Abstract
Despite being the most significant arboviral disease worldwide, dengue has no antiviral treatment or reliable severity predictors. It has been shown that apoptotic cells from blood and tissues may be involved in the complex pathogenesis of dengue. However, very little is known about the interplay between proapoptotic and antiapoptotic mediators in this disease. Therefore, plasma levels of the three proapoptotic mediators Fas ligand (FasL), tumor necrosis factor‐α (TNF‐α), and TNF‐related apoptosis‐inducing ligand (TRAIL) were measured in dengue patients. Patients were classified according to the World Health Organization classification of dengue revised in 2009. Additionally, inhibitors of apoptosis protein (IAPs) were determined in plasma (Survivin) and peripheral blood mononuclear cells (PBMCs) lysates (cIAP‐1, cIAP‐2, XIAP). Levels of apoptotic proteins in plasma were correlated with counts of blood cells. FasL and TRAIL levels were elevated in dengue patients without warning signs when compared to patients with severe dengue and controls. Dengue patients with warning signs showed decreased levels of Survivin compared to patients with severe dengue and controls. TRAIL was inversely correlated with counts of lymphocyte subsets. In contrast, Survivin was positively correlated with leukocyte counts. There was a trend of elevated IAPs levels in PBMCs of patients with severe dengue. The results suggest a likely antiviral effect of TRAIL in dengue. It appears that TRAIL might be involved with apoptosis induction of lymphocytes, whereas IAPs might participate in protecting leukocytes from apoptosis. Further research is needed to explore the interactions between pro and antiapoptotic molecules and their implications in dengue pathogenesis. J. Med. Virol. 86:1437–1447, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: apoptosis, pathogenesis, lymphocytes, monocytes
INTRODUCTION
Dengue is the most prevalent mosquito‐transmitted viral disease in the world and is caused by any of the four closely related, but antigenically distinct, serotypes of dengue virus (DENV‐1–4), of the genus Flavivirus, family Flaviviridae. Very recently, a map of dengue risk on the basis of the global population in 2010 estimated 390 million infections worldwide, of which 96 millions manifested apparently [Bhatt et al., 2013]. This estimate is more than three times higher the WHO predicted burden. In particular, Brazil has reported the largest number of dengue cases, accounting for more than 70% in the Americas. However, reliable biomarkers of dengue severity, specific antiviral therapy and a licensed antidengue vaccine are not available [World Health Organization, 2009; Guzman et al., 2010; Simmons et al., 2012].
The World Health Organization (WHO) classification of dengue, dengue fever (DF), and dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) has proven to be useful for more than 30 years. Still, a revised classification was proposed after a recent international multicenter study: dengue without warning signs, dengue with warning signs and severe dengue [World Health Organization, 2009; Alexander et al., 2011].
The pathogenesis of dengue is multifactorial and not fully understood. Thus far, a complex interplay among viral, immunological, and host factors has been demonstrated. This interplay includes antibody‐mediated immune enhancement in secondary dengue infections which increases uptake of virus–antibody complexes in monocytes and macrophages [Kliks et al., 1989]. Studies also have shown involvement of the storm of cytokines and other soluble mediators released by immune cells [Braga et al., 2001; Bozza et al., 2008], hyperactivation of lymphocytes [Rothman, 2010], and the influence of the infecting viral serotype [Balmaseda et al., 2006]. Comorbidities [Limonta et al., 2009] and genetic background [Sierra et al., 2007] also are associated with the pathophysiology of this viral disease.
Apoptotic cell death has been proposed to play a role in dengue pathophysiology. Previous studies of dengue infection have shown apoptosis in lymphocytes [Mongkolsapaya et al., 2003; Myint et al., 2006], monocytes [Torrentes‐Carvalho et al., 2009; Levy et al., 2010], and peripheral blood mononuclear cells (PBMCs) [Jaiyen et al., 2009]. Apoptotic cells also have been found in a variety of tissues from fatal dengue cases [Huerre et al., 2001; Limonta et al., 2007, 2009].
Caspases (cysteinyl‐aspartate‐cleaving proteases) are essential cellular proteins involved in apoptosis, a programmed cell death. Caspase activation is inhibited by inhibitors of apoptosis proteins (IAPs) [Hotchkiss et al., 2009]. Survivin is considered a fundamental IAPs that plays a role in tumorigenesis and inflammation [Altieri, 2010]. However, the role of Survivin has been poorly studied in viral diseases [Zhu et al., 2003; Bokarewa et al., 2007].
The extrinsic or death receptor pathway is one of the two apoptotic pathways, and is mediated by transmembrane receptors. The extrinsic pathway is activated when ligands that are members of the tumor necrosis factor (TNF) superfamily TNF‐α, TNF‐related apoptosis‐inducing ligand (TRAIL), and Fas ligand (FasL) also known as CD95L bind to the death receptors. These ligands display proapoptotic functions in membrane‐bound forms or in soluble forms [Locksley et al., 2001; Galluzzi et al., 2012].
Although TNF‐α has been extensively studied in dengue [Cardier et al., 2005; Chen et al., 2007; Levy et al., 2010] and an in vitro anti‐DENV action of TRAIL has been described [Warke et al., 2008b], no data appear to be available on the interplay among circulating pro and antiapoptotic mediators in dengue patients. Furthermore, to our knowledge, the revised WHO classification for dengue [World Health Organization, 2009] has not been used in studies of apoptosis in dengue cases.
In the present work, apoptotic mediators were measured in plasma and PBMCs lysates from Brazilian patients with dengue. For the first time, these apoptotic proteins were measured in patients with the WHO classification for dengue revised in 2009. The three plasma molecules evaluated (FasL, TNF‐α, and TRAIL) are fundamental apoptosis inducers. The other proteins studied, plasma Survivin and PBMCs lysate proteins, are IAPs. Our data suggest that TRAIL could be involved with apoptosis induction of blood lymphocytes and could also contribute to the antiviral response. Conversely, IAPs may participate in apoptosis protection of blood leukocytes. FasL may display a role in dengue pathophysiology, and especially in fatal cases of dengue.
METHODS
Patients and Sample Collection
Venous blood samples were obtained from 107 febrile dengue‐suspected cases during dengue outbreaks in the southwest of Brazil in 2010. Seventy‐two (67.3%) of the 107 cases had the diagnosis of dengue confirmed. Patients were assisted at the Centro de Referência em dengue in Campos dos Goytacazes, Rio de Janeiro state, and at the Hospital Professora Esterina Corsini in the Universidade Federal do Mato Grosso do Sul, Mato Grosso do Sul state. PBMCs and plasma were collected at these health care facilities, then transported in liquid nitrogen to the Viral Immunology Laboratory at the Oswaldo Cruz Institute, and stored in liquid nitrogen until use.
PBMCs and plasma samples from 10 healthy adults were collected as controls. Inclusion criteria for controls were absence of fever history in the preceding three months, absence of chronic diseases, and negative anti‐DENV IgM by ELISA (PanBio Diagnostics, Brisbane, Australia).
