Abstract
PHOTOPERIODIC CONTROL OF HYPOCOTYL 1 (PCH1) and PCH1-LIKE (PCHL) were shown to directly bind to phytochrome B (phyB) and suppress phyB thermal reversion, resulting in plants with dramatically enhanced light sensitivity. Here, we show that PCH1 and PCHL also positively regulate various light responses, including seed germination, hypocotyl gravitropism, and chlorophyll biosynthesis, by physically interacting with PHYTOCHROME INTERACTING FACTOR 1 (PIF1) and CONSTITUTIVE PHOTOMORPHO-GENIC 1 (COP1). PCH1 and PCHL interact with PIF1 both in the dark and light, and regulate PIF1 abundance. Moreover, PCH1 and PCHL facilitate the physical interaction between phyB and PIF1 in vivo to promote the light-induced degradation of PIF1. PCH1 and PCHL also inhibit the DNA-binding ability of PIF1 to negatively regulate the expressions of PIF1 target genes. In addition, PCH1 and PCHL interact with COP1 and undergo degradation through the 26S proteasome pathway in the dark. Consistently, pch1 suppresses cop1 phenotype in darkness. Collectively, our study reveals a novel mechanism by which PCH1 and PCHL regulate diverse light responses not only by stabilizing phyB Pfr form but also by directly interacting with PIF1 and COP1, providing a molecular understanding of the control of hypocotyl growth by these proteins.
Keywords: Arabidopsis, phytochrome, phytochrome interacting factor (PIF), photoperiodic growth, protein degradation
INTRODUCTION
Phytochromes (phys) are evolutionarily conserved photoreceptors in bacteria, fungi, algae, and plants (Rockwell and Lagarias, 2019). Phys regulate almost all aspects of plant development and growth, including germination, de-etiolation, shade avoidance, plant defense, floral induction, and senescence (Casal, 2013; Legris et al., 2019; Paik and Huq, 2019). Phys are red and far-red light photoreceptors that can be photoconverted between two relatively stable forms: the red-light-absorbing inactive Pr form localized in cytoplasm and the far-red-light-absorbing biologically active Pfr form, which migrates into nucleus (Huq and Quail, 2005; Legris et al., 2019). In addition to light-induced Pfr / Pr reversion, the active Pfr state can also revert to the inactive Pr state in a light-independent thermal relaxation process referred to as thermal reversion (Medzihradszky et al., 2013; Klose et al., 2020). Phytochrome A (phyA) and phytochrome B (phyB) play a major role in seed plants at the seedling stage. PhyA is required for sensing far-red light and weak light of any wavelength, while phyB is the primary receptor for red light (Legris et al., 2019). The physiological activity of phyB, the primary phytochrome in light-grown and adult plants, is strongly affected by thermal reversion. Increased rates of phyB thermal reversion in Arabidopsis seedlings exposed to high temperature reduce both the abundance of the biologically active Pfr-Pfr dimer pool and the size of the associated nuclear bodies under daylight and dark conditions. Mathematical analysis of stem growth for seedlings expressing wild-type phyB or thermally stable variants under various combinations of light and temperature revealed that phyB is physiologically responsive to both signals (Jung et al., 2016; Legris et al., 2016; Quint et al., 2016). Therefore, thermal reversion is an important factor that determines how plants respond to light and temperature.
Within the nucleus, the activated Pfr physically interacts with multiple proteins, including a small group of basic helix-loop-helix (bHLH) transcription factors called PHYTOCHROME INTERACTING FACTORS (PIFs; PIF1 to PIF8) (Leivar and Quail, 2011; Pham et al., 2018b; Oh et al., 2019). Phytochromes regulate light responses partly by inhibiting these PIFs, which negatively regulate various light responses. PIFs constitutively accumulate in the nucleus in the dark and inhibit photomorphogenesis (Leivar and Quail, 2011; Pham et al., 2018b; Oh et al., 2019). Upon light exposure, the physical interaction between Pfr and PIFs triggers a cascade of events, including light-induced phosphorylation, ubiquitination, and 26S proteasome-mediated degradation of PIFs (Bauer et al., 2004; Shen et al., 2005; Paik et al., 2019). The removal of PIFs after light exposure results in large-scale changes in gene expression that promote photomorphogenic development (Leivar et al., 2009; Shin et al., 2009; Leivar and Monte, 2014). Consistently, the reduction in PIF level in the pifq (pif1 pif3 pif4 pif5) quadruple mutant or the overexpression of a truncated form of PIF1 results in photomorphogenic development in the dark (Leivar et al., 2008; Shen et al., 2008; Shin et al., 2009; Pham et al., 2018c).
Among the negative regulators in light signaling, COP1 is a RING-type E3 ubiquitin ligase that forms multiple complexes with SUPPRESSOR OF PHYA-105 family members (SPA1 to SPA4) (Deng et al., 1992; Lau and Deng, 2012; Xu et al., 2015). These complexes, either by themselves or by forming CUL4COP1–SPA E3 ubiquitin ligases, degrade the positively acting transcription factors through the ubiquitin proteasome system to repress photomorphogenesis in the dark (Chen et al., 2010). Therefore, cop1 and spa quadruple-mutant seedlings (spaq) exhibit constitutive photomorphogenesis when grown in darkness (Deng et al., 1992; Laubinger et al., 2004; Hoecker, 2017). In wild-type seedlings grown in the dark, nuclear-localized COP1 ubiquitinates HY5, promoting its degradation (Osterlund et al., 2000; Lau and Deng, 2012), whereas light exposure reduces nuclear COP1 to a level that permits the accumulation of HY5 (Subramanian et al., 2004; Pacín et al., 2014). Light also represses COP1 activity by activating the photoreceptors phyA, phyB, CRYPTOCHROME 1 (CRY1), and CRY2 to modulate the complex between COP1 and SPA proteins, ultimately leading to HY5 accumulation (Lian et al., 2011; Zuo et al., 2011; Lu et al., 2015; Sheerin et al., 2015). Phytochromes directly interact with SPA proteins and inhibit the function of the COP1/ SPA ubiquitin E3 ligase complex by either disrupting the interaction between COP1 and SPAs, or by promoting the degradation of SPA2 (Balcerowicz et al., 2011; Lu et al., 2015; Sheerin et al., 2015). In response to light, the inhibition of the COP1/SPA activity by phytochromes results in the accumulation of its target substrates such as ELONGATED HYPOCOTYL 5 (HY5), LONG HYPOCOTYL IN FAR-RED 1 (HFR1), and B-BOX ZINC FINGER PROTEINs (BBXs) (Osterlund et al., 2000; Jang et al., 2005; Xu et al., 2015, 2017; Pham et al., 2018c). These, in turn, reprogram gene expression to promote photomorphogenesis.
PHOTOPERIODIC CONTROL OF HYPOCOTYL 1 (PCH1) has been identified as one of the proteins interacting with the light-activated phyB (Huang et al., 2016; Enderle et al., 2017). Seedlings lacking functional PCH1 display elongated hypocotyls compared with the wild type under short days. Under these conditions, PCH1 transcript and protein levels peak at dusk, enhancing phyB-dependent inactivation of the growth-promoting transcription factor PHYTOCHROME INTERACTING FACTOR 4 (PIF4). In contrast, PCH1 levels are low toward the end of the night, leading to increased PIF4 activity and hypocotyl growth. Thus, PCH1 has been suggested to integrate clock and light signals through modulation of diurnal phyB activity (Huang et al., 2016). We showed in our previous report that the pch1 pchl double mutant, which lacks functional PCH1 and a homolog, PCH1-LIKE (PCHL), displays strongly accelerated phyB thermal reversion. Moreover, PCH1 and PCHL stabilize phyB in the active state and inhibit phyB thermal reversion. We also showed that PCH1/PCHL accumulates in response to various lights and additional signaling pathways control the expression of PCH1 and PCHL, thereby affecting the activity of phyB (Enderle et al., 2017). Thus, PCH1 and PCHL fine-tuned light signaling by integrating environmental signals to regulate phyB thermal reversion.
Recently, Huang et al. (2019) further provided biochemical and genetic evidence that PCH1 is sufficient to slow down the process of thermal reversion of phyB from Pfr to Pr. They also showed that PCH1 enhances the assembly of phyB into the subnuclear foci known as photobodies, which are positively connected with phyB activity (Buskirk et al., 2014; Klose et al., 2015; Huang et al., 2019). Using the constitutively active phyB allele phyB Y276H tagged with YFP, they showed that the loss of photobodies in phyB Y276H-YFP pch1 seedlings represses the constitutive photomorphogenic phenotype of phyB Y276H, alleviating the thermo-insensitivity caused by the presence of phyB Y276H, and abolishes the phyB Y276H-mediated light input into the circadian clock of dark-grown seedlings (Huang et al., 2019). Collectively, these data indicate that PCH1 acts as a positive regulator of phyB photobody formation and multiple phyB-controlled physiological processes.
Although it is known that PCH1 and PCHL positively regulate the function of phyB by promoting phyB photobody formation, their relationship and functions in regulating other light-signaling components as well as the mechanism underlying their protein stability have not yet been shown. Here, we show that PCH1 and PCHL positively regulate various light responses, such as seed germination, negative gravitropism, and chlorophyll biosynthesis by directly interacting with PIF1 and COP1. In this process, PCH1 and PCHL inhibit PIF1 function by either directly inhibiting PIF1 DNA binding and/or promoting PIF1 degradation by facilitating the formation of phyB–PIF1 and COP1–PIF1 complexes. Moreover, like other positive components in light signaling, PCH1 and PCHL are also the substrates of COP1 and are targeted for degradation in darkness.
RESULTS
PCH1 and PCHL Positively Regulate Various Light Responses and Expression of Many Light-Responsive Genes
To further understand the functions of PCH1 and PCHL in light-signaling pathways, we examined multiple light-related phenotypes using the previously described pch1, pchl, and pch1 pchl double mutants, as well as 35Spro:HA-YFP-PCH1 (PCH1OE) and 35Spro:HA-YFP-PCHL (PCHLOE) overexpression transgenic lines. As phyB is known to promote seed germination in response to red light, we examined whether PCH1 and PCHL positively regulate seed germination. As shown in Figure 1A, PCH1OE and PCHLOE showed higher levels of seed germination under increasing amount of red light, whereas pch1, pchl, and pch1 pchl mutants displayed much lower germination compared with wild type. To assess whether PCH1 and PCHL promote seed germination through inhibition of PIF1 function, we created pch1 pif1, pchl pif1, and pch1 pchl pif1 mutant combinations and examined the seed germination phenotype. The results show that the reduced seed germination of pch1 and pchl mutants in response to light is eliminated in the pif1 background. The double- and triple-mutant seeds germinated similarly to the pif1 single mutant (Supplemental Figure 1), suggesting that pif1 is epistatic to pch in regulating seed germination.
Figure 1. PCH1 and PCHL Positively Regulate Various Light Responses and Expression of Light-Regulated Genes.
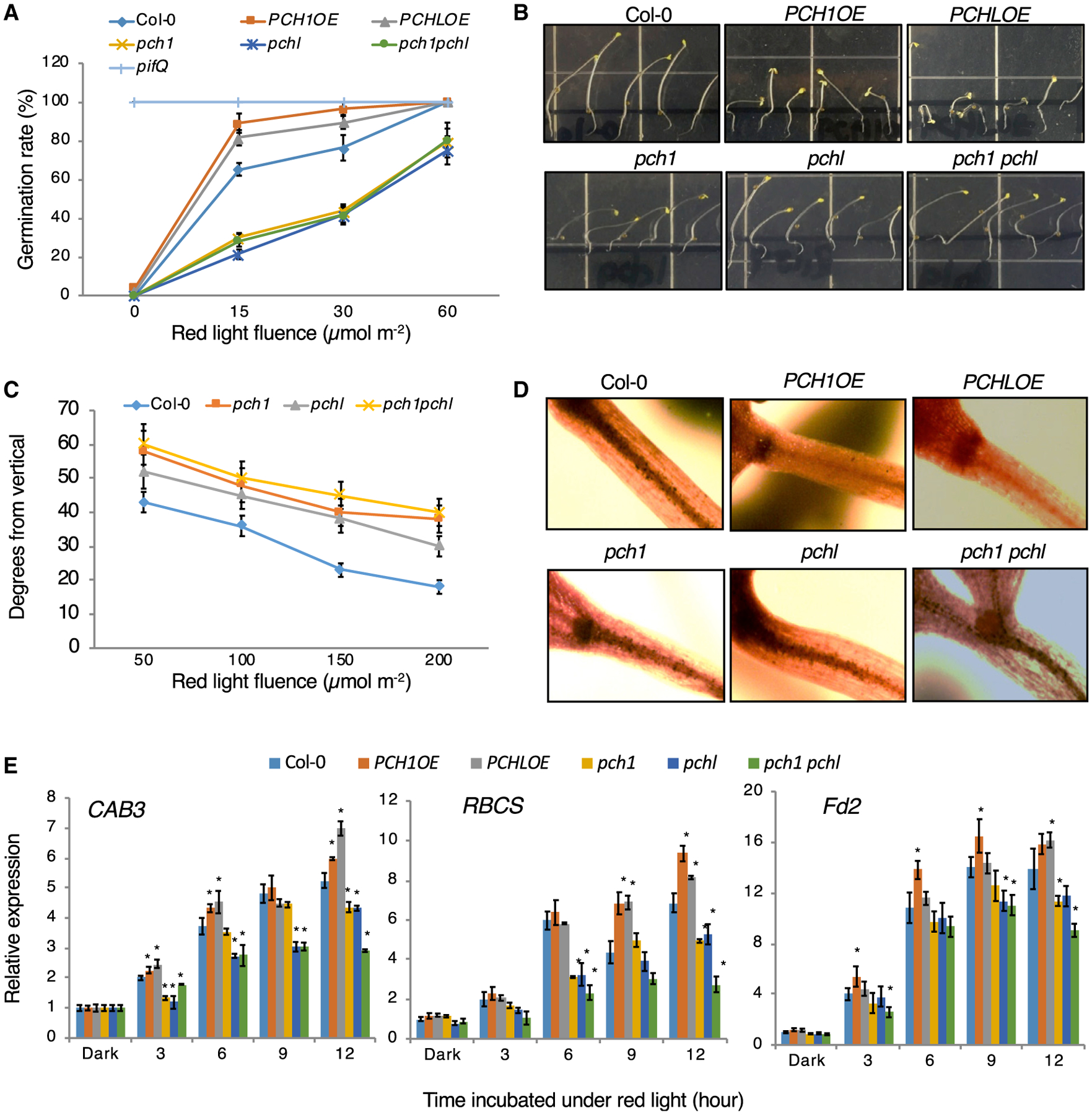
(A) Seed-germination phenotypes of Col-0, PCH1 and PCHL overexpression lines, pch1, pchl, and pch1 pchl double mutants in response to an increasing fluence of red light. Col-0 and pifQ were used as controls. Error bars indicate SEM (n = 3 biological repeats).
