Abstract
Aim The Kakamega Forest, western Kenya, has been biogeographically assigned to both lowland and montane forest biomes, or has even been considered to be unique. Most frequently it has been linked with the Guineo‐Congolian rain forest block. The present paper aims to test six alternative hypotheses of the zoogeographical relationships between this forest remnant and other African forests using reptiles as a model group. Reptiles are relatively slow dispersers, compared with flying organisms (Aves and Odonata) on which former hypotheses have been based, and may thus result in a more conservative biogeographical analysis.
Location Kakamega Forest, Kenya, Sub‐Saharan Africa.
Methods The reptile diversity of Kakamega Forest was evaluated by field surveys and data from literature resources. Faunal comparisons of Kakamega Forest with 16 other African forests were conducted by the use of the ‘coefficient of biogeographic resemblance’ using the reptile communities as zoogeographic indicators. Parsimony Analysis of Endemism and Neighbour Joining Analysis of Endemism were used to generate relationship trees based on an occurrence matrix with paup*.
Results The analysis clearly supports the hypothesis that the Kakamega Forest is the easternmost fragment of the Guineo‐Congolian rain forest belt, and thus more closely related to the forests of that Central–West African complex than to any forest further east, such as the Kenyan coastal forests. Many Kenyan reptile species occur exclusively in the Kakamega Forest and its associated forest fragments.
Main conclusions The Kakamega Forest is the only remnant of the Guineo‐Congolian rain forest in the general area. We assume that the low degree of resemblance identified for the Guineo‐Congolian forest and the East African coastal forest reflect the long history of isolation of the two forest types from each other. Kenyan coastal forests may have been historically connected through forest ‘bridges’ of the southern highlands with the Congo forest belt, allowing reptile species to migrate between them. The probability of a second ‘bridge’ located in the region of southern Tanzanian inselbergs is discussed. Although not particularly rich in reptile species, the area should be considered of high national priority for conservation measures.
Keywords: Conservation biogeography, East Africa, forest refugia, Guineo‐Congolian rain forest, Kakamega forest, Kenya, reptiles, Sub‐Saharan Africa, zoogeographical relationships
Introduction
The African tropical rain forests, extending from southern Senegal in the west to the East African coastal forests of Kenya and Tanzania in the east (Collins, 1992), harbour an estimated 105 snake species (Hughes, 1983), as well as 95 lizard, 16 chelonian and 3 crocodilian species (Bauer, 1993). Compared with other tropical forest regions this total is not impressively high, but certainly constitutes an underestimate due to a combination of the historical, logistical and political problems associated with herpetological research in Africa. These African forests are also seriously threatened by exploitation. For instance, Uganda has lost about 86% of its original forest cover in the past two decades, and the remaining forests are isolated fragments only (Vonesh, 2001). Compared with other vertebrate groups, reptiles in East Africa have been poorly studied and little is known about them. There is an urgent need for more baseline data to enable the establishment of efficient herpetological conservation programmes.
In general, East African rain forests may be classified into four different biogeographical units: the coastal forests; the Eastern Arc mountain forests; isolated mountain forest (e.g. Mt Elgon, Mt Kenya and Aberdare forests); and the easternmost outliers of the Congo basin (Guineo‐Congolian) forest belt. Howell (1993) reviewed the herpetofauna of the Eastern Arc mountain forests, but little is known about the amphibians and reptiles of the rain forests associated with the Congo forest belt (e.g. Budongo, Bwamba, Kibale, Bwindi and Mabira in Uganda) or isolated mountain forests. Species inventories are available only for the Bwindi (Drewes & Vindum, 1991) and Kibale Forests (Vonesh, 2001). The Kakamega Forest in westernmost Kenya was suggested as being the easternmost fragment of the equatorial rain forest system (Zimmermann, 1972; Drewes, 1976; Hamilton, 1976; Schiøtz, 1976; Kokwaro, 1988; Vonesh, 2001; Fashing & Gathua, 2004; Köhler, 2004; Clausnitzer, 2005) and although its amphibian fauna has recently been surveyed (Schick et al., 2005), its zoogeographical relationships with other forests and fragments of the equatorial rain forest have not been thoroughly analysed.
Several analyses indicate that the Kakamega Forest supports a considerable faunal diversity (e.g. Odonata: Clausnitzer, 1999, 2005; Amphibia: Schick et al., 2005; Wagner & Böhme, 2007; Reptilia: Wagner & Böhme, 2007; Aves: Zimmermann, 1972) and its value to Kenya is underlined by the fact that many species are not found elsewhere in the country (Wass, 1995; Köhler et al., 2006; Wagner & Böhme, 2007). Today the forest is becoming more and more fragmented and in some areas it is already reduced to isolated patches (Mitchell, 2004).
Several authors have analysed the relationships between Kakamega Forest and other forest types (Zimmermann, 1972; Kokwaro, 1988; Clausnitzer, 1999; Fashing & Gathua, 2004; Mitchell, 2004). It has been considered to represent both lowland and montane forest, as well as being considered to be unique. Therefore, as a zoogeographical unit the highland rain forest of Kakamega is of particular interest. Its relationship with the Guineo‐Congolian rain forest system was discussed on the basis of the avifauna by Zimmermann (1972) and the Odonata by Clausnitzer (1999), although both of these groups are highly vagile (as most of them possess the ability to fly) and are able to bridge forest gaps easily.
The aim of the present paper is to analyse the reptile fauna of Kakamega Forest with a focus on its zoogeographical affinities. Reptiles are excellent zoogeographical indicators as they are comparatively slow dispersers and are less prone to drift events. Thus they can present clear‐cut evidence on the relationships between Kakamega Forest and other African forests. We therefore use reptiles as a model group to test the previously proposed hypothesis of the classification of Kakamega Forest as part of the Guineo‐Congolian rain forest system (H0 hypothesis), which was mainly based on highly vagile organisms (e.g. Moreau, 1966; Zimmermann, 1972; Clausnitzer, 1999). The analysis was conducted by setting up and testing six alternative hypotheses which cover all possible relationships between Kakamega Forest and other African rain forests.
Materials and methods
Study area
Kakamega Forest (see Fig. 1) is situated in the Kakamega District, Western Province, Kenya, and extends from 0°10′ to 0°21′ N and 34°47′ to 34°58′ E, covering an area of approximately 240 km2. Only 44.55 km2 of the forest is protected by law (Mitchell, 2004). The forest is situated between 1500 and 1700 m a.s.l. and is part of a stratified landscape of the East African Rift Valley, which is situated only 150 km eastwards. The eastern margin of Kakamega Forest is marked by the 2200 m high Nandi Escarpment, which appears to be a montane habitat because the tree fern Cyathea manniana occurs there. The annual precipitation ranges from 1500 to 2300 mm. The annual average temperature is 27°C in the day and 15°C at night (Köhler, 2004). Two major rivers cross the forest: the Isiukhu River in the north and the Yala River in the south; both drain into the nearby Lake Victoria. The main forest block is surrounded by several smaller forest fragments, which differ in size and in the degree of human disturbance. The most important of these are the protected Kisere forest fragment to the north, and the unprotected but better known Kaimosi fragment to the south. In addition to these forest fragments, two large forest systems of the Nandi escarpment were connected with the Kakamega Forest system until recently. The North Nandi Forest was not connected with Kakamega Forest in the 20th century but there was ‘dense forest’ in the 1960s between the South Nandi Forest and the Kakamega Forest (Mitchell, 2004).
Figure 1.