The study protocol was approved by the Institutional Review Board of Oswaldo Cruz Foundation (FIOCRUZ, number 061/2008) from the Ministry of Health in Brazil (recognized by the Brazilian National Ethics Committee), and by the Institutional Review Board of each Brazilian hospital involved in this study. Enrollment of study participants, patients under the age of 15 required parents/legal guardians consent, and control subjects was conditional on obtaining appropriate written informed consent.
Laboratory Confirmation of Dengue Infection
A case was considered positive for dengue when diagnostic assays met one or more of the following criteria: (1) IgM detection in acute samples by DENV‐specific capture IgM enzyme‐linked immunosorbent assay (ELISA) (PanBio Diagnostics), (2) DENV RNA detection in acute‐phase serum by a serotype‐specific RT‐PCR assay as described elsewhere [Lanciotti et al., 1992], (3) DENV isolation in acute samples by inoculation onto Aedes albopictus C6/36 cells and subsequent immunoflourescent detection of viral antigens as described previously [Igarashi, 1978], and (4) detection of non‐structural 1 (NS1) protein using NS1 antigen strip (Bio‐Rad, Hercules, CA) in acute serum following the manufacturer's instructions.
Criteria for WHO Case Definition for Dengue
The new WHO dengue classification criteria from 2009 consisting of dengue without warning signs, dengue with warning signs, and severe dengue were used for each case. Dengue without warning signs comprises fever with at least two of the following: nausea, vomiting, rash, aches and pain, positive tourniquet test, and leucopenia. Dengue with warning signs includes dengue patients with any of the following warning signs: abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy or restlessness, liver enlargement >2 cm, and an increase in hematocrit concurrent with rapid decrease in platelet count. Severe dengue includes at least one of the following: severe plasma leakage (leading to shock and fluid accumulation with respiratory distress), severe bleeding evaluated by clinicians, severe involvement of liver by aspartate aminotransferase (AST) or alanine transferase (ALT) > 1,000 U, central nervous system with impaired consciousness, and severe involvement of the heart and other organs [World Health Organization, 2009].
Clinical Data Collection
Upon enrollment during consultation or hospitalization, standardized forms for data collection were completed by study clinicians experienced with dengue. The forms included demographic information, vital signs, physical examinations, complete blood counts, liver enzyme values (ALT, alanine transaminase; AST, aspartate transaminase), findings of ultrasounds and X‐rays.
Blood Mononuclear Cell Preparation
About 20 ml of peripheral venous blood were collected in Na‐heparin anticoagulated tubes from patients and control subjects. PBMCs were separated from blood samples using the Ficoll‐Hypaque procedure (d = 1.077 g/ml; Sigma‐Aldrich, St. Louis, MO) and centrifuged at 400g for 30 min. The PBMCs layer was washed twice in RPMI 1640 medium. The viability of PBMCs was greater than 95% after Trypan blue exclusion. Approximately 10,000,000 PBMCs were resuspended in 1 ml of freezing solution, which consisted of 80% inactivated FBS (Invitrogen, Carlsbad, CA) plus 20% DMSO (Sigma‐Aldrich), and stored at −70°C for 24 hr before transfer to liquid nitrogen. Aliquots were cryopreserved for later study.
Reagents and Monoclonal Antibodies
Lymphocyte subpopulations (CD4+ and CD8+ T cells) and monocytes (CD14+) were enumerated by a five or six‐color flow cytometry method using the following monoclonal antibodies: allophycocyanin cyanine dye (APC Cy7) conjugated anti‐CD4 (BioLegend, San Diego, CA), phycoerythrin cyanine dye (PE Cy7) conjugated anti‐CD8 (BD Biosciences, San Jose, CA), and phycoerythrin cyanine dye (PE Cy5.5) conjugated anti‐CD14 (Southern Biotech, Birmingham, AL). Appropriate matched‐isotype control antibodies were used to discriminate between positive and negative populations/subsets.
Flow Cytometric Determination of Peripheral Blood Mononuclear Cells
Subpopulations and subsets of leukocytes were determined by staining 100,000 cells of peripheral blood with appropriate combinations of monoclonal antibodies. PBMCs were suspended in PBS. The cells were then incubated on ice for 40 min in the dark with fluorochrome‐conjugated monoclonal antibodies, washed twice with PBS, and fixed in 1% paraformaldehyde. Fixed cells were kept in the dark until use. A minimum of 10,000 T cells' gated events were acquired using a CyAn flow cytometer (Dako, Glostrup, Denmark), and analysis was performed using Flow Jo v.7.6.1 software (Tree Star, Ashland, OR).
Determinations of Plasma Apoptosis Related Proteins
Plasma concentrations of the proapoptotic proteins FasL and TRAIL, and the antiapoptotic Survivin were measured by ELISA (Quantikine, R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. TNF‐α concentration in plasma was determined by ELISA (Pepro Tech, Inc., Rocky Hill, NJ) following the manufacturer's instructions.
Inhibitors of Apoptosis Protein Expression in Peripheral Blood Mononuclear Cell Lysates
Analysis of cellular inhibitor of apoptosis protein 1 (cIAP‐1), cellular inhibitor of apoptosis protein 2 (cIAP‐2), and X‐linked inhibitor of apoptosis protein (XIAP) expression profiles was performed on lysates of PBMCs obtained from dengue patients and controls. Following the manufacturer's instructions (Human Apoptosis Array Kit, Proteome Profiler™ Array, R&D Systems), apoptosis array data on developed X‐ray film were quantified using Quantity One image analysis software (Version 4.6.3, Bio‐Rad). A template that analyzed pixel density in each spot of the array was created and background values subtracted. A comparison of corresponding signals on different arrays was carried out to determine the relative change in IAPs levels between patients and control samples.
Statistical Analysis
Data were entered and analyzed using EpiData Software version 3.1 (Odense, Denmark). Differences and comparison analysis were carried out with non‐parametric Mann–Whitney and Spearman tests using the Prism software v5.0 (GraphPad, San Diego, CA). The Kolmogorov–Smirnov test was applied with P < 0.05 considered as significant.
RESULTS
Patients With Dengue
Sixty‐two patients with dengue and 10 control individuals were included in the apoptosis‐related proteins analysis (Table I). The mean age of patients and control subjects was 40 years old. The female/male gender distribution for patients was 30/32. Patients infected with DENV were recruited between the first and eleventh day after fever onset. Most of the dengue infections (80.8%) were caused by DENV‐2.
Table I.
Demographic and Clinical Data of Patients With Dengue and Demographic Data of Control Subjects
| Classification | ||||
|---|---|---|---|---|
| Controls (n = 10) | Dengue without warning signs (n = 27) | Dengue with warning signs (n = 20) | Severe dengue (n = 15) | |
| Demographic data | ||||
| Age in years (mean ± SD) | 38.4 ± 16.5 | 38.4 ± 16.5 | 40.7 ± 16.1 | 40.4 ± 19.6 |
| Gender (F:M) | 8:2 | 10:17 | 13:7 | 7:8 |
| Illness day (mean ± SD)a | — | 4.2 ± 2.4 | 3.8 ± 2.6 | 4.5 ± 2.2 |
| DENV‐1:DENV‐2b | — | 1:10 | 4:7 | 0:4 |
| Clinical findings | ||||
| Bleedingc (%) | 0 | 0 | 35 | 66.7 |
| Plasma leakaged (%) | 0 | 0 | 0 | 46.2 |
| Hypotensione (%) | 0 | 0 | 0 | 25 |
SD, standard deviation.
