hospitalization.
No significant difference was found in CRP levels in relation to the various clinical features.
Virus Detected in Nasopharyngeal Aspirates
Nasopharyngeal aspirate collection was performed in 221 patients (99.5%) (Table III).
Table III.
Virus Detected in Nasopharyngeal Aspirates (n = 217)
| Virus | n (%) |
|---|---|
| Respiratory syncytial virus | 166 (76) |
| Picornaviridae | 74 (34) |
| Human Rhinovirus | 72 (33) |
| A type | 32 (52) |
| B type | 2 (3) |
| C type | 28 (45) |
| Enterovirus | 4 (2) |
| CA6 type | 1 (25) |
| CA8 type | 1 (25) |
| B4 type | 2 (50) |
| Human metapneumovirus | 15 (7) |
| Adenovirus | 20 (9) |
| Bocavirus | 17 (8) |
| Coronavirus | 23 (10) |
| Parainfluenzae | 10 (4.5) |
At least one respiratory virus was detected in 217 samples (98%). A single infection was detected in 131 (60%) patients and two or more viruses were identified with our testing panel in 86 (39%) patients.
The most common pathogen was RSV, which was found in 166 samples (76%), followed by Picornaviruses (HRVs and EVs), which were found in 74 samples (34%). HRVs were found in 72 of these samples (33%) and hence were the second most common virus involved. ADV, BoV, hMPV, PIVs, and the IV A virus each represented <10% of the virus found. Of the 74 HRV/EV strains, genotyping based on VP4/VP2 sequences identified 4 EVs (Coxsackieviruses A6, A8, and B4) and 72 HRVs distributed as follows: HRV‐A (n = 32), HRV‐C (n = 28), and HRV‐B (n = 2). Eleven strains could not be assigned because of detection of mixed sequences, suggesting double HRV infection in patients.
Co‐infection was detected in 86 patients. The most common co‐infection was RSV and HRV (n = 41). Three viruses were involved in 17 patients, four in three patients and five in one.
KL‐6 Determinations
No significant difference in KL‐6 levels (P = 0.9) was found between the cases (mean 231 IU/mL ± 106) and the controls (230 IU/mL ± 102), nor between children who were hospitalized or not (P = 0.066), or who needed intensive care (P = 0.1). Serum KL‐6 levels were similar irrespective of the type of virus (P = 0.22). No correlation was found between serum KL‐6 levels and the disease severity score (Spearman's rank correlation coefficient 0.08 [−0.05 to 0.22]; P = 0.29) (Figs. 1 and 2).
Figure 1.
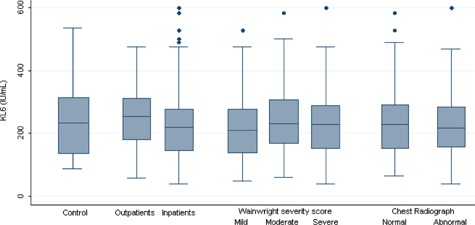
Serum KL‐6 levels in control groups, inpatients and outpatients groups, according to severity score and chest radiograph abnormality. No significant difference was found between the different groups.
Figure 2.
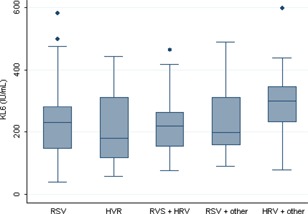
Serum KL‐6 levels according to the type of virus. RSV, Respiratory syncytial virus; HRV, Human Rhinovirus, other, other virus
Analysis of the Factors Related to Hospitalization
Univariate analysis
Hospitalization rate (P < 0.001), duration of hospitalization (P = 0.02), and need for intensive care (P < 0.001) were correlated with initial severity score (Table IV).
Table IV.
