General principles in biology have often been elucidated from the study of bacteria. This is true for the bacterial mechanosensitive channel of large conductance, MscL, the channel highlighted in this review. This channel functions as a last-ditch emergency release valve discharging cytoplasmic solutes upon decreases in osmotic environment.
KEYWORDS: drug targets, ion channels, mechanosensitive channels, membrane biophysics, membrane channel proteins, membrane transport, osmoregulation, physiology
SUMMARY
General principles in biology have often been elucidated from the study of bacteria. This is true for the bacterial mechanosensitive channel of large conductance, MscL, the channel highlighted in this review. This channel functions as a last-ditch emergency release valve discharging cytoplasmic solutes upon decreases in osmotic environment. Opening the largest gated pore, MscL passes molecules up to 30 Å in diameter; exaggerated conformational changes yield advantages for study, including in vivo assays. MscL contains structural/functional themes that recur in higher organisms and help elucidate how other, structurally more complex, channels function. These features of MscL include (i) the ability to directly sense, and respond to, biophysical changes in the membrane, (ii) an α helix (“slide helix”) or series of charges (“knot in a rope”) at the cytoplasmic membrane boundary to guide transmembrane movements, and (iii) important subunit interfaces that, when disrupted, appear to cause the channel to gate inappropriately. MscL may also have medical applications: the modality of the MscL channel can be changed, suggesting its use as a triggered nanovalve in nanodevices, including those for drug targeting. In addition, recent studies have shown that the antibiotic streptomycin opens MscL and uses it as one of the primary paths to the cytoplasm. Moreover, the recent identification and study of novel specific agonist compounds demonstrate that the channel is a valid drug target. Such compounds may serve as novel-acting antibiotics and adjuvants, a way of permeabilizing the bacterial cell membrane and, thus, increasing the potency of commonly used antibiotics.
INTRODUCTION
Mechanosensitive channel activities were discovered in bacterial cell membranes in 1987 (1). Since that time, researchers have defined the physiological function of some of these channels, constructed structural models, and determined the features responsible for sensing and responding to stimuli.
The channel in the focus of this review, the mechanosensitive channel of large conductance, MscL, appears to play a consistent role in every species in which it has been studied: a biological emergency release valve used to prevent cell lysis when exposed to extreme decreases in the osmotic environment. However, the findings from many studies of this channel seem to go far beyond explaining the mechanisms of this single channel and may give insight into several other channel families.
Evolutionarily, channels and sensors appear to have common gene ancestors and, thus, share common mechanisms and features. One study even found a consensus motif seen in many channel families, N-h-h-D, where “h” letters are hydrophobic amino acids (2); indeed, it appears that this motif plays important functional roles in many channels (3), including MscL (2, 4). These and many other findings led researchers to propose MscL as a paradigm for mechanosensitive channels as well as channels gated by other means.
While conventional high-tech approaches such as X-ray crystallography have been applied for the study of MscL, as we describe in this review, many of the significant findings, and resolutions of disputes in the field, were produced by low-tech basic and traditional bacteriological genetic, physiological, and in vivo approaches. We propose that such methods could be used for the numerous other putative channels whose genes were discovered in the bacterial and archaeal kingdoms by genome sequencing and whose protein products subsequently may have been used by structural biologists seeking high-resolution structures.
Finally, the physiological and mechanistic studies of MscL have also led researchers to speculate on alternative applications for these channels in the medical field, including for enhanced or “smart” magnetic resonance imaging contrast or drug-targeting nanodevices and as a drug target for potentially novel antibacterial reagents or adjuvants.
Here, we review the history, physiology, structure, molecular mechanisms, potential medical applications, and prospects of the bacterial MscL channel, and we draw attention to areas where MscL may give insight into the molecular mechanisms of other channels.
A BRIEF HISTORY OF MECHANOSENSITIVE AND BACTERIAL CHANNELS: CORRELATING PATCH CLAMP WITH PHYSIOLOGY
Channels, Mechanosensitive Channels, and the Tether Hypothesis, a Historical Perspective
The first intracellular electrical recordings from a living organism were obtained from a microbe. In 1934, the protozoon Paramecium was impaled by a glass microelectrode to record the resting membrane potential (5). This work predates the classic experiments of Hodgkin and Huxley in 1945 on the resting and action potentials in the squid giant axon (6), which eventually won them the Nobel Prize in Physiology or Medicine in 1963. The next major advancement in electrophysiology was the development of the patch clamp by Neher and Sakmann in 1976 (7), for which they were awarded the Nobel Prize in 1991. Patch clamping allowed for the accurate measurement of the electrical activity of single channels, the probability of channel opening, the current per channel opening (including partial openings or “substates”), the length of time of channel opening, and the duration between channel openings. By the 1980s and 1990s, this approach was employed by researchers around the world and led to breakthroughs in understanding channel physiology, including mechanosensitive (MS) channels.
By the 1980s, it was well known that many senses were based in sensing mechanical forces; these included touch, hearing, balance, and even aspects of cardiovascular regulation. Thus, it was inevitable that MS ion channels would be identified by patch clamp in some system. Indeed, the first mechanosensory activities were measured in 1983 and 1984 (8–10). With some assumptions and mathematical modeling, it was postulated in 1986 that an MS channel should concentrate energy from a large area of membrane (ca. 500-nm diameter), and this observation was interpreted to indicate that the channel must be extremely large or in series with a component of the cytoskeleton (11), a model that would later be called the tether hypothesis for MS channel gating. Several years later, genetic experiments with Caenorhabditis elegans found several alleles associated with development and function of mechanosensory neurons (12). Consistent with the tether hypothesis of MS channel gating, a stomatin-like protein (13), as well as transmembrane proteins thought to contribute to an MS channel (14), were among the proteins predicted to be encoded by the genes identified; a model was proposed that the proteins were linked together, with the stomatin-like protein serving as a tether. Were the assumptions that led to the tether hypothesis correct, and could there be an alternative? Bacterial channels were destined to provide the surprising answer.
The Discovery of Bacterial Mechanosensitive Channels
It was within this historical context that Ching Kung’s group in Madison, Wisconsin, patch-clamped native bacterial membranes. Using an approach to make giant cells, or spheroplasts, that had been described previously (15) (and has subsequently been modified and standardized [16]), patchable-sized cells were generated from Escherichia coli (1). Because the electrodes and tubing attached are hollow, suction could be applied by mouth or syringe to generate negative pressure and thereby tension in the patch. Using this as a stimulus, surprisingly, the major channel activity observed was mechanosensitive, opening in response to suction. A subsequent study demonstrated that these channels could also be activated by amphipaths (17), suggesting that they were sensing changes in their lipid environment. Studies in other bacterial species suggested that MS channels are common in both Gram-negative and -positive organisms (18–22). A subsequent study in E. coli revealed two types of mechanosensitive activities; the second required a larger stimulus than had been previously applied (23). This less sensitive channel opened a larger pore but for a shorter amount of time and was eventually named the mechanosensitive channel of large conductance (MscL), while the initially observed smaller one was named MscS, S for small conductance. The channel conductance is a function of the size and shape of the channel open pore, and these are truly large conducting channels, 3.6 nS for MscL and 1.0 nS for MscS, which is 1 to 2 orders of magnitude larger than that of most eukaryotic channels. These were not porin-like activities, and the channels appeared to be in the cytoplasmic membrane (24).
We now know that most species of bacteria contain one highly conserved gene copy of mscL (25). On the other hand, the MscS channel family is diverse, paralogues are common, and some species contain members with cyclic nucleotide binding sites (26–28). Several paralogues of MscS exist even in E. coli; these include MscM for miniconductance (29), MscK for K+ regulated (30), and others not observed by patch clamp at normal expression levels (31); they play little role in survival from the normal osmotic downshock protocols, but some changes have been noted when the rate or magnitude of the shock is altered (32). In contrast to MscL, which is only found in bacteria and some fungi and oomycetes (33), the MscS family extends all the way into the plant kingdom (34–36). Several structural models for MscS exist (37–42), and a mechanistic model for E. coli MscS gating has recently been proposed: filling of a lipid pocket in the protein appears to stabilize the closed structure while the removal of the lipids upon membrane tension leads to gating (42–45), a model not unlike that proposed for a eukaryotic K2P mechanosensitive channel, TREK-2 (46). A similar model has also been proposed for MscL (47); however, there are three limitations to the study: first, the Mycobacterium tuberculosis channel was studied in lipids that did not contain phosphatidylinositol, which have been shown to be necessary for normal function for this homologue (48); second, the conclusions relied largely on one mutational site (L89) with no other sites, contributing to a “pocket”; and third, modification of the equivalent site in the E. coli channel (M94), or even any residues surrounding it, with bulky hydrophobic R groups do not appear to have the same effect on channel function (49). However, the study does support the notion that significant modifications in protein-lipid interactions occur upon channel thinning within the membrane prior to and during gating (discussed below).
The study that first reported MscL activity also demonstrated that both the MscL and MscS activities survived solubilization with detergent, enrichment over a size exclusion column, and reconstitution into purified lipids. The activities were found in different fractions, demonstrating that the channel activities were the products of at least two distinct entities (23). This also suggested patch clamp as an assay for the purification of the channel. This extremely laborious approach, which required multiple enrichments using columns with different fractionation properties and the identification and pooling of channel activity-enriched fractions, was in fact performed for the enrichment of MscL. To our knowledge, this approach has never been used since, but it did work and was fundamental to the development of the field by allowing the purification and microsequencing of the N terminus of the protein (50). At the time, the E. coli genome sequencing was far from complete, but a gene called trkA had been sequenced, and the 5-prime portion of the neighboring gene had incidentally also been sequenced and matched the MscL protein N-terminal sequence. These data indicated the location on the chromosome of the mscL gene and allowed for the cloning and sequencing of mscL. Knocking out the gene, subsequently expressing it in trans, and expressing it heterologously in yeast membranes all confirmed the gene encoded the MscL activity (50). Later it was shown that single-residue substitutions changed the gating properties of the channel (51), confirming mscL as the pore-forming gene. The mscL gene was the first from any organism to be definitively shown to encode a mechanosensitive channel activity.
The Tether versus the Force-from-Lipid Hypothesis
The results from studies of the bacterial MS channels did not fit the tether hypothesis. MscL was a relatively small protein (ca. 15 kDa). The migration of the solubilized channel in the size exclusion column suggested that the complex contained a few identical subunits, but it was still much smaller than predicted from the tether model. Channel activity did not require tethering proteins: all that was needed was purified protein and lipids (52, 53). These findings, along with the sensitivity of bacterial MS channels to amphipaths (17) and lipid-like molecules that add stresses in the membrane (54), suggested that MscL sensed the lateral pressure profiles within the lipid membrane. Consistent with the latter notion, one comprehensive study determined that the stimulus that gates MscL is tension in the membrane rather than either the pressure across it or the curvature within it, and no specific lipid headgroup was essential for E. coli MscL channel activity; it even worked in phosphatidylcholine lipids, which are not synthesized by this organism (55). This notion, that an MS channel can sense and respond to the changes of lateral pressures within the membrane, has now evolved into the force-from-lipid (FFL) hypothesis. The FFL hypothesis appears to be true not only for bacterial MS channels but also for the majority of eukaryotic MS channels (56–60), including TRAAK and TREK-1 (61, 62), TREK-2 (46), Piezo1 (63, 64), and OSCA (65); even channels not gated by membrane tension are often modulated by it, presumably because they increase (or decrease) their external radius in the membrane; examples include the voltage-gated shaker K+ channel (66–69) and the ligand-gated NMDA receptors (70). See Figure 1 for a graphical comparison of the tether and force-from-lipid models.
FIG 1.
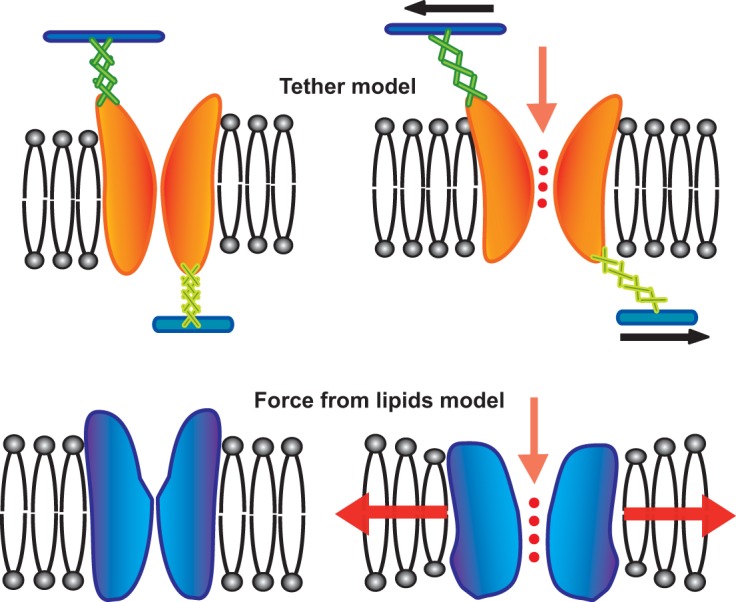
Two opposing models for gating mechanosensitive channels. (Top) In the tether model, the channel is tethered to the cytoskeleton and/or the extracellular matrix or cell wall, and it is the force from these tethers that opens the channel. (Bottom) In the force-from-lipids, or FFL, model, the channel directly senses biophysical changes in the membrane lipid bilayer, and it is these forces that gate the channel.
To put the identification of MscL into historical context, at about the same time mscL was cloned, kch from E. coli (71) and kcsA from Streptomyces lividans (72) were identified; the latter channel would be the first channel resolved by X-ray crystallography and helped to define how a channel can be K+ specific, a scientific leap that would earn the Nobel Prize in Chemistry in 2003. However, the physiological functions of these channels, as well as many others that have been identified from the genomic sequencing of bacterial species, remain mysterious. On the other hand, ion fluxes recently have been implicated in electrical spiking in E. coli, as determined by a fluorescent voltage-indicating protein (73), in electrical communication of B. subtilis communities discovered by using a fluorescent cationic dye (74), and Ca2+ fluxes observed in E. coli by using an ion-specific dye (75); in none of these instances have the channels ultimately responsible for the behavior been identified. The linking of channel genes with physiological phenomena is a frontier waiting to be conquered.