Illness day corresponds to the day the patient was enrolled after fever onset.
DENV‐1:DENV‐2 corresponds to a proportion of dengue virus isolations and/or dengue virus genome amplifications.
Bleeding includes skin hemorrhage, epistaxis, gingival hemorrhage, gastrointestinal hemorrhage, urinary tract hemorrhage, and metrorrhagia.
Plasma leakage manifested by pleural effusion, pericardial effusion, and ascites.
Hypotension defined by pulse pressure below 20 mm Hg and/or hypotensive for age.
WHO Dengue Definition Based on Clinical Findings and Laboratory Parameters
Twenty‐seven of the 62 patients with dengue were classified into dengue without warning signs, 20 into dengue with warning signs and 15 into severe dengue following the WHO dengue classification revised in 2009 [World Health Organization, 2009]. One fatal case was recorded in the severe dengue group. Patients with severe dengue more commonly had bleeding, vascular leakage manifestations, and hypotension than the rest of the studied dengue patients (Table I). Hematocrit values did not increase with disease severity. Decreased platelet counts were significantly related to severity and all patient groups showed values below those of control subjects. Values of liver enzymes (AST, ALT) were increased several fold in patients with severe dengue and the increase was statistically significant (P < 0.05).
Leukocyte and Mononuclear Cell Subpopulations in Peripheral Blood From Patients With Dengue
The total number of leukocytes and the three cells subpopulations (CD14+, CD4+, and CD8+) increased with disease severity (Table II). However, the frequency and the absolute numbers of leukocytes did not differ significantly among the three groups of patients with dengue. In particular, CD4+ and CD8+ T lymphocyte counts were significantly higher in the control group as compared to dengue with and without warning signs groups.
Table II.
Laboratory Data of Patients With Dengue and Control Subjects
| Classification | ||||
|---|---|---|---|---|
| Controls (n = 10) | Dengue without warning signs (n = 27) | Dengue with warning signs (n = 20) | Severe dengue (n = 15) | |
| Laboratory parameters | ||||
| Platelets × 103/mm3 (mean ± SD) | 296.8 ± 34.9a | 128 ± 73.7 | 88.2 ± 64.8b | 61.4 ± 48.1c , d |
| Hematocrit % (mean ± SD) | 39.9 ± 3.0 | 40.5 ± 3.8 | 39 ± 4.7 | 37.3 ± 4.4c |
| AST (IU/L) (mean ± SD) | — | 64.3 ± 63.9 | 80.2 ± 78.3 | 1592.3 ± 545.6c |
| ALT (IU/L) (mean ± SD) | — | 56.6 ± 48.8 | 71.5 ± 69.2 | 2055.0 ± 6791.0c |
| Leukocyte count/mm3 (mean ± SD) | 6073.0 ± 635.2 | 4130.0 ± 2148.0 | 4318.0 ± 2470.0 | 5813.0 ± 3545.0 |
| Lymphocyte count/mm3 (mean ± SD) | 1845.3 ± 349.3 | 1469.0 ± 993.0 | 1694.0 ± 1515.0 | 2253.0 ± 1591.0 |
| Monocyte count/mm3 (mean ± SD) | 432.7 ± 99.8 | 518.9 ± 377.8 | 409.7 ± 240.9 | 606.8 ± 387.0 |
| Monocyte % (mean ± SD) | 7.8 ± 1.7a | 12.3 ± 4.5 | 9.8 ± 4.0 | 12.7 ± 6.0 |
| Total lymphocyte % (mean ± SD) | 30.8 ± 7.6 | 36.0 ± 16.8 | 35.3 ± 17.0 | 43.8 ± 16.8 |
| CD14+ monocyte count/mm3 (mean ± SD) | 263.9.8 ± 35.7 | 254.5 ± 226.6 | 236.6 ± 136.7 | 317.4 ± 265.6 |
| CD4+ T lymphocyte count/mm3 (mean ± SD) | 616.2 ± 113.4a | 296.8 ± 192.5 | 500.7 ± 438.7 | 690.0 ± 486.0 |
| CD8+ T lymphocyte count/mm3 (mean ± SD) | 428.9 ± 205.5a | 199.1 ± 181.2 | 512.6 ± 331.7 | 345.2 ± 308.6 |
| CD14+ monocyte % (mean ± SD) | 60.8 ± 11.5 | 60.0 ± 21.1 | 57.0 ± 17.9 | 53.3 ± 18.9 |
| CD4+ T lymphocyte % (mean ± SD) | 30.2 ± 10.0 | 33.0 ± 14.3 | 31.7 ± 11.8 | 32.8 ± 12.1 |
| CD8+ T lymphocyte % (mean ± SD) | 19.9 ± 4.4 | 20.8 ± 9.3 | 20.7 ± 8.9 | 17.4 ± 8.0 |
Note SD, standard deviation; AST, aspartate transaminase; ALT, alanine transaminase.
Statistical differences were assessed by the Mann–Whitney test.
P values <0.05 of controls versus group(s) of patients with dengue.
Dengue without warning signs versus dengue with warning signs.
Dengue without warning signs versus severe dengue.
Dengue with warning signs versus severe dengue.
Differential Levels of Apoptotic Mediators in Patients With Dengue
As shown in Figure 1A, a significantly elevated level of FasL was found in patients with dengue without warning signs (362.8 ± 111.1 pg/ml) and in patients with severe dengue (298.6 ± 183.7 pg/ml) than in control subjects (248.3 ± 85.4 pg/ml). Remarkably, the more elevated value of FasL was recorded in a single fatal dengue case (824 pg/ml) on the day of death. In addition, TRAIL levels in patients with dengue without warning signs (667.7 ± 528 pg/ml) were statistically more elevated when compared to patients with severe dengue (275 ± 121.3 pg/ml) and control individuals (317.7 ± 74.6 pg/ml) (Fig. 1B). TRAIL was the only apoptosis mediator that differentiated patients with dengue with warning signs (761.5 ± 616.2 pg/ml) and those with severe dengue. Survivin levels in the plasma of dengue patients showed variability. Namely, these results showed decreased Survivin levels (Fig. 1C) in patients with dengue with warning signs (17.5 ± 32.8 pg/ml) compared to control individuals (175.2 ± 91 pg/ml) and patients with severe dengue (148.5 ± 157.3 pg/ml).
Figure 1.