Risk Factors Related to Hospitalization: Univariate and Multivariate Analysis
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR [CI 95%] | P‐value | OR [CI 95%] | P‐value | |
| Sex ratio | 1.05 [0.56–2] | 0.87 | ||
| Agea | 0.72 [0.64–0.82] | <0.001 | 0.65 [0.55–0.77] | <0.001 |
| Severity score (moderate to severe versus mild)a | 5.3 [2.7–10.5] | <0.001 | ||
| Serum KL‐6 levela | 0.998 [0.9945–1.0006] | 0.12 | ||
| Oxygen saturationa | 0.76 [0.65–0.90] | 0.001 | 0.67 [0.54–0.85] | 0.001 |
| Respiratory ratea | 1.07 [1.04–1.10] | <0.001 | 1.07 [1.03–1.11] | 0.001 |
| Presence of SRV | 2.39 [1.21–4.73] | 0.01 | 0.92[0.36–2.32] | 0.85 |
| Absence of HRV | 2.77 [1.44–5.32] | 0.002 | 3.4 [1.4–7.9] | 0.006 |
| Absence of Coronavirus | 3.86 [1.59–9.41] | 0.003 | 3.1 [0.9–10.4] | 0.06 |
| Absence of Parainfluenzae | 3.80 [1.05–13.7] | 0.04 | 2.5 [0.4–15] | 0.30 |
| Absence of co‐infection | 1.59 [0.83–3.07] | 0.17 | ||
RSV, Respiratory syncytial virus; HRV, Human Rhinovirus.
Variables considered as continuous variables.
Predictive factors for hospitalization were a moderate to severe bronchiolitis according to severity score (OR 5.3 [2.7–10.5]; P < 0.0001), a high respiratory rate (OR 1.07 [1.04–1.10]; P < 0.0001), the presence of SRV in the nasopharyngeal sample (OR 2.39 [1.21–4.73]; P = 0.01) and the absence of HRV (OR 2.77 [1.44–3.52]; P = 0.002). No difference was found whether a co‐infection was present or not (P = 0.17). Older age and a higher SpO2 were protective factors (OR 0.72 [0.64–0.82]; P < 0.0001 and OR 0.76 [0.65–0.90]; P = 0.001, respectively). Similar factors were found to be protective for hospitalization in the PICU (OR 0.31 [0.14–0.65]; P = 0.002 and OR 0.7 [0.59–0.83]; P < 0.0001, respectively). The predictive factor for hospitalization in the PICU was occurrence of a respiratory complication (OR 13.6 [3.6–51.1]; P < 0.0001).
Multivariate analysis
The absence of HRV (OR 3.4 [1.4–7.9]; P = 0.006) was a predictive factor for hospitalization. Older age was and a higher SpO2 were protective factors for hospitalization (OR 0.65 [0.55–0.77]; P < 0.0001 and, OR 0.67 [0.54–0.85] P < 0.001, respectively). This was equally true for hospitalization in PICU (OR 0.4 [0.1–0.9]; P = 0.03 and OR 0.76 [0.61–0.95]; P = 0.02, respectively). Predictive factors of hospitalization in the PICU were a higher respiratory rate (OR 1.07 [1.03–1.11]; P = 0.01) and occurrence of a respiratory complication (OR 8.9 [1.4–55.7]; P = 0.02).
DISCUSSION
In this prospective single‐centre French study of infants with a first episode of acute bronchiolitis, no significant difference in KL‐6 levels was found between case and control groups. KL‐6 is classified as cluster 9 (Mucine 1‐MUC1) of lung tumor proteins [Ishikawa et al., 2012]. These proteins are expressed predominantly on alveolar type II cells, respiratory bronchiolar epithelial cells, and bronchiolar gland serous cells. High serum KL‐6 levels are associated with alveolar–epithelial barrier dysfunction as they have been shown to correlate with indices of alveolar–capillary permeability [Nathani et al., 2008]. The measurement of serum KL‐6 levels is now a widely accepted diagnostic test in Japan to monitor the activity of interstitial lung disease [Kobayashi and Kitamura, 1995; Kawase et al., 2011; Kondo et al., 2011] and seems to be a useful tool in monitoring interstitial disease in European populations [Horimasu et al., 2012]. A few reports of serum KL‐6 measurements in chronic lung disease in newborns [Ogihara et al., 2000, 2006; Kim et al., 2008] or in children [Kobayashi et al., 2001] have appeared in pediatric journals with standard upper limit concentration in a group of children with non‐respiratory disease [Imai et al., 2002]. It would seem therefore that some degree of alveolar damage must be present in cases with elevated serum concentrations of KL‐6 in bronchiolitis. This alveola damage can be explained by an increase in alveolar pressure or by alveolar hyperinflation.