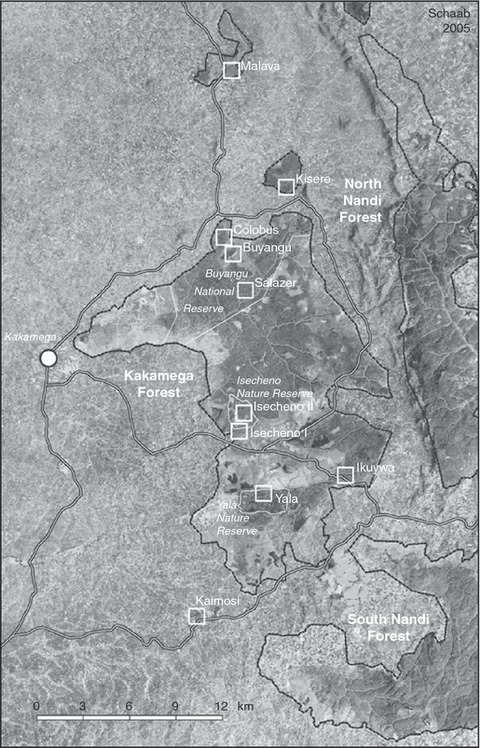
Map of the main study areas investigated by the BIOTA East Africa project in the Kakamega Forest with a subset of a Landsat 7 (ETM+) scene from 5 February 2001 in the background. (Contrast‐enhanced band combination 5/4/3 for within‐forest differentiation but printed in black and white. Squares show BIOTA East Africa research plots; forest borders are marked with a dashed black line; within these markings, darker shadings show the primary‐like forest, lighter shadings disturbed areas and younger secondary forest. Courtesy of G. Schaab, BIOTA‐E02.)
Comparative data
The 16 forests used for comparisons comprise various habitat types that cover a broad altitudinal range. Mount Nlonako, Korup (Cameroon) and Virunga (Democratic Republic of Congo) are primary forests and part of the equatorial rain forest block. Korup is clearly a lowland rain forest because 97% of it is located below 850 m a.s.l., whereas Mt Nlonako and Virunga are mostly montane rain forests, ranging between 710 and 5000 m a.s.l. Kakamega Forest is a mixture of forest and savanna elements, as is the Garamba NP (Democratic Republic of Congo), which, in contrast to Kakamega, still contains gallery forests. Kibale and Bwindi (Uganda) are situated on the Central African plateau between the western and the eastern rift valley branches. Usambara and Udzungwa forests (Tanzania) are submontane to montane. Arabuko‐Sokoke (Kenya) is a comparatively dry East African coastal rain forest. Loango and Moukalaba are lowland forests on the West African coast in Gabon with, in the case of Loango, a mixture of forest, savanna, swamps and lagoons. Lopé, Monts de Crystal and Massif de Chaillu are Parks of medium altitude, also in Gabon. Ziama (Guinea) and Mount Nimba (Guinea, Côte d’Ivoire, Liberia) forests are primary montane forests of the extreme western equatorial rain forest block. Together, all these forests contain a total (as referenced below) of 12 turtle, 124 lizard and 171 snake species.
Details of only two historical reptile collections from Kakamega have been published. Loveridge (1935, 1936) reported on the Kaimosi fragment and described three reptile taxa from there. Drewes (1976) collected at 12 sites in 2 weeks in the main forest, finding about 15 amphibian, seven lizard and eight snake species. Subsequently, little has been published on the Kakamega reptiles, save the mentioning of single voucher specimens (Spawls et al., 2002; Köhler et al., 2003).
Survey method
Most specimens were collected during a 3‐month herpetological survey by the senior author between March and June 2004 (Wagner & Böhme, 2007). The main forest and the isolated forest fragments were surveyed by both day and night. Specimens were obtained by drift‐fences with pitfall traps, road patrolling and opportunistic searching. Specimens were fixed in 98% ethanol and subsequently transferred to 70% ethanol. For final deposition, they were equally partitioned between the National Museums of Kenya, Nairobi (NMK) and the Zoologisches Forschungsmuseum A. Koenig, Bonn (ZFMK). Additional material analysed in this study is deposited in the Muséum d′Histoire Naturelle, Genève (MHNG). Data were supplemented by existing voucher material from Kakamega Forest in the MHNG and NMK; and ZFMK specimens that were collected by H.W. Herrmann, D. Modry and P. Neças. Species were arranged into ecological groups using the following literature: Loveridge (1935, 1936, 1942a, 1942b), Pitman (1974), Hughes (1983), Spawls et al. (2002), Uetz (2001), Broadley & Howell (1991), Chippaux (1991). Additional data, a complete species list and ecological characteristics of the reptiles found in Kakamega Forest, are provided in Appendices S1 and S2 (see Supplementary Material).
Faunal comparisons
In order to compare the reptile fauna of Kakamega Forest with those of the 16 other tropical African rain forests (as listed above), Parsimony Analysis of Endemism (PAE), first described by Rosen (1988) and Rosen & Smith (1988), was used. Under ideal conditions, when faunas are known completely, this technique produces dendrograms that link sites on the basis of shared species. PAE resembles cladistic phylogenetic analysis, except that the operational taxonomic units are geographic areas rather than taxa, and the characters used in PAE are species distributions. The character state for each species distribution is either present or absent. Shared presence of species provides evidence of biogeographic affinity between different sites, and is used to produce a hierarchical pattern of endemism. Dendrograms resulting from PAE might demonstrate historical relationships among the faunas. However, linkage between sites might simply reflect shared environmental conditions that result in colonization by similar faunas.
Parsimony analyses were carried out using paup* version 4.0b10 (Swofford, 1998). Heuristic searches were performed using the TBR (tree bisection reconnection) branch swapping algorithm with 100 random additions. When more than one most parsimonious tree was found, a strict consensus of all trees was calculated. Trees were rooted using a hypothetical outgroup area devoid of all species (see Rosen & Smith, 1988). All characters were analysed unordered, without differential character weighting. No upper limit was imposed on the maximum number of trees saved. To get an indication of the robustness of the topologies produced, bootstrap analyses were performed (Felsenstein, 1985) as implemented in paup*. A total of 50,000 tree pseudoreplicates were calculated.
To support the results of the PAE analysis, Neighbour Joining Analysis of Endemism (NJAE) was used as a second method to calculate the relationships between the forests (see Köhler, 2000). The NJAE was conducted using exactly the same data sets and options as in the PAE.
As a third method, the coefficient of biogeographic resemblance (CBR) (see Duellman, 1990) was calculated. The formula of the coefficient is CBR = 2C/(N 1 + N 2), where C is the number of species in common to two areas, N 1 is the number of species in the first area and N 2 is the number of species in the second area.
To strengthen the results, six alternative hypothetical tree topologies were tested using the nonparametric Templeton test (Wilcoxon‐signed ranks) and the Winning‐sites test as implemented in paup*. These tests measure the degree that a data set favours one tree over another. To cover all alternative hypothetical possible relationships, the following hypotheses were tested: Hypothesis 1 (H1): Arabuko‐Sokoke Forest is part of the East African forest block clade; Hypothesis 2 (H2): Kakamega Forest is closely related to Arabuko‐Sokoke Forest and both are closely related to the Eastern Arc forest block; Hypothesis 3 (H3): all forests of the Guinean forest block build one non‐resolved group; Hypothesis 4 (H4): Kakamega Forest is part of the Gold coast forest block clade; Hypothesis 5 (H5): Kakamega Forest is the sister group to the whole Guinean forest block; Hypothesis 6 (H6): Kakamega Forest is the sister group to the East African forest block clade.
Data for the PAE, NJAE and CBR analysis of sites were taken from the following sources: Forêt de Ziama (Guinea): Böhme (1993, 1994a, 1994b, 2000); Mount Nimba (Guinea, Côte d′Ivoire, Liberia): Ineich (2003); Korup National Park (Cameroon): Lawson (1993); Mt Nlonako (Cameroon): Herrmann et al. (2005); Monts de Crystal (Gabon): Pauwels et al. (2002b); Massif du Chaillu (Gabon): Pauwels et al. (2002a); Loango National Park (Gabon): Pauwels et al. (2006); Lopé National Park (Gabon): Pauwels et al. (2006); Moukalaba National Park (Gabon): Pauwels et al. (2006); Parc National des Virunga (DR Congo): DeWitte (1941); Parc National de la Garamba (DR Congo): DeWitte (1966); Kibale National Park (Uganda): Vonesh (2001); Bwindi Impenetrable National Park (Uganda): Drewes & Vindum (1997); Usambara and Udzungwa Mountains (Tanzania): Howell (1993); and Arabuko‐Sokoke Forest (Kenya): Kifcon (Kenyan indigenous forest conservation project) (1995). The forests are located between 9° 22′ (Ziama) in the west and 39° 30′ in the east (Arabuko‐Sokoke) and between 8° 22′ in the north (again Ziama) and 7° 50′ in the south (Udzungwa). For the exact position of each of these forests see Fig. 2. Other forests with apparently incomplete reptile species lists were not included because of the resulting artefacts in the PAE and NJAE methods.