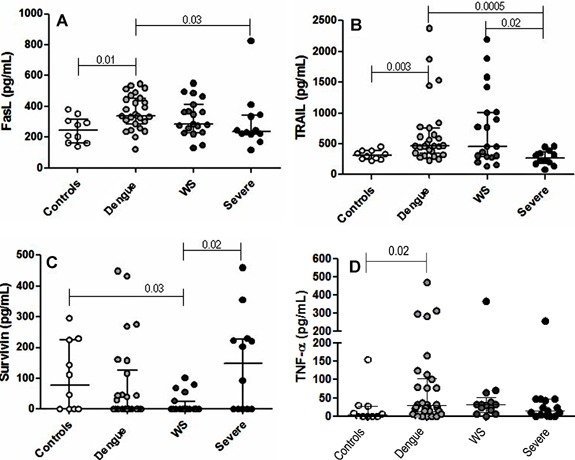
Plasma levels of TNF‐related apoptosis‐inducing ligand or TRAIL (A), Fas ligand or FasL (B), Survivin (C), and TNF‐α (D) in Controls (solid white) and patients classified as dengue without warning signs or dengue (solid bright gray), dengue with warning signs or WS (solid dark gray), and severe dengue or Severe (solid black). The Mann–Whitney test was used for statistical analysis by means of GraphPad Prism v5.0 program. Horizontal lines represent the median and the vertical lines correspond to the interquartile variation (25–75%). P‐value indicates the statistical significance.
No significant differences were found in TNF‐α levels among the dengue patients studied (Fig. 1D). However, this cytokine was significantly more elevated in dengue patients without warning signs than in control subjects.
TRAIL Was Inversely Correlated With CD4+ and CD8+ T Lymphocyte Counts Whereas Survivin Was Positively Correlated With CD14+ Monocyte Counts
TRAIL levels and platelet counts were positively associated in the dengue patients (Table III). A negative relationship was found between TRAIL levels and total lymphocytes. This negative correlation also occurred in both subpopulations of CD4+ and CD8+ T lymphocytes. In contrast to TRAIL, Survivin levels were positively related to peripheral leukocyte and lymphocyte counts, especially CD14+ monocyte counts. No relationship was found between Survivin levels and platelet counts. FasL correlated positively with TRAIL (r = 0.343, P = 0.009) (data not shown).
Table III.
Correlations Among Plasma Apoptotic Mediators and Blood Cell Counts
| FasL (pg/ml) | TRAIL (pg/ml) | Survivin (pg/ml) | |
|---|---|---|---|
| Platelets × 103/mm3 | ns | R = 0.443 (n = 54), P = 0.0008 | ns |
| Leukocyte count/mm3 | ns | ns | R = 0.333 (n = 48), P = 0.02 |
| Lymphocyte count/mm3 | ns | R = (−) 0.525 (n = 44), P = 0.0003 | R = 0.376 (n = 44), P = 0.01 |
| Lymphocyte % | ns | R = (−) 0.572 (n = 44), P < 0.0001 | ns |
| CD14+ monocyte count/mm3 | ns | ns | R = 0.428 (n = 27), P = 0.03 |
| CD4+ T lymphocyte count/mm3 | ns | R = (−) 0.647 (n = 27), P = 0.0003 | ns |
| CD8+ T lymphocyte count/mm3 | ns | R = (−) 0.727 (n = 27), P < 0.0001 | ns |
Note Correlations were explored using Spearman's rho test. No significant is represented by ns.
Cellular Expression of IAPs Appears Increased in Severe Dengue
Preliminary profiling data of the IAPs family members (cIAP‐1, cIAP‐2, and XIAP) were evaluated in PBMCs lysates from some control subjects (n = 02) and some patients with dengue (n = 06). Two of the patients had dengue without warning signs and four had severe dengue. A trend of increased levels in the expression of cIAP‐1, cIAP‐2, and XIAP was detected in patients with severe dengue, but not in the patients with dengue without warning signs and control subjects (Fig. 2). This trend was not statistically significant.
Figure 2.
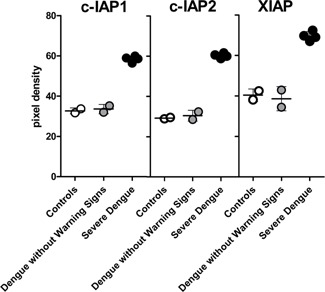
Inhibitors of apoptosis protein cIAP‐1 (cellular inhibitor of apoptosis protein 1), cIAP‐2 (cellular inhibitor of apoptosis protein 2), and XIAP (X‐linked inhibitor of apoptosis protein) expression in peripheral blood mononuclear cell lysates from Controls (solid white), dengue without warning signs patients (solid bright gray) and severe dengue patients (solid black). No statistical significance was found using the Mann–Whitney test for statistical analysis and by means of GraphPad Prism v5.0 program. Horizontal lines represent the median and the vertical lines correspond to the standard deviation.
DISCUSSION
In the present observational study, levels of apoptosis‐related mediators were measured in plasma and in PBMCs lysate samples from Brazilian patients with severe and non‐severe dengue. Patients were infected with either DENV‐1 or DENV‐2. The goal was to explore the interplay between proapoptotic and antiapoptotic mechanisms in dengue disease.
The findings in severe dengue patients of more hypotension, hemorrhagic and vascular leakage manifestations, decreased platelet counts and increased values of hepatic enzymes are in agreement with previous studies that used the WHO dengue clinical classification revised in 2009 [Barniol et al., 2011; Narvaez et al., 2011; de‐Oliveira‐Pinto et al., 2012].
On the other hand, in several cell lines infected by DENV, the Fas/FasL mechanism is involved in apoptotic‐induced cell death [Liew and Chow, 2006; Limjindaporn et al., 2007; Torrentes‐Carvalho et al., 2009; Liao et al., 2010]. Modulation of the immune response by apoptosis during dengue has been reported previously in Thai children who showed higher levels of plasma soluble Fas in DHF than DF [Myint et al., 2006]. However, the present study was performed mainly in an adult population, and the measured circulating mediator was the FasL. Therefore, this is the first reported study of circulating FasL molecule in dengue.
In this study, FasL values in patients with dengue without warning signs were elevated compared to those in patients with severe dengue and control subjects. However, the premortem detection of FasL in the only fatal dengue case showed the highest FasL value measured in the study. It is very likely this highest value was due to the plasma sample being obtained on the day of the death. More dengue fatalities should be studied to understand if the elevation of this proapoptotic mediator in the critical phase of dengue is related to fatal outcome and to explore the probable mechanisms of action involved.
In the present study, elevated TRAIL levels were observed in patients with dengue without warning signs compared to those with severe dengue and control subjects. In addition, TRAIL levels discriminated patients with dengue with warning signs from patients with severe dengue. Previous in vitro studies have demonstrated an increase of TRAIL production by DENV infection in a variety of human primary cells including umbilical vein endothelial cells [Warke et al., 2008b], muscle satellite cells [Warke et al., 2008a], dendritic cells, monocytes, and B cells [Warke et al., 2008b; Becerra et al., 2009; Balas et al., 2011; Wong et al., 2012]. In these human primary cells was not demonstrated an apoptotic activity of TRAIL. Conversely, antiviral action of TRAIL was shown by recombinant TRAIL pretreatment of monocytes and dendritic cells [Warke et al., 2008b].
