Electronic cigarettes (e-cigarettes) have been used in many countries for >10 years and in this time, there has been a division of opinions amongst both the general public and health professionals regarding the benefit or harms of e-cigarettes. Prior to the reporting of a new phenomenon known as vaping-associated pulmonary injury (VAPI), public opinion about the relative harm of e-cigarettes were increasing but they were perceived as less harmful than cigarettes by one third of people [1]. The recent cases of severe illness and death attributable to VAPI were first described in September 2019 [2]. VAPI appears to be related to either the addition of cannabis/cannabis derivates or vitamin E acetate [3], and as such has not caused radical swing away from the use of e-cigarettes without cannabis or cannabis derivates.
Short abstract
Thirdhand exposure to e-cigarette residue is likely to have harmful effects in children http://bit.ly/38a2umw
To the Editor:
Electronic cigarettes (e-cigarettes) have been used in many countries for >10 years and in this time, there has been a division of opinions amongst both the general public and health professionals regarding the benefit or harms of e-cigarettes. Prior to the reporting of a new phenomenon known as vaping-associated pulmonary injury (VAPI), public opinion about the relative harm of e-cigarettes were increasing but they were perceived as less harmful than cigarettes by one third of people [1]. The recent cases of severe illness and death attributable to VAPI were first described in September 2019 [2]. VAPI appears to be related to either the addition of cannabis/cannabis derivates or vitamin E acetate [3], and as such has not caused radical swing away from the use of e-cigarettes without cannabis or cannabis derivates.
Thirdhand cigarette exposure occurs when the toxicants found in cigarette smoke are absorbed by the environment (clothing, surfaces, dust, etc.). These then become a new environmental source of cigarette smoke toxicants, and are of particular concern to nonsmokers such as babies crawling on surfaces or exposed to the clothing of smokers. E-cigarettes have been shown to be a potential source of thirdhand exposure to nicotine [4], and therefore need to be evaluated in the same context as thirdhand cigarette smoking.
We have a long-standing interest in the potential health effects of e-cigarettes in children, and for example, we were the first group to show that e-cigarette use in pregnancy is likely to have detrimental effects upon the respiratory, renal and neurological health of the offspring [5–9]. One area of e-cigarette research where experimental data are lacking is thirdhand exposure. A search of PubMed (18 December 2019) found only 13 papers using the search term “e-cigarettes” and “thirdhand exposure”, and of the 13, four were studies measuring nicotine in thirdhand environmental e-cigarette samples and the rest were reviews on the topic.
In the current study, we have chosen to explore the potential harms of thirdhand e-cigarette exposure during early-life development. For obvious ethical reasons, we have explored this in a novel mouse model of exposure. We hypothesised that like thirdhand exposure to cigarettes, thirdhand exposure to e-cigarettes would have detrimental effects on childhood health.
To mimic the potential exposure that a human infant would have if they crawled on contaminated carpets or came into contact with the clothing of a vaper, we exposed a new towel (15×10 cm, 100% cotton) to the e-vapour generated by 20×5-s bursts of nicotine-free or nicotine-containing (18 mg·mL−1) e-liquid (tobacco flavour; Vaper Empire, Sydney, Asutralia) by a human e-cigarette machine (30 W, 0.5 Ω, 20 puffs) (NEBOX; KangerTech, Shenzhen, China) in a sealed 9-L container for 2 h. Each day, a freshly exposed towel was placed in the mouse cage (male, Balb/C, 4 weeks old). At the end of the 8 days of exposure, mice were sacrificed, and body and organ weights were assessed. Ethical approval was given by the Animal Care and Ethics Committee at the University of Technology Sydney (ETH18-2890), following the guidelines of the National Health and Medical Research Council, Australia. Serum cytokines and chemokines were assessed by a MAGPIX (Luminex, Austin, TX, USA) multiplexing system, and lung inflammatory cell counts were assessed in bronchoalveolar lavage fluid (BALF). Chemicals in the towel were extracted using methanol and analysed using gas chromatography–mass spectrometry (GC-MS).