Defining the Physiological Function of Bacterial Mechanosensitive Channels
To our knowledge, the bacterial MS channels remain the only bacterial channels with a well-defined physiological function. However, the path to firmly demonstrate their function was meandering. At the time of their discovery, bacterial MS channels were speculated to play a role in osmotic regulation. In high-osmolarity environments, compatible solutes such as proline, betaine, trehalose, and potassium accumulate (often close to 1 M concentration) within the bacterial cytoplasm to keep cell turgor high (for E. coli, it is estimated to be 2 to 4 atmospheres [76], but see also reference 77). Upon an acute decrease in osmolarity, called downshock, the cytoplasmic pressure can theoretically reach more than 10 atmospheres (see references 78–80 for reviews of bacterial osmotic regulation), but before cell lysis, the solutes are rapidly released (Fig. 2). In the older literature, efflux was thought to be due to multiple solute-specific channels (81, 82). After their discovery, the MscS activity and recently cloned MscL channel seemed likely candidates, although they seemed more likely to be size rather than solute selective. Unfortunately, the mscL null strain had no obvious phenotype, and it was speculated that this was due to a redundancy of function with MscS. On the other hand, early mutagenesis studies did demonstrate that changes to the MscL protein could lead to osmotic phenotypes (83). Marine organisms often do not contain MscL (84), presumably because they do not see osmotic shifts. One early study showed that the expression of E. coli MscL in trans in the MscL-lacking Vibrio alginolyticus marine bacterium alleviated the osmotic downshock cell lysis normally observed for this organism (85). These data suggested MscL truly did play a role in osmoregulation. The last nail was finally driven into the coffin when the gene responsible for MscS channel activity was identified and an mscS mscL double null was found to have an osmotic-sensitive phenotype, decreasing viability upon osmotic downshock (86). As shown in Fig. 2, we now have firm evidence for MscS and MscL playing the major role in adapting to acute, rapid decreases in osmotic environment by allowing fast efflux of accumulated osmoprotectants and other cytoplasmic solutes.
FIG 2.
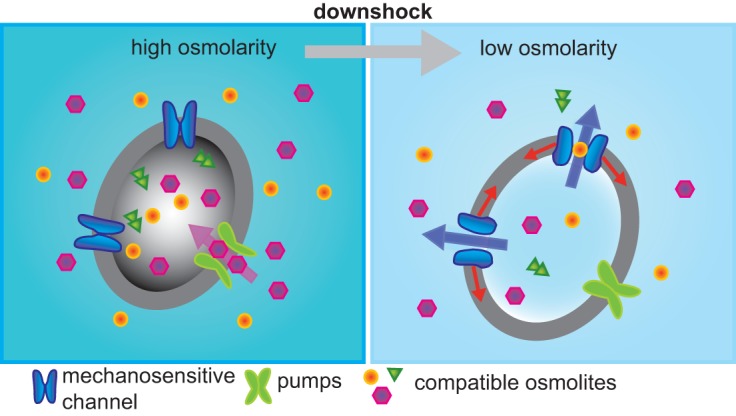
Bacterial adaptations to osmotic environment. (Left) At high osmolarity, bacteria accumulate osmoprotectants by either pumping them in or synthesizing them. (Right) Upon an osmotic downshock, the cell swells, membrane tension increases, and osmoprotectants and other solutes rapidly efflux from the cell, restoring osmotic equilibrium.
MscL CHANNEL STRUCTURAL STUDIES
Computational analysis of the MscL protein predicted that the channel possessed two helical transmembrane domains (TM1 and TM2). Both transmission Fourier transform infrared spectroscopy and circular dichroism (CD) spectra indicated that the protein is highly helical in detergents as well as in liposomes (87). The topology was determined by the genetic strategy of making fusions to the alkaline phosphatase gene phoA. PhoA requires modification in the periplasm to achieve a mature form and, thus, is inactive unless transported across the cytoplasmic membrane (88). A gene fusion to a phoA gene lacking its signal sequence for secretion will only yield active PhoA protein if the sequence preceding the phosphatase gene is transmembrane with the junction located in the periplasm. Hence, PhoA insertions have been used extensively to study the topology of membrane proteins, including those involved in transport (89–91). When the approach was applied to MscL, the data were consistent with the two-transmembrane-domain hypothesis, with the C and N termini both being cytoplasmic (53).
A breakthrough came when the MscL channel from Mycobacterium tuberculosis was crystallized (92) and subsequently further evaluated to resolve the N terminus of the protein (93) (Fig. 3) (PDB code 2OAR).
FIG 3.

Structure of M. tuberculosis MscL derived from X-ray crystallography. (A) MscL pentamer shown from a periplasmic view (left) and a side view (right) in which the approximate membrane location is indicated by gray lines. (B) A single isolated subunit is represented in which different MscL domains are indicated: N-terminal helix, called S1 helix (green), first transmembrane domain TM1 (blue), periplasmic loop (cyan), second transmembrane domain TM2 (red), and C-terminal helix (orange).
The MscL structure helped to resolve two issues in the field. The first was the structure and function of the N-terminal region of the protein, called S1. One earlier study suggested that this region was folded in a helical bundle that formed a “second gate” of the channel (94). However, the resolved structure of S1 clearly showed an α-helix running parallel to the cytoplasmic membrane surface (93), consistent with its amphipathic nature. A subsequent study of this region (95) utilized multiple approaches, including cysteine scanning, in vivo functional characterization, in vivo SCAM (substituted cysteine accessibility method [96]), which had previously been used with other regions of MscL (97, 98), electrophysiological studies, and in vivo disulfide-trapping experiments (95), and the data from these assays are consistent with the S1 helix being parallel to the cytoplasmic surface of the membrane. Furthermore, a molecular dynamics study performed at about the same time used the S1-resolved structure, assessed movements of this and other regions (99), and came to similar conclusions. These studies, using multiple approaches, supplied overwhelming evidence that S1 was indeed an amphipathic helix running along the cytoplasmic membrane, resolved the issue; it has even been further supported by subsequent studies using other approaches (49, 100, 101). Remarkably, in one study one mutant, K5C, was found not to be functional by patch clamp after it was solubilized and reconstituted (101), but K5C was functional when studied in native membranes (95). This discrepancy is presumably due to the channel losing function upon solubilization/reconstitution, suggesting that experiments performed closer to in vivo conditions better reflect true physiology. In summary, overwhelming evidence now shows that S1 is not a second gate but a “slide helix” running along the cytoplasmic membrane, stabilizing the TM1 domain, similar to the slide helix studied in the bacterial KirBac channel (102, 103), and indicating the function of slide helix-like structures observed in several other channels (see reference 95 for a discussion).
The second puzzle that solving the MscL structure helped to answer was the stoichiometry of the channel. An early study proposed that subunits in vivo were largely monomers, and this led the authors to suggest that generating an open pore was solely dependent upon assembly, which occurred only upon membrane stretch, as some bacterial toxins generate a pore upon assembly (104). However, this interpretation relied heavily on negative results, i.e., the inability to cross-link the channels efficiently in vivo. Other findings suggested the MscL channel was a homohexamer (53, 105). However, the resolved crystal structure clearly showed a pentamer. Another study strongly supported the latter stoichiometry (106). Thus, the channel was assumed to be a pentamer, that is, until another crystal structure of the Staphylococcus aureus MscL was solved and observed as a tetramer in what was interpreted as a partial-open state (107). The structure, however, did not fit the wealth of the mutagenic and other data. Moreover, a subsequent study suggested that in detergents multiple oligomeric structures of MscL could exist (108), further confusing the issue. However, disulfide trapping approaches where the disulfide bridging occurred in vivo, as well as several other approaches, made it clear that the vast majority, if not all, of the MscL channels are pentamers (109, 110). It appears that when solubilized with detergents, especially lauryldimethylamine oxide (LDAO), the channel can reassemble into different multimeric structures, but in vivo and under physiological conditions the channel is a pentamer. These conflicting results emphasize a need for the verification of all findings, including undisputed structures, by independent approaches, and also for using methods that are as close to the physiological in vivo conditions as practical. Testing hypotheses using functional studies is fundamental for elucidating accurate models.
FUNCTIONAL-STRUCTURAL STUDIES IN E. COLI
The Largest Gated Pore
The fully open pore of the MscL channel is huge. The first clue for this was that the conductance of the channel electrophysiologically is 3.6 nS, which is, as we mentioned above, about 2 orders of magnitude larger than most eukaryotic channels. The predicted pore size is 25 to 30 Å in diameter, as determined by molecular sieving experiments (111) and Förster resonance energy transfer (FRET) spectroscopy (112). To put this in perspective, this channel forms a pore larger than either OmpC or OmpF, in the outer membrane, which are on the order of 10 to 12 Å (113). Flux studies confirmed that the MscL pore was truly large and allowed the efflux of functional proteins and metabolites. Thioredoxin, elongation factor Tu, and DnaK have all been shown to be fluxed out of cells in an MscL-dependent manner upon osmotic downshock (114, 115). Some studies, using alternative approaches, have disputed exactly what does and does not exit from the cell upon osmotic downshock (116, 117), but one study suggests these discrepancies are simply due to the protocols used in the studies (118). Note that given the proportions of the channel pore, MscL must be very tightly regulated, and the controls for opening and closing are deeply rooted in its physiological function: keep shut when the osmolarity does not fluctuate and open rapidly when the cells are challenged by a sudden osmotic downshift.
Early Forward Genetics and the Pore
One of the first functional studies of the MscL channel involved a forward genetics approach (4). The mscL gene was placed in a plasmid under the transcriptional control of the lacUV5 promoter. The open reading frame was randomly mutagenized, and cells were replica plated without and then with isopropyl β-d-1-thiogalactopyranoside (IPTG), which induced mscL expression. Cells whose growth was compromised when the mutated MscL was expressed were identified. Analyses showed that these growth-defective bacteria leaked K+ and contained MscL channels more sensitive to mechanical forces, as determined by patch clamp (4). Many of the mutations leading to slowed-growth phenotypes were found in the first transmembrane domain, and many of them were mutations to more hydrophilic residues, including charged amino acids. After the structure of the M. tuberculosis MscL channel was resolved later that year (92), it became clear that many of the mutations were in the pore constriction point, and a model was proposed of a hydrophobic lock within the pore and vestibule (119). According to the model, the energy barrier to gating the channel was the transient exposure of hydrophobic TM1 residues to the aqueous environment. Making these residues more hydrophilic leads to channels that are more sensitive to membrane tension (119); simply stated, protein interactions break before the lipids tear apart. This hypothesis has been supported and refined by experiments in which charges were introduced at different locations within the pore/vestibule, and the number of charges needed to significantly change channel sensitivity were estimated (120, 121). At least one study suggests that membrane tension changes are still required to fully open the channel, even in the presence of charges in the pore (122). Modifications have even been made in vivo using a unique approach in which cysteine mutants are posttranslationally modified with thiol reagents, thereby adding side chains with different properties (charges or hydrophobic structures) at specific positions and assessing their influence on growth or other aspects of physiology (49, 95, 123).
How the Channel Opens: Speculations and Resolutions
Soon after the crystal structure was resolved, researchers were beginning to speculate on models for the structure of the MscL channel open state. To accommodate such a large pore size, huge structural changes must occur. An early model predicted that the channel thins in the plane of the membrane and opens by separating the TM1 domains like the iris of an old-fashion camera (94, 124); this is opposed to the notion that the channel expanded and all 10 of the transmembrane domains (TM1 and TM2 from 5 subunits) contributed to the pore. Under tension, membranes can compress only a few percent prior to rupture (125, 126), but it appears that MscL can compress and, thus, expand within the membrane prior to and during channel gating, restructuring the surrounding lipids and changing protein-lipid interactions (47). The tilting occurs prior to the iris opening, leading to a closed-expanded state (94, 124, 127, 128). This iris model fit many of the mutagenesis studies of the time, including the observation that the random mutagenesis study discussed above found TM1 was a hot spot for mutations causing modified gating, with only a few mutations occurring in TM2 (4). The model was further supported by structural studies using electron paramagnetic resonance and FRET spectroscopy combined with site-directed spin and fluorophore labeling, respectively (112, 129, 130).
There were indications of a twisting movement of the TM1 domain as well. For example, some studies involving disulfide trapping could only be explained if there were asymmetric movements or corkscrewing of the TM1 domains (131, 132). There are now numerous studies, using multiple approaches, supporting a corkscrewing movement of TM1 of probably over 100° in a clockwise direction, as observed from the periplasm (95, 97, 100, 129, 133, 134), with one study suggesting that this rotation occurs early in the gating process, before ion permeation (133).
More recently, two crystallographic structures of MscL from Methanosarcina acetivorans (Ma-MscL) have been resolved. They appear to reflect a closed and an expanded-intermediate state (PDB codes 4Y7K for the closed state and 4Y7J for the expanded state) (Fig. 4) (135). When these structures are compared with each other, the tilting of the TM domains and the beginnings of the corkscrewing movement of TM1 are clearly observed in the more open structure. Thus, multiple lines of evidence support the tilting of both TM domains and the rotation of TM1 tangential to the plane of the membrane, and it seems likely that the S1 slide helix stabilizes the protein as these movements occur (59).
FIG 4.

Structural changes during MscL gating determined by X-ray crystallography. Ma-MscL models derived from X-ray crystallography, showing the channel in closed (left) and partially open (right) states, shown from the side (top) and from the periplasm (bottom). Approximate membrane sites are shown as gray bars in the top panels; note the thinning of the protein and surrounding lipids in the top-right panel.