Another study showed serum elevation of TRAIL in Venezuelan dengue patients compared to febrile patients without dengue and control subjects. However, this previous study involved only two DHF cases and did not analyze the dengue severity [Becerra et al., 2009]. The findings in the present work, which used the revised WHO dengue classification, suggest that the antiviral action of TRAIL that was shown previously in cell culture [Warke et al., 2008b], may also occur in vivo and hence the likely increased severity of dengue in patients lacking an elevated TRAIL production.
In a previous study [Warke et al., 2008b], TRAIL inhibited DENV in infected dendritic cells not by an apoptosis‐dependent mechanism but by a mechanism linked to type I IFN signaling pathways. This finding suggests that the antiviral action of TRAIL could be mediated by known or novel cellular proteins induced by TRAIL. The TRAIL hypothesis, that TRAIL antiviral action is not direct, in dengue infection is supported by recent findings of Shrestha et al. [2012]. These authors demonstrated that TRAIL secreted by CD8+ T cells provides antiviral signals to neurons infected by West Nile virus (a flavivirus closely related to DENV). The effect was studied in the central nervous system of a mouse model and in primary culture of mouse cortical neurons. This work suggests that TRAIL produced by CD8+ T cells is necessary but not sufficient to confer an antiviral effect, and that such an effect requires additional secreted cytokines and/or cytolytic activities by the CD8+ T cells.
Recent work by this research group [Gandini et al., 2013] found that DENV infection activates plasmacytoid dendritic cells, from PBMCs of healthy donors, and leads to the subsequent expression of TRAIL in the cell membrane. These researchers also found that in dengue patients, the activation of plasmacytoid dendritic cells by membrane TRAIL is associated with less severe dengue. This report also confirms the in vitro antiviral action of recombinant TRAIL in monocytes infected with DENV and that TRAIL production in plasmacytoid dendritic cells is associated with IFN‐α production.
Previous studies have shown that TRAIL regulates megakaryocytopoiesis [Zauli and Secchiero, 2006] and promotes maturation of megakaryoblasts into platelets [Melloni et al., 2005]. In addition, it has been shown in vitro that activated platelets release TRAIL [Crist et al., 2004]. However, more studies are needed to determine whether TRAIL is related to platelet increase or TRAIL is released by platelets during dengue infection.
The inverse correlation among the levels of the proapoptotic TRAIL and the counts of CD4+ and CD8+ T lymphocyte subpopulations supports the hypothesis that TRAIL is related to lymphocytic cell death. A similar phenomenon has been reported in patients infected with HIV [Cummins and Badley, 2010], respiratory syncytial virus [Roe et al., 2004], SARS coronavirus [Law et al., 2009], measles virus [Okada et al., 2000], and in primate models of Ebola virus disease [Hensley et al., 2002]. Therefore, apoptosis might be a contributing factor that modulates the number of lymphocytes in dengue infection, as documented previously [Myint et al., 2006].
TRAIL expression is increased in primary hepatocytes and HepG2 cells infected with dengue [Suksanpaisan et al., 2007]. However, the significance of TRAIL action in hepatic cells remains unclear after the retraction of the work by Matsuda et al. [2005]. In the current investigation the liver cells were not studied. However, it cannot be ruled out that expressed TRAIL in liver cells after infection by DENV is released to the circulation during the clearance of infected liver cells.
In the present study, the plasma levels of TNF‐α were significantly higher in dengue patients without warning signs when compared with controls. No differences were found in TNF‐α levels among the three groups of dengue patients studied. Although TNF‐α is one of the most studied cytokines in dengue infection, the association of TNF‐α with disease severity is still debated. On one hand, some studies have found an association of TNF‐α with DHF [Hober et al., 1993; Braga et al., 2001; Levy et al., 2010]. On the other hand, some studies have not found such association [Laur et al., 1998; Bozza et al., 2008; Hoang et al., 2010; Priyadarshini et al., 2010].
The discrepancies in TNF‐α detection in dengue studies might be related to several factors. Previous reports in dengue patients have found differences of TNF‐α production in association with genetic polymorphisms of TNF‐α [Fernandez‐Mestre et al., 2004; Vejbaesya et al., 2009; Perez et al., 2010]. In addition, in vitro TNF‐α induction can be serotype‐specific [Azizan et al., 2006], and the highest plasma levels of TNF‐α occur before the sixth day of dengue disease [Hober et al., 1993].
The TNF‐α level was not elevated in the single fatal case of dengue in this study. This finding is in agreement with previous work that did not show differences in TNF‐α levels among fatal cases of dengue and survivors of DF and DHF [Chen et al., 2006].
The IAPs participate in multiple intracellular pathways comprising cell death and survival [Altieri, 2010]. For example, Survivin expression is related to in vitro lymphocyte division and clonal expansion [Song et al., 2005]. On the other hand, a previous study has shown an association of Survivin up‐regulation in the circulation with the induction of adhesion molecules on the surface of blood leukocytes [Mera et al., 2008].
The presence of antiapoptosis pathway proteins during dengue infection may counteract the role of proapoptosis signaling molecules. The antiapoptotic protein Survivin is studied in dengue infection for the first time in the present study. This molecule showed variable levels with reduced levels in patients with dengue without warning signs as compared to control subjects and patients with severe dengue. Survivin was positively correlated with counts of leukocytes, lymphocytes, and CD14+ monocytes in the dengue patients studied. Although this work did not verify experimentally the exocytosis of Survivin and the extracellular action of Survivin on white blood cells, these correlation results suggest that Survivin may be associated with proliferation of leukocytes, and specially monocytes during dengue. Additionally, elevated Survivin levels may provide more protection against apoptosis in these immunological blood cells from the circulation of the dengue patients studied.
IAPs are known for their ability to regulate caspases and cell death [Silke and Meier, 2013]. Although preliminary, the present study showed that the expression of IAPs family members in PBMCs was markedly higher in patients with severe dengue than in patients with dengue without warning signs and control subjects. This result suggests that the IAPs may be involved with apoptosis inhibition in PBMCs, however, it is not clear whether this antiapoptotic effect of IAPs might counteract the probable induction of apoptosis by TRAIL in circulating lymphocytes. Thus, the pro‐ and antiapoptotic mechanisms may be interacting but the real impact needs to be investigated further.
Even though the current study had novel findings of proteins related to apoptosis in dengue patients, there are still several limitations. For instance, patients were not stratified by day of infection which could explain the large standard deviation of the mean values for lymphocyte counts. Measuring the numbers of lymphocytes and plasma concentrations of apoptotic proteins daily would be a more accurate way to compare groups of dengue patients. However, the higher counts of total lymphocyte subpopulations in patients with severe dengue than in patients without the severe clinical form of dengue is in agreement with previous studies [Kadhiravan et al., 2010; de‐Oliveira‐Pinto et al., 2012].