However, the results presented here suggest that acute viral bronchiolitis in previously healthy children is correlated with their immune system and inflammatory reaction to viral aggression rather than with epithelial injury. In support of this, a recent study in a small sample of hospitalized patients showed that innate immune response is inversely correlated with disease severity in children with acute RSV or HRV lower respiratory tract infection [Garcia et al., 2012]. RSV infection of respiratory epithelial cells enhances neutrophil epithelial adhesion and activation and increases the cleavage and detachment of epithelium infected with the virus [Wang et al., 1998]. RSV infection of airway epithelium in an in vitro model was found to be non‐destructive and non‐invasive [Collins and Melero, 2011]. Rhinoviruses can infect cultures of human tracheal or bronchiolar epithelial cells, tracheal submucosal gland cells, and alveolar epithelial cells by binding to the InterCellular Adhesion Molecule 1 (ICAM‐1) and low density lipoprotein receptors and producing pro‐inflammatory cytokines [Jartti and Korppi, 2011; Kennedy et al., 2012].
Only a few published histopathology studies of acute RSV diseases are available, but it would seem that symptoms are caused by small airway obstruction by plugs composed of mucus, fibrin, and cellular debris from leukocytes and dead bronchial epithelial cells. Peri‐bronchiolar infiltrate is composed of morphonuclear cells [Bern et al., 2011]. Alveolar epithelial injury is clearly more present for RSV‐associated interstitial pneumonia. This suggests that the mechanisms that lead to respiratory failure are more related to bronchiolar obstruction and the host's immune response to the virus than they are to interstitial dysfunction, although this can be present in cases of acute respiratory distress syndrome or underlying alveolar disease, such as chronic heart disease. Polymorphonuclear neutrophil products have been shown to play an important role in the inflammatory response to RSV infection [Abu‐Harb et al., 1999], correlated to the severity of the first acute episode of bronchiolitis in a study with a small sample size [Marguet et al., 2009]. These results suggest that cytokines play a key role and partly mediate the respiratory tract inflammation elicited during the acute infection. Eosinophil degranulation products may also play a role in the pathogenesis of RSV bronchiolitis [Harrison et al., 1999; Kim et al., 2010], and have been found to be involved in RV‐induced asthma exacerbations in many in vitro models [Papadopoulos et al., 2001; Tsuji et al., 2005]. Viral infection may induce eosinophil chemoattractants to be released form airway epithelial cells, such as eotaxin or interleukine‐8 (IL‐8), particularly in atopic individuals [Papadopoulos et al., 2001]. Eosinophil activation, which involves the release of eosinophil granul proteins (eosinophil‐derived neurotoxin and eosinophil cationic protein), cysteinyl leukotrienes and cytolines, may lead to airway damage. The assessment of levels of cytokine products, particularly IL‐8, in the nasopharyngeal aspirates of atopic and non‐atopic patients could yield further significant findings. Studies on the release of KL‐6 and other markers of cell injury, oxidative stress molecules and cytokines in the respiratory tract would lead to a better understanding of the complex interaction between the host and the etiologic viral agent of bronchiolitis [Kratzer et al., 2012]. It would also be interesting to investigate the risk of developing recurrent wheezing and asthma according to the causative virus, the release of various cytokines and atopic status in a future work.
Whether concomitant infection increases the severity of bronchiolitis is a matter of debate. In one study with a small sample size, the occurrence of mechanical ventilation increased 10‐fold when a dual RSV and hMPV infection was present [Semple et al., 2005]. Conversely, the results of the present study are consistent with those of a French study which observed that a dual RSV/RV infection had no additional effect on the severity of bronchiolitis [Marguet et al., 2009].
