Abstract
The current study aimed to describe the molecular epidemiology of mixed respiratory viral infections during consecutive winter seasons in a tertiary care hospital. Patients with symptoms of respiratory tract infection were evaluated during the 2009‐2011 and 2013‐15 winter seasons. A clinical microarray technique was used for viral detection. Clinical and epidemiological data were correlated with mixed viral detection and the need for hospitalization. In 332 out of 604 (54.4%) evaluated patients (17.6% children) a respiratory virus was identified. Mixed viral infections were diagnosed in 68/332 (20.5%) patients with virus detection (66.2% mixed Influenza‐RSV infections). Mixed viral infections were more commonly detected in children (OR 3.7; 95%CI 1.9‐5.6, P < 0.01) and patients with comorbidities. In logistic regression analyses, mixed viral infections were associated with younger age (mean age 30.4 years vs. 41.8 years, P ≤ 0.001) and increased rates of fever (OR: 2.7; 95%CI 1.04‐7.2, P < 0.05) but no adverse outcomes or increased rates of hospitalization. High rates of mixed viral infections were noted during all winter seasons (especially Influenza and RSV) and were more common in younger patients. The clinical significance of mixed respiratory viral infection needs further elucidation.
Keywords: epidemiology, influenza, respiratory syncytial virus (RSV)
1. INTRODUCTION
Seasonal patterns of respiratory viruses circulation and detection in human infections are hard to explain. It has been suggested that based on seasonal variation and the clinical syndrome seen, one can predict what type of virus is implicated.1, 2 Limited data exist, with regards to the clinical importance of mixed respiratory viral infections during the winter season. Influenza and respiratory syncytial virus (RSV) are well known to circulate in winter.1, 3 The extent of overlap, the possibility of dual infections and associations of mixed respiratory viral infections with clinical and epidemiological data needs further elucidation. Point of Care testing and newer available molecular techniques have improved our ability to detect diverse viruses causing respiratory infections.4 In a recent study evaluating two different multiplex kits 14.2% of samples tested, harbored viral co‐infections.4 Limited studies have evaluated the presence of mixed viral infections at the clinical setting for adult populations. Significant rates of mixed infections have been reported in several studies mainly involving pediatric populations5, 6, 7, 8, 9, 10, 11, 12, 13, 14 while very little data exist regarding adult patients.15, 16, 17, 18 The clinical significance of mixed viral infections is a matter of debate with some data supporting a role for increased illness severity and adverse outcomes including hospitalizations5, 15 and some not.9, 10, 16 Further, most of the studies are biased towards studying hospitalized subjects9, 10, 19, 20 and very little data exist regarding outpatients.16, 17, 18
The current study aimed to describe the molecular epidemiology of mixed respiratory viral infections in both adults and children within the context of acute respiratory illness. The study was part of a molecular epidemiology study of respiratory viruses detection in patients presenting with acute respiratory symptoms during consecutive winter seasons, in a tertiary care hospital Emergency Room (ER), in a large metropolitan area.
The study's main aim was to identify the prevalence of such infections within the population sampled. An additional aim was to correlate the detection of mixed respiratory viral infection with baseline clinical and epidemiological data, including the decision to hospitalize due to severe illness.
2. MATERIALS AND METHODS
Patients with symptoms of respiratory tract infection (RTI) (i.e., fever and/or cough and/or other symptoms suggestive of a respiratory infection e.g., nasal congestion, rhinorrhea, sore throat) presenting to the Emergency Room (ER) of the tertiary care university hospital Attikon (Athens, Greece) during the winter season (i.e., November to March each season) of 2009‐11 (two seasons) and 2013‐2015 (two seasons) were evaluated during the study. Data were not collected for the seasons 2011‐12 and 2012‐13. After informed consent was provided, a respiratory sample was collected from each patient through a standardized technique (sputum sample obtained after a 5 s observed mouth gargle with normal saline involving the entire oropharyngeal area) into ThinPrep CytoLyt® solution (Cytyc Corporation, Malborough, MA) and was immediately submitted to the collaborating research laboratory. Basic clinical and epidemiological characteristics for each patient were collected through a structured case report form. All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation (Scientific council of the University Hospital Attikon) and with the Helsinki Declaration of 1975, as revised in 2008.
Viral RNA was extracted using 400 µL of ThinPrep CytoLyt solution using the MagMAX™ Viral RNA Isolation Kit and eluted in 50 µL elution buffer. The CLART® PneumoVir kit was used (GENOMICA, Madrid, Spain) that detects the presence of the 17 most frequent types of human viruses causing respiratory infections. The molecular procedure was followed according to the manufacturer's instructions. Virus amplification was performed via two RT (reverse transcriptase) multiplex PCR reactions and the detection/visualization was performed based on low‐density micro‐arrays. Negative controls were included in each experiment.