In addition, the lack of data on viremia, immune status, and co‐morbidities may limit the interpretation of the preliminary results of this study. The inclusion of both healthy subjects and febrile patients without dengue as the control group may have been preferable. Additionally, genetic polymorphisms might affect the circulating levels of these apoptotic mediators, as previously shown in studies of TRAIL [Korner et al., 2012], FasL [Nasi et al., 2005], and Survivin [Borges Bdo et al., 2011] in infectious and non‐infectious conditions.
In conclusion, FasL may exhibit a function in the different clinical forms of dengue disease and in fatal dengue. It is hypothesized that TRAIL has a role in inducing apoptosis of lymphocytes in dengue patients. Survivin seems likely to promote survival and proliferation of blood leukocytes whereas other IAPs family members may be involved in antiapoptotic pathways of PBMCs during dengue. More research should be done to characterize the complex interplay of pro and antiapoptotic mediators during the different phases of dengue disease to identify implications for dengue severity.
ACKNOWLEDGMENTS
The authors are truly thankful to Hermann G. Schatzmayr, a worldwide distinguished professor from FIOCRUZ, who inspired our investigation from the very beginning. We highly regret he could not participate in the final work because he died. We thank Jaqueline Bastos for the dengue diagnostics in the Flavivirus Laboratory at FIOCRUZ. We also acknowledge the staff from the Plínio Bacelar Laboratory headed by Dr. Carlos Bacelar and Prefeitura Municipal de Campos de Goytacazes. We are grateful to the clinical and nursing staff of the participating healthcare facilities. The preparation of this manuscript was improved by the intensive course in research writing at Texas A&M University (TAMU). We thank the main instructor of the course, Dr. Barbara Gastel, and her university colleagues. Dr. Daniel Limonta received a scholarship to attend this international course.
The authors declare that they have no competing interests.
REFERENCES
- Alexander N, Balmaseda A, Coelho IC, Dimaano E, Hien TT, Hung NT, Janisch T, Kroeger A, Lum LC, Martinez E, Siqueira JB, Thuy TT, Villalobos I, Villegas E, Wills B. 2011. Multicentre prospective study on dengue classification in four South‐east Asian and three Latin American countries. Trop Med Int Health 16:936–948. [DOI] [PubMed] [Google Scholar]
- Altieri DC. 2010. Survivin and IAP proteins in cell‐death mechanisms. Biochem J 430:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizan A, Sweat J, Espino C, Gemmer J, Stark L, Kazanis D. 2006. Differential proinflammatory and angiogenesis‐specific cytokine production in human pulmonary endothelial cells, HPMEC‐ST1.6R infected with dengue‐2 and dengue‐3 virus. J Virol Methods 138:211–217. [DOI] [PubMed] [Google Scholar]
- Balas C, Kennel A, Deauvieau F, Sodoyer R, Arnaud‐Barbe N, Lang J, Guy B. 2011. Different innate signatures induced in human monocyte‐derived dendritic cells by wild‐type dengue 3 virus, attenuated but reactogenic dengue 3 vaccine virus, or attenuated nonreactogenic dengue 1–4 vaccine virus strains. J Infect Dis 203:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmaseda A, Hammond SN, Perez L, Tellez Y, Saborio SI, Mercado JC, Cuadra R, Rocha J, Perez MA, Silva S, Rocha C, Harris E. 2006. Serotype‐specific differences in clinical manifestations of dengue. Am J Trop Med Hyg 74:449–456. [PubMed] [Google Scholar]
- Barniol J, Gaczkowski R, Barbato EV, da Cunha RV, Salgado D, Martinez E, Segarra CS, Pleites Sandoval EB, Mishra A, Laksono IS, Lum LC, Martinez JG, Nunez A, Balsameda A, Allende I, Ramirez G, Dimaano E, Thomacheck K, Akbar NA, Ooi EE, Villegas E, Hien TT, Farrar J, Horstick O, Kroeger A, Jaenisch T. 2011. Usefulness and applicability of the revised dengue case classification by disease: Multi‐centre study in 18 countries. BMC Infect Dis 11:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra A, Warke RV, Martin K, Xhaja K, de Bosch N, Rothman AL, Bosch I. 2009. Gene expression profiling of dengue infected human primary cells identifies secreted mediators in vivo. J Med Virol 81:1403–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokarewa M, Tarkowski A, Magnusson M. 2007. Pathological survivin expression links viral infections with pathogenesis of erosive rheumatoid arthritis. Scand J Immunol 66:192–198. [DOI] [PubMed] [Google Scholar]
- Borges Bdo N, Burbano RR, Harada ML. 2011. Survivin ‐31C/G polymorphism and gastric cancer risk in a Brazilian population. Clin Exp Med 11:189–193. [DOI] [PubMed] [Google Scholar]
- Bozza FA, Cruz OG, Zagne SM, Azeredo EL, Nogueira RM, Assis EF, Bozza PT, Kubelka CF. 2008. Multiplex cytokine profile from dengue patients: MIP‐1beta and IFN‐gamma as predictive factors for severity. BMC Infect Dis 8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga EL, Moura P, Pinto LM, Ignacio SR, Oliveira MJ, Cordeiro MT, Kubelka CF. 2001. Detection of circulant tumor necrosis factor‐alpha, soluble tumor necrosis factor p75 and interferon‐gamma in Brazilian patients with dengue fever and dengue hemorrhagic fever. Mem Inst Oswaldo Cruz 96:229–232. [DOI] [PubMed] [Google Scholar]
- Cardier JE, Marino E, Romano E, Taylor P, Liprandi F, Bosch N, Rothman AL. 2005. Proinflammatory factors present in sera from patients with acute dengue infection induce activation and apoptosis of human microvascular endothelial cells: Possible role of TNF‐alpha in endothelial cell damage in dengue. Cytokine 30:359–365. [DOI] [PubMed] [Google Scholar]
- Chen LC, Lei HY, Liu CC, Shiesh SC, Chen SH, Liu HS, Lin YS, Wang ST, Shyu HW, Yeh TM. 2006. Correlation of serum levels of macrophage migration inhibitory factor with disease severity and clinical outcome in dengue patients. Am J Trop Med Hyg 74:142–147. [PubMed] [Google Scholar]
- Chen HC, Hofman FM, Kung JT, Lin YD, Wu‐Hsieh BA. 2007. Both virus and tumor necrosis factor alpha are critical for endothelium damage in a mouse model of dengue virus‐induced hemorrhage. J Virol 81:5518–5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist SA, Elzey BD, Ludwig AT, Griffith TS, Staack JB, Lentz SR, Ratliff TL. 2004. Expression of TNF‐related apoptosis‐inducing ligand (TRAIL) in megakaryocytes and platelets. Exp Hematol 32:1073–1081. [DOI] [PubMed] [Google Scholar]