(B) Photograph showing that the hypocotyls of PCH1 and PCHL overexpression seedlings do not respond to changes in the direction of gravity. The direction of gravity was altered by turning plates 90° after the seedlings were grown for 2 days in pulsed red light on vertical agar plates. The plates were incubated for another 2 days under the same light conditions.
(C) Quantification of hypocotyl negative gravitropism in response to an increasing fluence of red light pulse. Data are mean with 95% confidence intervals indicated (n = 20 seedlings).
(D) I2-KI staining patterns of starch granules for the wild-type (Col-0), PCH1 and PCHL overexpression lines (PCH1OE and PCHLOE), pch1, pchl, and pch1 pchl double mutant.
(E) Light-responsive gene expression in Col-0, PCH1 and PCHL overexpression lines, pch1, pchl, and pch1 pchl double mutants in response to an increasing fluence of red light. Error bars indicate SEM (n = 3 biological repeats). *P < 0.01 by Student’s t-test; asterisk indicates significant difference of each genotype compared with Col-0 in each condition.
Phytochromes suppress the hypocotyls’ negative gravitropism by inhibiting PIFs in red or far-red light (Kim et al., 2011). We next investigated how PCH1 and PCHL regulate hypocotyl negative gravitropism by examining whether gravity sensing is disrupted in PCH1OE and PCHLOE. Wild-type hypocotyls curved against the direction of gravity upon alteration of the gravity vector, whereas PCH1OE and PCHLOE seedlings grew randomly in all directions (Figure 1B and Supplemental Figure 2). Moreover, pch1, pchl, and pch1 pchl mutants displayed hypersensitive phenotypes in response to gravity as they have higher curvature compared with wild-type hypocotyls after changing the direction of gravity, and the degrees of the curvature gradually decreased along with the increase in the red light fluence (Figure 1B and 1C; Supplemental Figure 2). We also examined the gravitropism phenotype of pch1 and pchl mutants in the pif1 mutant background. The hypersensitive phenotypes of pch1 and pchl mutants are slightly rescued by pif1 mutation (Supplemental Figure 3), suggesting that pif1 is epistatic to pch mutants. Starch granules containing amyloplasts of hypocotyl endodermal cells are responsible for gravity sensing in hypocotyls. We next examined endodermal amyloplasts by staining them with I2-KI. Consistent with the agravitropic phenotypes, PCH1OE and PCHLOE displayed little to no staining of amyloplasts in hypocotyl endodermal cells compared with dark-stained hypocotyls of wild-type seedlings. However, the stain intensity in pch1, pchl, and pch1 pchl hypocotyls was comparable with that of wild-type seedlings (Figure 1D). We also compared this gravitropism phenotype in transgenic seedlings overexpressing PCH1 and PCHL in phyB mutant backgrounds. As shown in Supplemental Figure 4, the negative gravitropism phenotype is completely rescued by phyB mutation, suggesting that the phenotype of PCH1OE and PCHLOE plants is phyB dependent.
To determine the function of PCH1 and PCHL at the molecular level, we examined the expression levels of a selected group of genes that are usually expressed in light-grown seedlings, including CHLOROPHYLL A/B BINDING PROTEIN 3 (CAB3), RIBULOSE BISPHOSPHATE CARBOXYLASE SMALL CHAIN 1A (RBCS), and FERREDOXIN 2 (Fd2). As expected, PCH1OE and PCHLOE seedlings displayed higher expression of these genes compared with the wild type under red light treatment (Figure 1E), whereas pch1, pchl, and pch1 pchl mutant showed lower expression compared with wild-type seedlings. These data suggest that PCH1 and PCHL positively regulate various light responses both at the morphological and molecular levels.
PCH1 and PCHL Regulate Chlorophyll Biosynthesis at the Early Stages
phyB has been shown to positively regulate chlorophyll synthesis and cotyledon greening (Huq et al., 2004), whereas PIF1 and PIF3 play a negative role in these responses (Huq et al., 2004; Moon et al., 2008; Stephenson et al., 2009). To prepare for exposure to light, seedlings accumulate a small pool of the immediate precursor of chlorophyll, called protochlorophyllide, to permit rapid assembly of functional photosynthetic machinery. However, overaccumulation of free protochlorophyllide leads to lethal photo-oxidative damage and bleaching (Huq et al., 2004). As PCH1 and PCHL are positive regulators in phyB-mediated light signaling, we also examined their cotyledon greening and chlorophyll biosynthesis phenotypes. Interestingly, 4-day etiolated PCH1OE and PCHLOE seedlings showed delayed cotyledon greening, whereas pch1, pchl, and pch1 pchl double mutants showed enhanced greening phenotype (Supplemental Figure 5A and 5B). We also compared this greening phenotype in transgenic seedlings overexpressing PCH1 and PCHL in phyA and phyB mutant backgrounds. As shown in Supplemental Figure 6A and 6B, the delayed greening phenotype is partially rescued by phyA and phyB mutations. In other words, this delayed greening of PCH1OE and PCHLOE plants is phyA and/or phyB dependent. On the other hand, we also checked the greening phenotype in pch1 and pchl mutants in pif1 mutant background. The results showed that the enhanced greening phenotype of pch mutants are eliminated by pif1 mutation (Supplemental Figure 6A and 6B), meaning that pif1 is also epistatic to pch in regulating cotyledon greening. To determine whether PCH1/LOE and mutants have altered levels of Pchlide, we performed spectrofluorometric analyses of acetone extracts of 4-day-old dark-grown seedlings of each genotype. The results show that PCH1OE and PCHLOE seedlings have higher relative fluorescence peaks at 632 nm, indicative of Pchlide, and pch1, pchl, and pch1 pchl mutants have lower relative fluorescence compared with wild-type seedlings (Supplemental Figure 7). These data suggest that the delayed cotyledon greening phenotypes of overexpression lines might result from photobleaching.
To examine whether PCH1 and PCHL regulate the expressions of the key genes involved in the regulation of the tetrapyrrole pathway (Supplemental Figure 8), we performed quantitative RT–PCR (qRT–PCR) assays. As shown in Supplemental Figure 9A and 9B, the expressions of genes involved in the early steps of the tetrapyrrole pathway, such as HEMA1 and GLUTAMATE-1-SEMIALDEHYDE 2 (GSA2), were significantly upregulated in PCH1OE and PCHLOE seedlings and downregulated in pch1, pchl, and pch1 pchl mutants. However, the expression of PROTOCHLOROPHYLLIDE OXIDOREDUCTASE A, B, and C (PORA, PORB, PORC), which regulate the later steps of the pathway, is downregulated in the overexpression lines and slightly upregulated in the mutants (Supplemental Figure 9B).
To further understand whether the delayed cotyledon greening phenotype in PCH1OE and PCHLOE seedlings is caused by photobleaching, we tested several antioxidant-related genes in these backgrounds. COPPER/ZINC SUPEROXIDE DISMUTASE 2 (CSD2) and Fe SUPEROXIDE DISMUTASE 1 (FSD1) are genes encoding superoxide dismutases; STROMAL ASCORBATE PEROXIDASE (SAPX) and CATIONIC AMINO ACID TRANSPORTER 2 (CAT2) encode enzymes catalyzing the degradation of H2O2 (Mittler, 2002). As a result, all these genes showed higher expression in the dark and also after white light treatment in dark-grown PCH1OE and PCHLOE seedlings except for FSD1. FSD1 had lower expression in the dark but higher expression after light treatment in the overexpression seedlings. The expressions of these genes were similar or slightly lower in pch1 and pchl mutants compared with wild type (Supplemental Figure 10). Taken together, these results suggest that PCH1 and PCHL are subtle regulators that control a small set of key genes involved in the early steps of chlorophyll biosynthetic and antioxidative pathways.
PCH1 and PCHL Promote Degradation of PIF1 and Negatively Regulate Expression of PIF1 Target Genes
Because light-induced seed germination is mainly regulated by PIF1 (Oh et al., 2004), and PCH1 and PCHL positively regulate the seed germination (Figure 1A), we examined the native PIF1 levels in the wild-type, PCH1OE and PCHLOE, pch1, pchl, and pch1 pchl double-mutant seeds under dark and red light pulse (Rp) treatment. The results show that the light-induced degradation of native PIF1 is strongly enhanced in PCH1OE and PCHLOE seeds (Figure 2E and 2F), where much weaker PIF1 band was detected in PCH1/LOE after Rp treatment compared with wild type. Conversely, this degradation was reduced in pch1, pchl, and pch1 pchl double mutants after Rp treatment, where a stronger PIF1 band was observed compared with wild-type seeds (Figure 2A and 2B). PIF1 mRNA levels remain unchanged under the same conditions in these genotypes (Supplemental Figure 11). To test whether the reduction in PIF1 is phyB dependent or phyB independent, we also examined the PIF1 protein levels in PCH1/LOE and pch1 pchl in phyB-9 mutant backgrounds. The results show that the PIF1 levels were partly restored to the wild-type levels in the PCH1OE/phyB-9 and PCHLOE/phyB-9 (Figure 2E and 2F). On the other hand, the reduced degradation of PIF1 in pch1 pchl double mutant remained similar in phyB-9 mutant background (Figure 2C and 2D). These data suggest that PCH1 and PCHL regulate PIF1 degradation in part in a phyB-dependent manner.
Figure 2. PCH1 and PCHL Promote the Degradation of PIF1.
