Abstract
Human adenovirus (HAdV) infection can result in a severe respiratory disease. The aim of this study was to identify HAdV types detected in patients hospitalized for severe respiratory illness. The study population consisted of 743 patients with severe respiratory disease admitted to four major hospitals in Kuwait between January 2013 and December 2016. Respiratory specimens were retrospectively screened for 20 respiratory viruses by real‐time PCR. The HAdV hexon gene was amplified and directly sequenced, and HAdV types were identified by performing Bayesian phylogenetic analysis. HAdV DNA was detected in 27 (3.6%) patients, with peaks in November and March. Most patients were infants and young children suffering from pneumonia or acute bronchiolitis. The detected HAdV types were C1, C2, C5, B3, and B7. Clusters of HAdV C1, C2, and C5 were observed with high posterior probability. All patients infected with HAdV C5 and 50% of patients infected with HAdV C2 or B7 were admitted to the intensive care unit (ICU). Co‐infection with other viruses was detected in 44.4% of patients. The most common co‐infecting virus was rhinovirus (HRV). HAdV/HRV co‐infection was detected in two children who presumably developed disseminated HAdV infection and died. This is the first report describing the circulation of HAdV types associated with severe outcomes in Kuwait. These findings highlight the need for a national surveillance system to monitor changes in predominant HAdV types and increased numbers of severe respiratory infections.
Keywords: adenovirus, Kuwait, severe respiratory infections, types
1. INTRODUCTION
Acute lower respiratory tract infections are caused by different viruses, including human adenoviruses (HAdVs). HAdVs are transmitted via direct contact, small droplet aerosols, the water system, and the fecal‐oral route.1, 2 Very young children, elderly persons with underlying respiratory or cardiac disease, or immunocompromised persons are at high risk for severe HAdV infection, leading to multi‐system organ failure and death.3, 4, 5, 6 More than 70 HAdV types divided into seven species (A to G) have been described7, 8; however, most common respiratory infections are associated with species B, C, and E.9 The most commonly detected HAdV types in the in the United States are C1, C2, B3, and C5.10 Clinical symptoms and epidemiological settings vary with HAdV type. Mild respiratory symptoms are often associated with species C4, whereas severe respiratory illness and disseminated infection have been reported with several types of species HAdV B,9, 11, 12, 13 particularly in infants and persons with underlying diseases. HAdV types E4 and B7 were also associated with outbreaks of acute respiratory disease in new non‐vaccinated military recruits in the United States.14
The prevalence of HAdV infection varies geographically.15 In Kuwait, where two‐thirds of the population consists of expatriates, HAdV was reported for approximately 3% of upper respiratory viral infections and 5% of lower respiratory viral infections during the period between September 2010 and April 2013.16 However, there are no reports describing the HAdV types involved in severe respiratory disease in Kuwait. In particular, the circulation of HAdV types (eg, B3, B7, and E4) associated with prolonged hospitalization has not previously been investigated; consequently, no control measures have been taken to stop their spread. In this study, we examined the molecular epidemiology of HAdV respiratory infection requiring hospitalization between 2013 and 2016.
2. MATERIALS AND METHODS
2.1. Study population
Clinical specimens were sent from different hospitals to the Virology Unit of the Department of Microbiology, Faculty of Medicine, Kuwait University, for daily routine screening for viruses. The study population consisted of 743 patients with severe respiratory disease admitted to Mubarak Al‐Kabeer, Farwania, Al‐Adan, or Al‐Sabah hospital for at least 3 days between January 2013 and December 2016. These four hospitals are the major hospitals in Kuwait. The diagnosis of viral pneumonia was based on the results of chest radiography17 and bacterial culture. Acute bronchiolitis was defined as signs and symptoms of respiratory distress associated with symptoms of a viral respiratory tract infection (cough, runny nose, blocked nose, tachypnea, recessions, nasal flaring, and cyanosis).18, 19 The following data were retrospectively collected for each patient: age, sex, clinical diagnosis, and preexisting chronic conditions. The study followed the ethical standards recommended by the Ethical Decision Committee of the Research Administration, Faculty of Medicine, Kuwait University.
