Highlights
-
•
Reported cases may not reflect true epidemic growth due to limited testing capacity.
-
•
Numbers of hospitalized and severe cases may be less biased.
-
•
Case series by date of symptoms onset can eliminate the bias from testing capacity.
Keywords: COVID-19, Basic Reproduction Number, Growth rate, Models, Statistical
Abstract
Since the novel coronavirus disease (COVID-19) emerged in December 2019 in China, it has rapidly spread around the world, leading to one of the most significant pandemic events of recent history. Deriving reliable estimates of the COVID-19 epidemic growth rate is quite important to guide the timing and intensity of intervention strategies. Indeed, many studies have quantified the epidemic growth rate using time-series of reported cases during the early phase of the outbreak to estimate the basic reproduction number, R0. Using daily time series of COVID-19 incidence, we illustrate how epidemic curves of reported cases may not always reflect the true epidemic growth rate due to changes in testing rates, which could be influenced by limited diagnostic testing capacity during the early epidemic phase.
Since the novel coronavirus disease (COVID-19) emerged in December 2019 in China, it rapidly spread globally, and soon after a pandemic was declared by the World Health Organization on 11th March 2020 (WHO, 2019, WHO, 2020). During the initial growth phase of epidemics unfolding in entirely susceptible populations and in the absence of interventions or behavior changes, the rate of change in the number of new cases is proportional to the number of currently infectious individuals. As a result, the trajectory of the epidemic grows exponentially so long as the fraction of the total cases in the population being detected does not change over time as a result of temporal changes in testing rates or testing capacity. In this setting, the trajectory of the epidemic reflects the growth profile of the epidemic despite the presence of delays in test results. However, changes in testing rates could artificially distort the true growth rate, which could result when the testing capacity is ramped up during the early epidemic's growth phase. For instance, when the number of new cases per day surpasses testing capacity, the maximum number of positive test results per day will appear to be constant, leading to a cumulative incidence curve that artificially grows linearly. This issue has important implications since the scope and intensity of public health interventions are largely guided by metrics that rely on the growth rate of the epidemic, such as the reproduction number that quantifies the average number of secondary cases per case. Hence, it is key to assess up to what extent the time-series of reported cases reflects the true trajectory of the epidemic. For illustration, we analyzed daily series of COVID-19 reported laboratory confirmed cases (CFS and SEJHU, 2020) using a linear growth model as well as a non-linear growth model describing exponential growth. To explore the extended phase of the epidemic, we employed regression by kernel-window analysis with a 2-week window.
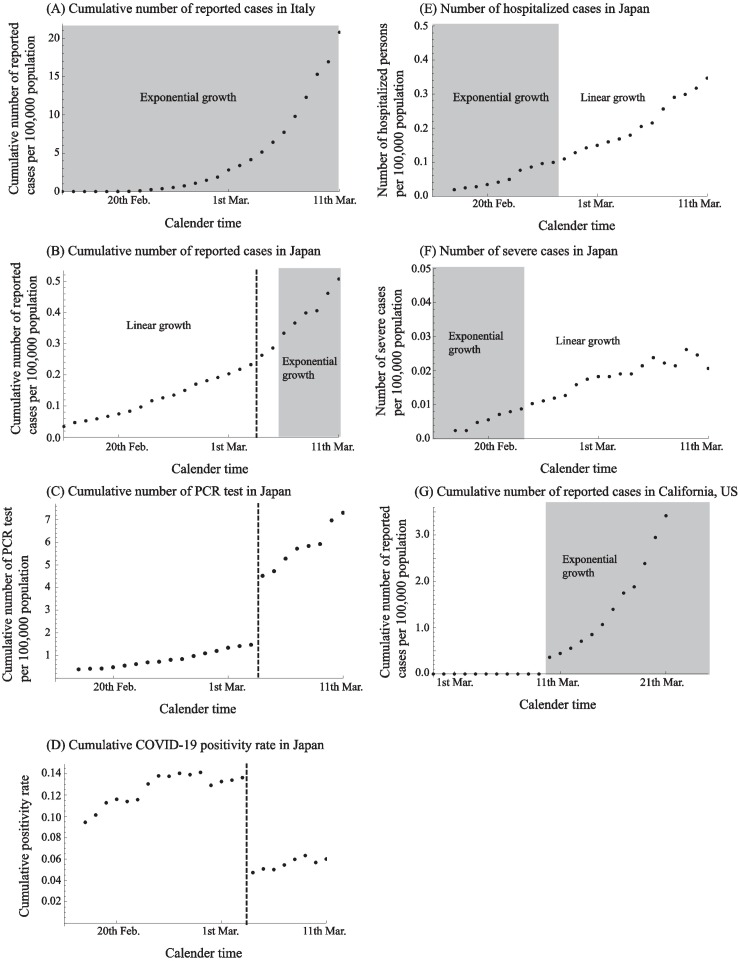
Our findings reveal an exponential growth phase for the COVID-19 epidemic in Italy during the entire study period (Fig. 1(A)), while the epidemic in Japan shows linear growth during the first few weeks. At face value, this pattern would suggest that Japan was facing a constant infection risk in spite of a growing epidemic (Fig. 1(B)). Linear growth is apparent until 5th March 2020 in Japan, a trend that suggests that the number of cases exceeded testing capacity during that period. Indeed, a drastic increase in testing rate was observed on 4th March 2020 (Fig. 1(C)), which in turn changes the cumulative positivity rate drastically (Fig. 1(D)) (MOH, 2020). Furthermore, the growth trends in the numbers of both hospitalized and severe cases (requiring intubation or admission to ICU), which may be less biased from limited testing capacity, show signs of exponential growth (Fig. 1(E) and (F)) (MOH, 2020).