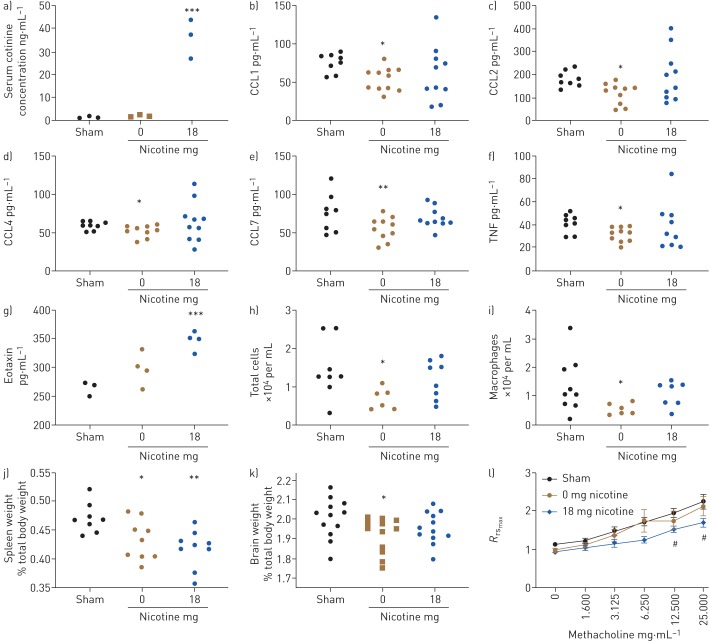
As shown in figure 1, nicotine was transferred from the towel to the mouse, leading to the hypothesis that other toxic components of the e-cigarette vapour would also be transferred to the animal. To assess the general development of the animal, both body weight and the weights of the spleen, liver, kidneys and brain were measured at the end of the exposure period. Body weight was not affected by thirdhand exposure to e-cigarettes and neither was the weight of the liver or kidneys (not shown). However, both the spleen and the brains were smaller in the groups exposed to thirdhand e-vapour with or without nicotine (figure 1). This suggests that the development of these organs is impaired by thirdhand exposure to e-vapour. 33 different cytokines and chemokines were assessed in the serum of sham and thirdhand e-vapour (with and without nicotine) exposed animals. Thirdhand e-vapour without nicotine decreased CCL1, CCL2, CCL4, CCL7 and tumour necrosis factor in the serum, and surprisingly, thirdhand e-vapour with nicotine had no effect on these cytokines. The only mediator that was found to be elevated in serum was CCL11 (eotaxin), which was significantly induced by nicotine-containing thirdhand e-vapour.
FIGURE 1.
a) Serum cotinine (n=3), b) CCL1, c) CCL2, d) CCL4, e) CCL7, f) tumour necrosis factor (TNF) (n=8–10) and f) eotaxin (n=3–4)) from mice with thirdhand exposure to tobacco flavoured e-vapour without (0 mg) or with (18 mg) nicotine, or sham exposed for 8 days. Bronchoalveolar h) total cell and i) macrophage counts (n=7–9), j) spleen and k) brain weights (n=8–12), and l) airway reactivity (Rrsmax) (n=8–9) to inhaled methacholine from the same groups. Data were analysed by one- or two-way ANOVA with an appropriate post hoc test (Fisher's least significant difference, Welch's correction or Tukey as appropriate). *: p<0.05 versus sham; **: p<0.01 versus sham; ***: p<0.001 versus sham; #: p<0.05 for 18 mg versus sham.
In this study, we were principally interested in the effects of thirdhand e-vapour exposure on the lungs. To assess inflammation, inflammatory cell counts in the BALF were carried out, and total cell numbers were markedly decreased in the thirdhand e-vapour without nicotine group, which was caused by a reduction in macrophage numbers in the BALF. Similar to what was observed in serum, thirdhand e-vapour with nicotine did not affect macrophage numbers. Lung function was also assessed, and it was found that upon thirdhand exposure to e-cigarettes with nicotine, the lungs were hyporesponsive to methacholine (figure 1).
Our data demonstrate, for the first time, that toxicants found in thirdhand e-cigarette vapour are biologically relevant. Our model is relatively acute, in that exposure only occurred for 8 days. Even with this short-term exposure, cotinine levels in the serum are equivalent to what might be found in a light cigarette smoker. One of the most startling findings of our study was that thirdhand e-cigarettes without nicotine suppressed the immune system by inhibiting several chemokines. CCL1, CCL2, CCL4 and CCL7 are chemokines that attract macrophages, and therefore the reduction in macrophage numbers in BALF is in accordance with these changes. Thirdhand exposure to e-vapour with nicotine reversed the suppression of the immune system, consistent with the anti-inflammatory effect of nicotine [10]. Airway hyporesponsiveness in animals exposed to e-vapour with nicotine might be caused by downregulation or occupancy of the nicotinic acetylcholine receptors, and interestingly, has occurred in animal models of cigarette smoking [11]. The suppression of the development of the brain following thirdhand exposure of e-cigarettes, especially in the group without nicotine, suggests a paradigm shift in the proposed roles of nicotine as a suppressor of brain development in smoker's offspring [12]. This suggests that other chemical(s) may play a critical role. To identify the chemicals in the towel, chemicals were extracted using methanol and analysed using GC-MS. Although formaldehyde, acetaldehyde, benzene, phenol and benzaldehyde were detected in the e-vapour, only nicotine and propylene glycol were detected in the towel. Interestingly, propylene glycol has been shown to be detrimental to the developing brain [13]. However, another method is still needed in future studies to identify other toxic chemical(s) responsible for the suppression of organ development.