The periplasmic loop between the TM1 and TM2 domains (Fig. 3), which appears to play the role of a spring element for the channel, is among the least conserved regions in MscL. Early work showed that enzymatic digestion of the periplasmic loop, or the reconstitution of separately synthesized TM1 and TM2 domains, led to channels that retained their mechanosensitive nature, but they were more sensitive to tension (136, 137). These studies suggested that the periplasmic loop tuned the mechanosensitivity level of the channel. Further modifications (49, 138) and the use of chimeras (139, 140) were consistent with this hypothesis. One study found that the periplasmic loop is a major spring that can control open dwell time of the channel (140). It also found that mutations in the loop can lead to gating hysteresis, i.e., when closed resisting opening and when open resisting closure. This observation led to a proposal that the region can act like a resistor in a “clasp knife” (140).
A cluster of polar and charged residues at the cytoplasmic end of TM2 is an interesting feature in MscL. In one study, a residue that seems cytoplasmic in the closed state (N103 in the E. coli MscL channel), located just a few amino acids away from the proposed lipid-aqueous interface (141–145), was found to alternate between the membrane and the cytoplasmic membrane-aqueous interface upon gating (146). A series of charged residues further cytoplasmic to this region have been shown to be critical for channel function (51). Taken together, the data described above led to what was referred to as the knot-in-the-rope hypothesis. According to this model, the charged residues cytoplasmic to N103 are thought to stabilize the movement between the lipid-aqueous interface, inhibiting additional residues from going into the membrane and, thus, preventing unwanted structural changes (146). The reconstitution of MscL orthologues and their chimeras in defined lipids complemented this finding by showing that one residue, K101, just prior to N103 and the knot in the rope, appeared to interact with negatively charged lipid headgroups and played a role in setting the mechanosensitivity of the protein and the stability of the open state (147). Thus, it appears that charges in this region play roles both in interacting with lipids to modify gating properties and in fine-tuning conformational changes upon gating; these functions are somewhat analogous to that of the S1 domain stabilizer for TM1. Figure 5 shows a schematic of a single subunit within the complex, based on the Ma-MscL structures. Movements of S1, TM1, and TM2, as well as the knot in the rope at the cytoplasmic end of TM2, are illustrated. Finding charged residues at a TM domain-cytoplasmic interface is not uncommon in channels. This is also observed in the TRP and K2P eukaryotic channel superfamilies, including those members that are thought to be mechanosensitive (146). Hence, both S1 and the knot in the rope may share a function with several other channels with similar structural motifs.
FIG 5.
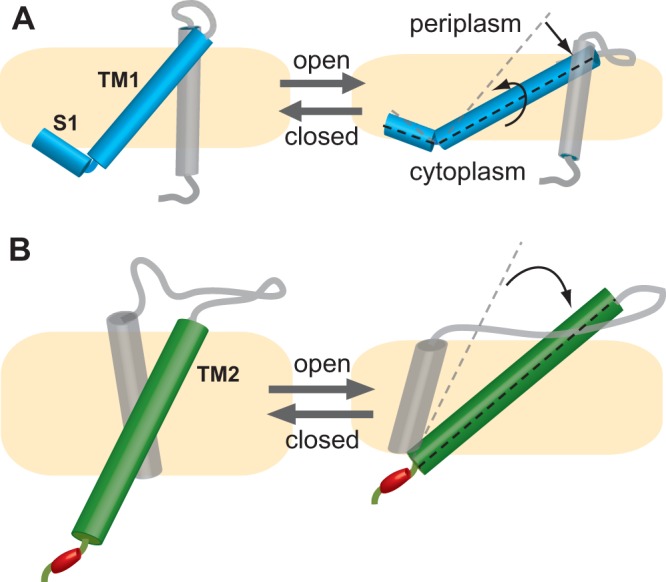
Schematic of a single subunit and its movements upon gating based on the Ma-MscL structures. (A) The movements of S1, which is parallel to the cytoplasmic surface of the membrane, and TM1 are shown (blue). Upon channel opening, TM1 tilts in the membrane and corkscrews within it in a clockwise rotation when observed from the periplasmic side. (B) The tilting of TM2 (green) is shown. The charged residue cluster (red), sometimes called the knot in the rope, at the cytoplasm is thought to prevent unwanted structural movements of TM2 into the membrane upon gating. Due to the tilting of both TM1 and TM2, the membrane (yellow) thins within the plane of the membrane in the open structures.
The C-terminal region of the MscL protein ends in a helical bundle (Fig. 3). This region of the protein appears to stay at least partially intact upon channel gating (148). Subsequent studies have upheld this notion (149) and shown that shortening or constraining the loop between TM2 and the cytoplasmic bundle can influence the conductance of the pore, which may have implications for using the channel in nanotechnological devices (150). Together, these studies imply that solutes must pass through the five portals formed between the loops connecting TM2 and the C-terminal bundle; permeation through cytoplasmic portals is yet another recurring theme for channels and is also observed for the bacterial MscS channel (40, 151), as well as several eukaryotic channels (152–158).
A recent study suggests the MscL C-terminal bundle also plays a role in determining mechanosensitivity as well as conductance (159); this study was performed using the M. tuberculosis MscL. Functional studies in additional bacterial species have given insight into the physiological role MscL plays in different bacteria, as discussed below.
FUNCTIONAL STUDIES IN OTHER BACTERIAL SPECIES
If MscL is an MS channel that truly plays a role in osmoregulation in E. coli, then this function is likely to be conserved among bacterial species. Indeed, a number of homologues from other species, heterologously expressed in E. coli, have channel activities as assayed by patch clamp (160, 161), although some were less mechanosensitive or had different kinetics for gating. Ironically, the M. tuberculosis MscL channel, which was the first to be crystalized (described above) (Fig. 3), was nonfunctional at physiological ranges when heterologously expressed in E. coli (161). A later study demonstrated that the M. tuberculosis MscL channel requires the lipid phosphatidylinositol, which is produced in Mycobacterium but not in most other bacterial species, to gate at normal tensions (48).
MscL-null strains have also been generated and studied in a number of species. A relatively early study showed not only that the MscL channel in Lactococcus lactis was an MS channel that played a role in osmoregulation but also that in this organism MscL is the main MS solute release system that protects the cells under conditions of osmotic downshock (162).
A study of the Bacillus subtilis MscL showed that it was expressed at measurable levels at logarithmic growth, during which it played a significant role in surviving osmotic downshock (163). One study suggests that MscL shunts efflux of other proteins that otherwise would be secreted (164). While MscL seems to play a major role in protection against osmotic downshock, MscS-like proteins have also been shown to contribute (165, 166). MscL expression increased 1.5-fold in high-salt medium but dropped beginning in late log and during sporulation (163). This contrasts with results in E. coli, where MscL is expressed at higher levels in stationary cultures and appears to be regulated by RpoS (167). Although some MscL was found in the B. subtilis spore, the MscL null, as well as MscS-like nulls, showed no deficiencies in sporulation or subsequent spore germination (163).
MscL channels have also been studied in Vibrio cholerae (168). Measurements suggest that MscL is present at a density three times higher than that in E. coli, yet the organism is less tolerant to large osmotic downshocks. Interestingly, when assayed by patch clamp, the addition of trehalose, a compatible solute, significantly decreases the sensitivity of both V. cholerae and E. coli MscL channels. However, it is important to note that trehalose has been shown to efflux from the cytoplasm upon osmotic downshock in an MscL-dependent manner (114). Whether trehalose plays a modulatory or regulatory role in MS channels in vivo, and what that role may be, has yet to be determined.
Pseudomonas aeruginosa has also been studied (169). The authors used a stopped flow, allowing them to better measure the kinetics of efflux upon downshock. P. aeruginosa showed faster osmolyte release rates during large osmotic dilutions than E. coli, which correlated with better survival for the former. P. aeruginosa also exhibited a more gradual response that is dominated by MscL-type channels. MscS channels from P. aeruginosa were also characterized and found to strongly inactivate. The authors therefore concluded that this organism relies primarily on MscL for defining a rapid release upon downshock, but the MscS channels may be responsible for terminating more massive permeability responses.
In sum, studies of different species reveal some general properties of the MscL channel. First, MscL is the more relied-upon channel for surviving osmotic downshock, while the MscS channel family, when involved in osmoregulation, is for smaller shocks or modulating the MscL response. Second, MscL seems to serve the common function of an osmotic emergency release valve in all organisms in which it is observed; no one has yet found another role for the MscL protein, not even in sporulation or spore germination (163).
POTENTIAL APPLICATIONS IN NANOTECHNOLOGY AND NANODEVICES
The huge pore size of the MscL channel has tempted researchers to consider engineering this biological channel for nanotechnological devices. For example, the MscL channel has been placed in a microchip to potentially allow future screening of chemicals or for other purposes (170, 171). It has also been reconstituted as a potential signaling pathway in compartmentalized artificial cells (172). MscL has been expressed in cells for controlled delivery of bioactive molecules (173). It has even been used as a pore for DNA passage and designing potential nano-DNA-sequencing devices (174, with erratum [175]). Finally, MscL has been shown to gate in response to ultrasound when expressed in primary cultures of rat hippocampal neurons (176) but not when expressed in liposomes (177); this discrepancy is due either to the use of a mutant in the former study, or, perhaps more likely, to additional tension in the membrane due to cytoskeletal and extracellular elements pushing and pulling on the membrane when in a cellular environment.
Researchers have also investigated the possibility of changing the stimulus that activates MscL. In one instance, the reversible activation of the MscL channel was achieved via a light-sensitive lipid mimic (178). Briefly, MscL from L. lactis and E. coli were reconstituted in lipid bilayers composed of light-sensitive lipids. Light-induced isomerization of an azobenzene moiety of 4-Azo-5P on one of the lipids from trans to cis was used to add stresses in the membrane and activate MscL (178). However, most studies modified the MscL channel directly to change its modality. As discussed above, random mutagenesis studies demonstrated that a charge in the pore can gate the channel inappropriately (4). Using this observation, given the critical placement within the pore of reactive chemicals, has allowed researchers to engineer channels that are actuated by light (179) and pH (180) and even modulated by magnetic force (181). The pH-triggered MscL-based nanovalve may be of special interest, given that many malignant and inflamed tissues decrease the pH of their immediate environment. Thus, modified MscL channels may be used as pH-triggered nanovalves that release a drug cargo from a liposome near a proliferating tumor for targeted release. Preliminary experiments have even been reported testing these devices in a rodent model system (182). Recent experiments in this area have centered on improving the design of the MscL-based triggered nanovalve by controlling what can pass through the nanovalve (150, 183) and modifying the labeling sites for better efficiency (123, 184).
Although these studies are still in their infancy, the large pore size, tractable nature of the MscL channel, and the promising nature of the experiments thus far suggest that someday we will have a series of practical MscL-based nanodevices.
MscL AS A DRUG TARGET
Rationale for Targeting MscL
There are good reasons to believe that MscL is a drug target for a novel class of antibiotics. (i) MscL is unique to microbes; no family members are found in mammals (185). (ii) MscL is found in almost every bacterial species, including many pathogenic strains, and even in some fungal species. It is highly conserved; thus, any resulting antibiotic is likely to be broad spectrum. This finding also suggests an intolerance to significant structural changes in its design. It therefore may be less prone to single-amino-acid changes that can lead to resistance (95, 132, 186). (iii) There is extensive evidence that forcing MscL channels to open inappropriately, by mutagenesis or posttranslational modifications, leads to impaired bacterial growth or even cell death (4, 49, 97, 146, 187, 188). Cell death associated with frequent gating of MscL is consistent with the loss of important metabolites (18, 81, 114, 189) as well as with membrane depolarization and inhibition of ATP synthesis (18). These factors may prevent multidrug resistance pumps from functioning properly and, thus, diminish the probability of resistance. (iv) MscL channels are expressed in all phases of bacterial growth and even upregulated in stationary phase (167). (v) Because the channel has a large pore (111), it may essentially permeabilize the cell to some degree; thus, MscL agonists may serve as adjuvants to allow access of antibiotics or toxins into the bacterial cytoplasm (190). (vi) The MscL channels do not require cellular metabolism or any cellular energy source; consequently, stationary-phase cultures are susceptible (191). Therefore, points iv to vi support the notion that MscL remains a viable target even during biofilm growth and for microbes that remain dormant for long periods of time, such as M. tuberculosis. Finally, MscL was identified as one of the top 20 potential drug targets using a comparative genomics approach to look at the CMN group of human pathogens, which includes Corynebacterium diphtheriae and C. pseudotuberculosis, Mycobacterium tuberculosis, and numerous pathogenic species in the genus Nocardia (185).
Unfortunately, there are no known endogenous ligands or specific toxins targeting MscL that could be useful for the study of chemical structures that directly bind to and modulate MscL gating. However, some chemicals, such as a spider toxin that intercalates in the membrane and modifies several MS channels (192), and even xenon gas (193), which can modify many membrane proteins, have been found to modulate MscL activity. In addition, gadolinium has been shown to inhibit the vast majority of mechanosensitive channels, including MscL, but appears to do so by altering the packing of negatively charged lipids (194). As mentioned above, researchers also knew early on that amphipathic molecules that added stresses in the membrane, including lysolipids (54), could increase the sensitivity of osmosensors and channels, including MscL (see reference 80 for a discussion of the effects of amphipaths such as procaine on osmotically regulated sensors and pumps).
Amphipaths and Other Compounds
One class of amphipathic compounds that has been studied for their effects on bacterial MS channels is the parabens. Parabens have been used in the food and cosmetic industry for years as antimicrobials. One study using a liposomal reconstitution system showed influences on both MscS and MscL, suggesting that parabens work by altering the lipid forces as they intercalate into the membrane bilayer (195). A later, more detailed study used spheroplasts to assess the effects in a native-membrane environment, which allowed the authors to look at the effects of different parabens on MscS when applied to different sides of the membrane (196). Based on the results of this study, the effects of parabens on MscS depend upon which leaflet of the bilayer they incorporate into. Butyl paraben added through the pipette (equivalent to the periplasmic face of the membrane) makes the channel more sensitive, while placing it in the bath (targeting the cytoplasmic face of the membrane) leads to a less sensitive channel, most likely due to increased lateral pressure in the cytoplasmic leaflet surrounding the gate. This study also found that more hydrophobic parabens partitioned more completely into the lipid and consequently could exert larger effects on channel gating through changes in lateral pressure. The approach used in the latter paper was further developed into a sensitive system to measure lipid partitioning of compounds and lateral pressure perturbations by amphipathic drugs (197). However, the authors noted that the effects parabens have on MS channel activity did not appear to fully explain the antimicrobial effects of parabens, since bacteria null for MS channels were still susceptible to them (196).