2.2. Microbiogical screening
The isolation of viral nucleic acid from respiratory samples was performed using an automated MagNa Pure LC 2.0 system (Roche Diagnostic Systems, Branchburg, NJ). The Fast‐Track Diagnostics Respiratory Pathogen 21 Assay (Fast‐Track Diagnostics Ltd., Sliema, Malta), based on multiplex one‐step reverse transcription polymerase chain reaction (RT‐PCR), was performed using an Applied Biosystems 7500 Fast Real‐Time PCR System (Applied Biosystems, Foster City, CA) to detect 21 respiratory pathogens (20 viruses and Mycoplasma pneumoniae). These 20 respiratory viruses included influenza virus A (Flu A); influenza virus A H1N1 (H1N1); influenza virus B (Flu B); human coronaviruses (HCoV) NL63, 229E, OC43, and HKU1; parainfluenza viruses (PIV) 1, 2, 3, and 4; human metapneumoviruses (hMPVs) A and B; human rhinovirus (HRV); respiratory syncytial viruses (RSV) A and B; human adenovirus (HAdV); enterovirus (EV); human parechovirus (HPeV); and human bocavirus (HBoV).
2.3. Adenovirus typing
Nested PCR amplification of a ∼1‐kb partial coding sequence of the adenovirus hexon hypervariable regions HVR1 to HVR‐6, which correlate closely with virus serotype, was carried out using two outer primers (Adhex‐GT3F and Adhex‐GT2R) and two inner primers (Adhex‐GT2F and Adhex‐GT1R), as previously described.20 Nested PCR products of ∼900 bp were purified using a Wizard SV GEL and PCR Clean‐Up System Kit (Promega Corporation, Madison, WI), and the nucleotide sequences of both DNA strands were then determined by performing direct double‐strand DNA cycle sequencing using the inner primers (Adhex‐GT2F and Adhex‐GT1R) and an ABI PRISM® BigDye® Terminator Cycle Sequencing v3.1 Kit (Applied Biosystems). Post‐sequencing PCR purification was carried out to remove unbound fluorescent dye deoxy terminators using a BigDye XTerminator™ Purification Kit (Applied Biosystems). The samples were then denatured for 2 min at 95°C, immediately chilled on ice, and loaded on an ABI 3500 Genetic Analyzer (Applied Biosystems). DNA sequences were subjected to electrophoresis on a 50‐cm 8‐capillary array using POP‐7™ polymer (Applied Biosystems) as a separation medium and analyzed using 3500 Series Data Collection Software version 3 and Sequencing Analysis Software version 6 (Applied Biosystems).
The obtained nucleotide sequences were searched against the NCBI GenBank database using the Basic Local Alignment Search Tool (BLAST), and then aligned with all adenovirus reference sequences available in the NCBI GenBank database using the ClustalW method with Molecular Evolutionary Genetics Analysis (MEGA) software version 4.02.21 Phylogenetic trees were reconstructed using the Bayesian Markov Chain Monte Carlo (MCMC) approach implemented in the BEAST v.1.8.2 program.22 The resulting Bayesian Skyline (BSL) plot was run under a relaxed uncorrelated log normal molecular clock using the best model for nucleotide substitution (HKY) that was most closely related to the model obtained with MODELTEST. Convergence of the parameters during the MCMC was inspected with Tracer v.1.5,22 with uncertainties designated as the 95% highest probability density intervals. Ten million chains were sufficient to achieve convergence of all parameters with an effective sampling size (ESS >200). The trees were sampled every 1000 steps, resulting in a final file of 10 000 trees. These trees were summarized in a maximum a posteriori (MAP) tree using the TreeAnotator v.1.7.4 program (part of the BEAST package) and visualized with FigTree v.1.4.0 (http://beast.bio.ed.ac.uk/FigTree). The accession number of each adenovirus reference sequence was added to the taxon label in the phylogenetic tree. Adenovirus sequences resulting from this study were uploaded to the NCBI GenBank database (accession numbers MF085378 to MF085404).