Study prevalence rates together with 95% Confidence Intervals were calculated for each virus (as denominator, the entire population sampled was used). Data on influenza detection were compared with National influenza surveillance data (for the respective years) available through the Hellenic Center for Disease Control and Prevention. Correlation between type of virus, mixed virus detection and age status (children if age <18 years old) was performed using chi‐square test. Univariate and multivariate analysis was used to evaluate associations between specific virus detection, mixed virus detection, clinical characteristics, for example, fever and the outcome of hospitalization. SPSS version 22.0 for Windows software (SPSS, Inc., Chicago, IL) was used for data analysis. All tests were two tailed.
3. RESULTS
Overall 604 consecutive patients with RTI were evaluated during the study period. Demographics of the study population according to age, gender, comorbidities, and type of virus detected are shown in Tables 1 and 2. The median age for the entire group was 42.4 years (IQR 25‐62 years) and 296 (49%) were female. The vast majority of the population were adults (n = 498, 82.5%) whereas 106 (17.5%) children were examined. Of 402 patients with available data 206 (51.2%) had comorbidities, like Chronic Obstructive Pulmonary Disease (COPD) (n = 125/402, 31.1%) and cardiovascular disease (n = 105/402, 26.1%, Table 1).
Table 1.
Demographics of the study population
| Variables, n (%) | Any virus, n (%) N = 332/604 (55) | Influenza, n (%) n = 194/604 (32.1) | RSV, n (%) n = 142/604 (23.5) | Mixed virus, (%) n = 68/604 (11.3) | Mixed RSV/Flu, n (%) n = 45/604 (7.5) |
|---|---|---|---|---|---|
| Female gender, n (%) | 169/332 (50.9) | 98/194 (50.5) | 70/142 (49.3) | 33/68 (48.5%) | 20/45 (44.4) |
| Age 0‐4 yrs, n = 37 (6.1) | 31/332 (9.3) | 5/194 (2.6) | 27/142 (19) | 10/68 (14.7) | 4/45 (8.9) |
| Age 5‐17 yrs, n = 69 (11.4) | 53/332 (16) | 27/194 (13.9) | 34/142 (23.9) | 15/68 (22.1) | 11/45 (24.4) |
| Age 18‐40 yrs, n = 208 (34.4) | 101/332 (30.4) | 65/194 (33.5) | 41/142 (28.9) | 23/68 (33.8) | 16/45 (35.6) |
| Age 41‐65 yrs, n = 161 (26.7) | 84/332 (25.3) | 56/194 (28.9) | 27/142 (19) | 14/68 (20.6) | 11/45 (24.4) |
| Age >65 yrs, n = 129 (21.4) | 63/332 (19) | 41/194 (21.1) | 13/142 (9.2) | 6/68 (8.8) | 3/45 (6.7) |
| Hospitalization, n = 165 (27.3) | 92/332 (27.7) | 62/194 (32) | 22/142 (15.5) * | 9/68 (13.2) | 6/45 (13.3) * |
| Any comorbidities, n = 206/402 (51.2) | 180/402 (44.8) | 127/402 (31.6) * | 22/402 (5.5) | 10/402 (2.5) | 3/402 (0.7) |
| Age ≩18 yrs | 165/180 (91.7) * | 114/127 (89.8) * | 22/22 (100) | 10/10 (100) | 3/3 (100) |
| COPD, n = 125 (31.1) | 67/180 (37.2) * | 50/127 (39.3) * | 8/22 (36.4) | 5/10 (50) | 2/3 (66.7) |
| CVD, n = 105 (26.1) | 58/180 (32.2) * | 43/127 (33.9) * | 5/22 (22.7) | 4/10 (40) | 1/3 (33.3) |
| Diabetes, n = 62 (15.4) | 30/180 (16.7) | 23/127 (18.1) | 3/22 (13.6) | 2/10 (20) | 1/3 (33.3) |
| Malignancy, n = 21 (5.2) | 10/180 (5.6) | 6/127 (4.7) | 0/22 (0) | 0/10 (0) | 0/3 (0) |
| ESRD, n = 7 (1.7) | 3/180 (1.7) | 2/127 (1.6) | 0/22 (0) | 0/10 (0) | 0/3 (0) |
| Allergy, n = 44 (10.9) | 17/180 (9.4) | 13/127 (10.2) | 2/22 (9.1) | 1/10 (10) | 0/3 (0) |
| Smoking, n = 174 (43.3) | 69/180 (38.3) | 46/127 (36.2) | 9/22 (40.9) | 6/10 (60) | 3/3 (100) |
| Influenza vaccine, n = 84 (20.9) | 41/180 (22.8) | 27/127 (21.3) | 6/22 (27.3) | 3/10 (30) | 1/3 (33.3) |
| Hospitalization, n = 142 (35.3) | 76/180 (42.2) * | 56/127 (44.1) | 10/22 (45.5) * | 5/10 (50) | 3/3 (100) |
Patients with Influenza‐like illness but no virology data were excluded. For comorbidities data are shown only for patients with available data (n = 402). Statistical significance for comparisons between groups with positive results and the rest of the group are flagged with an asterisk.
COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; ESRD, end stage renal disease.
Statistically significant associations (P < 0.05) are flagged.