The potential for disease progression has led workers to look for risk factors for severe bronchiolitis. The main clinical predictors of hospitalization evaluated in several outpatient groups were age under 3 months, a history of prematurity, oxygen saturation below 94%, a high respiratory rate for the infant's age, severe retraction signs and abnormal chest radiograph results [Zorc and Hall, 2010]. The need for supplemental oxygen, with or without intravenous fluids, has the greatest influence by the far on the severity‐of‐disease ranking and is a reliable predictor of the duration of hospital stay [Wainwright et al., 2003; Corneli et al., [Link]]. The results presented here are in agreement with these findings and confirm that clinical tools are required for the identification of previously healthy children at risk of developing acute respiratory insufficiency.
A strong point of this study is that both hospitalized children and outpatients applying the same protocols of respiratory virus detection and epithelial dysfunction analysis were included. All the significant viruses that are currently associated with bronchiolitis were studied.
However, some limitations of the study deserve to be mentioned. First, it was a single‐center study and results need to be confirmed in a larger population sample. Secondly, only a few patients required PICU hospitalization and mechanical ventilation. However, in a previous study performed in Japan in 2009 [Kawasaki et al., 2009], only seven cases required artificial respiration. Thus, the discrepancy observed between the results is not fully explained. It may have been due to differences in the inclusion criteria in the Japanese study, which probably allowed the inclusion of far more subjects with interstitial disease, that is, children with underlying congenital heart disease. The mechanism of epithelial injury is likely to be different in cases of acute bronchiolitis in infants without underlying disease. Another limitation is that we did not perform a bacteriological analysis of lower airways secretions which could have introduced bias, since a high incidence of pulmonary bacterial co‐infection with severe RSV bronchiolitis has been documented elsewhere [Thorburn et al., 2006]. However, the patient population studied by Thorburn et al. was different because they only included severe cases requiring mechanical ventilation, with all the samples obtained by aspirates through the endotracheal tube. Obtaining aspirates from lower airway secretions is an invasive procedure and cannot be performed with good quality results in children who are not intubated. Moreover, a more recent study performed in a large hospital‐based cohort concluded that serious bacterial infection appeared to occur infrequently in children hospitalized for RSV or non‐RSV bronchiolitis [Garcia et al., 2010].
CONCLUSION
The findings of this prospective study of French infants who were hospitalized or not for a first episode of acute bronchiolitis indicate that this respiratory infection is a well‐characterized clinical entity that can be associated with various viral antigens.
The results suggest that the clinical severity of bronchiolitis is not correlated with serum KL‐6 levels in previously healthy children indicating that the various clinical outcomes depend on the adaptive capacities of the host rather than on the intensity of epithelial dysfunction. This highlights the fact that many of the features of bronchiolitis are directly affected by comorbidity (underlying illness) and treatment (oxygen therapy and mechanical ventilation). Using a computed score with SpO2, respiratory rate and chest retraction signs could be of great use in helping diagnose infants with acute bronchiolitis and in treating them effectively.
ACKNOWLEDGMENTS
We thank Mr. Bruno Pereira for his invaluable advice and comments on this study, Clinical Research Assistants Emmanuelle Rochette, Nadège Rabiau, and Isabelle Petit for selecting controls and for the management of the data collection, Mr. Jeffrey Watts for his advice, Dr. Amélie Brebion, and all the members of the Pediatric Emergency Department staff for their involvement in the study.
The authors declare no conflict of interest.
REFERENCES
- Abu‐Harb M, Bell F, Finn A, Rao WH, Nixon L, Shale D, Everard ML. 1999. IL‐8 and neutrophil elastase levels in the respiratory tract of infants with RSV bronchiolitis. Eur Respir J 14:139–143. [DOI] [PubMed] [Google Scholar]
- ANAES . Consensus conference: Management of bronchiolitis in infants. September 21, 2000. Available on: http://www.has-sante.fr.