One of the more interesting possible modulators of MscL is a peptide produced by a bacterium. Lantibiotics are small posttranslationally modified peptides with antimicrobial activity that are produced by Gram-positive bacteria (198). The B. subtilis strain 168 is known to produce an extremely stable lantibiotic, referred to as sublancin 168. This substance exhibits bactericidal activity against other Gram-positive bacteria, including important pathogens, such as Streptococcus pyogenes, Bacillus cereus, and Staphylococcus aureus (199, 200). Interestingly, sublancin 168 activity appears to be dependent on MscL expression (201). Its effects are also osmolarity dependent; at high salt concentrations sublancin 168 is less effective. However, there has been no electrophysiological characterization showing sublancin 168 modulation of MscL activity, and no direct interaction or binding has been shown. It also remains unclear if the activity is due to gating the MscL channel or using the MscL channel as a passageway into the cell cytoplasm, as has been shown for other antibiotics (202), as discussed below.
Another compound that influences MscL was found through in silico screening (203, 204). Briefly, some evidence suggested that triphenyl methyl (TPM) dyes that are antimicrobial bind to the channel pore and modify MscL activity (205). In an attempt to improve the binding abilities of these dyes, a spatial map was designed between the exposed oxygen atoms of amino acids lining the constriction area of the MscL channel. This three-dimensional spatial map was used for the de novo design of several potential ligands capable of hydrogen bonding to the MscL channel amino acids in the pore (203, 204). An in silico screening led to additional compounds, and one, compound 10, later renamed Ramizol, was found to have a low docking energy and was further studied. Ramizol inhibits growth of MscL-expressing cells, but it also has some influence on cells expressing MscS, suggesting that at least some of its action is nonspecific, presumably through adding tension in the membrane. Ramizol also inhibited the growth of cells not expressing MscL or MscS, albeit only at higher concentrations, demonstrating that it has at least one other mode of action (204). As discussed below, one possibility is that Ramizol uses MscL as a primary passageway into the cell cytoplasm, as has been shown for other antibiotics (202).
Streptomycin Specifically Binds to, Opens, and Uses MscL as a Major Pathway to the Cytoplasm
An in vivo high-throughput screen (HTS) approach has recently been used in an attempt to find compounds that specifically bind to, and activate, MscL. A mild gain-of-function MscL mutant (K55T) expressed in E. coli cells was used in hopes of increasing the sensitivity of the assay. Compounds that decreased bacterial growth of cells expressing K55T were identified. A secondary screen was performed to test for MscL dependency and the ability to affect E. coli cells expressing mscL orthologues. Candidates that also effected slowed growth of MscS-expressing cells were discarded on the assumption that they primarily worked as amphipaths, increasing tension in the membrane (202). Surprisingly, some known antibiotics were identified in these screens. Subsequent studies confirmed that the influences on growth and viability of the aminoglycosides spectinomycin and dihydrostreptomycin (DHS) were specific to MscL; MscS expression had no influence. However, MscL expression increased only the potency of these antibiotics, consistent with the known primary mode of action being the inhibition or disruption of protein synthesis. Because of its tractability and interesting properties, DHS was further studied. In the 1960s, when the mechanism of action of streptomycin was just being elucidated, it was discovered that upon drug treatment K+ loss from cells preceded the decrease in viability (206). Moreover, there appears to be a significant increase in streptomycin uptake (207) and an increased potency (208) if the bacteria are prediluted into a lower-osmolarity media upon streptomycin incubation, suggesting a role for the absolute osmolarity of the bathing medium. The streptomycin-induced K+ flux does not lead to a decrease in the steep bacterial membrane potential (normally ca. 165 mV), suggesting that anions as well as K+ are fluxed from the cell (209; see reference 210 for a review on bacterial regulation of pH and membrane potential). MscL appears to be a major player in these events. Upon treatment with DHS, two osmoprotectants with opposing charges, K+ and glutamate, have been observed to be lost from the cell prior to any decrease in viability, and this efflux is MscL expression dependent (202). In a subsequent study, it was demonstrated that DHS binds directly to, modulates, and passes through the MscL channel pore, which appears to be one of the major pathways for access of DHS to the cytoplasm (211). In sum, the data suggest that DHS binds and opens MscL, allowing solutes to leak out of the cell and DHS into it. MscL seems to be a major, but not the only, pathway into the cell. It remains a question whether sublancin 168 and Ramizol (discussed above) also use MscL to traverse the cell membrane.
Discovery of the First MscL-Specific Agonists
More novel compounds have been identified from the HTS, and these have recently begun to be described and characterized. One of them, compound 120, is a sulfonamide compound that genetic experiments have confirmed to have the primary mode of action common for antimicrobial sulfonamides: inhibiting folate synthesis. Presumably compound 120 was identified by the HTS because, like DHS, it uses MscL as a primary pathway for cytoplasmic access (191). However, another sulfonamide compound identified, 011, did not appear to have any action through the folate pathway; rather, it had increased potency when the sulfonamide portion of the structure was removed, yielding a compound named 011A (191). Therefore, the 011A compound was further characterized.
A binding site for 011A has been confirmed by several independent approaches, including molecular docking, searching for partial suppressors in a cysteine library previously generated (49, 212), performing a competition assay between the compound and a thiol reaction using methoxypoly(ethyleneglycol)-5000-amidopropionylmethanethio-sulfonate (MTS-PEG5000), and assaying and engineering the proposed binding site of orthologues. Interestingly, the binding site for 011A is cytoplasmic, suggesting access of 011A through the membrane. It is located at the interface of two subunits, where the S1 helix slides along the cytoplasmic region of TM2, a region previously studied for its movements during the gating process and speculated to serve an important functional role (213). Interestingly, the finding of the binding site for compound 011A at a subunit interface is reminiscent of the observed location of the binding of acetylcholine to the muscle nicotinic receptor (214); indeed, many channel agonists have since been shown to bind at subunit interfaces (215).
Further study of 011A has demonstrated that it has many of the unique properties predicted for an MscL agonist: it decreases viability of stationary/quiescent cultures, is effective on other species in an MscL expression-dependent manner (Staphylococcus aureus and Mycobacterium smegmatis), and specifically permeabilizes bacterial cell membranes, allowing common antibiotics into the cell and, thus, increasing their potency (190, 191). While MscL agonists are not yet of high enough potency or efficacy to be useful as drugs, these studies demonstrate a proof of concept for the value of MscL as a drug target; with luck, time, and some work, more robust compounds will be found.
The role of MscL agonists as potential adjuvants needs to be more fully explored. One could foresee MscL agonists that selectively allow antibiotics to effectively cross only the membranes of bacteria. For example, MscL agonists could be used with potential antibiotics that are extremely effective when tested in in vitro assays but that have never been marketed because of a lack of penetration across the membrane (perhaps because of a required charged moiety). The MscL agonist could allow crucial access to the bacterial cytoplasm. In contrast, hydrophobic antibiotics that cause significant side effects could be modified so they no longer cross the membrane (e.g., addition of a charge or polar group). They could then be used in conjunction with an MscL agonist. The modified antibiotic then would have cytoplasmic access exclusively to bacterial cells, potentially decreasing or eliminating the unwanted side effects.
In summary, as shown in Fig. 6, MscL agonists are not only potential novel antimicrobials but also may serve as adjuvants that work in combination with other antibiotics; in either use they would be advantageous in otherwise intractable infections.
FIG 6.

Schematic of MscL gating by agonists and the potential for their use as novel antibiotics or adjuvants to be used with conventional antibiotics. Shown is the MscL channel (blue) in the membrane (lipids shown in gray). In the left panel, the channel is at rest and closed. In the center panel, upon treatment with an agonist (orange pentamers) that binds on the cytoplasmic side, solutes are lost from the cell, leading to decreased cell growth. As depicted in the right panel, such an agonist will also allow an unrelated antibiotic (green stars) to pass into the cytoplasm; thus, the agonist serves as an adjuvant.
CONCLUSIONS AND FINAL THOUGHTS
The bacterial MscL channel is one of the best understood channel proteins within any microbe. It has three high-resolution structures as well as the well-defined function of serving as an osmotic emergency release valve that prevents cell lysis upon acute decreases in osmotic environment. MscL is perhaps the only microbial channel where an activity, protein structure, protein dynamics, and invariant physiological function among homologues have all been integrated. Even with MscS, it is unclear if findings for the E. coli MscS will apply to other homologues. For the vast majority of MscS family members, a physiological function has yet to be found. It is unclear which other family members are involved in adaptation to osmotic environments and under what conditions, although there have been initial efforts to determine if different channels play slightly different roles (30, 78, 216, 217). A deeper understanding of the physiological roles of MS channels in bacteria will have implications not only for pathogenesis but also for other disease states, as well as for general health, as we are now learning from studies of the microbiome (218, 219).
Because MscL opens a huge pore of 25 to 30 Å (111), the conformational changes that occur upon gating are greatly exaggerated, allowing their study using a variety of approaches. The microbial system has allowed many experiments to be performed in vivo. These include traditional bacterial approaches, including forward genetics (4, 220) and isolation of intragenic suppressors (221) and temperature-sensitive mutants (222). However, unique approaches have also been used. These include in vivo disulfide trapping (109, 110, 134, 213) and an electrostatic repulsion test (ESReT) used to decrease the probability of two regions of the protein approaching each other (134). There was even the development of an in vivo SCAM (substituted cysteine accessibility method) (97, 98, 188), which evolved into a way of posttranslationally modifying residues, thereby affecting different properties at specific sites (49, 146). All of these assays significantly advanced the field and resolved controversies of how structure related to function. Many of the findings appear to have relevance for some other mechanosensitive channels as well as for channels gated by other means across the biological kingdoms because of conserved structural features; these are especially obvious when one looks at protein-protein and protein-lipid interactions at the lipid-cytoplasmic interface (59).
Finally, the uniquely large pore of MscL makes it readily amenable to potential applications. One direction is nanotechnology, where the modality of the channel can be changed and the resulting triggered nanovalve potentially used in an array of nanodevices. Another direction is drug discovery. Recent studies have confirmed that MscL is a valid pharmacological target for novel antibiotics, with agonist causing the detrimental release of bacterial cytoplasmic solutes (190, 191), as had been predicted from mutagenesis and physiological studies (4). Because MscL agonists essentially open holes in the membrane, they may also serve as adjuvants, facilitating passage of conventional antibiotics into the cytoplasm (190, 202, 211). Characterization of the binding site of the first true MscL-specific agonists further substantiates the functional importance of the lipid-cytoplasmic interface region (211) and suggests that the pharmacological targeting of interactions at this region will allow further refining of the structure-activity relationship of these agonists for the discovery of compounds with greater potency and higher efficacy.
ACKNOWLEDGMENTS
We thank Robin Wray, Ian Booth, Limin Yang, Paul Moe, and Gyorgyi Szebenyi for critical reading of the manuscript.
This work was supported by grant GM121780 from the National Institutes of Health and grant I-1420 of the Welch Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funding organizations.
Biographies

Paul Blount received a BA in microbiology from UCSD and a Ph.D. in neuroscience from Washington University in Saint Louis. He has a long-standing interest in channels and receptors. Initially he worked on the processingl and assembly of subunits of the mammalian muscle nicotinic acetylcholine receptor. He was the first to propose that the agonist binding sites were at subunit interfaces of the pentameric complex. He then performed structure/function analyses and found a functional nonequivalence of structurally homologous domains of two G-protein-coupled receptors in the tachykinin receptor subfamily. In Ching Kung’s laboratory, he was involved in cloning and characterizing mscL from E. coli, the first gene definitively shown to encode a mechanosensitive channel activity. His research since then has defined many features of bacterial mechanosensitive channels, where he has largely focused on the molecular mechanisms of MscL and defining how the channel senses and responds to mechanical force.

Irene Iscla currently holds an Assistant Professor position in the Physiology Department at the University of Texas Southwestern Medical Center. She pursued her undergraduate studies at the University of Buenos Aires (UBA), Argentina, where she obtained a bachelor degree in biology. The research for her Ph.D. thesis in neurobiology (UBA) introduced the complex modulatory role of nonspiking neurons and serotonin in sensory-motor neural circuits in the leech. Over 15 years ago, she transitioned to investigating the structure-function relationship of bacterial mechanosensitive channels. Irene’s work has contributed to a detailed understanding of MscL gating mechanisms and exploring its potential medical applications, including pharmacologically targeting the protein for the development of antibiotics as well as the use of MscL in nanotechnological devices. She has authored 17 original publications and two book chapters in the field.