Table 2.
Prevalence of identified viruses in the total population and in adults and children for patients with available virology data
| Virus | Total population, n = 604 | Adults, n, % (95%CI) n = 498 | Children, n, % (95%CI) n = 106 |
|---|---|---|---|
| Any virus (+) | 332/604, 55% (50.9‐59%) | 248/498, 49.8% (45.3‐54.3%) * | 84/106, 79.2% (70.1‐86.3%) * |
| Influenza, total | 194/604, 32.1% (28.4‐36%) | 162/498, 32.5% (28.5‐36.9%) | 32/106, 30.2% (21.9‐40%) |
| Influenza A, n (%) | 120/604, 19.9% (16.8‐23.3%) | 101/498, 20.3% (16.9‐24.1%) | 19/106, 17.9% (11.4‐26.8%) |
| Influenza B | 75/604, 12.4% (9.9‐15.4%) | 62/498, 12.4% (9.7‐15.7%) | 13/106, 12.3% (7‐20.4%) |
| RSV, total | 142/604, 23.5% (20.2‐27.1%) | 81/498, 16.3% (13.2‐19.9%) * | 61/106, 57.5% (47.6‐67%) * |
| RSV A | 78/604, 12.9% (10.4‐15.9%) | 40/498, 8% (5.9‐10.9%) * | 38/106, 35.8% (26.9‐45.8%) * |
| RSV B | 110/604, 18.2% (15.3‐21.6%) | 59/498, 11.8% (9.2‐15.1%) * | 51/106, 48.1% (38.4‐58%) * |
| Parainfluenza viruses | 18/604, 3% (1.8‐4.8%) | 17/498, 3.4% (2.1‐5.5%) | 1/106, 0.9% (0.05‐5.9%) |
| Rhinovirus | 16/604, 2.6% (1.6‐4.4%) | 12/498, 2.4% (1.3‐4.3%) | 4/106, 3.8% (1.2‐9.9%) |
| Human metapneumovirus | 13/604, 2.2% (1.2‐3.8%) | 7/498, 1.4% (0.6‐3%) * | 6/106, 5.7% (2.3‐12.4%) * |
| Adenovirus | 6/604, 1% (0.4‐2.3%) | 3/498, 0.6% (0.2‐1.9%) | 3/106, 2.8% (0.7‐8.7%) |
| Bocavirus | 2/604, 0.3% (0.1‐1.3%) | 0/498, 0% (0‐%) * | 2/106, 1.9% (0.3‐7.3%) * |
| Coronavirus | 1/604, 0.2% (0.0‐1.1%) | 1/498, 0.2% (0.01‐1.3%) | 0/106, 0% (0‐4.3%) |
| Echovirus | 1/604, 0.2% (0.01‐1.1%) | 1/498, 0.2% (0.01‐1.3%) | 0/106, 0% (0‐4.3%) |
| Mixed RSV and influenza | 45/604, 7.5% (5.5‐9.9%) | 30/498, 6% (4.2‐8.6) * | 15/106, 14.25 (8.4‐22.6%) * |
| Mixed viral infections | 68/604, 11.3% (8.9‐14.1%) | 43/498, 8.6% (6.4‐11.5%) * | 25/106, 23.6% (16.1‐33%) * |
N over total population in each category is depicted, followed by prevalence rate and 95%CI in brackets.
Denotes P < 0.05.
In 332 out of 604 (55%, 95%CI: 50.9%‐59%) patients a respiratory virus was identified (Tables 1 and 2). Rates of specific respiratory virus detection, as well as, mixed virus infections according to adult or child status are shown in Tables 1 and 2. The identification of viral infection was more common in children in all seasons (OR 3.8, 95% CI 2.3‐6.4, P < 0.001, Table 2). Confirmed influenza had a prevalence of 32.1% (95%CI: 28.4‐36%) while Respiratory Syncytial Virus (RSV) was detected in 23.5% (95%CI: 20.2‐27.1%) of all patients (Table 2). Virus positivity was more common in patients with comorbidities (OR 1.5, 95%CI 1.04‐2.3, P = 0.03). This association was driven by influenza virus positivity (OR 1.7, 95%CI 1.1‐2., P ≤ 0.01) and was not found for other viruses. Any virus positivity was more common in patients with COPD (OR 1.7, 95%CI 1.1‐2.6, P ≤ 0.01) or cardiovascular disease (OR 1.8, 95%CI 1.1‐2.8, P ≤ 0.01). In sub‐analyses, this was shown again to be specific for influenza detection (OR 1.7, 95%CI 1.1‐2.7, P = 0.02 and OR 1.8, 95%CI 1.1‐2.8, P = 0.02, respectively).
Table 3 depicts variability of study results according to study year and available data through the National sentinel Influenza‐like illness (ILI) and influenza surveillance network. Influenza strains identified during our study for the most part correlated with those circulating at the National level during the years of the study. During the 2009‐10 and 2010‐11seasons the predominant virus identified during the study was RSV (Table 2). In both instances study samples were collected during periods of high influenza activity according to national data (Table 3).