- Bern RA, Domachowske JB, Rosenberg HF. 2011. Animals models of human respiratory syncytial virus disease. Am J Physiol Lung Cell Mol Physiol 301:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PL, Melero JA. 2011. Progress in understanding and controlling respiratory syncytial virus: Still crazy after all these years. Virus Res 162:80–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corneli HM, Zorc JJ, Holubkov R, Bregstein JS, Brown KM, Mahajan P, Kuppermann N. Bronchiolitis Study Group for the Pediatric Emergency Applied Research Network. 2012. Bronchiolitis: Clinical characteristics associated with hospitalization and length of stay. Pediatr Emerg Care 28:99–103. [DOI] [PubMed] [Google Scholar]
- Feuillet F, Lina B, Rosa‐Calatrava M, Boivin G. 2012. Ten years of human metapneumovirus research. J Clin Virol 53:97–105. [DOI] [PubMed] [Google Scholar]
- Garcia C, Bhore R, Soriano‐Fallas A, Trost M, Chason R, Ramilo O, Mejias A. 2010. Risk factors in children hospitalized with RSV bronchiolitis versus non‐RSV bronchiolitis. Pediatrics 126:e1453–e1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia C, Soriano‐Fallas A, Lozano J, Leos N, Gomez AM, Ramilo O, Mejias A. 2012. Decreased innate immune cytokine responses correlate with disease severity in children with respiratory syncytial virus and human rhinovirus bronchiolitis. Pediatr Infect Dis 31:86–88. [DOI] [PubMed] [Google Scholar]
- Gern JE. 2010. The ABCs of rhinoviruses, wheezing and asthma. J Virol 84:7418–7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AM, Bonville CA, Rosenberg HF, Domachowske JB. 1999. Respiratory syncytial virus‐induced chemokine expression in the lower Airways: Eosinophil recrutment and degranulation. Am J Respir Crit Care Med 159:1918–1924. [DOI] [PubMed] [Google Scholar]
- Henquell C, Mirand A, Deseubis AL, Regagnon C, Archimbaud C, Chambon M, Bailly JL, Gourdon F, Hermet E, Dauphin JB, Labbé A, Peigue‐Lafeuille H. 2012. Prospective genotyping of human rhinoviruses in children and adults during the winter of 2009–2010. J Clin Virol 53:280–284. [DOI] [PubMed] [Google Scholar]
- Hirasawa Y, Kohno N, Yokohama A, Inoue I, Abe M, Hiwada K. 1997. KL‐6, a human MUCI mucin, is chemotactic for human fibroblast. Am J Respir Cell Miol Biol 17:501–507. [DOI] [PubMed] [Google Scholar]
- Horimasu Y, Hattori N, Ishikawa N, Kawase S, Tanaka S, Yoshioka K, Yokoyama A, Kohno N, Bonella F, Guzman J, Ohshimo S, Costanel U. 2012. Different MUC1 polymorphisms in German and Japanese ethnicities affect serum KL‐6 levels. Respir Med 106:1756–1764. [DOI] [PubMed] [Google Scholar]
- Imai T, Takase M, Takeda S, Kougo T. 2002. Serum KL‐6 levels in pediatric patients: Reference values for children and levels in pneumonia, asthma, and measles patients. Pediatr Pulmonol 33:135–141. [DOI] [PubMed] [Google Scholar]
- Ioannidis I, McNally B, Willette M, Peeples ME, Chaussabel D, Durbin JE, Ramilo O, Mejias A, Flano E. 2012. Plasticity and virus specificity of the airway epithelial cell immune response during respiratory virus infection. J Virol 86:5422–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa N, Hattoria N, Yokoyama A, Kohno N. 2012. Utility of KL‐6/MUC1 in the clinical management of interstitial lung diseases. Respir Invest 50:3–13. [DOI] [PubMed] [Google Scholar]