REFERENCES
- 1.Martinac B, Buechner M, Delcour AH, Adler J, Kung C. 1987. Pressure-sensitive ion channel in Escherichia coli. Proc Natl Acad Sci U S A 84:2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumánovics A, Levin G, Blount P. 2002. Family ties of gated pores: evolution of the sensor module. FASEB J 16:1623–1629. doi: 10.1096/fj.02-0238hyp. [DOI] [PubMed] [Google Scholar]
- 3.Balleza D, Rosas ME, Romero-Romero S. 2019. Voltage vs. ligand I: structural basis of the intrinsic flexibility of S3 segment and its significance in ion channel activation. Channels 13:455–476. doi: 10.1080/19336950.2019.1674242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ou X, Blount P, Hoffman RJ, Kung C. 1998. One face of a transmembrane helix is crucial in mechanosensitive channel gating. Proc Natl Acad Sci U S A 95:11471–11475. doi: 10.1073/pnas.95.19.11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamada T. 1934. Some observations on potential differences across the ectoplasm membrane of Paramecium. J Exp Biol 11:94–102. [Google Scholar]
- 6.Hodgkin AL, Huxley AF. 1945. Resting and action potentials in single nerve fibres. J Physiol 104:176–195. doi: 10.1113/jphysiol.1945.sp004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neher E, Sakmann B. 1976. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature 260:799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- 8.Hamill O. 1983. Potassium and chloride channels in red blood cells, p 451–471. In Sakmann B, Neher E (ed), Single-channel recording. Springer, Boston, MA. [Google Scholar]
- 9.Brehm P, Kullberg R, Moody-Corbett F. 1984. Properties of non-junctional acetylcholine receptor channels on innervated muscle of Xenopus laevis. J Physiol 350:631–648. doi: 10.1113/jphysiol.1984.sp015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guharay F, Sachs F. 1984. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol 352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sachs F. 1986. Biophysics of mechanoreception. Membr Biochem 6:173–195. doi: 10.3109/09687688609065448. [DOI] [PubMed] [Google Scholar]
- 12.Chalfie M, Au M. 1989. Genetic control of differentiation of the Caenorhabditis elegans touch receptor neurons. Science 243:1027–1033. doi: 10.1126/science.2646709. [DOI] [PubMed] [Google Scholar]
- 13.Huang M, Gu G, Ferguson EL, Chalfie M. 1995. A stomatin-like protein necessary for mechanosensation in C. elegans. Nature 378:292–295. doi: 10.1038/378292a0. [DOI] [PubMed] [Google Scholar]
- 14.Huang M, Chalfie M. 1994. Gene interactions affecting mechanosensory transduction in Caenorhabditis elegans. Nature 367:467–470. doi: 10.1038/367467a0. [DOI] [PubMed] [Google Scholar]
- 15.Ruthe HJ, Adler J. 1985. Fusion of bacterial spheroplasts by electric fields. Biochim Biophys Acta 819:105–113. doi: 10.1016/0005-2736(85)90200-7. [DOI] [PubMed] [Google Scholar]
- 16.Blount P, Sukharev SI, Moe PC, Martinac B, Kung C. 1999. Mechanosensitive channels of bacteria. Methods Enzymol 294:458–482. [DOI] [PubMed] [Google Scholar]
- 17.Martinac B, Adler J, Kung C. 1990. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature 348:261–263. doi: 10.1038/348261a0. [DOI] [PubMed] [Google Scholar]
- 18.Berrier C, Coulombe A, Szabo I, Zoratti M, Ghazi A. 1992. Gadolinium ion inhibits loss of metabolites induced by osmotic shock and large stretch-activated channels in bacteria. Eur J Biochem 206:559–565. doi: 10.1111/j.1432-1033.1992.tb16960.x. [DOI] [PubMed] [Google Scholar]
- 19.Zoratti M, Petronilli V. 1988. Ion-conducting channels in a gram-positive bacterium. FEBS Lett 240:105–109. doi: 10.1016/0014-5793(88)80348-x. [DOI] [PubMed] [Google Scholar]
- 20.Zoratti M, Petronilli V, Szabo I. 1990. Stretch-activated composite ion channels in Bacillus subtilis. Biochem Biophys Res Commun 168:443–450. doi: 10.1016/0006-291x(90)92341-v. [DOI] [PubMed] [Google Scholar]
- 21.Szabo I, Petronilli V, Zoratti M. 1992. A patch-clamp study of Bacillus subtilis. Biochim Biophys Acta 1112:29–38. doi: 10.1016/0005-2736(92)90250-p. [DOI] [PubMed] [Google Scholar]
- 22.Szabo I, Petronilli V, Zoratti M. 1993. A patch-clamp investigation of the Streptococcus faecalis cell membrane. J Membr Biol 131:203–218. doi: 10.1007/bf02260109. [DOI] [PubMed] [Google Scholar]
- 23.Sukharev SI, Martinac B, Arshavsky VY, Kung C. 1993. Two types of mechanosensitive channels in the Escherichia coli cell envelope: solubilization and functional reconstitution. Biophys J 65:177–183. doi: 10.1016/S0006-3495(93)81044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berrier C, Coulombe A, Houssin C, Ghazi A. 1989. A patch-clamp study of ion channels of inner and outer membranes and of contact zones of E. coli, fused into giant liposomes. Pressure-activated channels are localized in the inner membrane. FEBS Lett 259:27–32. doi: 10.1016/0014-5793(89)81486-3. [DOI] [PubMed] [Google Scholar]
- 25.Balleza D, Gomez-Lagunas F. 2009. Conserved motifs in mechanosensitive channels MscL and MscS. Eur Biophys J 38:1013–1027. doi: 10.1007/s00249-009-0460-y. [DOI] [PubMed] [Google Scholar]
- 26.Malcolm HR, Maurer JA. 2012. The mechanosensitive channel of small conductance (MscS) superfamily: not just mechanosensitive channels anymore. Chembiochem 13:2037–2043. doi: 10.1002/cbic.201200410. [DOI] [PubMed] [Google Scholar]
- 27.Malcolm HR, Elmore DE, Maurer JA. 2012. Mechanosensitive behavior of bacterial cyclic nucleotide gated (bCNG) ion channels: insights into the mechanism of channel gating in the mechanosensitive channel of small conductance superfamily. Biochem Biophys Res Commun 417:972–976. doi: 10.1016/j.bbrc.2011.12.049. [DOI] [PubMed] [Google Scholar]
- 28.Malcolm HR, Heo YY, Caldwell DB, McConnell JK, Hawkins JF, Guayasamin RC, Elmore DE, Maurer JA. 2012. Ss-bCNGa: a unique member of the bacterial cyclic nucleotide gated (bCNG) channel family that gates in response to mechanical tension. Eur Biophys J 41:1003–1013. doi: 10.1007/s00249-012-0855-z. [DOI] [PubMed] [Google Scholar]
- 29.Berrier C, Besnard M, Ajouz B, Coulombe A, Ghazi A. 1996. Multiple mechanosensitive ion channels from Escherichia coli, activated at different thresholds of applied pressure. J Membr Biol 151:175–187. doi: 10.1007/s002329900068. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Moe PC, Chandrasekaran S, Booth IR, Blount P. 2002. Ionic regulation of MscK, a mechanosensitive channel from Escherichia coli. EMBO J 21:5323–5330. doi: 10.1093/emboj/cdf537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards MD, Black S, Rasmussen T, Rasmussen A, Stokes NR, Stephen TL, Miller S, Booth IR. 2012. Characterization of three novel mechanosensitive channel activities in Escherichia coli. Channels 6:272–281. doi: 10.4161/chan.20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bialecka-Fornal M, Lee HJ, Phillips R. 2015. The rate of osmotic downshock determines the survival probability of bacterial mechanosensitive channel mutants. J Bacteriol 197:231–237. doi: 10.1128/JB.02175-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Booth IR, Blount P. 2012. Microbial emergency release valves: the MscS and MscL families of mechanosensitive channels. J Bacteriol 194:4802–4809. doi: 10.1128/JB.00576-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton ES, Schlegel AM, Haswell ES. 2015. United in diversity: mechanosensitive ion channels in plants. Annu Rev Plant Biol 66:113–137. doi: 10.1146/annurev-arplant-043014-114700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson ME, Maksaev G, Haswell ES. 2013. MscS-like mechanosensitive channels in plants and microbes. Biochemistry 52:5708–5722. doi: 10.1021/bi400804z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haswell ES, Phillips R, Rees DC. 2011. Mechanosensitive channels: what can they do and how do they do it? Structure 19:1356–1369. doi: 10.1016/j.str.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang W, Black SS, Edwards MD, Miller S, Morrison EL, Bartlett W, Dong C, Naismith JH, Booth IR. 2008. The structure of an open form of an E. coli mechanosensitive channel at 3.45 A resolution. Science 321:1179–1183. doi: 10.1126/science.1159262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X, Wang J, Feng Y, Ge J, Li W, Sun W, Iscla I, Yu J, Blount P, Li Y, Yang M. 2012. Structure and molecular mechanism of an anion-selective mechanosensitive channel of small conductance. Proc Natl Acad Sci U S A 109:18180–18185. doi: 10.1073/pnas.1207977109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards MD, Li Y, Kim S, Miller S, Bartlett W, Black S, Dennison S, Iscla I, Blount P, Bowie JU, Booth IR. 2005. Pivotal role of the glycine-rich TM3 helix in gating the MscS mechanosensitive channel. Nat Struct Mol Biol 12:113–119. doi: 10.1038/nsmb895. [DOI] [PubMed] [Google Scholar]
- 40.Bass RB, Strop P, Barclay M, Rees DC. 2002. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science 298:1582–1587. doi: 10.1126/science.1077945. [DOI] [PubMed] [Google Scholar]
- 41.Lai JY, Poon YS, Kaiser JT, Rees DC. 2013. Open and shut: crystal structures of the dodecylmaltoside solubilized mechanosensitive channel of small conductance from Escherichia coli and Helicobacter pylori at 4.4 A and 4.1 A resolutions. Protein Sci 22:502–509. doi: 10.1002/pro.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rasmussen T, Flegler VJ, Rasmussen A, Bottcher B. 2019. Structure of the mechanosensitive channel MscS embedded in the membrane bilayer. J Mol Biol 431:3081–3090. doi: 10.1016/j.jmb.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Malcolm HR, Blount P, Maurer JA. 2015. The mechanosensitive channel of small conductance (MscS) functions as a jack-in-the box. Biochim Biophys Acta 1848:159–166. doi: 10.1016/j.bbamem.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pliotas C, Dahl AC, Rasmussen T, Mahendran KR, Smith TK, Marius P, Gault J, Banda T, Rasmussen A, Miller S, Robinson CV, Bayley H, Sansom MS, Booth IR, Naismith JH. 2015. The role of lipids in mechanosensation. Nat Struct Mol Biol 22:991–998. doi: 10.1038/nsmb.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rasmussen T, Rasmussen A, Yang L, Kaul C, Black S, Galbiati H, Conway SJ, Miller S, Blount P, Booth IR. 2019. Interaction of the mechanosensitive channel, MscS, with the membrane bilayer through lipid intercalation into grooves and pockets. J Mol Biol 431:3339–3352. doi: 10.1016/j.jmb.2019.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong YY, Pike AC, Mackenzie A, McClenaghan C, Aryal P, Dong L, Quigley A, Grieben M, Goubin S, Mukhopadhyay S, Ruda GF, Clausen MV, Cao L, Brennan PE, Burgess-Brown NA, Sansom MS, Tucker SJ, Carpenter EP. 2015. K2P channel gating mechanisms revealed by structures of TREK-2 and a complex with Prozac. Science 347:1256–1259. doi: 10.1126/science.1261512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kapsalis C, Wang B, El Mkami H, Pitt SJ, Schnell JR, Smith TK, Lippiat JD, Bode BE, Pliotas C. 2019. Allosteric activation of an ion channel triggered by modification of mechanosensitive nano-pockets. Nat Commun 10:4619. doi: 10.1038/s41467-019-12591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhong D, Blount P. 2013. Phosphatidylinositol is crucial for the mechanosensitivity of Mycobacterium tuberculosis MscL. Biochemistry 52:5415–5420. doi: 10.1021/bi400790j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iscla I, Wray R, Eaton C, Blount P. 2015. Scanning MscL channels with targeted post-translational modifications for functional alterations. PLoS One 10:e0137994. doi: 10.1371/journal.pone.0137994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sukharev SI, Blount P, Martinac B, Blattner FR, Kung C. 1994. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature 368:265–268. doi: 10.1038/368265a0. [DOI] [PubMed] [Google Scholar]
- 51.Blount P, Sukharev SI, Schroeder MJ, Nagle SK, Kung C. 1996. Single residue substitutions that change the gating properties of a mechanosensitive channel in Escherichia coli. Proc Natl Acad Sci U S A 93:11652–11657. doi: 10.1073/pnas.93.21.11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Häse CC, Le Dain AC, Martinac B. 1995. Purification and functional reconstitution of the recombinant large mechanosensitive ion channel (MscL) of Escherichia coli. J Biol Chem 270:18329–18334. doi: 10.1074/jbc.270.31.18329. [DOI] [PubMed] [Google Scholar]
- 53.Blount P, Sukharev SI, Moe PC, Schroeder MJ, Guy HR, Kung C. 1996. Membrane topology and multimeric structure of a mechanosensitive channel protein of Escherichia coli. EMBO J 15:4798–4805. [PMC free article] [PubMed] [Google Scholar]
- 54.Perozo E, Kloda A, Cortes DM, Martinac B. 2002. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat Struct Biol 9:696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- 55.Moe P, Blount P. 2005. Assessment of potential stimuli for mechano-dependent gating of MscL: effects of pressure, tension, and lipid headgroups. Biochemistry 44:12239–12244. doi: 10.1021/bi0509649. [DOI] [PubMed] [Google Scholar]
- 56.Anishkin A, Loukin SH, Teng J, Kung C. 2014. Feeling the hidden mechanical forces in lipid bilayer is an original sense. Proc Natl Acad Sci U S A 111:7898–7905. doi: 10.1073/pnas.1313364111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teng J, Loukin S, Anishkin A, Kung C. 2015. The force-from-lipid (FFL) principle of mechanosensitivity, at large and in elements. Pflugers Arch 467:27–37. doi: 10.1007/s00424-014-1530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kung C. 2005. A possible unifying principle for mechanosensation. Nature 436:647–654. doi: 10.1038/nature03896. [DOI] [PubMed] [Google Scholar]