Table 3.
Variability in viral detection per season studied and comparison to national data regarding influenza surveillance available from the Hellenic Center for Disease Control and Prevention
| 2009‐10 | 2010‐11 | 2013‐14 | 2014‐15 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Onset | Peak | End | Onset | Peak | End | Onset | Peak | End | Onset | Peak | End | |
| Influenza season, week/year, national data | 48/09 | 04/10 | 15/10 | 52/10 | 05/11 | 13/11 | 52/13 | 09/14 | 16/14 | 51/14 | 09/15 | 20/15 |
| Study period, week/year | 48/09‐04/10 | 03/11‐11/11 | 47/13‐20/14 | 43/14‐13/15 | ||||||||
| National (N)/Study (S) data | National | Study n = 94 | National | Study n = 106 | National | Study n = 133 | National | Study n = 271 | ||||
| Influenza (+) in ILI | 56.4% | 43.6% | 55.2% | 23.6% | 30.7% | 16.5% | 39.9% | 39.1% | ||||
| Influenza A | 100% | 100% | 95.4% | 92% | 95.8% | 95.5% | 41% | 32.1% | ||||
| Influenza A (H1N1) pdm09 in total A | 99.8% | 100% | 98.9% | 95.7 | 79.4% | 100% | 12.5% | 14.3% | ||||
| Influenza A (H3N2) in total A | 0.2% | 0% | 1.1% | 4.3% | 20.6% | 0% | 86.7% | 85.7% | ||||
| Influenza B | 0% | 0% | 4.6% | 0.9% | 4.2% | 0.8% | 59% | 26.9% | ||||
| RSV (+) study data | 45/94 (47.9%) | 75/106 (70.8%) | 12/133 (9%) | 10/271 (3.7%) | ||||||||
| Mixed virus infection | 27/94 (28.7%) | 31/106 (29.2%) | 1/133 (0.8%) | 9/271 (3.3%) | ||||||||
| Mixed Influenza and RSV | 23/94 (24.5%) | 19/106 (17.9%) | 0/133 (0%) | 3/271 (1.1%) | ||||||||
RSV, respiratory syncytial virus.
RSV positivity ranged from 3.7% during the 2014‐15 season to 70.8% during the 2010‐11 season (P < 0.01) (Table 3). Variability of virus detection per collected samples, per week of study during the 2010‐11 season, is shown in Figure 1. Eighty‐one out of 498 (16.3%) adults had an RSV infection compared to 61 out of 106 (57.5%) children (OR 7, 95%CI 4.4‐11, P < 0.001, Tables 1 and 2). RSV positivity according to comorbidity status is shown in Table 1.
Figure 1.
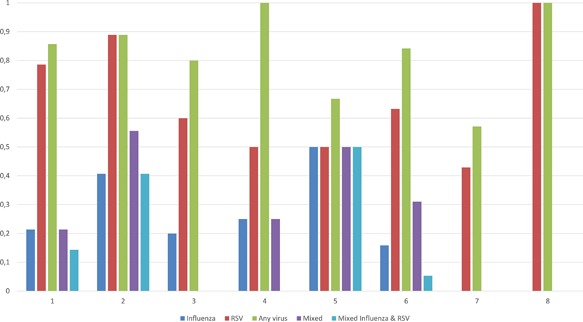
Variability of virus detection per collected samples (in %, one equals 100%) during the 2010‐11 season per week of study
Mixed viral infections were diagnosed in 68/332 (20.5%) patients where any virus was detected (Table 4); 45 out of the 68 (66.2%) represented mixed influenza and RSV infections. Mixed viral infections were more common in children than adults (OR 3.7; 95%CI 1.9‐5.6, P < 0.01). In logistic regression analyses, mixed infections were associated with younger age (mean age 30.4 yrs vs. 41.8, beta −0.02, OR: 0.98, 95%CI 0.97‐0.99, P ≤ 0.001) and increased rates of fever (63/68 [92.6%] pts vs. 217/264 [82.2%] pts, OR: 2.7; 95%CI 1.04–7.2, P < 0.05). Although, no statistical association was detected mixed viral infections were more frequent than single infections in patients with comorbidities (7 out of 206 [3.4%] patients vs. 3/196 [1.5%]), patients with COPD (50% vs. 36.5%), cardiovascular disease (40% vs. 31.8), diabetes mellitus (20% vs. 16.5%); the association approached statistical significance in smokers (60% vs. 37.1%, P = 0.19). Patients with mixed infections were less frequently hospitalized compared to patients with single infections (13.2% vs. 31.4%, P = 0.002).
Table 4.