- Cummins NW, Badley AD. 2010. Mechanisms of HIV‐associated lymphocyte apoptosis: 2010. Cell Death Dis 1:e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de‐Oliveira‐Pinto LM, Marinho CF, Povoa TF, de Azeredo EL, de Souza LA, Barbosa LD, Motta‐Castro AR, Alves AM, Avila CA, de Souza LJ, da Cunha RV, Damasco PV, Paes MV, Kubelka CF. 2012. Regulation of inflammatory chemokine receptors on blood T cells associated to the circulating versus liver chemokines in dengue fever. PLoS ONE 7:e38527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Mestre MT, Gendzekhadze K, Rivas‐Vetencourt P, Layrisse Z. 2004. TNF‐alpha‐308A allele, a possible severity risk factor of hemorrhagic manifestation in dengue fever patients. Tissue Antigens 64:469–472. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El‐Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nunez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G. 2012. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19:107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandini M, Gras C, Azeredo EL, Pinto LM, Smith N, Despres P, da Cunha RV, de Souza LJ, Kubelka CF, Herbeuval JP. 2013. Dengue virus activates membrane TRAIL relocalization and IFN‐alpha production by human plasmacytoid dendritic cells in vitro and in vivo. PLoS Negl Trop Dis 7:e2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martinez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. 2010. Dengue: A continuing global threat. Nat Rev Microbiol 8:S7–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley LE, Young HA, Jahrling PB, Geisbert TW. 2002. Proinflammatory response during Ebola virus infection of primate models: Possible involvement of the tumor necrosis factor receptor superfamily. Immunol Lett 80:169–179. [DOI] [PubMed] [Google Scholar]
- Hoang LT, Lynn DJ, Henn M, Birren BW, Lennon NJ, Le PT, Duong KT, Nguyen TT, Mai LN, Farrar JJ, Hibberd ML, Simmons CP. 2010. The early whole‐blood transcriptional signature of dengue virus and features associated with progression to dengue shock syndrome in Vietnamese children and young adults. J Virol 84:12982–12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hober D, Poli L, Roblin B, Gestas P, Chungue E, Granic G, Imbert P, Pecarere JL, Vergez‐Pascal R, Wattre P, Maniez‐Montreuil M. 1993. Serum levels of tumor necrosis factor‐alpha (TNF‐alpha), interleukin‐6 (IL‐6), and interleukin‐1 beta (IL‐1 beta) in dengue‐infected patients. Am J Trop Med Hyg 48:324–331. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. 2009. Cell death. N Engl J Med 361:1570–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerre MR, Lan NT, Marianneau P, Hue NB, Khun H, Hung NT, Khen NT, Drouet MT, Huong VT, Ha DQ, Buisson Y, Deubel V. 2001. Liver histopathology and biological correlates in five cases of fatal dengue fever in Vietnamese children. Virchows Arch 438:107–115. [DOI] [PubMed] [Google Scholar]
- Igarashi A. 1978. Isolation of a Singh's Aedes albopictus cell clone sensitive to Dengue and Chikungunya viruses. J Gen Virol 40:531–544. [DOI] [PubMed] [Google Scholar]
- Jaiyen Y, Masrinoul P, Kalayanarooj S, Pulmanausahakul R, Ubol S. 2009. Characteristics of dengue virus‐infected peripheral blood mononuclear cell death that correlates with the severity of illness. Microbiol Immunol 53:442–450. [DOI] [PubMed] [Google Scholar]
- Kadhiravan T, Saxena A, Singh A, Broor S, Sharma SK, Mitra DK. 2010. Association of intracellular T(H)1‐T(H)2 balance in CD4+ T‐cells and MIP‐1alpha in CD8+ T‐cells with disease severity in adults with dengue. Immune Netw 10:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliks SC, Nisalak A, Brandt WE, Wahl L, Burke DS. 1989. Antibody‐dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg 40:444–451. [DOI] [PubMed] [Google Scholar]
- Korner C, Riesner K, Kramer B, Eisenhardt M, Glassner A, Wolter F, Berg T, Muller T, Sauerbruch T, Nattermann J, Spengler U, Nischalke HD. 2012. TRAIL receptor I (DR4) polymorphisms C626G und A638C are associated with an increased risk for hepatocellular carcinoma (HCC) in HCV‐infected patients. BMC Cancer 12:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. 1992. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase‐polymerase chain reaction. J Clin Microbiol 30:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laur F, Murgue B, Deparis X, Roche C, Cassar O, Chungue E. 1998. Plasma levels of tumour necrosis factor alpha and transforming growth factor beta‐1 in children with dengue 2 virus infection in French Polynesia. Trans R Soc Trop Med Hyg 92:654–656. [DOI] [PubMed] [Google Scholar]
- Law HK, Cheung CY, Sia SF, Chan YO, Peiris JS, Lau YL. 2009. Toll‐like receptors, chemokine receptors and death receptor ligands responses in SARS coronavirus infected human monocyte derived dendritic cells. BMC Immunol 10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A, Valero N, Espina LM, Anez G, Arias J, Mosquera J. 2010. Increment of interleukin 6, tumour necrosis factor alpha, nitric oxide, C‐reactive protein and apoptosis in dengue. Trans R Soc Trop Med Hyg 104:16–23. [DOI] [PubMed] [Google Scholar]
- Liao H, Xu J, Huang J. 2010. FasL/Fas pathway is involved in dengue virus induced apoptosis of the vascular endothelial cells. J Med Virol 82:1392–1399. [DOI] [PubMed] [Google Scholar]
- Liew KJ, Chow VT. 2006. Microarray and real‐time RT‐PCR analyses of a novel set of differentially expressed human genes in ECV304 endothelial‐like cells infected with dengue virus type 2. J Virol Methods 131:47–57. [DOI] [PubMed] [Google Scholar]
- Limjindaporn T, Netsawang J, Noisakran S, Thiemmeca S, Wongwiwat W, Sudsaward S, Avirutnan P, Puttikhunt C, Kasinrerk W, Sriburi R, Sittisombut N, Yenchitsomanus PT, Malasit P. 2007. Sensitization to Fas‐mediated apoptosis by dengue virus capsid protein. Biochem Biophys Res Commun 362:334–339. [DOI] [PubMed] [Google Scholar]
- Limonta D, Capo V, Torres G, Perez AB, Guzman MG. 2007. Apoptosis in tissues from fatal dengue shock syndrome. J Clin Virol 40:50–54. [DOI] [PubMed] [Google Scholar]