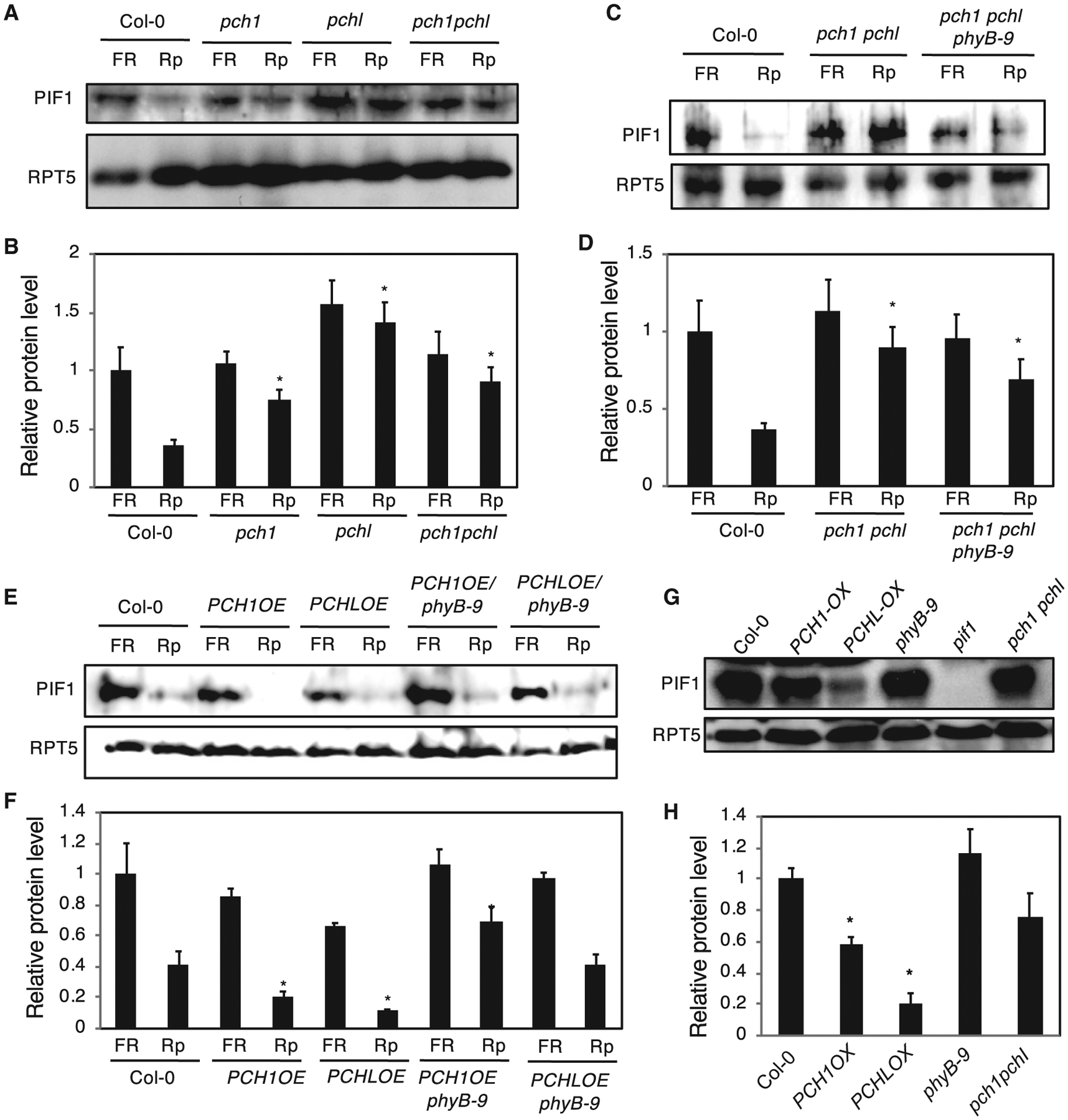
(A and C) Immunoblot shows that the light-induced degradation of native PIF1 is reduced in pch1, pchl, and pch1 pchl double mutants compared with wild-type seeds under far-red (FR) and red pulse (Rp) light treatment (A), and this effect remained similar in phyB-9 mutant background (C).
(E) Immunoblot shows that the light-induced degradation of native PIF1 is increased in PCH1 and PCHL overexpression lines compared with wild-type seeds under FR and Rp light treatment, but this effect is diminished in phyB-9 mutant background. Seeds (0.02 g) of all genotypes were surface sterilized within 1 h of imbibition and plated on MS plates (0.1 g/1 MS salt) with filter paper, exposed to 5 min of FR light (3.2 μmol m−2 s−1) or Rp light (20 μmol m−2 s−1), and these plates were kept in the dark for 24 h before harvesting.
(G) Immunoblot shows that the PCH1 and PCHL overexpression induces the degradation of PIF1 compared with wild type in etiolated seedlings under true dark conditions. Seeds of all genotypes were surface sterilized, plated on MS medium, and stratified at 4°C for 4 days. The plates were then exposed to white light for 3 h to induce the germination and then kept in the dark for 21 h at 22°C. The plates were then exposed to saturated FR light (FRp, 20 μmol m−2 s−1) for 5 min, wrapped in aluminum foil, and kept in darkness at 22°C for additional 3 days before being harvested for protein extraction. Total protein was extracted from all the samples and separated on a 10% SDS–PAGE gel, transferred to PVDF membrane, and probed with anti-PIF1 and anti-RPT5 antibodies.
(B, D, F, and H) Quantification of PIF1 levels according to (A), (C), (E), and (G) using western blot results from three independent experiments. RPT5 blots were used for normalization. The error bars indicate SEM (n = 4 biological repeats). *P < 0.01 by Student’s t-test; asterisk indicates significant difference of each genotype compared with Col-0 in each condition.
To confirm whether the PCH1/PCHL can induce degradation of PIF1 in a phyB-independent manner, we examined PIF1 levels in etiolated seedlings grown in true dark conditions, where phyB remains in inactive Pr form. The PIF1 level was still lower in the PCH1/LOE background compared with wild type (Figure 2G and 2H), suggesting that PCH1 and PCHL also regulate PIF1 abundance in darkness in a phyB-independent manner.
PCHs Interact with PIF1 Independent of phyB and Facilitate phyB–PIF1 Interaction In Vivo
Because PCH1 and PCHL interact with phyB and phyB interacts with PIF1, we tested whether PCH1 and PCHL also interact with PIF1. Yeast two-hybrid assays show that PCH1 and PCHL robustly interact with four major PIFs (Supplemental Figure 12A and 12B). phyB interacts with the N-terminal 150 amino acids containing the active phytochrome-binding (APB) domain of PIF1 (Shen et al., 2008). Domain-mapping analyses show that PCH1 might interact with the N-terminal APB-APA domain of PIF1 while PCHL might interact with the C-terminal 328 amino acids containing the bHLH domain of PIF1 (Supplemental Figure 13B and 13C). Conversely, the N-terminal 118-amino-acid fragment of PCH1 might interact with PIF1 while the C-terminal (184–279 amino acids) fragment of PCHL might interact with PIF1 (Supplemental Figure 13D–13F). However, these preliminary domain-mapping results need further verification.
To independently verify the physical interactions between PCH proteins and PIF1, we performed pull-down assays to test the interaction between the full-length PIF1 and PCH1/PCHL. In vitro pull-down assays show that glutathione S-transferase (GST)-PIF1 could be co-immunoprecipitated by PCH1-His, and His-PIF1 could be co-immunoprecipitated by GST-PCHL (Supplemental Figure 14A and 14B). These data suggest that PCH1 and PCHL physically interact with PIF1 in vitro.
To further demonstrate that PCH proteins interact with PIF1 in vivo, we performed co-immunoprecipitation (Co-IP) assays using protein extracts from PCH1OE and PCHLOE transgenic seedlings. HA-YFP-PCH1 and HA-YFP-PCHL were immunoprecipitated and PIF1 was detected using anti-PIF1 antibody. The results show that YFP-PCH1/PCHL could co-immunoprecipitate PIF1 under both dark and red light conditions (Figure 3A). Moreover, this interaction between PCH1/PCHL and PIF1 is phyA independent and/or phyB independent, as both PCH1 and PCHL also interact with PIF1 in phyB-9 and phyA-211 phyB-9 mutant backgrounds (Figure 3A and 3B).
Figure 3. PCH1 and PCHL Interact with PIF1 Independent of phyB and Facilitate phyB–PIF1 Interaction In Vivo.
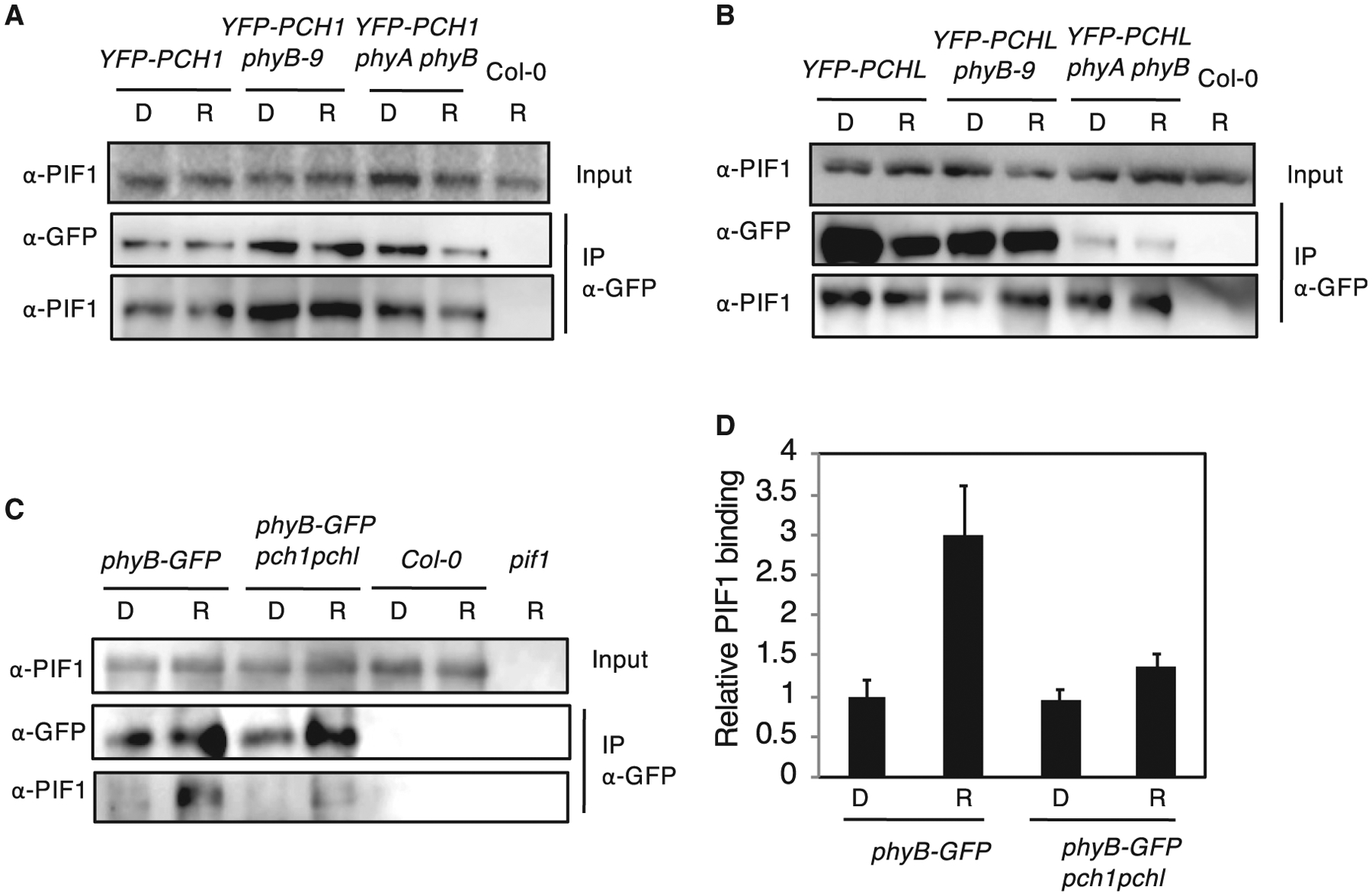
(A and B) PIF1 co-purified with PCH1 and PCHL from native plant extracts. Four-day-old dark-grown seedlings overexpressing HA-YFP-PCH1 (YFP-PCH1, A) or HAYFP-PCHL (YFP-PCHL, B) in Col-0 wild-type, phyB-9, and phyA-211 phyB-9 double-mutant backgrounds were either treated with red light (R, 7 μmol m−2 s−1) for 10 min or kept in darkness (D).
(C) PIF1 co-purified with phyB from native plant extracts. Four-day-old dark-grown seedlings overexpressing phyB-GFP in both Col-0 wild-type and pch1 pchl double-mutant background were either treated with red light (R, 7 μmol m−2 s−1) for 10 min or kept in darkness (D). Total protein extracts were used for Co-IP assays. Immunoprecipitation (IP) was performed using mouse α-GFP antibody. Rabbit α-PIF1 and rabbit α-GFP antibodies were used to detect endogenous PIF1 and YFP-tagged PCH1/PCHL or GFP-tagged phyB, respectively.
(D) Bar graph shows the quantitative results of the western blots shown in (C). The error bars indicate SEM. Relative levels of PIF1 proteins immunoprecipitated by phyB were normalized with the amount of phyB immunoprecipitated in the same experiments.
Because phyB interacts with PCH1 and PCHL as well as PIF1, we further tested whether phyB requires PCH1 and PCHL to interact with PIF1 in vivo. We performed Co-IP assays with phyB-GFP transgenic seedlings in both wild-type and pch1 pchl double-mutant backgrounds and detected native PIF1. As shown in Figure 3C and 3D, phyB-GFP interacts with PIF1 in the wild type but not efficiently in the pch1 pchl double-mutant background, indicating that phyB-GFP interacts with PIF1 preferentially in the presence of PCH1 and PCHL. Taken together, these data suggest that PCH1 and PCHL interact with PIF1 and might function in facilitating the phyB–PIF1 interaction in vivo.