Epidemiological characteristics of patients with mixed viral infections versus those with single viral infection
| Single virus, n = 264 | Mixed virus, n = 68 | P | |
|---|---|---|---|
| Age, mean ± SD in yrs | 41.8 ± 25.6 | 30.4 ± 23 | ≤0.001 |
| Child, n (%), n = 84/332 (25.3) | 59/264 (22.3) | 25/68 (36.8) | 0.01 |
| COPD, n (%), n = 67/180 | 62/170 (36.5) | 5/10 (50) | 0.5 |
| Cardiovascular disease, n (%), n = 58/180 (32.2) | 54/170 (31.8) | 4/10 (40) | 0.7 |
| Diabetes, n (%), n = 30/180 (16.7) | 28/170 (16.5) | 2/10 (20) | 0.7 |
| Neoplastic disease, n (%), n = 10/180 (5.6) | 10/170 (5.9) | 0/10 (0) | 1 |
| History of allergic condition, n (%), n = 17/180 (9.4) | 16/170 (9.4) | 1/10 (10) | 1 |
| Chronic renal diseases, n (%), n = 3/170 (1.7) | 3/170 (1.8) | 0 (0) | 1 |
| Tobacco use, n (%), n = 69/180 (38.3) | 63/170 (37.1) | 6/10 (60) | 0.19 |
| Influenza vaccination, n (%), n = 41/180 (22.8) | 38/170 (22.4) | 3/10 (30) | 0.7 |
| Fever, n (%), n = 280/332 (84.3) | 217/264 (82.2) | 63/68 (92.6) * | 0.039 |
| Hospitalization, n (%), n = 92/332 (27.7) | 83/264 (31.4) | 9/68 (13.2) * | 0.002 |
Data for comorbidities were available only for 180 people of the study population. N over total population in each category is depicted.
COPD, chronic obstructive pulmonary disease.
Denotes P < 0.05.
We identified very few cases of other respiratory viral pathogens (Table 2). Human metapneumovirus (hMPV) infections were more common in children (OR 5.9, 95%CI 1.8‐19.6, P = 0.006, Table 2). Both RSV infections and human metapneumovirus infections were more frequent in children aged <5 years old (P < 0.001 for all associations). Rates of other viruses were comparable between children and adults.
Thirty‐four (5.6%) patients were hospitalized. The presence of any comorbidity was significantly associated with hospitalization (P = 0.001). In logistic regression models adjusting for all potential confounders higher age (beta: 0.03, OR 1.03, 95%CI 1.009‐1.04, P < 0.01) and presence of a cardiovascular disease (beta: 0.99, OR 2.7, 95%CI 1.3‐5.5, P < 0.01) were associated with a higher chance of hospitalization.
4. DISCUSSION
A significant number of mixed viral respiratory infections was observed in the current study evaluating adults as well as children. Most mixed infections observed herein were RSV‐influenza co‐infections. Mixed viral infections were associated with younger age and increased rates of fever but were not associated with increased rates of hospitalization. We could not associate the presence of mixed infections with underlying comorbidities; however mixed viral infections appeared to be more frequent occurring than single infections in patients with comorbidities, patients with COPD and patients with cardiovascular disease. Nevertheless, it seems that our study did not have the power to elicit important differences in this regard.
In the current study, single as well as mixed viral infection was more common in children than adults. Multiple pediatric studies as well as a recent Greek study identified significant rates of mixed viral infections in the evaluated children population.5, 6, 7, 8, 9, 10, 11, 12, 13, 14 If higher percentages of pediatric population were included in our study mixed viral infection rates could have been higher.21 Studies evaluating adult populations are scarce.15, 16, 17, 18 It appears that mixed viral infections may represent a significant percentage of adult upper respiratory tract infections albeit lower than pediatric ones.17, 18 The exact role of each virus in contributing to the pathogenesis of such infections is unclear.
We could not associate the presence of mixed viral infections or mixed RSV and influenza infections with severity of disease, or increased rates of hospitalization as claimed previously in most pediatric and adult studies.5, 15 Only one childhood study reported an inverse relationship between viral detection and need for oxygen therapy and hospitalization days.14 Nevertheless, patients with mixed infections had higher rates of fever compared to the rest of the group. Other studies have not confirmed this association similarly to our findings.9, 10, 16 Of interest, in our study single infections were associated with higher rates of hospitalization. This could be attributed to a systemic bias of: a) mixed infections being more common in younger children that usually run a milder clinical disease course; b) selection bias regarding the presentation to the Emergency Room of a tertiary care Hospital of more severe cases of respiratory infection that most often had single rather than mixed infection, and; c) the possibility that the additional virus detected was accidentally discovered when it was actually having a colonizing role. Furthermore, in the last two seasons of the study we had very few cases of RSV or mixed infections. Whether the coexistence of RSV and influenza meant: a) establishment of RSV prior to influenza infection or vice‐versa; b) the presence of one virus as a colonizer/pathogen that facilitated the establishment of the other or; c) both acted as co‐pathogens at the same time, is a matter, future studies should try to elucidate.