Figure 2.
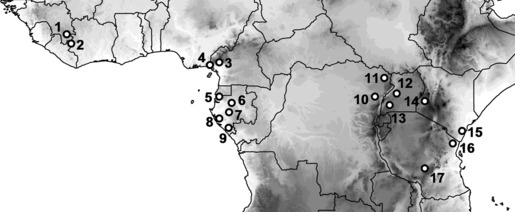
Map showing the rain forest localities used in the diversity coefficient analysis. The number of reptile species that the other forests have in common with Kakamega Forest is provided in bold below, followed by coefficient of biogeographic relationships in parentheses. Increasing elevation is shown by light to dark shading. Country borders are shown. 1 = ZIA, Ziama Forest, Guinea (15; 0.26); 2 = MTN, Mt Nimba, Guinea (14; 0.22); 3 = KOR, Korup NP, Cameroon (18; 0.27); 4 = MNL, Mt Nlonako, Cameroon (21; 0.29); 5 = MCR, Monts de Crystal, Gabon (13; 0.25); 6 = LOP, Lopé NP, Gabon (14; 0.31); 7 = MAC, Massif du Chaillu, Gabon (11; 0.22); 8 = LOA, Loango NP, Gabon (9; 0.20); 9 = MOK, Moukalaba‐Doudou NP, Gabon (11; 0.22); 10 = VIR, Virunga Mountains, DR Congo (37; 0.44); 11 = GAR, Garamba NP, DR Congo (25; 0.38); 12 = KIB, Kibale Forest, Uganda (28; 0.54); 13 = BWI, Bwindi Impenetrable Forest, Uganda (18; 0.39); 14 = KMF, Kakamega Forest, Kenya; 15 = ARA, Arabuko‐Sokoke Forest, Kenya (9; 0.15); 16 = USA, Usambara Mountains, Tanzania (5; 0.12); 17 = UZU, Udzungwa Mountains, Tanzania (0; 0.0).
Species composition
To get an impression of the degree of disturbance of the forests, the identified species were sorted according to their habitats (see Appendix S2) into: forest; clearings or forest edges; agricultural land; farmland, residential area and gardens; shores of water bodies; and bushland and open grassland. For referencing the habitats the following references were used: Hughes (1983), Pitman (1974) and Spawls et al. (2002).
Results
Field notes, species composition and comments
Based on our collection and literature records, a total of 58 reptile species (36 snake, 21 lizard, one turtle; see Appendix S2) are known from Kakamega Forest to date (Wagner & Böhme, 2007).
In the forest, lizards proved to be relatively rare and hard to find. Only Rhampholeon (Rhinodigitum) boulengeri, Adolfus africanus and ‘Mochlus’ aff. fernandi (P. Wagner et al., unpublished data) were found in leaf litter, whereas Feylinia currori inhabits the upper soil layer. The only tree‐dwelling lizards were the gekkonids Cnemaspis africana, C. elgonensis and Lygodactylus gutturalis. These forest‐dwelling species represent 32% of the recorded lizard fauna of the area.
Most lizards and the turtle species occur at the forest edges and in previously farmed areas. Dominant species here were Acanthocercus atricollis, Chamaeleo ellioti, Adolfus jacksoni and Trachylepis striata. Trachylepis quinquetaeniata obviously avoids human settlements and was found only on rocky outcrops some distance from human habitation. The following species were found to live only in farmbush and settled areas: Hemidactylus mabouia, Acanthocercus atricollis, Agama kaimosae (for the taxonomic status of this taxon see Wagner et al., in press), Chamaeleo gracilis, C. laevigatus, Eumecia anchietae, Trachylepis quinquetaeniata, Chamaesaura anguina and Varanus niloticus. Ubiquitous species, living not only in farmbush and settled areas, but in secondary forest and primary forest clearings and edges, were Pelomedusa subrufa, Chamaeleo ellioti, Trachylepis maculilabris, T. megalura, T. striata, and Adolfus jacksoni. These species represent 68% of the lizard fauna. Interestingly, the two latter species occur at open campsites within the forest but are absent from other clearings, which might argue for passive transport. However, the strongly anthropophilous gecko Hemidactylus mabouia is absent from the huts at the campsite clearing.
The structure of the snake fauna – as based on habitat preferences – gives a different picture. Thirty‐nine percent of the snake species were found to be typical forest dwellers; these are Hapsidophrys lineatus, Lycophidion ornatum, Philothamnus carinatus, P. nitidus, Psammophis phillipsi 1 , Thrasops aethiopissa, Toxicodryas blandingii, T. pulverulenta, Naja melanoleuca, Pseudohaje goldii, Causus lichtensteini, Atheris squamigera, A. hispida, and Bitis nasicornis. Fewer Kakamega snake species (only 22%) were typical of farmbush; these are Lamprophis fuliginosus, Lycophidion capense, L. depressirostre, Philothamnus battersbyi, P. heterolepidotus, P. hoplogaster, Psammophis mossambicus (sensu stricto) and P. rukwae. Snake species associated with secondary forests, clearings and forest edges which are also known to occur in primary forest were as numerous as typical primary forest species (see above) and represented also 39% of species; these are Typhlops angolensis, T. lineolatus, Crotaphopeltis hotamboeia, Dasypeltis atra, D. scabra, Dispholidus typus, Mehelya capensis, Natriciteres olivacea, Thrasops jacksoni, Polemon christyi, Dendroaspis jamesoni kaimosae, Elapsoidea loveridgei, Causus resimus and Bitis gabonica. Some of these taxa, e.g. Dasypeltis scabra, Dispholidus typus and Crotaphopeltis hotamboeia, are pan‐African savanna species, but were recorded from secondary forest during this study.
A summary of the reptilian fauna of the Kakamega Forest area including some distributional (including specific collecting and/or observation sites) and ecological (abundance, nutrition, habitat and microhabitat, activity patterns) characteristics is given in Appendix S2.
Faunal comparisons
The reptilian species richness of the African rain forests and their edges considered here ranges from 17 species in the Udzungwa Mountains to 109 in the Virungas. Among the forests, Korup and Mt Nlonako are most similar, with a CBR of 0.73, and 59 species in common. They are followed by the Ziama and Mt Nimba forests with a CBR of 0.70, Bwindi and Kibale with a CBR of 0.66, and Massif de Chaillu and Monts de Crystal with 0.63. The Ziama Forest in Guinea – despite being 2,040 km away and on the opposite side of the Dahomey gap (a savanna area in Benin separating the rain forest belt) (Salzmann & Hoelzmann, 2005) – has a coefficient of 0.46 with the Korup National Park in Cameroon, and a coefficient of 0.26 with Kakamega forest, even though they are separated by a geographical distance of 5000 km. But Kakamega has a similarity coefficient of only 0.15 with the Kenyan Arabuko‐Sokoke Forest, located at a distance of only 700 km. The Eastern Arc Mountains (Usambara, Udzungwa) form a homogenous unit, with a CBR of 0.27 between the two and lower coefficients with other forests.
In nearly all cases, the six alternative hypotheses tested gave a significantly worse fit than the null hypothesis (Kakamega Forest as part of the Guineo‐Congolian rain forest) and could therefore be rejected (for details see Table 1). Only in the case of the Winning‐sites test of hypothesis 6 was the fit not significantly different from that of the null hypothesis (P = 0.0703). The PAE and NJAE analyses both clearly grouped Kakamega Forest with other East African rain forests (Bwindi, Garamba, Kibale and Virunga), with a bootstrap support of 69% and 78%, respectively. This eastern Kakamega clade is sister to a clade formed by West and Central African forests, which together form a Guineo‐Congolian clade (bootstrap support 70%/83%). In both analyses, the geographically close Kenyan coastal forests, namely Arabuko‐Sokoke, are placed as a sister clade to this large Guineo‐Congolian forest clade with high support (bootstrap 84%/97%). The Eastern Arc Mountain forests are always grouped separately, although PAE and NJAE both failed to resolve their basal relationships (3, 4).
Table 1.