PCH1 and PCHL Impair the DNA-Binding and Transcriptional Activity of PIF1
To determine whether PCH1 and PCHL can block the DNA-binding ability of PIF1, we performed an in vitro DNA-immunoprecipitation assay using the DNA fragment of PIF1-binding region in PIL1 promoter. One microgram of biotin-labeled DNA was used for binding assay with GST-tagged recombinant PIF1 protein with or without PCH1 and PCHL. The results show that when increasing amount of PCH1 and PCHL were added, a reduced level of PIF1 was immunoprecipitated by PIL1 promoter fragment compared with without PCH1 and PCHL. This means that PCH1/PCHL prevents the binding of PIF1 to the PIL1 G-box fragment (Figure 4A). We also tested whether PCH1 and PCHL affect the DNA-binding ability of PIF1 in vivo by using chromatin immunoprecipitation (ChIP) assays in wild-type, PCH1OE, and pch1 pchl double-mutant seeds. The results show that PIF1 binding to the G-box region of RGA was decreased in PCH1OE but increased in pch1 pchl double mutant compared with wild type (Figure 4B). To further determine whether PCH1 and PCHL inhibit the transcriptional activation activity of PIF1, we performed in vivo transient transcription assays as described previously (Moon et al., 2008; Shi et al., 2013; Zhu et al., 2016). pPIL1:LUC and 35S:PIF1-HA were co-transformed with either GFP only, PCH1-GFP, or PCHL-GFP driven by the constitutively active 35S promoter into tobacco leaves. Renilla luciferase (LUC) driven by 35S promoter was used as an internal control. The results show that PIF1 activates pPIL1 driving LUC expression (Figure 4C); however, the addition of both and PCHL-GFP reduced the level of LUC activity, suggesting that PCH1/PCHL blocks PCH1-GFP PIF1 transcription activation from the PIL1 promoter. Together, our data suggest that PCH1/PCHL impaired the DNA-binding and transcriptional activation activity of PIF1.
Figure 4. PCH1 and PCHL Impaire the DNA-Binding and Transcriptional Activity of PIF1.
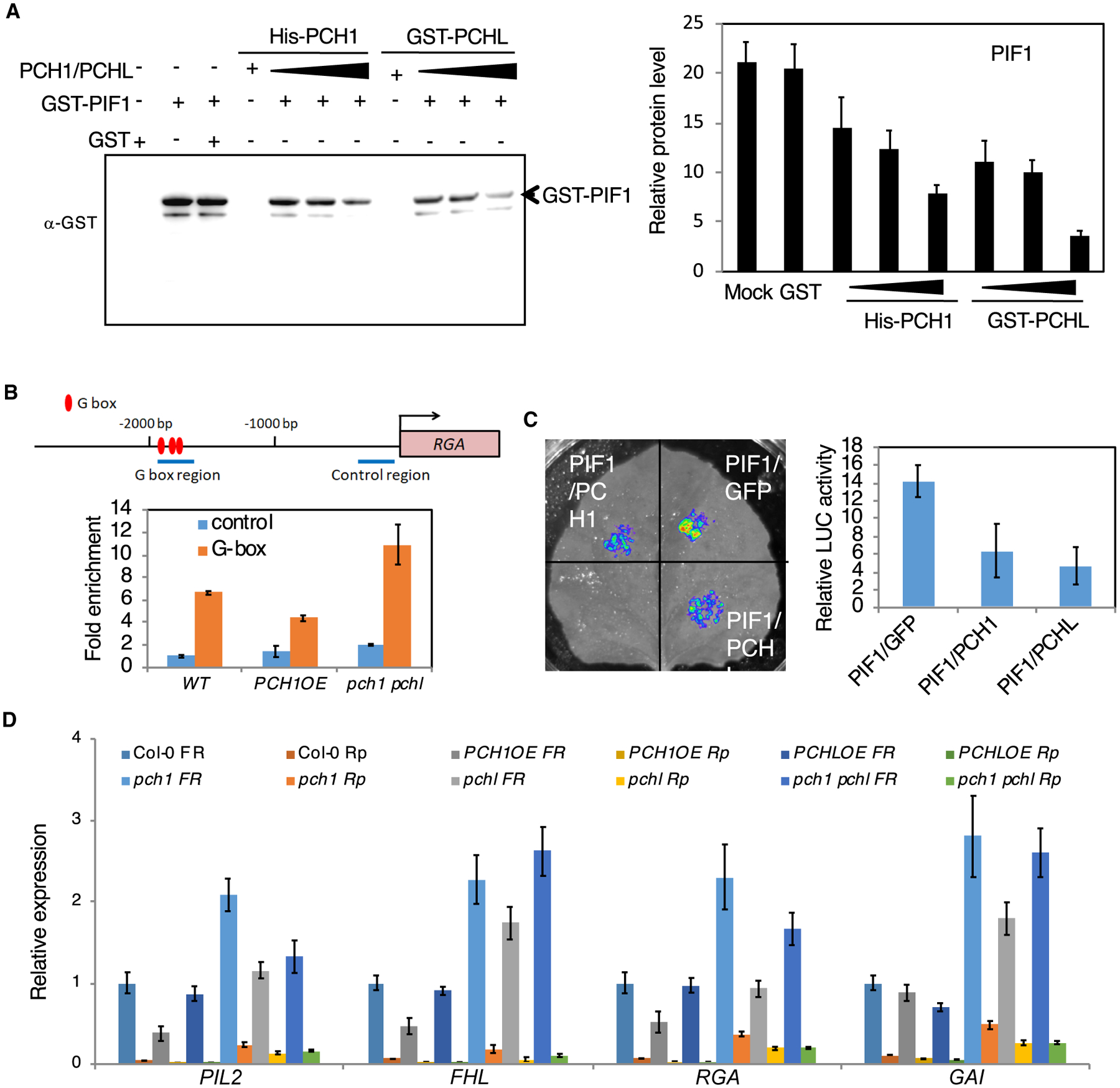
(A) DNA-IP assay. Left: PIF1 immunoprecipitated by PIL1 promoter fragment is gradually reduced when adding increasing amounts of PCH1 and PCHL. Three biological repeats were performed with similar results. Right: quantitative results of the left figure. Error bars represent SE (n = 5 biological repeats).
(B) Chromatin immunoprecipitation (ChIP) assays show PIF1 binding to the G-box motif of PIF1 target promoters. The ChIP assay was performed using wild-type, PCH1OE, and pch1 pchl mutant seeds exposed to 5 min of far-red light (3.2 μmol m−2s−1) and kept in the dark for 6 h as described by Oh et al. (2007). Anti-PIF1 antibodies were used to immunoprecipitate native PIF1 and associated DNA fragment. DNA was amplified using primers specific to the G-box fragments or control regions in RGA promoters as indicated by the arrows in the promoter structure above as designed by Oh et al. (2007). Error bars represent SE (n = 3 biological repeats).
(C) Transient transcriptional activity assay. Four-week-old tobacco plants were transiently transformed with the different combinations of constructs indicated below. Relative expression of LUC activity was observed and measured. The data were normalized by protein concentration. Error bars represent SE (n = 6 biological repeats).
(D) The expression of PIF1 target genes is decreased in PCH1 and PCHL overexpression lines and increased in pch1 and pchl mutants compared with wild-type seeds under far-red (FR) and red pulse (Rp) light. Bar graph shows expression of various PIF1 target genes in the indicated genotypes in far-red and red light. Error bars indicate SEM (n = 3 biological repeats).
To examine whether the PIF1 target gene expression is altered in these backgrounds, we performed qRT–PCR analyses of a few PIF1 target genes at the seed stage. The results show that the expression of the PIF1 target genes both under far-red light and red light is decreased in PCH1OE and PCHLOE seeds and increased in mutants compared with wild-type seeds, consistent with the PIF1 level in these backgrounds (Figure 4D). These data suggest that PCH1 and PCHL inhibit the expression of PIF1 target gene, possibly by reducing the DNA-binding and transcriptional activation activity of PIF1.
PCH1 and PCHL Are Unstable in the Dark, and This Degradation Is Mediated by COP1
PCH1 has been shown to be involved in photoperiodic control of hypocotyl length (Huang et al., 2016). To examine whether the protein stability of PCH1 and PCHL is regulated under dark and light conditions, we grew PCH1OE and PCHLOE seedlings in darkness for 4 days and transferred them to light over time, or exposed dark-grown seedlings to light for 4 h and transferred them back to darkness over time. Immunoblot analysis using anti-GFP antibody showed that PCH1 and PCHL proteins were increased after exposure to light for 1–24 h. Conversely, PCH1 and PCHL proteins became unstable when light-exposed plants were returned to darkness (Figure 5A–5C). To examine whether the 26S proteasome-dependent proteolysis is involved in the degradation of YFP-PCH1/PCHL under dark, we treated dark-grown seedlings with bortezomib, a proteasome inhibitor, and performed immunoblot analysis. The results show that both the YFP-PCH1 and YFP-PCHL fusion proteins remained abundant in darkness when treated with bortezomib (Supplemental Figure 15A and 15B). Although the expression of PCH1and PCHL transgenes was minimally affected under these conditions (Supplemental Figure 15C), the corresponding proteins were unstable and might have been degraded by the 26S proteasome pathway.
Figure 5. PCH1 and PCHL Are Unstable in the Dark and Their Degradation Is Mediated by COP1.
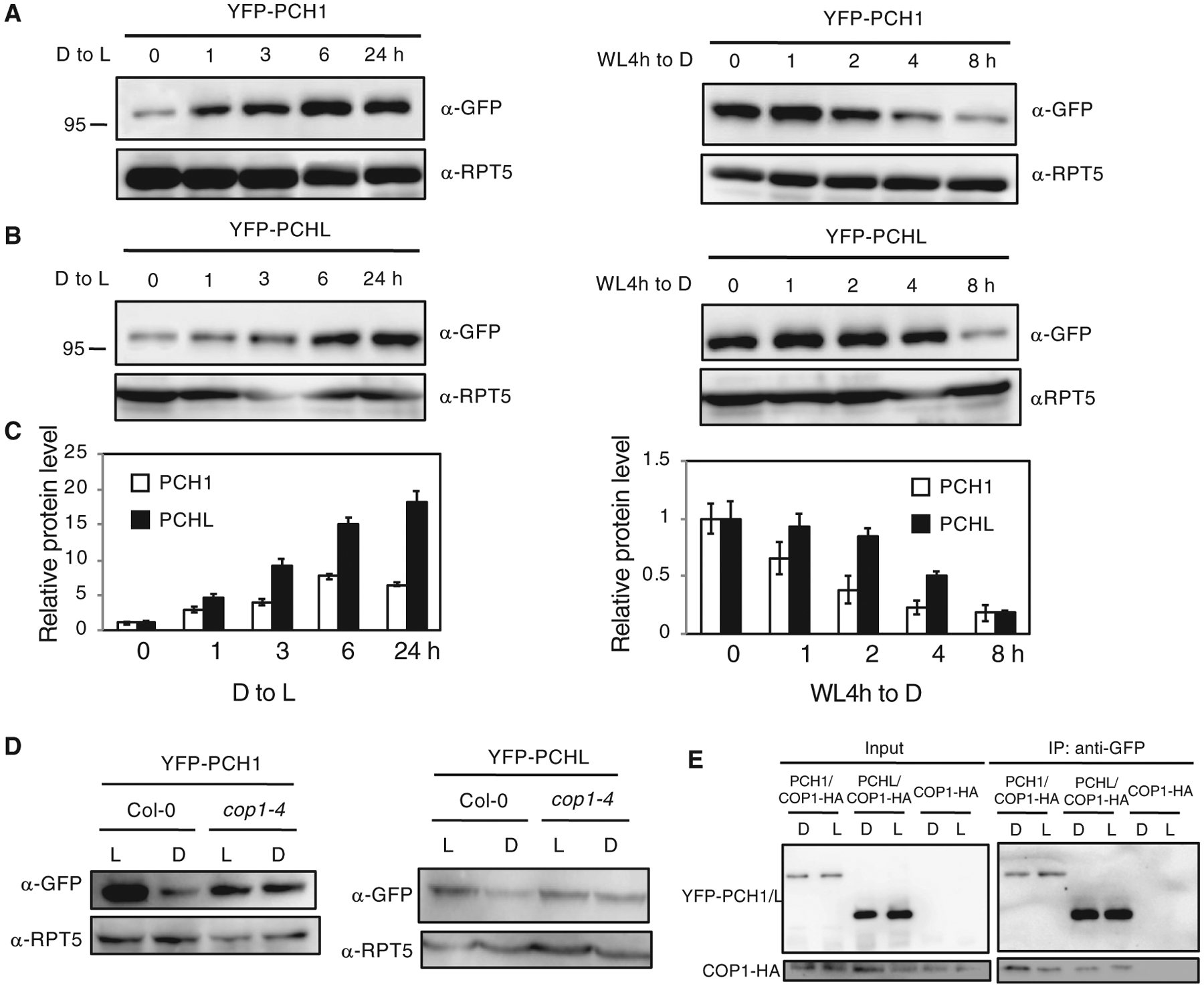
(A and B) Western blot analyses of PCH1 (A) and PCHL (B) protein (by GFP antibody) in PCH1OE and PCHLOE plants. The plants grown on MS were first illuminated for 4 h to induce germination. Four-day-old dark-grown seedlings were exposed to light for up to 24 h (both left panels) or first incubated in the light for 4 h and then left in darkness up to 8 h (both right panels). RPT5 was detected as loading control. Experiments were repeated three times with the same results, and a representative experiment is shown.
(C) Bar graph shows quantitative data from the western blots shown above. Error bars indicate SEM (n = 3 biological repeats).
(D) Western blots show that PCH1 (left) and PCHL (right) are degraded slower in cop1–4 mutants compared with wild-type background. Four-day-old dark-grown seedlings were illuminated for 4 h (L) or first illuminated for 4 h and then incubated in the dark for another 4 h (D).