Regarding single virus infections the most prevalent respiratory viruses identified during the time of the study were influenza and RSV. Significant rates of RSV infection were noted during the winter season co‐circulating with the influenza virus even during the 2009‐10 pandemic season. Our prevalence data regarding influenza types concurred with the National Influenza surveillance scheme data that is flawed by the lack of data availability on co‐circulation of other viruses; this co‐circulation has been also confirmed in other efforts.7
Our study results concur with these of other studies showing rates of respiratory virus detection from 38.8% to as high as 52.1% in adults with upper respiratory infection or influenza like illness.17, 18 The higher percent of viral infections in children was not unexpected.21, 22 In particular, infections due to RSV and hMPV were more frequently observed in children compared to adults. Regarding the higher rates of RSV detection in children, RSV is well known to be a common pathogen in respiratory tract infections in children. Although not seen in our study, RSV can be a devastating pathogen in children that are very young or that have underlying illnesses23, 24; more severe clinical outcomes and hospitalization have been described for children with RSV infection in our country.25
Human metapneumovirus was also more frequently seen in children in our study; as a virus it is known to affect younger children suffering from lower respiratory infections.23, 26, 27 Increased awareness for this pathogen in younger ages is required. The new molecular techniques have facilitated identification of previously unappreciated pathogens in respiratory infections.
We diagnosed RSV infections in a significant percentage of our adult population. The role of RSV in producing adult respiratory infection is increasingly recognized.28 RSV infections in adults are usually re‐infections and have a mild clinical picture.28 In our study, RSV detection was not associated with hospitalization. RSV may be an important pathogen for specific adult subgroups like immunocompromised individuals or patients with acute exacerbation of chronic obstructive pulmonary disease29; however we did not see this association in our cohort. The role of RSV in producing a more severe disease especially in the adult population is a matter of debate.30 In general, RSV appears to be associated with milder illness compared to influenza31 however it has been associated with more severe disease in older subjects.32
Our study is limited by the fact that it examined population from a single center. Despite the risk of sampling bias, for example, not all patients examined, lack of a significant number of pediatric patients, the results of our samples closely correlate to national data obtained by a Hellenic Center for Disease Control and Prevention coordinated sentinel network that operates in more than 200 sites across the country and is much more representative of the general population. Data from this network depict that seasonal influenza starts around the end of each year (weeks 51‐52) and lasts up until around week 16‐18 of the next year each season (Table 3, data available at http://www.keelpno.gr). Further, we could not adjust for any climatic effects in our viral detections. It has been reported that seasonality patterns for respiratory viruses circulation differ according to climatic conditions in different geographical areas,33, 34, 35 although some studies have failed to show this.36, 37 Another limitation is that we could not exclude the possibility of RSV community outbreaks during the first 2 years of the study that could have forced more RSV infected patients to seek medical assistance. Variability of virus detection per collected samples per week of study during the 2010‐11 season (the highest RSV rates were noted then) suggest such a possibility for the last weeks of the study, when only RSV was detected. In addition, the limited data on other viruses besides the influenza virus and RSV is indicative of the dominance of these viruses during the winter season. Larger studies performed throughout the year, are necessary to clarify clinic‐epidemiological associations for other viruses; for example, rhinoviruses (usually circulating in other seasons) or hMPV and bocavirus (both were more common in children in this study). Finally, we could not exclude the possibility of viral presence in patients with low viral loads in the upper airways as a reason for not detecting a virus in most of our carefully selected patients. It is well known that lower respiratory secretions have higher yield in viral detection in respiratory infections. Similarly, nasopharyngeal samples or pooled nasopharygeal and oropharyngeal samples have been reported as more appropriate for analyzing respiratory infections.38, 39 It is well known that nasopharyngeal sampling is more sensitive in detecting respiratory viruses. The detection of viruses by molecular technique in an oropharyngeal sample collected through a standardized technique has facilitated vastly in the performance of our study in a busy Emergency Room. In a subsample of our study concerning almost one third of our patients, the method described herein correlated well with nasopharyngeal swabbing results (manuscript under preparation). Nevertheless, it is possible, that our analysis was biased towards cases with higher viral loads that gave positive results more frequently than cases with lower loads. In a similar manner, we could not account for bacterial coinfections in our subjects as most of the patients unless seriously ill were not examined with specific testing for the presence of bacterial co‐infection. It is has been recently reported that viral‐ bacterial coinfections are associated with a more complicated clinical course and adversely affect the prognosis of severe community—acquired pneumonia.40 Nevertheless as shown in previous studies most of the acute respiratory infections in the general population are of viral origin.41
In conclusion, we describe for the first time in Greece, a large population based epidemiology study concerning the molecular detection of mixed viral respiratory infections in people presenting to a major tertiary care center. Newer molecular techniques assisted in diagnosing mixed viral infections in a significant fraction of our population. Although data from our study concur with data from several published pediatric studies, we believe that our study's main strength is that it is one of the very few epidemiological studies examining mixed respiratory viruses in adults. Further, as an epidemiological study it does not attempt to explain a hypothesis; rather it assists in generating hypotheses regarding the role of mixed viral infections in the adult population and points out the lack of data in this significant research area. Further work is necessary to elucidate the pathogenic role of mixed infections due to respiratory viruses and their local and systemic effects.