- 59.Iscla I, Blount P. 2012. Sensing and responding to membrane tension: the bacterial mscl channel as a model system. Biophys J 103:169–174. doi: 10.1016/j.bpj.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cox CD, Bavi N, Martinac B. 2019. Biophysical principles of ion-channel-mediated mechanosensory transduction. Cell Rep 29:1–12. doi: 10.1016/j.celrep.2019.08.075. [DOI] [PubMed] [Google Scholar]
- 61.Berrier C, Pozza A, de Lacroix de Lavalette A, Chardonnet S, Mesneau A, Jaxel C, Le Maire M, Ghazi A. 2013. The purified mechanosensitive channel TREK-1 is directly sensitive to membrane tension. J Biol Chem 288:27307–27314. doi: 10.1074/jbc.M113.478321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brohawn SG, Su Z, MacKinnon R. 2014. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc Natl Acad Sci U S A 111:3614–3619. doi: 10.1073/pnas.1320768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Syeda R, Florendo MN, Cox CD, Kefauver JM, Santos JS, Martinac B, Patapoutian A. 2016. Piezo1 channels are inherently mechanosensitive. Cell Rep 17:1739–1746. doi: 10.1016/j.celrep.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cox CD, Bae C, Ziegler L, Hartley S, Nikolova-Krstevski V, Rohde PR, Ng CA, Sachs F, Gottlieb PA, Martinac B. 2016. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat Commun 7:10366. doi: 10.1038/ncomms10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murthy SE, Dubin AE, Whitwam T, Jojoa-Cruz S, Cahalan SM, Mousavi SAR, Ward AB, Patapoutian A. 2018. OSCA/TMEM63 are an evolutionarily conserved family of mechanically activated ion channels. Elife 7:e41844. doi: 10.7554/eLife.41844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gu CX, Juranka PF, Morris CE. 2001. Stretch-activation and stretch-inactivation of Shaker-IR, a voltage-gated K+ channel. Biophys J 80:2678–2693. doi: 10.1016/S0006-3495(01)76237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laitko U, Juranka PF, Morris CE. 2006. Membrane stretch slows the concerted step prior to opening in a Kv channel. J Gen Physiol 127:687–701. doi: 10.1085/jgp.200509394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laitko U, Morris CE. 2004. Membrane tension accelerates rate-limiting voltage-dependent activation and slow inactivation steps in a Shaker channel. J Gen Physiol 123:135–154. doi: 10.1085/jgp.200308965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmidt D, del Marmol J, MacKinnon R. 2012. Mechanistic basis for low threshold mechanosensitivity in voltage-dependent K+ channels. Proc Natl Acad Sci U S A 109:10352–10357. doi: 10.1073/pnas.1204700109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paoletti P, Ascher P. 1994. Mechanosensitivity of NMDA receptors in cultured mouse central neurons. Neuron 13:645–655. doi: 10.1016/0896-6273(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 71.Milkman R. 1994. An Escherichia coli homologue of eukaryotic potassium channel proteins. Proc Natl Acad Sci U S A 91:3510–3514. doi: 10.1073/pnas.91.9.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schrempf H, Schmidt O, Kummerlen R, Hinnah S, Muller D, Betzler M, Steinkamp T, Wagner R. 1995. A prokaryotic potassium ion channel with two predicted transmembrane segments from Streptomyces lividans. EMBO J 14:5170–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kralj JM, Hochbaum DR, Douglass AD, Cohen AE. 2011. Electrical spiking in Escherichia coli probed with a fluorescent voltage-indicating protein. Science 333:345–348. doi: 10.1126/science.1204763. [DOI] [PubMed] [Google Scholar]
- 74.Prindle A, Liu J, Asally M, Ly S, Garcia-Ojalvo J, Süel GM. 2015. Ion channels enable electrical communication in bacterial communities. Nature 527:59–63. doi: 10.1038/nature15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bruni GN, Weekley RA, Dodd BJT, Kralj JM. 2017. Voltage-gated calcium flux mediates Escherichia coli mechanosensation. Proc Natl Acad Sci U S A 114:9445–9450. doi: 10.1073/pnas.1703084114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mitchell P, Moyle J. 1956. Osmotic function and structure in bacteria. Symp Soc Gen Microbiol 6:150–180. [Google Scholar]
- 77.Deng Y, Sun M, Shaevitz JW. 2011. Direct measurement of cell wall stress stiffening and turgor pressure in live bacterial cells. Phys Rev Lett 107:158101. doi: 10.1103/PhysRevLett.107.158101. [DOI] [PubMed] [Google Scholar]
- 78.Booth IR. 2014. Bacterial mechanosensitive channels: progress towards an understanding of their roles in cell physiology. Curr Opin Microbiol 18:16–22. doi: 10.1016/j.mib.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poolman B, Blount P, Folgering JH, Friesen RH, Moe PC, van der Heide T. 2002. How do membrane proteins sense water stress? Mol Microbiol 44:889–902. doi: 10.1046/j.1365-2958.2002.02894.x. [DOI] [PubMed] [Google Scholar]
- 80.Wood JM. 1999. Osmosensing by bacteria: signals and membrane-based sensors. Microbiol Mol Biol Rev 63:230–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Britten RJ, McClure FT. 1962. The amino acid pool in Escherichia coli. Bacteriol Rev 26:292–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsapis A, Kepes A. 1977. Transient breakdown of the permeability barrier of the membrane of Escherichia coli upon hypoosmotic shock. Biochim Biophys Acta 469:1–12. doi: 10.1016/0005-2736(77)90320-0. [DOI] [PubMed] [Google Scholar]
- 83.Blount P, Schroeder MJ, Kung C. 1997. Mutations in a bacterial mechanosensitive channel change the cellular response to osmotic stress. J Biol Chem 272:32150–32157. doi: 10.1074/jbc.272.51.32150. [DOI] [PubMed] [Google Scholar]
- 84.Booth IR, Miller S, Müller A, Lehtovirta-Morley L. 2015. The evolution of bacterial mechanosensitive channels. Cell Calcium 57:140–150. doi: 10.1016/j.ceca.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 85.Nakamaru Y, Takahashi Y, Unemoto T, Nakamura T. 1999. Mechanosensitive channel functions to alleviate the cell lysis of marine bacterium, Vibrio alginolyticus, by osmotic downshock. FEBS Lett 444:170–172. doi: 10.1016/s0014-5793(99)00054-x. [DOI] [PubMed] [Google Scholar]
- 86.Levina N, Totemeyer S, Stokes NR, Louis P, Jones MA, Booth IR. 1999. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J 18:1730–1737. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arkin IT, Sukharev SI, Blount P, Kung C, Brunger AT. 1998. Helicity, membrane incorporation, orientation and thermal stability of the large conductance mechanosensitive ion channel from E. coli. Biochim Biophys Acta 1369:131–140. doi: 10.1016/S0005-2736(97)00219-8. [DOI] [PubMed] [Google Scholar]
- 88.Derman AI, Beckwith J. 1991. Escherichia coli alkaline phosphatase fails to acquire disulfide bonds when retained in the cytoplasm. J Bacteriol 173:7719–7722. doi: 10.1128/jb.173.23.7719-7722.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boyd D, Manoil C, Beckwith J. 1987. Determinants of membrane protein topology. Proc Natl Acad Sci U S A 84:8525–8529. doi: 10.1073/pnas.84.23.8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ehrmann M, Boyd D, Beckwith J. 1990. Genetic analysis of membrane protein topology by a sandwich gene fusion approach. Proc Natl Acad Sci U S A 87:7574–7578. doi: 10.1073/pnas.87.19.7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Manoil C, Beckwith J. 1986. A genetic approach to analyzing membrane protein topology. Science 233:1403–1408. doi: 10.1126/science.3529391. [DOI] [PubMed] [Google Scholar]
- 92.Chang G, Spencer RH, Lee AT, Barclay MT, Rees DC. 1998. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science 282:2220–2226. doi: 10.1126/science.282.5397.2220. [DOI] [PubMed] [Google Scholar]
- 93.Steinbacher S, Bass R, Strop P, Rees DC. 2007. Structures of the prokaryotic mechanosensitive channels MscL and MscS. Curr Topics Membr 58:1–24. doi: 10.1016/S1063-5823(06)58001-9. [DOI] [Google Scholar]
- 94.Sukharev S, Betanzos M, Chiang C, Guy H. 2001. The gating mechanism of the large mechanosensitive channel MscL. Nature 409:720–724. doi: 10.1038/35055559. [DOI] [PubMed] [Google Scholar]
- 95.Iscla I, Wray R, Blount P. 2008. On the structure of the N-terminal domain of the MscL channel: helical bundle or membrane interface. Biophys J 95:2283–2291. doi: 10.1529/biophysj.107.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Akabas MH, Kaufmann C, Archdeacon P, Karlin A. 1994. Identification of acetylcholine receptor channel-lining residues in the entire M2 segment of the alpha subunit. Neuron 13:919–927. doi: 10.1016/0896-6273(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 97.Bartlett JL, Levin G, Blount P. 2004. An in vivo assay identifies changes in residue accessibility on mechanosensitive channel gating. Proc Natl Acad Sci U S A 101:10161–10165. doi: 10.1073/pnas.0402040101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Batiza AF, Kuo MM, Yoshimura K, Kung C. 2002. Gating the bacterial mechanosensitive channel MscL in vivo. Proc Natl Acad Sci U S A 99:5643–5648. doi: 10.1073/pnas.082092599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jeon J, Voth GA. 2008. Gating of the mechanosensitive channel protein MscL: the interplay of membrane and protein. Biophys J 94:3497–3511. doi: 10.1529/biophysj.107.109850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bavi N, Cortes DM, Cox CD, Rohde PR, Liu W, Deitmer JW, Bavi O, Strop P, Hill AP, Rees D, Corry B, Perozo E, Martinac B. 2016. The role of MscL amphipathic N terminus indicates a blueprint for bilayer-mediated gating of mechanosensitive channels. Nat Commun 7:11984. doi: 10.1038/ncomms11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Martinac AD, Bavi N, Bavi O, Martinac B. 2017. Pulling MscL open via N-terminal and TM1 helices: a computational study towards engineering an MscL nanovalve. PLoS One 12:e0183822. doi: 10.1371/journal.pone.0183822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kuo A, Domene C, Johnson LN, Doyle DA, Venien-Bryan C. 2005. Two different conformational states of the KirBac3.1 potassium channel revealed by electron crystallography. Structure 13:1463–1472. doi: 10.1016/j.str.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 103.Kuo A, Gulbis JM, Antcliff JF, Rahman T, Lowe ED, Zimmer J, Cuthbertson J, Ashcroft FM, Ezaki T, Doyle DA. 2003. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science 300:1922–1926. doi: 10.1126/science.1085028. [DOI] [PubMed] [Google Scholar]
- 104.Häse CC, Minchin RF, Kloda A, Martinac B. 1997. Cross-linking studies and membrane localization and assembly of radiolabelled large mechanosensitive ion channel (MscL) of Escherichia coli. Biochem Biophys Res Commun 232:777–782. doi: 10.1006/bbrc.1997.6370. [DOI] [PubMed] [Google Scholar]
- 105.Saint N, Lacapere JJ, Gu LQ, Ghazi A, Martinac B, Rigaud JL. 1998. A hexameric transmembrane pore revealed by two-dimensional crystallization of the large mechanosensitive ion channel (MscL) of Escherichia coli. J Biol Chem 273:14667–14670. doi: 10.1074/jbc.273.24.14667. [DOI] [PubMed] [Google Scholar]
- 106.Sukharev SI, Schroeder MJ, McCaslin DR. 1999. Stoichiometry of the large conductance bacterial mechanosensitive channel of E. coli. A biochemical study. J Membr Biol 171:183–193. doi: 10.1007/s002329900570. [DOI] [PubMed] [Google Scholar]
- 107.Liu Z, Gandhi CS, Rees DC. 2009. Structure of a tetrameric MscL in an expanded intermediate state. Nature 461:120–124. doi: 10.1038/nature08277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gandhi CS, Walton TA, Rees DC. 2011. OCAM: a new tool for studying the oligomeric diversity of MscL channels. Protein Sci 20:313–326. doi: 10.1002/pro.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Iscla I, Wray R, Blount P. 2011. The oligomeric state of the truncated mechanosensitive channel of large conductance shows no variance in vivo. Protein Sci 20:1638–1642. doi: 10.1002/pro.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dorwart MR, Wray R, Brautigam CA, Jiang Y, Blount P. 2010. S. aureus MscL is a pentamer in vivo but of variable stoichiometries in vitro: implications for detergent-solubilized membrane proteins. PLoS Biol 8:e1000555. doi: 10.1371/journal.pbio.1000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cruickshank CC, Minchin RF, Le Dain AC, Martinac B. 1997. Estimation of the pore size of the large-conductance mechanosensitive ion channel of Escherichia coli. Biophys J 73:1925–1931. doi: 10.1016/S0006-3495(97)78223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Y, Liu Y, Deberg HA, Nomura T, Hoffman MT, Rohde PR, Schulten K, Martinac B, Selvin PR. 2014. Single molecule FRET reveals pore size and opening mechanism of a mechano-sensitive ion channel. Elife 3:e01834. doi: 10.7554/eLife.01834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hancock RE, Schmidt A, Bauer K, Benz R. 1986. Role of lysines in ion selectivity of bacterial outer membrane porins. Biochim Biophys Acta 860:263–267. doi: 10.1016/0005-2736(86)90522-5. [DOI] [PubMed] [Google Scholar]
- 114.Ajouz B, Berrier C, Garrigues A, Besnard M, Ghazi A. 1998. Release of thioredoxin via the mechanosensitive channel MscL during osmotic downshock of Escherichia coli cells. J Biol Chem 273:26670–26674. doi: 10.1074/jbc.273.41.26670. [DOI] [PubMed] [Google Scholar]