(E)YFP-PCH1/PCHL interacts with COP1-HA in vivo. The input and pellet fractions are indicated. Co-IP was carried out using the anti-GFP antibody and then probed with anti-GFP and anti-HA antibodies.
Because COP1 is known to target various light-signaling components in darkness, we hypothesized that COP1 might be involved in regulating PCH1 and PCHL levels under dark conditions. To support this hypothesis, we crossed PCH1OE and PCHLOE with cop1–4 mutants and detected the PCH1 and PCHL protein levels in these backgrounds. Both PCH1 and PCHL proteins were more abundant in cop1–4 mutant background compared with controls under dark conditions (Figure 5D), suggesting that COP1 might mediate PCH1 and PCHL degradation in darkness.
To further test whether COP1 could interact with PCH1 and PCHL, we performed yeast two-hybrid analysis. Our results show that the PCH1 and PCHL full-length proteins robustly interact with COP1 (Supplemental Figure 16A). Domain-mapping analyses show that PCH1 has the highest interaction activity with the ZINC and coiled-coil domain of COP1. PCH1 and PCHL also have similar and relatively high interaction with the WD40 repeat domain (Gb) of COP1 and COP1 without the ZINC and coiled-coil domains (Supplemental Figure 16A). Conversely, the N-terminal 118-amino-acid fragment of PCH1 is both necessary and sufficient for the interaction with full-length COP1 (Supplemental Figure 16B). On the other hand, the C-terminal fragment of PCHL displayed a strong interaction with COP1 (Supplemental Figure 16B). We also performed pull-down assays to test the interaction between the full-length COP1 and PCH1/PCHL. The results show that both full-length PCH1-His and GST-PCHL could be pulled down by MBP-COP1 (Supplemental Figure 17A and 17B). We also used PCH1OE and PCHLOE in COP1-HA backgrounds to perform in vivo Co-IP assays. Figure 5E shows that COP1-HA robustly co-immunoprecipitates YFP-PCH1 and YFP-PCHL. Taken together, the yeast two-hybrid and in vitro/vivo Co-IP assays provide strong evidence that PCH1 and PCHL can associate with COP1.
COP1 has been previously reported to be the E3 ligase of PIF1 (Zhu et al., 2015). As PCH1 and PCHL interact with both COP1 and PIF1, and PIF1 is relatively more abundant in pch1 and pchl mutant seeds compared with wild type after Rp treatment (Figure 2), we further investigated whether PCH1 and PCHL promote PIF1 degradation by regulating the interaction between COP1 and PIF1. We performed in vitro Co-IP assays between COP1 and PIF1 in the absence and presence of increasing concentrations of PCH1 and PCHL. The results show that more PIF1 was immunoprecipitated by COP1 after adding increasing amounts of both PCH1 and PCHL (Supplemental Figure 18). These data suggest that the enhanced interaction between COP1 and PIF1 in the presence of PCHs might contribute to the enhanced degradation of PIF1 by COP1.
COP1 Mediates the Ubiquitination of Both PCH1 and PCHL
To further assess whether COP1 mediates the ubiquitination of PCH1 and PCHL, we first examined the in vivo ubiquitination levels of PCH1 and PCHL under dark and light conditions. PCH1OE and PCHLOE were grown in the dark for 4 days and the seedlings were pretreated with bortezomib for 4 h in darkness before being exposed to white light or remained in the dark for an additional 4 h. YFP-PCH1 and YFP-PCHL were immunoprecipitated from these samples and then detected with anti-GFP and anti-Ubi antibodies. The results show that the ubiquitination levels of YFP-PCH1 and YFP-PCHL are higher in darkness compared with light samples (Figure 6A and 6B), suggesting that PCH1 and PCHL degradation in the dark is polyubiquitination dependent. We also examined whether the ubiquitination of PCH1 and PCHL in the dark is affected in cop1–4 mutant. Four-day-old dark-grown seedlings overexpressing PCH1 and PCHL in both wild-type and cop1–4 mutant backgrounds were pretreated with bortezomib for 4 h in darkness and then remained in the dark for an additional 4 h. Strikingly, the level of PCH1 and PCHL ubiquitination is drastically reduced in the cop1–4 backgrounds compared with the wild type (Figure 6C).
Figure 6. COP1-Mediated PCH1/PCHL Degradation Is 26S Proteasome-Dependent.
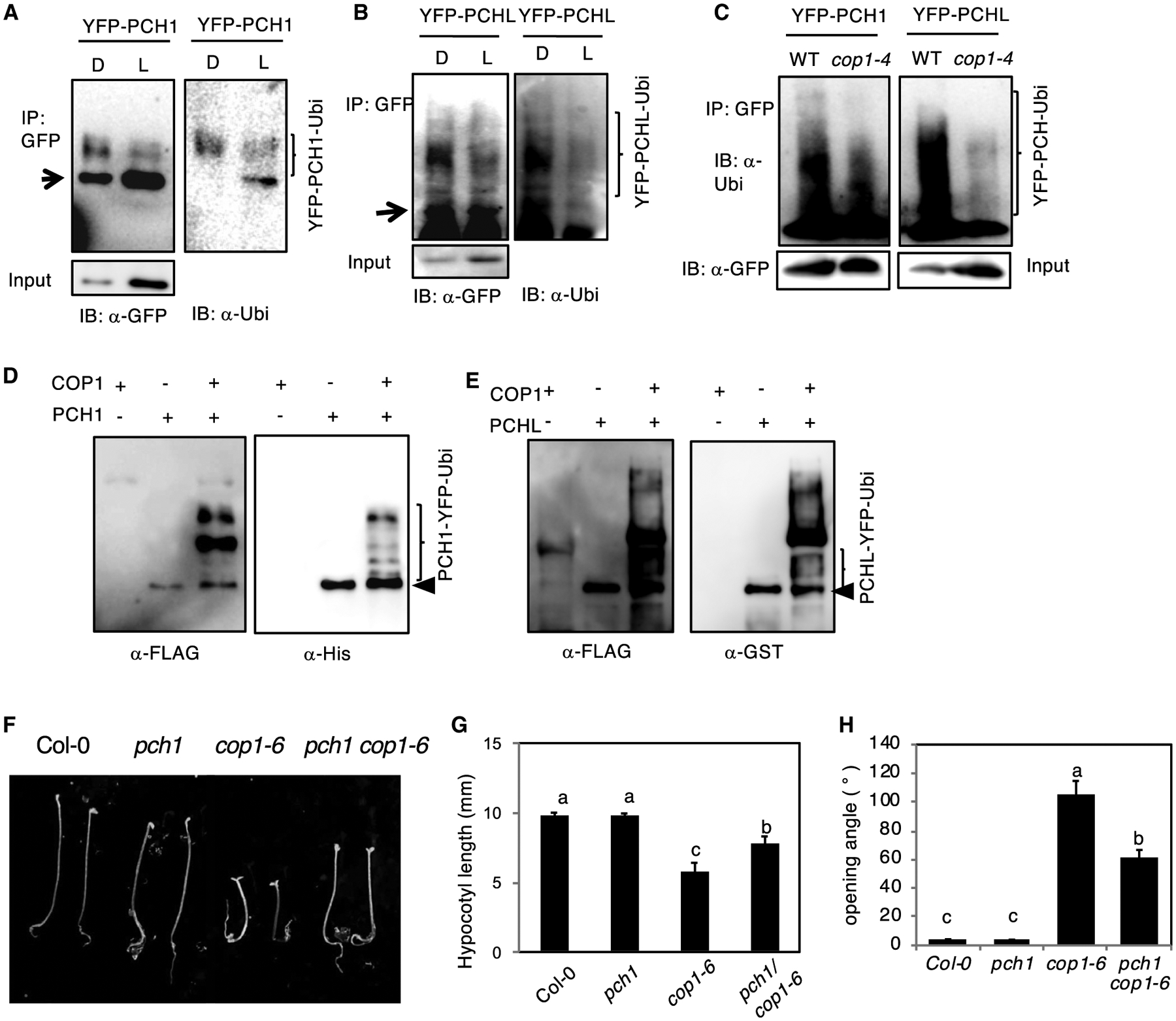
(A and B) Immunoblots show the ubiquitinated PCH1 (A) and PCHL (B) level under both dark and light.
(C) Immunoblots showing the relative ubiquitination status of YFP-PCH1 (left) and YFP-PCHL (right) in response to dark in cop1–4 mutants compared with the wild type. Total proteins were extracted from 4-day-old dark-grown seedlings and then immunoprecipitated with anti-GFP antibody (rabbit). The immunoprecipitated samples were then separated on 6.5% SDS–PAGE gels and probed with anti-GFP (mouse, left) or anti-Ubi antibodies (right). The upper smear bands are polyubiquitinated PCH1 and PCHL. Arrows indicates YFP-PCH1 and YFP-PCHL. Experiments were done three times with similar results, and a representative blot is shown.
(D and E) COP1 promotes ubiquitination of PCH1 and PCHL in vitro. An in vitro ubiquitination assay was performed for both PCH1 (D) and PCHL (E). FLAG-tagged ubiquitin was used. Left: immunoblot detected by anti-FLAG antibody. Right: immunoblot using anti-His for PCH1 and GST antibody for PCHL. Arrows indicate His-PCH1 and GST-PCHL.
(F) pch1 suppresses cop1–6 phenotype in darkness. Seedling morphology of seedlings grown under continuous darkness for 3 days. Scale bar, 5 mm.
(G and H) Bar graphs show the quantification of hypocotyl length (G) and the cotyledon opening angle (H) in (F). Error bars represent SD (n > 30 seedlings). Statistical significance among different genotypes was determined using one-way analysis of variance and Tukey’s honest significant difference tests, and is indicated by different letters.
To investigate the trans-ubiquitination activity of COP1 to PCH1 and PCHL, we performed in vitro ubiquitination assays as described previously (Saijo et al., 2003; Seo et al., 2003; Xu et al., 2014). In vitro, COP1 directly ubiquitinates PCH1 and PCHL (Figure 6D and 6E, left blots). Immunoblotting with anti-GST antibody also displayed the ubiquitinated GST-PCH1 and GST-PCHL (Figure 6D and 6E, right blots), suggesting that COP1 specifically mediates PCH1 and PCHL ubiquitination in vitro.
pch1 Partially Suppresses the Constitutive Photomorphogenic Phenotypes in cop1–6
To examine the biological significance of regulation of PCH1 and PCHL protein levels by COP1, we created pch1 cop1–6 double mutant and compared their phenotypes under dark conditions. The results show that pch1 partially suppresses the short hypocotyl and open cotyledon phenotypes of cop1–6 in darkness (Figure 6F–6H). These data, along with our biochemical evidence that COP1 directly targets PCH1 and PCHL for degradation, suggest that these two proteins are functioning positively in light-signaling pathways and that COP1 is targeting them to prevent photomorphogenesis in darkness.
DISCUSSION
In previous reports, PCH1 and PCHL were identified as phyB-interacting proteins that positively regulate phyB signaling by preventing its thermal reversion (Huang et al., 2016; Enderle et al., 2017). Recently, it was also shown that PCH1 promotes phyB photobody formation and interacts with phyB’s PASII domain to regulate light, temperature, and circadian signaling pathways (Huang et al., 2019). Here, we provide evidence that PCH1 and PCHL play a broader role in regulating light responses including seed germination, hypocotyl negative gravitropism, and chlorophyll biosynthesis not only by delaying phyB thermal reversion but also by interacting with other light-signaling components, PIFs and COP1. Several lines of evidence support this hypothesis that PCH1 and PCHL regulate these pathways by modulating either the stability and/or activity of PIF1, and possibly other PIFs, in a phyB-dependent and -independent manner. By examining the PIF1 protein levels in PCH1/LOE and pch1 and pchl mutant seeds, we provide molecular evidence that PCH1 and PCHL negatively regulate PIF1 level (Figure 2A–2H). PCH1 and PCHL directly interact with PIF1 and possibly other PIFs and might facilitate the interaction between phyB and PIF1, thereby promoting PIF1 degradation in response to light (Figure 3). Moreover, PCH1/PCHL impaired the DNA-binding and transcriptional activity of PIF1 (Figure 4A–4C). Consistently, the PIF1 target gene expressions were downregulated in PCH1/LOE and upregulated in pch1 and pchl mutants (Figure 4D). Thus, PCH1/PCHL and PIF1 are functioning antagonistically to regulate seed germination, hypocotyl negative gravitropism, and chlorophyll biosynthesis. Finally, we also demonstrate that, like other positive light-signaling regulators, PCH1 and PCHL are ubiquitinated by COP1 and are degraded through 26S proteasome pathway.