2.4. Statistical analysis
The results were expressed as percentages, medians and interquartile ranges (IQRs). A 2 × 2 or 2xk contingency table was generated to compare different proportions using the Chi‐square test and Fischer's exact test, as appropriate. The Chi‐square test for trend was used to assess trends in the proportion of HAdV detection during 2013‐2014. Age differences between different groups were assessed using the Kruskal Wallis test or Mann‐Whitney test, as appropriate. Statistical analysis was performed using IBM SPSS Statistics for Windows, version 23 (IBM Corp., Armonk, N.Y.).
3. RESULTS
HAdV DNA was detected in respiratory samples from 27 (3.63%) of 743 patients hospitalized for severe respiratory infection. The IQR of the threshold cycle (Ct) values was between 21 and 30. All HAdV‐positive cases were detected between August and May, with peaks in November and March (Figure 1). The majority of cases were reported from two main hospitals: Mubarak Al‐Kabeer (48%) and Farwaniya (37%). There was no significant increase in HAdV detection during the same period (P‐value = 0.62). The baseline characteristics of patients infected with HAdV are provided in Table 1. Most patients (n = 24, 88.89%) were infants and children under 4 years of age. Viral pneumonia and acute bronchiolitis had been diagnosed in 14 (51.85%) and 13 (48.15%) HAdV‐positive patients, respectively (Table 1). There was no significant difference in the distribution of males and females between patients with pneumonia and those with bronchiolitis (P‐value = 0.103). Fourteen (51.9%) patients were admitted to the intensive care unit (ICU) and 13 (48.1%) to pediatric or adult wards. None of the HAdV‐infected patients were immunocompromised, received immunosuppressive drugs, or had underlying chronic diseases.
Figure 1.
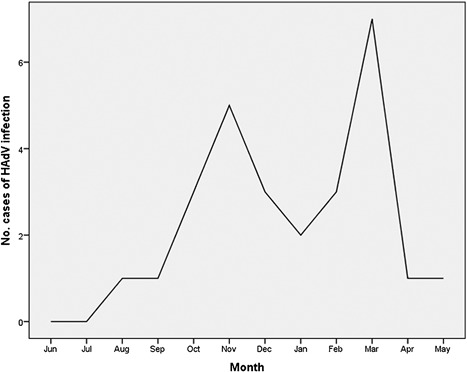
Number of cases of human adenovirus infection associated with severe respiratory diseases from 2013 to 2016 in Kuwait. HAdV, human adenovirus
Table 1.
Demographic and clinical characteristics for HAdV‐infected patients, January 2013‐December 2016
| Total | Viral pneumonia | Acute bronchiolitis | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Sex | |||
| Male | 9 (33.3) | 7 (50) | 2 (15.4) |
| Female | 18 (66.7) | 7 (50) | 11 (84.6) |
| Age median in years | 2 | 2 | 1 |
| Minimum | 1 month | 1 month | 1 month |
| Maximum | 36 years | 27 years | 36 years |
| Interquartile range | 5 months to 4 years | 5 months to 5 years | 3 months to 4 years |
| Sample type | |||
| Nasopharyngeal aspirate | 10 (37) | 6 (42.9) | 4 (30.8) |
| Nasopharyngeal swab | 15 (55.6) | 6 (42.9) | 9 (69.2) |
| Sputum | 2 (7.4) | 2 (14.3) | 0 |
| Hospital | |||
| Al‐Adan | 1 (3.7) | 0 | 1 (7.7) |
| Farwaniya | 10 (37) | 5 (35.7) | 5 (38.5) |
| Mubarak Al‐Kabeer | 13 (48.1) | 6 (42.9) | 7 (53.8) |
| Al‐Sabah | 3 (11.1) | 3 (21.4) | 0 |