- Limonta D, Gonzalez D, Capo V, Torres G, Perez AB, Rosario D, Roche‐Rodriguez R, Alvarez M, Guzman MG. 2009. Fatal severe dengue and cell death in sickle cell disease during the 2001–2002 Havana dengue epidemic. Int J Infect Dis 13:e77–e78. [DOI] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ. 2001. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 104:487–501. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Almasan A, Tomita M, Tamaki K, Saito M, Tadano M, Yagita H, Ohta T, Mori N. 2005. Dengue virus‐induced apoptosis in hepatic cells is partly mediated by Apo2 ligand/tumour necrosis factor‐related apoptosis‐inducing ligand. J Gen Virol 86:1055–1065. Retraction in: J Gen Virol 2010 Oct; 1091: 2658. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Melloni E, Secchiero P, Celeghini C, Campioni D, Grill V, Guidotti L, Zauli G. 2005. Functional expression of TRAIL and TRAIL‐R2 during human megakaryocytic development. J Cell Physiol 204:975–982. [DOI] [PubMed] [Google Scholar]
- Mera S, Magnusson M, Tarkowski A, Bokarewa M. 2008. Extracellular survivin up‐regulates adhesion molecules on the surface of leukocytes changing their reactivity pattern. J Leukoc Biol 83:149–155. [DOI] [PubMed] [Google Scholar]
- Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, Sawasdivorn S, Duangchinda T, Dong T, Rowland‐Jones S, Yenchitsomanus PT, McMichael A, Malasit P, Screaton G. 2003. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med 9:921–927. [DOI] [PubMed] [Google Scholar]
- Myint KS, Endy TP, Mongkolsirichaikul D, Manomuth C, Kalayanarooj S, Vaughn DW, Nisalak A, Green S, Rothman AL, Ennis FA, Libraty DH. 2006. Cellular immune activation in children with acute dengue virus infections is modulated by apoptosis. J Infect Dis 194:600–607. [DOI] [PubMed] [Google Scholar]
- Narvaez F, Gutierrez G, Perez MA, Elizondo D, Nunez A, Balmaseda A, Harris E. 2011. Evaluation of the traditional and revised WHO classifications of Dengue disease severity. PLoS Negl Trop Dis 5:e1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasi M, Pinti M, Bugarini R, Troiano L, Lugli E, Bellodi C, Mussini C, Borghi V, Trenti T, Balli F, Esposito R, Cossarizza A. 2005. Genetic polymorphisms of Fas (CD95) and Fas ligand (CD178) influence the rise in CD4+ T cell count after antiretroviral therapy in drug‐naive HIV‐positive patients. Immunogenetics 57:628–635. [DOI] [PubMed] [Google Scholar]
- Okada H, Kobune F, Sato TA, Kohama T, Takeuchi Y, Abe T, Takayama N, Tsuchiya T, Tashiro M. 2000. Extensive lymphopenia due to apoptosis of uninfected lymphocytes in acute measles patients. Arch Virol 145:905–920. [DOI] [PubMed] [Google Scholar]
- Perez AB, Sierra B, Garcia G, Aguirre E, Babel N, Alvarez M, Sanchez L, Valdes L, Volk HD, Guzman MG. 2010. Tumor necrosis factor‐alpha, transforming growth factor‐beta1, and interleukin‐10 gene polymorphisms: Implication in protection or susceptibility to dengue hemorrhagic fever. Hum Immunol 71:1135–1140. [DOI] [PubMed] [Google Scholar]
- Priyadarshini D, Gadia RR, Tripathy A, Gurukumar KR, Bhagat A, Patwardhan S, Mokashi N, Vaidya D, Shah PS, Cecilia D. 2010. Clinical findings and pro‐inflammatory cytokines in dengue patients in Western India: A facility‐based study. PLoS ONE 5:e8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe MF, Bloxham DM, White DK, Ross‐Russell RI, Tasker RT, O'Donnell DR. 2004. Lymphocyte apoptosis in acute respiratory syncytial virus bronchiolitis. Clin Exp Immunol 137:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman AL. 2010. Cellular immunology of sequential dengue virus infection and its role in disease pathogenesis. Curr Top Microbiol Immunol 338:83–98. [DOI] [PubMed] [Google Scholar]
- Shrestha B, Pinto AK, Green S, Bosch I, Diamond MS. 2012. CD8+ T cells use TRAIL to restrict West Nile virus pathogenesis by controlling infection in neurons. J Virol 86:8937–8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra B, Alegre R, Perez AB, Garcia G, Sturn‐Ramirez K, Obasanjo O, Aguirre E, Alvarez M, Rodriguez‐Roche R, Valdes L, Kanki P, Guzman MG. 2007. HLA‐A, ‐B, ‐C, and ‐DRB1 allele frequencies in Cuban individuals with antecedents of dengue 2 disease: Advantages of the Cuban population for HLA studies of dengue virus infection. Hum Immunol 68:531–540. [DOI] [PubMed] [Google Scholar]
- Silke J, Meier P. 2013. Inhibitor of apoptosis (IAP) proteins‐modulators of cell death and inflammation. Cold Spring Harb Perspect Biol 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons CP, Farrar JJ, Nguyen VV, Wills B. 2012. Dengue. N Engl J Med 366:1423–1432. [DOI] [PubMed] [Google Scholar]
- Song J, So T, Cheng M, Tang X, Croft M. 2005. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity 22:621–631. [DOI] [PubMed] [Google Scholar]
- Suksanpaisan L, Cabrera‐Hernandez A, Smith DR. 2007. Infection of human primary hepatocytes with dengue virus serotype 2. J Med Virol 79:300–307. [DOI] [PubMed] [Google Scholar]
- Torrentes‐Carvalho A, Azeredo EL, Reis SR, Miranda AS, Gandini M, Barbosa LS, Kubelka CF. 2009. Dengue‐2 infection and the induction of apoptosis in human primary monocytes. Mem Inst Oswaldo Cruz 104:1091–1099. [DOI] [PubMed] [Google Scholar]
- Vejbaesya S, Luangtrakool P, Luangtrakool K, Kalayanarooj S, Vaughn DW, Endy TP, Mammen MP, Green S, Libraty DH, Ennis FA, Rothman AL, Stephens HA. 2009. TNF and LTA gene, allele, and extended HLA haplotype associations with severe dengue virus infection in ethnic Thais. J Infect Dis 199:1442–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warke RV, Becerra A, Zawadzka A, Schmidt DJ, Martin KJ, Giaya K, Dinsmore JH, Woda M, Hendricks G, Levine T, Rothman AL, Bosch I. 2008a. Efficient dengue virus (DENV) infection of human muscle satellite cells upregulates type I interferon response genes and differentially modulates MHC I expression on bystander and DENV‐infected cells. J Gen Virol 89:1605–1615. [DOI] [PubMed] [Google Scholar]
- Warke RV, Martin KJ, Giaya K, Shaw SK, Rothman AL, Bosch I. 2008b. TRAIL is a novel antiviral protein against dengue virus. J Virol 82:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2009. Dengue: Guidelines for diagnosis, treatment, prevention and control. Geneva: WHO Press. [PubMed] [Google Scholar]
- Wong KL, Chen W, Balakrishnan T, Toh YX, Fink K, Wong SC. 2012. Susceptibility and response of human blood monocyte subsets to primary dengue virus infection. PLoS ONE 7:e36435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauli G, Secchiero P. 2006. The role of the TRAIL/TRAIL receptors system in hematopoiesis and endothelial cell biology. Cytokine Growth Factor Rev 17:245–257. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Roshal M, Li F, Blackett J, Planelles V. 2003. Upregulation of survivin by HIV‐1 Vpr. Apoptosis 8:71–79. [DOI] [PubMed] [Google Scholar]