Test values for the six alternative hypotheses.
| Templeton test (Wilcoxon‐signed test) | Winning‐Sites test | Brief description of the hypothesis | |||
|---|---|---|---|---|---|
| n | P | Counts | P | ||
| H1 | 24 | 0.0143* | 18 | 0.0227* | Arabuko‐Sokoke Forest is part of the East African forest block clade |
| H2 | 40 | 0.0044* | 29 | 0.0072* | Kakamega Forest is closely related to Arabuko‐Sokoke Forest and both are closely related to the Eastern Arc forest block |
| H3 | 119 | < 0.0001* | 119 | < 0.0001* | All forests of the Guinean forest block build one non‐resolved group |
| H4 | 39 | < 0.0001* | 34 | < 0.0001* | Kakamega Forest is part of the Gold coast forest block clade |
| H5 | 30 | 0.0106* | 22 | 0.0161* | Kakamega Forest is the sister group to the whole Guinean forest block |
| H6 | 8 | 0.0339* | 7 | 0.0703 | Kakamega Forest is the sister group to the East African forest block clade |
*Significant difference from H0 (Kakamega Forest is part of the Guineo‐Congolian rain forest) at P < 0.05.
Figure 3.
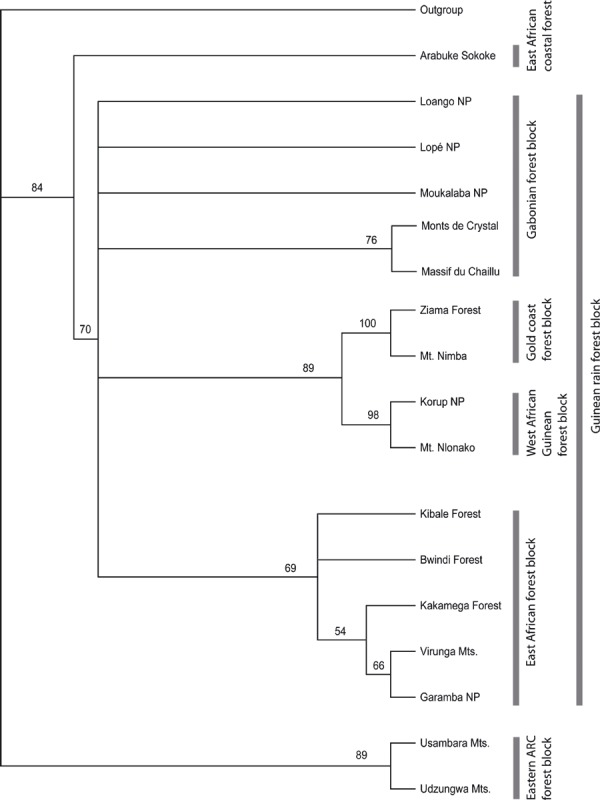
Parsimony Analysis of Endemism (PAE) tree of the relationships of the different forests based on a presence/absence matrix of their respective species compositions. Numbers at the nodes are bootstrap values in percent; values below 50% not shown. The black bars group related forests and their standard designation found in the literature.
Figure 4.
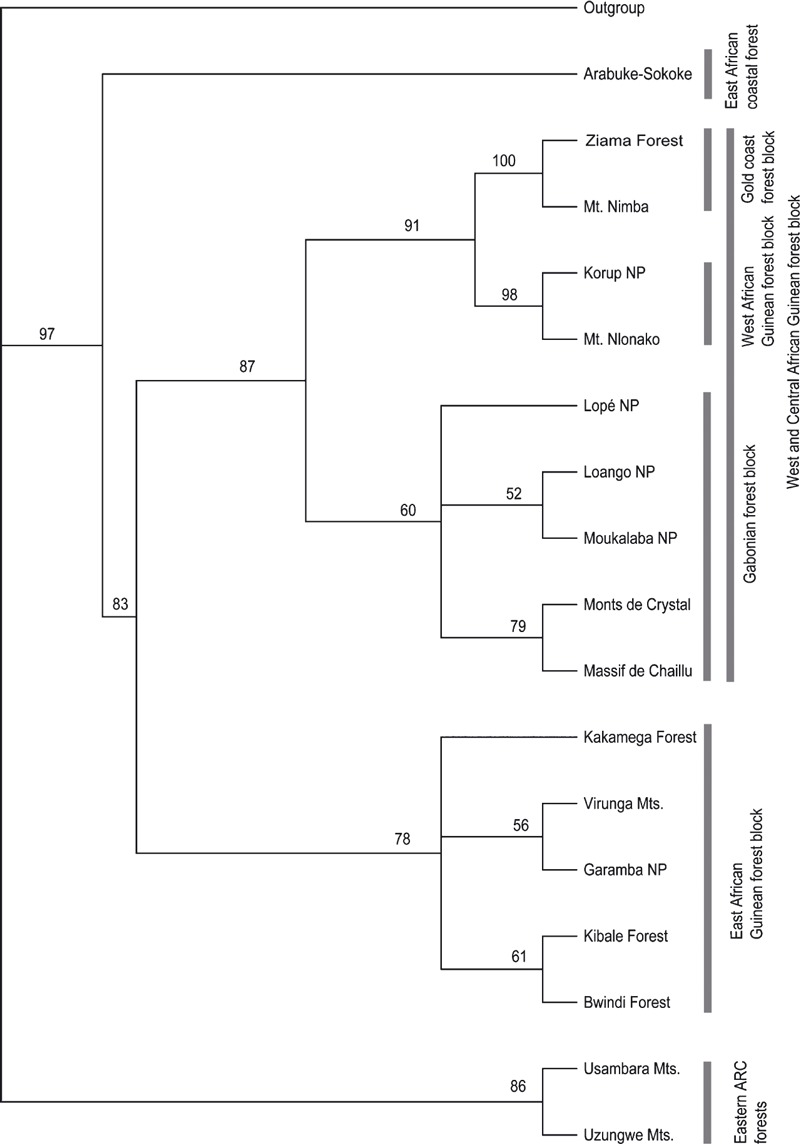
Neighbour Joining Analysis of Endemism (NJAE) tree of the relationships of the different forests based on a presence/ absence matrix of their respective species compositions. Numbers at the nodes are bootstrap values in percent; values below 50% not shown. The black bars group related forests and their standard designation found in the literature.
Discussion
Species composition
With its 58 reptile known so far, Kakamega Forest cannot be regarded as highly diverse and certainly does not represent a refugial area. Its reptile fauna may be classified into three groups: (1) forest species, (2) savanna and farmbush species, and (3) ubiquitous species occurring in forest clearings and forest edges as well as in farmbush areas, including human settlements. Groups 1 and 3 are dominant, whereas group 2 is of minor importance. However, ecological studies are still lacking for many species and their allocation to one of the three mentioned ecotypes is somehow hampered by the sparse knowledge (e.g. Schiøtz, 1975; Hughes, 1983; Howell, 1993).
Our experience with some group 3 species (ubiquitous) was as follows: Pelomedusa subrufa was found inside the forest in water bodies, on the forest floor, as well as in arid areas surrounding the forest. Chamaeleo ellioti and Adolfus jacksoni were both generally considered to be adapted to more arid situations, but both were found at Kakamega within the forest. The former was found in the grassy and wooded areas around the forest, in forest clearings and along the rivers inside the forest. The latter was only found within the forest at the campsite and it may well have been introduced by humans. The snake Bitis gabonica occurs both in secondary forests and at grassy forest edges.
Typical farmbush species (group 2) are Acanthocercus atricollis and Eumecia anchietae. Trachylepis quinquetaeniata seems to avoid not only forest but also intensively cultivated farmland so that it is mostly found in undisturbed fields, usually on rocks. In contrast, Hemidactylus mabouia is expressively anthropophilous, being confined to the houses within human habitations. Among snakes, Philothamnus battersbyi and Psammophis mossambicus (sensu Broadley et al., 2003) can be regarded as species characteristic for farmbush.
Rhampholeon boulengeri,‘Mochlus’ aff. fernandi and Adolfus africanus are typical forest‐dwelling lizards (group 1). Rhampholeon boulengeri is otherwise confined to eastern Democratic Republic of Congo, Rwanda and Burundi, i.e. to the eastern part of the Guineo‐Congolian forest. Originally believed to be a subspecies of Rhampholeon spectrum (‘R. s. boulengeri’: Loveridge, 1942a; DeWitte, 1965), it is not closely related to R. spectrum, a typical Congo forest block species, but is a member of a species group associated with Tanzanian mountain areas (Klaver & Böhme, 1997).