In conclusion, thirdhand exposure of e-cigarettes is highly likely to have detrimental effects on children, and in other high-risk groups such as pregnant women. We believe our data demonstrate that thirdhand exposure of e-cigarettes should be considered to be as dangerous as thirdhand cigarette exposure.
Footnotes
Conflict of interest: H. Chen has nothing to disclose.
Conflict of interest: G. Li has nothing to disclose.
Conflict of interest: V.S.R.R. Allam has nothing to disclose.
Conflict of interest: B. Wang has nothing to disclose.
Conflict of interest: Y.L. Chan has nothing to disclose.
Conflict of interest: C. Scarfo has nothing to disclose.
Conflict of interest: M. Ueland has nothing to disclose.
Conflict of interest: R. Shimmon has nothing to disclose.
Conflict of interest: S. Fu has nothing to disclose.
Conflict of interest: P. Foster has nothing to disclose.
Conflict of interest: B.G. Oliver has nothing to disclose.
References
- 1.Huang J, Feng B, Weaver SR, et al. Changing perceptions of harm of e-cigarette vs cigarette use among adults in 2 US national surveys from 2012 to 2017. JAMA Netw Open 2019; 2: e191047–e191047. doi: 10.1001/jamanetworkopen.2019.1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin — preliminary report. N Engl J Med 2020; 382: 903–916. doi: 10.1056/NEJMoa1911614 [DOI] [PubMed] [Google Scholar]
- 3.Bozier J, Zakarya R, Chapman DG, et al. How harmless are E-cigarettes? Effects in the pulmonary system. Curr Opin Pulm Med 2020; 26: 97–102. doi: 10.1097/MCP.0000000000000645 [DOI] [PubMed] [Google Scholar]
- 4.Goniewicz ML, Lee L. Electronic cigarettes are a source of thirdhand exposure to nicotine. Nicotine Tob Res 2015; 17: 256–258. doi: 10.1093/ntr/ntu152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H, Li G, Chan YL, et al. Maternal e-cigarette exposure in mice alters DNA methylation and lung cytokine expression in offspring. Am J Respir Cell Mol Biol 2018; 58: 366–377. doi: 10.1165/rcmb.2017-0206RC [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Li G, Chan YL, et al. Modulation of neural regulators of energy homeostasis, and of inflammation, in the pups of mice exposed to e-cigarettes. Neurosci Lett 2018; 684: 61–66. doi: 10.1016/j.neulet.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen T, Li GE, Chen H, et al. Maternal e-cigarette exposure results in cognitive and epigenetic alterations in offspring in a mouse model. Chem Res Toxicol 2018; 31: 601–611. doi: 10.1021/acs.chemrestox.8b00084 [DOI] [PubMed] [Google Scholar]
- 8.Nguyen T, Li GE, Chen H, et al. Neurological effects in the offspring after switching from tobacco cigarettes to e-cigarettes during pregnancy in a mouse model. Toxicol Sci 2019; 172: 191–200. doi: 10.1093/toxsci/kfz194 [DOI] [PubMed] [Google Scholar]
- 9.Li G, Chan YL, Nguyen LT, et al. Impact of maternal e-cigarette vapor exposure on renal health in the offspring. Ann NY Acad Sci 2019; 1452: 65–77. doi: 10.1111/nyas.14174 [DOI] [PubMed] [Google Scholar]
- 10.Piao WH, Campagnolo D, Dayao C, et al. Nicotine and inflammatory neurological disorders. Acta Pharmacol Sin 2009; 30: 715–722. doi: 10.1038/aps.2009.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Sanctis GT, Kelly SM, Saetta MP, et al. Hyporesponsiveness to aerosolized but not to infused methacholine in cigarette-smoking dogs. Am Rev Respir Dis 1987; 135: 338–344. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Morris MJ. Maternal smoking – a contributor to the obesity epidemic? Obes Res Clin Pract 2007; 1: 155–163. doi: 10.1016/j.orcp.2007.07.004 [DOI] [PubMed] [Google Scholar]
- 13.Lau K, Swiney BS, Reeves N, et al. Propylene glycol produces excessive apoptosis in the developing mouse brain, alone and in combination with phenobarbital. Pediatr Res 2012; 71: 54–62. doi: 10.1038/pr.2011.12 [DOI] [PMC free article] [PubMed] [Google Scholar]