Fig. 1.
Classifying the epidemic curve of novel coronavirus disease (COVID-19) into exponential or linear growth phases for different geographic areas and the time-series of the daily number of tests and the corresponding COVID-19 positivity rate in Japan. Time series data of the reported COVID-19 cases in Italy (A), Japan (B), California, US (G), hospitalized cases in Japan (E), severe cases in Japan (F). Shaded and white areas separate periods when the observed cumulative incidence curve grows exponentially or linearly, respectively. To classify the type of growth profile, linear regression and non-linear regression (exponential function) were utilized while the best model fit was selected using the Akaike Information Criteria (AIC). Time series data of cumulative number of PCR tests in Japan (C) and the cumulative COVID-19 positivity rate among tested samples (D). Dashed lines in (B), (C) and (D) denote the boundary changes the number of PCR test, between 3rd March and 4th March 2020.
Alternative explanations for the early linear growth phase other than saturated testing capacity could be considered. First, it could be the result of preferential testing for highly suspicious samples leading to a bias in the positive rate among samples. Another possibility that could be invoked is the effect of interventions, e.g., containment, on epidemic growth. Finally, transmission driven by sporadic outbreaks/clusters might lead to linear growth.
Furthermore, we also found that the trajectory of the epidemic in California, United States, started with a discontinuous increase from zero counts on 11th March, 2020, following an exponential growth (Fig. 1(G)). This abrupt increase is probably the result of testing delays, reporting delays or difficulties in identifying COVID-19 cases during the early phase of the outbreak, a pattern that is also misleading to the public.
Our results indicate that changes in testing rates, which could result from limited diagnostic testing capacity, could mask the epidemic's growth rate, which has public health implications. Specifically, the derivation of the basic reproduction number during the early phase of outbreak and the effective reproduction number during the course of the epidemic are key quantities that often rely on time-series of reported cases. Hence, biased epidemic trends can lead to incorrect inferences of metrics characterizing the transmission potential. Ideally, modelers are interested in case series by date of symptoms onset to mitigate this bias, but the date of symptoms onset becomes largely unavailable for epidemics of rapid dissemination such as COVID-19. Data on the testing strategy including testing rates as well as estimates of the reporting probability and ascertainment bias could help derive reliable inferences of epidemiological and transmission parameters (Ejima and Nishiura, 2018, Shim et al., 2020, Mizumoto and Chowell, 2020). Trends in numbers of hospitalized/severe cases may also capture the epidemic growth profile. However, linear growth was observed after a brief period of exponential growth, likely reflecting other mechanisms involved (highly variable times from symptoms onset to admission, heterogeneous severity levels in the exposed population) or other biases than testing capacity, e.g., highly specific testing among hospitalized individuals, limited hospital bed capacity. Further analysis is required to use the data.
Conflict of interest
We declare that we have no conflict of interest.
Acknowledgement
RO acknowledges support from the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 19K20393. KM acknowledges support from JSPS KAKENHI Grant Number 18K17368 and from the Leading Initiative for Excellent Young Researchers from the Ministry of Education, Culture, Sport, Science & Technology of Japan. GC acknowledges support from NSF grant 1414374 as part of the joint NSF-NIH-USDA Ecology and Evolution of Infectious Diseases Program.
References
- Center for Systems Science and Engineering Johns Hopkins University . March 2020. CSSEGISandData/COVID-19. Available from https://github.com/CSSEGISandData/COVID-19 [accessed 14.03.20] [Google Scholar]
- Ejima K., Nishiura H. Real-time quantification of the next-generation matrix and age-dependent forecasting of pandemic influenza H1N1 2009 in Japan. Ann Epidemiol. 2018;28(5):301–308. doi: 10.1016/j.annepidem.2018.02.010. [DOI] [PubMed] [Google Scholar]
- Mizumoto K., Chowell G. Estimating risk for death from 2019 novel coronavirus disease, China, January–February 2020. Emerg Infect Dis. 2020;26(6) doi: 10.3201/eid2606.200233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Documents for press release. Ministry of Health, Labour and Welfare, Japan. Available from https://www.mhlw.go.jp/stf/houdou/index.html (accessed 02.04.20) [in Japanese]
- Shim E., Tariq A., Choi W., Lee W., Chowell G. Transmission potential of COVID-19 in South Korea. Medrxiv. 2020 doi: 10.1101/2020.02.27.20028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, Coronavirus disease 2019 (COVID-19). Situation Report – 51. Available from https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10.
- World Health Organization, WHO Director-General's opening remarks at the media briefing on COVID-19-11 March 2020. Available from https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.