The following snake species are typical of forest: Atheris hispida, A. squamigera, Pseudohaje goldi, Causus lichtensteini and Psammophis phillipsi (meant here in the strict sense of the description by Hallowell, 1844). Of these, only Atheris hispida is confined to eastern Africa (Spawls et al., 2002); the others are largely distributed throughout the Congo forest block.
Faunal comparisons
The comparison between the reptile fauna of Kakamega Forest and the other African forests results in a hierarchical grouping. Remarkably, this area grouping is equivalent to distribution patterns exhibited by single forest species (P. Wagner et al., unpublished data). Consequently, these species can serve as indicators for the Guineo‐Congolian forest type, as has already been discussed for Adolfus africanus by Köhler et al. (2003) and for Feylinia currori by Wagner & Schmitz (2006). Similar results exist for ‘Mochlus’ aff. fernandi and Psammophis phillipsi; Pseudohaje goldi and many others are also to be assigned to this indicator group (P. Wagner et al., unpublished data).
The results are strongly supported by the testing of different hypotheses. Only the Winning‐sites test of hypothesis H6, setting Kakamega Forest as sister group to other East African forests, resulted in non‐significant differences of probability when compared with our H0 hypothesis (Table 1). Therefore, we cannot completely rule out this possibility, but this scenario is considered unlikely compared to the one contained in the H0 hypothesis. This assumption is supported by the fact that in the Wilcoxon‐signed test our null hypothesis was significantly better than hypothesis H6.
This strongly argues for a recent connection between Kakamega Forest and the forested parts of Uganda. The Virungas, the Garamba, Kibale, Bwindi and Kakamega forests have many reptile species in common (see Table 2), which may demonstrate that these forests were in contact for at least short periods. This also fits the hypotheses of Schiøtz (1976) and Poynton (1999), who argued that the forests of Uganda and western Kenya were former outliers of the Virunga Mountains, i.e. parts of the eastern Congo basin forest.
Table 2.
Coefficient of biogeographic resemblance (CBR) of the reptile communities of 17 African forests: Ziama (ZIA), Mt Nimba (MTN), Korup (KOR), Mt Nlonako (MNL), Loango (LOA), Lopé (LOP), Moukalaba (MOK), Monts de Crystal (MCR), Massif du Chaillu (MAC) Virungas (VIR), Garamba (GAR), Kibale (KIB), Bwindi (BWI), Usambara (USA), Udzungwa (UZU), Arabuko‐Sokoke (ARA), Kakamega (KMF). Numbers above the diagonal show the species number which two forests have in common; numbers below the diagonal show the values of the CBR between two forests; values > 0.50 are shown in bold.
| ZIA | MTN | KOR | MNL | LOA | LOP | MOK | MCR | MAC | VIR | GAR | KIB | BWI | USA | UZU | ARA | KMF | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZIA | – | 45 | 31 | 31 | 11 | 14 | 14 | 15 | 15 | 27 | 23 | 21 | 12 | 3 | 0 | 5 | 15 |
| MTN | 0.70 | – | 35 | 37 | 15 | 16 | 19 | 18 | 18 | 25 | 25 | 18 | 11 | 4 | 0 | 7 | 14 |
| KOR | 0.46 | 0.48 | – | 59 | 17 | 19 | 20 | 24 | 18 | 33 | 27 | 23 | 10 | 4 | 0 | 6 | 18 |
| MNL | 0.43 | 0.48 | 0.73 | – | 22 | 24 | 28 | 37 | 30 | 32 | 23 | 22 | 13 | 3 | 0 | 4 | 21 |
| LOA | 0.24 | 0.29 | 0.31 | 0.37 | – | 17 | 22 | 17 | 16 | 11 | 11 | 7 | 4 | 1 | 0 | 5 | 9 |
| LOP | 0.31 | 0.32 | 0.35 | 0.41 | 0.52 | – | 18 | 16 | 21 | 16 | 15 | 12 | 6 | 2 | 0 | 4 | 14 |
| MOK | 0.28 | 0.35 | 0.34 | 0.44 | 0.59 | 0.49 | – | 24 | 23 | 18 | 13 | 9 | 5 | 2 | 0 | 4 | 11 |
| MCR | 0.29 | 0.31 | 0.39 | 0.56 | 0.42 | 0.41 | 0.55 | – | 28 | 19 | 13 | 12 | 8 | 3 | 0 | 3 | 13 |
| MAC | 0.30 | 0.32 | 0.31 | 0.47 | 0.42 | 0.57 | 0.55 | 0.63 | – | 17 | 12 | 12 | 9 | 3 | 0 | 2 | 11 |
| VIR | 0.32 | 0.28 | 0.37 | 0.33 | 0.15 | 0.23 | 0.24 | 0.24 | 0.23 | – | 43 | 43 | 33 | 4 | 1 | 16 | 37 |
| GAR | 0.35 | 0.35 | 0.39 | 0.29 | 0.20 | 0.28 | 0.23 | 0.21 | 0.21 | 0.48 | – | 21 | 11 | 2 | 0 | 5 | 25 |
| KIB | 0.40 | 0.32 | 0.36 | 0.33 | 0.18 | 0.31 | 0.21 | 0.26 | 0.27 | 0.53 | 0.33 | – | 27 | 3 | 0 | 5 | 28 |
| BWI | 0.26 | 0.21 | 0.18 | 0.21 | 0.12 | 0.18 | 0.13 | 0.20 | 0.23 | 0.46 | 0.20 | 0.66 | – | 4 | 0 | 4 | 18 |
| USA | 0.07 | 0.08 | 0.08 | 0.05 | 0.03 | 0.07 | 0.06 | 0.08 | 0.09 | 0.06 | 0.04 | 0.08 | 0.13 | – | 6 | 4 | 5 |
| UZU | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.27 | – | 0 | 0 |
| ARA | 0.08 | 0.11 | 0.09 | 0.05 | 0.10 | 0.08 | 0.08 | 0.05 | 0.04 | 0.21 | 0.25 | 0.1 | 0.1 | 0.09 | 0.00 | – | 9 |
| KMF | 0.26 | 0.22 | 0.27 | 0.29 | 0.20 | 0.31 | 0.22 | 0.25 | 0.22 | 0.44 | 0.38 | 0.54 | 0.39 | 0.12 | 0.00 | 0.15 | – |
The comparatively low species richness of Kakamega Forest may be explained by its more elevated situation, moderate amount of rainfall, and its small size (and subsequent progressive destruction and habitat fragmentation). Fragmentation still continues and seriously threatens forest species in particular (classified in group 1 above), whereas common ubiquitous species are probably less affected. This underlines the necessity of prioritizing the protection of these forest remnants.
Our results also show, however, that the Eastern Arc Mountains form a zoogeographical unit of their own, indicating that they have long been isolated from equatorial forest. This is reinforced by their high rate of endemism. For instance, 87% of the amphibian and 55% of the reptile species of the Usambara Mountains are endemic to the Tanzanian highland forests (Howell, 1993). Kakamega Forest has more species in common with the Ziama Forest in south‐eastern Guinea (5000 km apart) than with the Usambara Mountains (1100 km apart), which reflects the obviously longer separation from the latter. The Udzungwa Mountains are even more isolated and they have only six reptile species in common with the Usambara Mountains, i.e. one species more than the species common to both Kakamega and Usambara forests. The faunal comparison also highlights the uniqueness of the East African coastal forests, which clearly form their own, distinct zoogeographic unit and which are in desperate need of efficient protection (Howell, 1993; Lovett, 1993b).