- Jartti T, Korppi M. 2011. Rhinovirus‐induced bronchiolitis and asthma development. Pediatr Allergy Immunol 22:350–355. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Aoyagi Y, Abe Y, Go H, Imamura T, Kaneko M, Ito M, Katayose M, Hashimoto K, Hosoya M. 2009. Serum KL‐6 levels as a biomarker of lung injury in respiratory syncytial virus bronchiolitis. J Med Virol 81:2104–2108. [DOI] [PubMed] [Google Scholar]
- Kawase S, Hattori N, Ishikawa N, Horimasu Y, Fujitaka K, Furonaka O, Isobe T, Miyoshi S, hamade H, Yamane T, Yokohama A, Kohno N. 2011. Change in serum KL‐6 level from baseline is useful for predicting life‐threatening EGFR‐TKIs induced interstitial lung disease. Respir Res 12:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy JL, Turner RB, Braciale T, Heymann PW, Borish L. 2012. Pathogenesis of rhinovirus infection. Curr Opin Virol 2:287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieninger E, Regamey N. 2012. Rhinoviruses: Markers of, or causative for, recurrent wheeze and asthma? Eur Respir J 39:238–239. [DOI] [PubMed] [Google Scholar]
- Kim DH, Kim HS, Shim SY, Lee JA, Choi CW, Kim EK, Kim BI, Choi JH. 2008. Cord blood KL‐6, a specific lung injury marker, correlates with the subsequent development and severity of atypical bronchopulmonary dysplasia. Neonatology 93:223–229. [DOI] [PubMed] [Google Scholar]
- Kim CK, Choi J, Kim HB, Shin BM, Kim JT, Fujiwasa T, Koh YY. 2010. A randomized intervention of montelukast for post‐bronchiolitis: Effect on eosinophil degranulation. J Pediatr 156:749–754. [DOI] [PubMed] [Google Scholar]
- Kobayashi J, Kitamura S. 1995. KL‐6: A serum marker for interstitial pneumonia. Chest 108:311–315. [DOI] [PubMed] [Google Scholar]
- Kobayashi I, Ono S, Kawamura N, Okano M, Miyazawa K, Shibuya H, Kobayashi K. 2001. KL‐6 is a potential marker for interstitial lung disease associated with juvenile dermatomyositis. J Pediatr 138:274–276. [DOI] [PubMed] [Google Scholar]
- Kondo T, Hattori N, Ishikawa N, Murai H, Haruta Y, Hirohashi N, Tanigawa K, Kohno N. 2011. KL‐6 concentration in pulmonary epithelial lining fluid is a useful prognostic indicator in patients with acute respiratory distress syndrome. Respir Res 12:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koponen P, Helminen M, Paassilta M, Luukkaala T, Korppi M. 2012. Preschool asthma after bronchiolitis in infancy. Eur Respir J 39:76–80. [DOI] [PubMed] [Google Scholar]
- Kratzer E, Tian Y, Sarich N, Wu T, Meliton A, Leff A, Birukova AA. 2012. Oxidative stress contributes to lung injury and barrier dysfunction via microtubule destabilization. Am J Respir Cell Mol Biol 47:688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marguet C, Lubrano M, Guedin M, Leroux P, Deschildre A, Forget C, Couderc L, Siret D, Donnou MD, Bubenheim M, Vabret A, Freymuth F. 2009. In very young infants severity of acute bronchiolitis depends on carried viruses. PLoS ONE 4:e4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midulla F, Pierangeli A, Cangiono G, Bonci E, Salvadei S, Scagnolari C, Moretti C, Antonelli G, Ferro V, Papoff P. 2012. Rhinovirus bronchiolitis and recurrent wheezing: 1‐year follow‐up. Eur Respir J 39:396–402. [DOI] [PubMed] [Google Scholar]