According to our phylogenetic analysis of the hexon gene (Figure 2), the most prevalent HAdV types detected in patients with viral pneumonia or acute bronchiolitis were C1, C2, and B3, followed by B7 and C5. The distribution of HAdV types between the two groups of patients was not significant (P‐value = 0.84, Table 2). The hexon gene sequences from HAdV B3‐positive samples showed 99.0‐100% sequence identity, while HAdV B7 sequences showed 99.6‐99.9%, HAdV C1 showed 99.4‐100%, HAdV C2 showed 99.2‐100%, and HAdV C5 showed 96.7‐98.5%. Some strains from the HAdV C1, C2, or C5 types formed clusters with high posterior probability (81‐100%) (Figure 2). Clusters formed by HAdV C1 or C2 strains were detected in different years at two different hospitals: Mubarak Al‐Kabeer hospital (strains 14403‐2, 15742‐2, and 18168) and Farwaniya hospital (strains 901325, 375, and 07187). There was no significant relationship between the admission facility (ICU vs ward) and HAdV type (P‐value = 0.44) or HAdV species (P‐value = 0.40). However, all patients infected with HAdV C5 and 50% of patients infected with HAdV C2 or B7 were admitted to the ICU (Table 2). The median age of the case‐patients did not vary among HAdV types (P‐value = 0.49), HAdV species (P‐value = 0.58), or admission facilities (P‐value = 0.39). However, HAdV C2 and C5 were not detected in adult patients.
Figure 2.
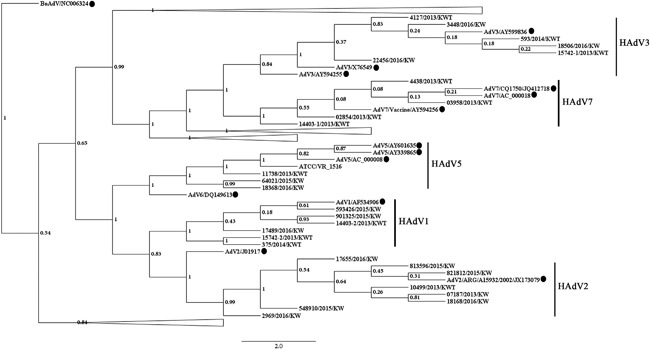
Bayesian phylogenic analysis of the adenovirus hexon gene. The values of posterior probability are depicted in the nodes of the trees. Each adenovirus reference sequence is labeled with its corresponding subtype followed by the GenBank accession number. Tree branches for adenovirus species not relevant to strains from this study are collapsed. Adenovirus sequences from this study are labeled with the strain number followed by the year of sample collection. Human adenovirus 5 (American Type Culture Collection, ATCC VR‐1516) was used as positive control in sequencing reactions. The black circles next to the taxa represent adenovirus sequences from the GenBank database. HAdV, human adenovirus
Table 2.
Distribution of HAdV types based on the final diagnosis and the hospital admission facility
| Department | |||||
|---|---|---|---|---|---|
| Total | Viral pneumonia | Acute bronchiolitis | Ward | ICU | |
| Adenovirus type | n (%) | n (%) | n (%) | n (%) | n (%) |
| C1 | 6 (22.2) | 4 (28.6) | 2 (15.4) | 4 (28.6) | 2 (15.4) |
| C2 | 8 (29.6) | 4 (28.6) | 4 (30.8) | 4 (28.6) | 4 (30.8) |
| C5 | 3 (11.1) | 2 (14.3) | 1 (7.7) | 0 | 3 (23.1) |
| B3 | 6 (22.2) | 2 (14.3) | 4 (30.8) | 4 (28.6) | 2 (15.4) |
| B7 | 4 (14.8) | 2 (14.3) | 2 (15.4) | 2 (14.3) | 2 (15.4) |
Co‐infection with other viruses was detected in 12 (44.4%) HAdV‐positive patient: 8 (29.6%) patients had a co‐infection with one virus and 4 (14.8%) with two viruses (Table 3). The most common co‐infecting virus was rhinovirus, which was detected in 4 (28.6%) patients with pneumonia and 5 (38.5%) patients with acute bronchiolitis. The Ct IQR for rhinovirus was between 26 and 33 cycles, while that for other viruses was between 24 and 31 cycles.