CONFLICTS OF INTEREST
No financial or other conflict of interest exists, that might be construed to influence the contents of the current manuscript, including the results or interpretation of publication.
ACKNOWLEDGMENT
This research received no specific grant from any funding agency, commercial or not‐for‐profit sectors.
Antalis E, Oikonomopoulou Z, Kottaridi C, et al. Mixed viral infections of the respiratory tract; an epidemiological study during consecutive winter seasons. J Med Virol. 2018;90: 663–670. 10.1002/jmv.25006
This study was presented in part in poster form: Abstract No 545, during the 2015 IDWEEK conference, October 7‐11, San Diego Convention Center, San Diego, CA.
REFERENCES
- 1. Monto AS. The seasonality of rhinovirus infections and its implications for clinical recognition. Clin Ther. 2002; 24:1987–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Monto AS. Epidemiology of viral respiratory infections. Am J Med. 2002; 112:4S–12S. [DOI] [PubMed] [Google Scholar]
- 3. Monto AS, Ohmit S. Respiratory syncytial virus in a community population: circulation of subgroups A and B since 1965. J Infect Dis. 1990; 161:781–783. [DOI] [PubMed] [Google Scholar]
- 4. Brotons P, Henares D, Latorre I, Cepillo A, Launes C, Munoz‐Almagro C. Comparison of NxTAG respiratory pathogen panel and Anyplex II RV16 tests for multiplex detection of respiratory pathogens in hospitalized children. J Clin Microbiol. 2016; 54:2900–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Essa S, Owayed A, Altawalah H, Khadadah M, Behbehani N, Al‐Nakib W. Mixed viral infections circulating in hospitalized patients with respiratory tract infections in kuwait. Adv Virol. 2015; 2015:714062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gooskens J, van der Ploeg V, Sukhai RN, Vossen AC, Claas EC, Kroes AC. Clinical evaluation of viral acute respiratory tract infections in children presenting to the emergency department of a tertiary referral hospital in the Netherlands. BMC Pediatr. 2014; 14:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pogka V, Kossivakis A, Kalliaropoulos A, et al. Respiratory viruses involved in influenza‐like illness in a Greek pediatric population during the winter period of the years 2005–2008. J Med Virol. 2011; 83:1841–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chonmaitree T, Alvarez‐Fernandez P, Jennings K, et al. Symptomatic and asymptomatic respiratory viral infections in the first year of life: association with acute otitis media development. Clin Infect Dis. 2015; 60:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calvo C, Garcia‐Garcia ML, Pozo F, et al. Respiratory syncytial virus coinfections with rhinovirus and human bocavirus in hospitalized children. Medicine (Baltimore). 2015; 94:e1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen YW, Huang YC, Ho TH, Huang CG, Tsao KC, Lin TY. Viral etiology of bronchiolitis among pediatric inpatients in northern Taiwan with emphasis on newly identified respiratory viruses. J Microbiol Immunol Infect = Wei mian yu gan ran za zhi. 2014; 47:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chung JY, Han TH, Kim SW, Hwang ES. Respiratory picornavirus infections in Korean children with lower respiratory tract infections. Scand J Infect Dis. 2007; 39:250–254. [DOI] [PubMed] [Google Scholar]
- 12. Jacques J, Bouscambert‐Duchamp M, Moret H, et al. Association of respiratory picornaviruses with acute bronchiolitis in French infants. J Clin Virol. 2006; 35:463–466. [DOI] [PubMed] [Google Scholar]
- 13. Freymuth F, Vabret A, Cuvillon‐Nimal D, et al. Comparison of multiplex PCR assays and conventional techniques for the diagnostic of respiratory virus infections in children admitted to hospital with an acute respiratory illness. J Med Virol. 2006; 78:1498–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martinez‐Roig A, Salvado M, Caballero‐Rabasco MA, Sanchez‐Buenavida A, Lopez‐Segura N, Bonet‐Alcaina M. Viral coinfection in childhood respiratory tract infections. Arch Bronconeumol. 2015; 51:5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poulakou G, Souto J, Balcells J, et al. First influenza season after the 2009 pandemic influenza: characteristics of intensive care unit admissions in adults and children in Vall d'Hebron Hospital. Clin Microbiol Infect. 2012; 18:374–380. [DOI] [PubMed] [Google Scholar]
- 16. Renois F, Talmud D, Huguenin A, et al. Rapid detection of respiratory tract viral infections and coinfections in patients with influenza‐like illnesses by use of reverse transcription‐PCR DNA microarray systems. J Clin Microbiol. 2010; 48:3836–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lu Y, Tong J, Pei F, et al. Viral aetiology in adults with acute upper respiratory tract infection in Jinan, Northern China. Clin Dev Immunol. 2013; 2013:869521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Noh JY, Song JY, Cheong HJ, et al. Laboratory surveillance of influenza‐like illness in seven teaching hospitals, South Korea: 2011–2012 season. PLoS ONE. 2013; 8:e64295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poulakou G, Perez M, Rello J. Severe acute respiratory infections in the postpandemic era of H1N1. Curr Opin Crit Care. 2012; 18:441–450. [DOI] [PubMed] [Google Scholar]