Chlorophyll and carotenoid biosynthesis are coordinately regulated in Arabidopsis in response to light to avoid photo-oxidative damage to seedlings upon illumination, and PIFs play a critical role in directly regulating both of these pathways (Moon et al., 2008; Stephenson et al., 2009; Toledo-Ortíz et al., 2010). It has been demonstrated that PIF1 and PIF3 directly and indirectly regulate the key genes to fine-tune the tetrapyrrole pathway (Moon et al., 2008; Stephenson et al., 2009). In these pathways, phyB and PIF1/PIF3 work antagonistically to regulate the greening process of seedlings. However, in our cotyledon greening analyses, PCH1 and PCHL did not promote the greening but instead led to the pale-green cotyledon phenotype, which is probably caused by photobleaching. Here, we provide molecular data that PCH1/PCHL positively regulates the expressions of HEMA1 and GSA2 gene, which are enzymes catalyzing the early stages of chlorophyll biosynthesis (Supplemental Figure 8), while PCH1/PCHL negatively regulates the expression of POR genes that catalyze the final steps of chlorophyll biosynthesis (Supplemental Figure 9). These genes are oppositely regulated by PIF1 and PIF3 (Moon et al., 2008; Stephenson et al., 2009), resulting in accumulation of free protochlorophyllide in excess due to an imbalance between the production of protochlorophyllide by HEMA1 and GSA2, and catabolism of protochlorophyllide by the POR enzymes. Consistent with our hypothesis, PCH1/PCHL shows higher expression levels of antioxidant-related genes including CSD2, FSD1, SAPX, and CAT2 compared with the wild type (Supplemental Figure 10). Because PCH1 and PCHL directly interact with PIF1 and other PIFs and regulate PIF1 abundance, it is possible that PCH1/PCHL might also prevent PIF function to fine-tune chlorophyll biosynthesis.
It is well accepted that phytochromes and PIFs have a “Yin–Yang” relationship. In response to light signal, phytochromes interact with PIFs to induce its phosphorylation, polyubiquitination, and subsequent degradation under both red and far-red light conditions (Leivar and Quail, 2011; Pham et al., 2018b). In our previous reports, we showed that CUL4COP1–SPA complex functions as a kinase-E3 ubiquitin ligase complex for the rapid light-induced degradation of PIF1 and PIF5 (Zhu et al., 2015; Pham et al., 2018b; Paik et al., 2019). Here, we show that PCH1 and PCHL promote PIF1 degradation (Figure 2) and also directly interact with PIF1 to inhibit the DNA-binding and transcriptional activation activity of PIF1 (Figures 3 and 4). Previously, other signaling components were shown to interact with PIFs through the bHLH domain and negatively regulate PIF function. For example, DELLAs and ELF3 interact with PIFs through the bHLH domain and prevent PIFs from binding to DNA (de Lucas et al., 2008; Feng et al., 2008; Nieto et al., 2014). Thus, PCH1/PCHL might regulate PIFs in multiple ways: (1) under light, PCH1/PCHL facilitates the binding of phyB to PIF1 and increases the phosphorylation and subsequent degradation of PIF1 (Figure 3C); (2) PCH1/PCHL increases the interaction of PIF1 with COP1–SPA1 complex to mediate PIF1’s polyubiquitination and subsequent degradation under both dark and light conditions (Supplemental Figure 18); and/or (3) PCH1 and PCHL interact with PIF1 to inhibit the DNA-binding and transcriptional activation activities of PIF1 (Figures 3 and 4). While the light-induced degradation of PIF1 is most likely a phyB-dependent process, the physical interaction between PCH and PIF1 along with inhibition of DNA-binding and transcriptional activation activities of PIF1 might be phyB independent.
Biochemical assays showed that PCH1 and PCHL proteins are unstable in the dark and are stabilized in response to light (Figure 5A). In 2016, Huang et al. reported that PCH1 is an evening-peaked protein and oscillates during a short-day time course. This is probably because PCH1 and PCHL are stabilized under light conditions and are degraded in the dark as observed in this study. When light is provided for up to 24 h, PCH1 and PCHL gradually accumulate regardless of the internal circadian clock (Figure 5A). Under light conditions, PCH1 and PCHL accumulate and act as stabilizers of phyB photobodies and positively regulate phyB-mediated light responses. However, in the dark PCH1 and PCHL are recognized by COP1 and are degraded through the 26S proteasome system (Figures 5 and 6). Many light-signaling components are reported as the substrates of COP1, including the bZIP transcription factor HY5 (Osterlund et al., 2000), the myb transcription factor LAF1 (Seo et al., 2003), the bHLH transcription factor HFR1 (Duek et al., 2004), and others (Lau and Deng, 2012; Xu et al., 2015). In general, COP1 interacts with its substrates through the WD40 repeats to mediate their degradation in plants (Holm and Deng, 1999; Holm et al., 2001). Our yeast two-hybrid results showed that PCH1 and PCHL do have modest interaction activity with the WD40 domain of COP1 (Supplemental Figure 16A). However, PCH1 also showed very high interaction activity with zinc-finger and coiled-coil domain only (Supplemental Figure 16A). It is possible that PCH1 has another role(s) in regulating the function of COP1.
Based on our data and those of others, we propose a model that summarizes our findings (Figure 7). In the dark, PCH1 and PCHL are associated with COP1 complex and are being degraded through the 26S proteasome pathway. However, PCH1 and PCHL also interact with PIF1 and regulate PIF1 either by inhibiting the DNA-binding and transcriptional activation activity of PIF1 and/or by inducing its degradation through the COP1–SPA complex, as this complex has been shown to degrade PIFs even in darkness (Xu et al., 2017; Pham et al., 2018a, 2018c). Under light conditions, PCH1 and PCHL facilitate phyB–PIF1 interaction and accelerate PIF1 degradation through the 26S proteasome system. Taken together, these data provide a novel mechanism by which PCH1 and PCHL fine-tune light responses under continuous light and dark and/or diurnal conditions.
Figure 7. A Proposed Model Showing PCH1- and PCHL-Mediated Regulation of Photomorphogenesis.
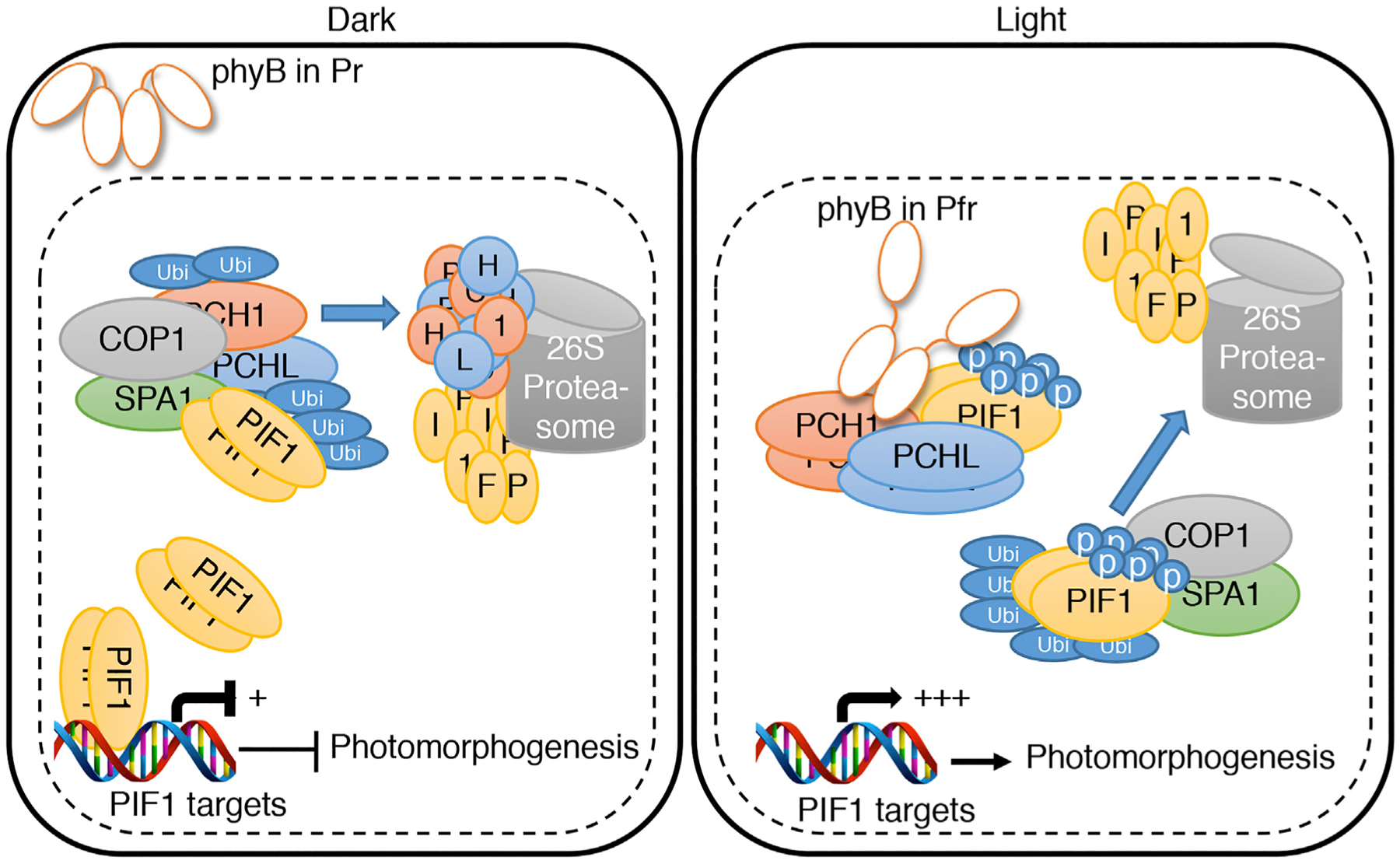
. (Left) In the dark, the biologically inactive Pr form of phytochrome is localized in the cytosol. The nuclear-localized PIF1 homodimers bind to the promoter region of light-regulated target genes and repress their expression to prevent photomorphogenesis. On the other hand, PCH1 and PCHL interact with PIF1 and negatively regulate PIF1 level and its downstream target gene expression. (Right) Upon light exposure, the biologically active Pfr form of phytochrome translocates into nucleus and interacts with PCH1 and PCHL. This interaction enables Pfr phyB to further interact with PIF1 and thus triggers the rapid light-induced phosphorylation of PIF1. The phosphorylated form of PIF1 is then recruited to the COP1–SPA1 complex for rapid ubiquitination and subsequent degradation through the 26S proteasome pathway. The destruction of the negative regulator, PIF1, derepresses the light-regulated gene expression and thereby promotes photomorphogenesis.
METHODS
Plant Materials and Growth Conditions
Arabidopsis thaliana plants were grown on Metro-Mix 200 soil (Sun Gro Hor-ticulture) (Shen et al., 2005). Light fluence rates were measured using a spectroradiometer (model EPP2000; StellarNet) as described by Shen et al. (2005). Seeds were surface sterilized and plated on Murashige and Skoog (MS) growth medium containing 0.9% agar without sucrose as described by Shen et al. (2005). After 3–4 days of moist chilling at 4°C in the dark, seeds were exposed to 3 h of white light at room temperature before placing them in the dark for another 4 days.
All A. thaliana lines used were in Columbia-0 (Col-0) background. The pch1 mutant (T-DNA insertion line; SALK_024229), pchl mutant (SALK_206946C; #N696800), pch1 pchl double mutant, phyB-9 mutant, and phyA-211 phyB-9 double mutant have been described previously (Enderle et al., 2017). The PCH1 and PCHL overexpression lines expressing HA-YFP-PCH1ox (p35S:HA-YFP-PCH1:terRbcS; plasmid pDS366) and HA-YFP-PCHLox (p35S:HA-YFP-PCHL:terRbcS; plasmid pBE52c) have also been described (Enderle et al., 2017). phyB-GFP overexpression line (p35S:PHYB-GFP) and phyB-GFP phyA-211 pch1 pchl used for Co-IP is in phyA-211 phyB-9 double-mutant background have been described previously (Enderle et al., 2017).
For PIF1 CRISPR lines, we transformed the pHEE2E-PIF1-PAM binary vector (Wang et al., 2015) into pch1, pchl, and pch1 pchl mutant plants via the floral dip method. T0 plants were screened on MS plates containing 25 mg/l hygromycin and transplanted to soil. We extracted genomic DNA from T1 plants and amplified fragments surrounding the target sites of PIF1 by PCR using PAM1-F and R primers, and the sequencing results showed an insertion of adenine (A) in pch1 or thymidine (T) in pchl and pch1 pchl backgrounds after G473 in the PIF1 open reading frame (Supplemental Figure 19), making them frameshift mutations. Homozygous lines were selected for the phenotypic assays. The CRISPR T-DNA has not yet been outcrossed in these lines.
Germination and Hypocotyl Negative Gravitropism Assay
For the phyB-dependent germination assay, triplicate sets of 60 seeds for each genotype were surface sterilized and plated on filter paper placed on agar medium (0.6% phytoagar, pH 5.7). At 1 h after the start of seed sterilization, the plated seeds were irradiated with far-red light (34 μmol m−2s−1) for 5 min. Seeds were then either directly incubated in the dark or first exposed to increasing amount of red light before being placed in the dark at 21°C. After 5 days of incubation in the dark, germinated seeds were determined by the emergence of radicles.