Table 3.
Frequency of viruses co‐detected with HAdV
| Total | Viral pneumonia | Acute bronchiolitis | |
|---|---|---|---|
| Virus | n (%) | n (%) | n (%) |
| HRV | 6 (22.2) | 3 (21.4) | 3 (23.1) |
| RSV | 1 (3.7) | 0 | 1 (7.7) |
| PIV | 1 (3.7) | 0 | 1 (7.7) |
| HRV + RSV | 1 (3.7) | 0 | 1 (7.7) |
| HRV + HCoV HKU1 | 1 (3.7) | 1 (7.1) | 0 |
| HRV + hMPV | 1 (3.7) | 0 | 1 (7.7) |
| HRV + HPeV | 1 (3.7) | 1 (7.1) | 0 |
Two children developed complications and died. One 2‐year‐old Syrian girl was diagnosed with bronchiolitis and developed left pleural effusion and hepatomegaly with abnormal levels of liver enzymes (aspartate transaminase [AST], 2300 IU/L; alanine transaminase [ALT], 891 IU/L); both HAdV B7 (strain 2854) and rhinovirus were detected in the nasopharyngeal aspirates. However, only HAdV B7 was detected in the blood, and the hexon region showed 100% sequence similarity with strain 2854. HAdV strain 2854 detected in Farwaniya hospital in March 2013 did not cluster with the other HAdV B7 strains detected later in the same hospital within the same year (Figure 2). A second 2‐year‐old Kuwaiti girl diagnosed with pneumonia developed encephalitis; HAdV C5 (strain 64021) and rhinovirus were both detected in the nasopharyngeal aspirate, whereas only HAdV C5 was detected in the blood and showed 100% hexon gene similarity with that detected in the respiratory sample. Interestingly, strain 64021 detected in March 2015 in Al‐Sabah hospital clustered with high posterior probability with strain 18368, which was detected in November 2016 in the same hospital in a 2‐year‐old male admitted to the pediatric ICU for severe respiratory symptoms. The 64021 strain shared 98.48% hexon gene sequence similarity with 18368 strain. No viruses other than HAdV C5 DNA were detected in the pharyngeal aspirate. The boy was discharged 4 days later.
4. DISCUSSION
Influenza A viruses, respiratory syncytial virus, and rhinoviruses are the most common viruses detected in patients suffering from pneumonia or bronchiolitis in Kuwait.16, 23 Low numbers of HAdV‐positive samples from patients suffering from severe respiratory illness and the absence of outbreaks of unusually severe respiratory disease that may elicit the development of a national sentinel system have hindered previous investigations of HAdV infections. Genomic variants of HAdV were shown to be associated with severe respiratory disease.24, 25 In this study, HAdV C1, C2, C5, B3, and B7 were identified as the predominant types detected in patients with severe respiratory infection. The detection of HAdV in a respiratory specimen should be interpreted with caution because asymptomatic HAdV shedding is known to occur. HAdV C persists in the adenoids and tonsils and is shed for prolonged periods after symptoms resolve.26, 27, 28 However, 75% of patients with HAdV infection had Ct values less than 30 cycles, suggesting acute infection rather than persistent shedding. Furthermore, several studies have suggested an association between mixed viral detection and disease severity.29, 30 Co‐infection with other respiratory viruses was observed in 44% of patients infected with HAdV, and rhinovirus was the most commonly detected co‐infecting virus. The frequency of mixed respiratory viral detection varies between 10 and 30% in hospitalized children. 31, 32, 33, 34 Previous results from Kuwait have shown that pneumonia and bronchiolitis are the most frequent reasons for hospitalization with mixed viral detection, and the most common co‐infecting viruses are HAdV and rhinovirus.35