- 115.Berrier C, Garrigues A, Richarme G, Ghazi A. 2000. Elongation factor Tu and DnaK are transferred from the cytoplasm to the periplasm of Escherichia coli during osmotic downshock presumably via the mechanosensitive channel mscL. J Bacteriol 182:248–251. doi: 10.1128/jb.182.1.248-251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vazquez-Laslop N, Lee H, Hu R, Neyfakh AA. 2001. Molecular sieve mechanism of selective release of cytoplasmic proteins by osmotically shocked Escherichia coli. J Bacteriol 183:2399–2404. doi: 10.1128/JB.183.8.2399-2404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van den Bogaart G, Krasnikov V, Poolman B. 2007. Dual-color fluorescence-burst analysis to probe protein efflux through the mechanosensitive channel MscL. Biophys J 92:1233–1240. doi: 10.1529/biophysj.106.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ewis HE, Lu CD. 2005. Osmotic shock: a mechanosensitive channel blocker can prevent release of cytoplasmic but not periplasmic proteins. FEMS Microbiol Lett 253:295–301. doi: 10.1016/j.femsle.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 119.Blount P, Moe PC. 1999. Bacterial mechanosensitive channels: integrating physiology, structure and function. Trends Microbiol 7:420–424. doi: 10.1016/s0966-842x(99)01594-2. [DOI] [PubMed] [Google Scholar]
- 120.Birkner JP, Poolman B, Kocer A. 2012. Hydrophobic gating of mechanosensitive channel of large conductance evidenced by single-subunit resolution. Proc Natl Acad Sci U S A 109:12944–12949. doi: 10.1073/pnas.1205270109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chandramouli B, Di Maio D, Mancini G, Barone V, Brancato G. 2015. Breaking the hydrophobicity of the MscL pore: insights into a charge-induced gating mechanism. PLoS One 10:e0120196. doi: 10.1371/journal.pone.0120196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mika JT, Birkner JP, Poolman B, Kocer A. 2013. On the role of individual subunits in MscL gating: “all for one, one for all”? FASEB J 27:882–892. doi: 10.1096/fj.12-214361. [DOI] [PubMed] [Google Scholar]
- 123.Iscla I, Eaton C, Parker J, Wray R, Kovacs Z, Blount P. 2013. Improving the design of a MscL-based triggered nanovalve. Biosensors 3:171–184. doi: 10.3390/bios3010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sukharev S, Durell S, Guy H. 2001. Structural models of the MscL gating mechanism. Biophys J 81:917–936. doi: 10.1016/S0006-3495(01)75751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Evans E, Hochmuth R. 1978. Topics in membrane and transport. Topics Membr Transport 10:1–64. [Google Scholar]
- 126.Evans E, Needham D. 1987. Physical properties of surfactant bilayer membranes: thermal transitions, elasticity, rigidity, cohesion and colloidal interactions. J Phys Chem 91:4219–4228. doi: 10.1021/j100300a003. [DOI] [Google Scholar]
- 127.Anishkin A, Chiang CS, Sukharev S. 2005. Gain-of-function mutations reveal expanded intermediate states and a sequential action of two gates in MscL. J Gen Physiol 125:155–170. doi: 10.1085/jgp.200409118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yoshimura K, Usukura J, Sokabe M. 2008. Gating-associated conformational changes in the mechanosensitive channel MscL. Proc Natl Acad Sci U S A 105:4033–4038. doi: 10.1073/pnas.0709436105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Perozo E, Cortes DM, Sompornpisut P, Kloda A, Martinac B. 2002. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature 418:942–948. doi: 10.1038/nature00992. [DOI] [PubMed] [Google Scholar]
- 130.Corry B, Hurst AC, Pal P, Nomura T, Rigby P, Martinac B. 2010. An improved open-channel structure of MscL determined from FRET confocal microscopy and simulation. J Gen Physiol 136:483–494. doi: 10.1085/jgp.200910376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shapovalov G, Bass R, Rees DC, Lester HA. 2003. Open-state disulfide crosslinking between Mycobacterium tuberculosis mechanosensitive channel subunits. Biophys J 84:2357–2365. doi: 10.1016/S0006-3495(03)75041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Iscla I, Levin G, Wray R, Blount P. 2007. Disulfide trapping the mechanosensitive channel MscL into a gating-transition state. Biophys J 92:1224–1232. doi: 10.1529/biophysj.106.090316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Iscla I, Levin G, Wray R, Reynolds R, Blount P. 2004. Defining the physical gate of a mechanosensitive channel, MscL, by engineering metal-binding sites. Biophys J 87:3172–3180. doi: 10.1529/biophysj.104.049833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li Y, Wray R, Eaton C, Blount P. 2009. An open-pore structure of the mechanosensitive channel MscL derived by determining transmembrane domain interactions upon gating. FASEB J 23:2197–2204. doi: 10.1096/fj.09-129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li J, Guo J, Ou X, Zhang M, Li Y, Liu Z. 2015. Mechanical coupling of the multiple structural elements of the large-conductance mechanosensitive channel during expansion. Proc Natl Acad Sci U S A 112:10726–10731. doi: 10.1073/pnas.1503202112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ajouz B, Berrier C, Besnard M, Martinac B, Ghazi A. 2000. Contributions of the different extramembranous domains of the mechanosensitive ion channel MscL to its response to membrane tension. J Biol Chem 275:1015–1022. doi: 10.1074/jbc.275.2.1015. [DOI] [PubMed] [Google Scholar]
- 137.Park KH, Berrier C, Martinac B, Ghazi A. 2004. Purification and functional reconstitution of N- and C-halves of the MscL channel. Biophys J 86:2129–2136. doi: 10.1016/S0006-3495(04)74272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tsai IJ, Liu ZW, Rayment J, Norman C, McKinley A, Martinac B. 2005. The role of the periplasmic loop residue glutamine 65 for MscL mechanosensitivity. Eur Biophys J 34:403–412. doi: 10.1007/s00249-005-0476-x. [DOI] [PubMed] [Google Scholar]
- 139.Zhong D, Yang L-M, Blount P. 2014. Dynamics of protein-protein interactions at the MscL periplasmic-lipid interface. Biophys J 106:375–381. doi: 10.1016/j.bpj.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yang LM, Zhong D, Blount P. 2013. Chimeras reveal a single lipid-interface residue that controls MscL channel kinetics as well as mechanosensitivity. Cell Rep 3:520–527. doi: 10.1016/j.celrep.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Perozo E, Kloda A, Cortes DM, Martinac B. 2001. Site-directed spin-labeling analysis of reconstituted Mscl in the closed state. J Gen Physiol 118:193–206. doi: 10.1085/jgp.118.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Powl AM, Carney J, Marius P, East JM, Lee AG. 2005. Lipid interactions with bacterial channels: fluorescence studies. Biochem Soc Trans 33:905–909. doi: 10.1042/BST20050905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Powl AM, East JM, Lee AG. 2003. Lipid-protein interactions studied by introduction of a tryptophan residue: the mechanosensitive channel MscL. Biochemistry 42:14306–14317. doi: 10.1021/bi034995k. [DOI] [PubMed] [Google Scholar]
- 144.Powl AM, East JM, Lee AG. 2007. Different effects of lipid chain length on the two sides of a membrane and the lipid annulus of MscL. Biophys J 93:113–122. doi: 10.1529/biophysj.107.105130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Powl AM, Wright JN, East JM, Lee AG. 2005. Identification of the hydrophobic thickness of a membrane protein using fluorescence spectroscopy: studies with the mechanosensitive channel MscL. Biochemistry 44:5713–5721. doi: 10.1021/bi047338g. [DOI] [PubMed] [Google Scholar]
- 146.Iscla I, Wray R, Blount P. 2011. An in vivo screen reveals protein-lipid interactions crucial for gating a mechanosensitive channel. FASEB J 25:694–702. doi: 10.1096/fj.10-170878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhong D, Blount P. 2014. Electrostatics at the membrane define MscL channel mechanosensitivity and kinetics. FASEB J 28:5234–5241. doi: 10.1096/fj.14-259309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Anishkin A, Gendel V, Sharifi NA, Chiang CS, Shirinian L, Guy HR, Sukharev S. 2003. On the conformation of the COOH-terminal domain of the large mechanosensitive channel MscL. J Gen Physiol 121:227–244. doi: 10.1085/jgp.20028768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bavi N, Martinac AD, Cortes DM, Bavi O, Ridone P, Nomura T, Hill AP, Martinac B, Perozo E. 2017. Structural dynamics of the MscL C-terminal domain. Sci Rep 7:17229. doi: 10.1038/s41598-017-17396-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang LM, Wray R, Parker J, Wilson D, Duran RS, Blount P. 2012. Three routes to modulate the pore size of the MscL channel/nanovalve. ACS Nano 6:1134–1141. doi: 10.1021/nn203703j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cox CD, Nomura T, Ziegler CS, Campbell AK, Wann KT, Martinac B. 2013. Selectivity mechanism of the mechanosensitive channel MscS revealed by probing channel subconducting states. Nat Commun 4:2137. doi: 10.1038/ncomms3137. [DOI] [PubMed] [Google Scholar]
- 152.Miyazawa A, Fujiyoshi Y, Stowell M, Unwin N. 1999. Nicotinic acetylcholine receptor at 4.6 A resolution: transverse tunnels in the channel wall. J Mol Biol 288:765–786. doi: 10.1006/jmbi.1999.2721. [DOI] [PubMed] [Google Scholar]
- 153.Basak S, Gicheru Y, Rao S, Sansom MSP, Chakrapani S. 2018. Cryo-EM reveals two distinct serotonin-bound conformations of full-length 5-HT3A receptor. Nature 563:270–274. doi: 10.1038/s41586-018-0660-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gharpure A, Teng J, Zhuang Y, Noviello CM, Walsh RM Jr, Cabuco R, Howard RJ, Zaveri NT, Lindahl E, Hibbs RE. 2019. Agonist selectivity and ion permeation in the alpha3beta4 ganglionic nicotinic receptor. Neuron 104:501. doi: 10.1016/j.neuron.2019.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Long SB, Campbell EB, Mackinnon R. 2005. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 156.El Hiani Y, Linsdell P. 2015. Functional architecture of the cytoplasmic entrance to the cystic fibrosis transmembrane conductance regulator chloride channel pore. J Biol Chem 290:15855–15865. doi: 10.1074/jbc.M115.656181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li MS, Cowley EA, El Hiani Y, Linsdell P. 2018. Functional organization of cytoplasmic portals controlling access to the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel pore. J Biol Chem 293:5649–5658. doi: 10.1074/jbc.RA117.001373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hassaine G, Deluz C, Grasso L, Wyss R, Tol MB, Hovius R, Graff A, Stahlberg H, Tomizaki T, Desmyter A, Moreau C, Li XD, Poitevin F, Vogel H, Nury H. 2014. X-ray structure of the mouse serotonin 5-HT3 receptor. Nature 512:276–281. doi: 10.1038/nature13552. [DOI] [PubMed] [Google Scholar]
- 159.Herrera N, Maksaev G, Haswell ES, Rees DC. 2018. Elucidating a role for the cytoplasmic domain in the Mycobacterium tuberculosis mechanosensitive channel of large conductance. Sci Rep 8:14566. doi: 10.1038/s41598-018-32536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Moe PC, Blount P, Kung C. 1998. Functional and structural conservation in the mechanosensitive channel MscL implicates elements crucial for mechanosensation. Mol Microbiol 28:583–592. doi: 10.1046/j.1365-2958.1998.00821.x. [DOI] [PubMed] [Google Scholar]
- 161.Moe PC, Levin G, Blount P. 2000. Correlating a protein structure with function of a bacterial mechanosensitive channel. J Biol Chem 275:31121–31127. doi: 10.1074/jbc.M002971200. [DOI] [PubMed] [Google Scholar]
- 162.Folgering JH, Moe PC, Schuurman-Wolters GK, Blount P, Poolman B. 2005. Lactococcus lactis uses MscL as its principal mechanosensitive channel. J Biol Chem 280:8784–8792. doi: 10.1074/jbc.M411732200. [DOI] [PubMed] [Google Scholar]
- 163.Wahome PG, Setlow P. 2006. The synthesis and role of the mechanosensitive channel of large conductance in growth and differentiation of Bacillus subtilis. Arch Microbiol 186:377–383. doi: 10.1007/s00203-006-0152-2. [DOI] [PubMed] [Google Scholar]
- 164.Kouwen TR, Antelmann H, van der Ploeg R, Denham EL, Hecker M, van Dijl JM. 2009. MscL of Bacillus subtilis prevents selective release of cytoplasmic proteins in a hypotonic environment. Proteomics 9:1033–1043. doi: 10.1002/pmic.200800483. [DOI] [PubMed] [Google Scholar]
- 165.Wahome PG, Setlow P. 2008. Growth, osmotic downshock resistance and differentiation of Bacillus subtilis strains lacking mechanosensitive channels. Arch Microbiol 189:49–58. doi: 10.1007/s00203-007-0292-z. [DOI] [PubMed] [Google Scholar]