For hypocotyl negative gravitropism assay, surface-sterilized seeds were plated on MS agar (half-strength [1/2] MS, 0.8% phytoagar, and 0.05% 2- (N-morpholino)ethanesulfonic acid [MES] [pH 5.7]) and imbibed for 3 days at 4°C in the dark, after which germination was induced by incubation in white light (70 μmol m−2 s−1) at 22°C for 4–8 h. Plates were incubated vertically in the dark. Following 16 h in darkness at 22°C, seedlings were given a far-red light pulse (5 min, 34 mmol m−2 s−1) to inactivate phytochromes before transfer to different fluences of red light pulse, which were given at the same time each day. After 2 days of incubation, plates were turned by 90° counter-clockwise and incubated for 2 more days under the same light conditions. Growth orientations of hypocotyls were determined by the degrees from vertical axis.
Cotyledon Greening and Chlorophyll Measurement
Surface-sterilized seeds were plated on MS agar (1/2 MS, 0.8% phytoagar, and 0.05% MES [pH 5.7]) and imbibed for 3 days at 4°C in the dark before germination was induced by incubation in white light (70 μmol m−2 s−1) at 22°C for 3 h. Following 16 h in darkness at 22°C, seedlings were given a far-red light pulse (5 min, 34 μmol m−2 s−1) to inactivate phytochromes before transfer to darkness for 3 days. After dark incubation, seedlings were transferred to white light (70 μmol m−2 s−1) at 22°C. Photographs were taken after 12 h of light incubation. Measurement of chlorophyll content was performed as described previously (Runge et al., 1995). In brief, Arabidopsis seedlings were weighed and ground in liquid nitrogen. Chlorophyll was extracted from powdered samples with 80% acetone in water, and chlorophyll concentration was calculated after measuring the absorption at 663 and 645 nm.
Pchlide were extracted as described by Runge et al. (1995) except that 4-day-old dark-grown seedlings for each genotype were used. Spectro-fluorometery (TimeMaster Pro; Photon Technologies International) was performed at an excitation wavelength of 440 nm and an emission wavelength of 600–700 nm, and data were curve-fitted by using PeakFit version 4.11 (Systat Software).
Visualization of Endodermal Amyloplasts
To visualize endodermal amyloplasts by iodine staining, we fixed seedlings in FAA (5% formaldehyde, 45% ethanol, 5% acetic acid) solution for 24 h at 4°C. After fixation, seedlings were rinsed in 50% (v/v) ethanol once and stained in I2-KI solution (2% [w/v] iodine, 5% [w/v] potassium iodine, and 20% [w/v] chloral hydrate) for 1 min. Samples were destained in trichloroacetic acid/phenol/lactic acid (1:1:1 ratio) for 5 min and carefully mounted on slides with a drop of destaining solution for the light microscopic observation.
RNA Isolation, RT–PCR, and qRT–PCR Assays
Total RNA was isolated from materials indicated in the figure legends using the Sigma-Aldrich (St. Louis, MO) plant RNA isolation kit as described by Pham et al. (2018c). One microgram of total RNA was reverse transcribed using SuperScript III (Invitrogen) as described in the manufacturer’s protocol. For RT–PCR, gene-specific primers listed in Supplemental Table 1 were used to detect mRNA levels. PP2A (At1g13320) was used as a control for normalization of the expression data. The qRT–PCR assays used the Power SYBR Green qRT–PCR Reagents Kit (Applied Biosystems).
Protein Extraction and Protein Gel Blotting
A total of 50 μg of seeds was plated on wet filter paper, incubated under phyB-dependent germination assay conditions (Oh et al., 2004; Zhu et al., 2015), and harvested at the times indicated. All seed tissues were collected in the dark and ground into powder in liquid nitrogen. For individual samples, total protein was extracted in 50 μl of urea extraction buffer (0.1 M phosphate buffer [pH 6.8], 0.01 M Tris–Cl [pH 6.8], 48% urea [w/v], 1 mM phenylmethylsulfonyl fluoride [PMSF], 40 μM bortezomib, and 1× protease inhibitor cocktail [Sigma-Aldrich, cat. no/ P9599]). Samples were centrifuged at 16 000 g for 10 min at 4°C. Supernatants were filtered through filtration columns (Sigma-Aldrich, cat. no. C6866) and boiled for 3 min with 6× SDS buffer added. Thirty-microliter supernatants of individual samples were loaded on 8% SDS–PAGE gels. The total protein was blotted onto polyvinylidene fluoride (PVDF) membranes and probed with native anti-PIF1 (1:5000 dilutions) (Shen et al., 2008) and anti-RPT5 (1:1000 dilutions; Enzo Life Sciences, Farmingdale, NY, cat. no. PW8375–0100) antibodies.
Yeast Two-Hybrid Analyses
The cloning of the full-length, different truncated forms and mutant versions of PIF1 and PCH1 have been described previously (Xu et al., 2014; Enderle et al., 2017). The full-length and various truncated forms of PCHL were PCR amplified using the primers listed in Supplementary Table 1. The PCR product and the vector pJG4.5 were digested with EcoRI and XhoI restriction enzymes, then ligated to produce AD-fusion constructs. All the constructs were verified by restriction enzyme digestion and sequencing. For the yeast two-hybrid assays, different combinations of prey and bait constructs were transformed into the yeast strain EGY48–0 and selected on His,-Ura,-Trp minimal synthetic medium at 30°C for 3–4 days. The quantitative β-galactosidase assay was performed according to the manufacturer’s instructions (Matchmaker Two-Hybrid System, Clontech Laboratories). Three independent repeats were performed for the β-galactosidase assays and the average values are shown with standard deviation.
In Vitro and In Vivo Co-immunoprecipitation Assays
The in vivo Co-IP assays were performed as described by Zhu et al. (2015). In brief, 4-day-old dark-grown seedlings were pretreated with 40 μM bortezomib (LC Laboratories, Woburn, MA) for at least 4 h. Total proteins were extracted from 0.4 g of tissue with 800 ml of native extraction buffer (100 mM phosphate buffer [pH 7.8], 150 mM NaCl, 0.1% NP-40, 1× protease inhibitor cocktail [Sigma-Aldrich, cat. no. P9599], 1 mM PMSF, 40 μM bortezomib, 25 mM β-glycerophosphate, 10 mM sodium fluoride [NaF], and 2 mM Na orthovanadate). After 15 min centrifugation at 16 000 g at 4°C in darkness, supernatants were incubated with Dynabeads Protein A (Life Technologies, Carlsbad, CA, cat. no. 10002D) bound with anti-GFP antibody (Abcam, Cambridge, MA, cat. no. ab9110). Twenty microliters of Dynabeads with 1 g of antibody were used for individual samples. After 2 h of incubation in the dark at 4°C, beads were washed three times with 1 ml of extraction buffer with 0.2% NP-40. Immunoprecipitated proteins were eluted with 1× SDS loading buffer and incubated at 65°C for 5 min. Samples were loaded on an 8% SDS–PAGE gel, blotted onto PVDF membranes, and probed with antibodies.
For in vitro coIP assay, His-PCH1 and GST-PCHL were used as bait protein to pull down GST-PIF1 and His-PIF1 (Huq et al., 2004), respectively. His-PCH1 was expressed from pDEST17 vector. The full-length open reading frames of PCH1 and PCHL were PCR amplified using the primers indicated in Supplementary Table 1. The PCH1 PCR product was cloned into pENTR and then recombined into pDEST17 vector. The PCHL PCR product was digested along with pGEX4t-1 vector with EcoRI and XhoI and then ligated to produce pGEX4t-1-PCHL construct. Bacterial extracts expressing His-PCH1 and GST-PCHL were purified with amylose resin and glutathione agarose resin, respectively, in 1× PBS buffer as described in the manufacturer’s protocol. The samples were boiled and analyzed by western blot onto PVDF membrane. Anti-His antibody (Santa Cruz Biotechnology, cat. no. SC-803, 1:500 dilutions) and anti-GST antibodies were used to detect bait and prey proteins.
In Vitro DNA Pull-Down Assay Using Biotinylated DNA
One microgram of biotin-labeled DNAs was used for binding assay with GST-tagged recombinant PIF1 protein (100 ng). His-PCH1 and GST-PCHL (2×, 5×, and 10×) were used for the inhibition of PIF1 DNA binding. After 2 h of incubation at 4°C in pull-down buffer (50 mM Tris–Cl [pH 7.5], 100 mM NaCl, 2 mM dithiothreitol, 0.05% NP-40, and 1× protease inhibitor), streptavidin agarose beads (Roche, IN, USA) were added to each binding reaction and further incubated for 30 min at 4°C. Beads were washed at 4°C briefly five times using pull-down buffer and boiled in SDS loading buffer, then loaded onto SDS–PAGE gel for further western blot analysis using anti-GST antibodies.
Chromatin Immunoprecipitation Assay
For the ChIP assay, wild-type, PCH1OE, and pch1 pchl double-mutant seeds were irradiated with far-red light (3.2 μmol m−2 s−1) for 5 min, incubated in the dark for 6 h, and crosslinked in 1% formaldehyde solution under vacuum for 1 h. The seeds were then ground to powder in liquid nitrogen, and chromatin complexes were isolated and sonicated as described by Oh et al. (2007). The sonicated chromatin complexes were precipitated with anti-PIF1 antibody. The crosslinking was then reversed, and DNA was extracted using the QIAEX II gel extraction kit (Qiagen, cat. no. 20051). qRT–PCR was performed to measure the amount of DNA immunoprecipitated at the different promoter regions of binding target genes (see Figure 4B).
In Vitro Ubiquitination Assays
The His-PCH1, GST-PCHL, MBP-COP1 (Saijo et al., 2003), and E2 At-UBC8 (Lee et al., 2009) were prepared as described previously. All in vitro ubiquitination assay procedures were performed as described by Saijo et al. (2003). In brief, 2 g of FLAG-ubiquitin (U120; Boston Biochem), 25 ng of E1 (UBE1, E-305; Boston Biochem), 100 ng of E2 (At-UBC8), 600 ng of MBP-COP1, and 400 ng of His-PCH1 or GST-PCHL were used in the reaction. The FLAG-ubiquitin-conjugated PCH1, PCHL, and COP1 were detected by immunoblot with anti-FLAG antibody (F1804; Sigma-Aldrich). Anti-His-horseradish peroxidase (HRP) conjugate and anti-GST-HRP conjugate were used for His-PCH1 and GST-PCHL detection, respectively.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ms. Madeleine Nagle for technical assistance, and the Huq lab members for technical support and critical reading of the manuscript. No conflict of interest declared.
FUNDING
This work was supported by grants from the National Institutes of Health (GM-114297) and National Science Foundation (MCB-1543813) to E.H. and by a grant from the German Research Foundation (DFG) to A.H. (HI 1369/7-1), and by the DFG under Germany’s Excellence Strategy (CIBSS - EXC-2189 - Project ID 390939984).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information is available at Molecular Plant Online.