HAdV B7 was detected in one 5‐month‐old infant, two 2‐year‐old children, and one 27‐year‐old adult. In the phylogenetic tree, the HAdV B7 strains did not cluster together, suggesting sporadic transmission of different strains in the population. One 2‐year‐old girl developed presumably disseminated HAdV B7 disease and passed away. Severe outcomes associated with HAdV B7 infection have previously been documented.9, 36 However, the contribution of HAdV B7 to the patient's death was not confirmed, and a role for rhinovirus, co‐detected in the nasopharyngeal aspirate, should not be excluded. Indeed, fatal respiratory infections associated with rhinovirus outbreaks have been described in previous reports.37, 38
The detection of HAdV B3 in five children and one adult is consistent with other studies showing outbreaks of severe respiratory diseases associated with HAdV B viruses.9, 11, 12, 13 HAdVs C1, C2, and C5 were mostly detected in children. Although the number of HAdV‐infected patients who fulfilled the inclusion criteria for the current study was very low, our findings agree with other studies showing that HAdVs C1, C2, and C5 are most frequently detected in young children.9, 10, 11, 39
HAdV C5 (strain 64021) was detected in the pharyngeal aspirate and blood of a 2‐year‐old girl who subsequently developed encephalitis and died. A retrospective review of adenovirus infections at Texas Children's Hospital during 1990‐1996 showed that disseminated adenovirus disease in children occurred in 11 (2.5%) out of 440 adenovirus‐infected patients, and the mortality rate was 83% and 60% among immunocompromised and immunocompetent children, respectively. In that study, HAdVs C5, B3, and B7 were associated with disseminated disease and multi‐organ involvement in immunocompetent children, whereas HAdVs C1 and C2 were the cause of severe outcomes in immunocompromised children.11 Again, a role for co‐detected rhinoviruses in patients’ deaths should not be excluded, as discussed above. Moreover, another HAdV C5 strain (18368) with high sequence similarity to the 64021 strain was detected in a 2‐year‐old boy suffering from pneumonia, who was admitted to the pediatric ICU of the same hospital 8 months later. No other viruses were found in the pharyngeal aspirate within the detection limit of the assay, and therefore, a role for co‐infection in the development of severe respiratory disease in that boy was excluded.
Interestingly, the hexon gene sequences of several HAdV C1, C2, and C5 strains formed clusters with high posterior probability, suggesting active transmission of these strains in the population. Notably, clusters were formed with HAdV C1 or C2 strains sampled from patients admitted to two different hospitals over a 1‐year period, indicating that the transmission of these virus strains in Kuwait is endemic. Full HAdV genome sequencing is required to confirm the similarity between strains.
The detection of HAdV B7 type and other related types associated with severe outcomes in Kuwait highlights the importance of establishing a national surveillance system to monitor the incidence of severe respiratory infections and generate alerts following the increased detection of HAdV B and changes in the relative prevalence of HAdV species/types. Furthermore, healthcare providers should undertake appropriate control measures to prevent secondary HAdV infections, particularly in vulnerable populations such as children, elderly persons, and immunocompromised persons.