Africa’s rain forests have been periodically affected by periods of dramatic expansion and contraction (Moreau, 1969; Hamilton, 1976, 1992; Martin, 1989). These oscillations are a consequence of the pluvial and interpluvial phases of the Pleistocene and supposedly led to a reduction of the rain forest to only two small refugia in the last interpluvial (18,000 yr bp): one in coastal southern Cameroon and Gabon, the other in the eastern the Democratic Republic of Congo and in the adjacent Rift Valley (Mayr & O’Hara, 1986). Some even smaller refugial forest patches persisted in West Africa and on the Kenyan coast. The subsequent global warming resulted in a comparatively rapid re‐colonization of the entire Congo basin, by the Guineo‐Congolian rain forest, with a maximal expansion of the Congo forest block, roughly 7000 yr bp (Hamilton, 1976, 1992). The long dynamic history and repeated former fragmentation and re‐extension of the Congolian rain forest is well reflected in its evolutionary history, i.e. the distribution patterns of many forest‐dwelling taxa, including amphibians and reptiles.
The low species richness of Kakamega Forest rejects the possibility that it served as a refugial area. This conclusion is reinforced when comparing its species numbers with the respective reptile species richness of Mt Nlonako (Herrmann et al., 2005), Korup National Park or of the Virunga Mountains (Hamilton, 1988), which probably provided relatively stable ecological conditions even in the Pleistocene. Moreover, it seems unlikely that the Kakamega Forest and the more elevated Nandi Forests ever had any faunal exchange with either the Kenyan montane forests or the Eastern Arc Mountains. Burgess et al. (1998) suggest that the coastal and eastern montane forests have been separated from the forest block for the last 10 Myr. However, a relationship between Kakamega Forest and the coastal forests does exist, and a forest ‘bridge’ through the southern Kenyan highlands may have connected them, allowing an exchange of forest taxa. This is supported by Wilfert et al.’s (2006) work on the damp‐wood termite Schedorhinotermes lamanianus (Isoptera: Rhinotermitidae). But Arabuko‐Sokoke and Kakamega forests only share typical savanna/forest dwellers such as Acanthocercus atricollis, Dispholidus typus and Naja melanoleuca. This indicates that either this bridge was not in place for long enough to allow for the migration of typical forest species, or the bridge consisted of a savanna/forest mosaic, and thus was too dry to allow typical forest animals to cross it.
The phylogenetic study of the genus Rhampholeon by Matthee et al. (2004), however, does provide evidence for a formerly closed pancontinental rain forest block because the sister taxon of the West and Central African R. spectrum is not the morphologically similar and geographically close R. boulengeri, but the geographically distant R. temporalis, a species endemic to the Usambara Mountains. This indicates a former connection between the Guineo‐Congolian forest and Eastern Arc Mountains. This connection must, of course, be much older than the last connection between Kakamega Forest and the Guineo‐Congolian forest block (as it currently exists) and might not have existed during the Pleistocene. Otherwise, the high rate of endemism in the isolated Eastern Arc Mountains would be difficult to explain. According to Matthee et al. (2004), the nearest relatives of R. boulengeri inhabit the forested southern foothills of the Albertine Rift, which indicates that their ancestors dispersed along a forested corridor through the Albertine Rift (see also Burgess et al., 1998). Since members of Rhampholeon are essentially forest animals with low migration/dispersal rates, such a bridge appears more likely than a northern bridge through the Kenyan highlands.
The closed pan‐African Guineo‐Congolian rain forest block dates back, according to Burgess et al. (1998), from 40 to 19 Myr bp (Oligocene to early Miocene) when a moist, tropical climate predominated (Axelrod & Raven, 1978). Around 18 to 17 Myr bp the northward drift of the African plate led to the closing of the Tethys Sea. The climate became drastically drier and the rift formations in East Africa started to rise. At this time the pan‐African forest block was divided into western (Guinea to Cameroon), central (Congo to Kenya) and eastern (East African coastal forests) isolates (Axelrod & Raven, 1978), which were further subdivided and fragmented by climate oscillations. During favourable climatic periods, 9 to 6.4 Myr bp and again 4.6 to 2.43 Myr bp, the pan‐African forest block may have been temporarily restored (Lovett, 1993a; b). It is not clear to what extent the coastal forests were connected with the forest relicts on the Eastern Arc Mountains (e.g. Usambara). According to Brenan (1978) and Lovett & Friis (1996), the Eastern Arc Mountains harbour a primitive flora that was more widely distributed in the past and which today cannot be found elsewhere in Africa; this argues for a long history of isolation of these montane habitats. However, the existence of a closed pan‐African rain forest system throughout the climate oscillations of the Pleistocene and the Holocene seems an unlikely possibility.
In conclusion, our results reveal clear biogeographical evidence that the Kakamega Forest is part of the Guineo‐Congolian rain forest block, although differences between the forest units within this block exist. The West African block is divided into the Gold Coast, West African and Gabonian blocks, while the East African block appears undivided. This phenomenon might be explained by the comparatively close geographical distances of the East African forests investigated, which may therefore connect more rapidly, given respective climatic conditions, and thus facilitate exchange of forest species. On the other hand, the East African coastal forests and the Arc Mountains form units which differ distinctly from the Guineo‐Congolian block.
All three latter mentioned forest types are still present in Kenya but are restricted to comparatively small remnants (Kakamega Forest, Arabuko‐Sokoke Forest, Shimba Hill and Taita Hill forests). All these forest types harbour unique species communities found nowhere else in the country. The rather recent and rapid loss of intact coastal rain forest regions is a tragic example of the significant reduction of a unique forest type, because of the low conservation priority given to it. Considering the very small size of Kakamega Forest, the last remnant of the Guineo‐Congolian forest in Kenya, the highest conservation priority should be applied to prevent its rapid disappearance, and that of its corresponding species communities.
Biosketches
Philipp Wagner is a PhD student at the Zoologisches Forschungsmuseum Alexander Koenig, Bonn, and has been working on the systematics and biogeography of African reptiles. His current research is focused on distribution patterns, especially of agamid lizards.
Jörn Köhler is Curator for Vertebrate Zoology at the Hessisches Landesmuseum Darmstadt. He has been working on the systematics and biogeography of the amphibians and reptiles of Africa and South America.
Andreas Schmitz is Research Officer for Herpetology at the Muséum d′Histoire Naturelle de la Ville de Genève, Geneva, and is working on the herpetofauna of Africa and Asia with special focus on the systematics and phylogeny of lizards.
Wolfgang Böhme is Professor of Zoology, Deputy Director, Head of the Vertebrate Department and Curator for Herpetology at the Zoologisches Forschungsmuseum Alexander Koenig in Bonn. He has specialized on the African and Asian herpetofauna, particularly on chameleons and monitor lizards.
Editor: Malte Ebach
Supporting information
Appendix S1 Reptile species from 10 African forests used for analysing the forest relationships.
Appendix S2 Ecological characteristics of the 58 reptile species recorded within the area of the Kakamega Forest.
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Acknowledgements
We are grateful to Stephen Spawls for his effort to improve our manuscript not only from a scientific point of view but also for a linguistic one. We are especially grateful to Robert J. Whittaker, Malte Ebach and two anonymous referees for their critical and very helpful comments on the manuscript. The study benefited from the support of the BIOLOG Programme/BIOTA project funded by the German Federal Ministry of Education and Research (BMBF). BIOTA East Africa is centred at the ZFMK. We are indebted to our Kenyan partners, the National Museums of Kenya (NMK) and the Kenya Wildlife Service (KWS), who kindly provided facilities and permissions. We are grateful to the Kakamega Environmental Education Programme (KEEP) for providing field assistance. We thank Stefan Lötters and his working group for providing unpublished data. Special thanks to Claudia Fink for joining P.W. in the field and to Sylvester Shirandula and Caleb Analo for assistance in the field. P.W. is indebted to his father, Rüdiger Wagner, for his support. We also thank the following sponsors: Deutsche Telekom, Fuji‐Films, Henkel, Kodak Films, Siemens, Unilever, Varta and Volkswagen, who supported the study in different ways.
Footnotes
There are taxonomic issues in the case of Psammophis phillipsi. Spawls et al. (2002) as well as Broadley et al. (2003) regard P. phillipsi as a synonym of P. mossambicus, whereas Loveridge (1940) considered it a subspecies of P. sibilans. All authors did not consider the condition of an entire vs. divided cloacal scale as a specific diagnostic character. However, there are more characters that separate the rain forest Psammophis phillipsi from the larger savanna species P. mossambicus, e.g. the lack of a juvenile colour pattern and a dark ventral pigmentation (see also Böhme et al., 1996; Brandstätter, 1996). Our voucher specimen (ZFMK 82050) has 17 scale rows around midbody, 173 ventral scales, 95 paired subcaudal scales and an entire cloacal scale. A colour pattern is lacking, but there are two fine spots on each ventral scale forming two fine longitudinal lines on the venter.