REFERENCES
- Balcerowicz M, Fittinghoff K, Wirthmueller L, Maier A, Fackendahl P, Fiene G, Koncz C, and Hoecker U (2011). Light exposure of Arabidopsis seedlings causes rapid de-stabilization as well as selective post-translational inactivation of the repressor of photomorphogenesis SPA2. Plant J. 65:712–723. [DOI] [PubMed] [Google Scholar]
- Bauer D, Viczian A, Kircher S, Nobis T, Nitschke R, Kunkel T, Panigrahi KC, Adam E, Fejes E, Schafer E, et al. (2004). Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell 16:1433–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buskirk EKV, Reddy AK, Nagatani A, and Chen M (2014). Photobody localization of phytochrome B is tightly correlated with prolonged and light-dependent inhibition of hypocotyl elongation in the dark. Plant Physiol. 165:595–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ (2013). Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol 64:403–427. [DOI] [PubMed] [Google Scholar]
- Chen HD, Huang X, Gusmaroli G, Terzaghi W, Lau OS, Yanagawa Y, Zhang Y, Li JG, Lee JH, Zhu DM, et al. (2010). Arabidopsis CULLIN4-damaged DNA binding protein 1 interacts with CONSTITUTIVELY PHOTOMORPHOGENIC1-SUPPRESSOR OF PHYA complexes to regulate photomorphogenesis and flowering time. Plant Cell 22:108–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lucas M, Davière JM, Rodríguez-Falcón M, Pontin M, Iglesias-Pedraz JM, Lorrain S, Fankhauser C, Blaezquez MA, Titarenko E, and Prat S (2008). A molecular framework for light and gibberellin control of cell elongation. Nature 451:480–484. [DOI] [PubMed] [Google Scholar]
- Deng X-W, Matsui M, Wei N, Wagner D, Chu AM, Feldman KA, and Quail PH (1992). COP1, an arabidopsis regulatory gene, encodes a protein with both a zinc-binding motif and a Gb homologous domain. Cell 71:791–801. [DOI] [PubMed] [Google Scholar]
- Duek PD, Elmer MV, van Oosten VR, and Fankhauser C (2004). The degradation of HFR1, a putative bHLH class transcription factor involved in light signaling, is regulated by phosphorylation and requires COP1. Curr. Biol 14:2296–2301. [DOI] [PubMed] [Google Scholar]
- Enderle B, Sheerin DJ, Paik I, Kathare PK, Schwenk P, Klose C, Ulbrich MH, Huq E, and Hiltbrunner A (2017). PCH1 and PCHL promote photomorphogenesis in plants by controlling phytochrome B dark reversion. Nat. Commun 8:2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng SH, Martinez C, Gusmaroli G, Wang Y, Zhou JL, Wang F, Chen LY, Yu L, Iglesias-Pedraz JM, Kircher S, et al. (2008). Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451:475–U479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoecker U (2017). The activities of the E3 ubiquitin ligase COP1/SPA, a key repressor in light signaling. Curr. Opin. Plant Biol 37:63–69. [DOI] [PubMed] [Google Scholar]
- Holm M, and Deng XW (1999). Structural organization and interactions of COP1, a light-regulated developmental switch. Plant Mol. Biol 41:151–158. [DOI] [PubMed] [Google Scholar]
- Holm M, Hardtke CS, Gaudet R, and Deng XW (2001). Identification of a structural motif that confers specific interaction with the WD40 repeat domain of Arabidopsis COP1. EMBO J. 20:118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, McLoughlin KE, Sorkin ML, Burgie ES, Bindbeutel RK, Vierstra RD, and Nusinow DA (2019). PCH1 regulates light, temperature, and circadian signaling as a structural component of phytochrome B-photobodies in Arabidopsis. Proc. Natl. Acad. Sci. U S A 116:8603–8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Yoo CY, Bindbeutel R, Goldsworthy J, Tielking A, Alvarez S, Naldrett MJ, Evans BS, Chen M, and Nusinow DA (2016). PCH1 integrates circadian and light-signaling pathways to control photoperiod-responsive growth in Arabidopsis. Elife 5:e13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq E, Al-Sady B, Hudson M, Kim C, Apel K, and Quail PH (2004). Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science 305:1937–1941. [DOI] [PubMed] [Google Scholar]
- Huq E, and Quail PH (2005). Phytochrome signaling. In Handbook of Photosensory Receptors, Briggs WR and Spudich JL, eds. (Weinheim, Germany: Wiley-VCH; ), pp. 151–170. [Google Scholar]
- Jang IC, Yang JY, Seo HS, and Chua NH (2005). HFR1 is targeted by COP1 E3 ligase for post-translational proteolysis during phytochrome A signaling. Genes Dev. 19:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J-H, Domijan M, Klose C, Biswas S, Ezer D, Gao M, Khattak AK, Box MS, Charoensawan V, Cortijo S, et al. (2016). Phytochromes function as thermosensors in Arabidopsis. Science 354:886–889. [DOI] [PubMed] [Google Scholar]
- Kim K, Shin J, Lee SH, Kweon HS, Maloof JN, and Choi G (2011). Phytochromes inhibit hypocotyl negative gravitropism by regulating the development of endodermal amyloplasts through phytochrome-interacting factors. Proc. Natl. Acad. Sci. U S A 108:1729–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose C, Nagy F, and Schäfer E (2020). Thermal reversion of plant phytochromes. Mol. Plant 13:386–397. [DOI] [PubMed] [Google Scholar]
- Klose C, Viczián A, Kircher S, Schäfer E, and Nagy F (2015). Molecular mechanisms for mediating light-dependent nucleo/ cytoplasmic partitioning of phytochrome photoreceptors. New Phytol. 206:965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau OS, and Deng XW (2012). The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci. 17:584–593. [DOI] [PubMed] [Google Scholar]
- Laubinger S, Fittinghoff K, and Hoecker U (2004). The SPA quartet: a family of WD-repeat proteins with a central role in suppression of photomorphogenesis in Arabidopsis. Plant Cell 16:2293–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Cho SK, Son O, Xu Z, Hwang I, and Kim WT (2009). Drought stress-induced Rma1H1, a RING membrane-anchor E3 ubiquitin ligase homolog, regulates aquaporin levels via ubiquitination in transgenic Arabidopsis plants. Plant Cell 21:622–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legris M, Ince YÇ, and Fankhauser C (2019). Molecular mechanisms underlying phytochrome-controlled morphogenesis in plants. Nat. Commun 10:5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legris M, Klose C, Burgie ES, Costigliolo C, Neme M, Hiltbrunner A, Wigge PA, Schäfer E, Vierstra RD, and Casal JJ (2016). Phytochrome B integrates light and temperature signals in Arabidopsis. Science 354:897–900. [DOI] [PubMed] [Google Scholar]
- Leivar P, and Monte E (2014). PIFs: systems integrators in plant development. Plant Cell 26:56–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Monte E, Oka Y, Liu T, Carle C, Castillon A, Huq E, and Quail PH (2008). Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr. Biol 18:1815–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, and Quail PH (2011). PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci. 16:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Tepperman JM, Monte E, Calderon RH, Liu TL, and Quail PH (2009). Definition of early transcriptional circuitry involved in light-induced reversal of PIF-imposed repression of photomorphogenesis in young Arabidopsis seedlings. Plant Cell 21:3535–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian H-L, He S-B, Zhang Y-C, Zhu D-M, Zhang J-Y, Jia K-P, Sun S-X, Li L, and Yang H-Q (2011). Blue-light-dependent interaction of cryptochrome 1 with SPA1 defines a dynamic signaling mechanism. Genes Dev. 25:1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X-D, Zhou C-M, Xu P-B, Luo Q, Lian H-L, and Yang H-Q (2015). Red-light-dependent interaction of phyB with SPA1 promotes COP1-SPA1 dissociation and photomorphogenic development in Arabidopsis. Mol. Plant 8:467–478. [DOI] [PubMed] [Google Scholar]
- Medzihradszky M, Bindics J,Ádám É, Viczián A, Klement É, Lorrain S, Gyula P, Mérai Z, Fankhauser C, Medzihradszky KF, et al. (2013). Phosphorylation of phytochrome B inhibits light-induced signaling via accelerated dark reversion in Arabidopsis. Plant Cell 25:535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7:405–410. [DOI] [PubMed] [Google Scholar]
- Moon J, Zhu L, Shen H, and Huq E (2008). PIF1 directly and indirectly regulates chlorophyll biosynthesis to optimize the greening process in Arabidopsis. Proc. Natl. Acad. Sci. U S A 105:9433–9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto C, López-Salmerón V, Davière J-M, and Prat S (2014). ELF3-PIF4 interaction regulates plant growth independently of the evening complex. Curr. Biol 25:187–193. [DOI] [PubMed] [Google Scholar]
- Oh E, Kim J, Park E, Kim JI, Kang C, and Choi G (2004). PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell 16:3045–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Yamaguchi S, Huc J, Yusukeb J, Jung B, Paik I, Leed H-S, Sun T-P, Kamiya Y, and Choi G (2007). PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by directly binding to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell 19:1192–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Park E, Song K, Bae G, and Choi G (2019). PHYTOCHROME INTERACTING FACTOR 8 inhibits phytochrome A-mediated far-red light responses in Arabidopsis. Plant Cell 32:186–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund MT, Hardtke CS, Wei N, and Deng XW (2000). Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405:462–466. [DOI] [PubMed] [Google Scholar]
- Pacín M, Legris M, and Casal JJ (2014). Rapid decline in nuclear COSTITUTIVE PHOTOMORPHOGENESIS1 abundance anticipates the stabilization of its target ELONGATED HYPOCOTYL5 in the light. Plant Physiol. 164:1134–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik I, Chen F, Pham VN, Zhu L, Kim J-I, and Huq E (2019). A phyB-PIF1-SPA1 kinase regulatory complex promotes photomorphogenesis in Arabidopsis. Nat. Commun 10:4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik I, and Huq E (2019). Plant photoreceptors: multi-functional sensory proteins and their signaling networks. Semin. Cell Dev. Biol 92:114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham VN, Kathare PK, and Huq E (2018a). Dynamic regulation of PIF5 by COP1-SPA complex to optimize photomorphogenesis in Arabidopsis. Plant J. 96:260–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham VN, Kathare PK, and Huq E (2018b). Phytochromes and phytochrome interacting factors. Plant Physiol. 176:1025–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham VN, Xu X, and Huq E (2018c). Molecular bases for the constitutive photomorphogenic phenotypes in Arabidopsis. Development 145:dev169870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint M, Delker C, Franklin KA, Wigge PA, Halliday KJ, and van Zanten M (2016). Molecular and genetic control of plant thermomorphogenesis. Nat. Plants 2:15190. [DOI] [PubMed] [Google Scholar]
- Rockwell N, and Lagarias J (2019). Phytochrome evolution in 3D: deletion, duplication, and diversification. New Phytol. 10.1111/nph.16240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge S, van Cleve B, Lebedev N, Armstrong G, and Apel K (1995). Isolation and classification of chlorophyll-deficient xantha mutants of Arabidopsis thaliana. Planta 197:490–500. [DOI] [PubMed] [Google Scholar]
- Saijo Y, Sullivan JA, Wang H, Yang J, Shen Y, Rubio V, Ma L, Hoecker U, and Deng XW (2003). The COP1-SPA1 interaction defines a critical step in phytochrome A-mediated regulation of HY5 activity. Genes Dev. 17:2642–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HS, Yang JY, Ishikawa M, Bolle C, Ballesteros ML, and Chua NH (2003). LAF1 ubiquitination by COP1 controls photomorphogenesis and is stimulated by SPA1. Nature 423:995–999. [DOI] [PubMed] [Google Scholar]
- Sheerin DJ, Menon C, zur Oven-Krockhaus S, Enderle B, Zhu L, Johnen P, Schleifenbaum F, Stierhof Y-D, Huq E, and Hiltbrunner A (2015). Light-activated phytochrome A and B interact with members of the SPA family to promote photomorphogenesis in Arabidopsis by reorganizing the COP1/SPA complex. Plant Cell 27:189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Ling Z, Castillon A, Majee M, Downie B, and Huq E (2008). Light-induced phosphorylation and degradation of the negative regulator PHYTOCHROME INTERACTING FACTOR 1 depends upon its direct physical interactions with photoactivated phytochromes. Plant Cell 20:1586–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Moon J, and Huq E (2005). PIF1 is regulated by light-mediated degradation through the ubiquitin-26S proteasome pathway to optimize seedling photomorphogenesis in Arabidopsis. Plant J. 44:1023–1035. [DOI] [PubMed] [Google Scholar]
- Shi H, Zhong S, Mo X, Liu N, Nezames CD, and Deng XW (2013). HFR1 sequesters PIF1 to govern the transcriptional network underlying light-initiated seed germination in Arabidopsis. Plant Cell 25:3770–3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Kim K, Kang H, Zulfugarov IS, Bae G, Lee CH, Lee D, and Choi G (2009). Phytochromes promote seedling light responses by inhibiting four negatively-acting phytochrome-interacting factors. Proc. Nat. Acad. Sci. U S A 106:7660–7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson PG, Fankhauser C, and Terry MJ (2009). PIF3 is a repressor of chloroplast development. Proc. Natl. Acad. Sci. U S A 106:7654–7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian C, Kim BH, Lyssenko NN, Xu X, Johnson CH, and von Arnim AG (2004). The Arabidopsis repressor of light signaling, COP1, is regulated by nuclear exclusion: mutational analysis by bioluminescence resonance energy transfer. Proc. Natl. Acad. Sci. USA 101:6798–6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Ortíz G, Huq E, and Rodríguez-Concepción M (2010). Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by Phytochrome-Interacting Factors. Proc. Natl. Acad. Sci. U S A 107:11626–11631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z-P, Xing H-L, Dong L, Zhang H-Y, Han C-Y, Wang X-C, and Chen Q-J (2015). Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol. 16:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Kathare PK, Pham VN, Bu Q, Nguyen A, and Huq E (2017). Reciprocal proteasome-mediated degradation of PIFs and HFR1 underlies photomorphogenic development in Arabidopsis. Development 144:1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Paik I, Zhu L, Bu Q, Huang X, Deng XW, and Huq E (2014). PHYTOCHROME INTERACTING FACTOR1 enhances the E3 ligase activity of CONSTITUTIVE PHOTOMORPHOGENIC1 to synergistically repress photomorphogenesis in Arabidopsis. Plant Cell 26:1992–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Paik I, Zhu L, and Huq E (2015). Illuminating progress in phytochrome-mediated light signaling pathways. Trends Plant Sci. 20:641–650. [DOI] [PubMed] [Google Scholar]
- Zhu L, Bu Q, Xu X, Paik I, Huang X, Hoecker U, Deng XW, and Huq E (2015). CUL4 forms an E3 ligase with COP1 and SPA to promote light-induced degradation of PIF1. Nat. Commun 6:7245. [DOI] [PubMed] [Google Scholar]
- Zhu L, Xin R, Bu Q, Shen H, Dang J, and Huq E (2016). A negative feedback loop between PHYTOCHROME INTERACTING FACTORs and HECATE proteins fine tunes photomorphogenesis in Arabidopsis. Plant Cell 28:855–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z, Liu H, Liu B, Liu X, and Lin C (2011). Blue light-dependent interaction of CRY2 with SPA1 regulates COP1 activity and floral initiation in Arabidopsis. Curr. Biol 21:841–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