Chehadeh W, Al‐Adwani A, John SE, et al. Adenovirus types associated with severe respiratory diseases: A retrospective 4‐year study in Kuwait. J Med Virol. 2018;90: 1033–1039. 10.1002/jmv.25059
REFERENCES
- 1. Leclerc H, Schwartzbrod L, Dei‐Cas E. Microbial agents associated with waterborne diseases. Crit Rev Microbiol. 2002; 28:371–409. [DOI] [PubMed] [Google Scholar]
- 2. Musher DM. How contagious are common respiratory tract infections? N Engl J Med. 2003; 348:1256–1266. [DOI] [PubMed] [Google Scholar]
- 3. Ison MG. Adenovirus infections in transplant recipients. Clin Infect Dis. 2006; 43:331–339. [DOI] [PubMed] [Google Scholar]
- 4. Lee J, Choi EH, Lee HJ. Clinical severity of respiratory adenoviral infection by serotypes in Korean children over 17 consecutive years (1991–2007). J Clin Virol. 2010; 49:115–120. [DOI] [PubMed] [Google Scholar]
- 5. Kandel R, Srinivasan A, D'Agata EMC, Lu X, Erdman D, Jhung M. Outbreak of adenovirus type 4 infection in a long‐term care facility for the elderly. Infect Control Hosp Epidemiol. 2010; 31:755–757. [DOI] [PubMed] [Google Scholar]
- 6. Moura PO, Roberto AF, Hein N, et al. Molecular epidemiology of human adenovirus isolated from children hospitalized with acute respiratory infection in Sao Paulo, Brazil. J Med Virol. 2007; 79:174–181. [DOI] [PubMed] [Google Scholar]
- 7. Hage E, Gerd Liebert U, Bergs S, Ganzenmueller T, Heim A. Human mastadenovirus type 70: a novel, multiple recombinant species D mastadenovirus isolated from diarrhoeal faeces of a haematopoietic stem cell transplantation recipient. J Gen Virol. 2015; 96:2734–2742. [DOI] [PubMed] [Google Scholar]
- 8. Espinola EE, Barrios JC, Russomando G, Mirazo S, Arbiza J. Computational analysis of a species D human adenovirus provides evidence of a novel virus. J Gen Virol. 2017; 10.1099/jgv.0.000947 [DOI] [PubMed] [Google Scholar]
- 9. Scott MK, Chommanard C, Lu X, et al. Human adenovirus associated with severe respiratory infection, oregon, USA, 2013–2014. Emerg Infect Dis. 2016; 22:1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gray GC, McCarthy T, Lebeck MG, et al. Genotype prevalence and risk factors for severe clinical adenovirus infection, United States 2004–2006. Clin Infect Dis. 2007; 45:1120–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Munoz FM, Piedra PA, Demmler GJ. Disseminated adenovirus disease in immunocompromised and immunocompetent children. Clin Infect Dis. 1998; 27:1194–1200. [DOI] [PubMed] [Google Scholar]
- 12. Lessa FC, Gould PL, Pascoe N, et al. Health care transmission of a newly emergent adenovirus serotype in health care personnel at a military hospital in Texas, 2007. J Infect Dis. 2009; 200:1759–1765. [DOI] [PubMed] [Google Scholar]
- 13. Gu L, Liu Z, Li X, et al. Severe community‐acquired pneumonia caused by adenovirus type 11 in immunocompetent adults in Beijing. J Clin Virol. 2012; 54:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gray GC, Goswami PR, Malasig MD, et al. Adult adenovirus infections: loss of orphaned vaccines precipitates military respiratory disease epidemics. For the Adenovirus Surveillance Group. Clin Infect Dis. 2000; 31:663–670. [DOI] [PubMed] [Google Scholar]
- 15. Lynch JP, 3rd , Fishbein M, Echavarria M. Adenovirus. Semin Respir Crit Care Med. 2011; 32:494–511. [DOI] [PubMed] [Google Scholar]
- 16. Essa S, Owayed A, Altawalah H, Khadadah M, Behbehani N, Al‐Nakib W. The prevalence of human bocavirus, human Coronavirus‐NL63, human metapneumovirus, human polyomavirus KI and WU in respiratory tract infections in Kuwait. Med Princ Pract. 2015; 24:382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsung JW, Kessler DO, Shah VP. Prospective application of clinician‐performed lung ultrasonography during the 2009 H1N1 influenza A pandemic: distinguishing viral from bacterial pneumonia. Crit Ultrasound J. 2012; 4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zorc JJ, Hall CB. Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 2010; 125:342–349. [DOI] [PubMed] [Google Scholar]
- 19. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014; 134:e1474–e1502. [DOI] [PubMed] [Google Scholar]