- 166.Hoffmann T, Boiangiu C, Moses S, Bremer E. 2008. Responses of Bacillus subtilis to hypotonic challenges: physiological contributions of mechanosensitive channels to cellular survival. Appl Environ Microbiol 74:2454–2460. doi: 10.1128/AEM.01573-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Stokes NR, Murray HD, Subramaniam C, Gourse RL, Louis P, Bartlett W, Miller S, Booth IR. 2003. A role for mechanosensitive channels in survival of stationary phase: regulation of channel expression by RpoS. Proc Natl Acad Sci U S A 100:15959–15964. doi: 10.1073/pnas.2536607100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rowe I, Elahi M, Huq A, Sukharev S. 2013. The mechanoelectrical response of the cytoplasmic membrane of Vibrio cholerae. J Gen Physiol 142:75–85. doi: 10.1085/jgp.201310985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cetiner U, Rowe I, Schams A, Mayhew C, Rubin D, Anishkin A, Sukharev S. 2017. Tension-activated channels in the mechanism of osmotic fitness in Pseudomonas aeruginosa. J Gen Physiol 149:595–609. doi: 10.1085/jgp.201611699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Andersson M, Okeyo G, Wilson D, Keizer H, Moe P, Blount P, Fine D, Dodabalapur A, Duran RS. 2008. Voltage-induced gating of the mechanosensitive MscL ion channel reconstituted in a tethered lipid bilayer membrane. Biosens Bioelectron 23:919–923. doi: 10.1016/j.bios.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 171.Urban M, Kleefen A, Mukherjee N, Seelheim P, Windschiegl B, Vor der Brüggen M, Koçer A, Tampé R. 2014. Highly parallel transport recordings on a membrane-on-nanopore chip at single molecule resolution. Nano Lett 14:1674–1680. doi: 10.1021/nl5002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hindley JW, Zheleva DG, Elani Y, Charalambous K, Barter LMC, Booth PJ, Bevan CL, Law RV, Ces O. 2019. Building a synthetic mechanosensitive signaling pathway in compartmentalized artificial cells. Proc Natl Acad Sci U S A 116:16711–16716. doi: 10.1073/pnas.1903500116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Doerner JF, Febvay S, Clapham DE. 2012. Controlled delivery of bioactive molecules into live cells using the bacterial mechanosensitive channel MscL. Nat Commun 3:990. doi: 10.1038/ncomms1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Farimani AB, Heiranian M, Aluru NR. 2015. Electromechanical signatures for DNA sequencing through a mechanosensitive nanopore. J Phys Chem Lett 6:650–657. doi: 10.1021/jz5025417. [DOI] [PubMed] [Google Scholar]
- 175.Farimani AB, Heiranian M, Aluru NR. 2015. Correction to “Electromechanical signatures for DNA sequencing through a mechanosensitive nanopore.” J Phys Chem Lett 6:3365. doi: 10.1021/acs.jpclett.5b01691. [DOI] [PubMed] [Google Scholar]
- 176.Ye J, Tang S, Meng L, Li X, Wen X, Chen S, Niu L, Li X, Qiu W, Hu H, Jiang M, Shang S, Shu Q, Zheng H, Duan S, Li Y. 2018. Ultrasonic control of neural activity through activation of the mechanosensitive channel MscL. Nano Lett 18:4148–4155. doi: 10.1021/acs.nanolett.8b00935. [DOI] [PubMed] [Google Scholar]
- 177.Babakhanian M, Yang L, Nowroozi B, Saddik G, Boodaghians L, Blount P, Grundfest W. 2018. Effects of low intensity focused ultrasound on liposomes containing channel proteins. Sci Rep 8:17250. doi: 10.1038/s41598-018-35486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Folgering JH, Kuiper JM, de Vries AH, Engberts JB, Poolman B. 2004. Lipid-mediated light activation of a mechanosensitive channel of large conductance. Langmuir 20:6985–6987. doi: 10.1021/la048942v. [DOI] [PubMed] [Google Scholar]
- 179.Koçer A, Walko M, Meijberg W, Feringa BL. 2005. A light-actuated nanovalve derived from a channel protein. Science 309:755–758. doi: 10.1126/science.1114760. [DOI] [PubMed] [Google Scholar]
- 180.Koçer A, Walko M, Bulten E, Halza E, Feringa B, Meijberg W. 2006. Rationally designed chemical modulators convert a bacterial channel protein into a pH-sensory valve. Angew Chem Int Ed Engl 45:3126–3130. doi: 10.1002/anie.200503403. [DOI] [PubMed] [Google Scholar]
- 181.Nakayama Y, Mustapic M, Ebrahimian H, Wagner P, Kim JH, Hossain MS, Horvat J, Martinac B. 2015. Magnetic nanoparticles for “smart” liposomes. Eur Biophys J 44:647–654. doi: 10.1007/s00249-015-1059-0. [DOI] [PubMed] [Google Scholar]
- 182.Pacheco-Torres J, Mukherjee N, Walko M, Lopez-Larrubia P, Ballesteros P, Cerdan S, Kocer A. 2015. Image guided drug release from pH-sensitive ion channel-functionalized stealth liposomes into an in vivo glioblastoma model. Nanomedicine 11:1345–1354. doi: 10.1016/j.nano.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 183.Yang LM, Blount P. 2011. Manipulating the permeation of charged compounds through the MscL nanovalve. FASEB J 25:428–434. doi: 10.1096/fj.10-170076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yang LM, Zheng H, Ratnakar JS, Adebesin BY, Do QN, Kovacs Z, Blount P. 2018. Engineering a pH-sensitive liposomal MRI agent by modification of a bacterial channel. Small 14:e1704256. doi: 10.1002/smll.201704256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Barh D, Jain N, Tiwari S, D’Afonseca V, Li L, Ali A, Santos AR, Guimaraes LC, de Castro Soares S, Miyoshi A, Bhattacharjee A, Misra AN, Silva A, Kumar A, Azevedo V. 2011. A novel comparative genomics analysis for common drug and vaccine targets in Corynebacterium pseudotuberculosis and other CMN group of human pathogens. Chem Biol Drug Des 78:73–84. doi: 10.1111/j.1747-0285.2011.01118.x. [DOI] [PubMed] [Google Scholar]
- 186.Maurer J, Elmore D, Lester H, Dougherty D. 2000. Comparing and contrasting Escherichia coli and Mycobacterium tuberculosis mechanosensitive channels (MscL). New gain of function mutations in the loop region. J Biol Chem 275:22238–22244. doi: 10.1074/jbc.M003056200. [DOI] [PubMed] [Google Scholar]
- 187.Yoshimura K, Batiza A, Schroeder M, Blount P, Kung C. 1999. Hydrophilicity of a single residue within MscL correlates with increased channel mechanosensitivity. Biophys J 77:1960–1972. doi: 10.1016/S0006-3495(99)77037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bartlett JL, Li Y, Blount P. 2006. Mechanosensitive channel gating transitions resolved by functional changes upon pore modification. Biophys J 91:3684–3691. doi: 10.1529/biophysj.106.088062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ajouz B, Berrier C, Ghazi A. 1998. Effluxes triggered by osmotic downshock in E. coli cells devoid of the mechanosensitive ion channel MscL. Biophys J 74:239a. [Google Scholar]
- 190.Wray R, Herrera N, Iscla I, Wang J, Blount P. 2019. An agonist of the MscL channel affects multiple bacterial species and increases membrane permeability and potency of common antibiotics. Mol Microbiol 112:896. doi: 10.1111/mmi.14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wray R, Iscla I, Kovacs Z, Wang J, Blount P. 2019. Novel compounds that specifically bind and modulate MscL: insights into channel gating mechanisms. FASEB J 33:3180–3189. doi: 10.1096/fj.201801628R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kamaraju K, Gottlieb PA, Sachs F, Sukharev S. 2010. Effects of GsMTx4 on bacterial mechanosensitive channels in inside-out patches from giant spheroplasts. Biophys J 99:2870–2878. doi: 10.1016/j.bpj.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Petrov E, Menon G, Rohde PR, Battle AR, Martinac B, Solioz M. 2018. Xenon-inhibition of the MscL mechano-sensitive channel and the CopB copper ATPase under different conditions suggests direct effects on these proteins. PLoS One 13:e0198110. doi: 10.1371/journal.pone.0198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ermakov YA, Kamaraju K, Sengupta K, Sukharev S. 2010. Gadolinium ions block mechanosensitive channels by altering the packing and lateral pressure of anionic lipids. Biophys J 98:1018–1027. doi: 10.1016/j.bpj.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nguyen T, Clare B, Guo W, Martinac B. 2005. The effects of parabens on the mechanosensitive channels of E. coli. Eur Biophys J 34:389–395. doi: 10.1007/s00249-005-0468-x. [DOI] [PubMed] [Google Scholar]
- 196.Kamaraju K, Sukharev S. 2008. The membrane lateral pressure-perturbing capacity of parabens and their effects on the mechanosensitive channel directly correlate with hydrophobicity. Biochemistry 47:10540–10550. doi: 10.1021/bi801092g. [DOI] [PubMed] [Google Scholar]
- 197.Rowe I, Guo M, Yasmann A, Cember A, Sintim HO, Sukharev S. 2015. Membrane affinity of platensimycin and its dialkylamine analogs. Int J Mol Sci 16:17909–17932. doi: 10.3390/ijms160817909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chatterjee C, Paul M, Xie L, van der Donk WA. 2005. Biosynthesis and mode of action of lantibiotics. Chem Rev 105:633–684. doi: 10.1021/cr030105v. [DOI] [PubMed] [Google Scholar]
- 199.Paik SH, Chakicherla A, Hansen JN. 1998. Identification and characterization of the structural and transporter genes for, and the chemical and biological properties of sublancin 168, a novel lantibiotic produced by Bacillus subtilis 168. J Biol Chem 273:23134–23142. doi: 10.1074/jbc.273.36.23134. [DOI] [PubMed] [Google Scholar]
- 200.Stein T. 2005. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol 56:845–857. doi: 10.1111/j.1365-2958.2005.04587.x. [DOI] [PubMed] [Google Scholar]
- 201.Kouwen TR, Trip EN, Denham EL, Sibbald MJ, Dubois JY, van Dijl JM. 2009. The large mechanosensitive channel MscL determines bacterial susceptibility to the bacteriocin sublancin 168. Antimicrob Agents Chemother 53:4702–4711. doi: 10.1128/AAC.00439-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Iscla I, Wray R, Wei S, Posner B, Blount P. 2014. Streptomycin potency is dependent on MscL channel expression. Nat Commun 5:4891. doi: 10.1038/ncomms5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Boulos RA, Man NY, Lengkeek NA, Hammer KA, Foster NF, Stemberger NA, Skelton BW, Wong PY, Martinac B, Riley TV, McKinley AJ, Stewart SG. 2013. Inspiration from old dyes: tris(stilbene) compounds as potent gram-positive antibacterial agents. Chemistry 19:17980–17988. doi: 10.1002/chem.201303119. [DOI] [PubMed] [Google Scholar]
- 204.Iscla I, Wray R, Blount P, Larkins-Ford J, Conery AL, Ausubel FM, Ramu S, Kavanagh A, Huang JX, Blaskovich MA, Cooper MA, Obregon-Henao A, Orme I, Tjandra ES, Stroeher UH, Brown MH, Macardle C, van Holst N, Ling Tong C, Slattery AD, Gibson CT, Raston CL, Boulos RA. 2015. A new antibiotic with potent activity targets MscL. J Antibiot 68:453. doi: 10.1038/ja.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Boulos RA. 2010. Mechanosensitive channels as novel targets for antimicrobial agents. BSc (honors) thesis. The University of Western Australia, Perth, Australia. [Google Scholar]
- 206.Dubin DT, Hancock R, Davis BD. 1963. The sequence of some effects of streptomycin in Escherichia coli. Biochim Biophys Acta 74:476–489. doi: 10.1016/0006-3002(63)91390-8. [DOI] [PubMed] [Google Scholar]
- 207.Plotz PH, Dubin DT, Davis BD. 1961. Influence of salts on the uptake of streptomycin by Escherichia coli. Nature 191:1324–1325. doi: 10.1038/1911324a0. [DOI] [PubMed] [Google Scholar]
- 208.Jiafeng L, Fu X, Chang Z. 2015. Hypoionic shock treatment enables aminoglycosides antibiotics to eradicate bacterial persisters. Sci Rep 5:14247. doi: 10.1038/srep14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Busse HJ, Wostmann C, Bakker EP. 1992. The bactericidal action of streptomycin: membrane permeabilization caused by the insertion of mistranslated proteins into the cytoplasmic membrane of Escherichia coli and subsequent caging of the antibiotic inside the cells due to degradation of these proteins. J Gen Microbiol 138:551–561. doi: 10.1099/00221287-138-3-551. [DOI] [PubMed] [Google Scholar]
- 210.Booth IR. 1985. Regulation of cytoplasmic pH in bacteria. Microbiol Rev 49:359–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wray R, Iscla I, Gao Y, Li H, Wang J, Blount P. 2016. Dihydrostreptomycin directly binds to, modulates, and passes through the MscL channel pore. PLoS Biol 14:e1002473. doi: 10.1371/journal.pbio.1002473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Levin G, Blount P. 2004. Cysteine scanning of MscL transmembrane domains reveals residues critical for mechanosensitive channel gating. Biophys J 86:2862–2870. doi: 10.1016/S0006-3495(04)74338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Iscla I, Wray R, Blount P. 2012. The dynamics of protein-protein interactions between domains of MscL at the cytoplasmic-lipid interface. Channels 6:255–261. doi: 10.4161/chan.20756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Blount P, Merlie JP. 1989. Molecular basis of the two nonequivalent ligand binding sites of the muscle nicotinic acetylcholine receptor. Neuron 3:349–357. doi: 10.1016/0896-6273(89)90259-6. [DOI] [PubMed] [Google Scholar]
- 215.Changeux JP, Christopoulos A. 2017. Allosteric modulation as a unifying mechanism for receptor function and regulation. Diabetes Obes Metab 19(Suppl 1):4–21. doi: 10.1111/dom.12959. [DOI] [PubMed] [Google Scholar]
- 216.Bialecka-Fornal M, Lee HJ, DeBerg HA, Gandhi CS, Phillips R. 2012. Single-cell census of mechanosensitive channels in living bacteria. PLoS One 7:e33077. doi: 10.1371/journal.pone.0033077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Boer M, Anishkin A, Sukharev S. 2011. Adaptive MscS gating in the osmotic permeability response in E. coli: the question of time. Biochemistry 50:4087–4096. doi: 10.1021/bi1019435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shaffer M, Armstrong AJS, Phelan VV, Reisdorph N, Lozupone CA. 2017. Microbiome and metabolome data integration provides insight into health and disease. Transl Res 189:51–64. doi: 10.1016/j.trsl.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lynch SV, Pedersen O. 2016. The human intestinal microbiome in health and disease. N Engl J Med 375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 220.Maurer JA, Dougherty DA. 2001. A high-throughput screen for MscL channel activity and mutational phenotyping. Biochim Biophys Acta 1514:165–169. doi: 10.1016/s0005-2736(01)00390-x. [DOI] [PubMed] [Google Scholar]
- 221.Li Y, Wray R, Blount P. 2004. Intragenic suppression of gain-of-function mutations in the Escherichia coli mechanosensitive channel, MscL. Mol Microbiol 53:485–495. doi: 10.1111/j.1365-2958.2004.04150.x. [DOI] [PubMed] [Google Scholar]
- 222.Owada N, Yoshida M, Morita K, Yoshimura K. 2019. Temperature-sensitive mutants of MscL mechanosensitive channel. J Biochem 166:281–288. doi: 10.1093/jb/mvz035. [DOI] [PubMed] [Google Scholar]