- 20. Nascimento‐Carvalho CM, Oliveira JR, Cardoso MR, et al. Respiratory viral infections among children with community‐acquired pneumonia and pleural effusion. Scand J Infect Dis. 2013; 45:478–483. [DOI] [PubMed] [Google Scholar]
- 21. Zhang D, He Z, Xu L, et al. Epidemiology characteristics of respiratory viruses found in children and adults with respiratory tract infections in southern China. Int J Infect Dis. 2014; 25:159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monto AS, Ullman BM. Acute respiratory illness in an American community. JAMA. 1974; 227:164–169. [PubMed] [Google Scholar]
- 23. Jain S, Williams DJ, Arnold SR, et al. Community‐acquired pneumonia requiring hospitalization among U.S. children. N Eng J Med. 2015; 372:835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rodriguez‐Auad JP, Nava‐Frias M, Casasola‐Flores J, et al. The epidemiology and clinical characteristics of respiratory syncytial virus infection in children at a public pediatric referral hospital in Mexico. Int J Infect Dis. 2012; 16:e508–e513. [DOI] [PubMed] [Google Scholar]
- 25. Kouni S, Karakitsos P, Chranioti A, Theodoridou M, Chrousos G, Michos A. Evaluation of viral co‐infections in hospitalized and non‐hospitalized children with respiratory infections using microarrays. Clin Microbiol Infect. 2013; 19:772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Principi N, Esposito S. Paediatric human metapneumovirus infection: epidemiology, prevention and therapy. J Clin Virol. 2014; 59:141–147. [DOI] [PubMed] [Google Scholar]
- 27. Panda S, Mohakud NK, Pena L, Kumar S. Human metapneumovirus: review of an important respiratory pathogen. Int J Infect Dis. 2014; 25:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walsh EE, Falsey AR. Respiratory syncytial virus infection in adult populations. Infect Disord Drug Targets. 2012; 12:98–102. [DOI] [PubMed] [Google Scholar]
- 29. Dimopoulos G, Lerikou M, Tsiodras S, et al. Viral epidemiology of acute exacerbations of chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2012; 25:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bont L, Baraldi E, Fauroux B, et al. RSV‐still more questions than answers. Pediatr Infect Dis. 2014; 33:1177–1179. [DOI] [PubMed] [Google Scholar]
- 31. Sundaram ME, Meece JK, Sifakis F, Gasser RA, Belongia EA, Jr. Medically attended respiratory syncytial virus infections in adults aged >/= 50 years: clinical characteristics and outcomes. Clin Infect Dis. 2014; 58:342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Widmer K, Griffin MR, Zhu Y, Williams JV, Talbot HK. Respiratory syncytial virus‐ and human metapneumovirus‐associated emergency department and hospital burden in adults. Influenza Other Respir Viruses. 2014; 8:347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pitzer VE, Viboud C, Alonso WJ, et al. Environmental drivers of the spatiotemporal dynamics of respiratory syncytial virus in the United States. PLoS Pathog. 2015; 11:e1004591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jaakkola K, Saukkoriipi A, Jokelainen J, et al. Decline in temperature and humidity increases the occurrence of influenza in cold climate. Environ Health. 2014; 13:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen Z, Zhu Y, Wang Y, et al. Association of meteorological factors with childhood viral acute respiratory infections in subtropical China: an analysis over 11 years. Arch Virol. 2014; 159:631–639. [DOI] [PubMed] [Google Scholar]
- 36. Alonso WJ, Laranjeira BJ, Pereira SA, et al. Comparative dynamics, morbidity and mortality burden of pediatric viral respiratory infections in an equatorial city. Pediatr Infect Dis J. 2012; 31:e9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Annan A, Ebach F, Corman VM, et al. Similar virus spectra and seasonality in paediatric patients with acute respiratory disease, Ghana and Germany. Clin Microbiol Infect. 2016; 22:340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lieberman D, Lieberman D, Shimoni A, Keren‐Naus A, Steinberg R, Shemer‐Avni Y. Identification of respiratory viruses in adults: nasopharyngeal versus oropharyngeal sampling. J Clin Microbiol. 2009; 47:3439–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lieberman D, Lieberman D, Shimoni A, Keren‐Naus A, Steinberg R, Shemer‐Avni Y. Pooled nasopharyngeal and oropharyngeal samples for the identification of respiratory viruses in adults. Eur J Clin Microbiol Infect Dis. 2010; 29:733–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Voiriot G, Visseaux B, Cohen J, et al. Viral‐bacterial coinfection affects the presentation and alters the prognosis of severe community‐acquired pneumonia. Crit care (London, England). 2016; 20:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Gageldonk‐Lafeber AB, Heijnen ML, Bartelds AI, Peters MF, van der Plas SM, Wilbrink B. A case‐control study of acute respiratory tract infection in general practice patients in The Netherlands. Clin Infect Dis. 2005; 41:490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]