References
- Axelrod, D.I. & Raven, P.H. (1978) Late Cretaceous and Tertiary vegetation history of Africa Biogeography and ecology of Southern Africa (ed. by Werger M.J.A.), pp. 77–130. Dr W. Junk Publishers, The Hague. [Google Scholar]
- Bauer, A. (1993) African–South American relationships: a perspective from the Reptilia Biological relationships between Africa and South America (ed. by Goldblatt P.), pp. 245–288. Yale University Press, New Haven, CT. [Google Scholar]
- Böhme, W. (1993) Mission d’études herpétologiques dans les forèts de Ziama et Diécké, Guinée forestière. Unpublished Report, Neu Isenburg, Germany. [Google Scholar]
- Böhme, W. (1994a) Frösche und Skinke aus dem Regenwaldgebiet Südostguineas, Westafrika. I. Einleitung; Pipidae, Arthroleptidae, Bufonidae. herpetofauna, 16, 11–16. [Google Scholar]
- Böhme, W. (1994b) Frösche und Skinke aus dem Regenwaldgebiet Südostguineas, Westafrika. II: Ranidae, Hyperoliidae, Scincidae; faunistisch‐ökologische Bewertung. herpetofauna, 16, 6–16. [Google Scholar]
- Böhme, W. (2000) Diversity of a snake community in a Guinean rain forest (Reptilia, Serpentes). Bonner zoologische Monographien, 46, 69–78. [Google Scholar]
- Böhme, W. , Meinig, H. & Rödel, M.‐O. (1996) New records of amphibians and reptiles from Burkina Faso and Mali. British Herpetological Society Bulletin, 56, 7–26. [Google Scholar]
- Brandstätter, F. (1996) Die Sandrennattern. Neue Brehm Bücherei, Magdeburg. [Google Scholar]
- Brenan, J.P.M. (1978) Some aspects of the phytogeography of tropical Africa. Annals of the Missouri Botanical Garden, 65, 437–478. [Google Scholar]
- Broadley, D.G. & Howell, K.M. (1991) A check list of the reptiles of Tanzania, with synoptic keys. Syntarsus, 1, 1–70. [Google Scholar]
- Broadley, D.G. , Doria, C.T. & Wigge, J. (2003) Snakes of Zambia. Chimaira Buchhandelsgesellschaft, Frankfurt am Main. [Google Scholar]
- Burgess, N.D. , Clarke, G.P. & Rodgers, W.A. (1998) Coastal forests of eastern Africa: status, endemism patterns and their potential causes. Biological Journal of the Linnaean Society, 64, 337–367. [Google Scholar]
- Chippaux, J.‐P. (1991) Les serpents d’Afrique occidentale et centrale, 1st edn Èditions de l’IRD, Paris. [Google Scholar]
- Clausnitzer, V. (1999) Dragonfly (Odonata) records of Kakamega Forest, Western Kenya, with notes on the ecology of rain forest species. Journal of East African Natural History, 88, 17–24. [Google Scholar]
- Clausnitzer, V. (2005) An updated checklist of the dragonflies (Odonata) of the Kakamega Forest, Kenya. Journal of East African Natural History, 94, 239–246. [Google Scholar]
- Collins, N.M. (1992) Introduction The conservation atlas of tropical forests in Africa (ed. by Sayer J.A., Harcourt C.S. and Collins N.M.), pp. 6–16. Macmillan Publishers Ltd, Basingstoke, UK. [Google Scholar]
- DeWitte, G.F. (1941) Batraciens et reptiles. Exploration du Parc National Albert, Mission G. F. DeWitte (1933–1935). Fascicle 33. Institut des Parcs Nationaux du Congo Belge, Bruxelles. [Google Scholar]
- DeWitte, G.‐F. (1965) Les caméléons de l’Afrique Centrale. Sciences Zoologiques, 142, 1–215. [Google Scholar]
- DeWitte, G.F. (1966) Reptiles. Exploration du Parc National de la Garamba, Mission H. De Saeger (1949–1952). Fascicle 48. Institut des Parcs Nationaux du Congo Belge, Bruxelles. [Google Scholar]
- Drewes, R.C. (1976) Report on an expedition to Kakamega Forest. East African National History Society Bulletin, 1976, 122–126. [Google Scholar]
- Drewes, R.C. & Vindum, J.V. (1991) Amphibians of the Impenetrable Forest, southwest Uganda. Journal of Zoology, 108, 55–70. [Google Scholar]
- Drewes, R.C. & Vindum, J.V. (1997) Amphibians and reptiles of the Bwindi Impenetrable National Park. Unpublished Report. Uganda Wildlife Authority, Kampala. [Google Scholar]
- Duellman, W.E. (1990) Herpetofaunas in Neotropical rainforests: comparative composition, history, and resource use Four Neotropical rain forests (ed. by Gentry A.H.), pp. 455–505. Yale University Press, New Haven, CT. [Google Scholar]
- Fashing, J.P. & Gathua, J.M. (2004) Spatial variability in the vegetation structure and composition of an East African rain forest. African Journal of Ecology, 42, 189–197. [Google Scholar]
- Felsenstein, J. (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution, 39, 783–791. [DOI] [PubMed] [Google Scholar]
- Hallowell, E. (1844) Description of new species of African reptiles. Proceedings of the Academy of Natural Science Philadelphia, 1844, 169–172. [Google Scholar]
- Hamilton, A.C. (1976) The significance of patterns of distribution shown by forest plants and animals in tropical Africa for the reconstruction of Upper Pleistocene palaeoenvironments: a review. Palaeoecology of Africa, 9, 63–97. [Google Scholar]
- Hamilton, A.C. (1988) Guenon evolution and forest history A primate radiation: evolutionary biology of the African guenons (ed. by Gautier‐Hior A., Bourliere F., Gautier J.‐P. and Kingdon J.), pp. 13–34. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Hamilton, A.C. (1992) History of forests and climate The conservation atlas of tropical forests: Africa (ed. by Sayer J.A., Harcourt C.S. and Collins N.M.), pp. 17–25. Macmillan Publishers Ltd, Basingstoke, UK. [Google Scholar]
- Herrmann, H.‐W. , Böhme, W. , Euskirchen, O. , Herrmann, P.A. & Schmitz, A. (2005) African biodiversity hotspots: the reptiles of Mt. Nlonako, Cameroon. Revue Suisse de Zoologique, 112, 1045–1069. [Google Scholar]
- Howell, K.M. (1993) Herpetofauna of the East African forests Biogeography and ecology of the rain forests of Eastern Africa (ed. by Lovett J.C. and Wasser S.), pp. 173–201. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Hughes, B. (1983) African snake faunas. Bonner zoologische Beiträge, 43, 311–356. [Google Scholar]
- Ineich, I. (2003) Contribution à la connaissance de la biodiversité des régions afro‐ montagnardes: les reptiles du mont Nimba Le peuplement animal du mont Nimba (Guinée, Côte d’Ivoire, Liberia) (ed. by Lamotte M. and Roy R.), pp. 597–637. Mémoires du Muséum national d’Histoire naturelle, Paris. [Google Scholar]
- Kifcon (Kenyan indigenous forest conservation project) (1995) Arabuko Sokoke Forest and Mida Creek: the official guide. Majestic Printing Works Ltd, Nairobi. [Google Scholar]
- Klaver, C.J.J. & Böhme, W. (1997) Chamaeleonidae. Das Tierreich, 112, 1–85. [Google Scholar]
- Köhler, J. (2000) Amphibian diversity in Bolivia: a study with special reference to montane forest regions. Bonner zoologische Monographien, 48, 1–243. [Google Scholar]
- Köhler, J. (2004) Was hat Biodiversitätsforschung mit nachhaltiger Nutzung zu tun? Tier und Museum, 8, 82–91. [Google Scholar]
- Köhler, J. , Wagner, P. , Visser, S. & Böhme, W. (2003) New country records of Adolfus africanus (Sauria: Lacertidae) – a rain forest lizard with disjunct distribution? Salamandra, 39, 241–248. [Google Scholar]
- Köhler, J. , Bwong, B. , Schick, S. , Veith, M. & Lötters, S. (2006) A new species of arboreal Leptopelis (Anura: Arthroleptidae) from the forests of western Kenya. Herpetological Journal, 16, 183–189. [Google Scholar]