- Nathani N, Perkins GD, Tunnicliffe W, Murphy N, Manji M, Thickett DR. 2008. Krebs von Lungen 6 antigen is a marker of alveolar inflammation but not of infection in patients with acute respiratory distress syndrome. Crit Care Med 12:R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogihara T, Hirano K, Morinobu T, Ogawa S, Hiroi M, ban R, Ogihara H, Tamai H. 2000. KL‐6, a mucinous glycoprotein, as an indicator of chronic lung disease of the newborn. J Pediatr 37:280–282. [DOI] [PubMed] [Google Scholar]
- Ogihara T, Hirano K, Morinobu T, Kim HS, Ogawa S, Hiroi M, Oue S, Ban R, Hira S, Hasegawa M, Yamaoka S, Yasui M. 2006. Plasma KL‐6 predicts the development and outcome of bronchopulmonary dysplasia. Pediatr Res 60:613–618. [DOI] [PubMed] [Google Scholar]
- Papadopoulos NG, Papi A, Meyer J, Stanciu LA, Salvi S, Holgate ST, Johnston SL. 2001. Rhinovirus infection up‐regulates eotaxin and eotaxin‐2 expression in bronchial epithelial cells. Clin Exp Allergy 31:1060–1066. [DOI] [PubMed] [Google Scholar]
- Pelletier AJ, Mansbach J, Camargo CA. 2006. Direct medical costs of bronchiolitis hospitalizations in the United States. J Pediatrics 118:2418–2423. [DOI] [PubMed] [Google Scholar]
- Semple MG, Cowell A, Dove W, Greensill J, McNamara PS, Halfhide C, Shears P, Smyth RL, Hart CA. 2005. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis 191:382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtatland ES, Cain E, Barton MB. 2001. The perils of stepwise logistic regression and how to escape using information criteria and the Output Pelivery System. SUGI'26 Proceedings. Available on: http://www2.sas.com.
- Steyerberg EW, Eijkemans MJC, Harrel FE Jr, Habbema JDF. 2000. Prognostic modelling with logistic regression analysis: A comparison of selection and estimation methods in small data sets. Stat Med 19:1059–1079. [DOI] [PubMed] [Google Scholar]
- Subcommittee on Diagnosis and Management of Bronchiolitis . 2006. Diagnosis and management of bronchiolitis. Pediatrics 118:1174–1703. [Google Scholar]
- Thorburn K, Harigopal S, Reddy V, Taylor N, van Saene HKF. 2006. High incidence of pulmonary bacterial co‐infection in children with severe respiratory syncytial virus (RSV) bronchiolitis. Thorax 61:611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji K, Yamamoto S, Ou W, Nishi N, Kobayashi I, Zaitsu M, Muro E, Sadakane Y, Ichimaru T, Hamasaki Y. 2005. dsRNA enhances eotaxin‐3 production through interleukin‐4 receptor upregulation in airway epithelial cells. Eur Respir J 26:795–803. [DOI] [PubMed] [Google Scholar]
- Valkonen H, Waris M, Ruohola A, Ruuskanen O, Heikkinen T. 2009. Recurrent wheezing after respiratory syncytial virus or non‐respiratory syncytial virus bronchiolitis in infancy: A 3‐year follow‐up. Allergy 64:1359–1365. [DOI] [PubMed] [Google Scholar]
- Wainwright C, Altamirano L, Cheney M, Cheney J, Barber S, Price D, Moloney S, Kimberley A, Woolfield N, Cadzow S, Fiumara F, Wilson P, Mego S, Van deVelde D, Sanders S, O'Rourke P, Francis P. 2003. A multicenter, randomized, double‐blind, controlled trial of nebulized epinephrine in infants with acute bronchiolitis. N Engl J Med 349:27–35. [DOI] [PubMed] [Google Scholar]
- Wang SZ, Xu H, Wraith A, Bowden JJ, Alpers JH, Forsyth KD. 1998. Neutrophils induce damage to respiratory epithelial cells infected with respiratory syncytial virus. Eur Respir J 12:612–618. [DOI] [PubMed] [Google Scholar]
- Zorc JJ, Hall C. 2010. Bronchiolitis: Recent evidence on diagnosis and management. Pediatrics 125:342–349. [DOI] [PubMed] [Google Scholar]