- 20. Okada M, Ogawa T, Kubonoya H, Yoshizumi H, Shinozaki K. Detection and sequence‐based typing of human adenoviruses using sensitive universal primer sets for the hexon gene. Arch Virol. 2007; 152:1–9. [DOI] [PubMed] [Google Scholar]
- 21. Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007; 24:1596–1599. [DOI] [PubMed] [Google Scholar]
- 22. Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012; 29:1969–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khadadah M, Essa S, Higazi Z, Behbehani N, Al‐Nakib W. Respiratory syncytial virus and human rhinoviruses are the major causes of severe lower respiratory tract infections in Kuwait. J Med Virol. 2010; 82:1462–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Calder JAM, Erdman DD, Ackelsberg J, et al. Adenovirus type 7 genomic‐type variant, new York city, 1999. Emerg Infect Dis. 2004; 10:149–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lebeck MG, McCarthy TA, Capuano AW, et al. Emergent US adenovirus 3 strains associated with an epidemic and serious disease. J Clin Virol. 2009; 46:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neumann R, Genersch E, Eggers HJ. Detection of adenovirus nucleic acid sequences in human tonsils in the absence of infectious virus. Virus Res. 1987; 7:93–97. [DOI] [PubMed] [Google Scholar]
- 27. Garnett CT, Erdman D, Xu W, Gooding LR. Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J Virol. 2002; 76:10608–10616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alkhalaf MA, Guiver M, Cooper RJ. Prevalence and quantitation of adenovirus DNA from human tonsil and adenoid tissues. J Med Virol. 2013; 85:1947–1954. [DOI] [PubMed] [Google Scholar]
- 29. Drews AL, Atmar RL, Glezen WP, Baxter BD, Piedra PA, Greenberg SB. Dual respiratory virus infections. Clin Infect Dis. 1997; 25:1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Franz A, Adams O, Willems R, et al. Correlation of viral load of respiratory pathogens and co‐infections with disease severity in children hospitalized for lower respiratory tract infection. J Clin Virol. 2010; 48:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harada Y, Kinoshita F, Yoshida LM, et al. Does respiratory virus coinfection increases the clinical severity of acute respiratory infection among children infected with respiratory syncytial virus? Pediatr Infect Dis J. 2013; 32:441–445. [DOI] [PubMed] [Google Scholar]
- 32. Lu Y, Wang S, Zhang L, et al. Epidemiology of human respiratory viruses in children with acute respiratory tract infections in Jinan, China. Clin Dev Immunol. 2013; 2013:210490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sentilhes A‐C, Choumlivong K, Celhay O, et al. Respiratory virus infections in hospitalized children and adults in Lao PDR. Influenza Other Respi Viruses. 2013; 7:1070–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tecu C, Mihai ME, Alexandrescu VI, et al. Single and multipathogen viral infections in hospitalized children with acute respiratory infections. Roum Arch Microbiol Immunol. 2013; 72:242–249. [PubMed] [Google Scholar]
- 35. Essa S, Owayed A, Altawalah H, Khadadah M, Behbehani N, Al‐Nakib W. Mixed viral infections circulating in hospitalized patients with respiratory tract infections in kuwait. Adv Virol. 2015; 2015:714062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beby‐Defaux A, Maille L, Chabot S, Nassimi A, Oriot D, Agius G. Fatal adenovirus type 7b infection in a child with Smith‐Lemli‐Opitz syndrome. J Med Virol. 2001; 65:66–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nguyen HL, Ruxrungtham K, Delaugerre C. Genetic barrier to the development of resistance to integrase inhibitors in HIV‐1 subtypes CRF01_AE and B. Intervirology. 2012; 55:287–295. [DOI] [PubMed] [Google Scholar]
- 38. Lupo J, Schuffenecker I, Morel‐Baccard C, et al. Disseminated rhinovirus C8 infection with infectious virus in blood and fatal outcome in a child with repeated episodes of bronchiolitis. J Clin Microbiol. 2015; 53:1775–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hong JY, Lee HJ, Piedra PA, et al. Lower respiratory tract infections due to adenovirus in hospitalized Korean children: epidemiology, clinical features, and prognosis. Clin Infect Dis. 2001; 32:1423–1429. [DOI] [PubMed] [Google Scholar]