- Kokwaro, L.O. (1988) Conservation status of the Kakamega Forest in Kenya: the eastern‐most relic of the equatorial rainforest in Africa. Monograph of Botany at the Missouri Botanical Gardens, 25, 471–489. [Google Scholar]
- Lawson, D.P. (1993) The reptiles and amphibians of the Korup National Park project, Cameroon. Herpetological Natural History, 1, 27–90. [Google Scholar]
- Loveridge, A. (1935) Scientific results of an expedition to rain forest regions in eastern Africa. I. New reptiles and amphibians from East Africa. Bulletin of the Museum of Comparative Zoology, 79, 3–19. [Google Scholar]
- Loveridge, A. (1936) Scientific results of an expedition to rain forest regions in eastern Africa. V. Reptiles. Bulletin of the Museum of Comparative Zoology, 79, 209–337. [Google Scholar]
- Loveridge, A. (1940) Revision of the snakes of the genera Dromophis and Psammophis . Bulletin of the Museum of Comparative Zoology, 87, 1–69. [Google Scholar]
- Loveridge, A. (1942a) Scientific results of a fourth expedition to forested areas in East and Central Africa. IV. Reptiles. Bulletin of the Museum of Comparative Zoology, 91, 237–373. [Google Scholar]
- Loveridge, A. (1942b) Scientific results of a fourth expedition to forested areas in East and Central Africa. V. Amphibians. Bulletin of the Museum of Comparative Zoology, 91, 377–436. [Google Scholar]
- Lovett, J.C. (1993a) Climatic history and forest distribution in eastern Africa Biogeography and ecology of the rain forests of Eastern Africa (ed. by Lovett J.C. and Wasser S.K.), pp. 23–33. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Lovett, J.C. (1993b) Eastern Arc moist forest flora Biogeography and ecology of the rain forests of Eastern Africa (ed. by Lovett J.C. and Wasser S.K.), pp. 33–55. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Lovett, J.C. & Friis, I. (1996) Patterns of endemism in the woody flora of north‐east and east Africa The biodiversity of African plants (ed. by Van Maesen L.J.G.), pp. 582–601. Kluwer Academic Publishers, Dordrecht, The Netherlands. [Google Scholar]
- Martin, C. (1989) Die Regenwälder Westafrikas. Ökologie, Bedrohung, Schutz. Birkhäuser, Basel, Switzerland. [Google Scholar]
- Matthee, C.A. , Tilbury, C.R. & Townsend, T. (2004) A phylogenetic review of the African leaf chameleons: genus Rhampholeon (Chamaeleonidae): the role of vicariance and climate change in speciation. Proceedings of the Royal Society B: Biological Sciences, 271, 1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr, E. & O’Hara, R.J. (1986) The biogeographic evidence supporting the Pleistocene forest refuge hypothesis. Evolution, 40, 55–67. [DOI] [PubMed] [Google Scholar]
- Mitchell, N. (2004) The exploitation and disturbance history of Kakamega Forest, Western Kenya. Bielefelder ökologische Beiträge, 20, 1–78. [Google Scholar]
- Moreau, R.E. (1966) The bird faunas of Africa and its islands. Academic Press, London, UK. [Google Scholar]
- Moreau, R.E. (1969) Climatic changes and the distribution of forest vertebrates in West Africa. Journal of Zoology, 158, 39–61. [Google Scholar]
- Pauwels, O.S.G. , Kamdem Toham, A. & Chimsunchart, C. (2002a) Recherches sur l′herpétofaune du Massif du Chaillu, Gabon. Bulletin de l'Institut Royal des Sciences Naturelles de Belgique, 72, 47–57. [Google Scholar]
- Pauwels, O.S.G. , Kamdem Toham, A. & Chimsunchart, C. (2002b) Recherches sur l′herpétofaune des Monts de Cristal, Gabon. Bulletin de l'Institut Royal des Sciences Naturelles de Belgique, 72, 59–66. [Google Scholar]
- Pauwels, O.S.G. , Christy, P. & Honorez, A. (2006) Reptiles and National Parks in Gabon, western Central Africa. Hamadryad, 30, 181–196. [Google Scholar]
- Pitman, C.R.S. (1974) A guide to the snakes of Uganda, revised edn. Wheldon & Wesley Ltd, Codicote, UK. [Google Scholar]
- Poynton, J.C. (1999) Distribution of amphibians in Sub‐Saharan Africa, Madagascar, and Seychelles Patterns of distribution of amphibians – a global perspective (ed. by Duellman W.E.), pp. 483–539. Johns Hopkins University Press, London, UK. [Google Scholar]
- Rosen, B.R. (1988) From fossils to earth history: applied historical biogeography Analytical biogeography: an integrated approach to the study of animal and plant distributions (ed. by Myers A. and Giller P.), pp. 437–481. Chapman & Hall, London, UK. [Google Scholar]
- Rosen, B.R. & Smith, A.B. (1988) Tectonics from fossils? Analysis of reef‐coral and sea‐urchin distributions from late Cretaceous to recent, using a new method. Geological Society Special Publications, 37, 275–306. [Google Scholar]
- Salzmann, U. & Hoelzmann, P. (2005) The Dahomey Gap: an abrupt climatically induced rain forest fragmentation in West Africa during the late Holocene. The Holocene, 15, 190–199. [Google Scholar]
- Schick, S. , Veith, M. & Lötters, S. (2005) Distribution patterns of amphibians from the Kakamega Forest, Kenya. African Journal of Herpetology, 54, 185–190. [Google Scholar]
- Schiøtz, A. (1975) The treefrogs of eastern Africa. Steenstrupia, Copenhagen. [Google Scholar]
- Schiøtz, A. (1976) Zoogeographical patterns in the distribution of East African treefrogs (Anura: Ranidae). Zoologica Africana, 11, 335–338. [Google Scholar]
- Spawls, S. , Howell, K.M. , Drewes, R. & Ashe, J. (2002) A field guide to the reptiles of East Africa. Academic Press, London, UK. [Google Scholar]
- Swofford, D.L. (1998) PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods), Version 4. Sinauer Associates, Sunderland, MA. [Google Scholar]
- Uetz, P. (2001) Classification of living reptiles [WWW document]. URL http://www.embl-heidelberg.de/~uetz/LivingReptiles.html [accessed on 30 December 2006]. [Google Scholar]
- Vonesh, J. (2001) Natural history and biogeography of the amphibians and reptiles of Kibale National Park, Uganda. Contemporary Herpetology, 4, 1–14. [Google Scholar]
- Wagner, P. & Böhme, W. (2007) Herpetofauna Kakamegensis – the amphibians and reptiles of Kakamega Forest, western Kenya. Bonner zoologische Beiträge, 55, 123–150. [Google Scholar]
- Wagner, P. & Schmitz, A. (2006) Feylinia currori Gray, 1845 (Squamata: Scincidae), a new genus and species record for Kenya. Salamandra, 42, 183–187. [Google Scholar]
- Wagner, P. , Burmann, A. & Böhme, W. (in press) Studies on African Agama II. Resurrection of Agama agama kaimosae Loveridge, 1935 (Squamata: Agamidae) from synonymy and its elevation to species rank. Russian Journal of Herpetology. [Google Scholar]
- Wass, P. (1995) Kenya’s indigenous forests: status, management and conservation. IUCN, Gland, Switzerland. [Google Scholar]
- Wilfert, L. , Kaib, M. , Durka, W. & Brandl, R. (2006) Differentiation between populations of a termite in eastern Africa: implications for biogeography. Journal of Biogeography, 33, 1993–2000. [Google Scholar]
- Zimmermann, D. (1972) The avifauna of the Kakamega Forest, western Kenya, including a bird population study. Bulletin of the American Museum of Natural History, 149, 257–339. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Reptile species from 10 African forests used for analysing the forest relationships.
Appendix S2 Ecological characteristics of the 58 reptile species recorded within the area of the Kakamega Forest.
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item


